Abstract
Depression is a debilitating chronic illness that affects around 350 million people worldwide. Current treatments, such as selective serotonin reuptake inhibitors, are not ideal because only a fraction of patients achieve remission. Tianeptine is an effective antidepressant with a previously unknown mechanism of action. We recently reported that tianeptine is a full agonist at the mu opioid receptor (MOR). Here we demonstrate that the acute and chronic antidepressant-like behavioral effects of tianeptine in mice require MOR. Interestingly, while tianeptine also produces many opiate-like behavioral effects such as analgesia and reward, it does not lead to tolerance or withdrawal. Furthermore, the primary metabolite of tianeptine (MC5), which has a longer half-life, mimics the behavioral effects of tianeptine in a MOR-dependent fashion. These results point to the possibility that MOR and its downstream signaling cascades may be novel targets for antidepressant drug development.
Introduction
Depression is the most disabling illness in the world today (Smith, 2014). It strikes in the young and most productive years of life and then persists as a recurring lifetime illness. Since around 350 million people worldwide suffer from depression, it is responsible for more years lost to disability than any other condition (Smith, 2014). Available treatments have historically ranged from electroshock therapy to antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs), which are some of the most prescribed drugs in the world. SSRIs were originally developed in the mid-1980s and replaced monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) as the first-line treatment for depression due to their more desirable side-effect profile. Since FDA approval of the SSRI fluoxetine in 1987, several other monoaminergic classes of drugs have been developed. However, it appears that monoaminergic drugs may have reached their limit in terms of effectiveness. Approximately one third of depressed patients do not respond to treatment with monoaminergic drugs, and nearly 2/3rd of patients do not remit to first-line treatments (Rush et al, 2006; Warden et al, 2007). Thus, in the last several years there has been a shift in research focus toward novel drug targets for development of new antidepressant medications. These potential new therapies include glutamate modulators (Berman et al, 2000; Sanacora et al, 2014; Zarate et al, 2006), anticholinergic agents (Furey and Drevets, 2006), and opioid modulators (Carlezon et al, 2009).
All three well-defined classes of opioid receptors (delta, kappa, and mu) have been implicated to varying degrees in the pathophysiology of depression and its treatment (Lutz and Kieffer, 2013; Richards et al, 2016). Mice lacking the delta opioid receptor (DOR) throughout life demonstrate increased levels of anxiety and depression-related behaviors (Filliol et al, 2000), and DOR agonists have antidepressant properties in preclinical models (Broom et al, 2002; Naidu et al, 2007; Saitoh et al, 2004; Tejedor-Real et al, 1998; Torregrossa et al, 2006). However, DOR agonists have limited efficacy in humans with comorbid anxiety and depression (Richards et al, 2016). Recent research into opioid modulators for depression has also focused on kappa opioid receptor (KOR) antagonists that block the actions of endogenous dynorphins, which are KOR-selective agonist peptides released during the stress response (Almatroudi et al, 2015; Carr et al, 2010; Chavkin, 2011; Mague et al, 2003; McLaughlin et al, 2003; Newton et al, 2002; Shirayama et al, 2004). More recent clinical studies suggest that the combined effects of buprenorphine, a mixed mu opioid receptor (MOR) partial agonist-KOR antagonist, and samidorphan, a MOR antagonist, show promise as an adjunctive treatment for inadequate response to antidepressant treatment (Ehrich et al, 2015; Fava et al, 2016). When combined, these drugs effectively form a dual MOR/KOR antagonist. However, this combination has shown mixed efficacy in clinical trials, which brings into question the potential of opioid antagonists as antidepressants.
Interestingly, our recent work demonstrates that the antidepressant tianeptine (Stablon, Coaxil and Tatinol) is a full agonist at MOR (Gassaway et al, 2014). When first developed, tianeptine was thought to be a TCA based on its chemical structure. However, further work demonstrated that tianeptine does not inhibit monoamine reuptake, but instead may elicit its effects via modulation of glutamatergic pathways (Kasper and McEwen, 2008; McEwen et al, 2010; Svenningsson et al, 2007). Some of tianeptine’s neurobiological effects include regulation of plasticity in the amygdala, attenuation of stress-induced glutamate release, and reversal of stress-induced dystrophy of hippocampal CA3 dendrites (McEwen et al, 2010). However, tianeptine’s direct molecular target remained elusive until we screened it against a broad panel of human brain receptors (Gassaway et al, 2014), which found that tianeptine binds to human MOR with a Ki of 383±183 nM. MOR is a Gi/o-coupled receptor, and functional assays revealed that tianeptine is a full agonist for G-protein activation and inhibition of cAMP accumulation (Gassaway et al, 2014). Tianeptine also binds weakly to human DOR with a Ki>10 μM and agonizes this receptor as well, albeit at much higher concentrations than at MOR. Tianeptine appears to be inactive at KOR, showing neither agonist nor antagonist activity. Interestingly, our screen found no other GPCR, transporter, or ion channel targets besides MOR and DOR (Gassaway et al, 2014).
On the basis of these studies, we sought to determine whether the behavioral effects of tianeptine are mediated by MOR, and to characterize its behavioral effects compared to classic MOR agonists, namely morphine. To this end, we assessed whether mice lacking MOR or pretreated with MOR antagonists were resistant to the effects of acute and chronic tianeptine treatment in behavioral tests sensitive to antidepressants.
Materials and methods
Mice
C57BL/6 mice were either acquired from The Jackson Laboratory (Bar Harbor, ME) or were bred in house (MOR-deficient mice (Schuller et al, 1999) and corresponding littermates). Experimental mice were housed in groups of three to five per cage and had ad libitum access to food and water. Mice were maintained on a 12 : 12 light/dark schedule; all testing was conducted during the light period. For the MOR-deficient mice, all cohorts contained littermates from several breeding cages. Mice of different genotypes, pretreatments, and treatments were all housed in the same cages and were handled identically. Mouse protocols were approved by the Institutional Animal Care and Use Committee of Columbia University, the Research Foundation for Mental Hygiene, and Rutgers University and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Mice. Care was taken to minimize the number of mice used and their suffering.
Drugs
Drugs were administered as indicated in the figures and legends. Tianeptine sodium salt was generously provided by Servier or purchased from Nyles7.com, in which case it was independently verified for purity and identity by NMR spectroscopy. For assessment of acute behavioral effects, tianeptine was administered by intraperitoneal (i.p.) injection at doses of either 10 or 30 mg/kg either 15 min or 1 h prior to behavioral testing. Morphine (morphine sulfate injection, USP from West-ward, Eatontown, NJ) was administered at 5 mg/kg. Cyprodime (10 mg/kg; Tocris Bioscience), naloxone (5 mg/kg; Sigma Aldrich), or naltrexone (10 mg/kg; Sigma Aldrich) were administered by subcutaneous (s.c.) injection 15 min prior to tianeptine administration. The MC5 metabolite (see synthesis procedure below) was administered at 30 mg/kg, 1 h prior to behavioral testing. For chronic experiments, corticosterone 4-Pregnene-11β,21-diol-3,20-dione (Sigma, St Louis, MO) was dissolved in 0.45% hydroxypropyl-β-cyclodextrin (β-CD, Sigma) at 35 μg/ml and was administered ad libitum in the drinking water for the duration of the experiment as previously described (David et al, 2009b). After 28 days, tianeptine (30 mg/kg) was administered b.i.d. via i.p. injection. Injections were given for 21 days and then behavioral testing began. Behavioral testing was performed 16–18 h after injections in order to avoid acute effects of tianeptine. All injections were given at a volume of 10 ml/kg body weight and 0.9% sterile saline was used as a vehicle.
