Abstract
Dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis has been suggested as a potential mechanism linking sleep and cardiometabolic disorders. However, the associations of two primary outputs of the HPA axis, cortisol and its antagonist dehydroepiandrosterone (DHEA), with sleep are less well studied. In the Nurses’ Health Study II, 233 postmenopausal women provided five timed saliva samples over one day (immediately upon waking, 45 minutes, 4 hours, and 10 hours after waking, and prior to going to sleep) to measure cortisol and DHEA. Of these, 209 completed assessment of their habitual sleep patterns using the Pittsburgh Sleep Quality Index (PSQI). We used piecewise linear mixed models to compare cross-sectional associations of slopes reflecting diurnal cortisol and DHEA rhythms with overall sleep quality and with seven sub-components. Overall, we observed no differences in the diurnal patterns of cortisol or DHEA between good versus poor sleepers as assessed by the global PSQI score. However, longer sleep latency was associated with significantly reduced cortisol awakening rise (p=0.02). Poorer subjective sleep quality (p=0.02), shorter sleep duration (p=0.02), and lower sleep efficiency (p=0.03) were associated with slower rate of cortisol decline later in the day. Women reporting daytime dysfunction had a sharper cortisol decline early in the day (p=0.03) but a flattened decline later in the day (p=0.01). The differences in diurnal patterns of DHEA between good versus poor sleepers, though less pronounced, were similar in direction to those of cortisol. Self-reported sleep duration, efficiency, latency and daytime dysfunction were associated with altered diurnal rhythms of cortisol and, to a lesser extent, DHEA. These findings provide support for the interplay between sleep and the HPA axis that may contribute to cardiometabolic disease.
Keywords: Sleep, Diurnal rhythms, Cortisol, DHEA
1. Introduction
Epidemiologic data provide strong evidence linking sleep quantity and quality with cardiometabolic outcomes, such as cardiovascular disease and diabetes (Cappuccio et al., 2011; Cappuccio et al., 2010). However, the biological alterations by which disrupted sleep leads to these adverse health outcomes are poorly understood. Sleep plays an essential role in modulating diurnal cycles of various human physiological and behavioral processes, including functioning of the hypothalamus-pituitary-adrenal (HPA) axis (Kalsbeek et al., 2012). It has been suggested that dysregulation of the HPA axis and its basal diurnal rhythms may be an important pathway through which sleep disturbances influence cardiometabolic risk.
The diurnal rhythmicity of the HPA axis is apparent in variations of cortisol secretion across the day, which are thought to optimize glucose utilization and energy metabolism. There is a marked, continued increase in the cortisol level prior to and immediately after awakening, with the peak reached 30–45 minutes after awakening (Edwards et al., 2001; Pruessner et al., 1997; Wust et al., 2000). After the awakening rise, cortisol secretion starts to decrease. Despite fluctuations in the release of cortisol due to varying environmental stimuli (e.g., exercise), basal cortisol secretion continues to decline over the day and such decline tends to get slower later in the day (Edwards et al., 2001; Wust et al., 2000). Prior work has identified specific alterations in cortisol rhythms (e.g., reduced awakening rise, flattened decline) as being unhealthy, given their associations with obesity, diabetes, coronary heart disease, and mortality (Champaneri et al., 2013; Hackett et al., 2014; Kumari et al., 2011; Smith et al., 2005). Dehydroepiandrosterone (DHEA), a precursor steroid for testosterone, provides resilience to the stress response by antagonizing the effect of cortisol (Charney, 2004). DHEA exhibits diurnal rhythms that parallel cortisol (Hucklebridge et al., 2005), and has previously been inversely associated with risk of cardiovascular disease and mortality in some, though not all, studies (Barrett-Connor et al., 1986; Tchernof and Labrie, 2004). The ratio of cortisol to DHEA has been used to indicate the biologic activity of the HPA axis, with higher ratios suggesting greater dysregulation of HPA axis (Phillips et al., 2010; Young et al., 2002).
Existing evidence is mixed regarding the associations between sleep and diurnal cortisol rhythms. Some studies have reported that poor sleep quality is associated with blunted cortisol awakening rise and slower cortisol decline over the day, yielding increased total diurnal cortisol output (Castro-Diehl et al., 2015; Kumari et al., 2009b; Leproult et al., 1997); others have reported no association (Rao et al., 2013; Zhang et al., 2011). However, most studies have focused on a limited characterization of sleep quality, considering either sleep duration or sleep efficiency. No study has evaluated DHEA or cortisol-DHEA ratio. Further, few studies considered these associations specific to women, who may be more vulnerable to sleep disturbances and associated consequences (Cappuccio et al., 2007; Santhi et al., 2016). Therefore, we investigated the associations of self-reported sleep quality, overall and by its sub-components (e.g., sleep latency, duration, disturbances, etc.), with diurnal rhythms of saliva cortisol, DHEA and their ratio in the Nurses’ Health Study II (NHSII).
2. Methods
2.1. Study population
NHSII is an ongoing, longitudinal study of US female registered nurses established in 1989. At baseline, 116,429 women, ages 25–42, returned a questionnaire regarding their health and lifestyle. Biennial follow-up questionnaires were mailed to all participants to update their information on disease diagnoses and exposures. We conducted a substudy with additional data collection to address research questions not covered by the main questionnaire. The study protocols for NHSII and nested substudy were approved by the institutional review board of the Brigham and Women’s Hospital.
