Abstract
Neurons in the adult visual cortex are capable of integrating signals over a large area that surrounds their classic receptive field (RF), and this ability of cortical neurons is thought to be intimately involved in perceptual binding. It is not known, however, at what age these long-range signal interactions emerge. Here, we report that qualitatively adult-like center/surround interactions are already present in the primary visual cortex as early as postnatal day 14 in macaque monkeys. However, the RF surrounds of visual area 2 (V2) neurons were largely absent until 4 weeks of age and, as late as 8 weeks of age, center/surround signal interactions in V2 neurons were immature. Our results suggest that the cortical circuits underlying the RF center/surround of individual neurons mature considerably later in V2 than in the primary visual cortex and give critical evidence for the hypothesis that the functional maturation of the primate visual brain proceeds in a hierarchical manner.
Keywords: monkey, postnatal development, primary visual cortex, long-range signal interaction, cortical circuits
Newborn infants are capable of detecting and discriminating local stimulus features such as contour orientation in visual scenes (1, 2). The cortical connections in macaque monkeys that support crude spatial vision are present in the primary visual cortex (V1) at birth or soon after birth (3, 4), and V1 neurons in neonatal monkeys are relatively well tuned to stimulus orientation, spatial frequency, and the direction of stimulus movement (5). However, the perceptual ability of infants to integrate local stimulus features over a large area (e.g., contour integration) does not emerge until relatively late in development (6, 7). The neural basis of subnormal perceptual binding in infants is not well understood.
In adult monkeys, timely integration of signals over a large area that surrounds the receptive field (RF) center is known to increase the saliency of weak stimuli in a context-dependent manner (8–10) and to mediate figure/ground segregation (11, 12). Implicit in these previous studies in infants and adults is that, in neonatal monkeys, the ability of individual neurons to integrate signals over a large area may not emerge until late in development. In this study, we examined whether individual V1 and visual area 2 (V2) neurons of infant monkeys were capable of integrating surround signals shortly after birth. We found that, as early as 14 days of age, the responses of RF centers and surrounds of V1 neurons were qualitatively adult-like, whereas V2 neurons exhibited considerable delays in the functional maturation of center/surround signal interactions.
Materials and Methods
Microelectrode recordings were made in seven anesthetized and paralyzed infant macaque monkeys (Macaca mulatta) by using our conventional methods described in ref. 5. All experimental and animal care procedures were in compliance with the Guiding Principles for Research Involving Animals and Human Beings (13) and were approved by the institutional animal care and use committee of the University of Houston.
Recorded action potentials were digitized at 25 kHz and sampled at a rate of 140 Hz (7.2-msec bin widths) and compiled into peristimulus time histograms that were equal in duration to and synchronized with the temporal cycle of the grating, by using a data acquisition system (System II, Tucker-Davis Technologies, Alachua, FL). The visual stimuli were generated on a monochrome monitor with ultrashort persistence (frame rate, 140 Hz; screen size, 800 × 600 pixels, 20° × 15° at 114 cm; mean luminance, 50 cd/m2). Cells were classified as either simple or complex (14), and responses to drifting sinusoidal gratings were measured to determine the preferred orientation and spatial frequency of each neuron.
After the center of the RF was determined by locating the position where the largest response was evoked by a 0.5° grating patch, responses were measured as a function of the diameter of the optimized circular grating patch that was positioned at the RF center (15) (Fig. 1). The measured area-response functions were fitted by using the following formula (15): R(x) = KcLc(x)/[1 + KsLs(x)]; Lc(x) = [wc × erf(x/wc)]2; Ls(x) = [ws × erf(x/ws)]2. x is the stimulus diameter, Kc and Ks are the gains of the center/surround, and Lc and Ls are the summed squared activities of the center/surround mechanisms. The spatial extents of the center/surround components are represented by wc and ws. During curve-fitting, we always constrained functions so that wc < ws.
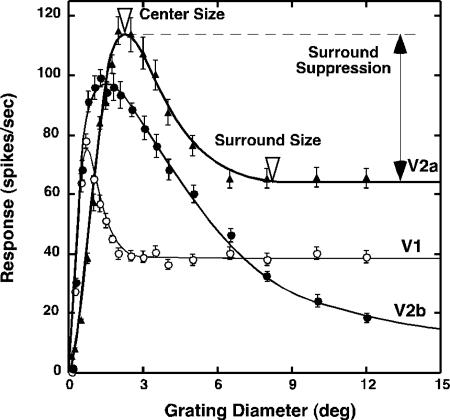
Fig. 1.
