Abstract
Background
Obesity and poor sleep are highly prevalent among Black women.
Purpose
We examined whether a weight gain prevention intervention improved sleep among Black women.
Methods
We conducted a randomized trial comparing a 12-month weight gain prevention intervention that included self-monitoring through mobile technologies and phone coaching to usual care in community health centers. We measured sleep using the Medical Outcomes Study Sleep Scale at baseline, 12 months, and 18 months. The scale examines quantity of sleep, sleep disturbance, sleep adequacy, daytime somnolence, snoring, shortness of breath and global sleep problems (sleep problem indices I and II).
Results
Participants (n=184) were on average 35.4 years, and obese (BMI:30.2kg/m2); 74% made <$30,000/y. At baseline, average sleep duration was 6.4(1.5) hours. Controlling for weight change and sleep medication, the intervention group reported greater improvements in sleep disturbance [−8.35 (−16.24, −0.45)] and sleep problems at 12 months: sleep problems index I [− 8.35 (−16.24, −0.45)]; sleep problems index II - [−8.35 (−16.24, −0.45)]. However, these findings did not persist at 18 months.
Conclusions
Preventing weight gain may afford clinical benefit on improving sleep quality.
Keywords: Sleep, obesity, weight gain prevention, minority health, primary care, digital health
Introduction
U.S. obesity rates remain high and are disproportionately high among racial/ethnic minority women; 57% of Non-Hispanic Black women are obese compared to 36% of NonHispanic White women.(1) Obesity is associated with a greater likelihood of experiencing a range of cardiometabolic abnormalities and associated with an increased risk of chronic disease.(2) Concurrent with the rise in obesity there has been a rise in reported sleep curtailment, both in sleep duration(3) and sleep quality.(4) Sleep quality assesses the domains of sleep continuity or efficiency, duration, timing, alertness and sleepiness, and satisfaction.(5) Insufficient sleep(6) and poor sleep quality(7) are associated with a host of adverse(8) cardiometabolic(9) and psychosocial outcomes such as high blood pressure, increased stress, poor quality of life, memory impairment, and accidental injury. (10–15)
Emerging evidence details a strong association between sleep and obesity,(16–18) although the causal direction is not well understood. Some studies indicate that reduced sleep duration and quality is associated with an increased risk of both obesity and associated cardiometabolic impairments.(19, 20) Conversely, obese individuals report fewer hours of sleep per night and poorer sleep quality.(21) As such, it is not clear whether obesity leads to poor sleep or whether poor sleep causes weight gain and subsequent obesity. Moreover, the association between obesity and sleep is particularly strong among women and racial/ethnic minorities.(22) Sleep disturbances are twice as common among women compared to men(23, 24) and the associations between BMI and sleep quality are stronger in women under 50 years of age.(25) Further, multiple studies using objective measures of sleep have shown that Black women have shorter sleep duration, poorer sleep efficiency, and higher sleep latency compared to White women, even after controlling for various socioeconomic and demographic factors.(26, 27) Therefore, Black women are a high-risk population in need of interventions to improve sleep outcomes.
Although the evidence supporting the association between sleep and obesity relies primarily on observational data, randomized trials show that weight loss can improve sleep quality and duration.(28, 29) Mechanistically, improvements in sleep duration and sleep disturbances are typically explained through better balance of hormones that regulate appetite and better glucose tolerance, as well as reductions in cortisol from increased exercise.(30, 31) Further, improvements in daytime sleepiness may be improved through reductions in sleep apnea as a result of reductions in body fat. (32, 33)
What is less clear, and generally under-examined, is whether preventing weight gain leads to improvements in sleep duration and sleep quality. Preventing weight gain is now recommended as part of national obesity guidelines for individuals not motivated or ready for weight loss. Black women in particular could benefit from weight gain prevention, as they tend to exhibit large gains between young adulthood and mid adulthood.(34)
Weight gain prevention approaches are similar to those for weight loss; albeit with a lower intensity. Small changes to diet and physical activity behaviors can result in a small caloric deficit to stave off weight gain, while larger changes are necessary to produce weight loss. It is not clear whether small changes to diet and physical activity, independent of weight change, would be beneficial for improving sleep health. It may be those changes, rather than weight loss per se, that can improve sleep. We recently conducted a 12-month randomized controlled trial of a weight gain prevention intervention (the “Shape Program”), relative to usual care, delivered in the primary care setting.(35) We found that the Shape intervention was efficacious in staving off weight gain at 12 and 18 months.(36) The Shape trial provides a unique opportunity to examine whether weight gain prevention – as achieved through small behavioral changes to stave off weight gains - can have a spillover effect of improved sleep in a population with a high risk for obesity and poor sleep.
