Abstract
Objective
Plasma cancer antigen (CA)-125 is a tumour marker recently shown to be associated with systolic heart failure and new-onset atrial fibrillation (AF) after myocardial infarction. However, no reports have described the relationship between CA-125 and new-onset AF in healthy postmenopausal women. The aim of the present study was to evaluate the relationship between CA-125 and new-onset AF in postmenopausal women.
Methods
Between 2005 and 2015, 2086 women, including 1012 postmenopausal women, visited our hospital for annual health check-ups. We excluded patients with systolic dysfunction, chronic inflammatory disease, chronic obstructive pulmonary disease, histories of AF or neoplastic diseases. A total of 746 postmenopausal women underwent thorough physical examinations, including those for biomarkers such as brain natriuretic peptide, high-sensitivity C-reactive protein (hs-CRP) and CA-125.
Results
During the 10-year observation period, AF was documented in 31 participants (4.2%). The mean age of participants developing AF (75±6 years) was higher than that of those without AF (68±8 years). Participants developing AF showed significantly higher CA-125 (11.4±6.3 U/mL) and hs-CRP (0.10±0.11 mg/dL) levels than did those without AF (7.7±3.2 U/mL, p<0.01; 0.07±0.08 mg/dL, p<0.05). Cox regression analyses revealed ageing (HR 1.3; 95% CI 1.08 to 1.57; p<0.01) and plasma CA-125 levels (HR 1.29; 95% CI 1.10 to 1.51; p=0.02) as independent predictors of AF.
Conclusions
High CA-125 levels might be associated with new-onset AF in healthy postmenopausal women.
Keywords: Atrial Fibrillation, Inflammatory markers, Epidemiology
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias and is a leading cause of mortality; its prevalence ranges from 0.5% to 1% in the general population.1 However, in the last decade, the AF prevalence has been commonly believed to be markedly higher, based on the number of hospitalisations,2 emergency room visits and outpatient visits due to AF.3 Some patients with AF have no symptoms; however, AF sometimes leads to serious complications, including cardioembolic stroke, heart failure (HF) and death. Paroxysmal AF may progress to permanent AF as electrical and structural remodelling of the atrium acts to perpetuate abnormal electrical rhythms.4 AF is commonly associated with ageing,5 male sex,6 obesity,7 hypertension (HTN),8 diabetes mellitus (DM),9 myocardial infarction,10 HF,10 or underlying structural heart diseases11 such as valvular heart disease or cardiomyopathy. In ageing societies, the AF prevalence is rapidly increasing; early detection and treatment of AF are, therefore, vital to the health of the patient. The use of biomarkers, such as high-sensitivity C-reactive protein (hs-CRP),12 interleukin-6 (IL-6),13tumour necrosis factor-alpha (TNF-α)13 and serum N-terminal pro-brain type natriuretic peptide (NT-proBNP),14 has been reported to detect AF.
Nagele et al 15 first reported elevated serum levels of cancer antigen-125 (CA-125) following heart transplantations. Now, CA-125 is a well-established tumour marker related to ovarian cancer and is used to monitor the efficacy of ovarian cancer therapy. Recently, several studies have reported the relationships between CA-125 and HF with reduced ejection fraction, HF with preserved ejection fraction16 and ischaemic heart disease.17 Yucel et al 18 reported that CA-125 levels are associated with the development of new-onset AF in patients hospitalised for systolic HF. However, no studies regarding the relationship between CA-125 and paroxysmal or persistent AF have been reported in healthy postmenopausal women. The present study aimed to evaluate the relationship between CA-125 and new-onset AF in this population.
Methods
Study design
All participants were Total Health Care (THC) members at Aoyama Hospital, Tokyo Women’s Medical University, or Health Mate members at the Institute of Geriatrics, Tokyo Women’s Medical University. THC and Health Mate are health check-up systems offered by our institution to more than 3000 registered members. The participants were admitted for annual health check-ups between April 2006 and August 2015. During these check-ups, we conducted detailed health examinations that included blood tests for several tumour makers, detailed metabolic profiles, two-dimensional echocardiography, pulmonary functional tests, chest CT scans, head MRI/angiography, gastrointestinal endoscopy and abdominal ultrasonography. Registered members are monitored for several years; the median follow-up period is 8.0 years.
