Abstract
Recent research has implicated the nucleus accumbens (NAc) in consolidating recently acquired goal-directed appetitive memories, including spatial learning and other instrumental processes. However, an important but unresolved issue is whether this forebrain structure also contributes to the consolidation of fundamental forms of appetitive learning acquired by Pavlovian associative processes. In addition, although dopaminergic and glutamatergic influences in the NAc have been implicated in instrumental learning, it is unclear whether similar mechanisms operate during Pavlovian conditioning. To evaluate these issues, the effects of posttraining intra-NAc infusions of D1, D2, and NMDA receptor antagonists, as well as d-amphetamine, were determined on Pavlovian autoshaping in rats, which assesses learning by discriminated approach behavior to a visual conditioned stimulus predictive of food reward. Intracerebral infusions were given either immediately after each conditioning session to disrupt early memory consolidation or after a delay of 24 h. Findings indicate that immediate, but not delayed, infusions of both D1 (SCH 23390) and NMDA (AP-5) receptor antagonists significantly impair learning on this task. By contrast, amphetamine and the D2 receptor antagonist sulpiride were without significant effect. These findings provide the most direct demonstration to date that D1 and NMDA receptors in the NAc contribute to, and are necessary for, the early consolidation of appetitive Pavlovian learning.
Keywords: consolidation, dopamine, learning, autoshaping, glutamate
The nucleus accumbens (NAc) is increasingly regarded as a key forebrain structure underlying appetitive learning and memory (1, 2), and there is widespread interest in understanding the involvement of NAc dopamine (DA) and glutamate in this process (3-7). Blockade of D1 and NMDA receptors in the NAc impairs the acquisition, but not the performance, of appetitive instrumental conditioning (4, 8). Likewise, antagonism of NAc NMDA and DA receptors (9), as well as selective NAc DA depletion (10, 11), impairs Pavlovian conditioned behavior. However, because all of these studies manipulated NAc DA and glutamate function prior to conditioning, it is possible that several processes relevant to learning and memory were affected, including acquisition, consolidation, and retrieval. Posttraining manipulations define more precisely the nature of the learning deficit, in addition to avoiding possible confounding effects of nonspecific performance factors on learning (12). Indeed, a number of studies report significant effects on radial maze and water maze learning after posttraining systemic (13, 14), intracaudate (15), and intra-NAc (3) administration of DA receptor agents. Furthermore, recent research indicates that early consolidation of instrumental learning requires new protein synthesis in the NAc (16).
However, there have been remarkably few studies investigating the involvement of DA and glutamate in the consolidation of appetitive Pavlovian learning. Although posttraining systemic (17) and intra-NAc (6) amphetamine administration facilitates Pavlovian conditioning, it is unknown whether NAc DA and NMDA receptors contribute to the consolidation of appetitive Pavlovian associative memories. We therefore investigated in rats the effects of posttraining intra-NAc infusions of D1 (SCH 23390) and D2 (sulpiride) receptor antagonists, the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (AP-5), and amphetamine on the acquisition of appetitive Pavlovian autoshaping (10, 11, 18). In this paradigm, learning is indexed by the emergence of a discriminated approach response to a visual conditioned stimulus (CS) that is either paired (CS+) or unpaired (CS-) with the presentation of food reward [unconditioned stimulus (US)]. The time dependency of any disruption in Pavlovian learning resulting from posttraining intra-NAc drug infusions was evaluated by administering compounds affecting DA or NMDA receptors either immediately after conditioning or after a delay of 24 h. A retention test followed 72 h later in which the CS+ and CS- were presented simultaneously during extinction. Finally, an omission test was used, whereby reward was made contingent on the nonoccurrence of a CS+ directed approach response (19, 20). A lack of sensitivity to this control procedure confirmed the degree to which Pavlovian, and not implicit instrumental contingencies, supported the conditioned appetitive response. Considerable evidence suggests that Pavlovian and instrumental associative processes jointly determine behavioral output in many learning situations (21, 22). Thus, a failure to maintain conditioned responding during an omission contingency test indicates the degree to which rats had learned an instrumental (action-outcome) association during conditioning when Pavlovian mechanisms had been insufficient to maintain that behavior.
Methods
Subjects. The subjects were 64 male Lister hooded rats (Charles River Breeding Laboratories) weighing between 360 and 420 g at the time of surgery. They were housed in pairs in a temperature-controlled room (22°C) under an alternating light/dark cycle (white lights off/red lights on from 7:30 a.m. to 7:30 p.m.). Each subject was given 18 g of food per day (Purina laboratory chow), sufficient to maintain body weight at no less than 85% of free feeding weight. Water was made freely available in the home cage. Animal procedures were conducted in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act (Project License PPL 80/1767).
