Abstract
Bisphenol A (BPA) is an environmental contaminant widely used in the plastic industry. BPA has been demonstrated to be an endocrine disruptor and has an adverse effect on the embryonic development of mammals. However, the mechanism of action of BPA is limited. In this study, we investigated the role and mechanism of BPA in porcine embryonic development. First, the parthenotes were treated with different concentrations of BPA. We found that blastocyst formation was impaired and the parthenotes were arrested at the 4-cell stage after treatment with 100 μm BPA. Second, ROS increased following the addition of BPA, which further caused mitochondrial damage, and cytochrome c was released from the mitochondria to induce apoptosis. The adaptive response was demonstrated through LC3 immunofluorescence staining and by assessing autophagy-related gene expression. In addition, BPA caused DNA damage through the p53-p21 signaling pathway. Thus, our results indicate that BPA displays an adverse effect on porcine early embryonic development through mitochondrial and DNA damage.
Introduction
Bisphenol A (BPA) is an environmental contaminant widely used in the plastic industry for the manufacture of bottles, containers, receipt paper, and so on. Widespread use of BPA-containing products leads to ubiquitous BPA exposure. Growing evidence indicates that environmental contaminants cause adverse health effects and poor developmental outcomes. BPA can affect the actions of endogenous hormones and disrupt endocrine function1, 2. Because of its toxic effect, some countries have banned BPA in feeding bottles for babies.
Research on the effect of BPA on reproduction is vast and has revealed altered hormone release3, 4, abnormal reproductive organs5. People are exposed to BPA mainly through skin contact and food ingestion. A study performed in Europe confirmed the presence of BPA in the urine of 97.7% of the participants6. BPA results in adverse reproductive outcomes. High BPA concentrations in urine are related to a decrease in ovarian response, number of fertilized oocytes, and blastocyst formation7.
The exposure of BPA to oocytes leads to the failure of maturation and occurrence of MII abnormalities, including spindle morphology, chromosome misalignment, and aberrant actin distribution8, 9. These abnormalities are associated with poor developmental outcomes. Affected embryos have shown decreased competency, increased apoptosis, and a skewed sex ratio10. In addition, exposure to BPA causes an adverse effect on sperm, such as a decrease in number and quality as well as DNA damage11. In animal models where BPA was administered orally or via injection, there were several abnormal reproductive effects, such as the early onset of puberty12 and development of reproductive tracts and organs13.
Several studies have shown that some cellular processes, including oxidative stress, DNA damage, and epigenetic modifications are essential for early embryonic development14–16. Many studies have demonstrated the negative effect of BPA exposure during early embryonic development10, 17. There is little research focused on the mechanism of action of BPA on porcine embryonic development. Therefore, the objective of this study was to evaluate the influence and mechanism of BPA on porcine early embryonic development, oxidative stress, DNA damage, apoptosis, autophagy, epigenetic modification, and inner cell mass formation.
Results
BPA exposure influences blastocyst formation during porcine early embryonic development
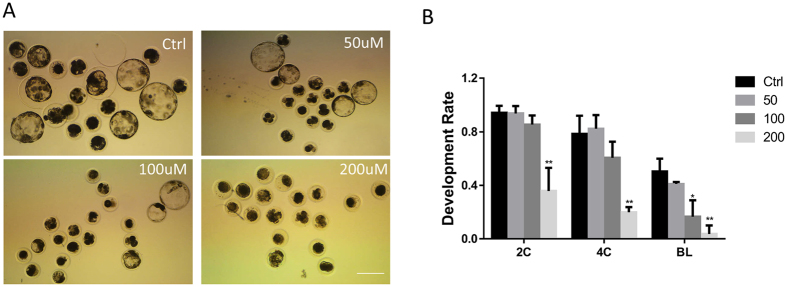
To detect the function of BPA on embryonic development, parthenotes were incubated in the in vitro culture (IVC) medium for 7 days in the presence of BPA at different doses (0, 50, 100, and 200 μM) and the blastocyst rates were examined. As shown in Fig. 1A,B, BPA exposure resulted in an effective decrease of blastocyst formation at concentrations of 100 and 200 μM (16.5 ± 7.1%, P < 0.05 and 3.7 ± 3.7%, P < 0.01), but not 50 μM (41.0 ± 1.0%) compared with the control (50.3 ± 5.5%). We also found that more treated parthenotes were arrested at the 4-cell stage. The proportion of parthenotes at the 2-cell stage with BPA treatment (50 and 100 μM) (93.7 ± 3.2% and 85.4 ± 3.9%) was not significantly different from that in the control group (94.1 ± 3%). However, there was a significant difference with 200 μM BPA (35.8 ± 9.9%, P < 0.01). Most of the parthenotes can develop to the 4-cell stage at concentrations of 0, 50, and 100 μM (78.4 ± 7.8%, 82.2 ± 7.2%, and 60.5 ± 7.0%, respectively), but not with 200 μM (19.8 ± 2.2%, P < 0.01). BPA concentration of 100 μM was used in all further experiments.