Area-summation functions of a typical V1 neuron and two V2 neurons (V2a and V2b) in an adult monkey. Center size was defined as the stimulus diameter that initiated the largest response (open triangle). Surround size was determined by measuring the stimulus diameter at which an increase in stimulus diameter did not change cell's firing rate (open triangle). Strength of surround suppression was quantified by calculating the SI, which expresses suppression as a fraction of the optimal response, by using the following equation (15): SI = (Ropt – Rsupp)/Ropt, where Ropt is the maximum response, and Rsupp is the suppressed response.
Based on the area-response function of each unit, we determined the extent and size of the RF center defined as the smallest circular grating that produced the maximum response (center size in Fig. 1). The surround stimuli in the center/surround interaction experiments were defined as an annulus with an inner diameter that did not initiate responses by itself and with an outer diameter that corresponded to the external edge of the surround, i.e., the point at which further increases in stimulus diameter did not alter response amplitudes (surround size in Fig. 1).
Results
We quantitatively investigated the center/surround organization of V1 (n = 257) and V2 (n = 344) units in seven infant and two adult monkeys. The RFs of all neurons were located within 6° of the center of the fovea.
Area-Summation Functions Are Similar in V1 and V2 of Adult Monkeys. Because we know very little about RF center/surround interactions in the V2 neurons of adult monkeys, we first compared the area-summation functions of mature V1 and V2 neurons. After optimizing the stimulus orientation/direction and spatial frequency for each neuron, we measured response amplitude as a function of the diameter of a patch of drifting sinusoidal gratings (contrast = 80%, temporal frequency = 3.1 Hz).
Area-summation functions of mature V1 neurons were similar to those reported in previous studies in anesthetized monkeys (e.g., unit V1 in Fig. 1) (15–19) and in awake, behaving monkeys (M. Sur, personal communication). Specifically, responses were low for the smallest patches but increased with stimulus diameters up to a point beyond which further increases in stimulus size resulted in suppression. The magnitude of surround suppression increased with stimulus diameters until further expansion caused no additional decreases in response amplitude. Area-summation functions of V2 neurons in mature monkeys (e.g., unit V2a in Fig. 1) were generally similar to those in V1 neurons. However, both the centers and surrounds of V2 neurons were substantially larger than those of V1, and surround suppression was generally greater in V2 neurons than in V1.
A group of V2 neurons had exceptionally large RF surrounds and very strong surround suppression (e.g., unit V2b in Fig. 1). We labeled these V2 neurons as “special” as opposed to “ordinary” (e.g., unit V2a in Fig. 1). Complete surround suppression was recently reported in some V2 units of adult monkeys by Solomon et al. (16). However, the surround sizes of these units were not nearly as large as those in our special V2 neurons. These special V2 neurons with large surrounds (e.g., surround diameters >13°) had disproportionately small centers (orange squares in Fig. 2a). In nearly all V1 units, the surround sizes were proportional to the center size (blue circles in Fig. 2a). The thick diagonal line in Fig. 2a is a regression line relating the center/surround sizes of V1 units, and the parallel dotted lines indicate the 99% confidence intervals. The majority of V2 units with measurable surrounds (57/82) fell within this confidence interval (open squares in Fig. 2a), whereas 25 units in V2 (orange squares) fell outside, indicating that these units may represent a functionally distinct group of neurons. In contrast to V1 and ordinary V2 units, the surrounds of special V2 units produced nearly complete suppression of the center responses [suppression index (SI) >0.9] except for the three units that exhibited SIs of ≈0.8 (orange squares in Fig. 2b).
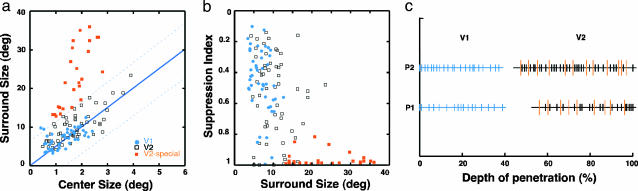
Fig. 2.