Surgery. Animals were anesthetized with 90 mg/kg (i.p.) ketamine (Ketaset, Vet Drug, Bury St. Edmunds, U.K.) and 6.7 mg/kg (i.p.) xylazine (Rompun, Vet Drug) and placed in a stereotaxic frame in a flat-skull position [incisor bar -3.3 mm relative to the interaural line (23)]. An ophthalmic ointment (Lacri-Lube, Allergan, High Wycombe, U.K.) was wiped over each eye to prevent desiccation of the corneal surfaces. A midline incision was made and the skin and underlying periostium retracted. Bilateral stainless steel guide cannulae (22 gauge; Plastics One, St. Albans, U.K.) were implanted above the NAc core (AP, +1.5 mm forward of bregma; L, ±1.9 mm; V, -2.0 mm relative to dura) and anchored to the skull with bone screws and dental cement. Stainless steel obdurators (28 gauge; Plastics One) were inserted into the guide cannulae to maintain patency. A plastic dust cap protected the guide cannulae assembly. Rats recovered from surgery in a separate humidity- and temperature-controlled room with free access to water. The next day, they were returned to the main stock holding room to recover for 5-7 days.
Autoshaping Apparatus. The autoshaping apparatus consisted of a test chamber and a touch-sensitive liquid crystal display (LCD) video unit (Intasolve, Colchester, U.K.) housed within a wooden sound attenuating box (Cambridge Cognition, Cambridge, U.K.). A schematic diagram of the test chamber is published elsewhere (18). The five test chambers measured 45 × 32 × 30-cm (length × width × height) and consisted of a metal frame and transparent Perspex walls with a perforated aluminum floor. They were each illuminated by a centrally located 3-W house light. The chamber was fitted with two food magazines and pellet dispensers, one in the rear of the chamber directly behind a pressure-sensitive floor panel (14.5 × 10 cm) and one located in front of the LCD screen. Stimuli were identical vertical white rectangles (6.5 × 14 cm), presented on either the right or the left of the LCD screen. Infrared photobeams to the left and right of the center food magazine allowed the detection of approach responses to either side of the LCD screen. A special feature of this task is that, because of the spatial separation of the CS and US (US, i.e., food), approach to the CS and US can be independently assessed, unlike other test settings (6, 24). The apparatus was controlled and monitored by whisker computer software (Version 2.2; R. N. Cardinal and M. R. F. Aitken, www.whiskercontrol.com). Closed-circuit television cameras (Tracksys, Nottingham, U.K.) were placed inside each chamber to monitor performance and ensure that food pellets were consumed on each trial.
Behavioral Training. Animals were trained on the autoshaping task during the dark phase of the activity cycle, between 8:30 a.m. and 5:30 p.m. On the day before being habituated to the apparatus (see below), animals were given one mock infusion. This involved removing the obdurator and inserting a bilateral injector (28 gauge; Plastics One) into the lumen of the guide cannula and leaving it in place for 1 min. The injector was not inserted into the brain during this procedure. The next day, subjects were given two 20-min habituation sessions where the house light was illuminated and pellets (45-mg Noyes dustless pellets; Sandown Scientific, Hampton, U.K.) were delivered to the central magazine on a variable time 0- to 30-s schedule. Five to 10 pellets were placed in and around the rear pressure-sensitive floor and, in addition, were delivered to the food magazine at the rear of the chamber following the depression of the rear floor panel. Animals were eliminated from further testing if they failed to consume all of the pellets. The magazine at the rear of the chamber was used only during the habituation sessions to encourage subjects to cross the pressure-sensitive floor panel. Activation of this panel during subsequent autoshaping triggered the onset of the CS- and CS+. Its position at the rear of the box enabled subjects to monitor both sides of the LCD unit.
On the next day (day 1), subjects were trained to associate 10-s visual stimuli with delivery of pellets in the central magazine. Stimuli were displayed on the left and right of the LCD; one was designated the CS+ and the other, the CS-, counterbalanced between subjects. The offset of the CS+ was occasioned by the delivery of a single food pellet in the center food magazine. No food was delivered after the offset of the CS-. A trial consisted of the presentation of both the CS+ and the CS- in a randomized order. After a variable interval (VI) of 10-40 s, the program waited for the subject to activate the floor panel. One stimulus was then presented for 10 s. After this, another VI of 10-40 s followed; the program then waited for the rat to return to the rear of the chamber, and the other stimulus was presented. This procedure made chance approach responses less likely by ensuring that subjects could attend to both sides of the LCD during any trial. It also ensured a minimum time between CS+ and CS- presentations of 10 s and that there were never more than two consecutive presentations of the CS+ and CS-. Over the course of training, rats developed a conditioned discriminative response of approaching the food-predictive CS+. An approach response was recorded if the subject broke the photocell beam directly in front of the stimulus on the LCD screen. Only the first approach was recorded for any given stimulus presentation. Data were analyzed in blocks of 10 trials as mean CS+ and CS- approach scores.