We analysed and compared several previously reported AF risk factors, including ageing, HTN, DM, and cardiac ultrasound parameters for left atrial diameter (LAD) and left ventricular ejection fraction (LVEF). We also measured several biomarkers, including hs-CRP, NT-proBNP and CA-125, using the data obtained from the records of our unique medical check-up system.
Study protocol
Participants and protocol
The participants were registered THC or Health Mate members who underwent annual medical check-ups between 2006 and 2015. All participants had their histories and the results of the following physical examination parameters recorded. (1) Blood sampling was performed after 12 hours of overnight fasting (plasma triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C); fasting plasma glucose (FPG); haemoglobin (Hb); serum albumin concentration (Alb); uric acid (UA); hs-CRP; and serum creatinine (Cr) were measured using standard laboratory techniques). (2) Blood pressure was measured after a 10 min rest, in sitting position, and expressed as the average of three consecutive measurements of each arm by using the conventional cuff method. (3) All participants underwent echocardiography. (4) Pulmonary functional tests were performed with the following parameters: the ratio of forced expiratory volume in 1 s (FEV1) and forced vital capacity (VC, expressed as the per cent of the predicted value for the individual’s age and sex). The annual health check-up items are listed in online Supplementary table 1.
heartjnl-2016-310272supp001.pdf (213.4KB, pdf)
The inclusion criteria were women older than 45 years having more than 12 months of amenorrhoea on first examination at our hospitals. The exclusion criteria included the following: (1) history or findings of cardiovascular disease, including HF symptoms or systolic dysfunction (LVEF <55%), coronary artery disease, significant valvular heart disease (ie, greater than mild valvular insufficiency or stenosis) and/or hypertrophic cardiomyopathy; (2) history of paroxysmal AF, persistent AF, long history of persistent AF, permanent AF or atrial flutter; (3) history of ovarian cancer; (4) bilateral oophorectomy; (5) chronic inflammatory disease; (6) chronic obstructive pulmonary disease; (7) severe renal and/or hepatic failure; (8) steroid therapy administration; and (9) neoplastic diseases.
The Human Research Protection Office of Tokyo Women’s Medical University approved this protocol (#2507), and informed consent was obtained from each participant before study enrolment.
Laboratory analyses and CA-125 measurements
CA-125 was determined from routine blood samples, taken during the annual health check-ups, using a chemiluminometric immunoassay (LSI Medience, Tokyo, Japan) at the time the patient underwent another blood test.
Detection of new-onset AF and definitions of paroxysmal, persistent and permanent AF
The primary outcome was the detection of new-onset AF at the annual observation. AF was considered to be present if it was identified in one 12-lead ECG for >30 s. Paroxysmal AF is defined as AF that spontaneously reverts to sinus rhythm within 7 days from onset, whereas persistent AF is defined as AF that fails to self-terminate within this period. Long-standing, persistent AF is defined as AF lasting for more than 12 months. Permanent AF is identified in individuals with persistent AF after a joint decision is made, between the patient and clinician, to no longer pursue rhythm control.
Statistical analysis
Participant demographics (ie, age, body mass index (BMI), and DM, HTN and dyslipidaemia status), metabolic syndrome risk factors (ie, waist circumference (WC), systolic blood pressure, diastolic blood pressure, TG, HDL-C and FPG), other cardiovascular risk factors (ie, LDL-C, HbA1c, UA, Alb, Hb, Cr, FEV1 and %VC) and biomarker (ie, hs-CRP, BNP and CA-125) levels were compared between participants developing AF and those without AF. The comparisons were made using Student’s t-test for normally distributed continuous variables or the Wilcoxon rank-sum test for skewed variables, and the χ2or Fisher’s exact tests for categorical variables. Continuous data were analysed using Student’s t-test; categorical data were compared using the χ2 test.
To reveal an association with AF variables, univariate and stepwise forward multiple logistic regression analyses were applied, examining the potential factors reported previously, such as ageing, HTN, low ejection fraction and biomarkers (hs-CRP, BNP and CA-125). The CA-125 values for a typical individual were identified at three different time points (on the first, middle and final medical check-ups) using repeated measures analysis of variance (ANOVA). The risk of new-onset AF was assessed using a Cox regression analysis and the following risk factors: age, LAD and CA-125 level. A receiver operating characteristic (ROC) curve was used to define the sensitivity and specificity of CA-125 for predicting new-onset AF, and the area under the curve was calculated. Survival analyses, using the Kaplan-Meier models, were also performed.
