ABSTRACT
Improving access to the carbohydrate content of lignocellulose is key to reducing recalcitrance for microbial deconstruction and conversion to fuels and chemicals. Caldicellulosiruptor bescii completely solubilizes naked microcrystalline cellulose, yet this transformation is impeded within the context of the plant cell wall by a network of lignin and hemicellulose. Here, the bioavailability of carbohydrates to C. bescii at 70°C was examined for reduced lignin transgenic switchgrass lines COMT3(+) and MYB Trans, their corresponding parental lines (cultivar Alamo) COMT3(−) and MYB wild type (WT), and the natural variant cultivar Cave-in-Rock (CR). Transgenic modification improved carbohydrate solubilization by C. bescii to 15% (2.3-fold) for MYB and to 36% (1.5-fold) for COMT, comparable to the levels achieved for the natural variant, CR (36%). Carbohydrate solubilization was nearly doubled after two consecutive microbial fermentations compared to one microbial step, but it never exceeded 50% overall. Hydrothermal treatment (180°C) prior to microbial steps improved solubilization 3.7-fold for the most recalcitrant line (MYB WT) and increased carbohydrate recovery to nearly 50% for the least recalcitrant lines [COMT3(+) and CR]. Alternating microbial and hydrothermal steps (T→M→T→M) further increased bioavailability, achieving carbohydrate solubilization ranging from 50% for MYB WT to above 70% for COMT3(+) and CR. Incomplete carbohydrate solubilization suggests that cellulose in the highly lignified residue was inaccessible; indeed, residue from the T→M→T→M treatment was primarily glucan and inert materials (lignin and ash). While C. bescii could significantly solubilize the transgenic switchgrass lines and natural variant tested here, additional or alternative strategies (physical, chemical, enzymatic, and/or genetic) are needed to eliminate recalcitrance.
IMPORTANCE Key to a microbial process for solubilization of plant biomass is the organism's access to the carbohydrate content of lignocellulose. Economically viable routes will characteristically minimize physical, chemical, and biological pretreatment such that microbial steps contribute to the greatest extent possible. Recently, transgenic versions of plants and trees have been developed with the intention of lowering the barrier to lignocellulose conversion, with particular focus on lignin content and composition. Here, the extremely thermophilic bacterium Caldicellulosiruptor bescii was used to solubilize natural and genetically modified switchgrass lines, with and without the aid of hydrothermal treatment. For lignocellulose conversion, it is clear that the microorganism, plant biomass substrate, and processing steps must all be considered simultaneously to achieve optimal results. Whether switchgrass lines engineered for low lignin or natural variants with desirable properties are used, conversion will depend on microbial access to crystalline cellulose in the plant cell wall.
KEYWORDS: Caldicellulosiruptor, switchgrass, lignocellulose deconstruction and conversion, extreme thermophiles, lignocellulose
INTRODUCTION
The intrinsic recalcitrance of plant biomass renders its deconstruction a major technical and economic hurdle to the development of conversion processes for fuels and chemicals (1–3). Lignocellulose is composed of three tightly interconnected biopolymers: cellulose, hemicellulose, and lignin (4, 5). For microbially based bioprocesses, the bioavailability of the carbohydrate content of the plant cell wall is a critical factor in achieving high yields and conversion efficiencies. Carbohydrate access to microbial attack varies considerably with plant properties (6), but this issue must be addressed to develop optimal bioprocesses for generating bio-based products from renewable resources.
Extremely thermophilic bacteria in the genus Caldicellulosiruptor can metabolize the carbohydrate content of plant cell walls (7, 8). These Gram-positive, anaerobic bacteria grow optimally at 70 to 78°C and coferment C5 and C6 sugars to generate acetate, lactate, H2, and CO2 as primary fermentation products (9–12). Thus, they offer a distinct advantage over microbes that can ferment only C6 sugars or that are subject to carbon catabolite repression (7). When encountering plant biomass, Caldicellulosiruptor responds by upregulating carbohydrate ABC transporters and unique multidomain extracellular glycoside hydrolases (6, 8), enzymes that are crucial to the degradation capacity of the microbe (13, 14). The model organism in this genus, Caldicellulosiruptor bescii, not only utilizes unpretreated plant biomass (8, 15, 16) but has been genetically modified for improved crystalline cellulose degradation (17), xylan degradation (18), and ethanol production (19). These characteristics make C. bescii a promising metabolic engineering platform for consolidated bioprocessing (CBP) of lignocellulosic feedstocks. Given that C. bescii can utilize pentose sugars (hemicellulose) and crystalline cellulose (15), lignin is the major barrier preventing microbial access to the entire store of plant carbohydrates.
Approaches for improving microbial accessibility to the plant's sugars, thereby making them more susceptible to degradation, have focused primarily on either developing genetically modified plants with reduced lignin composition or exposing the plant biomass to physical, chemical, and enzymatic treatments (1, 20, 21). In particular, genetic modification of feedstocks to reduce and/or modify lignin structure can improve fermentation yield by increasing enzymatic digestion and conversion of hemicellulose and cellulose (22–28). Switchgrass (Panicum virgatum L.), which is one of the most promising renewable feedstocks (29, 30) in part due to its geographic versatility, high biomass yields, and ability to grow on marginal land (low agronomic input requirements) (29, 31–33), has been the focus of extensive efforts to further optimize its use as a biofuel substrate through genetic engineering. Engineering strategies include modifying lignin (e.g., altering the syringyl/guaiacyl lignin monomer ratio), decreasing cellulose crystallinity, increasing plant polysaccharide content and overall plant biomass, and expressing recombinant cellulases and hemicellulases in the plant (34). Two such efforts of interest here are downregulation of the caffeic acid 3-O-methyltransferase (COMT3) (23) and overexpression of the R2-R3 MYB transcription factor PvMYB4 (MYB) (25). The COMT enzyme converts hydroxyconiferaldehyde and 5-hydroxyconiferyl alcohol to sinapaldehyde and sinapyl alcohol (35), respectively; thus, its downregulation reduces S-lignin content (36). With COMT3(+) switchgrass, the syringyl/guaiacyl lignin ratio was reduced and acetyl bromide (AcBr) lignin was reduced 13% (∼18.5% to ∼16%) relative to the unmodified control line (23). Saccharification efficiency with COMT3(+) switchgrass increased 29 to 38%, required a 3- to 4-fold-lower cellulase dosage for simultaneous saccharification and fermentation (SSF), and led to a higher ethanol yield (23). The R2-R3 MYB transcription factors comprise a large family of plant proteins that play a role in a variety of plant processes, including development, metabolism, and responses to biotic and abiotic stresses (37). Among other effects, MYB4 specifically represses the expression of the cinnamate 3-hydroxylase (C4H) gene (38), which encodes the enzyme responsible for the conversion of cinnamic acid to 4-coumaric acid in the lignin biosynthetic pathway (39). In the overexpressing switchgrass line (MYB4-OE), the ratio of ester-linked p-coumarate to ferulate (p-CA/FA ratio) was reduced ∼50%, together with a 40% reduction of AcBr lignin (∼25% to ∼15%), relative to the unmodified control line (25). These changes to lignin in the MYB Trans switchgrass led to a 3-fold increase in saccharification efficiency and ethanol yield (25, 26).
Here, we examined the bioavailability of the carbohydrate content of several switchgrass lines to C. bescii by assessing the bacterium's capacity to process unmodified Cave-in-Rock (CR) switchgrass and two reduced lignin switchgrass lines (and their wild-type [WT] parent strains) derived from the Alamo variety, each with and without hydrothermal treatment (180°C for 25 min). The results of this study show (i) that the combined action of hydrothermal treatment and reduced-lignin (genetically modified) switchgrass increases the bioavailability of plant carbohydrates to the fermenting microbe (C. bescii) relative to that for the unmodified parental switchgrass line, (ii) that hydrothermal treatment releases mostly hemicellulose (xylan and arabinan), which would be preserved and consumed in an industrial bioprocess, and improves C. bescii's access to glucan, and (iii) that certain unmodified natural variants (e.g., Cave-in-Rock, used in this study) may inherently have reduced recalcitrance relative to that of the genetically modified developed lines, enabling improved deconstruction and fermentation by C. bescii.
RESULTS
Biosolubilization of untreated transgenic switchgrass.
Cave-in-Rock (CR) switchgrass (field grown and unmodified), two transgenic switchgrass lines, and their respective WT parents were assessed for deconstruction by C. bescii at 12 days postinoculation at a loading of 5 g/liter. CR was included in our analysis as a reference genotype, given that it has been previously studied as a biofuel feedstock (8, 32, 33, 40). In one transgenic switchgrass line, COMT3(+), the caffeic acid 3-O-methyltransferase was downregulated, generating a transgenic switchgrass line with a 13% lower AcBr lignin level, an altered lignin composition (less S-lignin), and a normal growth phenotype (23, 27, 28). In the other transgenic line, MYB Trans, the R2-R3 MYB transcription factor PvMYB4 was overexpressed, resulting in switchgrass with a 40% lower AcBr lignin level, an altered lignin composition (lower p-CA/FA ratio), and an improved tillering growth phenotype (25, 26). The corresponding parental lines, COMT3(−) and MYB WT, were also examined.