In April 2013, 688 postmenopausal NHSII participants who had previously given blood were invited to participate in the Mind-Body Study (MBS), which aimed to characterize psychosocial stress and explore associated biological alterations using biomarkers. Briefly, women who reported childhood abuse were oversampled to increase the likelihood of experiencing chronic stress. Participants who expressed interest were mailed a consent form, discussing the extensive data collection at multiple time points over a 1-year period through questionnaires and biospecimens (including blood, urine, timed saliva, toenails, hair, stool, and oral microbiome sample). All collections were repeated once 6–12 months apart to assess within-person reproducibility. We used data from the first collection wave, in which 233 women returned the sample kits (including timed saliva collection over 1 day) and completed an online questionnaire on psychosocial stress (including habitual sleep quality). Here, we used data from 209 women who had no missing responses to sleep quality assessment and provided ≥4 timed saliva samples.
2.2. Collection and assay of timed saliva samples
MBS participants were mailed a kit containing equipment and instructions for collecting five timed saliva samples during one day: immediately upon waking (before getting out of bed), 45 minutes after waking, 4 hours after waking, 10 hours after waking, and just prior to bed. Participants filled out a log to record collection times and indicate whether they ate, drank, brushed the teeth or exercised and whether they felt excited or anxious near the time of each saliva collection (Supplemental Table 1) (Stalder et al., 2016). All samples were refrigerated overnight until shipping by overnight mail the next day with a cold pack to the laboratory. Assays were conducted in the Laboratory for Biological Health Psychology (Dr. Nicolas Rohleder) at Brandeis University using a competitive chemiluminescence immunoassay (CLIA-approved) with high sensitivity. Samples were measured in duplicate, and were repeated if CVs were >10%. The CVs of blinded QC pools were <6%. Cortisol is stable in samples with delayed freezing of up to 4 days (Inder et al., 2012).
2.3. Sleep Assessment
Within one month after saliva collection, MBS women were sent an link for an online, comprehensive assessment of their sleep patterns using the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). PSQI is a 19-item self-rated scale summarizing 7 components of sleep quality and patterns during the past month: subjective sleep quality; sleep latency; sleep duration; habitual sleep efficiency; sleep disturbances; use of sleep medication; and daytime dysfunction. Each component is equally weighted on a 0–3 scale, and the global PSQI score is scaled from 0 to 21, with higher scores indicating worse sleep quality. A global PSQI score >5 has been shown to have excellent sensitivity (0.896) and specificity (0.865) to indicate clinically relevant sleep disturbances as assessed using a combination of structured interviews, sleep logs and polysomnographic data; a sleep component score >1 suggests a moderate to severe difficulty in that area (Buysse et al., 1989). The psychometric properties of PSQI have been extensively validated in different populations (Mollayeva et al., 2016). Among MBS participants who completed 1-year follow-up psychosocial assessment, the intraclass correlation coefficient for PSQI was 0.64, consistent with prior evidence that sleep patterns are stable over time (Knutson et al., 2006).
2.4. Assessment of covariates
Birth date and height were assessed on 1989 baseline questionnaire. Information on weight, night shiftwork, smoking and diseases (hypertension, diabetes, sleep apnea) was used from the questionnaire in 2013. Alcohol and caffeine were assessed using validated semi-quantitative food frequency questionnaire (Willett et al., 1985); we used information collected in 2011. At the time of saliva collection, women completed a questionnaire regarding medication use in the past month, including antidepressants, beta-blockers, minor tranquilizers, oral steroids, and DHEA medication. At the time of sleep assessment, women were also evaluated for depressive symptoms using the short-form Center for Epidemiological Study–Depression (CES-D) scale (Irwin et al., 1999) and for anxiety symptoms using the Generalized Anxiety Disorder–7 items (GAD-7) scale (Spitzer et al., 2006).
2.5. Statistical analyses
Saliva collection time was centered at awakening; all subsequent measures were considered with respect to time since awakening. Both cortisol and DHEA were log-transformed due to their skewed distributions. To determine the optimal time points at which inflections in diurnal rhythms occurred, we fit mixed models with piecewise cubic spline functions with 3 knots chosen at the designated collection times (i.e., 45 min, 4 hours, and 10 hours after awakening), accounting for within-individual correlations and potential nonlinearity. Based on the exploratory curve (Figure 1), we removed the knot at 10 hours since awakening and added an additional knot at 16 hours to reflect the corresponding rhythm patterns (please see Supplemental Table 2 for additional information), including awakening response (i.e., change from awakening to 45 min after awakening), early decline (i.e., change from 45 min to 4 hours after awakening), late decline (i.e., change from 4 hours to 16 hours after awakening), and late night rise (i.e., change from 16 hours after awakening to bedtime). To quantify differences in diurnal cortisol/DHEA rhythms by habitual sleep quality (global PSQI >5 versus ≤5), we used mixed regression with piecewise linear splines and included cross-product interaction terms between the sleep variable and each of the three spline functions. Based on the mixed model, we calculated percent differences by exponentiating the corresponding coefficient or the linear combination of coefficients and subtracting one for the following measures: waking level; awakening response; early decline; late decline; late night rise; overall change (i.e., change from awakening to bedtime). We also calculated differences in diurnal output by comparing AUC from awakening to 16 hours after awakening. In the multivariable model, we adjusted for a number of factors that may potentially influence cortisol/DHEA rhythms, including whether they ate, drank, brushed teeth, or exercised before collection, whether they felt happy or worried at collection, age, wakeup time, body mass index, depressive symptoms (CES-D), anxiety symptoms (GAD-7), duration of past shiftwork, medication use (antidepressants, beta-blockers, minor tranquilizers, oral steroids, and DHEA medication), co-morbidities (hypertension, diabetes, and sleep apnea), caffeine intake, alcohol drinking, and smoking. To further explore more extreme comparisons of habitual sleep quality (e.g., women with very poor sleep quality versus women with very good sleep quality), we also evaluated differences between top versus bottom quintiles of PSQI (>12 versus ≤3).