Behavioral Testing
Behavioral tests were administered in the following order: open field, hypophagia or novelty suppressed feeding, forced swim test, hot plate, and then withdrawal for chronic experiments. Mice were given at least 2 days to recover between exposure to different behavioral tests.
Open Field/Hyperactivity
Open-field (16″ × 16″) activity was tested under high illumination (800 lux) and was collected and analyzed with MotorMonitor software (Kinder Scientific). Total distance traveled was documented. Mice were given a 1 h pre-injection habituation to the open field and then assessed for 3 h post-injection.
Hypophagia
Mice were deprived of food for 18 h and then placed into holding cages. After 30–60 min, individual mice were placed back into their home cage, which contained a single food pellet of known weight. After 5 min, the mouse and pellet were weighed. Home cage consumption was defined as the weight of pellet consumed divided by the weight of the mouse.
Novelty Suppressed Feeding
Mice were deprived of food for 18 h and then placed into holding cages. After 60 min, mice were placed into a novel, brightly lit (1200 lux) arena (16″ × 20″) with a pellet of chow placed in the center of the arena affixed to a circular platform of white filter paper (10 cm). The time taken to bite the chow was recorded as the latency to eat, at which point the pellet was immediately removed from the arena. Mice that did not eat were assigned a latency of 360 s. Immediately following the 6 min test, mice were placed into their home cage and the latency to eat from chow in the home cage was used as a control of overall hunger drive.
Forced Swim Test
The forced swim test (FST) was performed as previously described (David et al, 2009a). Briefly, mice were placed into clear plastic buckets 20 cm in diameter and 23 cm deep, filled two-thirds of the way with 26 °C water and were videotaped. Mice were in the forced swim buckets for 6 min, but only the last 4 min were scored. Scoring was automated using Videotrack software (ViewPoint, France). Two days of FST were performed. Variability is seen between experiments based on the background strain of the mice used (purchased from Jackson or bred in house), as previously seen (Voikar et al, 2001). Swimming and climbing behavior was analyzed using time-sampling methodology as previously described (Cryan and Lucki, 2000).
Hot Plate
Mice were placed into a plastic beaker on the center of a hot plate. The temperature at the inside edge of the beaker was 50 °C. An experimenter timed the latency of the mice to jump. The maximum amount of time was set as 30 s. For assessment of analgesia in comparison to morphine and for tolerance assays, mice were tested on a hot-plate apparatus set to 55 °C (Bioseb BIO-CHP, Vitrolles, France), using a 30 s cutoff to prevent tissue damage. Following a baseline test, animals were injected s.c. with escalating doses of drug and tested at 15, 30 and 60 min post-injection. Dose–response curves were fit with a nonlinear regression (GraphPad Prism, La Jolla, CA) for responses at the time of maximal effect (30 min for morphine and 15 min for tianeptine). Curves were then compared using an extra-sum-of-squares F test.
Withdrawal
Withdrawal-like behavior was assessed 4 h after the hot-plate test (during which mice had been administered either drug or saline). Mice were then administered naloxone sc (1 mg/kg) or saline and immediately placed into a beaker. Mice were observed for 15 min, and the number of jumps was counted by an observer blind to treatment condition.
Chemistry
Preparation of MC3 and MC5 metabolites
For the synthesis of MC5 and MC3 compounds, reagents and solvents were obtained from commercial sources and were used without further purification unless otherwise stated. All reactions were performed in flame-dried glassware under an argon atmosphere unless otherwise stated and monitored by TLC using solvent mixtures appropriate to each reaction. All column chromatography was performed on silica gel (40–63 μm). Nuclear magnetic resonance spectra were recorded on a Bruker 400 MHz instrument. Please see Supplementary Material for detailed methods on synthesis procedures.
Functional Assays
Materials: BRET
HEK-293T cells were obtained from the American Type Culture Collection (Rockville, MD) and were cultured in a 5% CO2 atmosphere at 37 °C in Dulbecco’s modified Eagle medium (high glucose #11965; Life Technologies; Grand Island, NY) supplemented with 10% FBS (Premium Select, Atlanta Biologicals; Atlanta, GA) and 100 U/ml penicillin and 100 μg/ml streptomycin (#15140, Life Technologies).
DNA constructs
The mouse MOR (mMOR) and mouse DOR (mDOR) were provided by Dr Lakshmi Devi at Mount Sinai Hospital. The human MOR (hMOR) and human DOR (hDOR) were obtained from the Missouri S&T Resource Center. The human G-protein constructs used here were previously described and were provided by C. Galés or were obtained from the Missouri S&T Resource Center unless otherwise noted (Negri et al, 2013; Rives et al, 2012). The G proteins used included GαoB with Renilla luciferase 8 (RLuc8) inserted at position 91 (GαoB-RLuc8), Gβ1 (β1), and Gγ2 fused to the full-length mVenus at its N-terminus via the amino acid linker GSAGT (mVenus-γ2). All constructs were sequence-confirmed prior to use in experiments.
Transfection
The following cDNA amounts were transfected into HEK-293T cells (5 × 106 cells/plate) in 10-cm dishes using polyethylenimine (PEI) in a 1 : 1 ratio (diluted in Opti-MEM, Life Technologies): 2.5 μg MOR/DOR, 0.125 μg GαoB-RLuc8, 6.25 μg β1, 6.25 μg mVenus-γ2. Cells were maintained in the HEK-293T media described above. After 24 h the media was changed, and the experiment was performed 24 h later (48 h after transfection).
BRET
Transfected cells were dissociated and re-suspended in phosphate-buffered saline (PBS). Approximately 200 000 cells per well were added to a black-framed, white well 96-well plate (#60050; Perkin Elmer; Waltham, MA). The microplate was centrifuged and the cells were re-suspended in PBS. Next, 5 μM of the luciferase substrate coelenterazine H was added to each well. After 5 min, ligands were added and the BRET signal was measured 5 min later on a PHERAstar FS plate reader. The BRET signal was quantified by calculating the ratio of the light emitted by the energy acceptor, mVenus (510–540 nm), over the light emitted by the energy donor, RLuc8 (485 nm). This drug-induced BRET signal was normalized using the Emax of [D-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO) or [D-Pen(2,5)]enkephalin (DPDPE) as the maximal response at MOR and DOR, respectively. Dose–response curves were fit using a three-parameter logistic equation in GraphPad Prism.
Pharmacokinetics
The pharmacokinetic study of tianeptine was conducted by Sai Life Sciences Limited (Hinjewadi, India). A group of 24 male C57BL/6 mice was administered tianeptine as a solution formulation in normal saline at a dose of 30 mg/kg given i.p. Blood samples (~60 μl) were collected under light isoflurane anesthesia from the retro orbital plexus at 0.08, 0.25, 0.5, 1, 2, 4, 8, and 24 h. Plasma samples were separated by centrifugation of whole blood and stored below −70 °C until analysis. Immediately after collection of blood, brain samples were collected from each mouse at 0.08, 0.25, 0.5, 1, 2, 4, 8, and 24 h (3 mice per time point). Brain samples were homogenized using ice-cold phosphate-buffered saline (pH 7.4), and homogenates were stored below −70 °C until analysis. Total brain homogenate volume was three times the tissue weight. All samples were processed for analysis by protein precipitation using acetonitrile and analyzed to determine the concentrations of both tianeptine and MC5 by a fit-for-purpose LC/MS/MS method (lower limit of quantification=2.02 ng/ml in plasma and 1.01 ng/ml in brain). Pharmacokinetic parameters were calculated using the non-compartmental analysis tool of Phoenix WinNonlin® (Version 6.3).