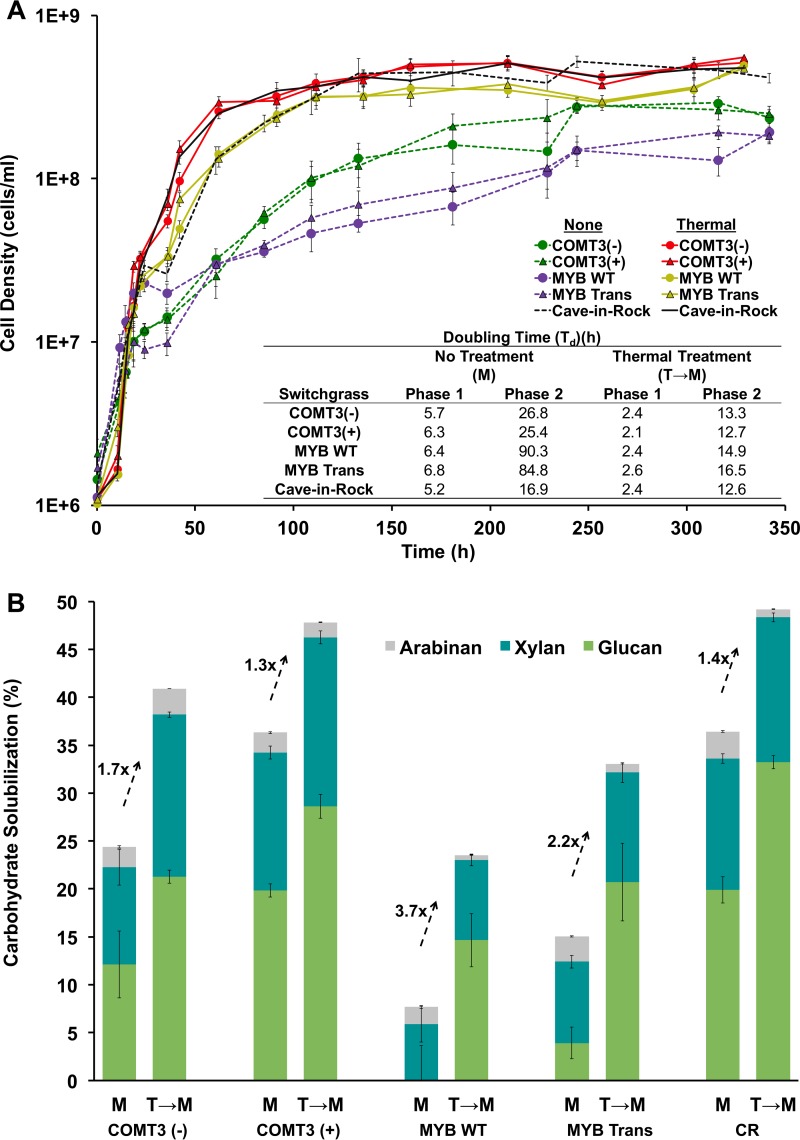
The recalcitrance of each switchgrass line was assessed by tracking C. bescii planktonic growth and the corresponding amount of carbohydrate that was solubilized. Growth and solubilization experiments were done at 70°C in small batch fermentations (closed serum bottles with 50-ml working volumes) with washed switchgrass at a loading of 5 g/liter. Low switchgrass loadings were chosen to focus on the recalcitrance of the switchgrass by minimizing the accumulation of inhibitory fermentative end products. C. bescii growth on all five untreated switchgrass lines was biphasic exponential, with initial rapid growth (phase 1, 11.5 to 18.5 h) followed by a markedly slower growth phase (phase 2, 24.0 to 60.5 h) (Fig. 1A). Small increases in the C. bescii growth rate were observed with the transgenic switchgrass lines [COMT3(+) or MYB Trans] relative to those of their corresponding parental line [COMT3(−) or MYB WT] in both phase 1 and phase 2 (Fig. 1A). Larger differences in growth rate were seen between the individual WT switchgrass genotypes (COMT3 versus MYB versus CR). For example, C. bescii planktonic doubling times during phase 2 (td2), from fastest to slowest, were CR (16.9 h), COMT3 [25.4 h for COMT3(+) and 26.8 h for COMT3(−)], and MYB (84.8 h for MYB Trans and 90.3 h for MYB WT) (Fig. 1A). The maximum cell density and the time it took to get there also varied between switchgrass lines. Fermentation of CR resulted in the highest cell density (5.2E+8 cells/ml at 244 h), followed by fermentation of COMT3(+) (2.8E+8 cells/ml at 244 h), COMT3(−) (2.9E+8 cells/ml at 316 h), MYB Trans (1.9E+8 cells/ml at 316 h), and MYB WT (1.9E+8 cells/ml at 342 h). The C. bescii growth rate was lowest on MYB switchgrass (WT and Trans), which corresponded to 10 to 30 percentage points lower carbohydrate solubilization than for the COMT3 lines and the CR line (Fig. 1B, microbe only [M]; see Table S1 in the supplemental material). The lower planktonic growth rate of COMT3(+) than of CR (reference genotype) did not reflect differences in solubilization, with both switchgrass lines decreasing by ∼36% in carbohydrate content (Fig. 1B). Though minor differences in C. bescii growth were observed between the respective transgenic and parental lines, transgenic modification (reduced lignin content) improved carbohydrate solubilization considerably (Fig. 1B, M; Table S1). Solubilization of COMT3(+) (36.3% ± 1.5%) was 1.5-fold higher than that of COMT3(−) (24.4% ± 5.4%), and solubilization of MYB Trans (15.0% ± 2.1%) was 2.3-fold higher than that of MYB WT (6.4% ± 5.4%) (Fig. 1B, M; Table S1). Taken together, the growth rate, cell density, and carbohydrate solubilization results indicate large recalcitrance differences between the individual genotypes (MYB [most recalcitrant] versus COMT versus CR [least recalcitrant]) and then among the transgenic versus parental lines [MYB WT versus MYB Trans and COMT3(−) versus COMT3(+)]. Interestingly, transgenic COMT3(+) and unmodified CR were considered to have comparable recalcitrance following one microbial pass (∼36% carbohydrate solubilization) (Fig. 1B, M; Table S1).
FIG 1.
(A) Planktonic growth of C. bescii on microbe-only-treated (M) switchgrass (purple and green symbols and dashed lines), hydrothermally treated (T→M) switchgrass (red and yellow symbols and solid lines), microbe-only-treated reference switchgrass (dashed black line), and hydrothermally treated reference switchgrass (solid black line). Phase 1 doubling times (td1) were calculated between 11.5 and 18.5 h, and phase 2 doubling times (td2) were calculated between 24.0 and 60.5 h. (B) Carbohydrate solubilized by C. bescii (percent arabinan, xylan, and glucan of total carbohydrate) after 12 days. CR, Cave-in-Rock.
Glucan, xylan, and arabinan solubilization across the less recalcitrant switchgrass lines (COMT and CR) showed that glucan and xylan in CR and all three polysaccharides in COMT3(+) were released in comparable amounts (Table S1). For the most recalcitrant genotype, MYB (WT and Trans), hemicellulose (xylan and arabinan) accounted for most of the carbohydrate solubilized, indicating that the glucan (cellulose) was highly inaccessible.
Effect of hydrothermal treatment on transgenic switchgrass biosolubilization.
Hydrothermal processing has been considered for biomass pretreatment, since it produces limited amounts of inhibitory degradation compounds (i.e., furfural and hydroxymethylfurfural) (41–44). Hydrothermal processing (with liquid hot water from 170 to 220°C [45]) acts to solubilize hemicellulose and causes structural changes in lignin, such that the cellulose becomes more accessible to hydrolytic enzymes and microbial attack (42, 46). To examine the effect of hydrothermal treatment on bioavailability to C. bescii, all five switchgrass lines were subjected to elevated temperatures (180°C for 25 min), followed by 12 days of C. bescii growth (Fig. 1B, T→M; Table S1). While each specific component (glucan, xylan, arabinan, and inert material) was solubilized uniformly when only microbial treatments were used (M and M→M) (see Fig. S1 in the supplemental material), hydrothermal treatment released primarily hemicellulose and lignin, as indicated by an increase in glucan content and a decrease in hemicellulose (xylan and arabinan) and inert material (Table 1). One of the clear advantages of hydrothermal treatment is the release of readily removed saccharides (e.g., hemicellulose [42, 46] and amorphous cellulose [47]) that could easily be consumed by C. bescii. Here, the treated plant material was washed so as not to obscure the effect of hydrothermal treatment on C. bescii's ability to access plant biomass carbohydrates that were not removed during heat treatment. The planktonic growth rate of C. bescii was 2-fold higher or more on hydrothermally treated switchgrass than on untreated material for all switchgrass lines (Fig. 1A), indicating that hydrothermal treatment improved the accessibility of the plant carbohydrates to C. bescii (Fig. 1B, T→M) relative to that with untreated switchgrass. Of particular note, the td2 for MYB WT decreased from 85 h to 16.5 h. Cell growth on all hydrothermally treated switchgrass lines was comparable during phase 1 (td1, ∼ 2.1 to 2.6 h), with only slight differences noted during growth phase 2 (td2, ∼12.6 to 16.5 h) (Fig. 1A). The higher growth rates on hydrothermally treated switchgrass corresponded to increased carbohydrate solubilization in all cases (Fig. 1B, T→M; Table S1). The largest increase in carbohydrate solubilization as a result of hydrothermal treatment was for the more recalcitrant MYB WT and MYB Trans. For MYB WT, solubilization increased from 6.4% to 23.5% (3.7-fold) due to hydrothermal treatment. Solubilization of hydrothermally treated and genetically modified MYB switchgrass (MYB Trans) resulted in greater total solubilization than that of MYB WT, increasing from 15.0% to 33.0% (2.2-fold), but the effect of hydrothermal treatment was smaller (Fig. 1B, T→M; Table S1). In contrast, COMT3(−) and COMT3(+) had the smallest improvements in carbohydrate solubilization following hydrothermal treatment, increasing from 24.4% to 40.9% (1.7-fold) and from 36.3% to 47.8% (1.3-fold), respectively (Fig. 1B, T→M; Table S1). Likewise, carbohydrate solubilization for the reference line CR increased from 36.4% to 49.2% (1.4-fold). Overall, the least recalcitrant lines, COMT3(+) and CR, resulted in the highest levels of carbohydrate solubilization, and transgenic modification led to improvements over the corresponding parental line; however, the impact of hydrothermal treatment on biosolubilization diminished with lower recalcitrance (Fig. 1B, T→M; Table S1).
TABLE 1.
Switchgrass composition before and after hydrothermal treatment
| Switchgrass | Treatmenta | % (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| Carbohydrate | Glucan | Xylan | Arabinan | Inert material | ||
| COMT3(−) | Raw | 66 ± 2.4 | 36 ± 0.8 | 26 ± 1.3 | 3.9 ± 0.3 | 34 ± 2.4 |
| T | 71 ± 1.8 | 43 ± 1.1 | 27 ± 0.7 | 1.9 ± 0.0 | 29 ± 1.8 | |
| COMT3(+) | Raw | 63 ± 0.9 | 34 ± 0.7 | 26 ± 0.3 | 3.5 ± 0.1 | 37 ± 0.9 |
| T | 73 ± 0.6 | 46 ± 0.6 | 25 ± 0.4 | 1.9 ± 0.1 | 28 ± 0.6 | |
| MYB WT | Raw | 63 ± 1.6 | 37 ± 1.4 | 24 ± 0.3 | 2.6 ± 0.1 | 37 ± 1.6 |
| T | 73 ± 1.0 | 54 ± 0.5 | 18 ± 0.5 | 0.9 ± 0.0 | 27 ± 1.0 | |
| MYB Trans | Raw | 65 ± 1.5 | 37 ± 1.6 | 25 ± 0.3 | 3.3 ± 0.4 | 35 ± 1.5 |
| T | 72 ± 1.0 | 50 ± 0.7 | 21 ± 0.4 | 1.2 ± 0.1 | 28 ± 1.0 | |
| Cave-in-Rock | Raw | 65 ± 2.1 | 37 ± 1.1 | 25 ± 0.8 | 3.2 ± 0.3 | 35 ± 2.1 |
| T | 65 ± 0.5 | 47 ± 0.3 | 18 ± 0.4 | 0.3 ± 0.5 | 35 ± 0.5 | |
Raw, before hydrothermal treatment; T, after hydrothermal treatment.