Figure 1.
Effect of BPA on porcine early embryonic development. (A) Effect of different BPA concentrations on embryonic development. (B) The rate of embryonic development. *P < 0.05, **P < 0.01. Ctrl, control; Treat, treatment. 2 C: 2-cell; 4 C: 4-cell; BL: Blastocyst. Scale bar: 200 μm.
BPA exposure results in reactive oxygen species (ROS) generation
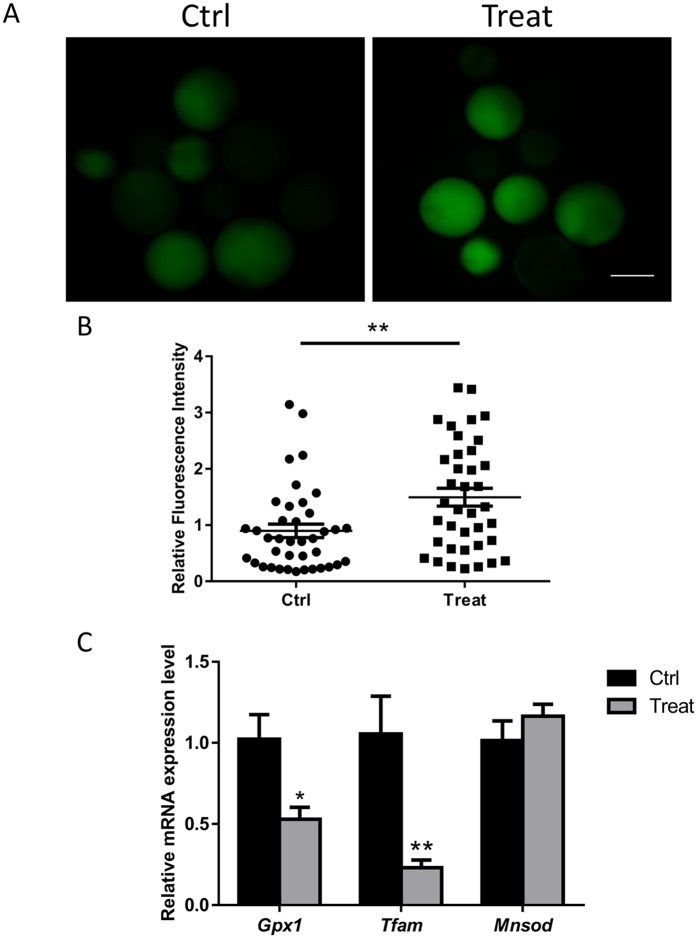
It has been demonstrated that ROS can be generated when exposed to environmental contaminants. Oxidative stress can also occur in a biological system when exposed to environmental contaminants and be defined as an imbalance between production and depletion of ROS18. Therefore, in this study, ROS levels were examined after BPA exposure. As shown in Fig. 2A, the generation of ROS increased in the treatment group compared with that in the control group. The relative green fluorescence intensity that induced ROS production was significantly increased (Fig. 2B). The mRNA expression level of oxidative stress-related genes (Gpx1, Tfam, and Mnsod) was also examined. The Gpx1 and Tfam mRNA levels significantly decreased compared with that in the control group. The Mnsod expression level showed no significant variation (Fig. 2C).
Figure 2.
BPA increases ROS. (A) Staining for ROS in porcine blastocysts in the control and BPA-exposed groups. (B) Green fluorescence intensity was measured. (C) mRNA expression of Gpx1, Mnsod, and Tfam in blastocysts with or without BPA. **P < 0.01. Ctrl, control; Treat, treatment. Scale bar: 200 μm.
Effect of BPA exposure on apoptosis
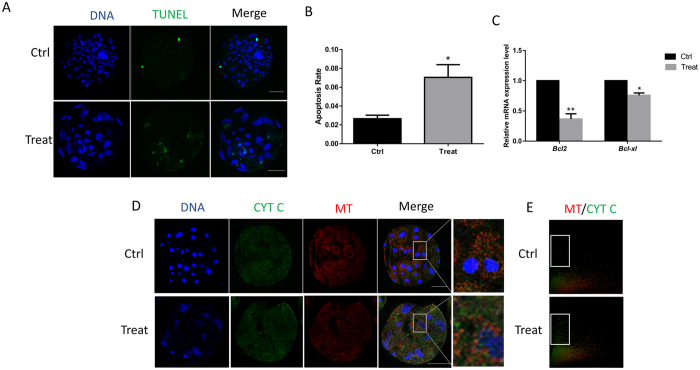
Apoptosis was examined after BPA exposure by using the TUNEL assay. The apoptotic effect was calculated as the ratio between the number of TUNEL-positive nuclei and the total cell number. As shown in Fig. 3A, more TUNEL-positive nuclei were observed in the treatment group. The ratio of the TUNEL-positive nuclei significantly increased after BPA exposure (Fig. 3B). The expression of apoptosis-related genes was also analyzed after treatment with BPA. The expression of both anti-apoptotic genes, Bcl2 and Bcl-xl, significantly reduced (Fig. 3C,D), suggesting that BPA exposure may induce apoptosis.