Results
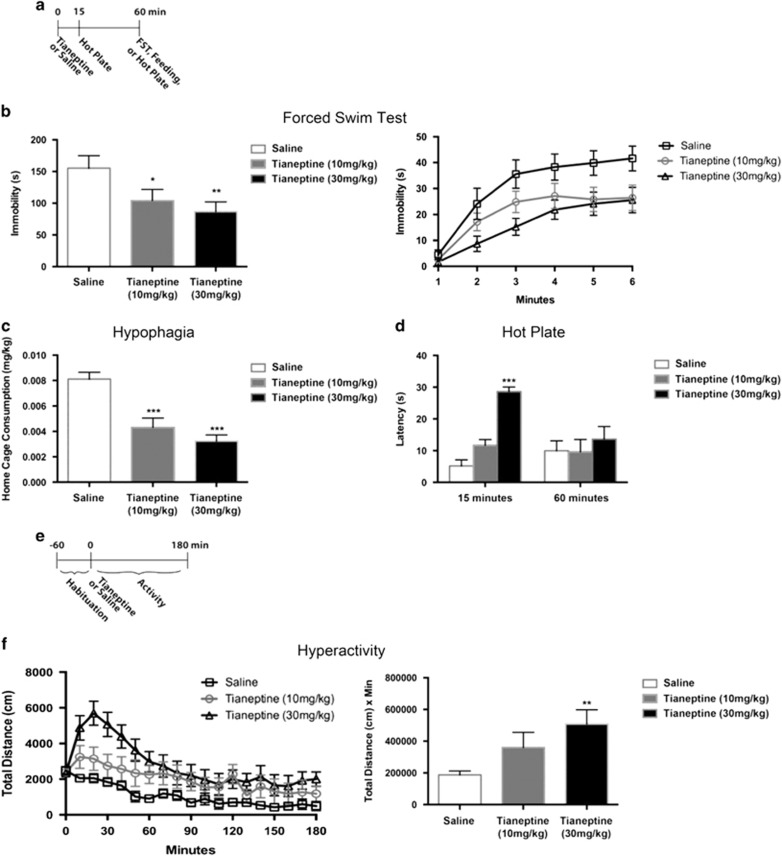
We first assessed the effects of tianeptine in a series of behavioral tests. We administered either saline, 10, or 30 mg/kg tianeptine via i.p. injection and then tested behavior 1 h later (Figure 1a). We assessed the effects of tianeptine in the FST, which is a predictive test for antidepressants (Porsolt et al, 1977). In the FST, tianeptine dose-dependently decreased immobility, consistent with an antidepressant effect (Figure 1b). We also assessed whether, in addition to this antidepressant-like effect, tianeptine displayed behavioral properties similar to morphine and other MOR agonists such as an acute hypophagia, analgesia, and hyperactivity (Belknap et al, 1989; Belknap et al, 1998; Brase et al, 1977; Levine et al, 1985; Oliverio and Castellano, 1974a,1974b; Wenger, 1989). We found that i.p. injection of tianeptine decreased food consumption in the home cage after an 18 h deprivation (Figure 1c), which is consistent with tianeptine having a morphine-like effect on food intake. To assess the analgesic effects of tianeptine, we also placed mice on a hot plate and measured their latency to jump (Figure 1d). Tianeptine dose-dependently increased the latency to jump off the hot plate 15 min after administration, consistent with an acute analgesic effect (Figure 1d). Interestingly, at 1 h after administration, there were no significant effects of tianeptine in the hot-plate assay. To determine whether tianeptine also increased locomotion, we placed mice into an open field for 4 h, including a 1 h habituation prior to drug administration (Figure 1e). We found that tianeptine dose-dependently increased total distance traveled by mice in the open field (Figure 1f). This hyperactivity effect was especially prominent in the first 30 min after administration of the higher dose of tianeptine (30 mg/kg), and then steadily decreased over the next few hours. AUC quantification for the entire 3 h of the open-field test indicated a significant effect of tianeptine at the higher dose (30 mg/kg) (Figure 1f). Taken together, these data suggest that tianeptine displays antidepressant and opioid-like properties.
Figure 1.
Dose response of tianeptine behavioral effects. (a) Timeline for (b–d). n=12–14 per group for (b and c) and 5 per group for (d). Tianeptine was administered by i.p. injection at doses of either 10 mg/kg or 30 mg/kg as indicated. (b) FST Day 1 results. Bar graph (left) shows combined immobility results of last four minutes and line graph (right) shows immobility per minute over the 6 min test. Immobility duration over the last 4 min was analyzed. One-way ANOVA: F(2,37)=3.966, p=0.0275. *p=0.0488, relative to saline; **p=0.0095, relative to saline (Fisher’s). (c) Home cage consumption over 5 min after an 18 h deprivation period was assessed as a measure of hypophagia. One-way ANOVA: F(2,37)=17.17, p<0.0001. ***p<0.0001, relative to saline. (d) Latency to jump after being placed on the hot plate was assessed. Two-way ANOVA: F(2,24)=6.063, time × treatment p=0.0074. ***p<0.0001 relative to 15 min/saline. (e) Timeline for (f). n=9–10 per group. Tianeptine was administered by i.p. injection at doses of either 10 mg/kg or 30 mg/kg as indicated. (f) Hyperactivity in an open field. Line graph (left) shows total distance traveled over 180 min (10 minute bins). Bar graph (right) shows AUC analysis of line graph. AUC analysis: one-way ANOVA: F(2,26)=4.601, p=0.0195. **p=0.0055, relative to saline (Fisher’s). Throughout the figure, all bar graphs indicate mean±SEM. For line graphs, each point indicates mean±SEM.