Relationships among center size, surround size, and surround suppression in adult monkeys. (a) Surround size as a function of center size for each V1 and V2 unit. For V1 units (blue circles) and ordinary V2 units (open squares), center sizes were generally proportional to surround sizes, as shown in the regression line (y = 4.985 × x, y is surround size, x is the center size, r = 0.75). Note that special V2 units with exceptionally large surround (orange squares) had disproportionately small centers. (b) SI as a function of surround size of each unit. Note that special V2 units (orange squares) exhibited nearly complete suppression (SI > 0.9). (c) Relative electrode depth at which each unit was recorded in V1 and V2 during two penetrations (P1 and P2). The discontinuities indicate when the electrode was traversing the white matter. Longer orange bars in V2 indicate the depth where special V2 units were recorded, and short black bars represent ordinary V2 units.
Relative recording depths of individual V2 units (Fig. 2c) indicate that these special neurons were encountered throughout V2 and were scattered among ordinary V2 neurons. These results suggest that there may be more than one source of input signals associated with the RF surrounds for adult V2 neurons, and that the input connections for surround mechanisms are not exactly the same for V1 and V2 neurons.
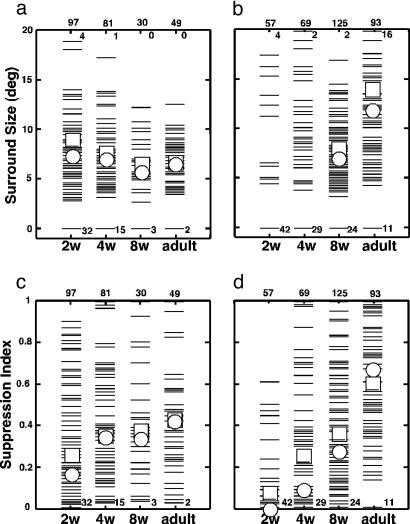
V2 Development Lags V1. Area-summation functions for representative V1 and V2 neurons at 2, 4, and 8 weeks of age and in adults are shown in Fig. 3. Three units were chosen for each group based on their quartile values for surround suppression. As early as 14 days of age, the area-summation functions for the majority of V1 neurons were qualitatively adult-like (Fig. 3a). However, a substantial number of V1 neurons (32/97) exhibited no inhibitory surrounds and/or considerably larger centers than neurons in adults (2/49) (Fig. 3d). V1 neurons having very weak or no surrounds were less frequently encountered at 4 weeks of age (15/81) (Fig. 2b) and were rare in 8-week-old monkeys (3/30) (Fig. 2c).
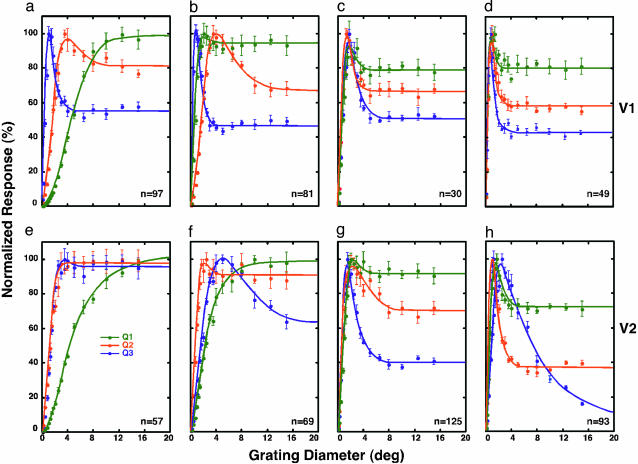
Fig. 3.
Developmental changes in area-summation functions in V1 and V2 neurons. Area-summation functions for representative V1 neurons at 2 weeks (a), 4 weeks (b), and 8 weeks (c) and in adult monkeys (d). Three units were chosen for each group based on their quartile values (Q1 = 25%, Q2 = 50%, and Q3 = 75%) for surround suppression (SI). (e–h) Comparable functions for V2 are illustrated.
