Abstract
γ-Aminobutyric acid (GABA) transporters (GATs) play an important role in inhibitory neurotransmission by clearing synaptically released GABA and by maintaining low resting levels of GABA in synaptic and extrasynaptic regions. In certain brain regions, vesicular zinc is colocalized and coreleased with glutamate and modulates the behavior of a number of channels, receptors, and transporters. We examined the effect of zinc on expressed GATs (GAT1, GAT2, GAT3, and GAT4) in Xenopus laevis oocytes by using tracer flux and electrophysiological methods. We show that zinc is a potent inhibitor of GAT4 (Ki of 3 μM). Immunolocalization of GAT4 in the hippocampus revealed dense localization in the CA1 and CA3 regions of the hippocampus, regions which are known to be heavily populated by zinc-containing glutamatergic neurons. The results suggest a physiological role of synaptically released zinc in the hippocampus, because zinc released from hyperactive glutamatergic neurons may simultaneously bring about elevated GABAergic inhibition. Therefore, this mode of zinc function signifies a link between excitatory and inhibitory neurotransmission and may play a neuroprotective role against glutamate-induced excitotoxicity.
Keywords: glutamate, transport, synapse, Xenopus oocytes
In most systems that involve synaptic transmission, the termination of the signal is achieved by rapid uptake of the released neurotransmitter into the synaptic terminal or the surrounding glial cells (1, 2). This uptake is mediated by specific, high-affinity neurotransmitter transporters using sodium electrochemical gradients generated by primary ion pumps. Both glutamate (the principal excitatory neurotransmitter) transporters and γ-aminobutyric acid (GABA, the principal inhibitory neurotransmitter) transporters (GATs) operate in this way. GATs are responsible for maintaining basal GABA levels in the brain, as well as for rapid transport of synaptically released GABA (3-6). Four GAT isoforms have been identified in mammalian tissues: GAT1, GAT2, GAT3, and GAT4. These correspond to the human genes SLC6A1, SLC6A12, SLC6A13, and SLC6A11, respectively. GAT1 and GAT4 are primarily localized to the central nervous system (CNS) and are widely distributed in the brain and spinal cord (4). Because of their abundance and colocalization with GABAergic synapses, GAT1 and GAT4 are thought to be the major players in regulating GABAergic neurotransmission in the nervous system. GAT2 localization does not overlap with GABAergic synapses and, given that it transports betaine with high affinity (7, 8), it is thought to serve an osmoregulatory role in the brain and in other tissues where it is found (e.g., liver and kidney). GAT3 is densely present in the leptomeninges and choroid plexus and is thus thought to mediate GABA fluxes between plasma and the cerebrospinal fluid (9).
Nerve cells mediate fast signaling between sensory systems and the CNS and between the CNS and effector systems. Within the CNS, nerve cells form complex circuits that serve the integration of inputs and the generation of specific activity patterns. One of the hallmarks of normal brain function is the exquisite balance between excitatory and inhibitory activity within neuronal networks, which is often achieved by convergence of excitatory and inhibitory input onto postsynaptic neurons (10). For example, hippocampal CA3 pyramidal neurons receive excitatory input from glutamatergic dentate projections (i.e., the mossy fibers) and inhibitory input from GABAergic interneurons (11-13). Given that a balance between excitation and inhibition is of paramount importance to the normal function of seizure-prone hippocampal neurons, the existence of neuromodulators that act as molecular links between excitatory and inhibitory neurotransmission is expected. Of particular interest in the hippocampus is a high concentration of chelatable zinc (Zn2+) in the synaptic vesicles of glutamatergic mossy fibers (14). This pool of free zinc is colocalized with glutamate to the synaptic vesicles and is coreleased with glutamate in a calcium- and impulse-dependent manner (15-17). Zinc is present at millimolar concentrations (3-30 mM) in the synaptic vesicles (14, 18, 19), and activity-dependent zinc release has been estimated to elevate synaptic zinc concentrations from ≈10 nM to 300 μM (15, 16). Thus, zinc may be a candidate neuromodulator of excitatory and inhibitory neurotransmission.
Here, we show that at physiologically relevant concentrations, zinc very potently inhibits GAT4 (Ki of 3 μM). We also show dense localization of GAT4 in the CA1 and CA3 regions of the hippocampus. Colocalization of GAT4 with zinc-containing glutamatergic terminals in the CA1 and CA3 regions points to zinc as a potentially important neuromodulatory link between excitatory and inhibitory neurotransmission. Thus, by blocking GABA uptake, zinc coreleased with glutamate may play a role in the enhancement of GABAergic inhibitory neurotransmission, which may have a protective role against glutamate-induced excitotoxicity of seizure-prone hippocampal neurons.
Materials and Methods
Expression of GATs in Xenopus Oocytes. Stage V-VI Xenopus laevis oocytes were injected with 50 ng of cRNA for mouse GAT1 (mGAT1), mGAT2, mGAT3, mGAT4, or mGAT3/4 chimeric protein (20, 21). The GAT nomenclature adopted here is that of Liu et al. (21). The mGAT3/4 chimeric protein was constructed by using a common BglII restriction site situated after 285 and 300 codons in the reading frames of GAT3 and GAT4, respectively. This position divides the two transporters into two equal parts and allows the construction of a chimeric protein consisting of half of each transporter in tandem (see Fig. 2B Inset). Like its parental proteins, the mGAT3/4 chimeric protein exhibited Na+-dependent GABA uptake, GABA-evoked inward currents, as well as voltage-induced presteady-state charge movements. After cRNA injection, oocytes were maintained in Barth's medium (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.5/50 μg/ml gentamicin/100 μg/ml streptomycin/100 units/ml penicillin) at 18°C for up to 7 days until used in experiments. Unless otherwise indicated, experiments were performed at 21°C ± 1°C.