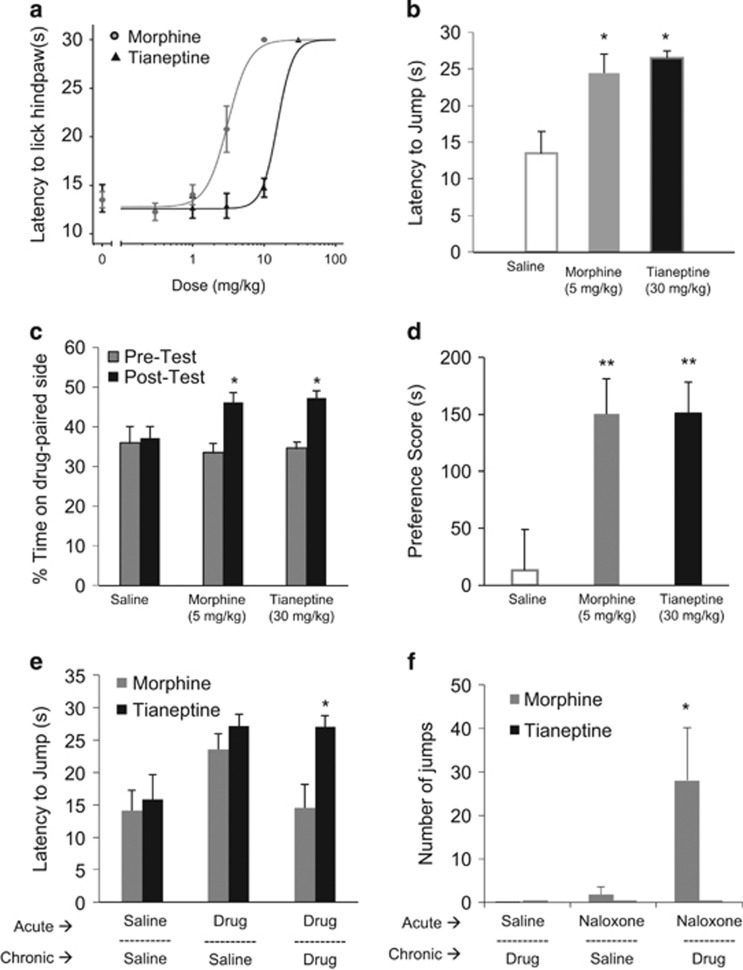
Given that tianeptine is a full MOR agonist, we were next interested in comparing its behavioral effects to other MOR agonists, particularly morphine, which has well-established analgesic and rewarding effects in mice. First, using a hot-plate analgesia test, the time point of maximal analgesic response was determined for tianeptine (15 min) and morphine (30 min), and then used for dose–response comparisons (Figure 2a). Tianeptine (ED50=15) showed a less potent analgesic response compared to morphine (ED50=3.1). Specifically, morphine showed a maximal analgesic response at 10 mg/kg a dose at which tianeptine produced minimal analgesic effects. In subsequent experiments comparing morphine and tianeptine, a dose of roughly two times the calculated ED50 was used, namely 5 mg/kg for morphine and 30 mg/kg for tianeptine. These doses had comparable analgesic effects on latency to jump in the hot-plate test 15 min after administration (Figure 2b). To test the rewarding effects of tianeptine, a conditioned place preference paradigm was used to compare the effects of tianeptine to morphine. Interestingly, tianeptine (at 30 mg/kg) produced a similar conditioned place preference to morphine (at 5 mg/kg) as mice significantly increased the amount of time spent in the context paired with administration of morphine or tianeptine (Figure 2c). Importantly, there was no difference in the preference for the drug-paired context following treatment with morphine or tianeptine (Figure 2d), suggesting that tianeptine elicits similar conditioned place preference to morphine, albeit at a higher dose.
Figure 2.
Tianeptine comparison to morphine. (a) Analgesic responses to morphine and tianeptine are shown over increasing doses as by latency to lick the hindpaw as measured in the hot-plate assay. The dose–response curve for morphine was measured 30 min post injection, and for tianeptine at 15 min post injection. The ED50s of these curves differ significantly: F(1,58)=53, p<0.0001. (b) The analgesic response to morphine and tianeptine is shown at 15 min post injection at roughly equianalgesic doses as measured by latency to jump in the hot-plate assay in a naïve group of animals. One-way ANOVA (drug): F(2, 31)=5.63, p=0.008; *p<0.05, compared to saline. (c) The percent of time spent on the drug-paired side before and after 8 days of context pairings with morphine at 5 mg/kg, tianeptine at 30 mg/kg, or saline is shown. One-way ANOVA for pre-test: F(2,27)=0.19, p=0.82; one-way ANOVA for post-test: F(2, 27)=5.01, p=0.014. *p<0.05 compared to post-test saline. (d) The preference score (total time on drug-paired side minus total time on saline-paired side) is shown for the 20 min post-pairing test. One-way ANOVA: F(2, 27)=6.47, p=0.0051. **p<0.01, compared to saline. (e) Tolerance was assessed by measuring the effect of acute drug treatment (saline, morphine at 5 mg/kg, or tianeptine at 30 mg/kg) using the hot-plate test following chronic exposure to saline, tianeptine (30 mg/kg twice daily for 34 days), or morphine (5 mg/kg twice daily, for 10 days). (f) Withdrawal was assessed through jumping behavior following acute administration of naloxone (1 mg/kg) following chronic exposure to saline, tianeptine (30 mg/kg twice daily for 34 days), or morphine (5 mg/kg twice daily, for 10 days). Two-way ANOVA: F(2, 47)=10.87, p<0.001 for drug × naloxone treatment. *p<0.05 compared to morphine-saline and tianeptine-naloxone. Throughout the figure, all bar graphs indicate mean±SEM. For line graphs, each point indicates mean±SEM.
Given that chronic administration of morphine leads to tolerance and withdrawal effects, we compared the effects of tianeptine to morphine in these parameters. As expected, acute administration of morphine following chronic administration (5 mg/kg twice daily for 10 days) produced no significant analgesic response in latency to jump in the hot-plate test (Figure 2e). Remarkably, mice did not display a similar tolerance to tianeptine. Following chronic administration of tianeptine (30 mg/kg twice daily for over 30 days), acute administration of tianeptine produced a robust analgesic response, which was not significantly different from mice treated chronically with saline (Figure 2e). Furthermore, assessment of withdrawal as measured by jumping behavior also revealed a difference between morphine and tianeptine. Mice treated chronically with morphine displayed the expected jumping behavior indicative of withdrawal following administration of naloxone (Figure 2f). Interestingly, mice chronically treated with tianeptine did not display jumping behavior following administration of naloxone. Overall, tianeptine produces similar rewarding effects as morphine when administered at equianalgesic doses, however unlike morphine, tianeptine does not appear to induce tolerance nor withdrawal.
To determine whether the behavioral effects of tianeptine were dependent on MOR, we next assessed acute administration of the higher dose (30 mg/kg) used in Figure 1 in MOR-deficient mice (Supplementary Figure 1). In the FST, tianeptine dramatically decreased immobility in wild-type (WT) mice but was ineffective in MOR-deficient littermates (Supplementary Figure 1B), indicating that the effects of tianeptine in the FST are mediated by MOR. We also measured swimming and climbing behavior during the FST. Tianeptine increased swimming, but not climbing behavior (Supplementary Figure 1B), which is more similar to the effects seen with serotonergic- rather than noradrenergic-acting antidepressants (Cryan et al, 2005). Tianeptine failed to increase swimming in the MOR-deficient mice (Supplementary Figure 1B). In the hypophagia test, tianeptine significantly decreased food intake in WT mice, but not in MOR-deficient mice (Supplementary Figure 1C). Similarly, in the hot-plate test, tianeptine administration 15 min prior to the test increased the latency to jump in WT mice but not in MOR-deficient littermates (Supplementary Figure 1D). Hyperactivity, as measured by total distance traveled in the open field for 30 min, was increased by tianeptine in WT but not MOR-deficient mice (Supplementary Figure 1E). Finally, the conditioned place preference to tianeptine was absent in MOR-deficient mice (Supplementary Figure 1F). Taken together, these results show that all measured acute antidepressant- and opioid-like behavioral effects of tianeptine are mediated by MOR.