Organic acid production (which reflects conversion of lignocellulose carbohydrates) correlated with carbohydrate solubilization for both untreated and hydrothermally treated material (see Fig. S3 in the supplemental material). The absence of any residual sugar in culture supernatants at the end of the fermentation indicated that sugars liberated from insoluble polysaccharides were completely converted to fermentation products (data not shown). Acetate was the primary fermentation product of both untreated and hydrothermally treated material, with lactate produced in small amounts in cultures grown on hydrothermally treated switchgrass; in Caldicellulosiruptor species, lactate production generally results from H2 inhibition, diverting carbon flux away from acetate to lactate (48). Organic acid production was variable, with the highest values for hydrothermally treated COMT3(+) and CR, at 329 ± 7 and 326 ± 4 mg per g carbohydrate, respectively, and the lowest for untreated MYB WT, at 103 ± 3 mg per g carbohydrate (Fig. S3).
Sequential hydrothermal and microbial treatments.
To determine if carbohydrate solubilization and conversion could be maximized, schemes involving multiple C. bescii microbial treatments (7 days each) and hydrothermal treatments of the switchgrass lines were evaluated. After two consecutive microbial treatments (M→M), fermentation of the transgenic switchgrass lines COMT3(+) and MYB Trans resulted in higher carbohydrate solubilization than that of their parental counterparts. For COMT3(+), carbohydrate solubilization reached 40.3% ± 2.4%, versus 34.6% ± 2.9% for COMT3(−). For MYB Trans, carbohydrate solubilization reached 27.5% ± 2.4%, versus 15.9% ± 3.3% for MYB WT (Fig. 2; see Table S2 in the supplemental material). CR had the highest carbohydrate solubilization at 43.3% ± 2.7%. Yet, after two consecutive C. bescii microbial treatments (M→M) of untreated switchgrass, bioavailable carbohydrate still remained (Fig. 2). Next, a hydrothermal treatment step, either before (T→M→M) (Fig. 2A) or between (M→T→M) (Fig. 2B) two rounds of microbial deconstruction, was included. Note that hydrothermally treated switchgrass was washed prior to each microbial step and that solubilization resulting from hydrothermal treatment was included in determining total carbohydrate solubilization (not included in Fig. 1). Hydrothermal treatment alone, before two rounds of microbial treatment (T→M→M), accounted for about ∼30% of the total carbohydrate released for all switchgrass lines, with the exception of MYB WT (22%) (Fig. 2A; Table S2). On the other hand, the contribution of hydrothermal treatment done between two rounds of microbial treatment (M→T→M) to carbohydrate solubilization ranged from 16.7% for CR to 25.1% for COMT3(+) (Fig. 2B; Table S2). Hydrothermal treatment before two rounds of microbial growth (T→M→M) led to higher carbohydrate solubilization for each switchgrass line than M→T→M. A single hydrothermal treatment prior to sequential microbial treatments (M→M), increased carbohydrate solubilization 1.6-fold (CR) to 2.9-fold (MYB WT) (Fig. 2; Table S2). Transgenic lines that were hydrothermally treated outperformed their corresponding parental lines by 18.9 percentage points for MYB Trans and 11.3 percentage points for COMT3(+). The transgenic COMT3(+) yielded the highest carbohydrate solubilization of all the lines with 75.1% (T→M→M), with its counterpart MYB Trans yielding 64.5%, which was comparable to that for COMT3(−) (63.8%) (Fig. 2; Table S2).
FIG 2.
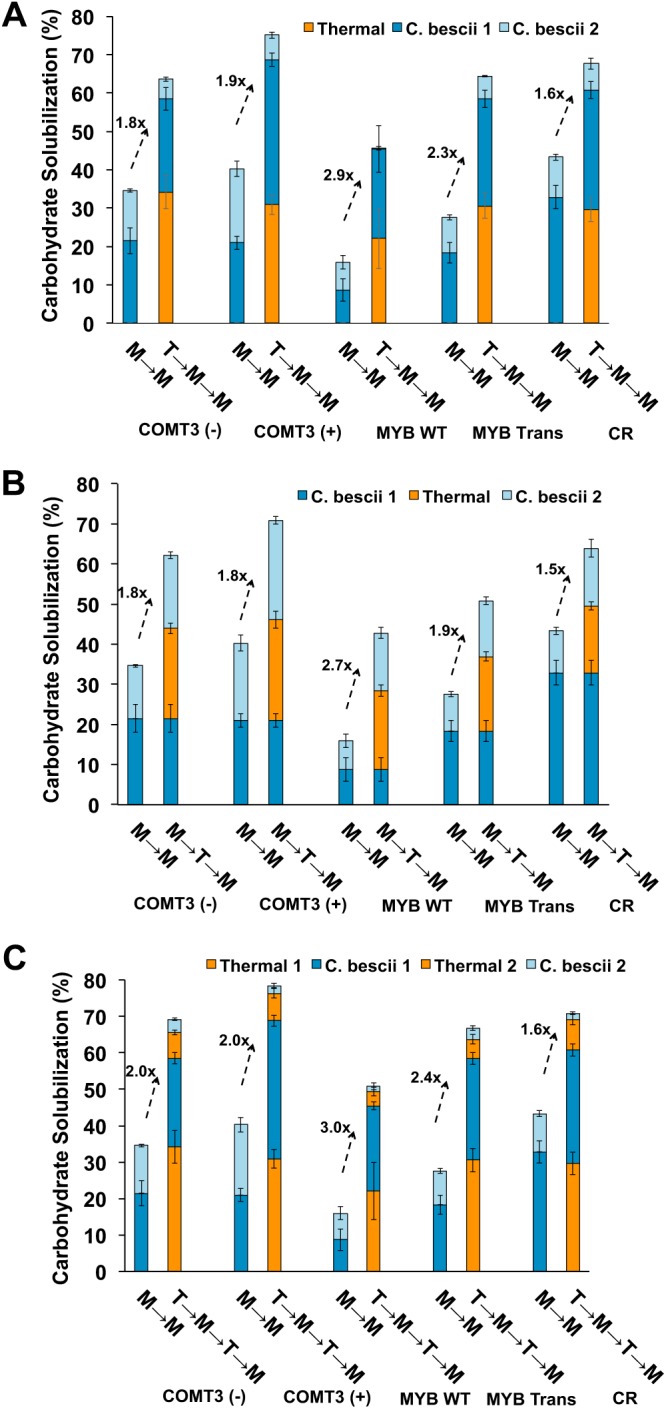
Carbohydrate solubilization after one hydrothermal treatment (thermal) either before (T→M→M) two rounds of microbial deconstruction (A) or between (M→T→M) two rounds of microbial deconstruction (B) or after a combination of two hydrothermal treatments and two rounds of microbial deconstruction (T→M→T→M) (C). Each fermentation with C. bescii was carried out for 7 days. The indicated fold change is relative to two consecutive rounds of microbial deconstructions (M→M) without hydrothermal treatment.
As a final scheme, hydrothermal treatment was carried out both before and between two rounds of microbial deconstruction (T→M→T→M) (Fig. 2C), resulting in small but meaningful increases in carbohydrate solubilization that ranged from 2.3 percentage points (MYB Trans) to 5.7 percentage points [COMT3(−)] higher than the best single hydrothermal treatment scheme (T→M→M) (Fig. 2A). Carbohydrate solubilization reached 78.5% ± 1.9% and 70.9% ± 2.4% for COMT3(+) and CR, respectively (Fig. 2C; Table S2); these were the highest levels achieved for any lines with multiple treatment steps. The amount of carbohydrate solubilized from MYB Trans (66.8%) was less than that from the unmodified COMT3(−) (69.5%).
Crystalline cellulose solubilization and glucan bioavailability in switchgrass.
After 7 days at 70°C, C. bescii is able to solubilize microcrystalline cellulose (Avicel) to near completion in a single microbial step at a loading of 1 to 2 g/liter in a closed bottle (see Fig. S2 in the supplemental material). When plant biomass was tested at 5 g/liter (∼1.7 to 1.8 g/liter glucan equivalency), C. bescii was able to solubilize only up to 36% of the glucan (Fig. 1B; Table S1). Not surprisingly, elements of the complex lignocellulosic matrix make access to glucan difficult in the plant cell wall versus when pure microcrystalline cellulose is available to C. bescii. However, improving microbial access to glucan (34 to 37% of switchgrass mass) will be essential for high conversion of plant biomass to product fuels and chemicals.
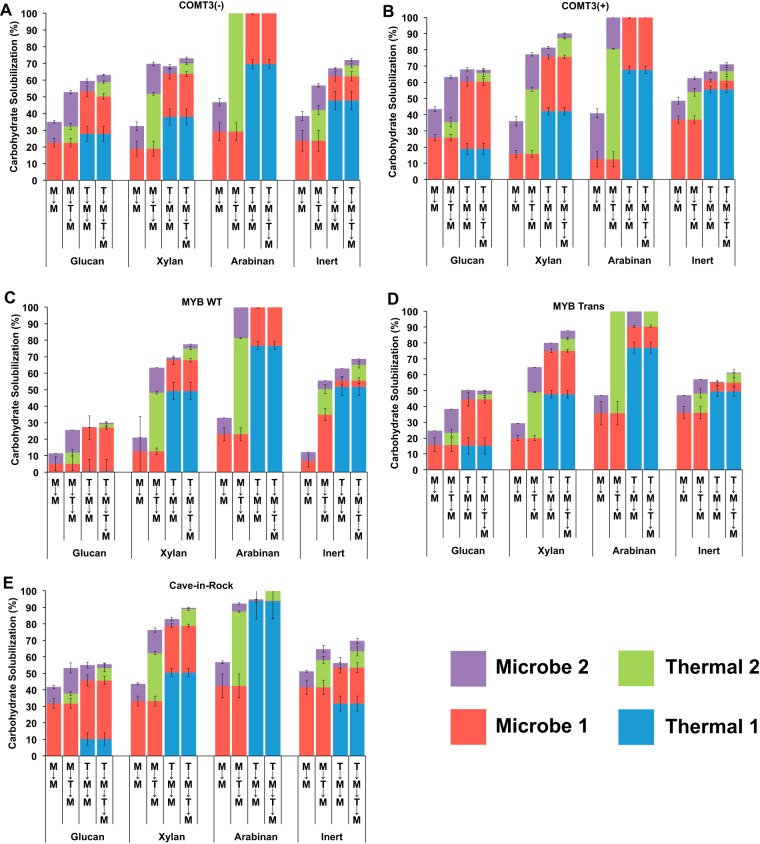
Hydrothermal treatment released primarily hemicellulose (xylan and arabinan) and led to higher total carbohydrate solubilization than microbial treatment alone (Fig. 3). It also improved microbial accessibility to glucan, but to various degrees across the switchgrass lines tested, and depended on the placement of the hydrothermal treatment within the treatment scheme (Fig. 3). A hydrothermal treatment before two microbial treatments (T→M→M) led to higher microbial glucan solubilization than in-between treatment (M→T→M) for only the MYB lines (Fig. 3), with MYB Trans resulting in higher microbial glucan solubilization than MYB WT for both treatment schemes (Fig. 3C and D). A hydrothermal treatment before two microbe passes (T→M→M) led to microbial glucan solubilization of 27% (MYB WT) and 35% (MYB Trans); in contrast, a hydrothermal treatment between two microbial passes (M→T→M) for MYB WT and MYB Trans resulted in microbial glucan solubilization of 19% and 31%, respectively (Fig. 3). A hydrothermal treatment between two microbial treatments (M→T→M) led to higher glucan solubilization for the COMT3 lines and CR, with the transgenic COMT3(+) resulting in the highest microbial glucan solubilization of all switchgrass genotypes. For COMT3(−), COMT3(+), and CR, microbial glucan solubilization for M→T→M was 43%, 54%, and 47% and that for T→M→M was 32%, 49%, and 45%, respectively (Fig. 3A, B, and E). Compared to successive microbial (M→M) treatments, the addition of a hydrothermal treatment (at the most effective placement) resulted in microbial glucan solubilization increases of 35% to 43% [COMT3(−)], 44% to 54% [COMT3(+)], 11% to 27% (MYB WT), 25% to 35% (MYB Trans), and 42% to 47% (CR) (Fig. 3). Transgenic COMT3(+) switchgrass resulted in the largest percentage of glucan solubilized by C. bescii, while the largest fold increase of microbial glucan solubilization from hydrothermal treatment came for the most recalcitrant line, MYB WT (Fig. 3). Hydrothermal treatment had the smallest effect on microbial glucan solubilization for CR (Fig. 3).