Methods
Study Overview
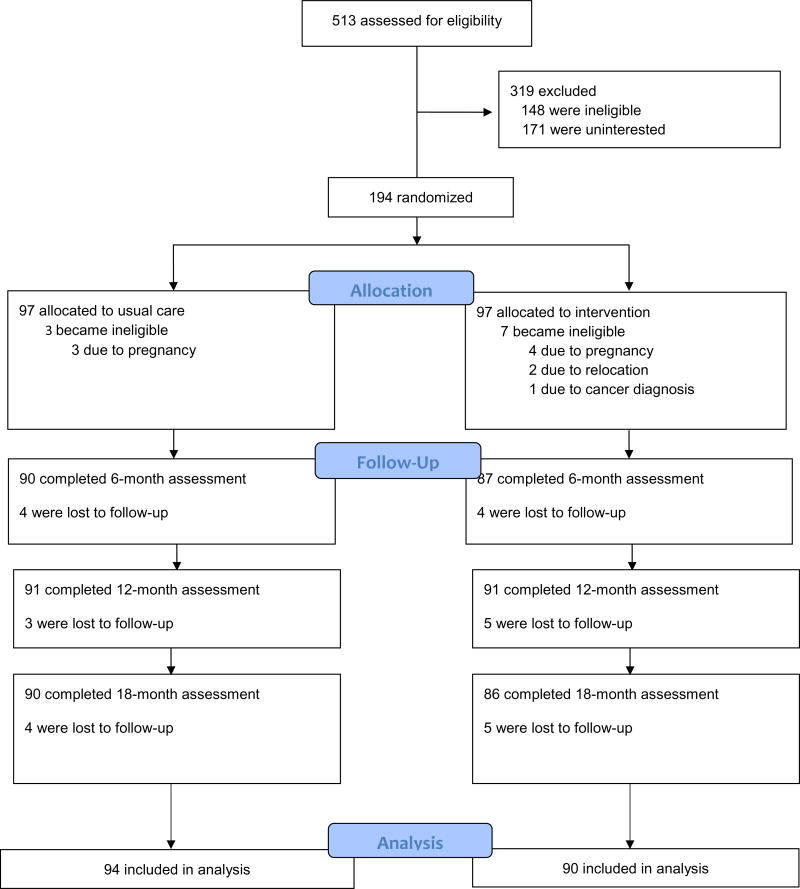
As described elsewhere,(35–37) the Shape Program was a randomized controlled trial between 2009–2012 that compared a 12-month weight gain prevention intervention to usual care in the primary care setting. Following eligibility screening and baseline measures, we randomized participants (n=194) to the Shape intervention (n=97) or to usual care (n=97). As seen in Figure 1, we excluded 10 participants after randomization. We re-evaluated all remaining participants (n=184) at 6, 12, and 18 months.(36) All study procedures were approved by the Duke University Institutional Review Board (protocol #2628) and the Piedmont Health Board of Advisors.
Figure 1.
Shape CONSORT
Participants
We recruited Black women, aged 25 to 44 years, with a body mass index (BMI) of 25 to 34.9 kg/m2. We recruited participants from 5 community health centers operated by Piedmont Health Inc. (PHS), a federally qualified community health center system that serves predominantly socioeconomically disadvantaged patients in central North Carolina. Additional inclusion criteria consisted of at least 1 visit to a Piedmont Health center in the previous 24 months and the ability to read and write in English. Exclusion criteria included current pregnancy, being less than 12 months postpartum, a history of myocardial infarction or stroke in the previous 2 years, and profound cognitive, developmental, or psychiatric disorders.
Intervention
The Shape Program intervention has been described in detail elsewhere.(35–37) Briefly, the year-long intervention included 5 primary components: (1) 3 personally tailored behavior change goals, (2) self-monitoring of these goals via weekly interactive voice response phone calls, (3) tailored skills training materials, (4) 12 monthly individual counseling calls with a registered dietitian, and (5) a 12-month YMCA membership. The intervention was designed to achieve weight gain prevention. As such, we did not recruit those with high weight loss motivation and intervention messages did not mention weight loss; rather, the primary intervention message concerned maintaining one’s weight and shape.
The intervention utilized the Interactive Obesity Treatment Approach,(38–40) which uses a computer algorithm to prescribe a series of tailored behavior change goals (e.g., 5 or more fruits and vegetables per day, no fast food, no sugar-sweetened beverages, walking 10,000 steps per day). The intervention emphasized achieving the behavioral goals as a means of creating a small caloric deficit to stave off weight gains. Healthy sleep habits were one of these goals. Participants reporting less than 7 or more than 9 hours of sleep per night were instructed to aim for 7–8 hours of sleep per night. However, not all participants were prescribed this goal. Our goal assignment algorithm considers self-efficacy, readiness, and the potential of the goal to produce a caloric deficit. We assigned 3 goals at baseline and updated them every 2 months over the 12-month intervention period. All intervention participants received walking up to 10,000 steps per day as one of their goals.
Participants self-monitored their adherence to the behavior change goals on weekly, 2-to 4-minute, automated, interactive voice response telephone calls that were issued by a computer database. After self-monitoring, participants received immediate, automated feedback regarding their progress and skills training tips from the interactive voice response system. Self-monitoring data, in part, informed the monthly coaching calls that were conducted by Piedmont Health registered dietitians. The 20-minute coaching calls used principles of motivational interviewing to help elicit patient-directed motivation for meeting behavioral goals.
Usual Care
Participants in the usual care arm received routine standard of care for obesity from providers at PHS. Current obesity treatment includes obesity screening and referral to medical nutrition therapy with a registered dietitian if available or referral to outside programs (eg., YMCA). Usual care participants also received semiannual newsletters that covered topics other than weight, nutrition, or physical activity (e.g., managing your finances) They also received the National Heart, Lung, and Blood Institute’s “Aim for a Healthy Weight” brochure at the baseline visit. Otherwise, no effort was made to impact their weight, or the care they received at the health center.
Measures
Sociodemographics
We collected a variety of demographic and psychosocial measures to help characterize the sample and understand if sleep duration and sleep problems vary by these characteristics. These included age, body mass index (kg/m2), gender, income, education, employment, whether children are living in the household, marital status, smoking status, self-reported minutes of moderate-vigorous activity as measured by the BRFSS physical activity questions, number of self-reported stressful negative life events (e.g., death in the family, foreclosure, divorce), and depression. We used the PHQ-8(41) to measure depressive symptoms at baseline, 12 and 18 months. Scores greater than or equal to 10 indicate a diagnosis of moderate to severe.
Sleep
To examine improvements in sleep we explored various dimensions, including; 1) duration of sleep in the past 24 hours; 2) sleep continuity, which is the ability to maintain sleep throughout the evening once the individual has fallen asleep; 3) alertness or sleepiness, which refers to daytime sleepiness or the ability to stay awake and; 4) satisfaction.