In contrast, the great majority of V2 neurons (42/57) at 2 weeks of age did not have measurable inhibitory surrounds (Fig. 3e). By 4 weeks of age (Fig. 3f), however, a substantial number of V2 neurons (29/69) exhibited qualitatively adult-like area-summation functions. By 8 weeks of age (Fig. 3g), the area-summation functions for the majority of V2 neurons (101/125) became qualitatively adult-like. However, special V2 neurons, common in adult monkeys (25/93), were less frequently encountered in 4- or 8-week-old infant monkeys (4/69 at 4 weeks and 7/125 at 8 weeks). These results suggest that the functional connections underlying the RF surrounds for special V2 neurons develop later than those for ordinary V2 units.
RF Center Responses in V2 Are Immature at 2 Weeks of Age. The RF center responses of V1 neurons were surprisingly adult-like at 2 weeks of age, whereas V2 neurons exhibited very immature center responses (Fig. 4). The median center size of V1 neurons in 2-week-old infants (open circles) was not substantially different from that in adults (1.88°, compared with 1.11° in adults) (Fig. 4a). In contrast, the median center size of V2 neurons in 2-week-old infants (5.8°) was nearly three times as large as that in adults (1.71°) (Fig. 4b). The median V2 centers became smaller in 4-week-old infants (2.8°), and by 8 weeks, the center size (1.58°) was similar to that in adults.
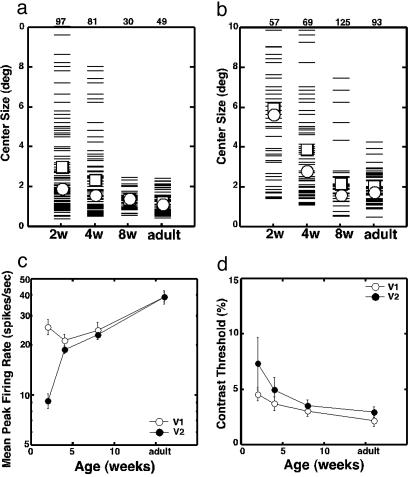
Fig. 4.
Developmental changes in the center responses of V1 and V2 units. (a) RF center size of V1 neurons. Each thin bar signifies an individual unit. Open squares and open circles indicate the mean and median values, respectively. The number at the top indicates the sample size for each group. (b) RF center sizes of V2 neurons. (c) The mean (±SE) peak firing rate of V1 (open circles) and V2 (filled circles) neurons as a function of age. Gratings were confined to the RF centers of individual neurons. (d) Average contrast thresholds (±SE) as a function of age in V1 (open circles) and V2 (filled circles).
The mean V1 center sizes (open squares) in 2- and 4-week-old infants (2.97° ± 0.24° and 2.31° ± 0.20°, respectively) were significantly larger than the mean center size in adults (1.22° ± 0.08°) (ANOVA, P < 0.01). Similarly, the mean center sizes in V2 showed parallel reductions over time, and the differences between 2-week-old infants (5.86° ± 0.41°) or 4-week-old infants (3.82° ± 0.30°) and adults (1.99° ± 0.12°) were statistically significant (ANOVA, P < 0.01).
The mean peak firing rate of V2 neurons in 2-week-old infants for gratings optimal for the RF centers (Fig. 4c) was only about one-third of the firing rate of V1 neurons and less than one-quarter of the adult value in V2 (ANOVA, P < 0.01). This dramatic difference in responsiveness between V1 and V2 neurons suggests that the feedforward connections between V1 and V2 are functionally immature at 2 weeks of age.
Although V1 neurons were quite responsive at 2 weeks of age, the mean peak firing rate was slightly but significantly lower than that in adults during the first 8 weeks of life (ANOVA, P < 0.01) (Fig. 4c). However, there were no significant differences among the three infant groups (ANOVA, P > 0.1). The responsiveness of V2 neurons dramatically increased with age, but at 4 and 8 weeks, the firing rates were still significantly lower than that in adults (ANOVA, P < 0.01). There were no differences between V1 and V2 at these ages.
The RF centers of V1 and V2 neurons at 14 days of age also exhibited reduced contrast sensitivities (Fig. 4d). The contrast thresholds of both V1 and V2 neurons, measured by determining the minimum stimulus contrast required to initiate responses that exceeded the 95% confidence limits for the baseline firing rate, were significantly elevated, compared with the adult threshold values (ANOVA, P < 0.02). At 4 and 8 weeks, however, contrast thresholds were not significantly different from those in adult V1 or V2. Also, there were no differences in mean contrast thresholds between V1 and V2 for any age group (ANOVA, P > 0.1). These results suggest that the observed subnormal contrast sensitivity of center responses at 2 weeks of age largely reflects immaturities in precortical structures.