FIG 3.
Percent glucan, xylan, arabinan, and inert material solubilized following various microbial and hydrothermal treatment schemes for COMT3(−) (A), COMT3(+) (B), MYB WT (C), MYB Trans (D), and Cave-in-Rock (E) switchgrasses.
In the final process scheme, the effect on glucan accessibility following alternating hydrothermal and microbial treatments (T→M→T→M) was examined in comparison to M→M (Fig. 3). The second hydrothermal treatment had a minimal or negative impact on total microbial glucan solubilization for the less recalcitrant lines COMT3(+) (0%), CR (−9%), and COMT3(−) (−23%). Microbial glucan solubilization increased, relative to that with M→M, for MYB WT (+139%) and MYB Trans (+28%). Although T→M→T→M yielded the highest total carbohydrate solubilization among all the switchgrass lines tested (Fig. 2C), the second hydrothermal treatment in comparison to the most effective single hydrothermal treatment (M→T→M or T→M→M) had a positive impact only on microbial glucan solubilization for MYB WT (increased 3%) and reduced microbial glucan solubilization for COMT3(−) (−37%), CR (−20%), COMT3(+) (−19%), and MYB Trans (−10%).
To examine recalcitrance more closely, switchgrass lines were tested for cellulose crystallinity and accessibility before and after microbial deconstruction and hydrothermal treatments (T→M→T→M) (Fig. 4). The mean cellulose crystallinity was reduced in all lines after exposure to extensive treatment (T→M→T→M), with the largest decrease in the lateral order index (LOI) seen with MYB WT (Fig. 4A). Furthermore, the cellulose accessibility (orange/blue [O/B] ratio) increased for all switchgrass lines following successive treatments (T→M→T→M) (Fig. 4B), presumably a result of hemicellulose removal. These increases in accessibility ranged from 3.8-fold for COMT3(−) to 12.7-fold for MYB Trans. Compositional analysis of this material found that the hemicellulose (xylan and arabinan) content decreased for each switchgrass line following treatments (Table 2). The arabinan composition was reduced to below detectable levels for all lines except COMT3(−), which was reduced from 3.9% ± 0.3% to 0.8% ± 0.0% (Table 2). The xylan composition was reduced the most in CR, with levels dropping from 25% ± 0.8% to 8.6% ± 0.2%. Thus, the composition of the remaining switchgrass after T→M→T→M was primarily glucan and inert material, with glucan concentrations that ranged from 45% [COMT3(+)] to 60% (MYB WT) and inert material concentrations that ranged from 27% to 44% (Table 2).
FIG 4.
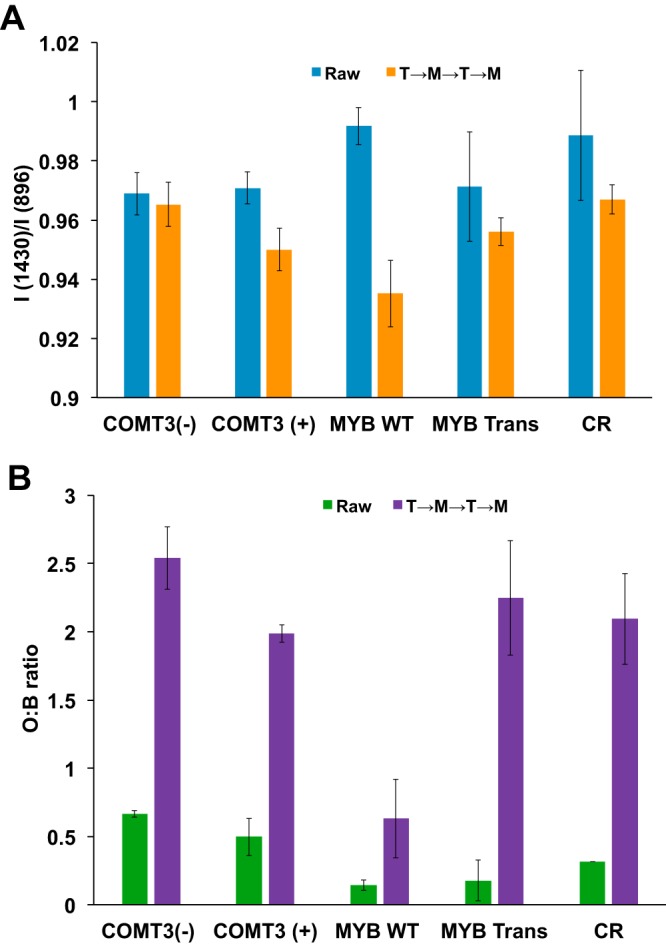
Cellulose crystallinity (A) and enzyme accessibility (B) of untreated switchgrass and switchgrass lines after two hydrothermal and microbial deconstructions. CR, Cave-in-Rock; I (1430)/I (896), the ratio of the peak intensity at 1,430 cm−1 to the peak intensity at 896 cm−1 (LOI) as measured using FTIR spectroscopy. O:B ratio, the ratio of direct orange 15 and direct blue 1 dyes.
TABLE 2.
Switchgrass composition for raw, nontreated material and material exposed to two microbial and hydrothermal treatments
| Switchgrass | Treatmenta | Glucan |
Xylan |
Arabinan |
Inert material |
||||
|---|---|---|---|---|---|---|---|---|---|
| Concn (%)b | Mass (g)c | Concn (%) | Mass (g) | Concn (%) | Mass (g) | Concn (%) | Mass (g) | ||
| COMT3(−) | Raw | 36 ± 0.8 | 1.8 | 26 ± 1.3 | 1.3 | 3.9 ± 0.3 | 0.2 | 34 ± 2.4 | 1.7 |
| T→M→T→M | 46 ± 1.3 | 0.7 | 17 ± 0.8 | 0.4 | 0.8 ± 0.0 | 0.0 | 37 ± 2.1 | 0.5 | |
| COMT3(+) | Raw | 34 ± 7.0 | 1.7 | 26 ± 0.3 | 1.3 | 3.5 ± 0.1 | 0.2 | 37 ± 0.9 | 1.9 |
| T→M→T→M | 45 ± 1.2 | 0.6 | 11 ± 0.2 | 0.1 | NDd | 0.0 | 44 ± 1.4 | 0.5 | |
| MYB WT | Raw | 37 ± 1.4 | 1.8 | 24 ± 0.3 | 1.1 | 2.6 ± 0.1 | 0.1 | 37 ± 1.6 | 1.9 |
| T→M→T→M | 60 ± 0.9 | 1.3 | 12 ± 0.4 | 0.3 | ND | 0.0 | 27 ± 1.3 | 0.6 | |
| MYB Trans | Raw | 37 ± 1.6 | 1.9 | 25 ± 0.3 | 1.3 | 3.3 ± 0.4 | 0.2 | 35 ± 1.5 | 1.7 |
| T→M→T→M | 53 ± 1.1 | 0.9 | 8.8 ± 0.4 | 0.2 | ND | 0.0 | 38 ± 1.3 | 0.7 | |
| Cave-in-Rock | Raw | 37 ± 1.1 | 1.9 | 25 ± 0.8 | 1.2 | 3.2 ± 0.3 | 0.2 | 35 ± 2.1 | 1.8 |
| T→M→T→M | 55 ± 1.4 | 0.9 | 8.6 ± 0.2 | 0.1 | ND | 0.0 | 36 ± 1.5 | 0.5 | |
M, microbial treatment; T, hydrothermal treatment. Switchgrass was washed following each treatment step, and the material after washing was used for the next planned treatment.
Values are means ± standard deviations.
The average mass compositional values were calculated based on a 5-g/liter initial loading.
ND, none detected.
Cellulose crystallinity and enzyme accessibility were also measured at every process step from untreated material through two microbial and hydrothermal treatments in the transgenic COMT3(+) (T→M→T→M) (Fig. 5); the results for untreated material corresponded to previous reports (49). Cellulose crystallinity was found to increase after one hydrothermal treatment (T), likely indicating the removal of amorphous cellulose (Fig. 5A). Correspondingly, cellulose accessibility only slightly increased after one hydrothermal treatment (Fig. 5B, T). Subsequent microbial treatments then lowered cellulose crystallinity from 0.99 (T) to 0.95 (T→M→T→M) (Fig. 5A). Cellulose accessibility increased 3-fold after the first microbial treatment (Fig. 5B, T→M). Compositional analysis of COMT3(+) found that the xylan content was reduced the most at this step, decreasing from 25% to 18% (Table 3, T→M). Further hydrothermal and microbial treatments had only minor effects on cellulose accessibility. For example, although the residual material after T→M→T→M had decreased cellulose crystallinity, it also had decreased accessibility (Fig. 5) and thus was more recalcitrant than the untreated switchgrass and still contained 45% glucan (Table 3).
FIG 5.