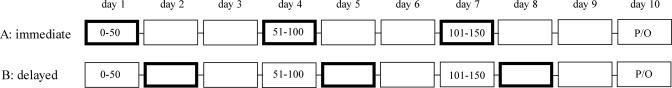
Study Design. The acquisition of autoshaping was conducted over three sessions with an intersession interval of 72 h (see Fig. 1). Sessions consisted of 50 trials and lasted generally 2 h. At the completion of each session, subjects were lightly restrained and administered vehicle or various drug treatments directly into the NAc within 5-10 min after completing the 50 trials (immediate condition). A separate group of animals received the same drug treatments 24 h later (delayed condition). The 28-gauge injectors (Plastics One) extended 5 mm below the end of the guide cannula. Infusions were given over 1 min in a different room from the autoshaping test room by using a microsyringe pump (Harvard Apparatus), in an injection volume of 0.5 μl, after an initial equilibration period of 1 min. Injectors were left in situ for 2 min after each infusion. Subjects were allocated randomly to 10 treatment groups: immediate vehicle [0.01 M PBS (PBS), pH 7.4] group (n = 7), delayed vehicle group (n = 6), immediate SCH 23390 (0.3 μg per side) group (n = 8), delayed SCH 23390 group (n = 5), immediate sulpiride (10 ng/side) group (n = 5), delayed sulpiride group (n = 5), immediate amphetamine (10 μg/side) group (n = 6), delayed amphetamine group (n = 5), immediate AP-5 (1 μg/side) group (n = 8), and delayed AP-5 group (n = 9). The actual doses were based on previous studies in rats showing them to be effective in other learning and memory paradigms (3, 4, 6, 9). The n values are final group sizes based on accurate injector placements in the NAc and the absence of gross nonspecific damage such as cavitation.
Fig. 1.
Schematic illustration of the experimental protocol. The acquisition of autoshaping was conducted over three sessions, each consisting of 50 trials with an intersession interval of 72 h. The highlighted boxes indicate the timing of the drug infusions in the NAc. These occurred either immediately after each conditioning session (condition A) or after a delay of 24 h (condition B). P denotes a probe retention test; O denotes omission training.
As a further index of discriminative learning, subjects were given a probe retention test 72 h after the last autoshaping session. This consisted of 20 trials in which the CS+ and CS- were presented simultaneously and approaches were measured. Food was not delivered during this short test. To confirm the extent to which Pavlovian contingencies maintained a conditioned approach to the CS+, an omission schedule was used 5-6 h after the probe test. In this schedule, the contingencies were reversed such that approach to the CS+ now prevented food delivery (19, 20). Approach to the CS- had no programmed consequence. Omission training consisted of 50 presentations of both the CS+ and CS-.
d-Amphetamine sulfate was obtained from Sigma. SCH 23390 hydrochloride, (S)-(-)-sulpiride, and d-AP5 were obtained from Tocris Cookson (Bristol, U.K.). Drug quantities were calculated from the free base weight. All drugs were dissolved in 0.01 M PBS except sulpiride, which was first dissolved in a small volume of 0.01 HCl.
At the end of the experiment, rats were administered 1.5 ml of sodium pentobarbitone (Euthatal, 200 mg/ml, Vet Drug) and perfused transcardially with PBS, followed by 4% paraformaldehyde. The brains were removed, stored in 4% paraformaldehyde for 24 h, and placed in 20% sucrose solution before sectioning and staining with Cresyl-violet to determine the position of the injector tips in the NAc based on their ventral most extent.
Data were subjected to repeated-measures ANOVA (spss for Windows, release 10, SPSS, Chicago). Homogeneity of variance was assessed by the Mauchly sphericity test. When data sets significantly violated this requirement, the Huynh-Feldt ε was used to calculate a more conservative P value for each F ratio. The probe data were compared by using a Welch-corrected t test. The probability of statistical significance was 0.05.