Figure 3.
Effect of BPA exposure on apoptosis. (A) TUNEL-positive cells were detected in the presence or absence of BPA. (B) The rate of TUNEL-positive and total cells are shown. (C) Relative mRNA expression level of Bcl2 and Bcl-xl in blastocysts with or without BPA exposure. (D) The localization of cytochrome c and mitochondria was detected using immunostaining. (E) The localization of cytochrome c and mitochondria in the whole blastocyst. *P < 0.05. Ctrl, control; Treat, treatment. Scale bar: 50 μm. MT: mitochondria; CYT C: cytochrome c.
The release of cytochrome c from the mitochondria into the cytoplasm initiates apoptosis. The co-localization of cytochrome c and mitochondria was examined using immunofluorescence staining. The release of cytochrome c from the mitochondria is shown in Fig. 3D after exposure to BPA. As shown in Fig. 3E, the control group showed obvious co-localization of mitochondria and cytochrome c in the whole blastocyst compared with the treatment group. The spots inside of white box displayed the cytochrome c which was released from the mitochondria. More cytochrome c was released from the mitochondria in the treatment group. Therefore, BPA exposure caused the release of cytochrome c and induced the initiation of apoptosis.
BPA exposure increases P53 and P21 expression in the blastocyst
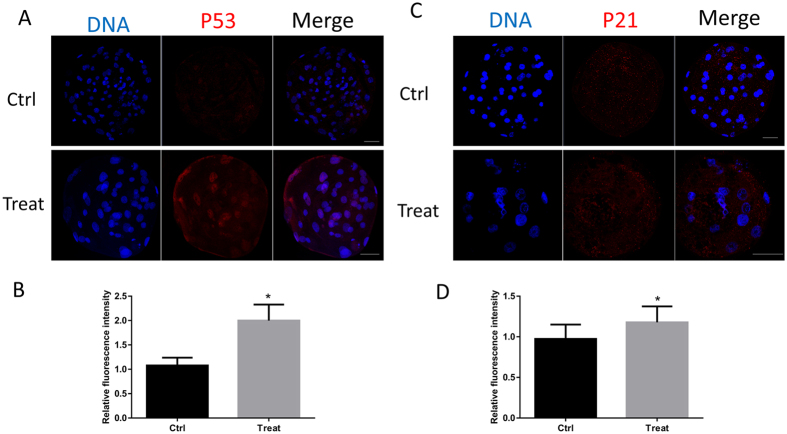
In order to detect the mechanism of the negative effects of BPA on embryonic development, the activation of the p53 pathway was examined. The activation of p53 can result in poor developmental potential19. Therefore, we showed that BPA treatment could cause an increased expression of p53 (Fig. 4A,B). The expression of p21, a known downstream target of p53, was also examined. The fluorescence intensity of p21 increased after embryos were treated with BPA (Fig. 4C,D). These results show that BPA exposure impaired the preimplantation embryo development through the p53-p21 pathway.
Figure 4.
BPA treatment triggers the activation of the p53 pathway. (A) Staining for p53 showed its activation and expression. (B) The relative intensity of p53 is shown as bars. (C) Laser scanning confocal microscopy images of immunostaining for p21. (D) The relative intensity of p21 was measured. *P < 0.05. Ctrl, control; Treat, treatment. Scale bar: 50 μm.
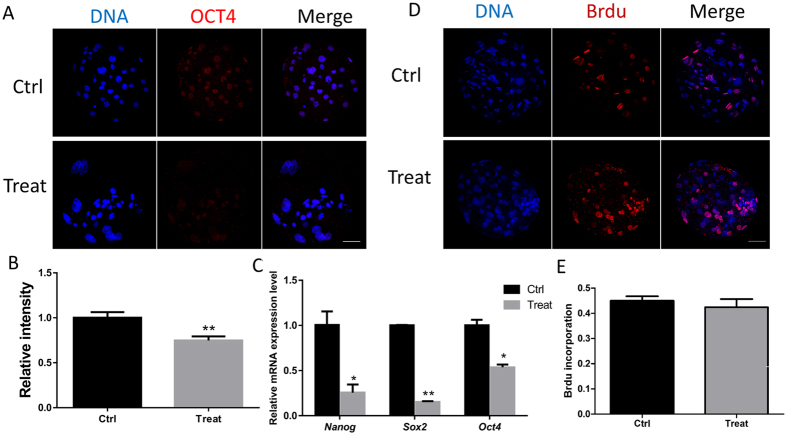
BPA exposure results in a decrease of pluripotency markers
Embryonic development is associated with the embryo quality. As the key regulator of pluripotency, OCT4 expression was examined using immunofluorescence staining. The relative fluorescent density of OCT4 decreased in the BPA-exposed group compared with that in the control group (P < 0.05) (Fig. 5A,B). To confirm the effect of BPA on pluripotency, the expression of three pluripotent-related genes was examined. As shown in Fig. 5C, the mRNA expression level of Oct4, Sox2, and Nanog significantly reduced (P < 0.05), which is consistent with the results of immunofluorescence staining. Next, we detected the differentiation where there was no difference between the control and BPA-exposed groups (Fig. 5D,E). These results indicate that BPA exposure can decrease embryo quality.