Maturation of V2 Surrounds Are Delayed. Surround size. No variation in mean (squares) or median (circles) surround size of V1 neurons was observed as a function of age (Fig. 5a). This result suggests that the cortical connections required for V1 surrounds are present and functional as early as 14 days after birth. In contrast, the great majority of V2 neurons (42/57) did not exhibit clear surrounds at 2 weeks of age, and therefore, meaningful comparisons of the mean or median size are not possible for these ages (Fig. 5b). However, the range of measurable surround size in V2 at 2 and 4 weeks of age was not different from that in adults. The mean surround size in 8-week-old infants was significantly lower than that in adults, because there were fewer special V2 units at this age (5.6%) than in adults (26.8%). Surround suppression. Although surround suppression in V1 was weaker at 2 weeks of age (SI = 0.27 ± 0.03, compared with 0.43 ± 0.03 in adults, ANOVA, P < 0.01) (Fig. 5c), by 4 weeks of age, suppression became nearly as strong as in adults. In V2, however, the median SI was quite low in all infant groups; specifically, 0.0, 0.1, and 0.3 in 2-, 4-, and 8-week-old infants, respectively, compared with 0.7 in adults (Fig. 5d). The average SI values for V2 neurons in all infant groups were significantly lower than that in adults (ANOVA, P < 0.01). The large difference in the average SI between 8-week-old infants and adults is associated with the low encounter rates of special V2 neurons in these infants (Figs. 1, 2, 3). Thus, the circuits mediating surround suppression in V2 were present and functional as early as 2 weeks of age in a small proportion of units, but even at 8 weeks of age, the functional connections underlying RF surrounds were considerably immature in V2.
Fig. 5.
Development of RF surrounds. (a) RF surround sizes in V1. Each thin bar signifies an individual unit. Open squares and open circles indicate the mean and median values, respectively. (b) RF surround sizes in V2. (c) Strengths of surround suppression in V1. (d) Strengths of surround suppression in V2. The number at the bottom indicates the number of neurons with “zero” values, i.e., no surround or SI = 0. The numbers at the top indicate the sample size (outside the panels) and the number of units that exceeded the maximum values on the ordinates (inside the panels), respectively.
Contrast-Response Functions Reveal the Nature of Immaturities. In adult V1, center/surround interactions are influenced by center stimulus contrasts and surround stimulus orientations (15–19). We determined at what age this contrast-dependent, orientation-sensitive property of long-range interactions emerged in V1 and V2.
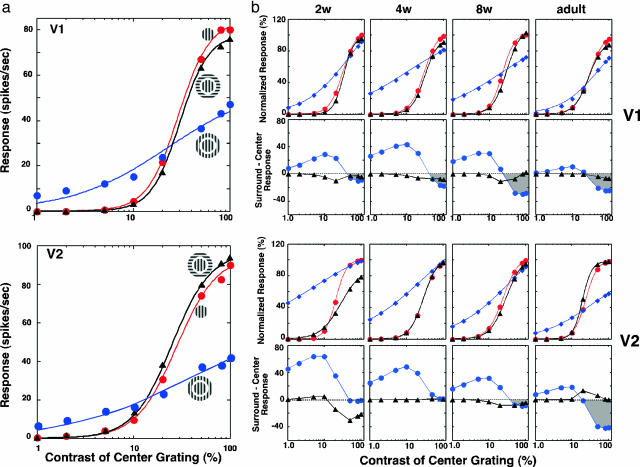
Initially, we investigated whether V2 neurons in adult monkeys exhibited similar response properties. In the representative units from V1 and V2 (Fig. 6a), surround stimulation reduced response gain, i.e., the slope of the fitted hyperbolic function (n) became flatter. Consequently, for stimulus contrasts >23% in V1 and >15% in V2, center responses were suppressed by surround stimulation, and this suppression became progressively larger with increasing contrast. However, at lower contrasts, surround stimulation exerted facilitatory effects on center responses. As previously reported in V1 (15), stimulating the surrounds with orthogonally oriented gratings had little or no effect on the center responses of V2 neurons.