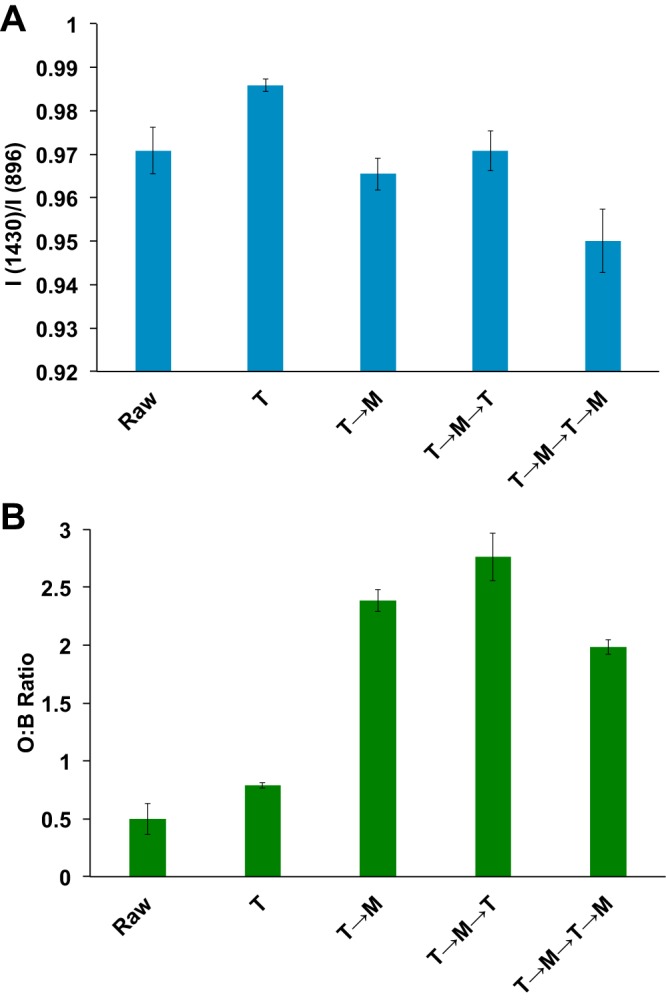
Cellulose crystallinity (A) and enzyme accessibility (B) of untreated COMT3(+) switchgrass after rounds of hydrothermal-treatment (T) and microbial deconstruction (M).
TABLE 3.
COMT3(+) composition for untreated, raw material and following microbial deconstruction and hydrothermal treatments
| Treatmenta | % (mean ± SD) |
|||
|---|---|---|---|---|
| Glucan | Xylan | Arabinan | Inert material | |
| Raw | 34 ± 0.7 | 26 ± 0.3 | 3.5 ± 0.1 | 37 ± 0.9 |
| T | 46 ± 0.6 | 25 ± 0.4 | 19 ± 1 | 28 ± 0.6 |
| T→M | 39 ± 2.0 | 18 ± 0.6 | NDb | 42 ± 2.5 |
| T→M→T | 43 ± 14 | 12 ± 0.4 | ND | 45 ± 1.9 |
| T→M→T→M | 45 ± 1.2 | 11 ± 0.2 | ND | 44 ± 1.4 |
M, microbial deconstruction; T, hydrothermal treatment. Switchgrass samples were washed following each treatment step, and the material after washing was used for the next planned treatment.
ND, none detected.
DISCUSSION
A major challenge to converting lignocellulose into fuels and chemicals is the lack of microbial access to the complex polysaccharides that comprise the plant cell wall. C. bescii is unique in its ability to degrade and coutilize sugars from both hemicellulose (C5) and cellulose (C6). Thus, for this extremely thermophilic bacterium, lignin is the major barrier to complete plant biomass conversion.
The transgenic switchgrass lines MYB Trans and COMT3(+), their respective parental lines (cv. Alamo), and a naturally occurring, low-recalcitrance switchgrass (cv. CR) were all solubilized by C. bescii but to different extents. Significant variations in solubilization were seen between the different parental lines (WT); this validates the observations previously reported (50) using CBP with Clostridium thermocellum. While the C. bescii growth rate and carbohydrate solubilization were higher on the transgenic lines than on their parental line, both MYB lines were especially resistant to microbial degradation. The low recalcitrance (higher carbohydrate bioaccessibility) of Cave-in-Rock (upland cultivar) when fermented by C. bescii compared to the Alamo lines (lowland cultivar) was unexpected given that lowland varieties typically have higher cellulose contents, higher biomass yields, lower ash contents, and lower fiber concentrations (51, 52). Agronomic factors such as precipitation, temperature, location, and harvest date may influence the plant in ways that impact carbohydrate bioavailability (53, 54). While the reason for differences in recalcitrance between the lines and resistance to C. bescii degradation is currently unknown, lignin structure, monolignol ratios, surface accessibility (55), and inhibitory compounds present in the plant are also likely to play a role (28, 56, 57).
The impact of hydrothermal treatment on carbohydrate solubilization was greatest for the most naturally recalcitrant switchgrass line, MYB (WT and Trans). Primarily hemicellulose, and very little glucan, was solubilized by C. bescii in both the untreated MYB WT and MYB Trans. However, following hydrothermal treatment, cellulose (glucan) solubilization dramatically increased for both (Fig. 1B). This suggests that hydrothermal treatment improved accessibility to cellulose (Fig. 4 and 5). This is consistent with reports that hydrothermal treatment removes/disrupts hemicellulose and lignin in the plant biomass matrix with a minimal effect on cellulose (42, 58). We would also expect that the greatest effect of pretreatment would be on the most recalcitrant line (thus benefiting most from the removal of some xylan and the lignin aggregation) (59, 60). Lignin was solubilized concomitantly with carbohydrate by C. bescii in all switchgrass lines and under all conditions examined here (see Fig. S1 in the supplemental material), consistent with previous studies that showed uniform degradation (“onion peeling” mechanism) of all cell wall components by C. bescii (16). The details of how this occurs are not yet clear, but possible contributions from carbohydrate esterases in C. bescii acting in concert with other carbohydrate-active enzymes (cellulases and hemicellulases) to liberate lignin and carbohydrate cannot be ruled out (61, 62).
Inhibition of microbial growth and fermentation processes is a concern when using biomass from transgenic plants that have been subjected to treatment technologies, given the release of a wide range of lignin-derived compounds (63). Previous studies with COMT3(+) switchgrass found that downregulation of caffeic acid O-methyltransferase resulted in the generation of a novel monolignol (64). The COMT3(+) line also showed no significant change in lignin molecular weight (65). The MYB Trans showed decreased levels of potential phenolic inhibitors (26). Yee et al. (28) found that acid-pretreated COMT3(+) switchgrass had an inhibitory effect on fermentation by C. bescii and two other thermophilic bacteria. The results presented here, however, show that C. bescii growth was apparently not inhibited by transgenic modification or by hydrothermal treatment, given that the growth rate increased on the transgenic lines and on hydrothermally treated switchgrass (Fig. 1A). This indicates that the fitness of C. bescii is not affected by modification of the lignin biosynthetic pathway in COMT3 and MYB and, furthermore, that hydrothermal treatment did not generate lignin by-products inhibitory to C. bescii, at least at these low-biomass solid loadings.
By successive hydrothermal and microbial steps (T→M→T→M), over 70% of the carbohydrate content of transgenic COMT3(+) (79%) and natural variant Cave-in-Rock (71%) could be achieved, with most attributed to the first hydrothermal and microbial treatment (T→M) (69% and 61%, respectively) (Fig. 2C). Additional hydrothermal and microbial treatments led to diminished benefits but still improved carbohydrate solubilization, modified the carbohydrate composition, and altered structural characteristics. Given that hemicellulose was easily removed following hydrothermal treatment, the final switchgrass material following T→M→T→M was primarily glucan and inert material (lignin and ash). The initial hydrothermal treatment was responsible for solubilizing the largest quantity of inert material {ranging from 31.6% (CR) to 55.5% [COMT3(+)]} but left an inert component that was highly resistant to additional hydrothermal and microbial solubilization attempts (Fig. 3). Thus, the increase of inert content in the final material after all treatments suggests that lignin content (type) and structure, in both the transgenic and parental switchgrass lines, likely become a limiting factor of microbial accessibility to the 30% [COMT3(+)] to 70% (MYB WT) glucan still remaining (66, 67) (Fig. 3). The inability of multiple microbial and hydrothermal treatment steps to overcome this recalcitrance barrier and achieve near-complete carbohydrate conversion suggests that further treatments and/or genetic modifications to either plant or microbe may be necessary to achieve 80 to 90% solubilization, typically the goal of industrial processes. Other physical, nonchemical attempts to improve switchgrass degradation achieved 68% carbohydrate solubilization (glucan, xylan, and arabinan) when switchgrass was ball milled between two fermentation stages with C. thermocellum (40). While C. thermocellum is unable to naturally metabolize the available pentose sugars (hemicellulose) (68), physical treatments (hydrothermal and other) should be considered in improving plant biomass degradation for eventual conversion into fuels and chemicals.
This study establishes a baseline for evaluating Caldicellulosiruptor species for conversion of transgenic and natural variant switchgrass feedstocks. Strategic combination of hydrothermal treatment, biomass with reduced recalcitrance, and extremely thermophilic bacteria is promising, especially if process heat integration can be optimized to minimize energy costs associated with cooling between treatment and fermentation. The work described here is consistent with hydrothermal treatment of other plant feedstocks, such as corn stover (69), prairie cord grass (70), and poplar (71), which effectively removed hemicellulose and lignin, improved sugar yields, and reduced recalcitrance. To date, hydrothermal treatment has been used primarily to improve SSF with yeast (27, 28, 72, 73) and conversion with thermophilic microorganisms (27, 74). Most recently, Yee et al. (28) in 2012 reported on the generation of fermentation products by C. thermocellum, C. bescii, and C. obsidiansis grown on hydrothermally treated wild-type and transgenic switchgrass (COMT3). When transgenic switchgrass was used as the feedstock, there were 10% and 4% increases in product generation for C. thermocellum and C. obsidiansis, respectively, yet with C. bescii, there was no increase and the fermentation product concentration was low (∼50 mg/g carbohydrate) (28). However, the results presented here with C. bescii showed 164 to 326 mg organic acids generated per g carbohydrates and increases in organic acid production of 25% [COMT3(+)] and 43% (MYB Trans) when grown on hydrothermally treated transgenic switchgrass, relative to that by their hydrothermally treated parental line (see Fig. S3 in the supplemental material). Given the recent improvements in C. bescii genetics tools (75), there is an opportunity to improve the capacity and use of this extremely thermophilic bacterium for lignocellulose deconstruction and conversion, as has been recently demonstrated for enhancing xylan utilization (18). In this work, the transgenic COMT3(+) led to the highest carbohydrate and highest microbial glucan solubilization, yet the results for unmodified CR highlight the need to identify and target natural variant genotypes with inherently low recalcitrance in future genetic modification efforts. Examination of the solid residues may also provide indications of the remaining polymers and linkages that require specific targeting in planta, microbially or via pretreatment. If plant and microbial metabolic engineering can lead to processes that require at most only a single hydrothermal treatment step, the prospects for an economically viable route to bio-based fuels and chemicals are promising.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Caldicellulosiruptor bescii was obtained as a freeze-dried culture from the German Collection of Microorganisms and Cell Cultures (DSMZ) (http://www.dsmz.de). C. bescii was grown anaerobically in a defined modified version of DSMZ medium 671 (671d), as described previously (8), containing the specified switchgrass type as the sole carbon source. Although the optimal growth temperature (Topt) for C. bescii is 75°C (9), Zurawski et al. (8) employed a growth temperature of 70°C for consistency during growth of three different Caldicellulosiruptor species (with different Topts). Thus, so that our work would be comparable to that in the earlier study (8), we grew C. bescii at 70°C. Switchgrass was prepared as described below. The pH of medium 671d was adjusted to 7.2 with 10 M NaOH, and the medium was filter sterilized through 0.2-μm filters prior to addition of switchgrass (nominally at 5 g liter−1) and prepared anaerobically under an N2-CO2 (80:20, vol/vol) headspace. Unless otherwise specified, cultures were grown as 50-ml batch cultures in 125-ml closed serum bottles at 70°C and agitated at 100 rpm. Prior to all experiments, C. bescii was passaged on the specified switchgrass 2 or 3 times at intervals of 1 to 2 days to allow for cell acclimation.