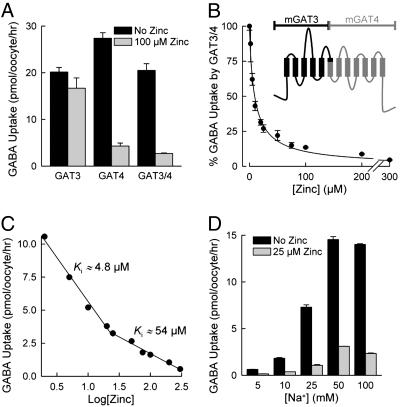
Fig. 2.
Zinc inhibition of a GAT3/4 chimeric transporter. (A) Shown is [3H]GABA uptake mediated by GAT3, GAT4, or the chimeric GAT3/4 transporter expressed in oocytes in the absence (black bars) and presence (gray bars) of 100 μM Zn2+. (B and C) Zinc inhibition of the GAT3/4 chimeric transporter also revealed high-affinity (Ki of ≈4.8 μM) and low-affinity (Ki of ≈54 μM) interaction with the protein. The data set in C is the transformed version of the data set in B. (B Inset) The predicted topology of the chimeric mGAT3/4 protein, in which the black region corresponds to the amino half of mGAT3 and the gray region corresponds to the carboxyl half of mGAT4 (see Materials and Methods). (D) GAT3/4 [3H]GABA uptake was examined at different NaCl concentrations in the absence (black bars) and presence (gray bars) of 25 μM Zn2+. Data are given as means (after subtraction of control oocyte values) ± SE (n = 20).
Experimental Solutions. Unless otherwise indicated, experiments were performed in a NaCl buffer containing 100 mm NaCl, 2 mm KCl, 1 mm CaCl2, 1 mm MgCl2,10mm Hepes, and 2 mm Mes (pH 7.4) at 21°C ± 1°C. In Na+-free solutions, NaCl was isosmotically replaced with choline chloride. In Cl--free solutions, NaCl, KCl, CaCl2, and MgCl2 were isosmotically replaced with corresponding gluconate salts. GABA, zinc, and other metals were added to the above solutions as indicated. For GABA uptake, control, mGAT1-, mGAT2-, mGAT3-, mGAT3/4-, or mGAT4-expressing oocytes were incubated for 30 min in uptake solution containing the indicated GABA concentrations and [3H]GABA (Amersham Pharmacia BioSciences). Zinc or other metals were added as indicated. At the end of the incubation period, oocytes were washed, solubilized in 10% SDS, and counted in a liquid scintillation counter (Beckman LS 7800).
Electrophysiological Measurements and Data Analysis. The two-microelectrode voltage-clamp technique was used for the recording of whole-cell transporter-mediated currents. Oocytes were voltage-clamped by using the Warner oocyte clamp (OC-725C, Warner Instruments, Hamden, CT). In the experimental recording chamber, oocytes were initially stabilized in the NaCl buffer, and the composition of the bath was changed as indicated. In all experiments, the reference electrodes were connected to the experimental oocyte chamber via agar bridges (3% agar in 3 M KCl). To examine the carrier-mediated presteady-state current transients, the pulse protocol consisted of voltage jumps (400 ms for GAT4 and 200 ms for GAT3/4) from the holding voltage (-50 mV) to test voltages ranging from +80 to -130 mV in 10-mV steps. Voltage pulses were separated by an interval of at least 4 s to allow for complete relaxation of the OFF transients (22, 23). Currents were low-pass filtered at 1 kHz and sampled at 12.5 kHz without averaging. The presteady-state transients were analyzed as previously detailed (22, 23). Where sample sizes are indicated (n), they refer to the number of oocytes in which the experiments were repeated. Reported errors represent the SE of the mean obtained from data from several oocytes.
Immunostaining of GAT4. Adult (60-day-old) rats were deeply anesthetized with avertin and perfused intracardially through the left ventricle with a 20-ml solution containing 0.9% NaCl, followed by perfusion with a solution containing 0.9% NaCl, 4% paraformaldehyde, and 0.5% zinc salicilate (pH 6.5). Brains were removed from the skull, postfixed for 1 h in the same fixative at room temperature, and cryoprotected in a solution containing 30% sucrose in PBS (pH 6.5) for 2 days at 4°C. Fixed brains were sectioned and decorated by the anti-GAT4 antibody as previously described (24). The primary antibody used was raised (in guinea pigs) against the last 103 aa of the GAT4 carboxyl terminus (24).
Results
Zinc has been found to modulate the activity of a number of receptors [NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and GABAA receptors], ion channels (K+ channels and voltage-gated Ca2+ channels), and transporters (glutamate, glycine, and dopamine transporters) known to be involved in synaptic activity (14, 19, 25-28). Physiological concentrations of zinc have been shown to exert neuroprotective and anticonvulsive effects, and these actions seem to be mediated through concurrent mitigation of excitatory neurotransmission and enhancement of inhibitory neurotransmission. An examination of the effect of zinc on the GATs is not present in refs. 14, 19, and 25-28. Given that GATs are strategically placed to remove GABA at GABAergic synapses (6, 29), and given the documented role of hippocampal GATs in seizure-related activity and temporal lobe epilepsy (30, 31), it is plausible to examine the effect of zinc on these transport proteins.