Fig. 6.
Developmental changes in center/surround interactions as a function of center stimulus contrast. (a) Center/surround interactions in representative units from V1 (Upper) and V2 (Lower) of an adult monkey. Contrast-response functions are illustrated for center only (red), center plus isooriented surround (blue), and center plus orthogonal surround (black). Responses are fitted into a hyperbolic function [R = RmC[supi]n/(C50[supi]n + Cn), in which C is the stimulus contrast, Rm is the maximum response, C50 refers to the stimulus contrast that elicits half the maximum response, and n, the exponent, signifies the rate at which response changes occur]. (b) Contrast-dependent center/surround interactions in infant and adult monkeys. (Top row) Normalized responses of V1 neurons as a function of center contrast for center only (red), center plus isooriented surround (blue), and center plus orthogonal surround (black). Surround contrasts were kept at 80%. The representative unit for each age group was chosen based on the median value of n, C50, and Rm of hyperbolic functions. (Second row) Differences (center/surround responses) between center only and center plus isooriented surround (filled circles) or between center only and center plus orthogonal surround (filled triangles). Positive values indicate facilitative center/surround interactions, and negative values indicate inhibitory interactions. (Third row) Comparable functions for representative V2 units as a function of age. (Bottom row) Comparable difference functions for V2 units.
As early as 2 weeks of age, the contrast-dependent surround effects were present in V1 and were qualitatively similar to those in adults (Fig. 6b). However, at 2 and 4 weeks of age, isooriented surround gratings had very small suppressive effects on center responses in the majority of units, and the surround facilitation at lower contrasts was much greater than that in adults. At 8 weeks of age, surround suppression at higher contrasts was adult-like in strength, whereas substantial surround facilitation was still present at lower contrasts.
With orthogonally oriented surround gratings, surround stimulation had minimal impact on the center responses of V1 units at any age.† This result substantially differs from the previous study of young infant monkeys in which orthogonally oriented surround stimuli were shown to suppress the center responses of V1 neurons more vigorously than isooriented surround stimuli.
In V2 of infant monkeys, suppression with isooriented surround stimuli was negligible in 2- and 4-week-old infants and was observed only at higher center contrasts in 8-week-old monkeys. Instead, robust facilitation dominated surround effects in all infant age groups. These facilitatory surround influences were largest in 2-week-old infants and became progressively smaller with age. However, unlike in V1, surround suppression at high contrasts was minimal as late as at 8 weeks of age.
Orthogonally oriented surround stimuli had no effects on the center responses of V2 neurons in all infant groups except in 2-week-old infants. Substantial surround suppression was observed at center contrasts >10%. This result indicates that additional immaturities exist in the V2 circuits shortly after birth.
Discussion
The most important finding of this study is that the functional connections underlying the RF center/surround mechanisms of individual neurons mature considerably later in V2 than in V1.
RF Center/Surround in Adults. Our results in V1 and V2 neurons of mature monkeys suggest that the basic circuits underlying the centers and surrounds of individual V2 neurons is qualitatively similar to that in V1. However, the origins of long-range surround signals may be different between V1 and V2 and also between ordinary and special neurons within V2. In adult monkeys, the details of the circuits underlying center/surround responses of V1 neurons are currently in dispute (15–22). A general consensus view is that the center responses of V1 neurons reflect the neural activity of feedforward connections and neighboring local neurons, whereas surround responses are mediated by the intrinsic long-range connections and/or the feedback connections from extrastriate visual areas.
The main unresolved issues are whether the feedback connections to V1 and V2 arise from the same extrastriate visual areas, and how much of the surround signals of individual V1 and V2 neurons depend on the intrinsic long-range connections as opposed to feedback connections. Our results in adult V2 and the slower functional development of RF surrounds in special V2 neurons, compared with ordinary V2 neurons (Figs. 2 and 3), suggest that there are multiple sources of extrastriate feedback connections that innervate different groups of cortical neurons, and that the maturational status of the intrinsic long-range connections is likely to differ between V1 and V2.
