Abstract
Objective
Today most patients with Lynch syndrome (LS) survive their first cancer. There is limited information on the incidences and outcome of subsequent cancers. The present study addresses three questions: (i) what is the cumulative incidence of a subsequent cancer; (ii) in which organs do subsequent cancers occur; and (iii) what is the survival following these cancers?
Design
Information was collated on prospectively organised surveillance and prospectively observed outcomes in patients with LS who had cancer prior to inclusion and analysed by age, gender and genetic variants.
Results
1273 patients with LS from 10 countries were followed up for 7753 observation years. 318 patients (25.7%) developed 341 first subsequent cancers, including colorectal (n=147, 43%), upper GI, pancreas or bile duct (n=37, 11%) and urinary tract (n=32, 10%). The cumulative incidences for any subsequent cancer from age 40 to age 70 years were 73% for pathogenic MLH1 (path_MLH1), 76% for path_MSH2 carriers and 52% for path_MSH6 carriers, and for colorectal cancer (CRC) the cumulative incidences were 46%, 48% and 23%, respectively. Crude survival after any subsequent cancer was 82% (95% CI 76% to 87%) and 10-year crude survival after CRC was 91% (95% CI 83% to 95%).
Conclusions
Relative incidence of subsequent cancer compared with incidence of first cancer was slightly but insignificantly higher than cancer incidence in patients with LS without previous cancer (range 0.94–1.49). The favourable survival after subsequent cancers validated continued follow-up to prevent death from cancer. The interactive website http://lscarisk.org was expanded to calculate the risks by gender, genetic variant and age for subsequent cancer for any patient with LS with previous cancer.
Keywords: INHERITED CANCERS, EPIDEMIOLOGY, CANCER GENETICS, COLORECTAL CANCER GENES, SCREENING
Significance of this study.
What is already known on this subject?
Inherited colorectal cancer may be caused by mismatch repair (MMR) gene variants and is then commonly referred to as Lynch syndrome.
Patients with Lynch syndrome are at risk for synchronous and metachronous cancers.
Endoscopic surveillance with removal of adenomas is recommended to prevent colorectal cancer.
What are the new findings?
This is the first comprehensive prospective study to provide empirically observed data on subsequent cancer incidence and survival in patients with Lynch syndrome who have survived previous cancer.
The cumulative incidences for any subsequent cancer were 73% for path_MLH1 and 76% for path_MSH2 carriers. The incidence was lower in MSH6 carriers.
Colorectal cancer occurred frequently despite continued colonoscopic surveillance with removal of adenomas.
Survival after subsequent cancer was good.
How might it impact on clinical practice in the foreseeable future?
The good survival of the prospectively detected cancers in our cohort supports continued medical follow-up including colonoscopy and promotion of cancer awareness, which may contribute to favourable outcomes in this high-risk group through early detection and treatment of cancers.
The high incidence of a subsequent colon cancer after a first colon cancer may be an argument in favour of subtotal colectomy as treatment for first colon cancer.
The freely available website http://lscarisk.org may be used to calculate risk for subsequent cancer in survivors of first cancer(s) using the patient's gender, pathogenic MMR gene variant and current age.
Introduction
Lynch syndrome (LS) is associated with a high probability of GI, gynaecological and other cancers.1 2 It is caused by germline pathogenic variants in any of the four DNA mismatch repair (MMR) genes. Such variants are here referred to as path_MSH2, path_MLH1, path_PMS2 or path_MSH6. Deletions in the EP CAM gene, which lead to methylation of the adjacent MSH2 promoter, are also referred to here as path_MSH2. To date, most patients with LS have been identified following investigation because of their family or personal history of multiple and/or early-onset cancers.
Screening by colonoscopy is recommended to prevent colorectal cancer (CRC) by removing preinvasive neoplasia (adenomas).1 2 Carriers of path_MLH1, path_MSH2, path_MSH6 or path_PMS2 require reliable information about their future cancer risk so that they can be offered appropriately targeted surveillance. However, a paucity of prospectively obtained information has led current clinical guidelines to rely heavily upon retrospective data from patient cohorts whose selection for molecular testing has been subject to diverse biases.
We have previously reported prospectively observed time to first cancer by mutated gene and gender in asymptomatic patients with LS undergoing follow-up including colonoscopy.3 CRC occurred with high incidence despite endoscopic surveillance with removal of adenomas. However, survival was excellent for patients with invasive CRC and for patients with endometrial and ovarian cancer.
The assumptions underlying current guidelines for healthcare for patients with LS, to which we have contributed,1 should be replaced by empirical observations whenever possible. However, there is limited empirical information from prospective studies on the outcomes for patients with LS who have survived a first cancer and are receiving continued surveillance according to existing guidelines.4
We designed the present study to address three questions in patients with LS who had survived previous cancer and were at risk of developing subsequent cancers: (i) what was the cumulative incidence of subsequent cancers, (ii) in which organs did subsequent cancers occur and (iii) what was survival following these subsequent cancers?
Methods
Patients and interventions
Database design and inclusion criteria have been described previously.3 The study was a case-based, open observational study without a control group. All patients were 20 years of age or older at inclusion. Age at inclusion was age at the first prospectively planned colonoscopy. We included all patients with LS who survived their previous cancer(s) and continued to have surveillance with the aim of preventing death from a subsequent cancer. Inclusion was from the day of the first prospectively planned and completed colonoscopy undertaken as part of the patient's follow-up surveillance.