Figure 5.
Effect of BPA treatment on pluripotency and differentiation. (A) Laser scanning confocal microscopy images of immunostaining for OCT4 in porcine blastocysts in the control and BPA treatment groups. (B) The relative intensity of OCT4 was measured. (C) The relative Oct4, Nanog, and Sox2 mRNA expression levels are shown. (D) BrdU incorporation in blastocysts after BPA exposure. (E) Graph summarizing the relative rate of proliferation. *P < 0.05, **P < 0.01. Ctrl, control; Treat, treatment. Scale bar: 50 μm.
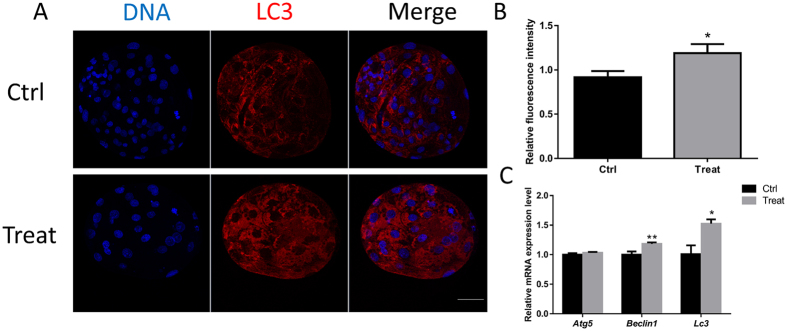
BPA exposure causes autophagy
Oxidative stress can induce cell autophagy. Therefore, the expression level of LC3 was examined after treatment with BPA. Immunofluorescence staining showed a significant increase in fluorescence intensity (Fig. 6A,B). Consistent with this, the mRNA expression level of autophagy-related genes also significantly increased (Fig. 6C).
Figure 6.
Effect of BPA exposure on autophagy. (A) Laser scanning confocal microscopy images of immunostaining for the LC3 protein in porcine blastocysts with or without BPA. (B) Relative fluorescence intensity of LC3. (C) Relative mRNA expression levels of autophagy-related genes, Atg5, Beclin1, and Lc3. *P < 0.05, **P < 0.01. Ctrl, control; Treat, treatment. Scale bar: 50 μm.
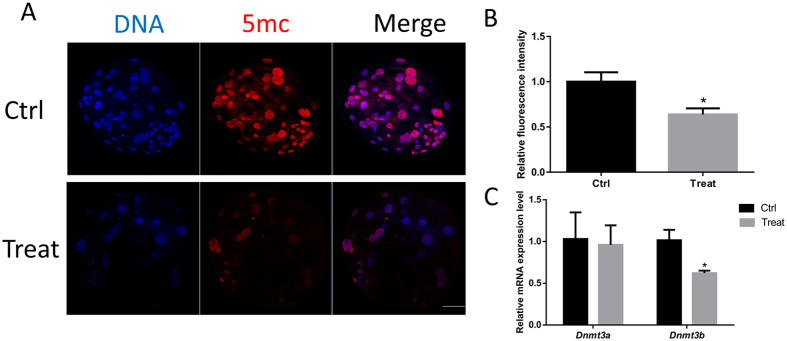
BPA exposure causes the alternation of methylation
To evaluate the effect of embryo exposure to BPA on epigenetic modification, the level of 5-methyl-cytosine (5mC) was examined. As shown in Fig. 7A,B, we found that 5mC was co-localized with DNA and the relative intensity decreased compared with that in the control (P < 0.05). The mRNA expression of DNA methyltransferases (Dnmt3a and Dnmt3b) was analyzed using RT-PCR. mRNA levels were found to be significantly decreased after exposure to BPA (Fig. 7C).
Figure 7.
BPA exposure results in the alternations of methylation. (A) Immunostaining for 5 mC was detected between the control and BPA treatment groups. (B) Relative fluorescence intensity of 5 mC is shown as bars. *P < 0.05. Ctrl, control; Treat, treatment. Scale bar: 50 μm.