Results
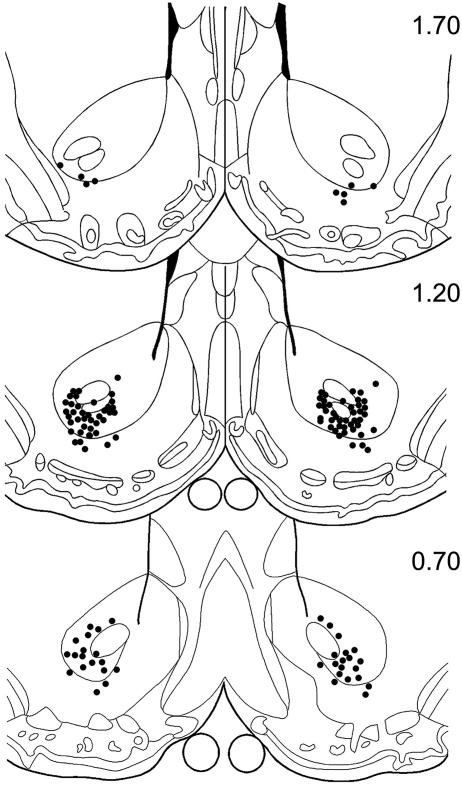
Cannula Placements. The location of the tips of injection cannulas within the NAc is shown in Fig. 2. The tips were located in the vicinity of the ventral border of anterior commissure between 0.7 and 1.7 mm forward of bregma. A small number of injector tips were located in the ventrolateral shell region of the NAc.
Fig. 2.
Diagrammatic sections modified from the atlas of Paxinos and Watson (23) showing injector tip placements in the vicinity of the NAc. The numbers to the right of each section are anterior coordinates forward of bregma (in mm).
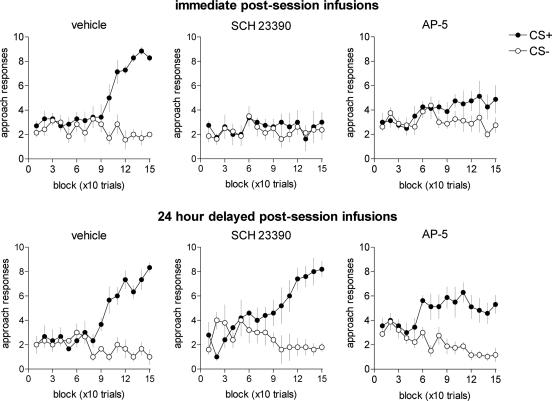
Autoshaping. Fig. 3 shows that all subjects receiving either immediate or delayed intra-NAc vehicle infusions acquired discriminated approach behavior to the appetitive CS. Thus, ANOVA revealed significant main effects of CS (F1,11 = 199.66; P < 0.01) and Block (F1,14 = 10.31; P < 0.01) and a significant CS × Block interaction (F14,154 = 27.98; P < 0.01), but there was no distinction between the timing of the infusions and learning (Timing, F1,11 = 1.95; P = 0.19; CS × Block × Timing, F14,154 = 1.05; P = 0.411). A later retention test confirmed that both the immediate and delayed control groups acquired this Pavlovian conditioned behavior (see Table 1).
Fig. 3.
Impaired Pavlovian learning as a consequence of immediate postsession intra-NAc infusions of the D1 receptor antagonist SCH 23390 (0.3 μg per side) and the NMDA receptor antagonist AP-5 (1 μg per side). Delayed infusions of the same compounds produced no significant effect. Discriminated approach to the visual stimulus predictive (CS+) and nonpredictive (CS-) of food reward was evaluated over 15 blocks, each consisting of 10 discrete trials. The maximum number of CS+ or CS- approach responses for each block was 10. Error bars represent ±1 SEM.
Table 1. Probe retention test of Pavlovian learning.
| Approach responses
|
||
|---|---|---|
| Treatment group | CS− | CS+ |
| Immediate infusions | ||
| Vehicle | 2.5 ± 0.7 | 9.0 ± 1.1** |
| SCH 23390 | 4.4 ± 1.2 | 5.0 ± 1.2 |
| Sulpiride | 3.4 ± 1.1 | 10.4 ± 1.6** |
| d-Amphetamine | 1.8 ± 0.3 | 11.7 ± 0.7** |
| AP-5 | 3.4 ± 0.9 | 12.0 ± 1.6** |
| 24-h delayed infusions | ||
| Vehicle | 3.7 ± 1.9 | 11.0 ± 1.2* |
| SCH 23390 | 2.1 ± 0.6 | 9.4 ± 1.3** |
| Sulpiride | 2.6 ± 0.5 | 10.6 ± 2.4** |
| d-amphetamine | 2.4 ± 0.6 | 11.8 ± 1.7** |
| AP-5 | 2.9 ± 0.9 | 11.2 ± 1.4** |
Subjects experienced 20 simultaneous presentations of the CS+ and CS− in extinction. The data are mean (±1 SEM) approach responses. *, P < 0.05; **, P < 0.01 CS+ vs. CS−.