The patients with LS included in this report were demonstrated or obligate carriers of pathogenic MMR variants who had been diagnosed with cancer before or at the same age as when included (referred to as ‘previous cancer’ later). All patients had at least one previous cancer before or at inclusion. Prevalent cancers including all cancers in the colorectum or any other organ that were diagnosed at the same age as the first prospectively planned and completed colonoscopy following a previous cancer were also scored as previous cancers. All incident cancers reported here were detected after the first prospectively planned colonoscopy. All patients were considered survivors of first cancer(s) and subjected to follow-up for prevention or early treatment of a possible subsequent new cancer. All patients were subjected to follow-up including surveillance colonoscopy according to international guidelines.1 2 Follow-up was conducted as previously reported (see details in online supplementary table S2 with references). In brief, the interval between colonoscopies was reduced circa 1996 to 2 years or less in keeping with new international guidelines by all reporting centres except those in Finland. A detailed analysis comparing outcomes in the Finnish series to the rest is in preparation and will be reported separately.
gutjnl-2016-311403supp001.pdf (361.1KB, pdf)
gutjnl-2016-311403supp002.pdf (213.5KB, pdf)
All patients had MMR variants that were considered pathogenic by the contributing centre at the time of reporting to the database. To validate this judgement, all reported genetic variants were independently searched for in the Leiden Open Variation Database (LOVD) (http://chromium.lovd.nl/LOVD2/colon_cancer/) in October 2015. Deletions in the EP CAM gene silencing MSH2 were scored as path_MSH2 variants. In sum 617 of the patients had pathogenic (class 5) variants, 14 patients had probably pathogenic (class 4) variants and the remaining 642 patients had variants that were not found in LOVD.
The patients were followed until the last update of information, and scored as alive or dead at last update. The following information was used for this report: gender, genetic variant, age at inclusion, age at last update, age at any cancer, months since last colonoscopy to CRC, cancers scored by the first three positions in the ICD9 diagnostic system and age of death. The ICD9 diagnoses were copied from the medical files. All cancer diagnoses were included to avoid assumptions on which cancers to be part of LS to bias the results. Cancer stage at diagnosis was not available. Data were complete for all patients included except for eight patients who lacked data on months since last colonoscopy to CRC. Cancers diagnosed after the first subsequent cancer were not considered. When calculating time to subsequent cancer and survival, each patient was scored once only, irrespective of how many synchronous subsequent cancers the patient might have had.
The extent of surgery for previous CRC before inclusion may influence the incidence of subsequent CRC. In general, patients with colon cancer prior to inclusion would not have been recognised as LS and would have been subjected to standard treatment. Also, subtotal colectomy as treatment for a first colon cancer in LS has not been advocated widely in Europe.1 The risk of a subsequent CRC may differ in patients subjected to subtotal colectomy compared with those who had less extensive surgery. We did not have access to details of surgical treatment of previous CRC, but we were interested to see whether the numbers observed in this study might allow planning of follow-up studies taking into account treatment for previous cancers, time from last colonoscopy to CRC and findings at last colonoscopy together with stage at diagnoses of CRC and the relations between these parameters and survival, all stratified by genetic variants. Additional confounding factors include modified risk for subsequent cancer following treatment with radiation or chemotherapy. Unfortunately, such information was not uniformly filed and/or not available from all the collaborating centres. We decided to analyse the dataset without discriminating between modes of treatment for any cancer, and the results must be interpreted accordingly.
Some centres have reported their prospective findings independently and their previously reported cases are included in the present series—see ref. 3 and online supplementary table S2 for a list of previous publications.
Statistical methods
Annual incidence rates (AIRs) by age were calculated in 5-year cohorts from 25 to 69 years of age. Cumulative incidence, denoted by Q, was computed starting at age 25, assuming zero incidence before age 25, using the formula Q(age)=Q(age−1)+(1−Q(age−1))·AIR(age) where AIR(age) is the AIR as estimated from the corresponding 5-year interval. SE for the AIR was estimated as SEAIR=sqrt(AIR·(1−AIR)/Yrs) where Yrs denotes the number of observation years in the 5-year age group for which the AIR is estimated. For cumulative incidence, the hazard rate H=−ln(1−AIR) was used with SE estimated as SEH=SEAIR/(1−AIR). The SE, denoted by SEQ, of the cumulative incidence Q(age) up to the given age is computed in two steps. First, for each 5-year age interval, having hazard rate H with SE SEH, the contribution to the cumulative hazard from that interval is N·H with SE N·SEH where N is the number of years from that 5-year interval: for example, the cumulative incidence up to age 32 contains all 5 years from the 25–29 age interval, but only 3 years from the 30–34 age interval. The accumulated hazard CH is computed by adding the N·H values across age intervals, while the corresponding SE, SECH, is found by setting SECH2 equal to the sum of (N·SEH) across age intervals. The accumulated hazard rate CH should now equal −ln(1−Q) with Q as computed above, while the SE of the cumulative incidence is computed as SEQ=SECH·(1−Q). We estimated 95% CIs as AIR±1.96 SEAIR and Q±1.96 SEQ.
In contrast to our former report that focused on the penetrance of the pathogenic variants of the different MMR genes3 this report focuses on cumulative cancer incidence from the age at which the previous cancer(s) was treated, and the results are presented by age at inclusion. In clinical practice, these figures provide the basis for improved advice for patients with LS who have been treated for cancer and now wish to know his/her risk of subsequent cancer. Cumulative incidences in this series relative to our former report3 on patients with LS who had not had cancer before inclusion were calculated as cumulative incidence in this report divided by corresponding cumulative incidence in the former report.
