Abstract
Background
The efficacy of opioid administration to reduce postoperative pain is limited by respiratory depression. We investigated whether clinically relevant opioid concentrations altered the respiratory pattern in the parabrachial nucleus (PBN), a pontine region contributing to respiratory pattern generation and compared these effects to a medullary respiratory site, the preBötzinger Complex.
Methods
Studies were performed in 40 young and 55 adult artificially ventilated, decerebrate rabbits. We identified an area in the PBN where α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) microinjections elicited tachypnea (tPBN). Two protocols were performed in separate sets of animals: 1) Bilateral microinjections of the mu-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO, 100 μM) into the tPBN determined the effect of maximal mu-opioid receptor activation. 2) Respiratory rate was decreased with continuous intravenous infusions of remifentanil. The opioid-antagonist naloxone (1mM) was then microinjected bilaterally into the tPBN to determine if the respiratory rate depression could be locally reversed.
Results
Average respiratory rate was 27±10 breaths/min. 1) DAMGO injections decreased respiratory rate by 62±20% in young and 45±26% in adult rabbits (both P<0.001). 2) During intravenous remifentanil infusion, bilateral naloxone injections into the tPBN reversed respiratory rate depression from 55±9% to 20±14% in young and from 46±20% to 18±27% in adult rabbits (both P<0.001). The effects of bilateral DAMGO injection and intravenous remifentanil on respiratory phase duration in the tPBN was significantly different from the preBötzinger Complex.
Conclusions
The tPBN is highly sensitive to mu-opioid receptor activation and mediates part of the respiratory rate depression by clinically relevant administration of opioids.
Keywords: parabrachial nucleus, respiratory depression, mu-opioid receptors, remifentanil
Introduction
Opioids are standard treatment to reduce perioperative and chronic pain, however, their use is limited by respiratory depression 1–5. Respiratory depression is primarily mediated by mu-opioid receptors 6,7, which are widely expressed throughout the brainstem respiratory network 3,4,8–16. The typical pattern of clinical, opioid-induced respiratory depression is a decrease in respiratory rate and even apnea. As respiratory rhythm is generated by the Central Pattern Generator (CPG) 17–20 in the brainstem, studies have looked for opioid effects in this area 4,10,14. Local application of mu-opioid receptor agonists at pharmacologic, micromolar concentrations cause significant depression of neuronal activity in several areas of the CPG, i.e., the ventral respiratory column 21,22 including the preBötzinger Complex (preBötC) 9,10,23,24 in the rostral medulla and the parabrachial nucleus (PBN) 14 and Kölliker-Fuse nucleus (KFN) 25 in the rostral pons.
There are conflicting results regarding the brainstem location where clinically relevant, nanomolar opioid concentrations depress respiratory rate 26–29. Since the discovery of pacemaker-like, opioid-receptor containing neurons in the preBötC, investigators have focused on this area 30. The importance of the preBötC regarding clinical opioid effects was called into question when in an in vivo decerebrate dog model local injection of naloxone into the preBötC did not reverse the bradypneic effects of an intravenous (IV) remifentanil infusion 9. In contrast, sequential injections of naloxone into the PBN region significantly reversed respiratory rate depression in the same model 14. Our previous study in the in vivo decerebrate rabbit model showed that IV remifentanil infusion indeed affected inspiratory and expiratory phase timing in the preBötC but that reversing these effects with local naloxone injections did not reverse the respiratory rate depression 23. These seemingly contradictory results suggest that systemic opioids at clinically relevant concentrations affect more than one area within the respiratory network but that not all effects necessarily lead to changes in respiratory rate.