To further confirm the underlying mechanism of tianeptine’s behavioral effects, we examined whether pretreatment with small-molecule opioid antagonists yielded resistance to tianeptine. We performed pretreatments with the non-selective opioid antagonists naloxone and naltrexone or the MOR-selective antagonist cyprodime (Marki et al, 1999). To this end, we injected the antagonist or saline 15 min before tianeptine administration (Supplementary Figure 2A). Overall, both non-specific opioid and specific MOR antagonists blocked the behavioral effects of tianeptine seen in the forced swim, feeding, analgesia, and open-field tests (Supplementary Figure 2). These data, in combination with the genetic loss-of-function experiments, confirm that the antidepressant- and opioid-like behavioral effects of tianeptine are mediated by MOR.
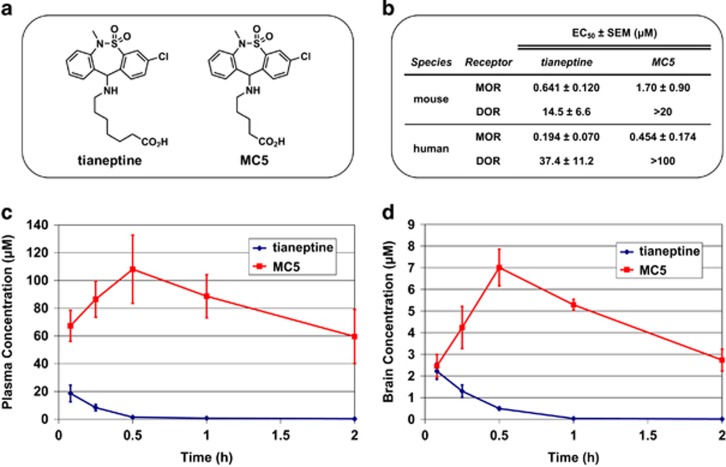
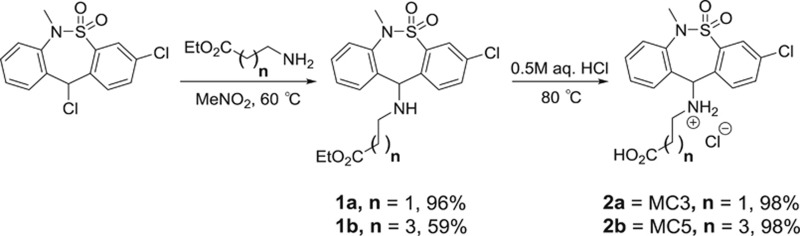
Tianeptine is significantly metabolized via β-oxidation of the heptanoic acid side chain to produce the MC5 metabolite (bearing a pentanoic acid side chain, Figure 3a) in both rodents and humans (Couet et al, 1990; Grislain et al, 1990). Accordingly, in order to more fully understand the mechanism of action for tianeptine, we evaluated the ability of MC5 to activate the opioid receptors. Using bioluminescence resonance energy transfer (BRET) functional assays (Gassaway et al, 2014; Negri et al, 2013; Rives et al, 2012) in HEK293 cells expressing MOR and DOR, we found that MC5 retains similar potency and efficacy as tianeptine at both human and mouse MOR (Figure 3b, Supplementary Figures 3 and 4). By contrast, the minimal DOR agonist activity of tianeptine was further reduced in the MC5 metabolite (Figure 3b, Supplementary Figures 3 and 4). We also evaluated another known tianeptine metabolite, MC3, which bears a propanoic acid side chain (Grislain et al, 1990). However, MC3 exhibited only very weak activity at MOR (EC50=16 μM at human MOR) and no agonist activity at DOR (Supplementary Figure 4). Therefore, it is likely that the tianeptine metabolite MC5, but not MC3, plays an active role in the behavioral effects of tianeptine at MOR.
Figure 3.
Pharmacokinetics of tianeptine and the MC5 metabolite. (a) Chemical structures of tianeptine and its MC5 metabolite. (b) In vitro activities of tianeptine and MC5 in G protein BRET assays measuring activation of mouse and human MOR and DOR, data points represent mean EC50±SEM (μM); Plasma (c) and brain (d) concentrations±STD of tianeptine and MC5 in C57BL/6 mice following a single administration of tianeptine (30 mg/kg).
In order to better frame and interpret our in vivo behavioral experiments, we also conducted a pharmacokinetic study to determine the plasma (Figure 3c) and brain (Figure 3d) concentrations of these compounds in mice (C57BL/6). Following i.p. administration, we found that tianeptine reaches peak concentrations within 5 min and is very rapidly metabolized. Tianeptine is nearly eliminated from both plasma and brain tissue after 1 h and is undetectable in the brain beyond the 2 h time point. By contrast, the MC5 metabolite resulting from this tianeptine dose reaches higher peak concentrations (albeit more slowly) and has a much longer elimination half-life compared to tianeptine, being detectable in brain tissue for at least 8 h. As a result, the overall exposure to MC5 is much higher in both the plasma and brain, as quantified by area under the curve (AUC), than that of tianeptine. Therefore, MC5 is expected to play a major role in mediating the behavioral effects of tianeptine in C57BL6 mice.
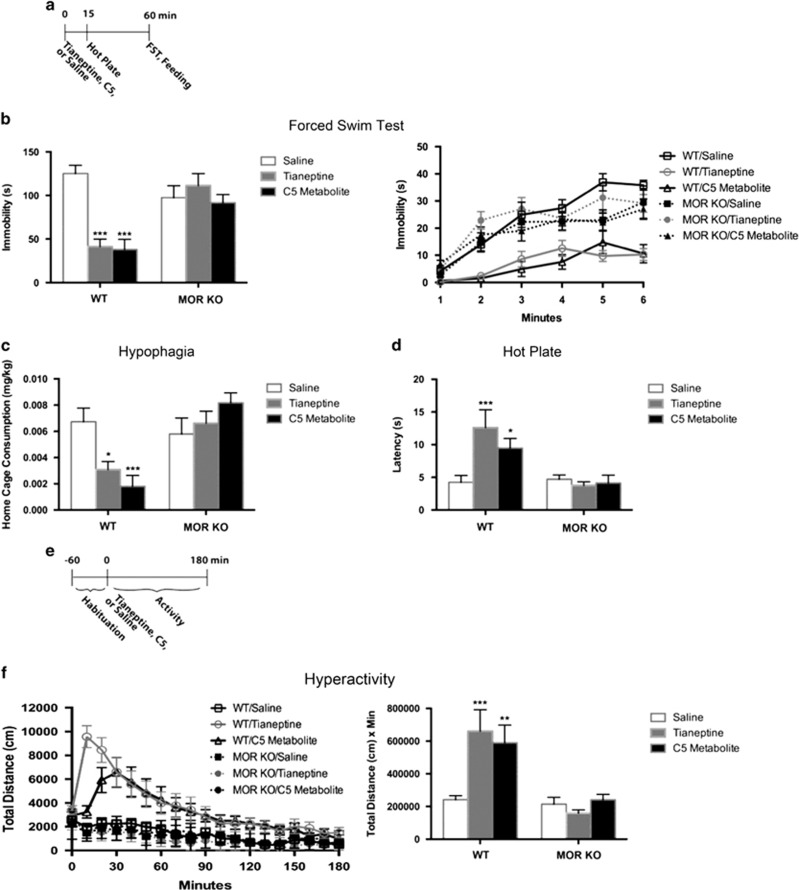
Considering the results of these experiments, we next sought to assess the behavioral effects following direct administration of the MC5 metabolite in WT and MOR-deficient mice (Figure 4a). In the FST, both tianeptine and its MC5 metabolite decreased immobility in WT mice but were ineffective in MOR-deficient littermates (Figure 4b). In the hypophagia test, both tianeptine and MC5 significantly decreased food intake in WT mice, but not in MOR-deficient mice (Figure 4c). Similarly, in the hot-plate test, both tianeptine and MC5 exhibited analgesic effects in WT mice but not in MOR-deficient mice (Figure 4d). Last, both tianeptine- and MC5-induced hyperactivity in the open field was abolished in MOR-deficient mice (Figure 4e and f). Taken together, these results demonstrate that the antidepressant- and opioid-like behavioral effects of both tianeptine and its MC5 metabolite are mediated by MOR. In addition, these results indicate that direct administration of the MC5 metabolite is sufficient to replicate the behavioral effects of tianeptine.