The cumulative incidences in this report may be considered prospective risks for cancer when discussing outcome of predictive genetic testing, and the interactive website http://lscarisk.org, which was established previously based on our former report, was expanded to display the future cancer risk for any patient with LS who has already had cancer, by age, sex and genetic variant.
Crude survival after the first subsequent cancer to be diagnosed was assessed by the Kaplan–Meier survivor function (K–M). Results of K–M analyses are given as point estimates (95% CI). Two-by-two tables were considered by Fisher's exact p. For comparisons of groups twosample t-test, χ2 test and Fisher’s exact p test were used as appropriate.
Ethics
All genetic tests were done with appropriate informed consent according to local and national requirements for healthcare and/or research. No named data were exported from any participating centre.
Results
A total of 1273 patients with LS including 555 males and 718 females, with cancer before or at inclusion, were observed for 4411 years (mean 6.1 years) in females and 3342 years (mean 6.0 years) in males. Mean age at inclusion was 51.6 years. Mean ages at inclusion were similar for path_MLH1 and path_MSH2 carriers (51.0 and 50.0, respectively), but older for path_MSH6 and path_PMS2 carriers (57.6 and 55.9, respectively). For details on numbers included, observation years, mean observation years and mean ages at inclusion by country, gene and gender see table 1. The number of path_PMS2 carriers was too small to allow detailed statistical calculations.
Table 1.
Number of cases, observation years and age at inclusion stratified on country of origin, genetic variant and gender
| Observation years |
Age at inclusion |
|||||
|---|---|---|---|---|---|---|
| Number | Total | Mean (range) | 95% CI of mean | Mean (range) | 95% CI of mean | |
| ALL | 1273 | 7753 | 6.1 (1–29) | ±0.24 | 51.6 (21–95) | ±0.68 |
| Females | 718 | 441 | 6.1 (1–29) | ±0.33 | 52.7 (21–95) | ±0.88 |
| Males | 555 | 3342 | 6.0 (1–27) | ±0.36 | 50.3 (21–91) | ±1.04 |
| Grouped by country | ||||||
| Finland | 365 | 2653 | 7.3 (1–24) | ±0.50 | 53.6 (25–95) | ±1.25 |
| UK | 195 | 988 | 5.1 (1–16) | ±0.51 | 51.0 (21–82) | ±1.71 |
| Denmark | 181 | 1004 | 5.5 (1–15) | ±0.49 | 52.0 (24–83) | ±1.70 |
| Spain | 141 | 743 | 5.3 (1–13) | ±0.60 | 51.4 (24–89) | ±2.08 |
| Germany | 126 | 650 | 5.2 (1–29) | ±0.73 | 45.1 (21–71) | ±1.88 |
| Norway | 108 | 684 | 6.3 (1–19) | ±0.92 | 55.6 (27–83) | ±2.20 |
| Sweden | 61 | 455 | 7.5 (1–27) | ±1.31 | 52.8 (29–77) | ±3.11 |
| Holland | 55 | 238 | 4.3 (1–19) | ±1.18 | 51.6 (22–82) | ±3.71 |
| Australia | 35 | 293 | 8.4 (1–26) | ±1.95 | 44.9 (22–70) | ±3.56 |
| Italy | 6 | 45 | 7.5 (2–11) | ±3.46 | 42.7 (33–55) | ±5.67 |
| Grouped by gene and gender | ||||||
| path_MLH1 | ||||||
| Females | 305 | 2172 | 7.1 (1–29) | ±0.55 | 52.0 (21–84) | ±1.42 |
| Males | 284 | 1906 | 6.7 (1–21) | ±0.52 | 49.8 (23–91) | ±1.45 |
| path_MSH2 | ||||||
| Females | 285 | 1502 | 5.3 (1–19) | ±0.45 | 51.6 (22–83) | ±1.27 |
| Males | 185 | 1085 | 5.9 (1–27) | ±0.65 | 47.5 (21–73) | ±1.56 |
| path_MSH6 | ||||||
| Females | 109 | 669 | 6.1 (1–19) | ±0.86 | 57.6 (27–95) | ±2.38 |
| Males | 58 | 265 | 4.6 (1–13) | ±0.75 | 57.6 (33–83) | ±3.44 |
| path_PMS2 | ||||||
| Females | 19 | 68 | 3.6 (1–13) | ±1.41 | 52.0 (37–68) | ±4.05 |
| Males | 28 | 86 | 3.1 (1–10) | ±0.93 | 58.5 (27–77) | ±4.80 |
Prior to inclusion, the 1273 patients had developed 1835 cancers (mean 1.4 per patient), with CRCs accounting for 1161 (63%) of all previous cancers. CRCs represented 83% of cancers diagnosed in males and 49% of the cancers in females. Endometrial (n=296) and ovarian (n=61) cancers accounted for 28% and 6%, respectively, of all previous cancers in females. Urinary tract (n=80), breast (n=46), upper GI tract (n=41) and prostate (n=21) cancers were also frequently reported. The separate diagnoses by gender are given in table 2. Out of the 1273 patients, 392 (31%) had had two or more previous tumours: 268 (21%) had two, 91 (7%) had three, 22 (2%) had four, 9 (1%) had five, one (0.1%) had six and one (0.1%) had seven cancers before inclusion.
Table 2.
