Abstract
Objective
This study aimed to examine the usefulness of each subscale score of the Clinical Dementia Rating (CDR) for predicting Alzheimer's disease (AD) dementia progression in amnestic mild cognitive impairment (MCI) elderly subjects.
Methods
Fifty-nine elderly MCI individuals were recruited from a university dementia and memory disorder clinic. Standardized clinical and neuropsychological tests were performed both at baseline and at the time of 2 years follow-up. Logistic regression analyses were conducted to examine the ability of various clinical measures or their combinations to predict progression to AD dementia in MCI individuals.
Results
MCIp individuals showed significantly higher CDR Orientation subscale and CDR sum-of-boxes (SOB) score than MCInp ones, while there were no significant differences in other CDR subscale scores between the two. MCIp individuals also showed marginally higher MMSE scores than MCInp ones. A series of logistic regression analyses demonstrated that the model including CDR Orientation subscale had better AD dementia prediction accuracy than either the model with either MMSE or CDR-SOB.
Conclusion
Our findings suggest that CDR Orientation subscale score, a simple and easily available clinical measure, could provide very useful information to predict AD dementia progression in amnestic MCI individuals in real clinical settings.
Keywords: Mild cognitive impairment; Alzheimer's disease; Clinical Dementia Rating,; Orientation; Prediction
INTRODUCTION
Mild cognitive impairment (MCI) has been defined as a transitional state between normal aging and dementia.1 Although MCI can present with a variety of symptoms, when memory loss is the predominant symptom it is termed “amnestic MCI” and is frequently seen as a prodromal stage of Alzheimer's disease (AD) dementia.2 The rate of AD dementia progression in amnestic MCI individuals is considerable according to estimates from various longitudinal studies: 12% per year,1 35% over 2 years,3 and 80% over 6 years.4 Due to its high risk for the progression to AD dementia,5 amnestic MCI has been becoming the focus of AD dementia prediction so as to start early optimal intervention.
However, amnestic MCI is a heterogeneous group. All amnestic MCI individuals do not progress to AD dementia, and substantial proportion remains stable or even reverts to cognitively normal state during longitudinal follow-up.6 Given this variability, it would be very useful to have reliable markers which can predict AD dementia progression among amnestic MCI individuals.7 There are many evidences supporting that various neuropsychological,8,9,10,11,12,13,14 neuroimaging,15,16,17,18,19 genetic 20,21,22 and biochemical markers23,24,25,26 or their combinations3,27 could predict the progression to AD dementia in MCI individuals. However, many of these markers have some limitations to be used in real clinical settings because they are complicated, expensive, invasive, or sometime unavailable.
For this practical reason, it is very important to find out a relatively simple and cost-effective predictor that is easily available in real clinical settings. A couple of studies suggested that Mini-Mental State Examination (MMSE),28 and Clinical Dementia Rating (CDR) Sum of Boxes (CDR-SOB)29 as such predictor for AD dementia progression.
The CDR30,31 is a representative clinical scale used to evaluate six domains of cognitive and functional performance applicable to AD and related dementias: Memory, Orientation, Judgment & Problem Solving, Community Affairs, Home & Hobbies, and Personal Care. The necessary information to make each rating is obtained through a semi-structured interview of the patient and a reliable informant or collateral source. It is frequently used to stage dementia severity and yields both CDR global score and CDR-SOB score in the clinical and research settings.31 The global CDR score is calculated via an algorithm with published scoring rules30 and the CDR-SOB score is obtained by summing each of the domain box scores.32 While the global CDR score is typically used for staging purposes with restricted ranges, CDR-SOB score not only provides more detailed information of dementia severity but also is regarded as good predictor for AD dementia progression in MCI individuals.29 Not all cognitive domains uniformly deteriorate during early AD process and the decline of non-memory areas and related functions follows episodic memory decline. Therefore, CDR subscale scores other than memory subscale score might be useful predictors for AD dementia progression in amnestic MCI individuals. However, little is known about this issue.
This study aimed to examine the usefulness of CDR subscale scores for predicting AD dementia progression within 2-year follow-up period in amnestic MCI elderly subjects, and to compare their predictive ability with those of other simple clinical cognitive or functional measures.