To measure sleep health, we administered the Medical Outcomes Study Sleep Scale(42) at baseline, 12-months and 18-months. This scale has been used in various clinical populations and validated in the general U.S. population.(42) The scale consists of 12 items that measure parameters of sleep across 6 domains : (1) sleep disturbance, which measures the ability to fall asleep and maintain restful sleep (e.g., In the past 4 weeks how often did you have trouble falling asleep?); (2) sleep adequacy, which measures the sufficiency of sleep in terms of sleeping enough to provide restoration of wakefulness (e.g., In the past 4 weeks did you get enough sleep to feel rested upon waking in the morning?); (3) sleep quantity, which measures (in hours) the amount of sleep an individual has had each night (On the average, how many hours did you sleep each night during the past 4 weeks?); (4) somnolence, which measures daytime drowsiness or sleepiness via 3 items (e.g., In the past 4 weeks, did you feel drowsy or sleepy during the day?); (5) snoring (In the past 4 weeks did you snore during your sleep?); and (6) shortness of breath, or headache (In the past 4 weeks did you awaken short of breath or with a headache?). The scale also includes two indices (sleep indices I and II) that assess general sleep problems. Sleep problems index I includes 6 items and sleep problems index II includes 9 items. Both indices include questions from the following domains: sleep disturbance, sleep adequacy, respiratory impairment, but neither include sleep duration. In addition, Sleep problems index II includes three questions which are exempt from sleep problems index I including, sleep continuity or efficiency, restful sleep, and alertness/sleepiness. To score these domains of sleep, 10 of the 12 items are based on a 6-point scale. One item (how long did it take for you to fall asleep during the past 4 weeks?) is scored using a 5-point Likert scale and sleep duration is quantified simply by hours. With the exception of sleep adequacy, score reductions indicate improvements in sleep problems.(43) Lower values for sleep quantity indicate fewer number of hours of sleep and less than 7 hours of sleep per night is considered insufficient sleep.
Weight and Height
Trained staff measured participants heights to the nearest 0.1 centimeter by using a calibrated wall-mounted stadiometer (Seca model 214; Hamburg, Germany).(44) Weights were measured to the nearest 0.1 kilogram with an electronic scale (Seca model 876; Hamburg, Germany).(44)
Medication Use
At 18 months, we collected self-reported medication use (i.e., In the past 18 months, has a doctor or medical professional prescribed a medication for you? If yes, which medication(s)?). We coded self-reported use of any drugs that are used to manage sleep based on the following six categories; 1) sleep agents (e.g., Ambien); 2) benzodiazepines (e.g., Lorazepam); 3) antipsychotics (e.g., Seroquel); 4) antidepressants (e.g., Elavil); 5) antihistamines (e.g., Benadryl); and 6) other (e.g., Melatonin). We then collapsed these categories to a dichotomous variable that examined whether a participant was on any vs. no medication used to improve sleep.
Engagement in the intervention
Intervention engagement was operationalized as percent of weeks of successful self-monitoring through IVR. We dichotomized this continuous measure to examine differences in sleep outcomes among those who completed at least 80% of weeks as compared to those who completed a fewer percentage of weeks. As indicated above, optimal sleep duration was included in the intervention as a behavioral goal to prevent weight gain. Thirty-four participants were assigned the sleep goal based on our computer algorithm.
Statistical Analysis
We used Chi-square (χ2) and analysis of variance (ANOVA) models to examine differences in baseline characteristics and attrition between groups. A binary variable was created to identify the participants who self-reported at least 7 hours of sleep per night (i.e. meeting sleep duration recommendations) and those who did not meet the recommended hours of sleep. We examined differences in baseline characteristics between these groups using two sample independent t-tests (continuous) and chi-square tests (categorical) for normally distributed data. For non-normal data we used Wilcoxon’s Rank Sum (continuous) or Cochran-Mantel-Haenszel (categorical) tests. Along with appropriate p-value for group differences, mean (standard deviation) or frequency (percentage) are reported overall and by group. We also examined whether there were any associations between baseline characteristics and scores of the sleep problems indices I and II using both continuous and median split of the scores.
To examine the effect of treatment on continuous outcomes over time, we conducted intent-to-treat analyses using linear mixed models with random intercepts and maximum likelihood estimates. We included all participants in the models, with the assumption that missing values were missing at random. We also fit the analogous models that controlled for weight change and medication use to determine the effect of the intervention on sleep beyond any effect that is attributable to change in weight and medication use. From the mixed models, we report mean(SE) at baseline, and mean change (SE) at 12-and 18-months are reported along with mean difference (95% confidence interval) between treatment arms. Lastly, we examined using ANOVA models whether there were any differences in sleep outcomes by engagement in the intervention, assignment of the sleep goal, meeting physical activity recommendations, and depression. We conducted all analyses in 2013 with SPSS for Mac (version 19, IBM, Somers, NY), with an alpha<0.05 to assess statistical significance.
Results
Baseline characteristics have been reported in detail elsewhere.(35, 36) Briefly, participants had a mean age of 35.4 (±5.5) years and most (80%) had less than a college degree. The majority (71%) were currently employed and 74% reported an annual income of less than $30,000. At baseline, 20% reported depressive symptomatology consistent with moderate to severe depression. There were no significant differences in baseline characteristics between treatment arms. We retained 96% of participants at 12 months and 18 months after randomization and there were no statistically significant differences in attrition between groups.(36)
The Shape trial main weight change outcomes have been presented in detail elsewhere.(36) Briefly, relative to usual care, the intervention was successful in mitigating weight gain over 12 months (−0.97 ±0.51 kg vs. 0.48 ±0.49 kg; mean difference: −1.45 kg; 95% confidence interval CI = 2.84, −0.05; P =.04) and 18 months (−0.91 ±0.58 kg vs. 0.83 ±0.55 kg; mean difference: −1.74 kg; 95% CI = 3.31, −0.15; P=.03) post randomization. In addition, at 12 months, a larger proportion of intervention participants (62%) were at or below their baseline weight, compared with those in usual care (45%; P=.02). We observed similar findings at 18 months. (36)
Baseline sociodemographic characteristics among all participants by sleep duration at baseline are reported in Table 1. Insufficient sleep was defined as not meeting the minimum recommended 7 hours of sleep per night for adults.(45) At baseline, 60% reported poor short sleep. Compared with those who reported at baseline sleeping at least 7 hours of sleep per night (n=75), a greater portion of participants who reported poor sleep (n=104) also reported experiencing 3 or more negative life events (60.6% vs. 41.1%; p=.01). There were no differences by sleep duration in age, BMI, income, education, employment, number of kids in the home, marital status, smoking status, physical activity, or depression. We found the same results when examining baseline characteristics using the median split for sleep problems index I and sleep problems index II and when sleep problem index scores were assessed as continuous scores. At 18 months, 6% (n=11) of all participants reported taking any medication that may be used as a sleep aid over the past 18 months. Of those 11, 55% (n=6) reported using sleep agents such as Ambien; 27% (n=3) reported using benzodiazepines and; 18% (n=2) reported using antidepressants. No other categories of sleep medications were reported.