Location of cancers diagnosed prior to inclusion
| ICD9 | Organ | Males | Females | All |
|---|---|---|---|---|
| 153 | Colon | 535 | 446 | 981 |
| 154 | Sigmoideum/rectum | 96 | 84 | 180 |
| 182 | Endometrium | 296 | 296 | |
| 183 | Ovary | 61 | 61 | |
| 151 | Stomach | 5 | 5 | 10 |
| 152 | Duodenum | 11 | 8 | 19 |
| 156 | Gall bladder/bile duct | 3 | 1 | 4 |
| 157 | Pancreas | 2 | 2 | 4 |
| 189 | Urinary bladder | 23 | 27 | 50 |
| 188 | Kidney/ureter | 16 | 14 | 30 |
| 173 | Skin | 21 | 28 | 49 |
| 174 | Breast | 46 | 46 | |
| 185 | Prostate | 20 | 4 | 24 |
| 191 | Brain | 2 | 7 | 9 |
| 140 | Lip | 1 | 1 | |
| 146 | Pharynx | 1 | 1 | |
| 150 | Oesophagus | 2 | 2 | |
| 155 | Liver | 1 | 1 | |
| 159 | Abdomen unspecified | 1 | 1 | |
| 161 | Mouth | 1 | 1 | |
| 162 | Lung | 1 | 1 | 2 |
| 171 | Soft tissue sarcoma | 3 | 7 | 10 |
| 172 | Melanoma | 1 | 7 | 8 |
| 180 | Cervix | 20 | 20 | |
| 186 | Testes | 6 | 6 | |
| 190 | Eye | 2 | 2 | |
| 193 | Thyroid | 2 | 2 | |
| 194 | Endocrine tumour | 1 | 1 | 2 |
| 199 | Unknown origin | 3 | 1 | 4 |
| 200 | Haematological malignancies | 7 | 2 | 9 |
| Sum | 764 | 1071 | 1835 |
A total of 1273 patients with Lynch syndrome including 555 males and 718 females were considered.
Spectrum of cancers diagnosed during follow-up
During follow-up 318 (25.7%) patients developed a subsequent cancer. In a minority of cases (n=18; 6%) two synchronous cancers were diagnosed: one patient had both a duodenal cancer and two skin cancers, and one had one colon cancer and three skin cancers. Skin cancers (excluding malignant melanomas) are often not reported and figures for skin cancers in the current report should be interpreted with caution. ICD9 diagnosis and gender of the 341 subsequent cancers are detailed in table 3. CRCs (n=147; 43%), mainly colonic, were the most frequent cancers and were diagnosed in males and females at similar frequencies. Other frequent cancers included urinary tract (n=32), stomach (n=14), duodenum (n=9), pancreas (n=8), gall bladder (n=6) and brain tumours (n=7).
Table 3.
First subsequent cancers diagnosed
| ICD9 | Organ | Males | Females | All |
|---|---|---|---|---|
| 153 | Colon | 57 | 57 | 114 |
| 154 | Rectum/sigmoid | 20 | 13 | 33 |
| 182 | Endometrium | 30 | 30 | |
| 183 | Ovary | 7 | 7 | |
| 151 | Stomach | 9 | 5 | 14 |
| 152 | Duodenum | 5 | 4 | 9 |
| 156 | Gall bladder/bile duct | 4 | 2 | 6 |
| 157 | Pancreas | 4 | 4 | 8 |
| 188 | Urinary bladder | 5 | 9 | 14 |
| 189 | Kidney/ureter | 8 | 10 | 18 |
| 173 | Skin* | 13 | 14 | 27 |
| 174 | Breast | 15 | 15 | |
| 185 | Prostate | 20 | 20 | |
| 191 | Brain | 2 | 5 | 7 |
| 141 | Tongue | 1 | 1 | |
| 145 | Mouth | 1 | 1 | |
| 155 | Liver | 1 | 1 | 2 |
| 159 | Abdomen unspecified | 1 | 1 | |
| 162 | Lung | 3 | 1 | 4 |
| 164 | Mediastinum | 1 | 1 | |
| 170 | Osteosarcoma | 2 | 2 | |
| 172 | Melanoma | 1 | 1 | |
| 180 | Cervix | 1 | 1 | |
| 199 | Unknown origin | 2 | 2 | 4 |
| 202 | Lymphoma | 1 | 1 | |
| Sum | 155 | 186 | 341 |
A total of 1273 patients with Lynch syndrome including 555 males and 718 females were followed up.
*Includes both epithelial skin cancer that is often not reported, and sebaceous gland invasive cancer. This specific diagnosis may not have been uniformly reported from the different centres.
Prostate cancer (n=20, 4%) was the second most frequent cancer in males. In females, endometrial (n=30) and breast (n=15) cancers were also frequently detected. Of note, 336 of 718 (47%) females had endometrial and/or ovarian cancer before inclusion and an additional 59 (8%) had undergone hysterectomy and/or oophorectomy without having had endometrial or ovarian cancer. In addition, 20 (3%) had been treated for cervical cancer. Because of low numbers of gynaecological cancers, as well as possible selection artefacts due to previous hysterectomies, we estimated neither cumulative incidence nor survival of endometrial or ovarian cancers.
The mean age at diagnosis for the first subsequent cancer was 58.8 years (range 28–90 years). Notably, 48 of these subsequent cancers (15%) occurred after 70 years of age. Age at inclusion was similar for path_MLH1 and path_MSH2 carriers and similar for males and females. Because of the similar frequencies of CRC in males and females (table 3), both genders were considered as one group for calculation of cumulative CRC incidences.