METHODS
Subjects
Fifty-nine elderly MCI individuals were recruited from a dementia and memory disorder clinic at Seoul National University Hospital. MCI was diagnosed accordingto current consensus criteria for amnestic MCI:33 1) memory complaint corroborated by an informant, 2) objective memory impairment for age, education and gender, 3) essentially preserved general cognitive function, 4) largely intact functional activities, 5) not demented. In terms of criterion 2), a performance score for at least one of the four episodic memory tests included in the Korean version ofthe Consortium to Establish a Registry for Alzheimer's disease (CERAD) neuropsychological battery [namely, Word List Memory (WLM), Word List Recall (WLR), Word List Recognition (WLRc) and Constructional Recall (CR) test] was 1.5 SD below the respective age-, education- and gender-specific normative mean.34,35 All MCI individuals had an overall CDR index30 of 0.5.
The following exclusion criteria were applied to all subjects: any evidence of present serious medical, psychiatric, or neurological disorders that might affectmental function; any evidence of focal brain lesions other than white matter changes on MRI; illiteracy; severe visual or hearing loss; no reliable informants. The Institutional Review Board of Seoul National University Hospital approved the study and written informed consent was obtained from each participant (IRB No. H-1506-131-683).
Baseline clinical and neuropsychological assessments
All subjects were examined by neuropsychiatrists with advanced training in dementia research and CDR assessment30 according to the protocol of the CERAD, and received MRI and laboratory tests. To acquire accurate information, a reliable informant was necessarily interviewed as well as the participant. A panel consisting four neuropsychiatrists made clinical decisions including the assignment of CDR. The CERAD neuropsychological battery,35,36 including Verbal fluency (VF), 15-item Boston naming test (BNT), MMSE, WLM, WLR, WLRc, Constructional Praxis (CP), and CR test, were also applied by experienced clinical psychologists who were blinded to the neuropsychiatrist's clinical assessment. In addition to the CERAD battery, the Neuropsychiatric Inventory (NPI)37 and a structured, anchored version of the 17-item Hamilton Rating Scale for Depression (HRSD)38 were administered to evaluate non-cognitive neuropsychiatric symptoms or depression.
Follow-up assessment
Each subject underwent subsequent clinical assessments by a neuropsychiatrist according to the CERAD protocol at 24-month intervals. After each follow-up evaluation, the consensus panel reviewed all the available clinical data obtained current follow-up evaluation and made a clinical diagnosis and assigned a CDR rating. Separately from the clinical assessment process, at each visit, the subjects were administered the same neuropsychological tests as in the baseline evaluation by clinical psychologists blinded to the neuropsychiatrist's clinical evaluation.
The subject's condition was considered to have progressed to AD dementia if he or she met the diagnostic and statistical manual of mental disorders (DSM-IV TR) criteria for dementia39 and the National Institute of Neurobiological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable or possible AD40 within 2 years.
Statistical analysis
The subjects were classified as 2 groups according to the clinical state at the time of 2 years follow-up evaluation: those who progressed to AD dementia (MCIp) and those who did not progress to AD dementia (MCInp). Between-group comparisons for baseline continuous data including demographic and clinical data were done by two-tailed t tests. Baseline categorical data were analyzed by the χ2 test.
Logistic regression analyses were conducted to examine the ability of various clinical measures or their combinations to predict progression to AD dementia in MCI individuals. We used the differences of -2 log likelihood (-2LL) to statistically compare the predictive ability of various models with different numbers of independent variables.41 The -2LL is aquantity generated by the logistic regression procedure and is directly proportional to the contribution of variables to the separation of groups. A smaller -2LL means abetter predictive ability of the model. The probability distribution of -2LL difference between simple (model 1) and more complex model (model 2) can be approximated by a chi-square distribution with (df2-df1) degrees of freedom, where df1 and df2 are the degrees of freedom of models 1 and 2 respectively. Therefore, the -2LL difference allows the direct comparison of prediction models of different complexities.42 The level of statistical significance was set as two-tailed p<0.05.
RESULTS
Presence of AD dementia progression within 2 years follow-up period
All subjects (n=59) were in amnestic MCI at baseline assessments. After the 2 years follow-up period, 22 (37.3%) progressed to AD dementia and 37 (62.7%) did not (Table 1). Among MCInp group, 32 were in amnestic MCI, 5 were in non-amnestic MCI, and 2 were in non-specific cognitive impairment (CDR=0.5, all Z-score ≥-1.5 in the CERAD neuropsychological battery).