Our current study focused on the rabbit PBN: Previous studies had achieved respiratory rate depression with a mu-opioid receptor agonist bath applied to the dorsal surface of the pons in cats31 and with grid-wise injections of mu-opioid receptor agonists into an area caudal of the inferior collicle and several millimeters lateral from midline in dogs 14. We sought to determine 1) whether there is a subregion of the PBN where mu-opioid receptor agonists depress respiratory rate, 2) whether this location can be identified by the tachypneic response to local injection of the glutamate agonist α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), 3) the maximal respiratory rate depression that pharmacological concentrations of mu-opioid receptor agonists can achieve in this area, and 4) the degree by which respiratory rate depression from systemic, clinically relevant concentrations of mu-opioid receptor agonists can be reversed in this area. Since the contribution of the pons to the respiratory pattern seems to change throughout development 32 experiments were performed in young and adult rabbits. Finally, we compared the results from the PBN with our previous study investigating opioid effects on the preBötC 23 to determine any differences in opioid effects on the two brainstem sites.
Methods
Surgical Procedures
This research was approved by the subcommittee on animal studies of the Zablocki VA Medical Center, Milwaukee, WI, in accordance with provisions of the Animal Welfare Act, the Public Health Service Guide for the Care and Use of Laboratory Animals, and VA policy. The surgical procedures were similar to previous studies in this laboratory 23. In short, adult (> 6 months; 3–4 kg) and young (14–25 days; 200–500g) New Zealand White rabbits of either sex were induced with 5% sevoflurane via facemask. Animals were tracheotomized and then ventilated with an anesthesia machine ventilator (Ohmeda CD, GE Datex Ohmeda, USA) or at a weight <400g, with a small animal ventilator (SAR-830 ventilator, CWE, Colorado Springs, CO). Anesthesia was maintained with 1–2% (young) or 1.5–3% (adult) isoflurane 33. Anesthetic depth was increased for signs of inadequate anesthesia, such as increased heart rate, blood pressure or lacrimation. FiO2, expiratory carbon dioxide and expiratory isoflurane concentration were continuously recorded with an infrared analyzer (POET II, Criticare Systems, USA). Skin was infiltrated with 1% lidocaine before each skin incision. Femoral arterial and venous lines were used for blood pressure monitoring and infusion of solutions, respectively. Lactated Ringer’s solution with 2μg/ml epinephrine was continuously infused at 1ml/h. This infusion rate did not result in appreciable changes in heart rate and blood pressure. Infusion rate was increased as needed for hypotension in response to drug injections or from blood loss. Rectal temperature was monitored and maintained at 37.0 ± 0.5 °C with a warming blanket. The animal was placed in a stereotaxic frame (David Kopf Instruments, Tujunga, USA), and a pneumothorax was performed to prevent ventilator artifact during neuronal recording. Blunt precollicular decerebration with complete removal of the forebrain was performed through a parietal craniotomy. After decerebration, isoflurane was discontinued or continued at subanesthetic levels (0.3–0.4%) for additional blood pressure control. In the latter case, isoflurane concentration was not changed throughout the experimental protocol. The brainstem was exposed via occipital craniotomy and partial (young) or complete (adult) removal of the cerebellum was performed. Animals were paralyzed with vecuronium (initially 1 mg/kg and re-dosed as needed) to avoid motion artifacts during neural/neuronal recording. The vagal nerves were left intact. Phrenic nerve activity was recorded with fine bipolar electrodes through a posterior neck incision. Throughout the experiment animals were ventilated with a hyperoxic gas mixture (FiO2 0.6) to achieve functional denervation of the peripheral chemoreceptors and at mild hypercapnia (expiratory CO2: 45–55 mmHg) to ensure sufficient respiratory drive (i.e., above the apneic threshold) including during systemic opioid infusion. At the end of the experiment the animals were euthanized with intravenous KCl. In a subgroup of animals the brainstem was fixed via transcardial perfusion for histological analysis. The brainstem tissue was cryo-protected, frozen and serially sectioned for Nissl staining and identification of fluorescent tracer injection sites.