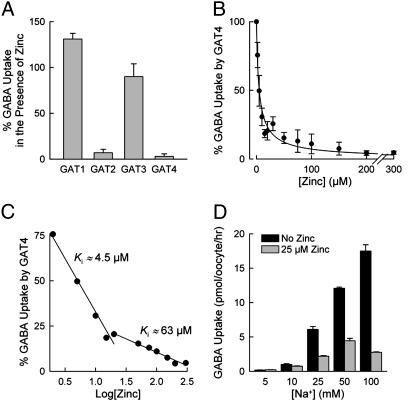
Isoform-Specific Inhibition of the GATs by Zinc. The effect of zinc on the GATs was dependent on the examined isoform. Zinc led to a complete inhibition of GAT4 (Fig. 1 A and B) and nearly complete inhibition of GAT2 (93 ± 6%; Fig. 1 A), but only partial inhibition of GAT3 (Fig. 1 A). Zinc (up to 200 μM) had no significant effect on GABA uptake mediated by GAT1 (Fig. 1 A). Because GAT4 exhibited the greatest sensitivity to Zn2+ and because in certain brain regions (e.g., hippocampus) GAT4 is colocalized with zinc-containing glutamatergic neurons (29) (see below), we have carried out a complete examination of the inhibitory effect of Zn2+ on GAT4. We have not fully investigated the inhibition of GAT2 by Zn2+ in this study.
Fig. 1.
Effect of zinc on [3H]GABA uptake by the four GAT isoforms. (A and B) Zinc nearly completely inhibited GABA uptake mediated by GAT2 and GAT4. (A) However, it only partially inhibited GABA uptake mediated by GAT3 and had no effect on GABA uptake by GAT1. Zinc concentrations were 50 μM (GAT2) and 100 μM (GAT1, GAT3, and GAT4). The values are given as a percentage of GABA uptake for each transporter in the absence of zinc. (C) Zinc inhibition of GAT4 revealed high-affinity (Ki of ≈4.5 μM) and low-affinity (Ki of ≈63 μM) interaction of zinc with GAT4. This data set is the transformed version of the data set shown in B.(D) The effect of Na+ concentration on Zn2+ inhibition of mGAT4. Shown is GAT4 [3H]GABA uptake at different NaCl concentrations in the absence (black bars) and presence (gray bars) of 25 μM Zn2+. Data are given as means (after subtraction of control oocyte values) ± SE (n = 20).
Closer examination of zinc inhibition of GAT4 revealed high-affinity (4.5 ± 0.3 μM) and low-affinity (63 ± 5 μM) interaction of zinc with GAT4 (Fig. 1C). Zinc interaction with GAT4 appeared to be influenced by Na+ as an increase of the Na+ concentration enhanced the zinc inhibition (Fig. 1D). Zinc (25 μM) inhibited 64% of GAT4 activity in the presence of 25 mM Na+ and 84% of GAT4 activity in the presence of 100 mM Na+.
Zinc also inhibited a GAT3/4 chimeric protein (Fig. 2 A and B). In this chimeric protein, the first 300 residues of mGAT4 have been replaced by the corresponding 285 residues from the closely related mGAT3 isoform. This manipulation results in a 12-transmembrane domain transporter in which approximately equal halves are contributed by the parental proteins; the amino half is contributed from the mGAT3 sequence, and the carboxyl half is contributed from the mGAT4 sequence (Fig. 2B Inset). Comparable chimeric protein composed of GAT1 and GAT4 segments was not active. Similar to that seen with GAT4, zinc exhibited high-affinity (4.8 ± 0.6 μM) and low-affinity (54 ± 7 μM) interaction with GAT3/4 (Fig. 2C). The data therefore suggest that zinc interaction with GAT4 involves two sites located in the carboxyl half of the transporter. In contrast to GAT4, zinc inhibition of the GAT3/4 chimeric protein seemed not to be influenced by the Na+ concentration (Fig. 2D).
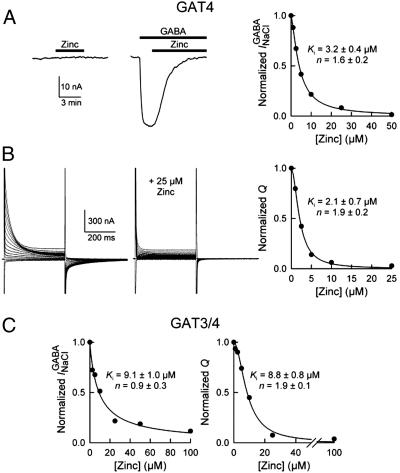
Similar to the inhibition of GABA uptake mediated by GAT4 and GAT3/4, zinc inhibited the GABA-evoked steady-state inward current, as well as the voltage-induced presteady-state charge movements of these transporters (Fig. 3). The zinc concentration for 50% inhibition of GAT4 GABA-evoked inward current was 3.2 ± 0.4 μM (n = 3) (Fig. 3A), and that for inhibition of the presteady-charge movements was 2.1 ± 0.7 μM (n = 3) (Fig. 3B). The corresponding values for inhibition of GAT3/4 were 9.1 ± 1.0 μM (n = 3) and 8.8 ± 0.8 μM (n = 3), respectively (Fig. 3C). Zinc inhibition of GAT4 GABA-evoked current was consistent with inhibition of GABA uptake, because the two events are coupled. Importantly, zinc alone did not evoke a response in oocytes expressing GAT4 (Fig. 3A) or GAT3/4, thus suggesting that zinc did not inhibit any uncoupled current in the absence of GABA. The transporter presteady-state charge movements were obtained in the absence of GABA and indicate transporter conformational changes that are induced by voltage pulses. Although zinc reduced the total charge moved, it did not lead to an alteration of the effective valence of the transporter or the half-point of the charge-voltage relationship (data not shown). In addition, zinc did not alter the time constants for the presteady-state current relaxations (data not shown). Thus, zinc interacts with GAT4 in the absence of GABA and restricts the conformational changes necessary for function.