Cumulative incidences of subsequent cancers
AIR of cancer in 5-year cohorts from age 25–70 years were calculated for any cancer and for CRC (table 4). The 10 patients included aged 20–24 years had no subsequent cancers before 25 years of age. AIRs at >70 years were not calculated since patients aged >70 years might not have been subjected to systematic colonoscopic surveillance in all centres.
Table 4.
Calculated cumulative incidence from current age to 70 years, for any subsequent cancer or for colorectal cancer as a subsequent cancer at 70 years by patient's current age and genetic variant, and comparison with corresponding incidences previously reported in patients who had not had cancer before inclusion
| Cumulative incidence for any subsequent cancer at 70 years (95% CI) | Cumulative incidence for colorectal as subsequent cancer at 70 years (95% CI) | |||||
|---|---|---|---|---|---|---|
| Current age (years) | path_MLH1 | path_MSH2 | path_MSH6 | path_MLH1 | path_MSH2 | path_MSH6 |
| Patients with LS having had previous cancer(s) (this report) | ||||||
| 40 | 73% (66.9% to 79.2%) | 76% (68.8% to 82.6%) | 50% (33.8% to 66.5%) | 46% (37.7% to 54.4%) | 48% (38.1% to 58.1%) | 23% (7.4% to 37.9%) |
| 50 | 65% (57.9% to 72.1%) | 63% (54.1% to 71.8%) | 47% (30.6% to 62.5%) | 38% (29.2% to 45.9%) | 35% (24.5% to 44.9%) | 23% (7.4% to 37.9%) |
| 60 | 47% (38.2% to 56.0%) | 42% (30.8% to 52.8%) | 31% (15.4% to 47.0%) | 24% (15.8% to 32.2%) | 18% (8.2% to 27.3%) | 13% (0.7% to 25.1%) |
| Patients with LS without previous cancer* | ||||||
| 40 | 66% (57.8% to 74.2%) | 67% (55.8% to 77.8%) | 53% (38.6% to 68.6%) | 37% (30.1% to 49.4%) | 28% (15.2% to 41.2%) | 20% (4.4% to 35.4%) |
| 50 | 53% (41.9% to 63.6%) | 55% (40.4% to 69.0%) | 43% (26.4% to 60.2%) | 25% (18.0% to 39.6%) | 21% (6.9% to 34.1%) | 18% (2.8% to 33.8%) |
| 60 | 32% (17.7% to 45.4%) | 33% (14.4% to 51.5%) | 24% (5.6% to 42.2%) | 13% (4.5% to 27.1%) | 14% (0.6% to 27.6%) | 11% (2.8% to 33.8%) |
| Relative cumulative incidence† patients with LS having had previous cancer(s) | ||||||
| 40 | 1.11 (0.96 to 1.29) | 1.13 (0.95 to 1.38) | 0.94 (0.60 to 1.44) | 1.26 (0.93 to 1.77) | 1.70‡ (1.09 to 3.24) | 1.14 (0.33 to 5.47) |
| 50 | 1.23 (0.99 to 1.59) | 1.15 (0.87 to 1.60) | 1.09 (0.63 to 1.91) | 1.50 (0.98 to 2.65) | 1.69 (0.91 to 5.16) | 1.23 (0.35 to 8.40) |
| 60 | 1.49 (0.98 to 2.71) | 1.27 (0.74 to 2.97) | 1.29 (0.53 to 5.76) | 1.80 (0.88 to 8.62) | 1.26 (0.46 to 30.82) | 1.17 (0.06 to ∞) |
*Calculated from online table in Møller et al 2015.3
†Cumulative incidence in this report divided by cumulative incidence in patients with LS without previous cancer.3
‡None of the cumulative incidences compared besides this one were significantly different (p<0.05).
LS, Lynch syndrome.
In patients who had cancer before the age of 40, the cumulative incidences for any further cancer by 70 years of age were high, being 73% for path_MLH1, 76% for path_MSH2 and 52% for path_MSH6 carriers (table 4). The cumulative incidences for CRC as a next cancer were 46% for path_MLH1, 48% for path_MSH2 and lower 23% (p<0.05) for path_MSH6 carriers. Most of these incidences were higher than we have previously reported in patients with LS without cancer before inclusion, but not significantly so (table 4). Patient numbers did not allow for reliable calculations of cumulative incidence of subsequent cancers for path_PMS2 carriers. The limited numbers of late-occurring LS-associated cancers in the upper GI tract, urinary tract and brain precluded calculation of cumulative incidences of extra-CRCs stratified by genetic variants.
Next, we considered whether or not a previous history of colon cancer increased the incidence of CRC cancer in patients with LS. The cumulative incidences from 40 to 70 years were 36% in those having been diagnosed previously with colon cancer and 39% in those with non-CRCs only before inclusion (table 5).
Table 5.
Calculated cumulative incidences for colorectal cancer from current ages as indicated in left column to 70 years of age for patients having had colon cancer before inclusion and for patients not having had colon cancer before inclusion
| Calculated cumulative incidences of cancers from current age (in left column) to 70 years |
|||
|---|---|---|---|
| Current age (years) | Colon cancer prior to inclusion (95% CI) | Extracolonic cancers prior to inclusion (95% CI) | Relative cumulative incidence* |
| 40 | 36% (29.0% to 43.8%) | 39% (28.5% to 50.2%) | 0.92 (0.61% to 1.32%) |
| 50 | 31% (24.3% to 38.9%) | 25% (16.7% to 35.3%) | 1.24 (0.78% to 1.90%) |
| 60 | 19% (13.1% to 27.0%) | 15% (6.6% to 23.0%) | 1.26 (0.70% to 3.06%) |
(95% CI) for all patients irrespective of genetic variant.