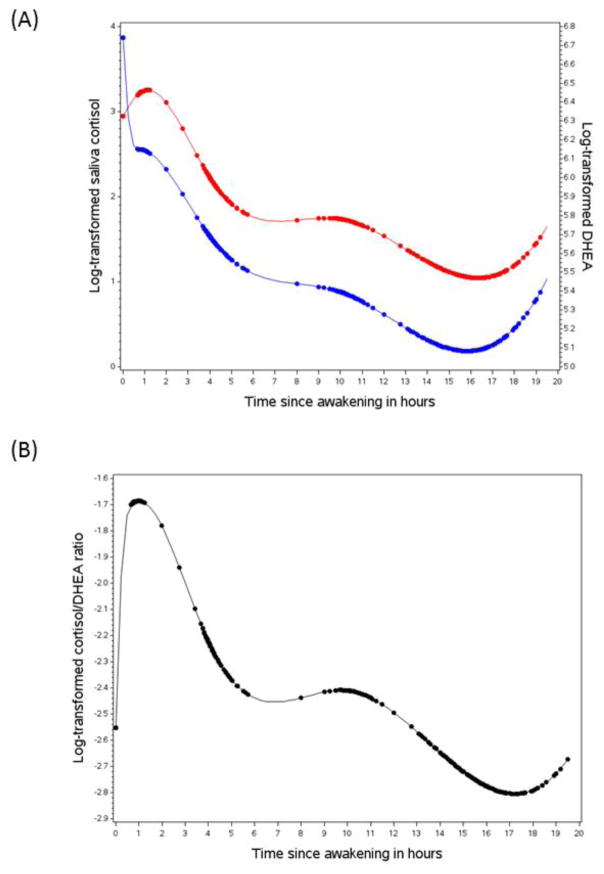
Figure 1.
Diurnal rhythms of the hypothalamus-pituitary-adrenal axis activity. (A) Saliva cortisol (red) and DHEA (blue). (B) Saliva cortisol to DHEA ratio. The rhythm curves were generated based on predictive values from mixed model with piecewise cubic splines of the time variable. Dots represent the predictive biomarker values at the actual saliva collection time since awakening.
Pearson correlations were used to assess the association of individual sleep components with overall sleep quality, and partial correlations were used to assess the relationships among individual components. We performed similar analyses to examine whether diurnal rhythms of cortisol and DHEA differed by individual sleep dimensions (severe/moderate versus mild/minimum [component score >1 versus ≤1]), with mutual adjustment of different sleep components to evaluate independent associations.
Since we observed an increase in cortisol and DHEA before bedtime, which was not reported in prior studies, we conducted a sensitivity analysis excluding women who went to bed at least 30 minutes later on the day of saliva collection (based on saliva collection log) than their usual bed time (based on PSQI questionnaire). We also repeated the analyses excluding women who reported use of oral steroids or DHEA medication to assess their potential influences on the results. All analyses were performed in SAS 9.4 for UNIX (SAS Institute).
3. Results
Among the 209 eligible participants, global sleep quality was 6.5 (SD: 3.3), with 125 (59.8%) classified as having poor habitual sleep quality (PSQI>5). Compared with good sleepers, poor sleepers had higher levels of depressive or anxiety symptoms (Table 1). Women with poor sleep quality tended to have higher BMI, consume more caffeine and have done more shiftwork in the past, and were more likely to have had a clinical diagnosis of hypertension, diabetes, sleep apnea or depression. Use of antidepressants, beta-blockers, minor tranquilizer and oral steroids was more prevalent in poor versus good sleepers. As expected by design, history of early life abuse was common in this sample of women, and the prevalence was even higher in women with poor sleep quality.
Table 1.
Age and age-adjusted sample characteristics of participants in the MBS by habitual sleep quality, as assessed in 2013.*
| Sleep quality assessed by PSQI | P | ||
|---|---|---|---|
| Good (PSQI≤5) | Poor (PSQI>5) | ||
| N | 84 | 125 | |
| Age, yrs | 61.2 (3.9) | 60.4 (4.0) | 0.15 |
| Body mass index, kg/m2 | 24.9 (4.1) | 27.2 (6.1) | 0.007 |
| Duration of past shiftwork, yrs | 2.7 (3.7) | 4.4 (4.9) | 0.001 |
| Shiftwork in the past 4 yrs, % | 2 | 10 | 0.009 |
| Hypertension, % | 29 | 36 | 0.24 |
| Type 2 diabetes, % | 3 | 8 | 0.27 |
| Sleep apnea, % | 3 | 6 | 0.37 |
| Abuse in childhood/adolescence, % | 25 | 35 | 0.13 |
| 10-item CES-D | 3.1 (2.8) | 7.5 (4.7) | <0.0001 |
| Elevated depressive symptoms, %1 | 3 | 29 | <0.0001 |
| GAD-7 | 1.2 (2.5) | 3.6 (3.7) | <0.0001 |
| Elevated anxiety symptoms, %2 | 5 | 31 | <0.0001 |
| Antidepressant use, % | 16 | 30 | 0.02 |
| Beta-blocker use, % | 10 | 13 | 0.65 |
| Minor tranquilizer use, % | 1 | 12 | 0.004 |
| DHEA medication, % | 3 | 2 | 0.69 |
| Oral steroid, % | 2 | 5 | 0.48 |
| Caffeine intake, mg/day | 172 (125) | 186 (157) | 0.44 |
| Alcohol drinking, g/day | 8.6 (12.5) | 7.9 (11.3) | 0.85 |
| Current smokers, % | 2 | 2 | 0.99 |
Abbreviations: PSQI, Pittsburg Sleep Quality Index; CES-D, Center for Epidemiologic Studies Depression Scale; GAD-7, Generalized Anxiety Disorder 7-item Scale
Values are means (SD) or percentages
Defined as CES-D≥10
Defined as GAD-7≥5
The majority of women followed the protocol to collect saliva sample at designated times, as indicated by the clustering of data points at awakening, and 45 minutes, 4 hours, and 10 hours after awakening (Figure 1). We observed an increase in the cortisol level after awakening (median awakening time: 06:35; range: 04:00, 10:15), which reached the peak within about 1 hour of awakening. During the first 4 hours after awakening, there was a sharp decrease in cortisol, and then the rate of decline was less rapid over the rest of the day until about 16 hours after awakening. From then, there was a cortisol rise until bedtime (median bedtime: 22:15; range: 20:10, 01:55). DHEA showed a parallel declining pattern throughout most of the day and an increase before bedtime; however, a marked decrease in DHEA was observed at awakening. As a result, compared to cortisol alone, the cortisol-DHEA ratio increased more sharply at awakening, and then progressively declined over the day until it began to increase again before bedtime.