Discussion
People increasingly focus on the adverse effect of BPA because of its worldwide use and being detected in food and water consumed by people and animals. Accumulating evidence implicates that environmental toxicity impacts metabolism, the immune system, and reproduction. Several environmental factors that can perturb embryonic development have been reported. These factors have an effect on conditions or techniques used in assisted reproductive technologies (ART). Although the number of babies that are conceived through ART is growing, these individuals represent a relatively small percentage (1–4%) of babies born in industrialized countries20. Despite a number of studies have reported the toxic effect of BPA, the mechanism of BPA adverse effect is unknown. In this study, the mechanism of the negative effects of BPA on porcine early embryonic development was determined. Our results demonstrate that BPA treatment leads to the arrest of embryonic development at the 4-cell stage. In addition, the mechanism of BPA toxicity was explored by assessing DNA damage, apoptosis, autophagy, and epigenetic modification.
Previously, some studies have shown that BPA elicits its function through estrogen-mediated pathways by binding estrogen receptors, therefore, regulating gene expression. Sometimes, BPA shows a dual effect, which can exert pro- and anti-estrogenic effects21, 22. In the present study, we found that BPA causes the embryonic development arrest. Other previous studies have demonstrated that BPA reduced blastocyst development and metabolism in bovine and mouse embryos17, 23. The adverse effect of BPA exposure during oocyte maturation leads to alterations in subsequent embryonic development10. Most studies have focused on the function of BPA and regarded it as a xenoestrogen; therefore, the mechanism of BPA is via the estrogen-mediated effect.
In this study, we found that BPA modulates oxidative stress in porcine embryonic development. In vitro produced embryo displays low quality and small scale production. The major obstacles in in vitro embryo development are the production of excessive free radicals and exposure to oxidative stress. When ROS production exceeds the antioxidant capacity of embryos, oxidative stress occurs24, 25. The production of ROS is particular critical in early embryonic development, and excessive ROS will induce apoptosis and metabolic disorders26, 27. Several studies have implicated a close relationship between BPA toxicity and the generation of ROS, which results in oxidative stress in tissues28, 29. We also showed that embryos suffered oxidative stress, which was demonstrated by the increased production of ROS and oxidative stress-related gene mRNA expression of Gpx1 and Tfam. Gpx1 plays a role in the detoxification of hydrogen peroxide, and Tfam can stabilize mitochondria and thus affects oxidative stress30, 31.
Excessive ROS production leads to different kinds of injuries including mitochondria. In the present study, we observed that BPA causes the generation of ROS. It also resulted in mitochondrial damage, as observed by the release of cytochrome c from mitochondria. Cytochrome c is typically localized between the inner and outer membranes of mitochondria. However, during apoptosis, it is released into the cytoplasm, where it binds to apoptotic protease activating factor 1 (APAF1). The release of cytochrome c from mitochondria is, therefore, an important event in apoptosis initiation32. The apoptosis related genes (Bcl-2, Bcl-xl) mRNA expression confirms the effect of BPA on apoptosis The anti-apoptotic Bcl-2 members prevent mitochondrial protein release33, 34. The result of Bcl2 and Bcl-xl mRNA expression analysis confirmed the effect of BPA on apoptosis.
BPA also can cause DNA damage, which may occur via BPA-induce ROS production in porcine parthenotes35, 36. However, the effect of BPA exposure on DNA damage has not been extensively investigated. In this study, we found that the possible mechanism of BPA-induce DNA damage may be from the p53 pathway. DNA damage-induced phosphorylation of p53 results in the growth suppression. An increase in p53 and p21 levels were observed in BPA-treated embryos. These results suggest that BPA exposure results in DNA damage through oxidative stress.
As described above, BPA induced an increase in ROS levels, which leads to detrimental effects, such as mitochondrial and DNA damage. Generation of ROS is critical for cell survival and death, as well as autophagy. Among the various molecular components and signaling cascades related to autophagy induction, cellular redox status has been regarded as a critical mediator or regulator of autophagy37, 38. Our results implicate that BPA-induced autophagy, which was demonstrated by the increased level of LC3 and alteration of the mRNA expression of autophagy-related genes. Autophagy is a cellular mechanism, through which cells digest organelles, part of the cytoplasm, or proteins to circumvent nutrient deprivation or accumulation of damaged proteins/organelles. The importance of autophagy has mostly been attributed to its ability to remove damaged organelles and altered proteins resulting from programmed cell death39–41. Autophagy regulation and development are closely related to cell death machinery, especially in the regulation of apoptosis. In this study, BPA exposure led to mitochondrial and DNA damage, this causes autophagy as a protective effect.
Accumulating evidence indicates that BPA treatment may alter the epigenome, including DNA methylation and histone modification. Previous studies have suggested that BPA treatment disrupts the methylation status22, 42. The reduced expression of 5mC and Dnmt genes observed in the present study was caused by BPA exposure. DNA methylation is modulated by DNMTs, which results in the formation of 5mC. DNA methylation can correlate with gene transcription.
The embryonic developmental potential is influenced by pluripotency43. In addition, excessive DNA damage can induce apoptosis and decrease pluripotency-related gene expression in pigs44. In this study, the reduced expression of pluripotency-related genes might be induced by DNA damage and apoptosis.