Table 1.
Baseline Characteristics Across Entire Sample and by Meeting Sleep Duration Recommendations (N=184)
| Characteristic | Total (n=184)* | Meeting Recommended Hours of Sleep (n=75) | Not Meeting Recommended Hours of Sleep (n=104) | p-value | |||
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 35.41 | (5.50) | 35.04 | (5.64) | 35.55 | (5.47) | 0.55 |
| Body mass index, mean (SD), kg/m2 | 30.19 | (2.53) | 30.22 | (2.45) | 30.07 | (2.55) | 0.69 |
| Household income/y, No. (%) | 0.97 | ||||||
| 1=Under $10,000 | 35 | (19.77) | 15 | (20.27) | 20 | (19.42) | |
| 2=$10,000–$19,999 | 50 | (28.25) | 20 | (27.03) | 30 | (29.13) | |
| 3=$20,000–$29,999 | 45 | (25.42) | 20 | (27.03) | 25 | (24.27) | |
| 4=$30,000+ | 47 | (26.55) | 19 | (25.68) | 28 | (27.18) | |
| Education, No. (%) | 0.64 | ||||||
| 1=< High School | 18 | (10.23) | 8 | (10.96) | 10 | (9.71) | |
| 2=High school or GED | 44 | (25.00) | 22 | (30.14) | 22 | (21.36) | |
| 3=Vocational or trade school after high school | 16 | (9.09) | 6 | (8.22) | 10 | (9.71) | |
| 4=Some college or university | 62 | (35.23) | 25 | (34.25) | 37 | (35.92) | |
| 5=Associate degree/College graduate/post graduate | 36 | (20.45) | 12 | (16.44) | 24 | (23.30) | |
| # Kids in household under 5, mean (SD) | 0.47 | (0.82) | 0.44 | (0.60) | 0.50 | (0.96) | 0.63 |
| # Children living at home (2 categories), No. (%) | 0.27 | ||||||
| 1=no children at home | 109 | (65.27) | 43 | (60.56) | 66 | (68.75) | |
| 2=some children at home | 58 | (34.73) | 28 | (39.44) | 30 | (31.25) | |
| Marital status, No. (%) | 0.21 | ||||||
| 1=currently married/partnered | 47 | (26.86) | 16 | (21.92) | 31 | (30.39) | |
| 2=other | 128 | (73.14) | 57 | (78.08) | 71 | (69.61) | |
| Smoker, No. (%) | 0.44 | ||||||
| 0=Not at all | 123 | (69.10) | 48 | (64.00) | 75 | (72.82) | |
| 1=Some days | 15 | (8.43) | 7 | (9.33) | 8 | (7.77) | |
| 2=Every day | 40 | (22.47) | 20 | (26.67) | 20 | (19.42) | |
| Minutes of Moderate-Vigorous Activity, mean (SD) | 23.47 | (38.54) | 29.39 | (44.38) | 19.07 | (33.83) | 0.09 |
| Physical Activity Met the guideline of >=150 Min/Week, No. (%) | 0.54 | ||||||
| No | 70 | (44.59) | 28 | (41.79) | 42 | (46.67) | |
| Yes | 87 | (55.41) | 39 | (58.21) | 48 | (53.33) | |
| Three or more negative life events, (excluding missing) No. (%) | 0.01 | ||||||
| No | 82 | (47.67) | 43 | (58.90) | 39 | (39.39) | |
| Yes | 90 | (52.33) | 30 | (41.10) | 60 | (60.61) | |
| Employment status, No. (%) | 0.25 | ||||||
| Employed | 126 | (71.19) | 50 | (66.67) | 76 | (74.51) | |
| Unemployed | 51 | (28.81) | 25 | (33.33) | 26 | (25.49) | |
| Moderate-severe depression, No (%) | 0.48 | ||||||
| No | 135 | (79.41) | 59 | (81.94) | 76 | (77.55) | |
| Yes | 35 | (20.59) | 13 | (18.06) | 22 | (22.45) | |
Subgroup numbers may not sum to sample n due to missing data
As shown in Table 2, relative to the usual care group, intervention participants reported significantly greater improvements at 12 months in sleep disturbance (−9.94 ±2.82 vs. −0.85 ±2.73; mean difference: −9.10; 95% CI = −16.79, −1.41), snoring (−8.08 ±3.22 vs 1.38 ±3.08; mean difference: −9.46; 95% CI = −18.19, −0.74), somnolence (−7.04 ±2.30 vs 0.60 ±2.21; mean difference: −7.64; 95% CI = −13.89, −1.39), sleep problems index I (−8.18 ±2.18 vs −0.94 ±2.10; mean difference: −7.24; 95% CI = −13.18, −1.30), and sleep problems index II (−8.80 ±2.13 vs − 0.89 ±2.06; mean difference: −7.91; 95% CI = −13.71, −2.10). After adjustment for weight change and medication use at 12 months (Table 2), significant, but slightly attenuated, effects across groups remained for sleep disturbance (−9.42 ±2.88 vs. −1.08 ±2.80; mean difference: −8.35; 95% CI = −16.24, −0.45), sleep problems index I (−7.64 ±2.10 vs −1.43 ±2.03; mean difference: −6.21; 95% CI = −11.93, −0.48, and sleep problems index II (−8.27 ±2.11 vs −1.29 ±2.05; mean difference: −6.99; 95% CI = −12,77, −1.20, but not snoring (−7.44 ±3.14 vs 0.79 ±3.02; mean difference: −8.23; 95% CI = −16.79, 0.33) or somnolence (−6.29 ±2.51 vs −0.02 ±2.42; mean difference: −6.26; 95% CI = −13.11, 0.58). For all outcomes, there were no significant effects between treatment arms at 18-months post randomization. Moreover, we did not find any differences in sleep outcomes by intervention engagement or assignment of the sleep goal (data not shown). We also did not find any differences in sleep outcomes over time by meeting recommended levels of physical activity. We did, however, find a significant association between depression and sleep problems among intervention participants at 12 months; participants with depression (n=9) had higher scores on sleep disturbance [66.3 (28.4) vs 25.3 (22.2); p<.001], sleep problems index I [53.3 (22.5) vs 29.1 (16.6); p<.001], and sleep problems index II [61.0 (23.3) vs 29.4 (17.2); p<.001], compared to those without depression (n=70).