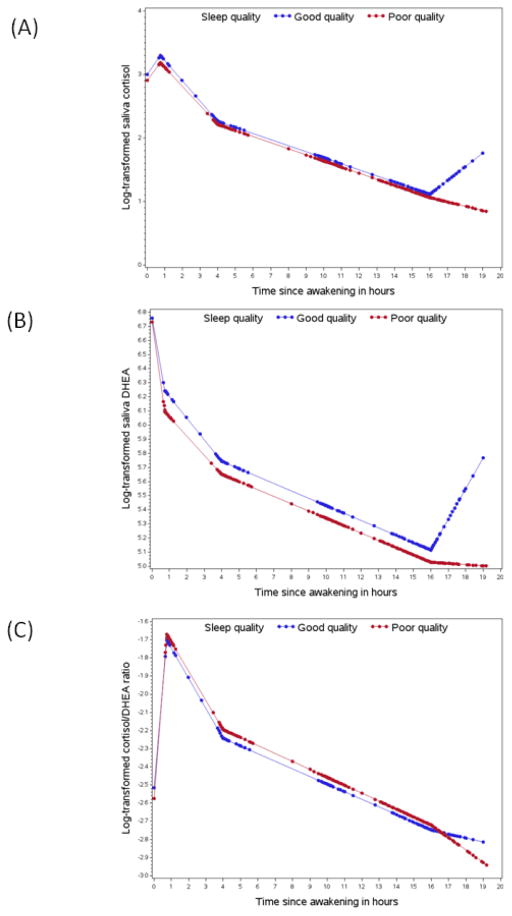
Overall, we observed no significant differences in the diurnal rhythms of cortisol, DHEA and their ratio between women with poor sleep quality (PSQI>5) versus those with good quality (PSQI≤5; Figure 2), except that the cortisol increase before bedtime was more pronounced for good versus poor sleepers. When comparing the top versus bottom quintile of the PSQI score (PSQI>12 versus PSQI≤3), we observed an even larger difference in bedtime cortisol rise. Adjustment for covariates did not change the results substantially (Table 2). The multivariable-adjusted percent differences in the slope of bedtime cortisol rise were −26.2% (95% CI: −43.0, −4.4; p=0.02) comparing PSQI>5 versus≤5 and −51.1% (95% CI: −72.4, −13.4; p=0.01) comparing PSQI>12 versus ≤3. Additionally, very poor sleepers (PSQI>12) had significantly lower DHEA levels at awakening (percent difference: −51.9; 95% CI: −75.5, −5.6; p=0.03) and slower late decline in cortisol (percent difference in slope: 6.3; 95% CI: 1.4, 11.5; p=0.01) compared with very good sleepers (PSQI≤3).
Figure 2.
Diurnal rhythms of the hypothalamus-pituitary-adrenal axis activity by habitual sleep quality. Good sleep quality was defined as PSQI≤5 (blue) and poor quality as PSQI>5 (red). (A) Saliva cortisol; (B) Saliva DHEA; (C) Saliva cortisol to DHEA ratio. The rhythm curves were generated based on predictive values from mixed model with piecewise linear terms of the time variable. Dots represent the predictive biomarker values at the actual saliva collection time since awakening
Table 2.
Percent differences (95% CIs) in diurnal rhythms (waking level, awakening response, early decline, late decline, night rise, overall slope and AUC) of cortisol, DHEA and their ratio according to overall sleep quality measured by Pittsburgh Sleep Quality Index
| Cortisol | DHEA | Ratio | |
|---|---|---|---|
| Percent differences (95% CI)1 | |||
| PSQI>5 versus PSQI≤5 | |||
| Waking level | −7.9 (−20.4, 6.6) | −5.2 (−27.5, 23.9) | −1.3 (−24.4, 28.8) |
| Awakening response2 | −1.8 (−23.6, 26.3) | −13.2 (−32.8, 12.0) | 11.6 (−13.5, 43.8) |
| Early decline2 | 1.7 (−3.9, 7.7) | 1.5 (−4.2, 7.6) | 0.3 (−5.3, 6.3) |
| Late decline2 | 0.1 (−1.9, 2.1) | −0.1 (−2.1, 2.0) | 0.1 (−1.9, 2.1) |
| Night rise2 | −26.2 (−43.0, −4.4) | −19.9 (−39.0, 5.1) | −7.2 (−28.6, 20.6) |
| Overall slope2 | −0.4 (−1.7, 0.9) | −0.7 (−2.1, 0.7) | 0.2 (−1.1, 1.5) |
| AUC | −0.7 (−2.6, 1.2) | −1.9 (−6.0, 2.2) | 1.3 (−3.0, 5.5) |
| Top versus bottom quintile of PSQI3 | |||
| Waking level | 8.8 (−25.1, 58.0) | −51.9 (−75.5, −5.6) | 99.2 (−0.7, 299.7) |
| Awakening response2 | −26.8 (−59.8, 33.3) | −8.4 (−50.3, 68.7) | −18.9 (−55.9, 49.0) |
| Early decline2 | −4.6 (−16.6, 9.2) | 0.7 (−12.3, 15.5) | −5.3 (−17.4, 8.6) |
| Late decline2 | 6.3 (1.4, 11.5) | 3.3 (−1.7, 8.6) | 3.2 (−1.6, 8.3) |
| Night rise2 | −51.1 (−72.4, −13.4) | −32.7 (−63.2, 23.0) | −32.8 (−62.4, 20.1) |
| Overall slope2 | 1.0 (−2.2, 4.2) | 1.5 (−1.9, 5.0) | −0.6 (−3.6, 2.6) |
| AUC | 0.5 (−4.4, 5.3) | −8.1 (−19.0, 2.7) | 8.4 (−2.6, 19.5) |
Adjusted for age, saliva sample collection characteristics, depression, anxiety, beta-blocker use, minor tranquilizer use, oral steroids use, DHEA medication, duration of shiftwork, BMI, hypertension, type 2 diabetes, sleep apnea, smoking, caffeine intake, and alcohol intake
Meaured by percent differences in the corresponding slope
The boundary for the top and bottom quintile was PSQI>12 and PSQI≤3, respectively. The corresponding sample size was 44 and 37, respectively.