All values are expressed as means±SD, with p values <0.05 being considered statistically significant. Data analyses were performed using SPSS Statistics V.22.0.
Results
Participant characteristics
The study participants had an overall mean age of 68.0±7.8 years. Table 1 shows the baseline characteristics of the participants who developed AF and those without AF during follow-up. During the 10-year observation period (median, 8.0 years), AF was documented in 31 participants (4.2%).
Table 1.
Baseline characteristics of study participants
| Characteristics | AF (n=31) | Non-AF (n=715) | p Value |
| Clinical characteristics | |||
| Age, years | 74.5±5.6 | 67.7±7.7 | <0.01 |
| BMI | 23.2±3.5 | 21.7±3.1 | 0.01 |
| Diabetes, n (%) | 5 (16.1) | 93 (13.0) | 0.61 |
| Hypertension, n (%) | 23 (74.2) | 361 (50.4) | 0.01 |
| Dyslipidaemia, n (%) | 24 (63.0) | 483 (67.6) | 0.25 |
| Metabolic syndrome risk factors | |||
| Waist circumference (cm) | 86±10 | 81±9 | 0.03 |
| Systolic blood pressure (mm H2O) | 126±14 | 121±15 | 0.72 |
| Diastolic blood pressure (mm H2O) | 75±8 | 73±9 | 0.26 |
| Triglyceride (mg/dL) | 95±33 | 94±49 | 0.97 |
| High-density lipoprotein (mg/dL) | 71±17 | 71±17 | 0.91 |
| Fast plasma glucose (mg/dL) | 101±15 | 99±16 | 0.55 |
| Other cardiovascular risk factors | |||
| Low-density lipoprotein (mg/dL) | 128±28 | 131±29 | 0.54 |
| HbA1c, % | 5.5±0.5 | 5.4±0.5 | 0.70 |
| UA, mg/dL | 5.7±1.4 | 4.9±1.1 | <0.01 |
| Serum albumin; Alb, g/L | 4.6±0.2 | 4.6±0.3 | 0.91 |
| Haemoglobin concentration, g/L | 13.6±1.0 | 13.2±1.0 | 0.03 |
| Ccr, mL/min | 78.4±15.9 | 77.3±16.7 | 0.71 |
| %VC | 93.0±3.0 | 102.0±17.3 | <0.01 |
| FEV 1.0% | 78.8±8.8 | 79.4±6.7 | 0.59 |
| Echocardiographic findings | |||
| Left atrial diameter (mm) | 40.2±6.4 | 32.5±5.0 | <0.01 |
| Left ventricular ejection fraction (%) | 68.8±4.3 | 69.2±6.2 | 0.72 |
AF, atrial fibrillation; BMI, body mass index; Ccr, creatinine clearance; FEV, forced expiratory volume; HbA1c, haemoglobin A1c; UA, uric acid; VC, vital capacity.
The mean age of participants with AF was higher than that of participants without AF. Similarly, WCs and BMIs were also higher in participants with AF than in those without AF. Participants developing AF also had higher serum UA and Hb concentrations than did participants without AF. The frequency of HTN was higher in participants with AF than in those without, whereas the %VC was lower in those with AF than in participants without AF.
Participants with AF had larger LADs than did those without AF. No significant difference was found in the LVEF between the two groups.
Biomarkers and AF incidence
The baseline biomarker levels are shown in table 2. The hs-CRP level in participants with AF was higher than in those without AF. Participants with AF were also observed to have higher CA-125 levels than did those without AF (figure 1). However, BNP levels were not significantly different between the two groups.
Table 2.
Baseline biomarkers of participants with atrial fibrillation vs those without AF
| Biomarkers | AF (n=31) | Non-AF (n=715) | p Value |
| hs-CRP (mg/dL) | 0.099±0.106 | 0.068±0.084 | <0.05 |
| BNP (pg/dL) | 25.0±12.6 | 24.7±17.7 | 0.40 |
| CA-125 (U/mL) | 11.4±6.3 | 7.7±3.2 | <0.01 |
AF, atrial fibrillation; BNP, brain natriuretic peptide; CA-125, cancer antigen-125; hs-CRP, high-sensitivity C-reactive protein.