Figure 4.
Behavioral effects of tianeptine and its MC5 metabolite in MOR-deficient mice. (a) Timeline for (b–d). n=7–9 per group. Tianeptine (30 mg/kg) or MC5 (30 mg/kg) were administered by i.p. injection. (b) FST Day 1 results. Bar graph (left) shows combined immobility results of last 4 min, and line graph (right) shows immobility per minute over the 6 min test. Immobility duration over the last 4 min was analyzed. Two-way ANOVA: F(2,46)=9.756, genotype × treatment p=0.0003. ***p<0.0001, relative to WT/saline. (c) Home cage consumption over 5 min after an 18-h deprivation period was assessed as a measure of hypophagia. Two-way ANOVA: F(2,46)=7.726, genotype × treatment p=0.0013. *p=0.0101, relative to WT/saline; ***p=0.0007, relative to WT/saline (Fisher’s). (d) Latency to jump after being placed on the hot plate was assessed. Two-way ANOVA: F(2,46)=4.652, genotype × treatment p=0.0145. ***p=0.0005, relative to WT/saline; *p=0.0240 relative to WT/saline (Fisher’s). (e) Timeline for (f). n=7–9 per group. Tianeptine (30 mg/kg) or MC5 (30 mg/kg) were administered by i.p. injection. (f) Hyperactivity in an open field. Line graph (left) shows total distance traveled over 180 min (10 min bins). Bar graph (right) shows AUC analysis of line graph. AUC analysis: Two-way ANOVA: F(2,46)=4.701, p=0.0139. ***p=0.0006, relative to WT/saline; **p=0.0038, relative to WT/saline (Fisher’s). Throughout the figure, all bar graphs indicate mean±SEM. For line graphs, each point indicates mean±SEM.
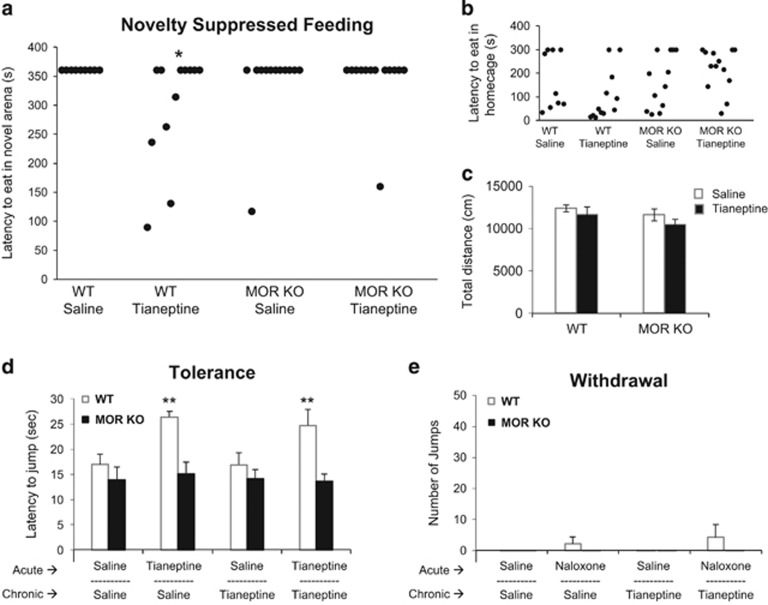
Assessment of chronic antidepressant effects in mice includes manipulations, such as administration of chronic glucocorticoids, that yield increased depressive-like behaviors associated with negative valence constructs that can then be reversed with treatment. Therefore, we sought to assess the behavioral effects of chronic tianeptine administration in WT and MOR-deficient mice exposed to chronic glucocorticoids. Following 28 days of corticosterone administration via the drinking water, mice were treated with tianeptine (30 mg/kg twice daily) or saline i.p. injections. The chronic antidepressant/anxiolytic effects of tianeptine were then assessed in Novelty Suppressed Feeding (NSF). Chronic, but not acute, antidepressant treatment decreases the latency to feed in this novel arena (Santarelli et al, 2003; Samuels and Hen, 2012). While chronic tianeptine reduced latency to feed in WT mice compared to saline-treated controls, tianeptine did not have an effect in MOR-deficient mice (Figure 5a). Chronic tianeptine treatment did not affect feeding behavior in the home cage tested at this time point, 18 h following tianeptine injection, suggesting that the decreased latency observed in the NSF was not due to a direct effect on feeding (Figure 5b). In addition, there was no effect of chronic tianeptine treatment in either genotype on total distance traveled in the open field at this time point, suggesting that the effects of tianeptine on NSF behavior are not due to any residual hyperactivity effects of tianeptine (Figure 5c). Lastly, the effects of tianeptine on tolerance and withdrawal were assessed. Similar to the results in Figure 2, an analgesic effect was seen in WT mice treated with acute tianeptine (even when preceded by chronic treatment with tianeptine; Figure 5d), while all analgesic effects were absent in MOR-deficient mice. Similarly, there was minimal jumping behavior observed in mice treated with chronic tianeptine following naloxone administration, and there was no jumping behavior observed in MOR-deficient mice (Figure 5e). These data demonstrate that chronic tianeptine treatment results in antidepressant-like effects in a MOR-dependent fashion.
Figure 5.
Chronic effects of tianeptine in WT and MOR-deficient mice. (a) Latency to feed in the novel arena is shown from the novelty suppressed feeding assay 18 h post tianeptine injection, following chronic treatment with tianeptine (30 mg/kg twice daily for 21 days) in chronic corticosterone treated mice. Each dot represents an individual mouse. Logrank (Mantel–Cox Survival): X3=7.57, p=0.056, saline vs tianeptine—*p=0.035 for WT; p=0.12 for MOR KO. (b) A control measure of latency to feed in the home cage is also shown measured immediately following the novelty suppressed feeding assay. Each dot represents an individual mouse. Logrank (Mantel–Cox): X3=4.41, p=0.22, planned comparisons: saline vs tianeptine—p=0.20, for WT; p=0.75 for MOR KO. (c) Total distance traveled in the open-field arena measured 16–18 h post tianeptine injection shows no significant effect of chronic tianeptine on locomotion. Two-way ANOVA: F(1,44)=0.11, p=0.74 treatment × genotype. (d) Tolerance was assessed by measuring the effect of acute tianeptine using the hot-plate test following chronic exposure. Three-way ANOVA (genotype × acute × chronic): main effect of genotype F(1,38)=18.16, **p<0.001. (e) Withdrawal was assessed through jumping behavior following acute administration of naloxone (1 mg/kg) following chronic exposure to saline or tianeptine. Three-way ANOVA (genotype × chronic × naloxone): F(1,38)=0.14, p=0.71. For (c–e), all bar graphs indicate mean±SEM.