The correlations of individual sleep quality components with overall PSQI score ranged from 0.45 (daytime dysfunction) to 0.72 (subjective sleep quality). Except for a moderate correlation between sleep duration and sleep efficiency (partial correlation=0.57), individual sleep components showed relatively weak interrelationships (partial correlation<0.30; Supplemental Table 3). When examining associations with individual sleep components (Table 3), shorter habitual sleep duration (component score >1 versus ≤1 corresponding to <6 versus ≥6 hours) was associated with a flattened cortisol decline later in the day (percent difference in slope: 3.1; 95% CI: 0.4, 5.8; p=0.02). Similar associations with late cortisol decline were observed for lower sleep efficiency defined as <75% versus ≥75% (percent difference in slope: 3.3; 95% CI: 0.4, 6.2; p=0.03) and poorer subjective sleep quality defined as fairly/very bad versus fairly/very good (percent difference in slope: 3.1; 95% CI: 0.4, 5.9; p=0.02). Daytime dysfunction (i.e., trouble staying awake) was associated with a more rapid early decline (percent difference in slope: −6.3; 95% CI: −11.7, −0.6; p=0.03) but a less pronounced late decline (percent difference in slope: 3.4; 95% CI: 0.7, 6.1; p=0.01) in cortisol. Compared with women with short sleep latency, women with long sleep latency had a significantly reduced cortisol awakening rise (percent difference in slope: −30.6; 95% CI: −50.6, −2.4; p=0.04). Most of the associations with DHEA rhythms were similar in both direction and magnitude, although few were statistically significant. We did not note any clear associations between sleep quality measures and cortisol-DHEA ratio.
Table 3.
Percent differences (95% CIs) in diurnal rhythms (waking level, awakening response, early decline, late decline, night rise, overall slope and AUC) of cortisol, DHEA and their ratio according to individual sleep quality components measured by Pittsburgh Sleep Quality Index
| Sleep quality components1 | Cortisol | DHEA | Ratio |
|---|---|---|---|
| Percent differences (95% CI)2 | |||
| Subjective sleep quality | |||
| Waking level | 15.2 (−2.5, 36.0) | 23.2 (−10.7, 70.1) | −4.8 (−30.9, 31.0) |
| Awakening response | −11.7 (−32.6, 15.8) | −5.7 (−28.5, 24.4) | −8.3 (−30.4, 20.7) |
| Early decline | −2.8 (−8.7, 3.5) | −5.0 (−10.9, 1.3) | 2.4 (−3.9, 9.1) |
| Late decline | 3.1 (0.4, 5.9) | 2.5 (−0.3, 5.4) | 0.5 (−2.2, 3.2) |
| Night rise | −12.8 (−34.3, 15.8) | −31.8 (−49.4, −8.1) | 25.9 (−5.6, 67.9) |
| AUC | 1.8 (−0.6, 4.1) | 2.2 (−2.9, 7.2) | −0.4 (−5.5, 4.7) |
| Overall slope | −1.0 (−2.5, 0.4) | −0.6 (−2.2, 0.9) | −0.4 (−1.8, 1.0) |
| Sleep latency | |||
| Waking level | 15.4 (−5.2, 40.6) | 3.5 (−27.8, 48.5) | 13.8 (−20.5, 62.8) |
| Awakening response | −30.6 (−50.6, −2.4) | −17.6 (−41.8, 16.6) | −16.7 (−41.1, 17.8) |
| Early decline | 3.7 (−4.0, 12.1) | 1.6 (−6.2, 10.0) | 1.6 (−6.2, 9.9) |
| Late decline | 1.4 (−1.6, 4.5) | 2.0 (−1.2, 5.3) | −0.7 (−3.7, 2.4) |
| Night rise | −11.1 (−36.0, 23.4) | −11.7 (−37.6, 25.0) | −1.4 (−29.3, 37.5) |
| AUC | 0.6 (−2.0, 3.3) | 0.4 (−5.2, 6.0) | 0.1 (−5.6, 5.8) |
| Overall slope | −1.0 (−2.7, 0.8) | −0.1 (−2.0, 1.9) | −1.0 (−2.7, 0.8) |
| Sleep duration | |||
| Waking level | 5.3 (−10.2, 23.6) | 17.7 (−12.6, 58.5) | −10.7 (−33.5, 19.9) |
| Awakening response | −12.2 (−32.8, 14.8) | −18.4 (−37.9, 7.3) | 7.2 (−18.3, 40.6) |
| Early decline | 0.7 (−5.3, 7.1) | −0.4 (−6.4, 6.1) | 1.0 (−5.0, 7.5) |