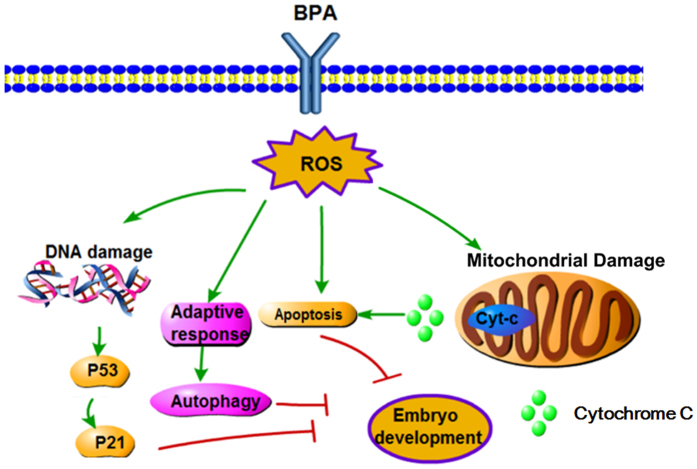
In conclusion, the toxic effect of BPA on porcine embryonic development was confirmed in this study. BPA exposure to embryos caused increased levels of ROS resulting in oxidative stress. The oxidative stress caused mitochondrial and DNA damage, which leads to autophagy. In addition, BPA exposure can result in the alteration of DNA methylation, which may affect the health of offspring (Fig. 8).
Figure 8.
Schematic representation of the adverse effect of BPA during porcine early embryonic development. BPA exposure causes the generation of oxidative stress, which results in DNA and mitochondrial damage. Apoptosis was induced by the release of cytochrome c from the mitochondria because of mitochondrial damage. The DNA damage led to the activation of the p53-p21 pathway. In addition, the oxidative stress induced the adaptive response, autophagy.
Materials and Methods
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Oocyte collection, in vitro maturation, and embryo culture
Ovaries from prepubertal gilts were obtained from a local slaughterhouse, maintained in saline at 37 °C, and transported to the laboratory. Follicles that were 3–6 mm in diameter were aspirated. Cumulus-oocyte complexes (COCs) that were surrounded by more than three layers of cumulus cells were selected for culture. COCs were isolated from follicles and washed three times with TL-HEPES. COCs were cultured in tissue culture medium 199 (TCM 199) with 10% porcine follicular fluid, 0.1 g/L sodium pyruvate, 0.6 mM L-cysteine, 10 ng/mL epidermal growth factor, 10 IU/mL luteinizing hormone, and 10 IU/mL follicle stimulating hormone at 38.5 °C for 44 h in a humidified atmosphere of 5% CO2.
After maturation, cumulus cells were removed with 0.1% hyaluronidase and repeated pipetting. For activation of parthenogenesis, oocytes with polar bodies were selected. They were activated by two direct current pulses of 1.1 kV/cm for 60 µs and then incubated in porcine zygote medium (PZM-5) containing 7.5 μg/mL of cytochalasin B for 3 h. Finally, approximately 90 embryos were cultured in PZM-5 for 8 days at 38.5 °C in a humidified atmosphere of 5% CO2. On the fifth day, 10% fetal bovine serum was added to the medium. To determine the effect of BPA on early porcine embryonic development, BPA was added to the medium after activation at final concentrations of 100 or 200 μM. The 100 μM concentration was used in the following experiments as it represents the minimum concentration that induces an effect on blastocyst formation.
Reactive oxygen species (ROS) staining
Blastocysts (n = 39, 3 replicates) were incubated for 15 min in IVC medium containing 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) at 37 °C. After incubation, blastocysts were washed three times with IVC medium and transferred to PBS drops covered with paraffin oil in a polystyrene culture dish. The fluorescent signal was captured using an epifluorescence microscope (Nikon Corp., Tokyo, Japan). The fluorescence intensity in the control group was arbitrarily set at 1, and the fluorescence intensities in the treatment groups were then measured and expressed as relative values for the control group.
Terminal deoxynucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP) nick-end labeling (TUNEL) assay
After the embryos had been treated with BPA, the blastocysts were collected. The blastocysts (n = 36, 3 replicates) were then fixed in 3.7% paraformaldehyde for 15 min at room temperature and subsequently permeabilized by incubation in 0.5% Triton X-100 for 30 min at 37 °C. The embryos were incubated with fluorescein-conjugated dUTP and the terminal deoxynucleotidyl transferase enzyme (In Situ Cell Death Detection Kit, Roche; Mannheim, Germany) for 1 h at 37 °C, and then washed three times with PBS/PVA. Embryos were treated with Hoechst 33342 for 5 min, washed three times with PBS/PVA, and mounted onto glass slides. Images were captured using a confocal microscope (Zeiss LSM 710 META, Jena, Germany).