Immediate postsession intra-NAc infusions of the D1 receptor antagonist SCH 23390 produced a severe impairment in autoshaping that was not observed in subjects receiving 24-h delayed infusions of this compound. Thus, analysis of the difference scores between CS+ and CS- responses over Block (a measure of discriminative learning) confirmed that subjects in the delayed treatment group acquired autoshaping (vehicle vs. SCH 23390: Group, F1,13 = 0.01; P = 0.99; Block, F14,126 = 18.6; P < 0.01; Block × Group, F14,126 = 1.09; P = 0.37), whereas those in the immediate infusion group did not (vehicle vs. SCH 23390, Group, F1,13 = 16.9; P < 0.01; Block, F14,182 = 5.52; P < 0.01; Block × Group, F14,182 = 5.24; P < 0.01). These differential effects were confirmed by a main effect of CS for the delayed group (F1,4 = 15.9; P = 0.016) but not the immediate group (F1,7 = 0.52; P = 0.49) and by a probe test showing impaired and unimpaired discrimination in the immediate (P = 0.716) and delayed (P = 0.004) groups, respectively (see Table 1). By contrast, postsession infusions of the D2 receptor antagonist sulpiride and d-amphetamine were without significant effect on Pavlovian learning (see Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 3 shows that subjects receiving immediate postsession infusions of the NMDA receptor antagonist AP-5 failed to discriminate significantly between the CS+ and CS- during acquisition (CS, F1,7 = 3.92; P = 0.088; CS × Block, F14,98 = 1.25; P = 0.251). By contrast, subjects receiving delayed infusions of AP-5 showed significant CS-/CS+ discrimination (CS, F1,8 = 39.62; P < 0.01; CS × Block, F14,112 = 3.09; P < 0.01). Although subjects receiving immediate AP-5 infusions were significantly disrupted in acquiring the task (vehicle vs. AP-5, difference score; immediate condition, F1,13 = 8.56; P = 0.012; delayed condition, F1,13 = 0.02; P = 0.91), they nevertheless demonstrated significant learning during the subsequent probe retention test, similar to the delayed AP-5 group. Therefore, although immediate postsession intra-NAc infusions of AP-5 significantly retarded appetitive approach learning, they did not abolish it.
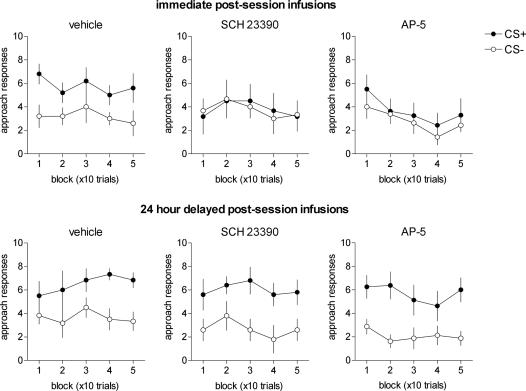
Omission Training. Fig. 4 shows the effects of postsession intra-NAc infusions of vehicle, AP-5, and SCH 23390 on performance of an omission schedule where reward was made contingent on the nonoccurrence of a CS+ approach response. Vehicle-treated rats continued to discriminate between the CS+ and CS- during this procedure (CS, F1,11 = 10.64; P = 0.01), and this did not alter as a function of training (CS × Block, F4,44 = 0.27; P = 0.895; Block, F4,44 = 0.744; P = 0.569) or timing of the infusions (Timing, F1,11 = 1.23; P = 0.296; CS × Timing, F1,11 = 0.027; P = 0.872). Rats receiving immediate postsession intra-NAc infusions of SCH 23390 were significantly impaired in discriminating between the CS+ and CS- during omission training (CS, F1,7 = 0.009; P = 0.93), unlike subjects that received delayed intra-NAc infusions of SCH 23390 (CS, F1,4 = 7.03; P < 0.05) that did not diminish over the course of the omission session (CS × Block, F4,16 = 0.27; P = 0.89). Despite exhibiting a significant preference for the CS+ during probe retention testing, rats receiving immediate AP-5 infusions failed to discriminate between the CS+ and CS- during omission training (CS, F1,7 = 0.48; P = 0.512; CS × Block, F4,28 = 0.24; P = 0.913). By contrast, rats in the delayed AP-5 group continued to approach the CS+ during this procedure (CS, F1,8 = 18.75; P < 0.01; CS × Block, F4,32 = 1.52; P = 0.22). Subjects receiving postsession infusions of sulpiride or amphetamine during acquisition were unimpaired during the omission contingency test (see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 4.