Baseline characteristics of AD dementia progression and non-progression group
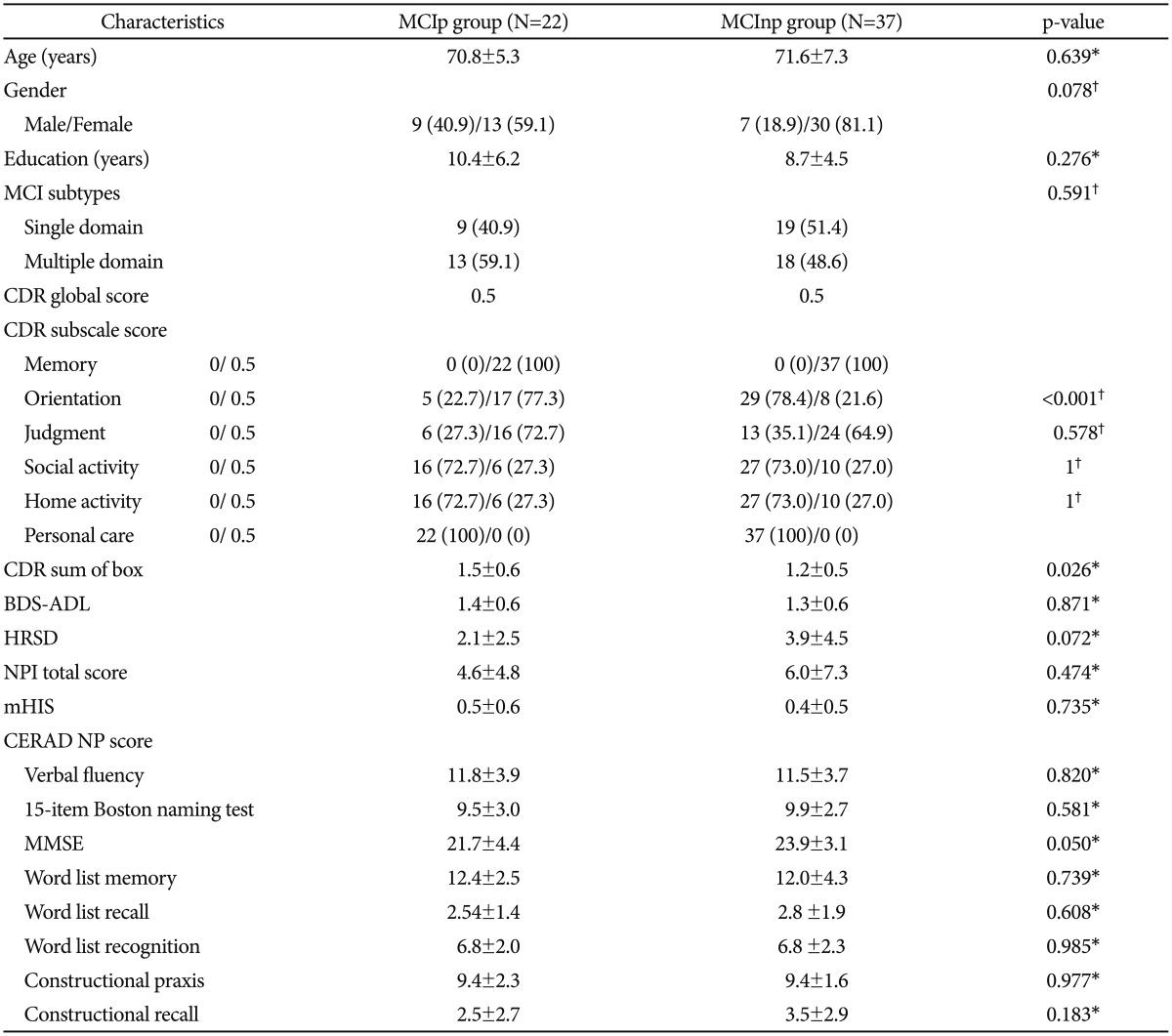
Baseline demographic and clinical characteristics of MCIpand MCInp individuals are shown in Table 1. There were no statistically significant differences between MCIp (n=22) and MCInp (n=37) in regard of age, gender, education, BDS-ADL, HRSD, NPI total score, and mHIS. MCIp had significantly greater mean CDR-SOB scores than MCInp. In terms of CDR subscale scores, MCIp individuals showed significantly higher CDR Orientation scores than MCInp ones, while there were no significant differences in other CDR subscale scores between the two. There were no significant differences in all CERAD neuropsychological scores between the two. MCIp showed marginally higher MMSE scores compared to MCInp.