*Cumulative incidence patients with colon cancer prior to inclusion divided by cumulative incidence in patients with Lynch syndrome with previous extracolonic cancers only; none of the differences between the cumulative incidences that we compared reached statistical significance (p>0.05).
Based on the AIRs in online supplementary table S1 and the algorithms described above, we included the cumulative future risk for any subsequent cancer or for CRC as a subsequent cancer for any age up to 70 years as a separate tab in the website available at http://lscarisk.org.
Time since last colonoscopy to CRC
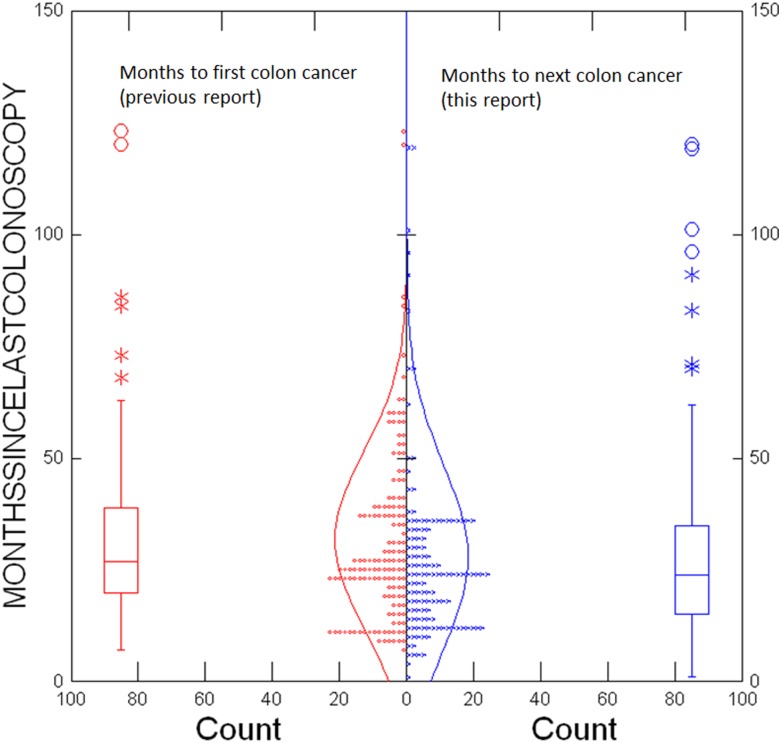
Time since last colonoscopy to CRC was available for 133 (94%) of the 141 patients in whom the first subsequent cancer was a CRC (table 6). Sixty (46%) of CRCs were diagnosed within 2 years, and 102 (78%) within 3 years of the last colonoscopy. The time distribution of CRC diagnoses did not differ significantly from that observed in patients with LS without previous cancer(s) (p=0.10; figure 1). We did note, however, that patients found to have subsequent CRC in this report had been colonoscopied according to protocol (with a 1-year, 2-year or 3-year interval) more consistently than were patients prior to their first CRC as reported in our former report.3 Only 9% of the CRCs were diagnosed 3.5 years or more since last colonoscopy.
Table 6.
Months elapsed between last complete colonoscopy and diagnosis of CRC
| Months since last colonoscopy | Number CRC | Cumulative number CRC | Cumulative (%) |
|---|---|---|---|
| <6 | 1 | 1 | 1 |
| 6–11 | 12 | 13 | 10 |
| 12–17 | 28 | 41 | 31 |
| 18–23 | 19 | 60 | 45 |
| 24–29 | 29 | 89 | 67 |
| 30–35 | 13 | 102 | 77 |
| 36–42 | 16 | 118 | 89 |
| 43–47 | 3 | 121 | 91 |
| 48–120 | 12 | 133 | 100 |
CRC, colorectal cancer.
Figure 1.
Result of two-sample t-test of months since last colonoscopy to colorectal cancer in previous report3 and in this report: p=0.10.