Discussion
We previously demonstrated that the antidepressant tianeptine is a full agonist of MOR (Gassaway et al, 2014). In the data presented here, we show that all of the behavioral effects of tianeptine examined, including acute antidepressant, hypophagic, analgesic, hyperactive, and conditioned place preference effects, as well as chronic antidepressant effects, are dependent upon MOR. These results have far-reaching implications. First, they extend our previous finding that MOR is a molecular target of tianeptine in the brain and demonstrate that tianeptine acts like a MOR agonist in vivo. Second, these data demonstrate that the effects of tianeptine are distinct from those of morphine since tianeptine does not produce tolerance or withdrawal. These results suggest that specific signaling pathway(s) downstream of MOR could be potential targets for novel antidepressant development. Third, our data with the MC5 metabolite indicate that this compound elicits behavioral effects comparable to tianeptine while having an improved pharmacokinetic profile.
MOR as a Novel Target for Antidepressants
Tianeptine is currently prescribed as an antidepressant in several countries in Europe, Asia, and Latin America. However, the cellular target(s) of tianeptine remained obscure for some 30 years. We recently reported that tianeptine is a MOR agonist in vitro (Gassaway et al, 2014) and our data here demonstrate that the acute and chronic antidepressant-like behavioral effects of tianeptine in mice are mediated by MOR. Importantly, we show that tianeptine exhibits some notable differences compared to morphine, perhaps explaining the lack of reported large-scale tianeptine abuse. This work also highlights the need to better understand MOR signaling cascades that are activated following tianeptine and morphine administration. In addition, given that MOR is a unique antidepressant target, it may be interesting to determine whether tianeptine or other MOR modulators are effective in subsets of depressed patients, such as those that suffer from resistance to current treatments such as SSRIs. Two studies of limited scale have already demonstrated efficacy in these patients resistant to SSRI monotherapy, and thus further studies in this patient population are now warranted (Woo et al, 2013). Furthermore, tianeptine or other MOR modulators may be more effective than current treatments for other specialized groups of depressed patients. For example, there are reports that tianeptine is effective in the treatment of depression observed in Parkinson’s disease (Levin, 2007), post-traumatic stress disorder (Aleksandrovskii et al 2005; Onder et al, 2006), and the elderly (Karpukhin, 2009). Interestingly, tianeptine treatment also avoids some of the negative side effects of SSRIs. For example, switching patients experiencing SSRI-induced sexual dysfunction to tianeptine was successful in alleviating this key side effect while maintaining high response rates (Atmaca et al, 2003). There is also evidence of genetic polymorphisms in MOR that could potentially be used to stratify patients most likely to respond to tianeptine (Lee and Smith, 2002; Lotsch and Geisslinger, 2005). Finally, an understanding of the brain circuits through which tianeptine mediates its antidepressant effects may also lead to stratifications based on imaging or neuropsychological studies (Hsu et al, 2015; Hsu et al, 2013a,2013b).
One possible concern for drug development may be the opioid-like effects of tianeptine. While commonly prescribed for the management of pain, opioids such as oxycodone have a very high abuse potential. Approximately 12.5 million Americans abused prescription opioids in 2012 (Brady et al, 2016). However, there have only been a few isolated case reports of addiction or withdrawal symptoms associated with tianeptine in the literature (Guillem and Lepine, 2003; Leterme et al, 2003; Saatcioglu et al, 2006; Vadachkoria et al, 2009; Vandel et al, 1999). While tianeptine did support a conditioned place preference, it did not lead to tolerance or withdrawal symptoms. These differences may contribute to the decreased abuse liability relative to other MOR agonists. In line with these results, a clinical trial found that even supertherapeutic doses of tianeptine showed little abuse potential (Bernard et al, 2011). This poses very interesting questions as to whether tianeptine engages different signaling machinery than classic opioids such as morphine. For example, it may be worthwhile to compare the effects of tianeptine relative to other opioids on GPCR activation and arrestin recruitment. Such approaches may guide the design of novel MOR modulators for the treatment of depression that have improved abuse and side-effect profiles compared to traditional agents in this class.
MC5 is an Active Metabolite of Tianeptine
We found that in mice, tianeptine is rapidly metabolized and is nearly absent from both the plasma and brain by 1 h after administration. However, the MC5 metabolite of tianeptine reaches much higher peak concentrations and has a much longer elimination half-life compared to tianeptine in mice (Figure 3). In addition, the behavioral effects of MC5 were comparable to tianeptine (Figure 4). We performed several of the behavioral tests (including FST and hypophagia) 1 h after drug administration. Since tianeptine is nearly absent from the brain by this time point, while MC5 is detectable in the brain for at least 8 h, in all likelihood MC5 is the active compound at the time of behavioral testing. This is also suggested by the observation that the time course for hyperactivity following tianeptine or MC5 administration is identical beyond 30 min, indicating that the effects of tianeptine administration are mostly MC5-mediated at longer time points. Therefore, active metabolites such as MC5 may be reasonable compounds for drug development that could potentially increase medication adherence by decreasing the three times a day dosing that is currently required for tianeptine treatment. However, metabolic conversion to MC5 is less efficient in man and thus, the role of this metabolite in mediating tianeptine’s antidepressant effects remains uncertain in humans.
Given the limited efficacy of widely prescribed antidepressants such as SSRIs, there is a clear need for novel treatments that do not rely on monoaminergic effects. The data presented here suggest that MOR modulators are a novel strategy for antidepressant drug development and treatment.