Fig. 3.
Zinc inhibition of transporter mediated currents. (A Left) Current traces are shown from an oocyte expressing mGAT4 [holding voltage (Vm)of -50 mV]. Introduction of 50 μM Zn2+ alone had no effect on the holding current. When added in the presence of GABA (200 μM), Zn2+ (50 μM) abolished the inward current evoked by GABA ( ). (Right) The Zn2+ concentration needed to achieve 50% inhibition of the GABA-evoked current was 3.2 ± 0.4 μM (n = 3). The smooth line is the fit of the data to the following equation: I/I0 = (Ki)n/[(Ki)n + (Zn2+)n], where I0 is the GABA-evoked current in the absence of Zn2+, I is the current in the presence of a given concentration of Zn2+, Ki is the Zn2+ concentration that gives rise to 50% I0, and n is a pseudo-Hill coefficient. (B) Zinc also inhibited the mGAT4 voltage-induced presteady-state current transients. The oocyte was voltage-clamped at -50 mV. (Left) Current traces were obtained in response to voltage pulses (400 ms) ranging from +80 to -70 mV in 10-mV steps. (Right) The total charge (Qmax) moved in response to the voltage pulses was quantified in the absence and presence of increasing concentrations of Zn2+. Zinc led to a concentration-dependent reduction in Qmax. The Zn2+ concentration for 50% inhibition of the charge was 2.1 ± 0.7 μM(n = 3). (C) Zinc also inhibited the GABA-evoked current (Ki of 9.1 ± 1.0 μM; n = 3) and the presteady-state currents (Ki of 8.8 ± 0.8 μM; n = 3) of the GAT3/4 chimeric transporter.
). (Right) The Zn2+ concentration needed to achieve 50% inhibition of the GABA-evoked current was 3.2 ± 0.4 μM (n = 3). The smooth line is the fit of the data to the following equation: I/I0 = (Ki)n/[(Ki)n + (Zn2+)n], where I0 is the GABA-evoked current in the absence of Zn2+, I is the current in the presence of a given concentration of Zn2+, Ki is the Zn2+ concentration that gives rise to 50% I0, and n is a pseudo-Hill coefficient. (B) Zinc also inhibited the mGAT4 voltage-induced presteady-state current transients. The oocyte was voltage-clamped at -50 mV. (Left) Current traces were obtained in response to voltage pulses (400 ms) ranging from +80 to -70 mV in 10-mV steps. (Right) The total charge (Qmax) moved in response to the voltage pulses was quantified in the absence and presence of increasing concentrations of Zn2+. Zinc led to a concentration-dependent reduction in Qmax. The Zn2+ concentration for 50% inhibition of the charge was 2.1 ± 0.7 μM(n = 3). (C) Zinc also inhibited the GABA-evoked current (Ki of 9.1 ± 1.0 μM; n = 3) and the presteady-state currents (Ki of 8.8 ± 0.8 μM; n = 3) of the GAT3/4 chimeric transporter.
Together, the data suggest that, by restricting transporter conformational changes, zinc very potently inhibits all functional features of GAT4 (GABA uptake, GABA-evoked current, and presteady-state charge movements) and that zinc's interaction with GAT4 likely involves low-affinity and high-affinity binding sites located at the carboxyl half of the transporter.
Inhibition of GATs by Metal Ions. The fact that similar Ki values were obtained for the inhibition of Na+/Cl-/GABA cotransport (in the presence of GABA) and the voltage-induced presteady-state charge movements (in the absence of GABA) suggests a noncompetitive inhibition of GAT4 by zinc. The fact that the Ki is in the low micromolar range suggests that three coordination sites may be involved in zinc binding to GAT4 (32). This mode of inhibition is reminiscent of the mechanism by which zinc inhibits the related dopamine transporter, in which residues in transmembrane domains 3, 4, and 7 have been implicated in zinc binding to the transporter (26, 32). Remarkably, mutation of homologous residues in rat GAT1 rendered this otherwise zinc-insensitive transporter sensitive to zinc (33). Our results suggest that zinc binding to GAT4 uses a zinc binding site that has not been previously described.
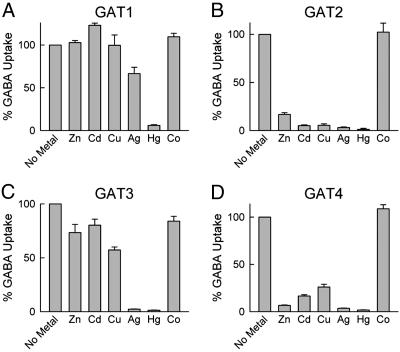
We examined the effect of a number of metal ions on GABA uptake by the four GAT isoforms (Fig. 4). GAT1 was resistant to most metal ions, and only Hg+ diminished its GABA uptake activity. GAT2 and GAT4 exhibited high sensitivity to metal ions, and Zn2+, Cd2+, Cu2+, Ag+, and Hg+ strongly inhibited their activity. GAT3 was quite resistant to the various metal ions but was highly sensitive to Ag+ and Hg+. The presence of relatively high (100 μM) Zn2+ concentration was also inhibitory. This inhibition was sensitive to the Na+ concentration in the medium because, at 50 mM Na+, the inhibition by Zn2+ was ≈50%, but, at 100 mM Na+ concentration, the inhibition by Zn2+ was only ≈20% (data not shown). Thus, of all of the metal ions tested, only Zn2+ inhibited GABA transport at physiological concentrations, and only GAT4 was sensitive enough to be significantly modulated by the Zn2+ concentrations that are present in and around synapses during glutamatergic neurosecretion. However, exposure to toxic levels of Cd2+, Ag+, and Hg+ might cause alteration in the balance of neurotransmission through the inhibition of neurotransmitter transporters.