Inflexible CS+ approach during omission training, where reinforcement was made contingent on the nonoccurrence of a CS+ directed response. A failure to extinguish responding to the CS+ during this procedure indicates that appetitive learning was supported mainly by Pavlovian associative mechanisms. Error bars represent ±1 SEM.
Discussion
The results reported here demonstrate that D1 receptors and NMDA receptors in the NAc contribute to the early consolidation of appetitive Pavlovian conditioning. Specifically, we have shown that learning associated with the normal emergence of a discriminated approach response to a CS predictive of food reward is abolished by the postsession intra-NAc administration of the D1 receptor antagonist SCH 23390. The inability of delayed 24-h infusions of SCH 23390 to interfere significantly with this form of appetitive Pavlovian learning argues strongly that D1 receptors in the NAc play a time-limited role in consolidating appetitive Pavlovian memories. These data thus support the predicted involvement of midbrain DA neurons in appetitive learning based on electrophysiological studies in monkeys (25) and, additionally, demonstrate a causal involvement of D1 receptors in this form of learning. Our findings also implicate NAc NMDA receptors in appetitive Pavlovian learning and therefore are consistent with the widely held concept that D1 and NMDA receptors within distributed corticostriatal networks are important targets underpinning long-term potentiation and other forms of neuronal plasticity hypothesized to underlie associative processes in learning (5, 26-29).
Previous work has established a critical and interactive role of D1 and NMDA receptors in the NAc in appetitive instrumental learning based on lever-press responding for food reward (4). The present results extend these findings in two important ways. First, we used postsession intra-NAc infusions of SCH 23390 and AP-5 to distinguish effects on learning from effects on performance (12). Second, we used what is a principally a Pavlovian task that is minimally sensitive to changes in instrumental contingencies (10, 11, 30). This was demonstrated most convincingly by the failure of control rats to extinguish autoshaped conditioned approach when reinforcement was made contingent on the nonoccurrence of a CS+ approach. In contrast, appetitive instrumental learning generally depends on interacting Pavlovian and instrumental processes that preclude simple inferences to be drawn on the nature of any learning deficit. For example, reinforcing a lever-press response in the presence of a discriminative stimulus such as the illumination of a light stimulus above the lever may, in some circumstances, be represented by a Pavlovian association between a light CS and food (21).
Much previous evidence has implicated the NAc in appetitive Pavlovian and instrumental learning (1, 2, 31) in ways that are readily distinguishable from its well known involvement in motivation, reinforcement, and motor activity (32). In particular, the NAc has been implicated in the acquisition and/or consolidation of spatial information but not in its retrieval (1, 33). In intact rats, place learning in the aversively motivated Morris water maze is impaired by pretraining intra-NAc infusions of haloperidol (34) and by posttraining intra-NAc infusions of sulpiride (3) and AP-5 (7), but not by posttraining intra-NAc infusions of the AMPA receptor antagonist DNQX (7). Consistent with these data, posttraining intra-NAc infusions of AP-5 in the present study retarded learning of a spatially conditioned appetitive approach response. In our hands, however, posttraining intra-NAc infusions of sulpiride neither impaired nor facilitated autoshaping, despite previous evidence that spatial escape learning is impaired by a comparable posttraining intra-NAc dose of sulpiride (3). The reasons for this are unclear but may be due to differences in underlying learning processes engaged by the two tasks, for example appetitive vs. aversive, in addition to apparent differences in the timing, frequency, and site of the intra-NAc sulpiride infusions. For example, Setlow and McGaugh (3) infused sulpiride into more rostral and dorsal sites of the NAc core compared with the present study. Moreover, posttraining infusions of d-amphetamine (10 μg) into the dorsomedial shell of the NAc but not the core, as here, have recently been shown to facilitate appetitive Pavlovian conditioning (6). Therefore, variations in the precise effects of DA receptor agents on appetitive learning and memory may be explained by the existence of functionally distinct neuroanatomical loci within the NAc, possibly consistent with the heterogeneous distribution of inputs to the NAc from the prefrontal cortex, hippocampus, and amygdala (35).
Learning-related plasticity has often been attributed to D1 and NMDA receptor interactions within broadly distributed but interconnected brain regions, including the NAc, basolateral amygdala, and prefrontal cortex (2, 29, 36). Recently, distinct and interactive effects of NMDA and D1 receptor antagonists on appetitive instrumental learning have been reported with impaired learning after pretraining intra-NAc infusions of AP-5 and SCH 23390 (4). In addition, pretraining systemic and intra-NAc NMDA and D1 receptor antagonists have been reported to impair Pavlovian learning in the context of the conditioned appetitive approach (9, 24). Because intra-NAc AP-5 infusions appear to have no significant effect on the expression of the appetitive Pavlovian approach (9), it is likely that NMDA receptors in the NAc contribute to the acquisition and/or consolidation of appetitive Pavlovian learning. The present data provide evidence that NMDA receptors in the NAc play a significant role in the early consolidation of discriminated approach learning. Intriguingly, however, a summary of recent unpublished findings suggests that posttrial infusions of either AP-5 or SCH 23390 do not interfere with appetitive instrumental learning (2). The basis for these discrepant results is unclear, but it is assumed that differences in the associative processes and/or distributed brain networks that support learning in these tasks may be contributory factors.