Experimental procedures including neuronal recording, drug application, measurement of respiratory variables and data analysis
The neuronal recording and microinjection techniques have been previously described in detail 34,35. In short, extracellular neuronal recordings were obtained using glass multibarrel micropipettes (20–40 μm tip diameter) consisting of three drug barrels and a recording barrel containing a 7μm thick carbon filament (~0.5 MΩ). Barrels were filled with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA, 50 μM), the mu-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO, 100 μM) and the opioid receptor antagonist naloxone (1 mM), which were dissolved in artificial cerebrospinal fluid (aCSF). The microinjected volume was determined via height changes in the meniscus in the respective pipette barrel with a 100× monocular microscope and calibrated reticule (resolution ~3.5 nl). Respiratory neurons were classified according to their discharge pattern and their temporal relationship relative to the phrenic neurogram. The neuronal and pressure microejection marker signals were continuously displayed and recorded along with the phrenic neurogram, respiratory rate-meter, arterial blood pressure, airway pressure and expiratory carbon dioxide on a computerized chart recorder (Powerlab/16SP; ADInstruments, Castle Hill, Australia).
Post hoc analysis averaged respiratory cycles from the phrenic neurogram. For each study protocol, steady-state conditions were obtained for respiratory parameters both pre- and post-drug injection. Based on the phrenic neurogram, between 10 and 50 consecutive respiratory cycles were averaged over 1–2 minutes with the number of cycles dependent on the respiratory rate (breaths/min). We determined peak phrenic activity (PPA), respiratory rate and inspiratory (TI) and expiratory (TE) duration. Respiratory drive was calculated as PPA/TI.
Exploratory study 1: Functional identification of an opioid- and AMPA-sensitive area (“tachypneic area”) in the rostral pons
Chamberlin and Saper described various changes of the respiratory pattern with glutamate injection into the PBN/KFN region in rats studied in-vivo with an increase in respiratory rate elicited mostly in the PBN region 15. Prkic et al. showed in dogs studied in-vivo that grid-wise injection of DAMGO into the PBN area, i.e., between the inferior collicle and superior cerebellar peduncle decreased respiratory rate 14. Guided by these studies we performed grid-wise microinjections of AMPA (70 nl) with step size 0.5 mm into the equivalent area in adult (n=12) and young (n=14) rabbits. Recorded neuronal activity was used to guide the depth of injection. We identified a location in the rostral pons, where AMPA microinjection caused an increase in respiratory rate (tachypnea). On average, in young rabbits this area was located 0.5 ± 0.5 mm caudal to the inferior collicle, 2.0 ± 0.5 mm lateral to midline, and 6.0 ± 1.0 mm below the dorsal surface of the brainstem/residual superior cerebellar peduncle (n=26). The cerebellum was not completely removed in animals <400g. In adult rabbits the area was located 0.5 ± 0.2 mm caudal to the inferior collicle, 2.7 ± 0.1 mm lateral of midline and 7.4 ± 0.2 mm below to the dorsal surface of the brainstem/residual superior cerebellar peduncle (n=35). Often a lesser tachypneic response was observed with AMPA injection 0.5mm rostral and/or caudal and 0.5mm medial to this area (Figure 1A). We injected the mu-opioid receptor agonist DAMGO bilaterally into the area of greatest tachypnea and found that an injection volume of 350nl in young and 700nl in adult rabbits was necessary to achieve maximal respiratory rate depression (n=6) but that with these volumes additional injections around this area did not produce any additional rate depression (n=12). The majority of neurons we recorded in this area had no modulation with respiratory phase, i.e., a tonic discharge pattern (young: tonic neurons n=411, expiratory neurons n=4; adult: tonic neurons n=444, expiratory neurons n=6, inspiratory neurons n=2). Postmortem histology located the area in the PBN region (Figure 1B). We will thus refer to this “tachypneic area” as the “tPBN”. Before each study protocol, the “tPBN” was located bilaterally according to stereotaxic coordinates, presence of neuronal discharge activity and maximal tachypneic response to AMPA injection.
Figure 1.