Survival
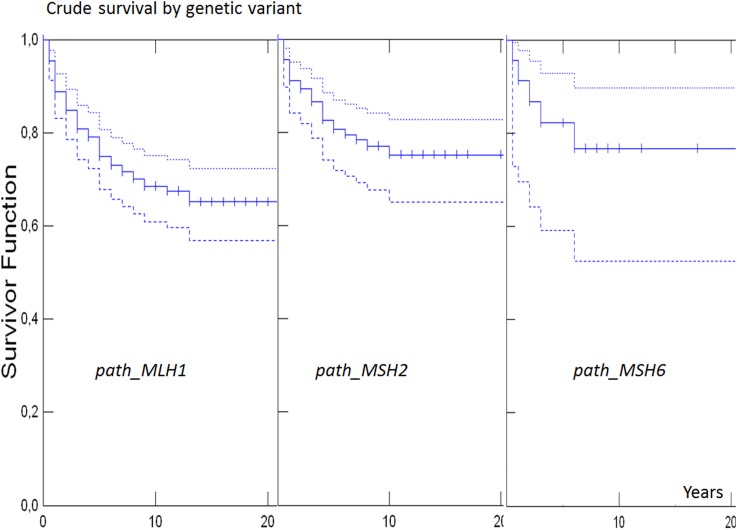
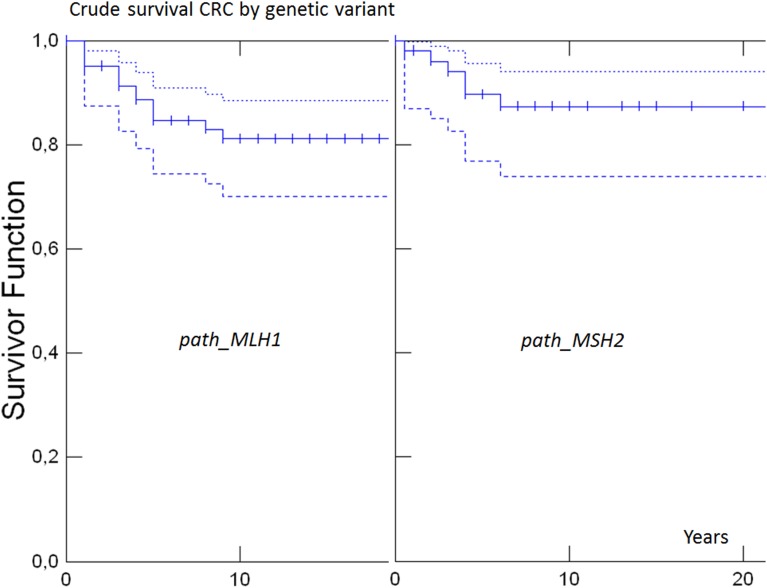
Without having access to causes of death, we could only calculate crude survival. Since crude survival is more meaningful in young patients, we restricted survival analysis to patients who had a subsequent cancer before 65 years of age. Crude 10-year survival in the whole cohort after any subsequent cancer was 82% (95% CI 76 to 87). In path_MLHI, path_MSH2 or path_MSH6 carriers, crude 10-year survival was 80% (95% CI 71% to 87%), 84% (95% CI 73% to 90%) and 84% (95% CI 51% to 96%), respectively, with no difference between genetic variants (p=0.63; figure 2). Crude 10-year survival in the whole series after CRC was 91% (95% CI 83% to 95%), and in path_MLHI, path_MSH2 or path_MSH6 carriers 89% (95% CI 76% to 95%), 92% (95% CI 77% to 97%) and 100%, respectively, and with no significant difference between the pathogenic variants of the different genes (p=0.44; figure 3).
Figure 2.
Survival after subsequent cancer by genetic variants (with 95% CIs as dotted line in same colour) when cancer diagnosed <65 years of age. Dotted lines indicate upper 95% CIs and broken lines indicate lower 95% intervals. There was no death observed in path_PMS2 carriers.
Figure 3.
Survival after subsequent colorectal cancer by genetic variant (with 95% CIs as dotted line in same colour) when cancer diagnosed <65 years of age. Dotted lines indicate upper 95% CIs and broken lines indicate lower 95% intervals. There was no death observed in path_MSH6 carriers.
Discussion
Our study addressed three critical clinical questions in a large cohort of LS carriers of pathogenic variants of the MMR genes who had a history of previous cancer: (i) what is the cumulative incidence of subsequent cancers, (ii) in which organs will these subsequent cancers occur; and (iii) what is the survival following a subsequent cancer? Family G reported by Warthin in 1913 (later demonstrated to carry a path_MSH2 variant) illustrates the dismal outcome of LS prior to its formal identification: 10 out of the 12 females with cancer had ‘cancer uterus’ and none of them lived to develop a subsequent cancer.5 There is now a need for better information on what is happening to the growing number of cancer survivors in order to further individualise their continued healthcare. Such data are in principle not obtainable from retrospective studies, because many patients in former generations died from their first cancers and because of the ascertainment biases inherent in retrospective studies. This study is the first to present prospective empirical observations from multiple centres and including sufficient numbers to meet these needs.
Both CRCs and extra-CRCs continued to occur, and with a similar or moderately higher incidence compared with patients with LS who had not had cancer before inclusion (our previous report, 3). The point estimates that showed a modest increase in the incidence of cancer in LS cancer survivors compared with asymptomatic carriers need independent validation. If confirmed, a number of possible causes could be considered, among which are the impact of distinct penetrance patterns of different pathogenic variants of the same gene as well as the role of genetic and/or environmental modifiers.6
Patients with previous colon cancer(s) were not at an increased risk for a subsequent colon cancer when compared with those with previous extracolonic cancers. Treatment of the first colon cancer is an obvious confounder in this observation: patients with LS with a first colon cancer treated with more extensive colonic resection reportedly have a lower risk of metachronous CRC than those receiving less extensive surgery.7 8 The current results indicate that we have enough cases of CRCs subsequent to a previous colon cancer to plan a study on examining relationship between treatment of previous colon cancer and incidence of subsequent CRC, and we will expand the database to do so. Also, for the current study we did not have information on adenoma identification and removal at surveillance colonoscopy that would enable us to consider the relation between adenomas detected/removed and subsequent CRC. The database is currently being expanded to include information on adenomas to this end, and the results will be reported separately.
In line with our observations in patients without a previous history of cancer, non-compliance with surveillance could not account for the majority of CRCs (table 6 and figure 1).
A more detailed description of the relationships between time since last colonoscopy, stage of CRC at diagnosis and survival is of interest and we are in process of carrying out a further study to address these questions.
Of note, the incidence of CRC was similar in females and males, probably reflecting a reduced number of endometrial cancer because of the high proportion of females who had hysterectomy for previous endometrial cancer. In female patients with LS without previous cancer(s), endometrial cancer may be the first cancer and mask the high incidence of colon cancer. As mentioned above, in retrospective studies of former generations (family history/segregation analysis) many patients died from their first cancer, some females might have died from a first endometrial cancer, the number of CRCs in females might have been lower than in males, and this may have been reported as lower incidence of CRC in females. Regarding other extracolonic cancers, cancers of the urinary tract, upper GI tract including pancreas and bile duct, and brain were more frequent in the present cohort than in MMR mutation carriers with no previous history of cancer.3 This higher incidence may be associated with their older age.