Fig. 4.
Effect of metals on [3H]GABA uptake by the four GAT isoforms. In all experiments, the metal concentration was 100 μM. The NaCl concentration was 100 mM for GAT1-expressing (A) and GAT2-expressing (B) cells, and 50 mM for GAT3-expressing (C) and GAT4-expressing (D) cells. The unlabeled GABA concentration was 1 μM (GAT1), 20 μM (GAT2), and 5 μM (GAT3 and GAT4). Uptake values in the presence of metals are reported as a percentage of uptake for each transporter in the absence of metal (No Metal). Five oocytes were used for each condition, and each experiment was repeated four times with oocytes from different donor frogs. Data are given as means (after subtraction of control oocyte values) ± SE.
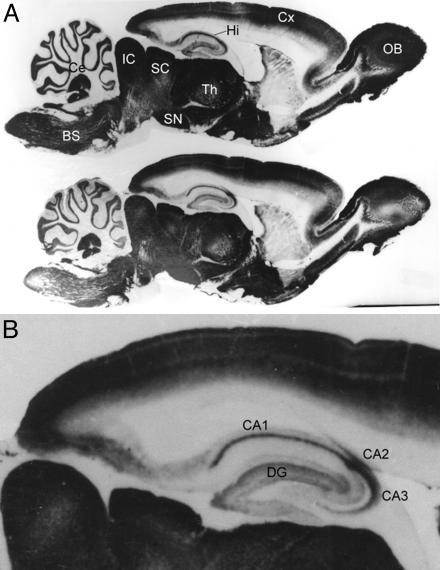
Immunolocalization of GAT4 in Brain Regions Rich in Zinc. Although all zinc-containing neurons are glutamatergic neurons, not all glutamatergic neurons contain zinc (14, 18). Thus, whether zinc plays a role in modulating synaptic neurotransmission depends on the location. Brain zinc concentrations are highest in the hippocampal CA1 and CA3 regions. Zinc is heavily localized to the granular cell mossy fiber terminals located in the CA3 region of the hippocampus, and its release at this mossy fiber pyramidal cell synapse is thought to play a number of important roles (11). To examine the CNS localization of GAT4 in the adult brain, we examined the localization of GAT4 in parasagittal sections of the adult rat brain. Intense GAT4 immunoreactivity was observed in numerous regions. Particularly important was the dense staining in the hippocampal CA1, CA2, and CA3 regions (Fig. 5).
Fig. 5.
Immunoreactivity of GAT4 in parasagittal sections of rat brain. Antibody against the last 103 aa of the GAT4 carboxyl terminus was raised in guinea pigs (20). Brain of a 60-day old female rat was sectioned and decorated by the anti-GAT4 antibody as previously described (20). (A) Two parasagittal sections are shown. Strong GAT4 immunostaining was detected in the olfactory bulb (OB), cerebral cortex (Cx), thalamus (Th), hippocampus (Hi), superior colliculus (SC), inferior colliculus (IC), substantia nigra (SN), cerebellum (Ce), and brainstem (BS). (B) Within the hippocampus, intense GAT4 immunostaining was observed in the dentate gyrus (DG) and CA1, CA2, and CA3 regions.
Most extracellular zinc is bound to metalloproteins, and, thus, the basal zinc concentration in the brain extracellular fluid is <500 nM (18, 34). Activation of zinc-containing neurons may raise the local synaptic zinc concentration to ≈300 μM (16), although, because of the presence of zinc-binding proteins in the extracellular fluid, the actual free zinc concentration at the activated mossy fiber CA3 synapse is 10-100 μM (35, 36). Our data show clearly that even this reduced concentration is sufficient to bring ≈92-100% inhibition of GAT4 (given a Ki of 3 μM and a Hill coefficient of 2).
Discussion
The Crosstalk of Zinc Between Excitatory and Inhibitory Neurotransmission. Zinc is the second most abundant transition element in the mammalian body (iron being the first). In the brain, zinc is most concentrated in the limbic system, particularly in the hippocampus and amygdala (18, 37). Zinc can be detected by Timm's sulfide-silver staining method, which localizes zinc to the presynaptic vesicles of some glutamatergic neurons (17, 38). Zinc-containing synaptic vesicles also contain glutamate and possess the vesicular transporters for zinc (ZnT-3) and glutamate (VGLUT-1) (39). This pool of zinc may be readily released into the synaptic cleft along with glutamate in a calcium- and impulse-dependent manner upon vesicle fusion with the presynaptic plasma membrane (15, 16). Because of its interaction with a number of receptors, channels, and transporters, zinc coreleased with glutamate is thought to play a neuromodulatory role in shaping synaptic events (18, 19).