Table 2.
Change in Sleep Outcomes by Study Group (N=184)
| Change from baseline Unadjusted models |
Change from baseline Adjusted models** |
||||
|---|---|---|---|---|---|
| Outcome Variable and Group | Baseline Score* | 12 Months | 18 Months | 12 months | 18 months |
| Hours of Sleep | |||||
| Intervention, mean (SE) | 6.66 (0.16) | −0.13 (0.26) | −0.15 (0.18) | −0.17 (0.23) | −0.17 (0.23) |
| Usual Care, mean (SE) | 6.20 (0.15) | 0.22 (0.25) | −0.31 (0.18) | 0.26 (0.22) | −0.29 (0.22) |
| Difference between arms, mean (95% CI) | −0.35 (−1.06, 0.35) | 0.17 (−0.33, 0.67) | −0.42 (−1.05, 0.20) | 0.12 (−0.51, 0.75) | |
| Sleep Disturbance | |||||
| Intervention, mean(SE) | 38.87 (2.79) | −9.94 (2.82) | −9.89 (2.93) | −9.42 (2.88) | −9.46 (2.87) |
| Usual Care, mean (SE) | 45.37 (2.73) | −0.85 (2.73) | −6.31 (2.86) | −1.08 (2.80) | −6.81 (2.82) |
| Difference between arms, mean (95% CI) | −9.10 (−16.79, −1.41) | −3.58 (−11.61, 4.44) | −8.35 (−16.24, −0.45) | −2.65 (−10.55, 5.25) | |
| Snoring | |||||
| Intervention, mean (SE) | 43.00 (3.65) | −8.08 (3.22) | −8.17 (3.07) | −7.44 (3.14) | −7.60 (3.14) |
| Usual Care, mean (SE) | 34.89 (3.55) | 1.38 (3.08) | −0.10 (2.94) | 0.79 (3.02) | −1.22 (3.03) |
| Difference between arms, mean (95% CI) | −9.46 (−18.19, −0.74) | −8.08 (−16.41, 0.26) | −8.23 (−16.79, 0.33) | −6.38 (−14.95, 2.19) | |
| Short of Breath | |||||
| Intervention, mean (SE) | 22.44 (2.92) | −7.12 (3.52) | −6.97 (3.26) | −6.86 (3.28) | −6.72 (3.26) |
| Usual Care, mean (SE) | 21.28 (2.86) | 1.46 (3.40) | −0.99 (3.17) | 1.00 (3.17) | −1.47 (3.19) |
| Difference between arms, mean (95% CI) | −8.57 (−18.16, 1.01) | −5.98 (−14.89, 2.93) | −7.86 (−16.80, 1.09) | −5.26 (−14.21, 3.70) | |
| Sleep Adequacy | |||||
| Intervention, mean (SE) | 43.22 (2.38) | 8.69 (2.88) | 10.88 (2.88) | 8.17 (2.87) | 10.46 (2.86) |
| Usual Care, mean (SE) | 40.74 (2.32) | 4.00 (2.77) | 4.14 (2.79) | 4.51 (2.78) | 4.76 (2.79) |
| Difference between arms, mean (95% CI) | 4.69 (−3.15, 12.54) | 6.74 (−1.12, 14.60) | 3.66 (−4.19, 11.50) | 5.70 (−2.16, 13.55) | |
| Somnolence | |||||
| Intervention, mean(SE) | 35.63 (2.32) | −7.04 (2.30) | −4.48 (2.75) | −6.29 (2.51) | −3.81 (2.49) |
| Usual Care, mean(SE) | 33.26 (2.27) | 0.60 (2.21) | −0.34 (2.66) | −0.02 (2.42) | −1.31 (2.44) |
| Difference between arms, mean (95% CI) | −7.64 (−13.89, −1.39) | −4.14 (−11.64, 3.35) | −6.26 (−13.11, 0.58) | −2.49 (−9.34, 4.36) | |
| Sleep Problems Index I | |||||
| Intervention, mean (SE) | 39.43 (1.94) | −8.18 (2.18) | −8.24 (2.18) | −7.64 (2.10) | −7.76 (2.09) |
| Usual Care, mean (SE) | 42.18 (1.90) | −0.94 (2.10) | −3.04 (2.11) | −1.43 (2.03) | −3.77 (2.04) |
| Difference between arms, mean (95% CI) | −7.24 (−13.18, −1.30) | −5.20 (−11.14, 0.75) | −6.21 (−11.93, −0.48) | −3.99 (−9.72, 1.74) | |
| Sleep Problems Index II | |||||
| Intervention, | 41.29 | ||||
| mean (SE) | (1.95) | −8.80 (2.13) | −9.23 (2.19) | −8.27 (2.11) | −8.76 (2.11) |
| Usual Care, mean | 43.56 | ||||
| (SE) | (1.91) | −0.89 (2.06) | −3.73 (2.13) | −1.29 (2.05) | −4.37 (2.06) |
| Difference between arms, mean (95% CI) | −7.91 (−13.71, −2.10) | −5.51 (−11.49, 0.48) | −6.99 (−12.77, −1.20) | −4.39 (−10.18, 1.40) |
There were no differences in baseline scores across study arms
Models were adjusted for weight change and medication use
Discussion
Our results indicate that a weight gain prevention intervention for black women with obesity improves sleep as compared to usual primary care for weight management; however, these improvements are no longer significant once the intervention stops. Specifically, the intervention group saw improvements in sleep disturbance, snoring, and general sleep problems. Improvements in these sleep problem indices are particularly telling because they indicate that the Shape intervention improved global sleep quality. Moreover, these improvements were independent of weight change or the use of sleep medications. This is important given that at baseline almost 60% reported insufficient sleep, indicating a population at high risk for the adverse outcomes as a result of poor sleep. It is notable that we did not find any change in sleep duration as a result of the intervention. We are not able to fully explain the discrepancy in our findings between sleep duration and sleep quality, however, it is possible that our study population had specific barriers (e.g., multiple jobs, young kids in the household) that might have impeded improvements in duration, but not sleep quality. Improvements in sleep quality afford similar clinical benefits as is seen for improvements in sleep duration. This includes impacts on cognitive function, cardiovascular disease, diabetes, metabolic syndrome and even mortality.(5) Overall, the implications of these findings are that a “maintain don’t gain” intervention is useful for improving sleep quality.