Table 1. Baseline characteristics of MCI group that progressed to AD dementia (MCIp) and the group that did not progress to AD dementia (MCInp) at two-year follow-up (N=59).
Data are presented as mean±SD or number (%). *by Student t-test, †by χ2 test. MCI: mild cognitive impairment, AD: Alzheimer's disease, CDR: Clinical Dementia Rating, BDS-ADL: Blessed Dementia scale-Activities of Daily Living, HRSD: Hamilton Rating Scale for Depression, NPI: neuropsychiatric inventory, mHIS: modified Hachinski ischemic score, MMSE: Mini-Mental State Examination, CERAD: the Consortium to Establish a Registry for Alzheimer's Disease, NP: neuropsychological assessment battery
Comparison of prediction models for the progression to AD dementia
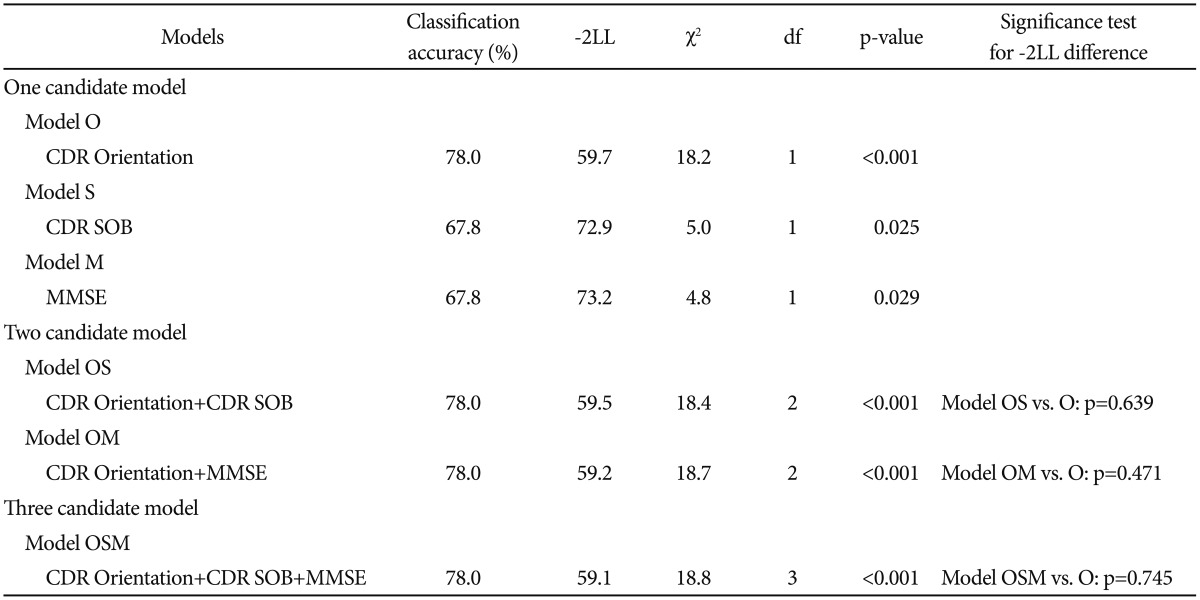
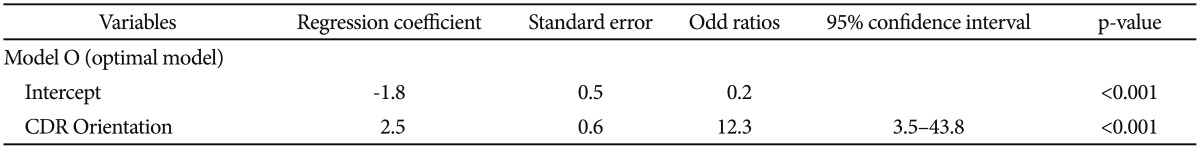
We first selected CDR Orientation score, CDR-SOB score, and MMSE score referring to the results from the comparison of baseline clinical characteristics between MCIp and MCInp as candidate-independent variables for logistic regression analyses for model comparison. A series of logistic regression analyses were conducted in three steps (Table 2). In the first step, we tested the following three “one candidate models”: “Model O including CDR Orientation”, “Model S including CDR-SOB”, and “Model M including MMSE”. All three models were statistically significant, and “Model O” had the highest classification accuracy and smallest -2LL among three “one candidate models”. In the second step, we compared the-2LL between each of the “one-candidate models” with the corresponding “two candidate models”, which included CDR Orientation and either CDR-SOB or MMSE. The AD dementia prediction accuracy of either “Model OM including CDR Orientation and MMSE” or “Model OS including CDR Orientation and CDR-SOB” was not significantly different from that of “Model O”, which had the highest classification accuracy and smallest -2LL among the three “one candidate models”. In the third step, the “three-candidate model” (Model OSM), which included all three variables, was compared with “Model O”. The “Model OSM” was not significantly different from “Model O”. Table 3 shows the finally selected logistic regression model (Model O) for AD dementia prediction in amnestic MCI at two-year follow-up.