Survival was excellent for subsequent CRC (figure 3), but slightly worse for any subsequent cancer (figure 2), suggesting a worse survival for some extracolonic cancers. Lifetime cumulative incidence of specific cancers and their associated survival will need a different set of material and methods, and the database is currently being expanded to report on this separately and at a later time. Furthermore, the good survival of all prospectively detected cancers in our cohort supports continued medical follow-up and promotion of cancer awareness, which may contribute to favourable outcomes in this high-risk group through early detection and treatment of cancers. In principle, we should have had a control group without any intervention to conclude that the favourable survival observed was associated with our interventions. How to construct such a control group, if possible, is a challenge for future research.
The present study design has several strengths. It is the first prospective study with power to give reliable estimates on risk for subsequent cancers in patients with LS and for survival when it occurs. The methods used adjust for unequal distributions of ages and are suitable to demonstrate variations related to age between and within the groups examined. The results reported here provide a solid basis for statistical power calculations that will inform the feasibility of relevant studies in this field. The observations analysed and discussed in this report are summarised in online supplementary table S1 and the corresponding observations in our former report on patients with LS without cancer prior to inclusion are given in online supplementary table S1 in our former report.3
Some limitations are also apparent, and the results presented must be interpreted accordingly. Detailed information on the management of first cancers or precancers that might have modified the incidences reported, as well as the number of adenomas removed at colonoscopy and their histopathology, are of interest and will be addressed in further studies.
Conclusions
We have previously reported that most patients with LS survive their first cancer(s).3 Here we report a high incidence of subsequent cancers, and that most patients survive their subsequent cancer(s) as well. While the implementation of successful follow-up strategies has produced a large and growing cohort of LS survivors, we still have limited information on what will happen next to these patients. We will expand our database to provide more information on what these patients have experienced so far, and we will continue our prospective observations. We welcome other centres to join us so that we can identify sufficient numbers of patients to address more questions of importance to patients and healthcare professionals.
The results reported here are empirical observations and may be used for genetic counselling based on the premises given. We have established an open access interactive website http://lscarisk.org that may now be helpful for patients with LS both without and with previous cancers. It includes our prospective observations and the algorithms described to calculate specific cancer risk estimates for any patient with LS. To date, this is the most comprehensive tool to assess age-related, gene-related and gender-related cancer risk in patients with LS.
For further information on the collaborating activities please visit http://insight-group.org/ and http://mallorca-group.org/. To tailor cancer risk prediction according a given patient's age, gender, MMR gene variant and history of previous cancer, visit http://lscarisk.org.
Footnotes
Twitter: Follow Toni Seppälä at @Adductor and Ignacio Blanco at @consejogenetico
Contributors: PM: Designed the study, managed the database and computed the results. PM, JRS and GC wrote the manuscript. EH, SN, EAR and KT calculated the CIs to the cumulative incidences. EH and SN constructed the website calculating individual risks. All: Participated in study design, interpreted the results, commenting the manuscript and approved the final manuscript.
Funding: The Finnish contribution was supported by The Finnish Cancer Foundation, The Sigrid Juselius Foundation, Mary and Georg Ehrnrooth foundation and State Research Funding. D Gareth Evans is an NIHR senior investigator. Mark Jenkins has a Fellowship from the National Health and Medical Research Council of Australia. The Spanish contribution has been funded by the Spanish Ministry of Economy and Competitiveness SAF2012-33636, SAF2015-68016-R (GC, MP); the Carlos III Health Institute; RTICC (RD12/0036/0031); the Scientific Foundation Asociación Española Contra el Cáncer; and the Government of Catalonia (2014 SGR 338) and the Welsh Contribution by the Wales Gene Park. The Swedish contribution was supported by the Swedish Cancer Society, the Swedish Research Council and the Stockholm Cancer Society.
Competing interests: JB has a patent for high-speed low-cost tumour profiling pending to JB and QuantuMDx.
Ethics approval: Each reporting centre local approval as mentioned in text.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812–23. 10.1136/gutjnl-2012-304356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol 2014;109:1159–79. 10.1038/ajg.2014.186 [DOI] [PubMed] [Google Scholar]
- 3.Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2015. 10.1136/gutjnl-2015-309675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pylvänäinen K, Lehtinen T, Kellokumpu I, et al. Causes of death of mutation carriers in Finnish Lynch syndrome families. Fam Cancer 2012;11:467–71. 10.1007/s10689-012-9537-3 [DOI] [PubMed] [Google Scholar]
- 5.Warthin AS. Classicsin oncology: Heredity with reference to carcinoma as shown by the study of the cases examined in the pathological laboratory of the University of Michigan, 1895–1913. CA Cancer J Clin 1985;35:348–59. 10.3322/canjclin.35.6.348 [DOI] [PubMed] [Google Scholar]
- 6.Talseth-Palmer BA, Wijnen JT, Grice DM, et al. Genetic modifiers of cancer risk in Lynch syndrome: a review. Fam Cancer 2013;12:207–16. 10.1007/s10689-013-9614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst 2012;104:1363–72. 10.1093/jnci/djs351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60:950–7. 10.1136/gut.2010.228056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2016-311403supp001.pdf (361.1KB, pdf)
gutjnl-2016-311403supp002.pdf (213.5KB, pdf)