The spillover effects of the Shape intervention are compelling, particularly because the intervention was not specifically designed to address sleep- or characteristics that are known to affect sleep quality and duration. Although getting the recommended number of hours per night was a behavioral goal assigned to some participants, this goal was assigned within the context of overall wellness and the need to make multiple behavior changes simultaneously. We gave participants basic information in how to achieve a sufficient amount of sleep, with limited information on strategies for addressing specific domains of sleep. For example, we did not discuss how to reduce the likelihood of snoring; yet we found improvements in this dimension of sleep health. In contrast, the intervention primarily emphasized the impact of diet and physical activity behaviors on weight maintenance given their direct relation to weight. We evaluated the impact of the sleep goal through sensitivity analyses and found no differential effects on sleep outcomes. This indicates other mechanisms through which our intervention was beneficial for sleep.
Although the benefits on sleep quality are compelling, these effects did not persist 6 months post intervention. It is important to note that trends showing greater improvements in sleep quality among intervention participants as compared to control persisted, however, these differences were not significant. The intervention was designed based on the RE-AIM framework with implementation in primary care as the ultimate goal. We worked closely with our community health center partners to utilize existing infrastructure (e.g., electronic health records) and personnel (e.g. clinic registered dietitians) to recruit, deliver and evaluate this intervention. As such, it is highly probable that the health center could adopt this or a similar intervention. Should that occur, we would suspect continued benefits on sleep. We are currently conducting a pragmatic intervention trial evaluating the effectiveness of a similar program fully implemented in the health center. We plan to evaluate the impact of that program on sleep.
In contrast to the existing literature on behavioral weight control trials and sleep outcomes, these findings are notable for several reasons. Emerging evidence indicates that weight loss improves sleep outcomes.(28) This evidence is largely derived from improvements in sleep post bariatric surgery;(46, 47) Only a few large-scale weight loss trials have shown that weight loss, among overweight and obese individuals, improves sleep outcomes. The POWER-UP(48) trial, which tested a behavioral weight loss intervention delivered in primary care, reported an increase in sleep duration, among participants who had lost ≥5% of their baseline body weight. Moreover, the Look AHEAD Study’s Sleep AHEAD(49) trial, widely considered one of the gold standard weight loss trials in participants with obstructive sleep apnea (OSA), showed that weight loss improved both sleep duration and quality. However, it is difficult to apply the results of the Sleep AHEAD trial to all overweight or obese individuals because participants with OSA experience anatomical and metabolic changes as a result of weight loss that may impact sleep in a unique manner. OSA patients may have a greater imbalance of hormones responsible for regulating hunger and satiety because of the chronic sleep restriction.(50–52) Although these studies are important for understanding the impact of weight loss on OSA severity, the current evidence base is lacking in how it is that weight loss improves sleep quality and duration among non-OSA patients. Even more limited is our understanding about the impact of preventing weight gain among non-OSA patients.
Beyond being one of the first studies to examine whether preventing weight gain can improve sleep, our study focused on young adult Black women with obesity. This population has a high risk of weight gain during young to middle adulthood(34) and generally reports shorter sleep duration and poorer sleep quality as compared to White women. Disparities in sleep by race are independent of and further exacerbated by socioeconomic factors.(26, 53–60) We found that the low-income Black women in our sample reported a similarly insufficient amount of sleep as is reported in previous studies. Moreover, the previous intervention trials examining whether weight control can improve sleep included predominantly moderate-to-high income, highly educated White women. Thus, our finding that weight gain prevention can improve sleep among low-income Black women is a strong contribution to the evidence base. To be able to impact sleep in this high-risk population is beneficial for preventing the numerous consequences of poor sleep that often co-occur with obesity.
Although this weight gain prevention approach among Black women was effective at improving global sleep quality, the intervention included multiple components and our study design does not allow us to dismantle their independent effects on sleep. However, we can only speculate about potential mechanisms.
First, evidence suggests a strong association between sleep and diet, (61, 62) but it is not clear whether insufficient sleep leads to consumption of calorie dense, nutritionally deficient foods or if a healthy diet improves sleep.(61–66) We did not measure diet in Shape so are not able to examine the impact of dietary changes on sleep. However, the intervention emphasized dietary patterns consistent with improved sleep and it is possible that adherence to these goals contributed to the success of Shape on both weight maintenance and improvements in sleep. Second, engaging in exercise or any degree of physical activity has been linked to improved sleep.(67–69) Given the strong link between exercise and weight maintenance,(70) physical activity was emphasized in Shape; however we found no significant differential effects of the intervention on physical activity and no association between physical activity and sleep health. Lastly, studies have shown that decreased sleep can increase the risk for depression.(71–73). In Shape, intervention participants saw large improvements in depression, while there was no change in depression among participants in the control arm.(37) Although the directionally is unknown, we can speculate that the improvements in sleep may be a cause of or determinant of the improvements in depression.