Figure 1.
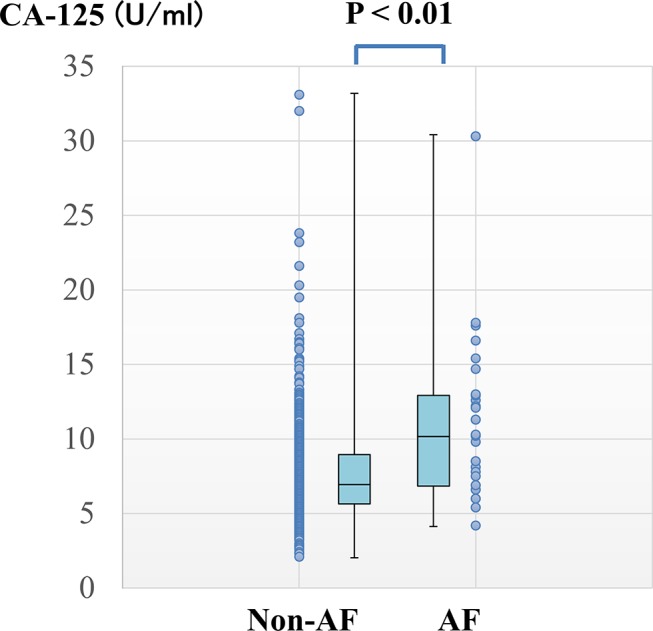
Distribution of baseline CA-125 levels between the two groups. Box plot representing baseline CA-125 levels in the non-AF (left) and AF groups (right). The dots depict scatter plots of baseline CA-125 levels, whereas the graph shows the comparison of CA-125 levels (median ± IQR) between the two groups; p<0.001. AF, atrial fibrillation; CA-125, cancer antigen-125.
CA-125 levels during the observation period
We checked the CA-125 levels at distinct time points to confirm differences in CA-125 levels in all individuals. For those developing AF, the mean CA-125 levels were 11.4±6.3 U/mL, 10.9±6.7 U/mL and 11.6±6.6 U/mL on the first, middle and final time points, respectively. The mean CA-125 levels were 7.7±3.2 U/mL, 7.3±2.7 U/mL and 7.5±4.8 U/mL on the first, middle and final time points, respectively, in the group without AF. One-factor repeated measures ANOVA did not reveal any differences in CA-125 values for a typical individual at the three time points. However, the CA-125 levels in the group of participants developing AF were significantly higher than those for participants in the non-AF group at all three time points (p<0.05).
Association between new-onset AF and risk factors
The results of the univariate and multivariate logistic regression analyses for new-onset AF risk factors are shown in table 3. Age, LAD, CA-125 level and HTN were associated with the AF burden in the univariate analysis. In the multivariate analysis, age, LAD and CA-125 remained significantly associated with new-onset AF.
Table 3.
Univariate and multivariate logistic regression analyses for atrial fibrillation risk factors
| Variable | Univariate | Multivariate | ||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (years) | 1.127 | 1.038 to 1.428 | 0.016 | 1.174 | 1.020 to 1.351 | 0.025 |
| Left atrial diameter (cm) | 1.277 | 1.147 to 1.423 | <0.001 | 1.266 | 1.052 to 1.523 | 0.012 |
| EF (%) | 0.992 | 0.916 to 1.075 | 0.849 | |||
| CA-125 (U/mL) | 1.176 | 1.100 to 1.256 | <0.001 | 1.386 | 1.047 to 1.834 | 0.022 |
| BNP (pg/mL) | 1.001 | 0.969 to 1.034 | 0.939 | |||
| hs-CRP(mg/dL) | 7.076 | 0.561 to 89.237 | 0.130 | |||
| HTN | 1.127 | 1.038 to 1.428 | 0.013 | |||
BNP, brain natriuretic peptide; EF, ejection fraction; hs-CRP, high-sensitivity C-reactive protein; HTN, hypertension.
Predictors of new-onset AF
We performed Cox regression analyses examining AF occurrence and previously reported AF risk factors.19 In the univariate analysis, three factors were significantly predictive of AF occurrence: ageing (HR 1.2; 95% CI 1.04 to 1.40; p=0.01), CA-125 level (HR 1.13; 95% CI 1.09 to 1.19; p<0.01) and LAD (HR 1.25; 95% CI 1.15 to 1.35; p<0.01). The other risk factors were not predictive of AF occurrence in this study population (table 4).