Table 2. Logistic regression analyses designed to select appropriate models for AD dementia prediction in MCI.
AD: Alzheimer's disease, MCI: mild cognitive impairment, CDR: Clinical Dementia Rating, SOB: sum of box, MMSE: Mini-Mental State Examination, -2LL: -2 log likelihood
Table 3. Final logistic regression model* for AD dementia prediction in MCI.
*χ2 of the model=18.2, df=1, p<0.001, Nagelkerke R2=0.4. AD: Alzheimer's disease, MCI: mild cognitive impairment, CDR: Clinical Dementia Rating
DISCUSSION
We followed up amnestic MCI individuals for two years to examine the usefulness of a CDR subscale scores for predicting AD dementia progression, and to compare their predictive ability with those of other simple clinical measures including CDR-SOB and MMSE. In this study, 22 (37.3%) of 59 amnestic MCI individuals progressed to AD dementia within the follow-up period. This progression rate is in line with the recent results from the analysis of two-year follow-up data for Alzheimer's Disease Neuroimaging Initiative (ADNI) amnestic MCI subjects, which showed that AD dementia progression rate was 35%.3
Our results from logistic regression analyses of one-candidate model showed that both CDR-SOB and MMSE could predict AD dementia progression with statistical significance. These results were consistent with those of previous studies.28,29 However, CDR Orientation had higher AD dementia prediction accuracy (78.0%) than either CDR-SOB model (67.8%) or MMSE model (67.8%). AD dementia prediction of either two- or three-candidate model including CDR-Orientation was not significantly different from that of CDR Orientation alone.
The excellent predictive ability of CDR Orientation subscale for AD dementia progression in amnestic MCI may be explained by the fact that orientation for time and place is closely related to attention and visuospatial perception as well as memory. In early clinical process of AD, amnesia, especially episodic memory decline, was the earliest symptom and frequently accompanied or followed by in attention and visuospatial dysfunction.41,43 Episodic memory decline itself is not useful as a predictor for AD dementia because all amnestic MCI individuals already have poor episodic memory in common as reflected in the name of the category.44 In contrast, the additional information about inattention and visuospatial perceptual decline may have predictive value for the progression from premorbid condition to clinical AD dementia.45
The usefulness of CDR Orientation subscale as well as CDR-SOB seems in part related with the method of CDR assessment.30 The CDR assessment relies on not only brief cognitive evaluation, but also information obtained from the interview with collateral informant about cognitive and functional changes compared to previous usual level. Due to this assessment process, CDR scores are less influenced by various factors, such as age, education, depression, and practice effect, which could affect cognitive test scores.30
There is an important discussion point in our study. As well as its advantage, it was important to rate CDR score with high reliability in clinical settings. The CDR has been widely used as a criterion standard in multicenter clinical trials in AD36,46,47 and its inter-rater reliability has been well established.48,49,50 One study demonstrates that high inter-rater reliability on the CDR subscale ratings by trained clinical personnel with supervision from highly trained monitors, i.e., agreement with gold standard ranged from 73% to 87% or agreement among raters ranged from 73% to 87%.50 From these points of view, our CDR ratings were highly reliable because final assignment of the rating were given by the consensus panel including 2 psychiatrists who had finished CDR training course at Washington University Alzheimer Disease Research Center and had got the certification as a CDR rater.41
In conclusion, our findings suggest that CDR Orientation subscale score, a simple and easily available clinical measure, could provide very useful information to predict AD dementia progression in amnestic MCI individuals in real clinical settings.
Acknowledgments
This study was supported by a grant from the Ministry of Science, ICT, and Future Planning, Republic of Korea (Grant No. NRF-2014M3C7A1046042).