In vitro examination of the effect of zinc on different proteins involved in synaptic activity has revealed a complicated picture of opposing effects (14, 19). Zinc inhibits the NMDA receptors (40, 41), voltage-gated calcium channels (42), GABAA receptors (43-45), and the transporters for glutamate (25, 46), dopamine (26, 32), and glycine (28). Interestingly, in many cases, the effectiveness of zinc depends on the isoform examined and/or subunit present. For example, GABAA receptors composed of the γ subunit are far less sensitive to Zn2+ inhibition than are the receptors with different subunits. Furthermore, it seems that synaptic GABAA receptors have evolved as less sensitive to Zn2+ than extrasynaptic GABAA receptors by including the γ subunit (44). In contrast, zinc seems to potentiate the AMPA subtype of glutamate receptors (47, 48). Despite these apparently conflicting effects, a general picture has emerged that zinc, depending on its free concentration, has opposing effects on neurons. Low levels of zinc protect neurons against seizure activity and glutamate-mediated excitotoxicity, whereas high levels of zinc induce seizures and lead to neuronal death (14). The cytotoxic effects of zinc are observed with concentrations as low as 100 μM and seem to result from zinc entry into neurons via zinc-permeable cation-selective channels (e.g., AMPA and NMDA receptors and voltage-gated calcium channels), with the ultimate consequence of disruption of intracellular enzymatic function (14). Such events may take place during stroke, and it is interesting that zinc chelation reduces excitotoxic neuronal injury caused by stroke and traumatic brain injury.
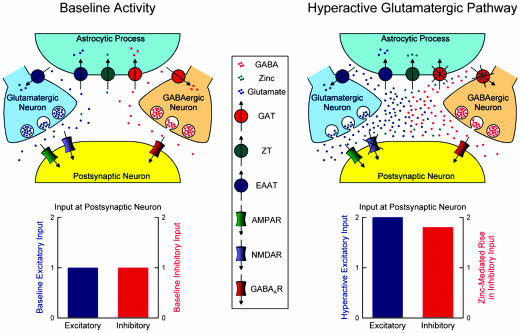
Our findings allow us to put forth a model for a potential physiological role of zinc in the hippocampus (Fig. 6). A typical hippocampal neuron receives both excitatory (glutamatergic) and inhibitory (GABAergic) input. At any given time, there is some balance between the excitatory and inhibitory inputs received by this neuron. Zinc is coreleased with glutamate and is known to modulate the function of a number of targets; in particular, zinc inhibits postsynaptic NMDA receptors, as well as GAT4 present in astrocytic processes. However, under normal conditions, zinc synaptic and extrasynaptic concentrations may be too low to exert an appreciable effect. When excitatory input is dramatically increased, overexcitation by glutamate may lead to excitotoxicity of the postsynaptic cell, but concurrent release of zinc elevates its synaptic and extrasynaptic concentrations to such levels as to inhibit the postsynaptic NMDA receptors as well as astrocytic GAT4. The ultimate consequence of GAT4 inhibition is an elevation of synaptic and extrasynaptic GABA levels in the region. Elevated GABA levels, together with inhibition of postsynaptic NMDA receptors, reduce the probability that the postsynaptic neuron may reach excitotoxic levels of activity. Thus, concurrent release of zinc with glutamate may have a protective effect against glutamate-induced excitotoxicity. Colocalization of GAT4 (see Fig. 5) and zinc-containing glutamatergic neurons (obtained by Timm's staining) suggest that this scenario may be likely in the hippocampus, where a delicate balance must exist between excitation and inhibition in the epilepsy-prone hippocampal cells. The zinc-containing glutamatergic neuron represents a mossy fiber terminal forming synaptic contact with a CA3 pyramidal neuron (11), whereas the GABAergic neuron may be either a GABAergic mossy fiber (49, 50) or a GABAergic interneuron (13, 50).
Fig. 6.
A model of the physiological role of zinc. (Upper) The scheme shows the mossy fiber CA3 pyramidal cell synapse under normal (Left) and hyperactive (Right) states. The postsynaptic cell is a CA3 hippocampal neuron. The glutamatergic terminal is that of a dentate mossy fiber, and the GABAergic terminal may be either a GABAergic mossy fiber or a GABAergic interneuron. Transport of zinc into the presynaptic vesicles is carried out by an unknown zinc transporter. The hyperactive glutamatergic pathway is generated by extensive excitation of glutamatergic neurons. (Bottom) The predicted relative excitatory and inhibitory input onto the postsynaptic neuron under normal (Left) and hyperactive (Right) states. ZT, zinc transporter; EAAT, excitatory amino acid (glutamate) transporter; AMPAR, AMPA receptor; NMDAR, NMDA receptor; GABAAR, GABAA receptor.
Possible Connection to Epileptic Seizures. A number of observations point to an anticonvulsive and neuroprotective role of low concentrations of zinc in the hippocampus. (i) Zinc-depleted animals have reduced hippocampal zinc levels and tend to exhibit a greater frequency of epileptiform activity than do normal controls (51). (ii) In mice genetically selected for ethanol-withdrawal seizure susceptibility, significant reductions in mossy fiber terminal zinc levels are found (52). (iii) An important observation that supports the role of vesicular zinc in setting a balance between excitatory and inhibitory neurotransmission has come from ZnT-3 knockout mice. These animals exhibit a lack of vesicular zinc in neurons and, remarkably, are more sensitive to kainate-induced seizures (53, 54). (iv) In the epilepsy-prone E1 mice, in which seizures seem to originate in the hippocampus, zinc concentration in the hippocampal dentate area is lower than in that of control animals, suggesting a role for zinc in the pathophysiology of seizures (55). (v) Zinc chelation experiments suggest that zinc released from mossy fibers induced by tetanic stimulation serves to prevent bursting behavior of CA3 pyramidal cells (56). (vi) Moreover, evidence has accumulated in favor of zinc modulation of GABAergic neurotransmission in the CA3 subfield of the hippocampus. Superfusion of CA3 pyramidal neurons with as little as 10 μM zinc leads to an increase in GABA levels in this region, and, conversely, chelation of endogenous zinc leads to a decrease in extracellular GABA (57).