Although AP-5 retarded significantly the acquisition of autoshaping after its immediate postsession intra-NAc administration, rats nevertheless displayed significant evidence of learning during a subsequent retention test involving simultaneous presentations of the CS+ and CS-. These findings suggest that the probe test is probably a less-sensitive method for detecting impairments in discriminative approach learning; indeed, simultaneous procedures have previously been shown to be more sensitive than successive procedures in revealing discrimination learning (22). AP5-treated rats, however, failed to maintain discriminative performance when an instrumental contingency was introduced that prevented food delivery after a CS+ approach. The most parsimonious explanation for this finding was that AP-5 infusions blocked the predominantly Pavlovian elements of this task to the point that they were no longer sufficient to maintain discriminated approach behavior. In view of the fact that many learning situations likely engage competing Pavlovian and instrumental influences (21, 22), it is also possible that a small residual instrumental component supported appetitive approach learning in AP-5 rats that was subsequently disrupted by the omission contingency. Due to the severe learning deficits induced by SCH 23390, no inferences can be drawn on the relative involvement of D1 receptors in this process. However, pretraining depletion of NAc DA produces effects on omission performance that are broadly opposite to the effects of AP-5. Specifically, DA-lesioned rats exhibit an inflexible CS+ approach rather than the normal fluctuations observed in sham rats that may indicate transient effects of instrumental contingencies (10).
Conclusion
It is hypothesized that converging afferent inputs to the NAc from glutamatergic neurons arising from limbic-cortical structures, as well as dopaminergic neurons from the midbrain (35), contribute to, and are necessary for, distinct aspects of memory consolidation relating to Pavlovian conditioned approach learning. Additional studies are required to understand how multiple interacting but dissociable neural systems involving the anterior cingulate cortex (18, 30, 37), orbitofrontal cortex (38), and amygdala (39) contribute to this learning process.
Supplementary Material
Acknowledgments
We are grateful to Professor Anthony Dickinson for invaluable discussion of the data. This study was supported by a Wellcome Trust Program grant and completed within the Cambridge Medical Research Council Centre in Behavioral and Clinical Neuroscience. P.R.C. holds a Wellcome Trust Studentship. Y.C. was supported by Cambridge Cognition.
Author contributions: J.W.D., Y.C., and T.W.R. designed research; J.W.D., K.L., D.E.T., H.C.A., P.R.C., and Y.C. performed research; J.W.D. analyzed data; and J.W.D. wrote the paper.
Abbreviations: NAc, nucleus accumbens; DA, dopamine; CS, conditioned stimulus; US, unconditioned stimulus; LCD, liquid crystal display.
References
- 1.Setlow, B. (1997) J. Neurosci. Res. 49, 515-521. [DOI] [PubMed] [Google Scholar]
- 2.Kelley, A. E. (2004) Neurosci. Biobehav. Rev. 27, 765-776. [DOI] [PubMed] [Google Scholar]
- 3.Setlow, B. & McGaugh, J. L. (1998) Behav. Neurosci. 112, 603-610. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Roe, S. L. & Kelley, A. E. (2000) J. Neurosci. 20, 7737-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley, A. E., Andrzejewski, M. E., Baldwin, A. E., Hernandez, P. J. & Pratt, W. E. (2003) Ann. N.Y. Acad. Sci. 1003, 159-168. [DOI] [PubMed] [Google Scholar]
- 6.Phillips, G. D., Setzu, E. & Hitchcott, P. K. (2003) Behav. Neurosci. 117, 675-684. [DOI] [PubMed] [Google Scholar]