Biomass feedstocks.
COMT3(+) and MYB Trans transgenic switchgrasses and their corresponding unmodified parental genotypes COMT(−) and MYB WT (Panicum virgatum L. cv. Alamo) were prepared as described previously (23, 25). These were obtained from collaborators in the BioEnergy Science Center (BESC) and were gathered from senesced material from the second year of field studies at the University of Tennessee (76–78). COMT3(+) and MYB Trans were identified as the two best lines in sugar release studies (23, 25, 26, 50) and thus were chosen for analysis in this work. In those studies, COMT3(+) is often referred to as COMT-KD and MYB Trans as Myb4-OE. Cultivar Cave-in-Rock (CR) (Panicum virgatum L.) was field grown in Monroe County, IA, seeded in 2000, harvested in 2010, and obtained from the National Renewable Energy Laboratory (NREL).
Biomass substrate preparation.
All varieties were mechanically ground and sieved to 20/80 mesh for closed-bottle experiments. Material from several replicate plant clones was combined after milling. Hydrothermal treatment was done by first soaking switchgrass overnight at 4°C in water (9 ml g−1). The switchgrass-water slurry was then centrifuged at 5,000 × g for 20 min, after which the supernatant was discarded and the switchgrass was loaded into stainless steel tubular reactors (4 or 6 by 0.5 in; McMaster Carr), as described in reference 45. The reactors were preheated in boiling water for 2 min and then transferred to a fluidized sand bath (SBS18; Techne) at 180°C for 25 min (23, 45, 79). The reactors were then immediately cooled in an ice bath. Hydrothermally treated and untreated switchgrasses were washed with water at 25°C by centrifugation at 6,000 × g for 10 min, and the supernatant was discarded. This was repeated (∼4 times) until no sugars were present in the wash, as measured by high-pressure liquid chromatography (HPLC) (Empire 1515 separations module; Waters) using a refractive index detector (model 2414; Waters). Washed switchgrass was oven dried overnight at 70°C and used as the growth substrate in all experiments. All switchgrass types were not autoclaved to eliminate the confounding effect of further treatment prior to use in experiments. At the elevated temperature of C. bescii growth, autoclaving to prevent growth by contaminants was not necessary.
Switchgrass solubilization experiments.
Batch cultures (50 ml) were prepared in triplicate on 671d medium with the specified switchgrass (5 g liter−1), inoculated with 1 × 106 cells/ml, agitated at 100 rpm, and incubated at 70°C for 12 days. Run in parallel to solubilization cultures, the growth curve cultures (to measure cell density) were carried out for an additional 2 days (14 days total). Solubilization cultures were harvested by centrifugation at 5,000 × g for 15 min. Residual substrate was washed with two volumes (100 ml) of 25°C sterile water and oven dried at 70°C until a constant mass was achieved. The extent of solubilization was determined from the mass difference between the switchgrass used to prepare cultures and insoluble substrate remaining after harvest. For the sequential microbial and hydrothermal cotreatment experiments, cultures were prepared and harvested as described above except with a 7-day incubation period. Residual switchgrass was then harvested, quantified for solubilization, and divided; approximately half was hydrothermally treated and washed, as described above. Solubilization by hydrothermal treatment was determined from the mass difference between the switchgrass loaded into the reactor treatment tubes and the switchgrass remaining after washing. A second round of batch cultures using fresh medium was prepared in triplicate, as described above, with either untreated or hydrothermally treated spent switchgrass, incubated for 7 days, and harvested for solubilization determination. All switchgrass loadings for these experiments were 5 g liter−1.
Determination of switchgrass composition.
The carbohydrate content of switchgrass, before and after incubation with C. bescii, was analyzed using a modified version of an NREL procedure (http://www.nrel.gov/biomass/analytical_procedures.html) (80). Sulfuric acid (600 μl of a 72% [wt/wt] solution) was added to 40 mg switchgrass and mixed using a glass stir rod. Samples were incubated in a 30°C constant-temperature water bath for 70 min; the samples were mixed with a glass rod every 10 min. The sulfuric acid was then diluted to 4% (wt/wt) with 16.8 ml deionized (DI) water. Tubes were sealed and autoclaved for 1 h on the liquid cycle. Sugar concentrations were determined using HPLC (Empire 1515 separations module; Waters) with a refractive index (model 2414; Waters) detector. Acetate, cellobiose, glucose, xylose, and arabinose were quantified using a Rezex-ROA column (300 mm by 7.8 mm; Phenomenex) operated with a mobile phase of 5 mM H2SO4 at 0.6 ml/min and 60°C. The inert components (lignin and ash) were determined as the difference between the mass of total carbohydrate and the total mass.
Characterization of switchgrass for cellulose accessibility and crystallinity.
Cellulose accessibility was completed using the Simons stain procedure, as described in reference 66. Direct orange 15 and direct blue 1 dyes were obtained from Pylam Products Company, Inc. (Tempe, AZ, USA) and used in working concentrations of 10 mg liter−1. For direct orange 15,-low-molecular weight components were removed by ultrafiltration through 100 K membranes (EMD Millipore Corp) on an Amicon ultrafiltration apparatus. Following filtration, 1 ml of direct orange 15 was dried on a petri dish in a 105°C oven over 3 days. The recovered solid was weighed, and the concentrated solution was diluted to the working concentration (10 mg liter−1).
Switchgrass (∼10 mg) was added to seven 2.5-ml centrifuge tubes. The dye mixture adsorption isotherm was determined by adding a series of 1:1 dye mixtures at increasing concentrations to each tube. Phosphate-buffered saline solution (0.3 M Na3PO4 and 1.4 mM NaCl at pH 6) was also added (0.1 ml) to each test tube, and the final volume was adjusted to 1.0 ml with deionized water. Samples were incubated with shaking at 70°C for 6 h and then centrifuged at 10,250 × g. The supernatant was recovered, and its absorbance was measured using a Lambda 35 UV-visible spectrophotometer (PerkinElmer, CT, USA). Calculation of the amount of dye absorbed (orange/blue [O/B] ratio) to the switchgrass was adapted from standard curves constructed from and equations located in reference 81.
For cellulose crystallinity (49), air-dried switchgrass (∼1 g) was placed inside Whatman cellulose extraction thimbles and Soxhlet extracted for 24 h in toluene-ethanol (2:1, vol/vol) under reflux. Following extraction, the biomass was air dried overnight in a fume hood to evaporate residual solvent. Peracetic acid (5 g peracetic acid/g biomass) was added to 0.6 g of the extractive-free biomass, and delignification proceeded at 25°C for 24 h. The delignified material was then refluxed in 2.5 M HCl at 100°C for 1.5 h to hydrolyze hemicellulose. The mixture was cooled to room temperature and filtered to recover cellulose. The retentate was washed with water (100 ml) through filtration. The cellulose was analyzed on an attenuated total reflectance Fourier transform infrared (FTIR) spectrometer (Spectrum 100N; PerkinElmer) equipped with a ZnSe crystal. The spectral width was 4,000 to 600 cm−1, and the spectra were acquired with 32 scans.
Analysis of fermentation products and soluble sugars.
Acetate and lactate were analyzed by HPLC using a Rezex-ROA column (300 mm by 7.8 mm; Phenomenex) operated with a mobile phase of 5 mM H2SO4 at 0.6 ml/min and 60°C. Samples were acidified to 0.05% (wt/wt) H2SO4 and detected by refractive index. The total saccharide content was determined by addition of 35 μl 72% (wt/wt) H2SO4 to 1 ml of supernatant. Screw-top tubes were sealed and autoclaved for 1 h to hydrolyze soluble oligosaccharides to their respective monosaccharides. Samples were then analyzed by HPLC as described above.
Supplementary Material
ACKNOWLEDGMENTS
The BioEnergy Science Center (BESC) is a U.S. Department of Energy (DOE) Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. L. L. Lee acknowledges support from an NIH Biotechnology Traineeship (NIH T32 GM008776-11) and an NSF Graduate Research Fellowship. J. M. Conway acknowledges support from a U.S. Department of Education GAANN Fellowship (P200A100004-12). H. O. Akinosho is grateful for financial support from the Paper Science & Engineering (PSE) fellowship program at the Renewable Bioproducts Institute at Georgia Institute of Technology.
The COMT3(+) knockdown and MYB Trans overexpression transgenic lines and controls were provided by BESC collaborators under a Material Transfer Agreement. We appreciate the lines of COMT from Zeng-Yu Wang of the Noble Foundation and MYB4 from Richard Dixon of the University of North Texas and the field-grown line from C. Neal Stewart of the University of Tennessee. NREL provided the initial milling of the samples, which was coordinated by Erica Gjersing. The Cave-in-Rock samples were from Dan Schell at NREL.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00969-17.