RF Center Development. At 2 weeks of age, the average peak firing rates and contrast sensitivities of V2 neurons for gratings confined to RF centers were substantially lower than those in older infants or adults. In contrast, V1 neurons were far more responsive at this age. These contrasting results between V1 and V2 suggest that the feedforward input connections underlying the RF centers of V2 neurons were not as well developed as those in V1 neurons at 14 days of age. This result is consistent with the view that neuronal responses mature later in higher-order visual areas than in V1 (4, 7, 23–25). However, there was a subtle but significant immaturity in the overall responsiveness of center responses in V1 at 2 weeks of age (Fig. 4 c and d). These subtle but significant subnormal responses in V1 are thought to reflect known immaturities in precortical structures, especially those in photoreceptors (26, 27).
RF Surround Development. Our results show that the functional connections that underlie V1 surrounds are qualitatively adult-like very early in life, possibly at birth. The moderate immaturities observed in V1 at 2 weeks of age suggest that the intrinsic long-range connections and/or feedback connections from extrastriate visual areas in V1 are functionally immature at this age. Obviously, another possibility is that there is a delay in the functional maturation of the extrastriate visual areas that send feedback signals to V1.
As early as 14 days of age, the basic connections for RF surrounds appear to exist in a small number of V2 neurons, because we could define a surround in these units, and the range of surround sizes was not different from that for older animals. This result is consistent with previous anatomical studies (4, 28, 29). Functionally, however, these surround connections were extremely immature in 2-week-old monkeys, and many subnormal responses persisted even at 8 weeks of age (Fig. 5 b and d and Fig. 6). Thus, the functional development of V2 surrounds is very much delayed relative to V1.
Perceptual Development. The responses of V2 neurons in adult monkeys have been directly linked to their ability to perform perceptual figure/ground segregation tasks (10, 11). Contour integration, another perceptual task that requires integration of signals over large areas, does not perceptually emerge until ≈8–10 weeks of age (7). The interpretation of our results in terms of postnatal perceptual development is somewhat limited. However, our study unambiguously demonstrated that the ability of V2 neurons to integrate local information over large areas is absent or subnormal at ≤8 weeks of age, suggesting that the basic machinery necessary for perceptual tasks such as contour integration or figure/ground segregation may be absent or functionally very immature in V2 or downstream in higher-order visual areas until later ages.
Conclusion
The present results provide support for the growing body of evidence indicating that, although the basic anatomical connections in higher-order visual areas are present at or before birth (3, 4, 28, 29), the maturation of the functional connections in the visual brain proceeds in a hierarchical order (4, 7, 23, 24). Also, our results support the hypothesis that the anatomical feedback connections from extrastriate visual areas to V1 or V2 mature much slower than the feedforward connections in these cortical areas (4).
Acknowledgments
This work was supported by National Institutes of Health Grants EY-08128, EY-03611, and RR-07146.
Author contributions: B.Z. and Y.C. designed research; B.Z., J.Z., I.W., I.M., H.B., and Y.C. performed research; B.Z., E.L.S., and Y.C. contributed new reagents/analytic tools; B.Z., J.Z., I.W., I.M., H.B., E.L.S., and Y.C. analyzed data; and B.Z. and Y.C. wrote the paper.
Abbreviations: V1, primary visual cortex; V2, visual area 2; RF, receptive field; SI, suppression index.
Footnotes
Movshon, A. J., Kiorpes, L., Cavanaugh, J. R. & Hawken, M. J. (2000) Invest. Ophthalmol. Visual Sci. 41, 751 (abstr.).