Despite the strengths of our study listed above, there are limitations worth noting. To begin, Shape was novel as it was a weight gain prevention trial, within a socioeconomically disadvantaged population. However, since the targeted population was low-income Black women, the findings may not be generalizable to other populations. This is important when considering whether these findings are applicable to clinical settings where they want to refer to programs shown to have broad population impact. Further, the MOS sleep scale was used because it is the most appropriate and reliable scale for populations in a large, primary care setting and outlines the multiple dimensions of sleep.(74) It is a viable measurement for studies in which general health is assessed and sleep parameters are of interest, however it has not been validated against objective measures of sleep.(74) Further, because we were collecting data in the clinical setting, we relied on the use of a limited, self-report assessment of medication use. The use of sleep aids is common,(75, 76) yet we only had 11 individuals report using any sleep aid. As such, it is possible that our self-report measure did not capture all possible medications or over-the-counter products. Moreover, because we only collected medication data at 18 months and our statistical models conservatively assumed that medication use was consistent at all evaluation points, we may have misclassifed the interaction between medication use and the intervention effect. It is also important to note that we did not collect data on all variables that may moderate the relationship between the Shape intervention and sleep such as type of employment or employment shifts. Future studies should consider including these variables if the intention is to examine the effect of behavioral interventions on sleep. Further, the attenuation of our findings after controlling for weight change suggests that it at least partially mediates the association between the intervention and improvements in sleep. As the primary aim of this analysis was to examine group differences in sleep outcomes, it is important for future studies to more robustly examine the potential mediating mechanisms.
Conclusions
In summary, we found that a weight gain prevention intervention among socioeconomically disadvantaged Black women led to significant improvements in sleep quality over 12 months, however 6 months later these improvements were no longer significant. Further, our findings suggest that weight gain prevention, rather than just weight loss, can be beneficial for improving sleep outcomes. More research is needed, however, to examine how to make such improvements persist post intervention, which components of interventions such as Shape contribute to improvements in sleep, and the potential impacts of a “maintain, don’t gain” weight-management approach on other patient-centered outcomes.
Acknowledgments
We express deep gratitude to the administration and staff of Piedmont Health for their continued collaboration and participation in the Shape Program. Most importantly, we would especially like to thank the women who participated in Shape.
Funding
This trial was funded by grant R01DK078798 from the National Institute for Diabetes and Digestive and Kidney Diseases. G.G. Bennett was supported by grant K22CA126992 from the National Cancer Institute. The funder had no role in study design, data collection, data analysis. and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Footnotes
Declaration of conflicting interests
Dori Steinberg and Gary G. Bennett have equity in Scale Down, LLC that produces mobile applications for weight loss. Dr. Bennett also has equity in Coeus Health and serves on Nutrisystem’s scientific advisory board. The remaining authors declare that they have no conflicting interests.
Trial Registration
The trial is registered with clinicaltrials.gov NCT00938535.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of internal medicine. 2001;161(13):1581. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults - United States, 2014. MMWR Morbidity and mortality weekly report. 2016;65(6):137–41. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 4.National Sleep Foundation. Adult Sleep Habits. Washington, DC: 2002. 2002 Report No. [Google Scholar]
- 5.Buysse DJ. Sleep health: can we define it? Does it matter. Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Hoevenaar-Blom MP, Spijkerman A, Kromhout D, van den Berg JF, Verschuren W. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyykkönen A-J, Isomaa B, Pesonen A-K, Eriksson JG, Groop L, Tuomi T, et al. Subjective Sleep Complaints Are Associated With Insulin Resistance in Individuals Without Diabetes The PPP-Botnia Study. Diabetes care. 2012;35(11):2271–8. doi: 10.2337/dc12-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction a population study. Circulation. 2011;124(19):2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 10.Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28(5):896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 11.Chaput JP, Despres JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50(11):2298–304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 13.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. SLEEP-NEW YORK THEN WESTCHESTER. 2003;26(2):117–29. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Metaanalysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring, Md) 2008;16(3):643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep medicine reviews. 2008;12(4):289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 20.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep medicine. 2014;15(1):42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep medicine. 2014;15(12):1456–62. doi: 10.1016/j.sleep.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Jean-Louis G, Youngstedt S, Grandner M, Williams NJ, Sarpong D, Zizi F, et al. Unequal burden of sleep-related obesity among black and white Americans. Sleep health. 2015;1(3):169–76. doi: 10.1016/j.sleh.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan V, Collop NA. Gender differences in sleep disorders. Current opinion in pulmonary medicine. 2006;12(6):383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 24.National Sleep Foundation. Summary of Findings: 2005 Sleep in America Poll. Washington, DC: 2005. [Google Scholar]
- 25.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30(1):41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 26.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. American journal of epidemiology. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 27.Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr, Buysse DJ, Kamarck TW, et al. Influence of race and socioeconomic status on sleep: Pittsburgh Sleep SCORE project. Psychosom Med. 2008;70(4):410. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoef SP, Camps SG, Gonnissen HK, Westerterp KR, Westerterp-Plantenga MS. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. The American journal of clinical nutrition. 2013;98(1):25–31. doi: 10.3945/ajcn.112.054650. [DOI] [PubMed] [Google Scholar]