Table 4.
Univariate and multivariate Cox regression analyses for atrial fibrillation occurrence
| Variable | Univariate | Multivariate | ||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (years) | 1.206 | 1.038 to 1.401 | 0.014 | 1.298 | 1.075 to 1.568 | <0.01 |
| CA-125 (U/mL) | 1.138 | 1.087 to 1.193 | <0.001 | 1.287 | 1.096 to 1.511 | 0.02 |
| Left atrial diameter (cm) | 1.248 | 1.151 to 1.354 | <0.001 | |||
CA-125, cancer antigen-125.
We confirmed the new-onset AF predictors using multivariate analysis. Cox regression analysis revealed ageing (HR 1.30; 95% CI 1.08 to 1.57; p=0.07) and CA-125 levels (HR 1.14; 95% CI 1.09 to 1.19; p<0.001) as independent predictors of new-onset AF (table 4).
CA-125 cut-off level
Based on the ROC curve analysis, the optimal CA-125 cut-off level for predicting new-onset AF was ≥9.8 U/mL, with a specificity and sensitivity of 82.0% and 54.8%, respectively (area under the curve=0.711; 95% CI 0.609 to 0.813) (figure 2).
Figure 2.
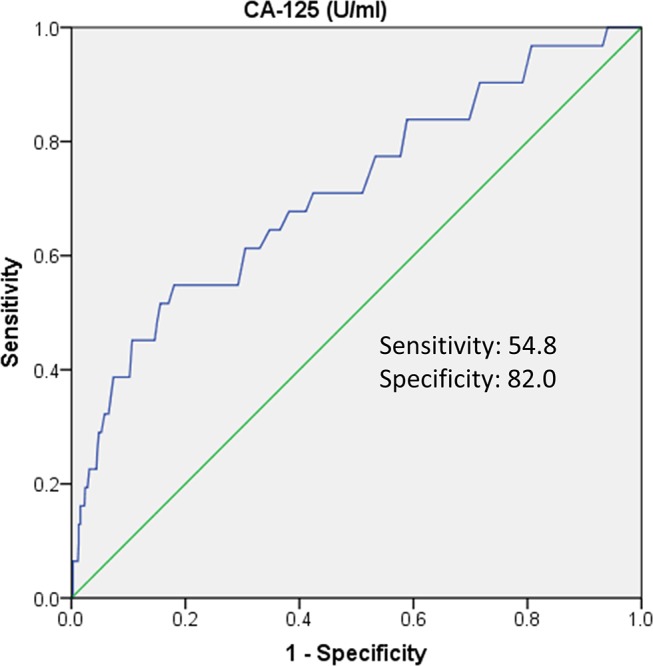
ROC curve of CA-125 to predict new-onset AF. The blue line represents CA-125, and the green line is the reference line. AF, atrial fibrillation; CA-125, cancer antigen-125; ROC, receiver operating characteristic.
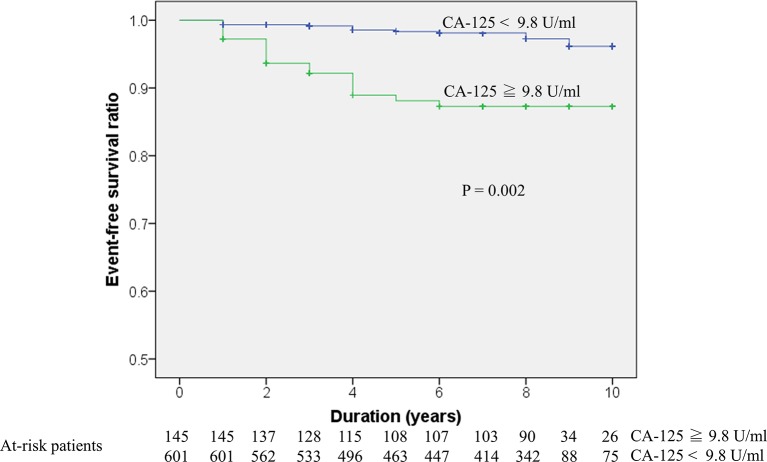
Long-term follow-up for new-onset AF
The event-free survival curve was also evaluated using the optimal CA-125 cut-off value (9.8 U/mL). The results indicated that individuals with high (≥9.8 U/mL) CA-125 levels had significantly higher risk of new-onset AF compared with individuals with lower CA-125 levels (CA-125 <9.8 U/mL; p=0.002) (figure 3).