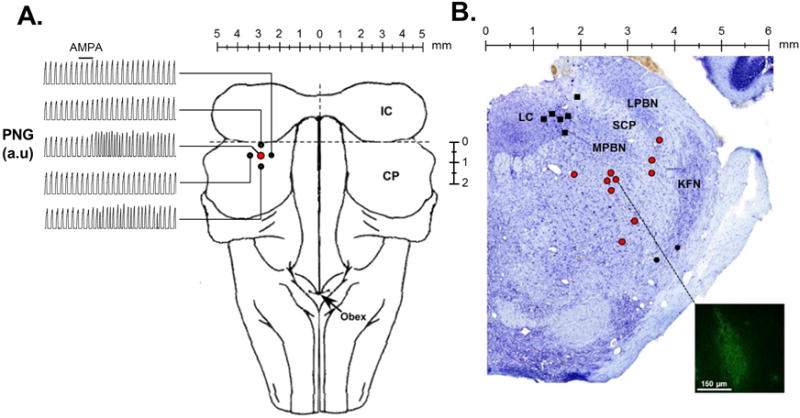
Injections of the glutamate receptor AMPA were used to functionally identify the “tachypneic area” in the parabrachial nucleus (tPBN). A: Dorsal brainstem image with representative records of the time-averaged phrenic neurogram (PNG, a.u.=arbitrary units). AMPA injection (solid bar) caused marked tachypnea in the tPBN (red circle), but little or no change in four surrounding sites 0.5 mm off-target (solid circles) in an individual adult rabbit. B: Location of fluorescent tracer or Chicago sky blue (700nl) injections into the LC (solid black squares), tPBN (red circles), and KFN (solid black circles) in Nissl stained tissue (Control Study). The inset depicts an example of the tracer spread which has been contrast enhanced to highlight the injection site. LC: locus coeruleus, LPBN: lateral parabrachial nucleus, MPBN: medial parabrachial nucleus, SCM: superior cerebellar peduncle, KFN: Kölliker Fuse nucleus.
Histological identification of the tPBN location
In four adult animals, the tPBN was functionally identified and then marked by injection of a fluorescent tracer (700nl, 5% Red Retrobeads, Lumafluor Inc, USA). The animals were then transcardially perfused with phosphate buffered saline (PBS) and 4% paraformaldehyde in PBS, followed by extraction of the brainstem tissue. The tissue was cryoprotected in 30% sucrose for at least 72 h, frozen and serially sectioned (25 μm) in the transverse plane from the superior cerebellar peduncle to 2 mm rostral to the caudal border of the inferior colliculus (~5 mm). Each section was adhered to electrostatically treated slides and was Nissl-stained for identification of gross anatomical structures and the relative location of fluorescent tracer (Figure 1B).
For Nissl-staining, after a 10-min drying period, the tissue was cleared in Histoclear (Sigma, USA) for 1 h followed by sequential rehydration in 100%, 95%, and 70% ethanol before a 10 min distilled, deionized water rinse. The tissue was exposed to 4% cresyl violet for 12 min followed by sequential dehydration in ethanol, including exposure to 0.5% acetic acid ethanol. The tissue was again cleared in Histoclear for 1 h followed by coverslipping. Tissue was stained at 100-μm intervals, and images were captured at 4000 DPI (Nikon Super Coolscan 9000). Metamorph imaging software was used to spatially calibrate each image.
To identify the location of the fluorescent tracer, each section was examined with a Nikon Eclipse E600 fluorescent microscope and photographed with a Hamamatsu ORCA-FLASH 4.0 LTS SCMOS camera. Images were acquired at a resolution of 2,048 × 2,048 pixels, 6.5 ×6.5 um pixel size. The location of the fluorescent tracer relative to midline and the ventral surface of the brainstem were recorded. Distance from the brainstem dorsal surface was not used due to variation in the amount of cerebellar tissue remaining in each animal. The medio-lateral and dorso-ventral coordinates of the tracer were marked on the spatially calibrated Nissl stained image.
Exploratory study 2: Verification that drugs injected into the tPBN do not affect the Locus Coeruleus (LC) and Kölliker-Fuse (KF) nuclei
The “tachypneic area” in the PBN is in close anatomical proximity to other respiratory-related areas that also contain opioid receptors, i.e., the LC 36,37 and the KF 25,38 nucleus. To verify that the drug effects observed with injection into the tPBN were not due to diffusion into these adjacent areas, we performed a separate set of experiments in 8 adult animals where we injected DAMGO into the bilateral tPBN and then attempted to reverse the DAMGO effect with naloxone injections into the bilateral LC and KF.