REFERENCES
- 1.Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. 2007. Biomass recalcitrance: engineering plants and enzymes for biofuel production. Science 454:1–18. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 2.Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CAWJF Jr, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski TJ. 2006. The path forward for biofuels and biomaterials. Science 311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 3.Loque D, Scheller HV, Pauly M. 2015. Engineering of plant cell walls for enhanced biofuel production. Curr Opin Plant Biol 25:151–161. doi: 10.1016/j.pbi.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Pauly M, Keegstra K. 2008. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 54:559–568. doi: 10.1111/j.1365-313X.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 5.Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi G, Gholami M, Ardjmand M. 2013. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renewable Sustainable Energy Rev 27:77–93. doi: 10.1016/j.rser.2013.06.033. [DOI] [Google Scholar]
- 6.Blumer-Schuette SE, Zurawski JV, Conway JM, Khatibi P, Lewis DL, Li Q, Chiang VL, Kelly RM. 2017. Caldicellulosiruptor saccharolyticus transcriptomes reveal consequences of chemical pretreatment and genetic modification of lignocellulose. Microb Biotechnol doi: 10.1111/1751-7915.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumer-Schuette SE, Brown SD, Sander KB, Bayer EA, Kataeva I, Zurawski JV, Conway JM, Adams MWW, Kelly RM. 2014. Thermophilic lignocellulose deconstruction. FEMS Microbiol Rev 38:393–448. doi: 10.1111/1574-6976.12044. [DOI] [PubMed] [Google Scholar]
- 8.Zurawski JV, Conway JM, Lee LL, Simpson HJ, Izquierdo JA, Blumer-Schuette S, Nookaew I, Adams MWW, Kelly RM. 2015. Comparative analysis of extremely thermophilic Caldicellulosiruptor species reveals common and unique cellular strategies for plant biomass utilization. Appl Environ Microbiol 81:7159–7170. doi: 10.1128/AEM.01622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SJ, Kataeva I, Wiegel J, Yin YB, Dam P, Xu Y, Westpheling J, Adams MWW. 2010. Classification of ‘Anaerocellum thermophilum’ strain DSM 6725 as Caldicellulosiruptor bescii sp. nov. Int J Syst Evol Microbiol 60:2011–2015. doi: 10.1099/ijs.0.017731-0. [DOI] [PubMed] [Google Scholar]
- 10.Svetlichnyi VA, Svetlichnaya TP, Chernykh NA, Zavarzin GA. 1990. Anaerocellum thermophilum gen. nov. sp. nov.—an extremely thermophilic cellulolytic eubacterium isolated from hot-springs in the valley of geysers Microbiology 59:598–604. [Google Scholar]
- 11.Zurawski JV, Blumer-Schuette SE, Conway JM, Kelly RM. 2014. The extremely thermophilic genus Caldicellulosiruptor: physiological and genomic characteristics for complex carbohydrate conversion to molecular hydrogen. Microb Bioenergy Hydrogen Prod 38:177–195. doi: 10.1007/978-94-017-8554-9_8. [DOI] [Google Scholar]
- 12.Blumer-Schuette SE, Lewis DL, Kelly RM. 2010. Phylogenetic, microbiological, and glycoside hydrolase diversities within the extremely thermophilic, plant biomass-degrading genus Caldicellulosiruptor. Appl Environ Microbiol 76:8084–8092. doi: 10.1128/AEM.01400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young J, Chung D, Bomble YJ, Himmel ME, Westpheling J. 2014. Deletion of Caldicellulosiruptor bescii CelA reveals its crucial role in the deconstruction of lignocellulosic biomass. Biotechnol Biofuels 7:142. doi: 10.1186/s13068-014-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA, Yang SJ, Resch MG, Adams MW, Lunin VV, Himmel ME, Bomble YJ. 2013. Revealing nature's cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513–1516. doi: 10.1126/science.1244273. [DOI] [PubMed] [Google Scholar]
- 15.Basen M, Rhaesa AM, Kataeva I, Prybol CJ, Scott IM, Poole FL, Adams MWW. 2014. Degradation of high loads of crystalline cellulose and of unpretreated plant biomass by the thermophilic bacterium Caldicellulosiruptor bescii. Bioresour Technol 152:384–392. doi: 10.1016/j.biortech.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Kataeva I, Foston MB, Yang SJ, Pattathil S, Biswal AK, Poole FL, Basen M, Rhaesa AM, Thomas TP, Azadi P, Olman V, Saffold TD, Mohler KE, Lewis DL, Doeppke C, Zeng YN, Tschaplinski TJ, York WS, Davis M, Mohnen D, Xu Y, Ragauskas AJ, Ding SY, Kelly RM, Hahn MG, Adams MWW. 2013. Carbohydrate and lignin are simultaneously solubilized from unpretreated switchgrass by microbial action at high temperature. Energy Environ Sci 6:2186–2195. doi: 10.1039/c3ee40932e. [DOI] [Google Scholar]
- 17.Chung D, Young J, Cha M, Brunecky R, Bomble YJ, Himmel ME, Westpheling J. 2015. Expression of the Acidothermus cellulolyticus E1 endoglucanase in Caldicellulosiruptor bescii enhances its ability to deconstruct crystalline cellulose. Biotechnol Biofuels 8:113. doi: 10.1186/s13068-015-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway JM, Pierce WS, Le JH, Harper GW, Wright JH, Tucker AL, Zurawski JV, Lee LL, Blumer-Schuette SE, Kelly RM. 2016. Multidomain, surface layer-associated glycoside hydrolases contribute to plant polysaccharide degradation by Caldicellulosiruptor species. J Biol Chem 291:6732–6747. doi: 10.1074/jbc.M115.707810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung D, Cha M, Guss AM, Westpheling J. 2014. Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc Natl Acad Sci U S A 111:8931–8936. doi: 10.1073/pnas.1402210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sticklen M. 2006. Plant genetic engineering to improve biomass characteristics for biofuels. Curr Opin Biotechnol 17:315–319. doi: 10.1016/j.copbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Badhan A, McAllister T. 2016. Designer plants for biofuels: a review. Curr Metabolomics 4:49–55. doi: 10.2174/2213235X03666141226213656. [DOI] [Google Scholar]
- 22.Chen F, Dixon RA. 2007. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 23.Fu C, Mielenz JR, Xiao X, Ge Y, Hamilton CY, Rodriguez M Jr, Chen F, Foston M, Ragauskas A, Bouton J, Dixon RA, Wang ZY. 2011. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc Natl Acad Sci U S A 108:3803–3808. doi: 10.1073/pnas.1100310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisano H, Nandakumar R, Wang ZY. 2009. Genetic modification of lignin biosynthesis for improved biofuel production. In Vitro Cell Dev Biol Plant 45:306–313. doi: 10.1007/s11627-009-9219-5. [DOI] [Google Scholar]
- 25.Shen H, He X, Poovaiah CR, Wuddineh WA, Ma J, Mann DG, Wang H, Jackson L, Tang Y, Stewart CN Jr, Chen F, Dixon RA. 2012. Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol 193:121–136. doi: 10.1111/j.1469-8137.2011.03922.x. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Poovaiah CR, Ziebell A, Tschaplinski TJ, Pattathil S, Gjersing E, Engle NL, Katahira R, Pu Y, Sykes R, Chen F, Ragauskas AJ, Mielenz JR, Hahn MG, Davis M, Stewart CN, Dixon RA. 2013. Enhanced characteristics of genetically modified switchgrass (Panicum virgatum L.) for high biofuel production. Biotechnol Biofuels 6:71. doi: 10.1186/1754-6834-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee KL, Rodriguez M Jr, Thompson OA, Fu C, Wang ZY, Davison BH, Mielenz JR. 2014. Consolidated bioprocessing of transgenic switchgrass by an engineered and evolved Clostridium thermocellum strain. Biotechnol Biofuels 7:75. doi: 10.1186/1754-6834-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yee KL, Rodriguez M Jr, Tschaplinski TJ, Engle NL, Martin MZ, Fu C, Wang ZY, Hamilton-Brehm SD, Mielenz JR. 2012. Evaluation of the bioconversion of genetically modified switchgrass using simultaneous saccharification and fermentation and a consolidated bioprocessing approach. Biotechnol Biofuels 5:81. doi: 10.1186/1754-6834-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin SB, Kszos LA. 2005. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenerg 28:515–535. doi: 10.1016/j.biombioe.2004.05.006. [DOI] [Google Scholar]
- 30.David K, Ragauskas AJ. 2010. Switchgrass as an energy crop for biofuel production: a review of its ligno-cellulosic chemical properties. Energy Environ Sci 3:1182–1190. doi: 10.1039/b926617h. [DOI] [Google Scholar]
- 31.Schmer MR, Vogel KP, Mitchell RB, Perrin RK. 2008. Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A 105:464–469. doi: 10.1073/pnas.0704767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouton JH. 2007. Molecular breeding of switchgrass for use as a biofuel crop. Curr Opin Genet Dev 17:553–558. doi: 10.1016/j.gde.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Keshwani DR, Cheng JJ. 2009. Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100:1515–1523. doi: 10.1016/j.biortech.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Abramson M, Shoseyov O, Shani Z. 2010. Plant cell wall reconstruction toward improved lignocellulosic production and processability. Plant Sci 178:61–72. doi: 10.1016/j.plantsci.2009.11.003. [DOI] [Google Scholar]
- 35.Louie GV, Bowman ME, Tu Y, Mouradov A, Spangenberg G, Noel JP. 2010. Structure-function analyses of a caffeic acid O-methyltransferase from perennial ryegrass reveal the molecular basis for substrate preference. Plant Cell 22:4114–4127. doi: 10.1105/tpc.110.077578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. 2010. Lignin biosynthesis and structure. Plant Physiol 153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends Plant Sci 15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J 19:6150–6161. doi: 10.1093/emboj/19.22.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen HC, Song J, Williams CM, Shuford CM, Liu J, Wang JP, Li Q, Shi R, Gokce E, Ducoste J, Muddiman DC, Sederoff RR, Chiang VL. 2013. Monolignol pathway 4-coumaric acid:coenzyme A ligases in Populus trichocarpa: novel specificity, metabolic regulation, and simulation of coenzyme A ligation fluxes. Plant Physiol 161:1501–1516. doi: 10.1104/pp.112.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paye JM, Guseva A, Hammer SK, Gjersing E, Davis MF, Davison BH, Olstad J, Donohoe BS, Nguyen TY, Wyman CE, Pattathil S, Hahn MG, Lynd LR. 2016. Biological lignocellulose solubilization: comparative evaluation of biocatalysts and enhancement via cotreatment. Biotechnol Biofuels 9:8. doi: 10.1186/s13068-015-0412-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobleter O. 1994. Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19:797–841. doi: 10.1016/0079-6700(94)90033-7. [DOI] [Google Scholar]