The available evidence is consistent with a role of zinc in modulating the balance between excitatory and inhibitory synaptic transmission. The data suggest that zinc exerts its anticonvulsive and neuroprotective effects by reducing excitatory neurotransmission while enhancing inhibitory neurotransmission. Zinc inhibition of GAT4 is entirely consistent with the hypothesis that zinc elevation of hippocampal GABA levels may be in part due to inhibition of GAT4. The balance between excitatory and inhibitory input may be most important in epilepsy-prone structures such as the hippocampus, where GABA-mediated inhibition is critical in suppressing overexcitation. We speculate that high frequency activation of dentate granule cells leads to the release of zinc into the mossy fiber CA3 pyramidal cell synapse. This zinc not only leads to the inhibition of pyramidal cell NMDA receptors but also inhibits the surrounding GAT4 molecules. The result is an increase in synaptic and extrasynaptic GABA levels, with the ultimate consequence of enhancing GABA-mediated inhibition of the CA3 pyramidal cell. Thus, by the concurrent mitigation of excitatory transmission and enhancement of inhibitory transmission, zinc may protect neurons against glutamate-induced neurotoxicity.
Acknowledgments
We thank Gail M. Drus and Michael J. Errico for technical assistance. This work was supported by National Institutes of Health Grant S06 GM53933 (to S.E.) and by United States-Israel Binational Science Foundation Grant 99-002 (to N.N.).
Author contributions: E.C.-K., W.L., S.E., and N.N. designed research; E.C.-K., W.L., S.E., and N.N. performed research; E.C.-K., W.L., S.E., and N.N. analyzed data; and E.C.-K., S.E., and N.N. wrote the paper.
Abbreviations: GAT, GABA transporter; mGATn, mouse GATn; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
References
- 1.Kanner, B. I. (1989) Curr. Opin. Cell Biol. 1, 735-738. [DOI] [PubMed] [Google Scholar]
- 2.Roettger, V. R. & Amara, S. G. (1999) Adv. Neurol. 79, 551-560. [PubMed] [Google Scholar]
- 3.Borden, L. A. (1996) Neurochem. Int. 29, 335-356. [DOI] [PubMed] [Google Scholar]
- 4.Nelson, N. (1998) J. Neurochem. 71, 1785-1803. [DOI] [PubMed] [Google Scholar]
- 5.Gadea, A. & López-Colomé, A. M. (2001) J. Neurosci. Res. 63, 461-468. [DOI] [PubMed] [Google Scholar]
- 6.Conti, F., Minelli, A. & Melone, M. (2004) Brain Res. Brain Res. Rev. 45, 196-212. [DOI] [PubMed] [Google Scholar]
- 7.Uchida, S., Kwon, H. M., Yamauchi, A., Preston, A. S., Marumo, F. & Handler, J. S. (1992) Proc. Natl. Acad. Sci. USA 89, 8230-8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jursky, F. & Nelson, N. (1999) J. Neurosci. Res. 55, 24-35. [DOI] [PubMed] [Google Scholar]
- 9.Jursky, F. & Nelson, N. (1999) J. Neurosci. Res. 55, 394-399. [DOI] [PubMed] [Google Scholar]
- 10.Kandel, E. R., Schwartz, J. H. & Jessell, T. M. (2000) Principles of Neural Science (McGraw-Hill, New York).
- 11.Henze, D. A., Urban, N. N. & Barrionuevo, G. (2000) Neuroscience 98, 407-427. [DOI] [PubMed] [Google Scholar]
- 12.Weiss, J. H., Sensi, S. L. & Koh, J. Y. (2000) Trends Pharmacol. Sci. 21, 395-401. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence, J. J. & McBain, C. J. (2003) Trends Neurosci. 26, 631-640. [DOI] [PubMed] [Google Scholar]
- 14.Frederickson, C. J. & Moncrieff, D. W. (1994) Biol. Signals 3, 127-139. [DOI] [PubMed] [Google Scholar]
- 15.Assaf, S. Y. & Chung, S. H. (1984) Nature 308, 734-736. [DOI] [PubMed] [Google Scholar]
- 16.Howell, G. A., Welch, M. G. & Frederickson, C. J. (1984) Nature 308, 736-738. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu, C., Dyck, R. & Cynader, M. (1992) NeuroReport 3, 861-864. [DOI] [PubMed] [Google Scholar]
- 18.Frederickson, C. J. (1989) Int. Rev. Neurobiol. 31, 145-238. [DOI] [PubMed] [Google Scholar]
- 19.Frederickson, C. J. & Bush, A. I. (2001) Biometals 14, 353-366. [DOI] [PubMed] [Google Scholar]
- 20.Nelson, H., Mandiyan, S. & Nelson, N. (1990) FEBS Lett. 269, 181-184. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Q.-R., López-Corcuera, B., Mandiyan, S., Nelson, H. & Nelson, N. (1993) J. Biol. Chem. 268, 2106-2112. [PubMed] [Google Scholar]
- 22.Sacher, A., Nelson, N., Ogi, J. T., Wright, E. M., Loo, D. D. F. & Eskandari, S. (2002) J. Membr. Biol. 190, 57-73. [DOI] [PubMed] [Google Scholar]