| Late decline | 3.1 (0.4, 5.8) | 1.4 (−1.3, 4.2) | 1.6 (−1.0, 4.4) |
| Night rise | −10.6 (−35.3, 23.6) | −20.6 (−43.5, 11.8) | 9.2 (−21.2, 51.4) |
| AUC | 1.8 (−0.4, 4.0) | 1.1 (−3.6, 5.7) | 0.6 (−4.1, 5.3) |
| Overall slope | 0.6 (−0.8, 2.1) | 0.1 (−1.4, 1.7) | 0.5 (−0.9, 1.9) |
| Sleep efficiency | |||
| Waking level | −13.3 (−25.6, 1.0) | 2.6 (−23.4, 37.4) | −14.9 (−36.3, 13.8) |
| Awakening response | 2.2 (−20.5, 31.5) | −4.5 (−26.1, 23.6) | 6.2 (−17.6, 37.0) |
| Early decline | −1.2 (−6.8, 4.7) | 3.3 (−2.7, 9.6) | −4.1 (−9.6, 1.7) |
| Late decline | 3.3 (0.4, 6.2) | 1.4 (−1.5, 4.4) | 1.8 (−1.1, 4.7) |
| Night rise | −18.6 (−39.1, 8.7) | −19.6 (−40.7, 8.9) | 0.0 (−25.4, 34.0) |
| AUC | −0.3 (−2.4, 1.9) | 2.3 (−2.3, 6.9) | −2.5 (−7.1, 2.2) |
| Overall slope | 0.2 (−1.1, 1.5) | 1.0 (−0.4, 2.4) | −0.8 (−2.1, 0.5) |
| Sleep disturbances | |||
| Waking level | 3.7 (−24.0, 41.6) | −10.4 (−49.5, 59.1) | 13.2 (−35.8, 99.7) |
| Awakening response | −10.3 (−47.7, 53.6) | 7.4 (−37.8, 85.4) | −16.7 (−51.5, 43.2) |
| Early decline | 2.2 (−9.6, 15.5) | −8.3 (−19.0, 3.9) | 11.0 (−1.8, 25.6) |
| Late decline | 3.1 (−1.5, 7.9) | 4.3 (−0.6, 9.4) | −1.2 (−5.6, 3.5) |
| Night rise | 8.9 (−39.9, 97.6) | −33.8 (−64.5, 23.5) | 59.7 (−12.4, 191.0) |
| AUC | 2.4 (−1.7, 6.5) | −1.7 (−10.6, 7.2) | 3.6 (−5.4, 12.7) |
| Overall slope | 1.6 (−1.3, 4.5) | 0.9 (−2.2, 4.0) | 0.8 (−2.0, 3.6) |
| Sleep medication use | |||
| Waking level | 1.7 (−12.5, 18.3) | −10.0 (−32.3, 19.6) | 12.2 (−15.3, 48.7) |
| Awakening response | −16.9 (−35.4, 6.8) | −14.6 (−34.0, 10.5) | −1.7 (−23.9, 26.9) |
| Early decline | 3.2 (−2.6, 9.4) | 1.3 (−4.6, 7.5) | 1.9 (−3.9, 8.1) |
| Late decline | 2.0 (−0.8, 5.0) | 0.3 (−2.6, 3.3) | 1.5 (−1.4, 4.5) |
| Night rise | −11.4 (−34.3, 19.6) | −2.0 (−28.6, 34.4) | −11.9 (−34.9, 19.3) |
| AUC | 1.0 (−1.2, 3.1) | −2.8 (−7.3, 1.7) | 3.6 (−1.0, 8.1) |
| Overall slope | −0.8 (−2.1, 0.5) | −0.8 (−2.2, 0.6) | −0.1 (−1.4, 1.2) |
| Daytime dysfunction | |||
| Waking level | 6.2 (−8.8, 23.7) | −13.9 (−35.2, 14.4) | 26.0 (−4.9, 66.9) |
| Awakening response | −4.8 (−26.4, 23.0) | −0.9 (−23.9, 28.9) | −5.5 (−27.2, 22.7) |
| Early decline | −6.3 (−11.7, −0.6) | −1.0 (−6.8, 5.2) | −5.2 (−10.7, 0.7) |
| Late decline | 3.4 (0.7, 6.1) | −0.3 (−3.0, 2.4) | 3.7 (1.0, 6.4) |
| Night rise | 31.6 (−2.9, 78.3) | 36.6 (−0.8, 88.1) | −5.0 (−30.1, 29.1) |
| AUC | −0.1 (−2.2, 2.0) | −3.2 (−7.6, 1.3) | 3.3 (−1.3, 7.8) |
| Overall slope | 0.4 (−1.0, 1.8) | −0.3 (−1.8, 1.2) | 0.7 (−0.6, 2.1) |
For each sleep quality component, compare the score >1 versus ≤1
Adjusted for age, saliva sample collection characteristics, depression, anxiety, beta-blocker use, minor tranquilizer use, oral steroids use, DHEA medication, duration of shiftwork, BMI, hypertension, type 2 diabetes, sleep apnea, smoking, caffeine intake, and alcohol intake, and mutually adjusted for different components of sleep quality
A total of 17 women had late sleep schedules (i.e., stayed up later than usual) on the saliva collection day. Excluding these women did not alter the diurnal rhythms of cortisol and DHEA during the day, but diminished the cortisol and DHEA rise during late night (Supplemental Figure 1); the association between overall sleep quality and bedtime cortisol rise was no longer significant after this exclusion (Supplemental Table 4). Exclusion of women reporting use of oral steroids or DHEA medication resulted in similar results as shown in Table 2 and 3 (data not shown).