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE; Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 5.Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:603–611. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 6.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 7.Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res. 2006;3:161–170. doi: 10.2174/156720506776383103. [DOI] [PubMed] [Google Scholar]
- 8.Gainotti G, Quaranta D, Vita MG, Marra C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2014;38:481–495. doi: 10.3233/JAD-130881. [DOI] [PubMed] [Google Scholar]
- 9.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 10.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 11.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 12.Small BJ, Herlitz A, Fratiglioni L, Almkvist O, Backman L. Cognitive predictors of incident Alzheimer’s disease: a prospective longitudinal study. Neuropsychology. 1997;11:413–420. doi: 10.1037//0894-4105.11.3.413. [DOI] [PubMed] [Google Scholar]
- 13.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- 14.Esteban-Santillan C, Praditsuwan R, Ueda H, Geldmacher DS. Clock drawing test in very mild Alzheimer’s disease. J Am Geriatr Soc. 1998;46:1266–1269. doi: 10.1111/j.1532-5415.1998.tb04543.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Paajanen T, Zhang Y, Westman E, Wahlund LO, Simmons A, et al. Analysis of regional MRI volumes and thicknesses as predictors of conversion from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2010;31:1375–1385. doi: 10.1016/j.neurobiolaging.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Chetelat G, Desgranges B, de la, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease. Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 17.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hanninen T, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging. 2008;29:31–38. doi: 10.1016/j.neurobiolaging.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 21.Blom ES, Giedraitis V, Zetterberg H, Fukumoto H, Blennow K, Hyman BT, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27:458–464. doi: 10.1159/000216841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 23.Samtani MN, Raghavan N, Shi Y, Novak G, Farnum M, Lobanov V, et al. Disease progression model in subjects with mild cognitive impairment from the Alzheimer’s disease neuroimaging initiative: CSF biomarkers predict population subtypes. Br J Clin Pharmacol. 2013;75:146–161. doi: 10.1111/j.1365-2125.2012.04308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 26.Smach MA, Charfeddine B, Ben Othman L, Lammouchi T, Dridi H, Nafati S, et al. Evaluation of cerebrospinal fluid tau/beta-amyloid(42) ratio as diagnostic markers for Alzheimer disease. Eur Neurol. 2009;62:349–355. doi: 10.1159/000241881. [DOI] [PubMed] [Google Scholar]
- 27.Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging. 2011;32:2322.e19–2322.e27. doi: 10.1016/j.neurobiolaging.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakata E, Kasai M, Kasuya M, Akanuma K, Meguro M, Ishii H, et al. Combined memory and executive function tests can screen mild cognitive impairment and converters to dementia in a community: the Osaki-Tajiri project. Neuroepidemiology. 2009;33:103–110. doi: 10.1159/000222092. [DOI] [PubMed] [Google Scholar]
- 29.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 32.O’Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee DY, Lee KU, Lee JH, Kim KW, Jhoo JH, Kim SY, et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc. 2004;10:72–81. doi: 10.1017/S1355617704101094. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57:47–53. doi: 10.1093/geronb/57.1.p47. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 37.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Lee DY, Youn JC, Choo IH, Kim KW, Jhoo JH, Pak YS, et al. Combination of clinical and neuropsychologic information as a better predictor of the progression to Alzheimer disease in questionable dementia individuals. Am J Geriatr Psychiatry. 2006;14:130–138. doi: 10.1097/01.JGP.0000192487.58426.d2. [DOI] [PubMed] [Google Scholar]
- 42.Hosmer D. Applied Logistic Regression. Toronto: John Wiley & Sons; 1989. [Google Scholar]
- 43.Hodges J. The amnestic prodrome of Alzheimer’s disease. Brain. 1998;121:1601–1602. doi: 10.1093/brain/121.9.1601. [DOI] [PubMed] [Google Scholar]
- 44.Tounsi H, Deweer B, Ergis AM, Van der Linden M, Pillon B, Michon A, et al. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:38–46. doi: 10.1097/00002093-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 47.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 48.Burke WJ, Miller JP, Rubin EH, Morris JC, Coben LA, Duchek J, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45:31–32. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- 49.McCulla MM, Coats M, Van Fleet N, Duchek J, Grant E, Morris JC. Reliability of clinical nurse specialists in the staging of dementia. Arch Neurol. 1989;46:1210–1211. doi: 10.1001/archneur.1989.00520470070029. [DOI] [PubMed] [Google Scholar]
- 50.Schafer KA, Tractenberg RE, Sano M, Mackell JA, Thomas RG, Gamst A, et al. Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis Assoc Disord. 2004;18:219–222. [PMC free article] [PubMed] [Google Scholar]