Funding and disclosure
This work was supported by the Hope for Depression Research Foundation (RH & JAJ), NIH Grant # MH068542 (RH), NIH Grant # DA022413 (JAJ), NIH Grant # MH106731 (KMN), NIH Grant # MH112861 (BAS), the New Jersey Health Foundation (JEP), the New Jersey Governor’s Council on Autism (JEP), the Interdisciplinary Research Initiatives Seed (IRIS) Fund Program of Columbia University College of Physicians & Surgeons (DS), and NARSAD Young Investigator Awards (BAS & KMN). RH is a consultant for Servier and Lundbeck. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aleksandrovskii Iu A, Avedisova AS, Boev IV, Bukhanovkskii AO, Voloshin VM, Tsygankov BD et al (2005). Efficacy and tolerability of coaxil (tianeptine) in the therapy of posttraumatic stress disorder. Zh Nevrol Psikhiatr Im S S Korsakova 105: 24–29. [PubMed] [Google Scholar]
- Almatroudi A, Husbands SM, Bailey CP, Bailey SJ (2015). Combined administration of buprenorphine and naltrexone produces antidepressant-like effects in mice. J Psychopharmacol 29: 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Buyukbayram A (2003). Switching to tianeptine in patients with antidepressant-induced sexual dysfunction. Hum Psychopharmacol 18: 277–280. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Noordewier B, Lame M (1989). Genetic dissociation of multiple morphine effects among C57BL/6J, DBA/2J and C3H/HeJ inbred mouse strains. Physiol Behav 46: 69–74. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Riggan J, Cross S, Young ER, Gallaher EJ, Crabbe JC (1998). Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol Biochem Behav 59: 353–360. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Bernard K, Penelaud PF, Mocaer E, Donazzolo Y (2011). Absence of psychostimulant effects of a supratherapeutic dose of tianeptine: a placebo-controlled study versus methylphenidate in young healthy volunteers. J Clin Psychopharmacol 31: 441–448. [DOI] [PubMed] [Google Scholar]
- Brady KT, McCauley JL, Back SE (2016). Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am J Psychiatry 173: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL (1977). Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther 201: 368–374. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH (2002). Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology 26: 744–755. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Beguin C, Knoll AT, Cohen BM (2009). Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I (2010). Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology 35: 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C (2011). The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology 36: 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet W, Girault J, Latrille F, Salvadori C, Fourtillan JB (1990). Kinetic profiles of tianeptine and its MC5 metabolite in plasma, blood and brain after single and chronic intraperitoneal administration in the rat. Eur J Drug Metab Pharmacokinet 15: 69–74. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I (2000). Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther 295: 1120–1126. [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29: 547–569. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I et al (2009. a). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al (2009. b). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R et al (2015). Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology 40: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M et al (2016). Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am J Psychiatry 173: 499–508. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F et al (2000). Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature genetics 25: 195–200. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC (2006). Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63: 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D (2014). The atypical antidepressant and neurorestorative agent tianeptine is a mu-opioid receptor agonist. Transl Psychiatry 4: e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grislain L, Gele P, Bertrand M, Luijten W, Bromet N, Salvadori C et al (1990). The metabolic pathways of tianeptine, a new antidepressant, in healthy volunteers. Drug Metab Dispos 18: 804–808. [PubMed] [Google Scholar]
- Guillem E, Lepine JP (2003). Does addiction to antidepressants exist? About a case of one addiction to tianeptine. Encephale 29: 456–459. [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ et al (2015). It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry 20: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H et al (2013. a). Response of the mu-opioid system to social rejection and acceptance. Mol Psychiatry 18: 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H et al (2013. b). Social feedback activates the endogenous opioid system. Mol Psychiatry 18: 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpukhin IB (2009). Use of Coaxil (tianeptine) in elderly patients with combined mild cognitive and depressive-anxiety disorders. Neurosci Behav Physiol 39: 53–56. [DOI] [PubMed] [Google Scholar]
- Kasper S, McEwen BS (2008). Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs 22: 15–26. [DOI] [PubMed] [Google Scholar]
- Lee NM, Smith AP (2002). Opioid receptor polymorphisms and opioid abuse. Pharmacogenomics 3: 219–227. [DOI] [PubMed] [Google Scholar]
- Leterme L, Singlan YS, Auclair V, Le Boisselier R, Frimas V (2003). Misuse of tianeptine: five cases of abuse. Ann Med Interne (Paris) 154 Spec No 2: S58–S63. [PubMed] [Google Scholar]
- Levin OS (2007). Coaxil (tianeptine) in the treatment of depression in Parkinson's disease. Neurosci Behav Physiol 37: 419–424. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE, Gosnell BA, Billington CJ, Bartness TJ (1985). Opioids and consummatory behavior. Brain Res Bull 14: 663–672. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Geisslinger G (2005). Are mu-opioid receptor polymorphisms important for clinical opioid therapy? Trends Mol Med 11: 82–89. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL (2013). Opioid receptors: distinct roles in mood disorders. Trends Neurosci36: 195–206.. [DOI] [PMC free article] [PubMed]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC Jr et al (2003). Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305: 323–330. [DOI] [PubMed] [Google Scholar]
- Marki A, Monory K, Otvos F, Toth G, Krassnig R, Schmidhammer H et al (1999). Mu-opioid receptor specific antagonist cyprodime: characterization by in vitro radioligand and [35S]GTPgammaS binding assays. Eur J Pharmacol 383: 209–214. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P et al (2010). The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry 15: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23: 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD (2007). NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur J Pharmacol 566: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri A, Rives ML, Caspers MJ, Prisinzano TE, Javitch JA, Filizola M (2013). Discovery of a novel selective kappa-opioid receptor agonist using crystal structure-based virtual screening. J Chem Inf Model 53: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N et al (2002). Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22: 10883–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio A, Castellano C (1974. a). Experience modifies morphine-induced behavioral excitation of mice. Nature 252: 229–230. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Castellano C (1974. b). Genotype-dependent sensitivity and tolerance to morphine and heroin: dissociation between opiate-induced running and analgesia in the mouse. Psychopharmacologia 39: 13–22. [DOI] [PubMed] [Google Scholar]
- Onder E, Tural U, Aker T (2006). A comparative study of fluoxetine, moclobemide, and tianeptine in the treatment of posttraumatic stress disorder following an earthquake. Eur Psychiatry 21: 174–179. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodynam Ther 229: 327–336. [PubMed] [Google Scholar]
- Richards EM, Mathews DC, Luckenbaugh DA, Ionescu DF, Machado-Vieira R, Niciu MJ et al (2016). A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology 233: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives ML, Rossillo M, Liu-Chen LY, Javitch JA (2012). 6'-Guanidinonaltrindole (6'-GNTI) is a G protein-biased kappa-opioid receptor agonist that inhibits arrestin recruitment. J Biol Chem 287: 27050–27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- Saatcioglu O, Erim R, Cakmak D (2006). A case of tianeptine abuse. Turk Psikiyatri Derg 17: 72–75. [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J (2004). Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci 95: 374–380. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Chapter 7: Novelty-suppressed feeding in the mouse. Mood and anxiety related phenotypes in mice: characterization using behavioral tests. Todd D. Gould (ed). Volume 2. Springer Protocols Neuromethods. 2012, p 107–121..
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ et al (2014). Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 19: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ et al (1999). Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2: 151–156. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS (2004). Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem 90: 1258–1268. [DOI] [PubMed] [Google Scholar]
- Smith K (2014). Mental health: a world of depression. Nature 515: 181. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M et al (2007). Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci 26: 3509–3517. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Mico JA, Smadja C, Maldonado R, Roques BP, Gilbert-Rahola J (1998). Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol 354: 1–7. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Jutkiewicz EM, Mosberg HI, Balboni G, Watson SJ, Woods JH (2006). Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res 1069: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadachkoria D, Gabunia L, Gambashidze K, Pkhaladze N, Kuridze N (2009). Addictive potential of Tianeptine—the threatening reality. Georgian Med News 174: 92–94. [PubMed] [Google Scholar]
- Vandel P, Regina W, Bonin B, Sechter D, Bizouard P (1999). Abuse of tianeptine. A case report. Encephale 25: 672–673. [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H (2001). Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 72: 271–281. [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR (2007). The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep 9: 449–459. [DOI] [PubMed] [Google Scholar]
- Wenger GR (1989). The role of control activity levels in the reported strain differences to the behavioral effects of drugs in mice. Pharmacol Biochem Behav 32: 241–247. [DOI] [PubMed] [Google Scholar]
- Woo YS, Bahk WM, Jeong JH, Lee SH, Sung HM, Pae CU et al (2013). Tianeptine combination for partial or non-response to selective serotonin re-uptake inhibitor monotherapy. Psychiatry Clin Neurosci 67: 219–227. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.