4. Discussion
In this sample of postmenopausal women with a relatively high level of psychosocial distress, about 60% reported poor sleep quality (mean=6.5) falling in between previously reported scores (2.7 in healthy individuals and 11.1 in patients with depression) (Buysse et al., 1989). We modeled diurnal rhythms of cortisol and DHEA over one day based on five timed saliva samples. A parallel declining pattern between cortisol and DHEA was observed across most of the day, with the distinction that there was a significant post-awakening rise for cortisol but not for DHEA, consistent with existing evidence (Hucklebridge et al., 2005). While women characterized as having good versus poor global sleep quality did not display appreciable differences in diurnal rhythms of cortisol and DHEA, we observed alterations in the HPA axis rhythms for specific aspects of sleep quality and patterns, particularly subjective sleep quality, sleep duration, sleep efficiency, sleep latency, and daytime dysfunction.
No prior studies have simultaneously evaluated the variations in multiple aspects of habitual sleep in relation to HPA diurnal rhythms. The reason for the null association by global sleep quality may be that women with moderate/severe problems in one or two sleep components may still be classified as having good sleep quality (PSQI≤5), although specific sleep problems may play more important roles in synchronizing sleep schedules with HPA axis rhythms (e.g., sleep duration, latency) or be more related to insomnia symptoms or stress levels (e.g., subjective sleep quality, efficiency, latency) that are directly relevant to HPA axis functioning.
We observed that longer sleep latency, an indicator for sleep onset insomnia, was associated with blunted cortisol awakening rise (30.4% difference in slope). Results from Multi-Ethnic Study of Atherosclerosis (MESA) suggested a similar flattened cortisol awakening rise with insomnia, particularly among short sleepers (37.7% difference in slope) (Castro-Diehl et al., 2015). By contrast, a more pronounced cortisol morning rise was observed for chronic insomnia symptoms in the Whitehall II study (Abell et al., 2016). Further, we observed that women reporting daytime dysfunction had more rapid early decline but slower late decline in cortisol, suggesting that early-onset low-cortisol profiles may relate to daytime dysfunction (Kumari et al., 2009a). Additional work to evaluate the role of insomnia and associated daytime dysfunction in HPA axis regulation is needed.
Our observation that shorter habitual sleep duration was associated with a flatter cortisol decline later in the day (3.1% difference) is consistent with prior studies (Castro-Diehl et al., 2015; Kumari et al., 2009b; Leproult et al., 1997). In MESA, shorter sleepers, measured by actigraphy, had a less pronounced late decline in cortisol compared to longer sleepers (2.2% difference) (Castro-Diehl et al., 2015). However, low sleep efficiency was associated with different phases of cortisol decline (flatter late decline in our study and flatter early cortisol decline in MESA), although the cutpoints and measurements used for defining low sleep efficiency differed between the two studies. Similarly, Whitehall II reported that self-reported sleep duration and sleep disturbance were associated with a shallower slope in cortisol decline over the day, although they did not differentiate between the early versus late phase decline; shorter sleep duration was also associated with increased cortisol awakening rise, which was not observed in our study (Kumari et al., 2009b). Another study in men reported elevated cortisol levels in the evening following partial or total sleep deprivation (Leproult et al., 1997), which may be the result of slower cortisol decrease during the day. Two other studies found no associations of objective sleep duration with salivary awakening cortisol (Zhang et al., 2011) or 24-hour urinary cortisol rhythm (Rao et al., 2013).
Interestingly, there was a cortisol/DHEA elevation prior to bedtime in our sample, particularly among those who stayed awake for more than 16 hours after awakening. Although such elevations were not modeled in previous studies, it is known that a delay in usual sleep time results in an increase in cortisol secretion (Weitzman et al., 1983). Our sensitivity analysis excluding those who stayed awake past their usual bedtime showed a flattened tail of the cortisol rhythm curve. Further, women with poor overall sleep quality appeared less likely to have a late night cortisol rise, partly because a late usual sleep schedule was more likely to occur among poor sleepers (range of bedtime in our sample: 8:30pm–12:00am for good sleepers and 8:30pm–2:00am for poor sleepers), possibly reducing the impact of staying up late on HPA responses. By contrast, given that regular sleep timing is fundamental to sleep hygiene, those reporting good global sleep quality were more likely to have more regular, earlier sleep times (Kang and Chen, 2009), which may precipitate tightly controlled HPA rhythms with sensitive responses to occasional changes in sleep schedules. Future studies with more frequent saliva sampling during evening and night will help elucidate cortisol/DHEA changes during this time period and their interactions with sleep schedule.
Most of the differences in DHEA associated with sleep quality were in the same direction as cortisol. This is consistent with existing knowledge that DHEA is secreted synchronously with cortisol in response to stress and provides potential protection against the adverse effects of sustained and excessive cortisol secretion (Charney, 2004; Rosenfeld et al., 1971). The stress-buffering effects of DHEA secondary to cortisol may help explain why the associations of sleep quality with DHEA were more modest compared to those with cortisol, with few reaching statistical significance. Because of the paralleled synchronization between cortisol and DHEA, we observed almost no differences in cortisol-DHEA ratio by overall sleep quality or its sub-components. However, the comparison of the extreme quintiles of PSQI noted a significantly elevated cortisol-DHEA ratio at awakening for women with very poor sleep quality, which suggests that the balance and synchronization between cortisol and DHEA may be interrupted by severely disturbed sleep.