- 42.Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M. 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Hendriks AT, Zeeman G. 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Nitsos CK, Matis KA, Triantafyllidis KS. 2013. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. Chemsuschem 6:110–122. doi: 10.1002/cssc.201200546. [DOI] [PubMed] [Google Scholar]
- 45.Yang B, Wyman CE. 2009. Dilute acid and autohydrolysis pretreatment. Methods Mol Biol 581:103–114. doi: 10.1007/978-1-60761-214-8_8. [DOI] [PubMed] [Google Scholar]
- 46.Hu ZJ, Ragauskas AJ. 2011. Hydrothermal pretreatment of switchgrass. Ind Eng Chem Res 50:4225–4230. doi: 10.1021/ie101886d. [DOI] [Google Scholar]
- 47.Yu Y, Wu HW. 2010. Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind Eng Chem Res 49:3902–3909. doi: 10.1021/ie901925g. [DOI] [Google Scholar]
- 48.Willquist K, van Niel EW. 2010. Lactate formation in Caldicellulosiruptor saccharolyticus is regulated by the energy carriers pyrophosphate and ATP. Metab Eng 12:282–290. doi: 10.1016/j.ymben.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Li M, Pu Y, Yoo CG, Gjersing E, Decker SR, Doeppke C, Shollenberger T, Tschaplinski TJ, Engle NL, Sykes RW, Davis MF, Baxter HL, Mazarei M, Fu C, Dixon RR, Wang Z-Y, Stewart CN Jr, Ragauskas AJ. 2017. Study of traits and recalcitrance reduction of field-grown COMT down-regulated switchgrass. Biotechnol Biofuels 10:12. doi: 10.1186/s13068-016-0695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumitrache A, Natzke J, Rodriguez M Jr, Yee KL, Thompson OA, Poovaiah CR, Shen H, Mazarei M, Baxter HL, Fu C, Wang ZY, Biswal AK, Li G, Srivastava AC, Tang Y, Stewart CN Jr, Dixon RA, Nelson RS, Mohnen D, Mielenz J, Brown SD, Davison BH. 2017. Transgenic switchgrass (Panicum virgatum L.) targeted for reduced recalcitrance to bioconversion: a 2-year comparative analysis of field-grown lines modified for target gene or genetic element expression. Plant Biotechnol J 15:688–697. doi: 10.1111/pbi.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemus R, Brummer EC, Moore KJ, Molstad NE, Burras CL, Barker MF. 2002. Biomass yield and quality of 20 switchgrass populations in southern Iowa, USA. Biomass Bioenergy 23:433–442. doi: 10.1016/S0961-9534(02)00073-9. [DOI] [Google Scholar]
- 52.Cassida KA, Muir JP, Hussey MA, Read JC, Venuto BC, Ocumpaugh WR. 2005. Biofuel component concentrations and yields of switchgrass in south central US environments. Crop Sci 45:682–692. doi: 10.2135/cropsci2005.0682. [DOI] [Google Scholar]
- 53.Bals B, Rogers C, Jin MJ, Balan V, Dale B. 2010. Evaluation of ammonia fibre expansion (AFEX) pretreatment for enzymatic hydrolysis of switchgrass harvested in different seasons and locations. Biotechnol Biofuels 3:1. doi: 10.1186/1754-6834-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parrish DJ, Fike JH. 2005. The biology and agronomy of switchgrass for biofuels. Crit Rev Plant Sci 24:423–459. doi: 10.1080/07352680500316433. [DOI] [Google Scholar]
- 55.Dumitrache A, Tolbert A, Natzke J, Brown SD, Davison BH, Ragauskas AJ. 2017. Cellulose and lignin colocalization at the plant cell wall surface limits microbial hydrolysis of Populus biomass. Green Chem 19:2275–2285. doi: 10.1039/C7GC00346C. [DOI] [Google Scholar]
- 56.Li Z, Zhao C, Zha Y, Wan C, Si S, Liu F, Zhang R, Li F, Yu B, Yi Z, Xu N, Peng L, Li Q. 2014. The minor wall-networks between monolignols and interlinked-phenolics predominantly affect biomass enzymatic digestibility in Miscanthus. PLoS One 9:e105115. doi: 10.1371/journal.pone.0105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y, Zhao S, Yang S, Ding SY. 2014. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:38–45. doi: 10.1016/j.copbio.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Laser M, Schulman D, Allen SG, Lichwa J, Antal MJ Jr, Lynd LR. 2002. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour Technol 81:33–44. doi: 10.1016/S0960-8524(01)00103-1. [DOI] [PubMed] [Google Scholar]
- 59.Langan P, Petridis L, O'Neill HM, Pingali SV, Foston M, Nishiyama Y, Schulz R, Lindner B, Hanson BL, Harton S, Heller WT, Urban V, Evans BR, Gnanakaran S, Ragauskas AJ, Smith JC, Davison BH. 2014. Common processes drive the thermochemical pretreatment of lignocellulosic biomass. Green Chem 16:63–68. doi: 10.1039/C3GC41962B. [DOI] [Google Scholar]
- 60.Pingali SV, O'Neill HM, Nishiyama Y, He LL, Melnichenko YB, Urban V, Petridis L, Davison B, Langan P. 2014. Morphological changes in the cellulose and lignin components of biomass occur at different stages during steam pretreatment. Cellulose 21:873–878. doi: 10.1007/s10570-013-0162-6. [DOI] [Google Scholar]
- 61.d'Errico C, Jorgensen JO, Krogh KB, Spodsberg N, Madsen R, Monrad RN. 2015. Enzymatic degradation of lignin-carbohydrate complexes (LCCs): model studies using a fungal glucuronoyl esterase from Cerrena unicolor. Biotechnol Bioeng 112:914–922. doi: 10.1002/bit.25508. [DOI] [PubMed] [Google Scholar]
- 62.Sethi A, Scharf ME. 2013. Biofuels: fungal, bacterial and insect degraders of lignocellulose. eLS doi: 10.1002/9780470015902.a0020374. [DOI] [Google Scholar]
- 63.Palmqvist E, Hahn-Hagerdal B. 2000. Fermentation of lignocellulosic hydrolysates. II. Inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. [Google Scholar]
- 64.Tschaplinski TJ, Standaert RF, Engle NL, Martin MZ, Sangha AK, Parks JM, Smith JC, Samuel R, Jiang N, Pu YQ, Ragauskas AJ, Hamilton CY, Fu CX, Wang ZY, Davison BH, Dixon RA, Mielenz JR. 2012. Down-regulation of the caffeic acid O-methyltransferase gene in switchgrass reveals a novel monolignol analog. Biotechnol Biofuels 5:71. doi: 10.1186/1754-6834-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samuel R, Pu Y, Jiang N, Fu C, Wang ZY, Ragauskas A. 2014. Structural characterization of lignin in wild-type versus COMT down-regulated switchgrass. Front Energy Res 14:1. [Google Scholar]
- 66.Dumitrache A, Akinosho H, Rodriguez M Jr, Meng X, Yoo CG, Natzke J, Engle NL, Sykes RW, Tschaplinski TJ, Muchero W, Ragauskas AJ, Davison BH, Brown SD. 2016. Consolidated bioprocessing of Populus using Clostridium (Ruminiclostridium) thermocellum: a case study on the impact of lignin composition and structure. Biotechnol Biofuels 9:31. doi: 10.1186/s13068-016-0445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida M, Liu Y, Uchida S, Kawarada K, Ukagami Y, Ichinose H, Kaneko S, Fukuda K. 2008. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci Biotechnol Biochem 72:805–810. doi: 10.1271/bbb.70689. [DOI] [PubMed] [Google Scholar]
- 68.Prawitwong P, Waeonukul R, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Deng L, Sermsathanaswadi J, Septiningrum K, Mori Y, Kosugi A. 2013. Direct glucose production from lignocellulose using Clostridium thermocellum cultures supplemented with a thermostable beta-glucosidase. Biotechnol Biofuels 6:184. doi: 10.1186/1754-6834-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohgren K, Bura R, Saddler J, Zacchi G. 2007. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour Technol 98:2503–2510. doi: 10.1016/j.biortech.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Cybulska I, Lei HW, Julson J. 2010. Hydrothermal pretreatment and enzymatic hydrolysis of prairie cord grass. Energy Fuels 24:718–727. doi: 10.1021/ef9009179. [DOI] [Google Scholar]
- 71.Bhagia S, Muchero W, Kumar R, Tuskan GA, Wyman CE. 2016. Natural genetic variability reduces recalcitrance in poplar. Biotechnol Biofuels 9:106. doi: 10.1186/s13068-016-0521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pessani NK, Atiyeh HK, Wilkins MR, Bellmer DD, Banat IM. 2011. Simultaneous saccharification and fermentation of Kanlow switchgrass by thermotolerant Kluyveromyces marxianus IMB3: the effect of enzyme loading, temperature and higher solid loadings. Bioresour Technol 102:10618–10624. doi: 10.1016/j.biortech.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Silva GM, Giordano RLC, Cruz AJG, Ramachandriya KD, Banat IM, Wilkins MR. 2015. Ethanol production from sugarcane bagasse using SSF process and thermotolerant yeast. Trans ASABE 58:193–200. [Google Scholar]
- 74.Hormeyer HF, Tailliez P, Millet J, Girard H, Bonn G, Bobleter O, Aubert JP. 1988. Ethanol production by Clostridium thermocellum grown on hydrothermally and organosolv-pretreated lignocellulosic materials. Appl Microbiol Biotechnol 29:528–535. doi: 10.1007/BF00260980. [DOI] [Google Scholar]
- 75.Lipscomb GL, Conway JM, Blumer-Schuette SE, Kelly RM, Adams MW. 2016. A Highly thermostable kanamycin resistance marker expands the tool kit for genetic manipulation of Caldicellulosiruptor bescii. Appl Environ Microbiol 82:4421–4428. doi: 10.1128/AEM.00570-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baxter HL, Mazarei M, Fu CX, Cheng QK, Turner GB, Sykes RW, Windham MT, Davis MF, Dixon RA, Wang ZY, Stewart CN. 2016. Time course field analysis of COMT-downregulated switchgrass: lignification, recalcitrance, and rust susceptibility. Bioenergy Res 9:1087–1100. doi: 10.1007/s12155-016-9751-1. [DOI] [Google Scholar]
- 77.Baxter HL, Mazarei M, Labbe N, Kline LM, Cheng Q, Windham MT, Mann DG, Fu C, Ziebell A, Sykes RW, Rodriguez M Jr, Davis MF, Mielenz JR, Dixon RA, Wang ZY, Stewart CN Jr. 2014. Two-year field analysis of reduced recalcitrance transgenic switchgrass. Plant Biotechnol J 12:914–924. doi: 10.1111/pbi.12195. [DOI] [PubMed] [Google Scholar]
- 78.Baxter HL, Poovaiah CR, Yee KL, Mazarei M, Rodriguez M, Thompson OA, Shen H, Turner GB, Decker SR, Sykes RW, Chen F, Davis MF, Mielenz JR, Davison BH, Dixon RA, Stewart CN. 2015. Field evaluation of transgenic switchgrass plants overexpressing PvMYB4 for reduced biomass recalcitrance. Bioenergy Res 8:910–921. doi: 10.1007/s12155-014-9570-1. [DOI] [Google Scholar]
- 79.Shi J, Pu Y, Yang B, Ragauskas A, Wyman CE. 2011. Comparison of microwaves to fluidized sand baths for heating tubular reactors for hydrothermal and dilute acid batch pretreatment of corn stover. Bioresour Technol 102:5952–5961. doi: 10.1016/j.biortech.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 80.Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D. 2008. Determination of structural carbohydrates and lignin in biomass. Technical Report NREL/TP-510-42618. National Renewable Energy Laboratory, Golden, CO: https://permanent.access.gpo.gov/lps94089/42618.pdf. [Google Scholar]
- 81.Chandra R, Ewanick S, Hsieh C, Saddler JN. 2008. The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis. 1. A modified Simons' staining technique. Biotechnol Prog 24:1178–1185. doi: 10.1002/btpr.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.