- 7.Sargolini, F., Florian, C., Oliverio, A., Mele, A. & Roullet, P. (2003) Learn. Mem. 10, 285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley, A. E., Smith-Roe, S. L. & Holahan, M. R. (1997) Proc. Natl. Acad. Sci. USA 94, 12174-12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Ciano, P., Cardinal, R. N., Cowell, R. A., Little, S. J. & Everitt, B. J. (2001) J. Neurosci. 21, 9471-9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalley, J.W., Chudasama, Y., Theobald, D. E., Pettifer, C. L., Fletcher, C. M. & Robbins, T. W. (2002) Psychopharmacology 161, 425-433. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson, J. A., Dalley, J. W., Cardinal, R. N., Bamford, A., Fehnert, B., Lachenal, G., Rudarakanchana, N., Halkerston, K. M., Robbins, T. W. & Everitt, B. J. (2002) Behav. Brain Res. 137, 149-163. [DOI] [PubMed] [Google Scholar]
- 12.McGaugh, J. L. (1966) Science 153, 1351-1358. [DOI] [PubMed] [Google Scholar]
- 13.White, N. M., Packard, M. G. & Seamans, J. (1993) Behav. Neural. Biol. 59, 230-241. [DOI] [PubMed] [Google Scholar]
- 14.Packard, M. G. & McGaugh, J. L. (1994) Psychobiology 22, 54-60. [Google Scholar]
- 15.Packard, M. G. & White, N. M. (1991) Behav. Neurosci. 105, 295-306. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez, P. J., Sadeghian, K. & Kelley, A. E. (2002) Nat. Neurosci. 5, 1327-1331. [DOI] [PubMed] [Google Scholar]
- 17.Oscos, A., Martinez, J. L. & McGaugh, J. L. (1988) Psychopharmacology 95, 132-134. [DOI] [PubMed] [Google Scholar]
- 18.Bussey, T. J., Everitt, B. J. & Robbins, T. W. (1997) Behav. Neurosci. 111, 908-919. [DOI] [PubMed] [Google Scholar]
- 19.Williams, D. R. & Williams, H. (1969) J. Exp. Anal. Behav. 12, 511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland, P. C. (1979) J. Exp. Psychol. 5, 178-193. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson, A. (1994) in Animal Learning and Cognition, ed. Mackintosh, N. J. (Academic, San Diego), pp. 45-76.
- 22.Mackintosh, N. J. (1974) The Psychology of Animal Learning, ed. Mackintosh, N. J. (Academic, San Diego).
- 23.Paxinos, G. & Watson, C. (1998) The Rat Brain in Stereotaxic Coordinates, eds. Paxinos, G. & Watson, C. (Academic, Sydney, Australia).
- 24.Eyny, Y. S. & Horvitz, J. C. (2003) J. Neurosci. 23, 1584-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz, W. & Dickinson, A. (2000) Annu. Rev. Neurosci. 23, 473-500. [DOI] [PubMed] [Google Scholar]
- 26.Pennartz, C. M., Ameerun, R. F., Groenewegen, H. J., Lopes da Silva, F. H. (1993) Eur. J. Neurosci. 5, 107-117. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell, P., Greene, J., Pabello, N., Lewis, B. L. & Grace, A. A. (1999) Ann. N.Y. Acad. Sci. 877, 157-175. [DOI] [PubMed] [Google Scholar]
- 28.Spencer, J. P. & Murphy, K. P. (2000) Exp. Brain Res. 135, 497-503. [DOI] [PubMed] [Google Scholar]
- 29.Floresco, S. B., Blaha, C. D., Yang, C. R. & Phillips, A. G. (2001) J. Neurosci. 21, 6370-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkinson, J. A., Willoughby, P. J., Robbins, T. W. & Everitt, B. J. (2000) Behav. Neurosci. 114, 42-63. [PubMed] [Google Scholar]
- 31.Parkinson, J. A., Olmstead, M. C., Burns, L. H., Robbins, T. W. & Everitt, B. J. (1999) J. Neurosci. 19, 2401-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins, T. W. & Everitt, B. J. (1996) Curr. Opin. Neurobiol. 6, 228-236. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland, R. J. & Rodriguez, A. J. (1989) Behav. Brain Res. 32, 265-277. [DOI] [PubMed] [Google Scholar]
- 34.Ploeger, G. E., Spruijt, B. M. & Cools, A. R. (1994) Behav. Neurosci. 108, 927-934. [DOI] [PubMed] [Google Scholar]
- 35.Zahm, D. S. (2000) Neurosci. Biobehav. Rev. 24, 85-105. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin, A. E., Sadeghian, K. & Kelley, A. E. (2002) J. Neurosci. 22, 1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardinal, R. N., Parkinson, J. A., Djafari Marbini, H., Toner, A. J., Bussey, T. J., Robbins, T. W. & Everitt, B. J. (2003) Behav. Neurosci. 117, 566-587. [DOI] [PubMed] [Google Scholar]
- 38.Chudasama, Y. & Robbins, T. W. (2003) J. Neurosci. 23, 8771-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkinson, J. A., Robbins, T. W. & Everitt, B. J. (2000) Eur. J. Neurosci. 12, 405-413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.