Location of the LC
Grid-wise injections of AMPA (50μM, 70nl) into the LC area to identify an area involved in respiratory rate control did not reveal consistent changes in respiratory rate (n=6). In 2 animals we observed transient slowing of respiratory rate in a location 1mm medial, 0.5 mm rostral and 3mm dorsal from the “tachypneic area” of the PBN, i.e., about 1.5mm lateral from midline, 0.5mm caudal from inferior collicle and 3.5mm ventral to the dorsal surface. DAMGO (100μM, 700nl) injection into this area did not have any effect. Dye injection confirmed that this area was within the LC. For the Control Protocol (see below) we thus determined the location of the tPBN first and used the coordinates 1mm medial, 0.5mm rostral and 3mm dorsal to this area for our LC injections. Dye injection into these areas after completion of the protocol confirmed that this area was always in the LC.
Location of the KF
We identified the KF by injecting AMPA (50μM, 70nl) in grid-wise fashion lateral, caudal and ventral to the tPBN where it was expected per histology. We found an area where AMPA injection caused bradypnea as described by Dutschmann 39 and Levitt 25, which was located on average 0.5mm caudal, 0.5–1mm lateral and 2mm ventral to the tPBN. Dye injection after completion of the Control Protocol confirmed that this area was in the KF.
Control Protocol
After determining the location of the tPBN, the LC and the KF, we performed bilateral DAMGO injections (100μM, 700nl) into the tPBN analogous to Protocol 1 (see below: Main Studies). Three minutes after the second DAMGO injection, i.e., when steady-state respiratory depression was reached and when we would inject naloxone into the tPBN in Protocol 1 (see below: Main Studies), we instead injected naloxone (1mM, 700nl) into the bilateral LC and the bilateral KF. Finally, we injected naloxone (1mM, 840nl) into the bilateral tPBN. Bilateral DAMGO injections into the tPBN decreased respiratory rate from 24±12 breaths/min to 12±7 breaths/min. Naloxone injections into the bilateral LC and KF did not have any reversal effect (10±8 breaths/min, P=0.503). However, final injection of naloxone into the bilateral tPBN reversed the depression (18±11 breaths/min, P=0.026, all 1-way RM ANOVA). We conclude that the respiratory depression achieved with local DAMGO injection in Protocol 1 (see below: Main Studies) as well as any local naloxone reversal of IV remifentanil-induced respiratory depression in Protocol 2 (see below: Main Studies) indicate opioid effects solely mediated by the PBN.
Main Studies
Protocol 1: Effects of pharmacological opioid concentrations on the tPBN in young and adult rabbits
The experimenters were not blinded to the experimental conditions. Animals were not randomized to the protocols as New Zealand White rabbits are a purebred strain with little physiological variation between animals. Only one complete protocol was performed per animal to avoid any confounding effects from residues of the locally injected drugs.
To determine the effect of maximal mu-opioid receptor activation 350 nl (young) or 700 nl (adult) DAMGO (100μM) was microinjected bilaterally into the tPBN. Injections were spaced three minutes apart, which was sufficient to accomplish steady-state respiratory rate depression. Subsequently, the effect was reversed with bilateral injections of the competitive opioid antagonist naloxone (1mM, young: 420 nl; adult: 840 nl) at the same coordinates.
Protocol 2: Effect of clinical opioid concentrations on the tPBN in young and adult rabbits
To determine the contribution of the tPBN to systemic opioid-induced respiratory depression we injected naloxone into the tPBN during continuous IV infusion of the potent mu-agonist remifentanil. The tPBN was identified on both sides of the brainstem as described above. Then remifentanil was infused intravenously at 0.08–0.5 μg/kg/min until respiratory rate was depressed by approximately 50%. These infusion rates match the “clinically relevant”, analgesic dose-rates described for rabbits 40. Remifentanil was chosen for its short onset time and short half-life (~4 min) 41 that remains independent of the duration of the infusion 42,43. After reaching steady-state respiratory depression for at least 5 minutes, 1mM naloxone (young: 420 nl; adult: 840 nl) was injected bilaterally into the tPBN with injections spaced three minutes apart. After respiratory pattern had again reached a steady-state, a single intravenous injection of naloxone (30–80 μg/kg) was given to completely reverse any residual systemic opioid effect. Only then was the remifentanil infusion discontinued.