Figure 3.
Kaplan-Meier survival curve of new-onset AF and CA-125. The blue line represents CA-125 levels ≥9.8 U/mL, and the green line represents CA-125 levels <9.8 U/mL. AF, atrial fibrillation; CA-125, cancer antigen-125.
Discussion
Main findings
This observational cohort study revealed that a high CA-125 level is an independent predictor of new-onset AF in healthy postmenopausal women. Our mean observation period was 8 years, with 31 participants developing AF within that period; this length of follow-up was beneficial for observing new-onset AF in individuals without HF.
For ovarian cancer detection, the usual CA-125 cut-off level is >35 U/mL.20 In contrast, Yucel et al 18 reported that the optimal cut-off level for CA-125 for predicting AF development was ≥68.49 U/mL in patients with systolic HF. Recently, Kaya et al 21 reported a retrospective cohort study where high CA-125 levels (≥91 U/mL) were predictors of the presence of permanent AF in patients with systolic HF. Based on these reports, the systolic HF patients had a mean BNP level of 100–200 pg/dL, and approximately 50% of the included patients were male. Additionally, CA-125 levels were elevated in HF patients, making the distinction between CA-125 elevation due to HF or AF difficult. Hence, in our study, we excluded male patients and only included postmenopausal women; this eliminated any effects of HF and ovarian function.
CA-125 is produced by the coelomic epithelial cells,22 such as those in the pericardium, pleura, peritoneum and Müllerian epithelium, following stimulation by mechanical stress and inflammation.23 The mean CA-125 level in our study was lower than that in the previously cited reports, likely because we did not include patients with HF or ovarian cancer in our population. However, the normal CA-125 range indicates that the level is not indicative of cancer, but only of new-onset AF. Thus, if CA-125 level is high, compared with the mean value for a patient’s age, or fails to decrease after menopause, an AF investigation may be warranted, including an assessment of the patient’s history of palpitations, echocardiography, radiography and/or ultrasonography findings. Moreover, CA-125 levels did not change significantly in individuals over the several months of follow-up, suggesting that CA-125 levels may be used as a biomarker for paroxysmal or persistent AF. Thus, CA-125 levels are beneficial for the screening of paroxysmal or persistent AF in high-risk AF groups, such as elderly men with multiple chronic diseases.
Inflammatory biomarkers for AF
The elevation of inflammatory biomarkers, such as CRP and IL-6, has been observed in patients with both paroxysmal and persistent AF.24 In addition, myocyte necrosis, fibrosis and inflammatory infiltrates have been observed in biopsied atrial tissue in patients with AF alone.25 However, in one population-based cohort of 1011 patients, followed for more than 4 years, high baseline CRP levels were not significantly associated with AF onset.26 Our results showed significant differences in the baseline hs-CRP levels between patients with paroxysmal AF and those without AF. However, hs-CRP level was not a risk factor for the development of paroxysmal AF in the multiple regression analysis. One reason for this discrepancy is that some patients were on medications such as statins, ACE inhibitors and angiotensin receptor blockers (ARBs). Statins reportedly reduce CRP levels by an anti-inflammatory effect;27 ACE inhibitors or ARBs have been reported to have similar effects.28 Presently, because many patients with AF have coexisting conditions, such as HTN, DM and DL, using inflammatory markers to predict AF occurrence might be difficult. CA-125 was recently reported to activate inflammatory responses by interacting with molecules such as IL-8, soluble IL-2 and TNF-α in patients with AF.29 If the release of CA-125 is affected by inflammation in patients with AF, hs-CRP and CA-125 were the confounding factors in the statistical analysis. This might be another reason for the discrepancy.
Natriuretic peptide for AF
BNP is a highly plausible candidate biomarker for AF risk because it is an indicator of cardiac stress. Several community-based studies have reported that NT-proBNP and BNP12 are predictors of incident AF. In the case of paroxysmal AF, BNP levels were elevated in patients with lone paroxysmal AF.30 However, NT-proBNP and BNP have some limitations as clinical biomarkers. Notably, patients with chronic renal failure, elderly individuals and females sometimes show non-specific BNP level elevations in the absence of HF. In our results, the mean BNP level was approximately 25.0 pg/mL in each group; thus, BNP levels may be non-specific in postmenopausal elderly women.