- 23.Whitlow, R. D., Sacher, A., Loo, D. D. F., Nelson, N. & Eskandari, S. (2003) J. Biol. Chem. 278, 17716-17726. [DOI] [PubMed] [Google Scholar]
- 24.Jursky, F. & Nelson, N. (1996) J. Neurochem. 67, 857-867. [DOI] [PubMed] [Google Scholar]
- 25.Vandenberg, R. J., Mitrovic, A. D. & Johnston, G. A. (1998) Mol. Pharmacol. 54, 189-196. [DOI] [PubMed] [Google Scholar]
- 26.Norregaard, L., Frederiksen, D., Nielsen, E. O. & Gether, U. (1998) EMBO J. 17, 4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tapiero, H. & Tew, K. D. (2003) Biomed. Pharmacother. 57, 399-411. [DOI] [PubMed] [Google Scholar]
- 28.Ju, P., Aubrey, K. R. & Vandenberg, R. J. (2004) J. Biol. Chem. 279, 22983-22991. [DOI] [PubMed] [Google Scholar]
- 29.Sperk, G., Schwarzer, C., Heilman, J., Furtinger, S., Reimer, R. J., Edwards, R. H. & Nelson, N. (2003) Hippocampus 13, 806-815. [DOI] [PubMed] [Google Scholar]
- 30.During, M. J., Ryder, K. M. & Spencer, D. D. (1995) Nature 376, 174-177. [DOI] [PubMed] [Google Scholar]
- 31.Williamson, A., Telfeian, A. E. & Spencer, D. D. (1995) J. Neurophysiol. 74, 378-387. [DOI] [PubMed] [Google Scholar]
- 32.Loland, C. J., Norregaard, L. & Gether, U. (1999) J. Biol. Chem. 274, 36928-36934. [DOI] [PubMed] [Google Scholar]
- 33.MacAulay, N., Bendahan, A., Loland, C. J., Zeuthen, T., Kanner, B. I. & Gether, U. (2001) J. Biol. Chem. 276, 40476-40485. [DOI] [PubMed] [Google Scholar]
- 34.Takeda, A. (2000) Brain Res. Brain Res. Rev. 34, 137-148. [DOI] [PubMed] [Google Scholar]
- 35.Li, Y., Hough, C. J., Suh, S. W., Sarvey, J. M. & Frederickson, C. J. (2001) J. Neurophysiol. 86, 2597-2604. [DOI] [PubMed] [Google Scholar]
- 36.Vogt, K., Mellor, J., Tong, G. & Nicoll, R. (2000) Neuron 26, 187-196. [DOI] [PubMed] [Google Scholar]
- 37.Sawashita, J., Takeda, A. & Okada, S. (1997) Brain Res. Dev. Brain Res. 102, 295-298. [DOI] [PubMed] [Google Scholar]
- 38.Ibata, Y. & Otsuka, N. (1969) J. Histochem. Cytochem. 17, 171-175. [DOI] [PubMed] [Google Scholar]
- 39.Sindreu, C. B., Varoqui, H., Erickson, J. D. & Pérez-Clausell, J. (2003) Cereb. Cortex 13, 823-829. [DOI] [PubMed] [Google Scholar]
- 40.Chen, N., Moshaver, A. & Raymond, L. A. (1997) Mol. Pharmacol. 51, 1015-1023. [DOI] [PubMed] [Google Scholar]
- 41.Paoletti, P., Ascher, P. & Neyton, J. (1997) J. Neurosci. 17, 5711-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busselberg, D., Platt, B., Michael, D., Carpenter, D. O. & Haas, H. L. (1994) J. Neurophysiol. 71, 1491-1497. [DOI] [PubMed] [Google Scholar]
- 43.Barberis, A., Cherubini, E. & Mozrzymas, J. W. (2000) J. Neurosci. 20, 8618-8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosie, A. M., Dunne, E. L., Harvey, R. J. & Smart, T. G. (2003) Nat. Neurosci. 6, 362-369. [DOI] [PubMed] [Google Scholar]
- 45.Ruiz, A., Walker, M. C., Fabian-Fine, R. & Kullmann, D. M. (2004) J. Neurophysiol. 91, 1091-1096. [DOI] [PubMed] [Google Scholar]
- 46.Spiridon, M., Kamm, D., Billups, B., Mobbs, P. & Attwell, D. (1998) J. Physiol. 506, 363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rassendren, F. A., Lory, P., Pin, J. P. & Nargeot, J. (1990) Neuron 4, 733-740. [DOI] [PubMed] [Google Scholar]
- 48.Lin, D. D., Cohen, A. S. & Coulter, D. A. (2001) J. Neurophysiol. 85, 1185-1196. [DOI] [PubMed] [Google Scholar]
- 49.Walker, M. C., Ruiz, A. & Kullmann, D. M. (2001) Neuron 29, 703-715. [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez, R. (2003) Prog. Neurobiol. 71, 337-358. [DOI] [PubMed] [Google Scholar]
- 51.Fukahori, M. & Itoh, M. (1990) Brain Res. 529, 16-22. [DOI] [PubMed] [Google Scholar]
- 52.Feller, D. J., Tso-Olivas, D. Y. & Savage, D. D. (1991) Brain Res. 545, 73-79. [DOI] [PubMed] [Google Scholar]
- 53.Cole, T. B., Wenzel, H. J., Kafer, K. E., Schwartzkroin, P. A. & Palmiter, R. D. (1999) Proc. Natl. Acad. Sci. USA 96, 1716-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole, T. B., Robbins, C. A., Wenzel, H. J., Schwartzkroin, P. A. & Palmiter, R. D. (2000) Epilepsy Res. 39, 153-169. [DOI] [PubMed] [Google Scholar]
- 55.Fukahori, M., Itoh, M., Oomagari, K. & Kawasaki, H. (1988) Brain Res. 455, 381-384. [DOI] [PubMed] [Google Scholar]
- 56.Xu, H. & Mitchell, C. L. (1993) Brain Res. 624, 162-170. [DOI] [PubMed] [Google Scholar]
- 57.Takeda, A., Minami, A., Seki, Y. & Oku, N. (2004) J. Neurosci. Res. 75, 225-229. [DOI] [PubMed] [Google Scholar]