Protocol 3: Control studies – Effects of naloxone or aCSF injection into the tPBN
To ensure that the naloxone effect represented reversal of remifentanil-induced respiratory depression rather than reversal of intrinsic opioidergic tone 15 we injected naloxone (1mM, 840 nl) into the bilateral tPBN without remifentanil infusion. Similarly, to rule out an independent effect of aCSF, which was used as solvent for all injected drugs, aCSF (840nl) was injected into the bilateral tPBN without remifentanil infusion. In the interest of reducing animal use, control experiments were only conducted in adult rabbits.
Statistical Analysis
Statistical analysis was performed using SigmaPlot 11 (Systat Software, Richmond, CA) for ANOVA with Tukey test for pairwise multiple comparisons in the Exploratory Study 2 and the paired t-tests in Protocol 3. Data sets were tested for normal distribution (Kolmogorov –Smirnov test). R software (R package version 3.1–128, URL: http://CRAN.R-project.org/package=nlme) was used for the linear mixed effect model with Bonferroni correction for multiple comparisons for the results for Protocols 1 and 2 and the comparison between tPBN and preBötC. We did not perform a formal a priori power analysis. Sample sizes for each protocol were based on previous studies after an initial review of the first 5 or 6 animals per protocol, and no adjustments were made for interim analyses. Comparable studies have used 8 to 15 rabbits 23 or 4 to 9 rats 10 or 10 to 21 dogs per protocol 9,14. For all protocols statistical tests were performed on raw data except for PPA and respiratory drive, where activity is measured in arbitrary units and normalization to control is necessary to allow for comparison between animals. The effects of opioid agonists/antagonists on all respiratory parameters were determined using a linear mixed model including fixed effects for age (adult/young), type of drug (Protocol 1: control, local DAMGO, local naloxone; Protocol 2: control, IV remifentanil, IV remifentanil + local naloxone, IV remifentanil + IV naloxone) and study (tPBN/preBötC). Interaction terms measured any interaction between type of drug, type of study and the factor age. Other interaction terms were considered but found to be non-significant. The intercept consisted of an overall intercept term, i.e., the average response for an adult control animal in the preBötC study, a random effect for each individual animal and the model noise term. Similarly, the inputs to inspiratory and expiratory duration as determined from the two studies were compared using a linear mixed effect model with fixed effects for age, study and input source and Bonferroni correction for multiple comparisons.
Results
Protocol 1: Effects of pharmacological opioid concentrations on the tPBN in young and adult rabbits
Average respiratory rate in all experiments was 27±10 breaths/min. Bilateral injection of DAMGO into the tPBN resulted in a significant decrease in respiratory rate from 26±10 breaths/min by 15.5 ± 9.1 breaths/min (62±20%) in young rabbits (n=14) and a decrease from 23±13 breaths/min by 11.0 ± 7.2 breaths/min (45±26%) in adults (n=11; P <0.001; Figure 2). This was due to an increase in both inspiratory duration (TI) (young: 1.2 ± 2.0 sec; adult: 1.0 ± 2.2; P=0.004) and TE (young: 6.3 ± 5.7 sec; adult: 3.1 ± 4.4 sec; P<0.001). Bilateral DAMGO injection also decreased PPA (young: −17± 32%; adult: −25 ± 29%; P=0.007) and respiratory drive (PPA/TI) (young: −49 ± 26%; adult: −38 ± 56%; P <0.001). All DAMGO effects were near-completely reversed by bilateral injection of naloxone at the same coordinates (P<0.05). There was no significant difference in DAMGO effect between young and adult rabbits (P>0.05) for any parameters. For full disclosure, one young animal (23d, 350g) was removed from above analysis as DAMGO injection resulted in severe, naloxone-reversible inspiratory apneusis with TI increased 50x more than average. The effect suggested incorrect injection into the KFN (see Discussion for KFN characteristics).