Study limitations
The strengths of our study include the use of detailed metabolic profiles and the high follow-up rate. However, this study also has several limitations. Our sample size and the number of outcomes were small. We also used only a single ECG to detect AF, rather than using a prolonged monitoring method, such as 48-hour Holter electrocardiography; this may have significantly underestimated the incidence of asymptomatic AF and AF in our study population. Participants were from urban areas in Japan, with a preponderance of middle-class individuals. However, the study population may represent the general populations of other developed countries. In addition, more than 99% of our cohort comprised Asian individuals; thus, generalisability to other ethnic or racial populations may not be valid. Similar research is necessary in other regions to assess the general application of our findings.
Conclusions
This is the first study to investigate the biomarkers predictive of new-onset AF during long-term follow-up in patients without HF. Our results show that CA-125 may be a novel biomarker for incident paroxysmal or persistent AF in healthy postmenopausal women. CA-125 levels should typically decrease after menopause; thus, elevated CA-125 levels in women without HF, ovarian cancer or other disease-related elevations may indicate the need for a careful evaluation for the presence of AF.
Key messages.
What is already known on this subject?
Cancer antigen (CA)-125 level is associated with the development of new-onset atrial fibrillation (AF) in patients hospitalised for systolic heart failure (HF).
What might this study add?
CA-125 levels predicted the onset of AF in postmenopausal women without HF.
How might this impact on clinical practice?
Healthy postmenopausal women with elevated CA-125 levels should be carefully evaluated for the presence of AF.
Acknowledgments
We would like to thank Editage (www.editage.jp) for the English language editing.
Correction notice Since this paper was first published online the information in figure 3 has been updated.
Footnotes
Contributors: HS led the study as the principal investigator, initiated the project, wrote the statistical analysis plan and drafted and revised the paper. KS wrote the statistical analysis plan, and cleaned and analysed the data. MT, YT, FT and EW collected the data. KJ designed the data collection tools and analysed the data. MK analysed the data and drafted and revised the paper. NH critically reviewed the draft of the manuscript. All authors approved the final version of the manuscript.
Competing interests: None declared.
Ethics approval: The Human Research Protection Office of Tokyo Women’s Medical University approved this protocol (#2507).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Go AS, Hylek EM, Phillips KA, et al. . Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) Study. Jama 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2. Ruskin JN, Singh JP. Atrial fibrillation endpoints: hospitalization. Heart Rhythm 2004;1:31–5. discussion B4–5 10.1016/j.hrthm.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 3. Santini M, De Ferrari GM, Pandozi C, et al. ; FIRE Investigators. Atrial fibrillation requiring urgent medical care. approach and outcome in the various departments of admission. data from the atrial fibrillation/flutter italian REgistry (FIRE). Ital Heart J 2004;5:205–13. [PubMed] [Google Scholar]
- 4. de Vos CB, Pisters R, Nieuwlaat R, et al. . Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. 10.1016/j.jacc.2009.11.040 [DOI] [PubMed] [Google Scholar]
- 5. Verdecchia P, Reboldi G, Gattobigio R, et al. . Atrial fibrillation in hypertension: predictors and outcome. Hypertension 2003;41:218–23. 10.1161/01.HYP.0000052830.02773.E4 [DOI] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Levy D, Vaziri SM, et al. . Independent risk factors for atrial fibrillation in a population-based cohort. the framingham heart study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 7. Tedrow UB, Conen D, Ridker PM, et al. . The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol 2010;55:2319–27. 10.1016/j.jacc.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas MC, Dublin S, Kaplan RC, et al. . Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 2008;21:1111–6. 10.1038/ajh.2008.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcus GM, Alonso A, Peralta CA, et al. ; Candidate-Gene Association Resource (CARe) Study. European ancestry as a risk factor for atrial fibrillation in african americans. Circulation 2010;122:2009–15. 10.1161/CIRCULATIONAHA.110.958306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Agarwal SK, Folsom AR, et al. . A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol 2011;107:85–91. 10.1016/j.amjcard.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Psaty BM, Manolio TA, Kuller LH, et al. . Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455–61. 10.1161/01.CIR.96.7.2455 [DOI] [PubMed] [Google Scholar]