- 29.Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiology & behavior. 2005;86(1–2):224–32. doi: 10.1016/j.physbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Chaput JP, Klingenberg L, Astrup A, Sjödin AM. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obesity Reviews. 2011;12(5):e12–e20. doi: 10.1111/j.1467-789X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 31.Passos GS, Poyares D, Santana MG, Teixeira AAdS, Lira FS, Youngstedt SD, et al. Exercise improves immune function, antidepressive response, and sleep quality in patients with chronic primary insomnia. BioMed research international. 2014:2014. doi: 10.1155/2014/498961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St-Onge M-P, Shechter A. Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Hormone molecular biology and clinical investigation. 2014;17(1):29–37. doi: 10.1515/hmbci-2013-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovin S, Bercea R, Cojocaru C, Rusu G, Mihăescu T. Body composition in obstructive sleep apneahypopnea syndrome bio-impedance reflects the severity of sleep apnea. Multidisciplinary respiratory medicine. 2010;5(1):1. doi: 10.1186/2049-6958-5-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens J, Cai J, Jones DW. The effect of decision rules on the choice of a body mass index cutoff for obesity: examples from African American and white women. The American journal of clinical nutrition. 2002;75(6):986–92. doi: 10.1093/ajcn/75.6.986. [DOI] [PubMed] [Google Scholar]
- 35.Foley P, Levine E, Askew S, Puleo E, Whiteley J, Batch B, et al. Weight gain prevention among black women in the rural community health center setting: the Shape Program. BMC public health. 2012;12:305. doi: 10.1186/1471-2458-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett GG, Foley P, Levine E, Whiteley J, Askew S, Steinberg DM, et al. Behavioral treatment for weight gain prevention among black women in primary care practice: a randomized clinical trial. JAMA internal medicine. 2013;173(19):1770–7. doi: 10.1001/jamainternmed.2013.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinberg DM, Askew S, Lanpher MG, Foley PB, Levine EL, Bennett GG. The effect of a “maintain, don’t gain” approach to weight management on depression among black women: results from a randomized controlled trial. American journal of public health. 2014;104(9):1766–73. doi: 10.2105/AJPH.2014.302004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Archives of internal medicine. 2012;172(7):565–74. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greaney ML, Quintiliani LM, Warner ET, et al. Weight management among patients at community health centers: The Be Fit Be Well Study. Obesity Management. 2009;5:222–8. [Google Scholar]
- 40.Bennett GG, Herring SJ, Puleo E, Stein EK, Emmons KM, Gillman MW. Web-based weight loss in primary care: a randomized controlled trial. Obesity (Silver Spring, Md) 2010;18(2):308–13. doi: 10.1038/oby.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders. 2009;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep) Sleep medicine. 2009;10(5):531–9. doi: 10.1016/j.sleep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Spritzer K, Hays R. MOS sleep scale: a manual for use and scoring, version 1.0. Los Angeles, CA: 2003. pp. 1–8. [Google Scholar]
- 44.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Protocol. Hyattsville, MD: US Department of Health and Human Services; 2007. Report No. [Google Scholar]
- 45.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep health. 2015;1(1):40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obesity surgery. 2013;23(3):414–23. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]
- 47.Toor P, Kim K, Buffington CK. Sleep quality and duration before and after bariatric surgery. Obesity surgery. 2012;22(6):890–5. doi: 10.1007/s11695-011-0541-8. [DOI] [PubMed] [Google Scholar]
- 48.Alfaris N, Wadden TA, Sarwer DB, Diwald L, Volger S, Hong P, et al. Effects of a 2-year behavioral weight loss intervention on sleep and mood in obese individuals treated in primary care practice. Obesity. 2015;23(3):558–64. doi: 10.1002/oby.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Archives of internal medicine. 2009;169(17):1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. European Respiratory Journal. 2003;22(2):251–7. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 51.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of internal medicine. 2004;141(11):846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 52.Lundahl A, Nelson TD. Sleep and food intake: A multisystem review of mechanisms in children and adults. Journal of health psychology. 2015;20(6):794–805. doi: 10.1177/1359105315573427. [DOI] [PubMed] [Google Scholar]
- 53.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. American journal of epidemiology. 2009;169(9):1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall M, Bromberger J, Matthews K. Socioeconomic Status as a Correlate of Sleep in African-American and Caucasian Women. Annals of the New York Academy of Sciences. 1999;896(1):427–30. doi: 10.1111/j.1749-6632.1999.tb08161.x. [DOI] [PubMed] [Google Scholar]
- 56.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of followup. Annals of epidemiology. 2007;17(12):948–55. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jean-Louis G, Kripke DF, Ancoli-Israel S, Klauber MR, Sepulveda RS. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biological psychiatry. 2000;47(10):921–7. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 58.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–11. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hale L, Troxel WM, Kravitz HM, Hall MH, Matthews KA. Acculturation and sleep among a multiethnic sample of women: The Study of Women’s Health Across the Nation (SWAN) Sleep. 2014;37(2):309–17. doi: 10.5665/sleep.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piccolo RS, Yang M, Bliwise DL, Yaggi HK, Araujo AB. Racial and socioeconomic disparities in sleep and chronic disease: results of a longitudinal investigation. Ethnicity & disease. 2013;23(4):499–507. [PMC free article] [PubMed] [Google Scholar]
- 61.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutrition research. 2012;32(5):309–19. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–59. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best practice & research Clinical endocrinology & metabolism. 2010;24(5):687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson RE, Emond JA, Natarajan L, Wesseling-Perry K, Kolonel LN, Jardack P, et al. Short sleep duration is associated with higher energy intake and expenditure among African- American and non-Hispanic white adults. The Journal of nutrition. 2014;144(4):461–6. doi: 10.3945/jn.113.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public health nutrition. 2011;14(05):889–95. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Youngstedt SD. Effects of exercise on sleep. Clinics in sports medicine. 2005;24(2):355–65. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Faulkner GE, Taylor AH. Exercise, health and mental health: Emerging relationships. Taylor & Francis; 2005. [Google Scholar]
- 69.Kubitz KA, Landers DM, Petruzzello SJ, Han M. The effects of acute and chronic exercise on sleep. Sports Medicine. 1996;21(4):277–91. doi: 10.2165/00007256-199621040-00004. [DOI] [PubMed] [Google Scholar]
- 70.Fogelholm M, Vainio H. Weight control, physical activity and cancer–strong links. Obes Rev. 2002;3(1):1–3. doi: 10.1046/j.1467-789x.2002.00051.x. [DOI] [PubMed] [Google Scholar]
- 71.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 72.Ford DE, Cooper-Patrick L. Sleep disturbances and mood disorders: an epidemiologic perspective. Depression and anxiety. 2001;14(1):3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- 73.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders. 2011;135(1–3):10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Smith MT, Wegener ST. Measures of sleep: the insomnia severity index, medical outcomes study (MOS) sleep scale, Pittsburgh sleep diary (PSD), and Pittsburgh sleep quality index (PSQI) Arthritis Care & Research. 2003;49(S5):S184–S96. [Google Scholar]
- 75.Pearson NJ, Johnson LL, Nahin RL. Insomnia, Trouble Sleeping, and Complementary and Alternative MedicineAnalysis of the 2002 National Health Interview Survey Data. Arch Intern Med. 2006;166(16):1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 76.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National Use of Prescription Medications for Insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]