Figure 2.
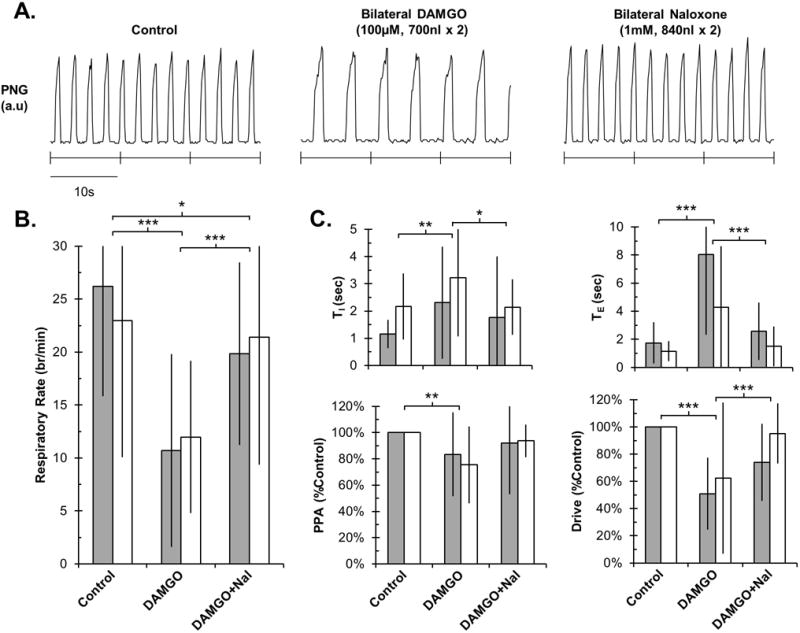
Bilateral DAMGO injection into the tPBN significantly reduced respiratory rate and drive in young and adult rabbits, which was reversible with local naloxone. A: PNG tracing during control and after drug injections in an individual adult rabbit. B: Summary data for changes in respiratory rate. C: Summary data for changes in other respiratory parameters. Grey bars: young (n=14). White bars: adult (n=11). Mean ± SD. * P<0.05, ** P<0.01 and *** P<0.001 indicate significant differences between drug application conditions (linear mixed model with factors: drug application and developmental age). There were no differences in drug effects between young and adult animals.
Protocol 2: Effect of clinical opioid concentrations on the tPBN in young and adult rabbits
In a separate group of 12 young and 12 adult rabbits, naloxone was injected bilaterally into the tPBN during systemic IV remifentanil infusion (rate: 0.08–0.5 μg/kg/min). Baseline respiratory rate was 31±4 breaths/min in young and 27±11 breaths/min in adult animals. IV remifentanil depressed respiratory rate by 17.0 ± 3.4 breaths/min (55±9%) in young rabbits and by 12.8 ± 7.0 breaths/min (46±20%) in adults (P <0.001; Figure 3). This was due to an increase in TI (young: 0.5 ± 0.4 sec; adult: 0.3 ± 0.6 sec; P <0.001) and TE (young: 2.3 ± 1.6 sec; adult: 2.5 ± 3.3 sec; P <0.001). IV remifentanil also decreased PPA (young: −24 ± 22%; adult: −38 ± 18%; P<0.001) and respiratory drive (young: −48 ± 21%; adult: −51 ± 15% (P<0.001).
Figure 3.