Immunofluorescence and confocal microscopy
Embryos (n = 38, 3 replicates) were fixed in 3.7% paraformaldehyde for 20 min at room temperature, permeabilized with PBS/PVA containing 0.5% Triton X-100 at 37 °C for 1 h, and then incubated in PBS/PVA containing 1.0% bovine serum albumin at 37 °C for 1 h. Subsequently, the embryos were incubated overnight at 4 °C with anti-LC3 (ab58610, 1:100; Abcam, Cambridge, UK), anti-cytochrome C (ab110325, 1:100; Abcam), anti-p53 (sc6243, 1:100; Santa Cruz Biotech, CA, USA) and anti-OCT4 (sc8628, 1:100; Santa Cruz Biotech) antibodies. To check the fluorescence signal of 5mC, blastocysts were denatured with 1 N HCl at room temperature for 30 min and neutralized with 0.1 M Tris-HCl, pH 8.0 for 15 min. Subsequently, blastocysts were incubated in PBS containing 1% BSA, and then incubated overnight at 4 °C with 5mC antibody (ab10805, 1:100, Abcam, Cambridge, UK). After washing three times with PBS/PVA, the oocytes and embryos were incubated at 37 °C for 1 h with either goat anti-rabbit IgG (A11011, 1:200, Invitrogen) or rabbit anti-goat IgG (A11079, 1:200, Invitrogen), anti-mouse IgG (A21202, 1:200, Invitrogen). The oocytes and embryos were then stained with Hoechst 33342 for 5 min, washed three times with PBS/PVA, mounted onto slides, and examined using a confocal microscope (Zeiss LSM 710 META, Jena, Germany). Images were processed using Zen software (version 8.0, Zeiss, Jena, Germany).
5-Bromo-deoxyuridine analysis
The rate of cell proliferation was analyzed using 5-bromo-deoxyuridine (BrdU). Briefly, day 7 blastocysts (n = 32, 3 replicates) were incubated with 100 μM BrdU for 6 h at room temperature and then washed three times with PBS/PVA. Blastocysts were fixed in 3.7% paraformaldehyde for 20 min, and then permeabilized with PBS/PVA containing 0.5% Triton X-100 for 30 min. They were treated with 1 N HCl at room temperature for 30 min. Blastocysts were incubated in PBS/PVA containing 3.0% bovine serum albumin at 37 °C for 1 h and then with anti-BrdU at 4 °C overnight. After washing with PBS/PVA three times, blastocysts were incubated at 37 °C for 1 h with goat anti-mouse antibody. Finally, blastocysts were stained with Hoechst 33342 for 5 min, mounted onto slides, and examined using a confocal microscope.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Day 8 blastocysts were collected and mRNA was extracted from 10 blastocysts per group using a DynaBeads mRNA Direct Kit (Dynal Asa, Oslo, Norway), according to the manufacturer’s instructions. cDNA was obtained via reverse transcription of mRNA using the Oligo (dT)12–18 primer and SuperScript III Reverse Transcriptase (Invitrogen Co., Grand Island, NY, USA). The amplification cycles were as follows: 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 20 s, with a final extension at 72 °C for 5 min. Relative gene expression was normalized to internal porcine Gapdh mRNA levels using the 2−ΔΔCt method
Statistical analysis
All data were analyzed using the one-way analysis of variance (ANOVA) and Chi-square test implanted in Statistical Package for the Social Sciences (SPSS). Differences among treatments were examined using the Duncan multiple range test. Data were expressed as mean ± standard error of the mean. Each experiment was performed in triplicate and differences were considered significant if P < 0.05.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2015R1D1A1A01057629), Republic of Korea.
Author Contributions
J.G. and M.-H.Z. designed and conceived the experiments; J.G. wrote and all authors reviewed the manuscript. N.-Y.K. and X.-S.C. provided professional advice on experimental design and paper writing. K.-T.S., Y.-J.N., Y.-D.A. provided assistance on experiment performance.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jing Guo and Ming-Hui Zhao contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nam-Hyung Kim, Email: nhkim@chungbuk.ac.kr.
Xiang-Shun Cui, Email: xscui@chungbuk.ac.kr.