References
- 1.Atkinson, J., Hood, B., Wattam-Bell, J., Anker, S. & Tricklebank, J. (1988) Perception 17, 587–595. [DOI] [PubMed] [Google Scholar]
- 2.Slater, A., Morison, V. & Somers, M. (1988) Perception 17, 597–602. [DOI] [PubMed] [Google Scholar]
- 3.Rakic, P. (1976) Nature 261, 467–471. [DOI] [PubMed] [Google Scholar]
- 4.Batardiere, A., Barone, P., Knoblauch, K., Giroud, P., Berland, M., Dumas, A. M. & Kennedy, H. (2002) Cereb. Cortex 12, 453–465. [DOI] [PubMed] [Google Scholar]
- 5.Chino, Y. M., Smith, E. L., III, Hatta, S. & Cheng, H. (1997) J. Neurosci. 17, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou, C., Pettet, M. W., Sampath, V., Candy, T. R. & Norcia, A. M. (2003) J. Neurosci. 23, 8630–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiorpes, L. & Bassin, S. A. (2003) Visual Neurosci. 20, 567–575. [DOI] [PubMed] [Google Scholar]
- 8.Dragoi, V. & Sur, M. (2000) J. Neurophysiol. 83, 1019–1030. [DOI] [PubMed] [Google Scholar]
- 9.Polat, U., Mizobe, K., Pettet, M. W., Kasamatsu, T. & Norcia, A. M. (1998) Nature 391, 580–584. [DOI] [PubMed] [Google Scholar]
- 10.Kapadia, M. K., Westheimer, G. & Gilbert, C. D. (2000) J. Neurophysiol. 84, 2048–2062. [DOI] [PubMed] [Google Scholar]
- 11.Knierim, J. J. & van Essen, D. C. (1992) J. Neurophysiol. 67, 961–980. [DOI] [PubMed] [Google Scholar]
- 12.Zipser, K., Lamme, V. A. & Schiller, P. H. (1996) J. Neurosci. 16, 7376–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Physiological Society (2002) Am. J. Physiol. 283, R281–R283. [DOI] [PubMed] [Google Scholar]
- 14.Skottun, B. C., De Valois, R. L., Grosof, D. H., Movshon, J. A., Albrecht, D. G. & Bonds, A. B. (1991) Vision Res. 31, 1079–1086. [DOI] [PubMed] [Google Scholar]
- 15.Cavanaugh, J. R., Bair, W. & Movshon, J. A. (2002) J. Neurophysiol. 88, 2530–2546. [DOI] [PubMed] [Google Scholar]
- 16.Solomon, S. G., Peirce, J. W. & Lennie, P. (2004) J. Neurosci. 24, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sceniak, M. P., Hawken, M. J. & Shapley, R. (2001) J. Neurophysiol. 85, 1873–1887. [DOI] [PubMed] [Google Scholar]
- 18.Sceniak, M. P., Ringach, D. L., Hawken, M. J. & Shapley, R. (1999) Nat. Neurosci. 2, 733–739. [DOI] [PubMed] [Google Scholar]
- 19.Angelucci, A., Levitt, J. B., Walton, E. J., Hupe, J. M., Bullier, J. & Lund, J. S. (2002) J. Neurosci. 22, 8633–8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitt, J. B. & Lund, J. S. (1997) Nature 387, 73–76. [DOI] [PubMed] [Google Scholar]
- 21.Somers, D. C., Todorov, E. V., Siapas, A. G., Toth, L. J., Kim, D. S. & Sur, M. (1998) Cereb. Cortex 8, 204–217. [DOI] [PubMed] [Google Scholar]
- 22.Li, W. & Gilbert, C. D. (2002) J. Neurophysiol. 88, 2846–2856. [DOI] [PubMed] [Google Scholar]
- 23.Barone, P., Dehay, C., Berland, M., Bullier, J. & Kennedy, H. (1995) Cereb. Cortex 5, 22–38. [DOI] [PubMed] [Google Scholar]
- 24.Distler, C., Bachevalier, J., Kennedy, C., Mishkin, M. & Ungerleider, L. G. (1996) Cereb. Cortex 6, 184–195. [DOI] [PubMed] [Google Scholar]
- 25.Rodman, H. R., Skelly, J. P. & Gross, C. G. (1991) Proc. Natl. Acad. Sci. USA. 88, 7572–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendrickson, A. & Kupfer, C. (1976) Invest. Ophthalmol. Visual Sci. 15, 746–756. [PubMed] [Google Scholar]
- 27.Packer, O., Hendrickson, A. E. & Curcio, C. A. (1990) J. Comp. Neurol. 298, 472–493. [DOI] [PubMed] [Google Scholar]
- 28.Coogan, T. A. & Van Essen, D. C. (1996) J. Comp. Neurol. 372, 327–342. [DOI] [PubMed] [Google Scholar]
- 29.Barone, P., Dehay, C., Berland, M. & Kennedy, H. (1996) J. Comp. Neurol. 374, 1–20. [DOI] [PubMed] [Google Scholar]