- 12. Schnabel RB, Larson MG, Yamamoto JF, et al. . Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010;121:200–7. 10.1161/CIRCULATIONAHA.109.882241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parthenakis FI, Patrianakos AP, Skalidis EI, et al. . Atrial fibrillation is associated with increased neurohumoral activation and reduced exercise tolerance in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol 2007;118:206–14. 10.1016/j.ijcard.2006.03.090 [DOI] [PubMed] [Google Scholar]
- 14. Latini R, Masson S, Pirelli S, et al. ; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011;269:160–71. 10.1111/j.1365-2796.2010.02287.x [DOI] [PubMed] [Google Scholar]
- 15. Nägele H, Bahlo M, Klapdor R, et al. . CA 125 and its relation to cardiac function. Am Heart J 1999;137:1044–9. 10.1016/S0002-8703(99)70360-1 [DOI] [PubMed] [Google Scholar]
- 16. D’Aloia A, Faggiano P, Aurigemma G, et al. . Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J Am Coll Cardiol 2003;41:1805–11. [DOI] [PubMed] [Google Scholar]
- 17. Li X, He M, Zhu J, et al. . Higher carbohydrate antigen 125 levels are associated with increased risk of coronary heart disease in elderly chinese: a population-based case-control study. PLoS One 2013;8:e81328 10.1371/journal.pone.0081328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yucel H, Kaya H, Zorlu A, et al. . Cancer antigen 125 levels and increased risk of new-onset atrial fibrillation. Herz 2015;40 Suppl 2(Suppl 2):119–24. 10.1007/s00059-014-4148-4 [DOI] [PubMed] [Google Scholar]
- 19. Kirchhof P, Lip GY, Van Gelder IC, et al. . Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options – a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. 10.1093/europace/eur241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bast RC, Klug TL, St John E, et al. . A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian Cancer. N Engl J Med 1983;309:883–7. 10.1056/NEJM198310133091503 [DOI] [PubMed] [Google Scholar]
- 21. Kaya H, Zorlu A, Yucel H, et al. . Higher Cancer antigen 125 level is associated with the presence of permanent atrial fibrillation in systolic heart failure patients. Acta Cardiol 2016;71:61–6. 10.2143/AC.71.1.3132099 [DOI] [PubMed] [Google Scholar]
- 22. Barbieri RL, Niloff JM, Bast RC, et al. . Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril 1986;45:630–4. 10.1016/S0015-0282(16)49333-7 [DOI] [PubMed] [Google Scholar]
- 23. Halila H, Stenman UH, Seppälä M. Ovarian Cancer antigen CA 125 levels in pelvic inflammatory disease and pregnancy. Cancer 1986;57:1327–9. [DOI] [PubMed] [Google Scholar]
- 24. Aviles RJ, Martin DO, Apperson-Hansen C, et al. . Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006–10. 10.1161/01.CIR.0000103131.70301.4F [DOI] [PubMed] [Google Scholar]
- 25. Frustaci A, Chimenti C, Bellocci F, et al. . Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180–4. 10.1161/01.CIR.96.4.1180 [DOI] [PubMed] [Google Scholar]
- 26. Dernellis J, Panaretou M. Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardiol 2006;97:245–8. 10.1016/j.amjcard.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 27. Nissen SE, Tuzcu EM, Schoenhagen P, et al. ; REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. Jama 2004;291:1071–80. 10.1001/jama.291.9.1071 [DOI] [PubMed] [Google Scholar]
- 28. Di Raimondo D, Tuttolomondo A, Buttà C, et al. . Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012;18:4385–413. 10.2174/138161212802481282 [DOI] [PubMed] [Google Scholar]
- 29. De Gennaro L, Brunetti ND, Montrone D, et al. . Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur J Clin Invest 2012;42:371–5. 10.1111/j.1365-2362.2011.02592.x [DOI] [PubMed] [Google Scholar]
- 30. Li J, Wang L. B-type natriuretic peptide levels in patients with paroxysmal lone atrial fibrillation. Heart Vessels 2006;21:137–40. 10.1007/s00380-005-0884-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2016-310272supp001.pdf (213.4KB, pdf)