References
- 1.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Frontiers in behavioral neuroscience. 2009;3 doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandari RK, et al. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. General and comparative endocrinology. 2015;214:195–219. doi: 10.1016/j.ygcen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The Environmental Estrogen Bisphenol A Stimulates Prolactin Release in Vitro and in Vivo 1. Endocrinology. 1997;138:1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 4.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine reviews. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki A, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reproductive Toxicology. 2002;16:107–116. doi: 10.1016/S0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 6.Pirard C, Sagot C, Deville M, Dubois N, Charlier C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environment international. 2012;48:78–83. doi: 10.1016/j.envint.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich S, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Human reproduction. 2012;27:3583–3592. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris J, Favetta LA, King WA. Bisphenol A Exposure during Oocyte Maturation in vitro Results in Spindle Abnormalities and Chromosome Misalignment in Bos taurus. Cytogenetic and genome research. 2015;145:50–58. doi: 10.1159/000381321. [DOI] [PubMed] [Google Scholar]
- 9.Wang T, et al. The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget. 2016;7:32554–32565. doi: 10.18632/oncotarget.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris J, Mahboubi K, MacLusky N, King WA, Favetta LA. BPA exposure during in vitro oocyte maturation results in dose-dependent alterations to embryo development rates, apoptosis rate, sex ratio and gene expression. Reproductive Toxicology. 2016;59:128–138. doi: 10.1016/j.reprotox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Dobrzyńska MM, Radzikowska J. Genotoxicity and reproductive toxicity of bisphenol A and X-ray/bisphenol A combination in male mice. Drug and chemical toxicology. 2013;36:19–26. doi: 10.3109/01480545.2011.644561. [DOI] [PubMed] [Google Scholar]
- 12.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, Vom Saal FS. Environmental toxins: exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 13.Vom Saal FS, et al. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicology and Industrial Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 14.Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Molecular Human Reproduction. 1996;2:717–724. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. Effect of ATM and HDAC inhibition on etoposide-induced DNA damage in porcine early preimplantation embryos. PloS one. 2015;10 doi: 10.1371/journal.pone.0142561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi, J., Zhang, X., Liu, Y. & Chen, Q. Epigenetic information in gametes: Gaming from before fertilization: Comment on “Epigenetic game theory: How to compute the epigenetic control of maternal-to-zygotic transition” by Qian Wang et al. Physics of Life Reviews (2017). [DOI] [PubMed]
- 17.Choi, B.-I., Harvey, A. J. & Green, M. P. Bisphenol A affects early bovine embryo development and metabolism that is negated by an oestrogen receptor inhibitor. Scientific reports6 (2016). [DOI] [PMC free article] [PubMed]
- 18.Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and environmental safety. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Current Biology. 1995;5:931–936. doi: 10.1016/S0960-9822(95)00183-7. [DOI] [PubMed] [Google Scholar]
- 20.de Mouzon J, et al. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod. 2010;25:1851–1862. doi: 10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- 21.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of steroid biochemistry and molecular biology. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Santangeli, S. et al. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. General and Comparative Endocrinology (2016). [DOI] [PubMed]
- 23.Takai Y, et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochemical and biophysical research communications. 2000;270:918–921. doi: 10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- 24.Ali I, et al. Reactive oxygen species-mediated unfolded protein response pathways in preimplantation embryos. Journal of veterinary science. 2017;18:1–9. doi: 10.4142/jvs.2017.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecological endocrinology. 2010;26:563–567. doi: 10.3109/09513591003686395. [DOI] [PubMed] [Google Scholar]
- 26.Yang HW, et al. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Human reproduction. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, et al. Peroxisome proliferator-activated receptor δ improves porcine blastocyst hatching via the regulation of fatty acid oxidation. Theriogenology. 2017;90:266–275. doi: 10.1016/j.theriogenology.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Bindhumol V, Chitra K, Mathur P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–124. doi: 10.1016/S0300-483X(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 29.Kovacic P. How safe is bisphenol A? Fundamentals of toxicity: metabolism, electron transfer and oxidative stress. Medical hypotheses. 2010;75:1–4. doi: 10.1016/j.mehy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Suarez J, et al. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. American Journal of Physiology-Cell Physiology. 2008;295:C1561–C1568. doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10 doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annual review of biochemistry. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 33.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 34.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes & development. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 35.Wu H-J, et al. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2013;752:57–67. doi: 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 36.De Flora S, et al. Upregulation of clusterin in prostate and DNA damage in spermatozoa from bisphenol A–treated rats and formation of DNA adducts in cultured human prostatic cells. Toxicological sciences. 2011;122:45–51. doi: 10.1093/toxsci/kfr096. [DOI] [PubMed] [Google Scholar]
- 37.Yuan H, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. American Journal of Physiology-Heart and Circulatory Physiology. 2009;296:H470–H479. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherz‐Shouval R, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO journal. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bursch, W. The autophagosomal–lysosomal compartment in programmed cell death. Cell Death & Differentiation8 (2001). [DOI] [PubMed]
- 40.Levine B, Yuan J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellot GL, Liu D, Pervaiz S. ROS, autophagy, mitochondria and cancer: Ras, the hidden master? Mitochondrion. 2013;13:155–162. doi: 10.1016/j.mito.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Wang, T. et al. The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget (2016). [DOI] [PMC free article] [PubMed]
- 43.Kehler J, et al. Oct4 is required for primordial germ cell survival. EMBO reports. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z-W, et al. Laser microbeam-induced DNA damage inhibits cell division in fertilized eggs and early embryos. Cell cycle. 2013;12:3336–3344. doi: 10.4161/cc.26327. [DOI] [PMC free article] [PubMed] [Google Scholar]