This study has several limitations. First, habitual sleep quality and patterns were assessed by self-report that is more susceptible to measurement error, although reflects the subjective experience of sleep, an essential aspect of sleep disorders. For example, previous studies have shown moderate correlations between self-reported and objectively-measured sleep duration (Cespedes et al., 2016; Lauderdale et al., 2008). Despite this, the validity and reliability of PSQI have been proved to be excellent in non-clinical settings, with utility as a screening tool for identifying sleep dysfunction (Mollayeva et al., 2016). The fact that our results were similar to those based on actigraphy-measured sleep studies suggests that the misclassification did not appreciably influence our findings (Castro-Diehl et al., 2015). Second, our study was cross-sectional. Thus, we cannot rule out the possibility that dysregulated HPA axis may precipitate sleep disturbance and lower sleep quality. Third, we cannot assess whether sleep patterns for some participants on the night before saliva collection deviated from habitual sleep patterns (e.g., acute sleep deprivation), which may have an effect on our measured diurnal rhythms of cortisol and DHEA (Omisade et al., 2010). Fourth, we did not account for multiple testing when we examined the associations with individual sleep quality components. Nevertheless, the consistency of associations across several subcomponents and with prior studies reduces the possibility of false positive findings. Notably, it would be of great interest to assay the second set of timed saliva samples collected at 1-year follow-up to internally replicate our findings in the future. This will also allow evaluation of the prospective associations between baseline sleep quality and changes in cortisol/DHEA rhythms over 1 year or the associations between concurrent changes in sleep quality and salivary cortisol/DHEA. Lastly, because of the high proportion of selected women with childhood abuse history, the results from our study may not be generalizable to women in general.
A notable strength of the study is the use of five timed saliva samples over one day to characterize the diurnal rhythms of cortisol and DHEA simultaneously. Because our cross-sectional study was embedded in a well-phenotyped prospective cohort with highly reliable self-reports on health and lifestyle, we were able to conduct a number of important secondary analyses to test the robustness of our results. Further, in our statistical modeling, we adjusted for a number of important factors to reduce residual confounding.
5. Conclusion
Our results suggest that specific components of sleep quality and patterns relate to HPA regulation. Sleep duration, efficiency, latency and daytime dysfunction are associated with altered diurnal rhythms of cortisol, particularly flattened late decline. The DHEA rhythm showed weaker associations with sleep quality. Given the elevated risk of cardiometabolic disorders associated with altered HPA rhythms, similar to those observed in the current study, our results provide further evidence that poor habitual sleep quality and patterns may increase cardiometabolic risk through influencing the HPA axis.
Supplementary Material
Highlights.
Overall habitual sleep quality was not associated altered diurnal rhythms of salivary cortisol and DHEA
Specific dimensions of sleep quality was associated with alterations in diurnal cortisol rhythms
Sleep duration, efficiency, daytime dysfunction and subjective quality were associated with flattened cortisol decline later in the day
Sleep latency was associated with reduced cortisol awakening response
Altered HPA axis may contribute to the link between sleep and cardiometabolic risk
Acknowledgments
Funding
This work was supported by the National Institute of Health (grant number UM1 CA176726, U54 CA155626, R01 CA163451). T.H. is a recipient of the American Heart Association Postdoctoral Fellowship (Founders Affiliate) Award (16POST27480007).
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Conflicts of interest: none
Disclosure Summary
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abell JG, Shipley MJ, Ferrie JE, Kivimaki M, Kumari M. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: A 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology. 2016;68:91–99. doi: 10.1016/j.psyneuen.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, Shrager SE, Shea S. Association of Sleep Duration and Quality With Alterations in the Hypothalamic-Pituitary Adrenocortical Axis: The Multi-Ethnic Study of Atherosclerosis (MESA) J Clin Endocrinol Metab. 2015;100:3149–3158. doi: 10.1210/jc.2015-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, Hall MH, Loredo JS, Mossavar-Rahmani Y, Ramos AR, Reid KJ, Shah NA, Sotres-Alvarez D, Zee PC, Wang R, Patel SR. Comparison of Self-Reported Sleep Duration With Actigraphy: Results From the Hispanic Community Health Study/Study of Latinos Sueno Ancillary Study. Am J Epidemiol. 2016;183:561–573. doi: 10.1093/aje/kwv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: the Multiethnic Study of Atherosclerosis. Obesity (Silver Spring) 2013;21:E56–63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J Clin Endocrinol Metab. 2014;99:4625–4631. doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 - laboratory techniques and clinical indications. Clin Endocrinol (Oxf) 2012;77:645–651. doi: 10.1111/j.1365-2265.2012.04508.x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Kang JH, Chen SC. Effects of an irregular bedtime schedule on sleep quality, daytime sleepiness, and fatigue among university students in Taiwan. BMC Public Health. 2009;9:248. doi: 10.1186/1471-2458-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep. 2006;29:1503–1506. doi: 10.1093/sleep/29.11.1503. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M. Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology. 2009a;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009b;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam Experience Study. Eur J Endocrinol. 2010;163:285–292. doi: 10.1530/EJE-10-0299. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Rao MN, Blackwell T, Redline S, Punjabi NM, Barrett-Connor E, Neylan TC, Stone KL. Association between sleep duration and 24-hour urine free cortisol in the MrOS Sleep Study. PLoS One. 2013;8:e75205. doi: 10.1371/journal.pone.0075205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld RS, Hellman L, Roffwarg H, Weitzman ED, Fukushima DK, Gallagher TF. Dehydroisoandrosterone is secreted episodically and synchronously with cortisol by normal man. J Clin Endocrinol Metab. 1971;33:87–92. doi: 10.1210/jcem-33-1-87. [DOI] [PubMed] [Google Scholar]
- Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci U S A. 2016;113:E2730–2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112:332–340. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wust S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. 2004;151:1–14. doi: 10.1530/eje.0.1510001. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, Li SX, Yu MW, Ho CS, Chan MH, Zhang B, Wing YK. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34:225–233. doi: 10.1093/sleep/34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.