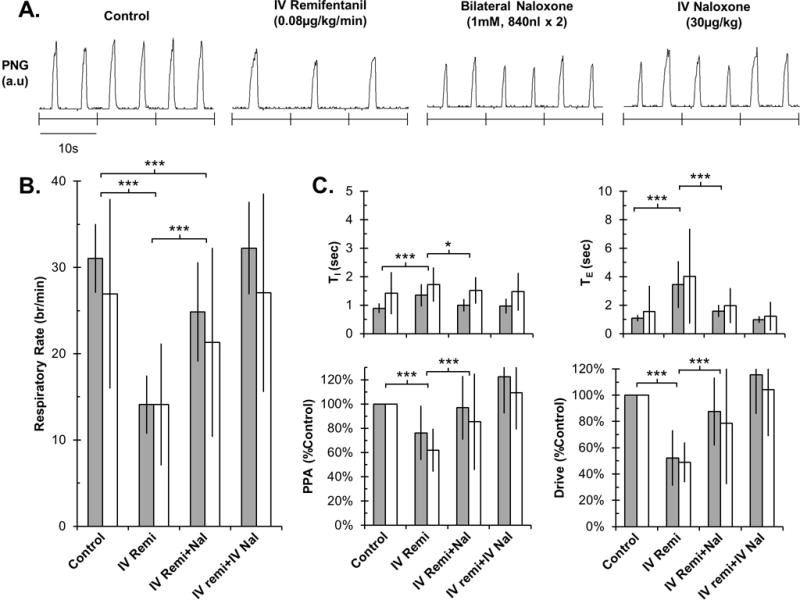
Bilateral naloxone injection into the tPBN significantly reversed IV remifentanil-induced respiratory rate depression in young and adult rabbits. Residual changes from control were completely reversed with IV naloxone injections. A: PNG tracing during control conditions and after drug injections in an individual adult rabbit. B: Summary data for changes in respiratory rate. C: Summary data for changes in other respiratory parameters. Grey bars: young (n=12). White bars: adult (n=12). Mean±SD. * P<0.05; *** P<0.001 indicate significant differences between drug application conditions (linear mixed model, factors: drug application and developmental age). There were no differences in drug effects between young and adult animals.
Bilateral injection of naloxone into the tPBN resulted in a partial recovery of respiratory rate by 10.8 ± 5.8 breaths/min, which was within 20±14% of control rate in young rabbits, and by 7.2 ± 11.0 breaths/min, which was within 18±27% of control rate in adults (P<0.001; Figure 3). This was due to a decrease in TI (young: −0.4 ± 0.2 sec; adult: −0.2 ± 0.5 sec; P=0.02) as well as TE (young: −1.8 ± 0.4 sec; adult: −2.0 ± 1.2 sec; P <0.001). Bilateral naloxone injection also resulted in a recovery in PPA (young: 21 ± 26%; adult: 24 ± 40%; P=0.001) and respiratory drive (young: 35 ± 26%; adult: 30 ± 47%; P<0.001). IV naloxone infusion (30–80 μg/kg) completely reversed any residual remifentanil effects. There were no significant differences between young and adult rabbits in either the IV remifentanil effect or the local naloxone reversal (P>0.05 for all parameters).
Protocol 3: Control studies – Effects of naloxone or aCSF injection into the tPBN
In 6 adult rabbits bilateral injection of naloxone into the tPBN under control conditions had no significant effect on respiratory rate TI, TE, PPA, or respiratory drive (all P>0.05; data not shown). In a separate set of animals, injection of 700 nl of aCSF into the tPBN did not have any effect on respiratory rate and pattern (n=8, all P>0.05; data not shown).
Comparison of the opioid effect on the tPBN versus the preBötC
The linear mixed model allowed comparison of our current data with data from our previous study that investigated opioid effects on the preBötC with similar protocols. Pharmacological concentrations of DAMGO affected the respiratory pattern in both areas, however, there were some distinct differences (Figure 4): DAMGO depressed respiratory rate significantly more in the tPBN than the preBötC (difference 7±3 breaths/min, P=0.012). While the increase in TE was similar (P=0.456), DAMGO caused an increase in TI in the tPBN but shortened it in the preBötC (difference 2.2±0.6 sec, P<0.001). DAMGO injection decreased respiratory drive in the tPBN but not in the preBötC (difference 60±14%, P<0.001).
Figure 4.
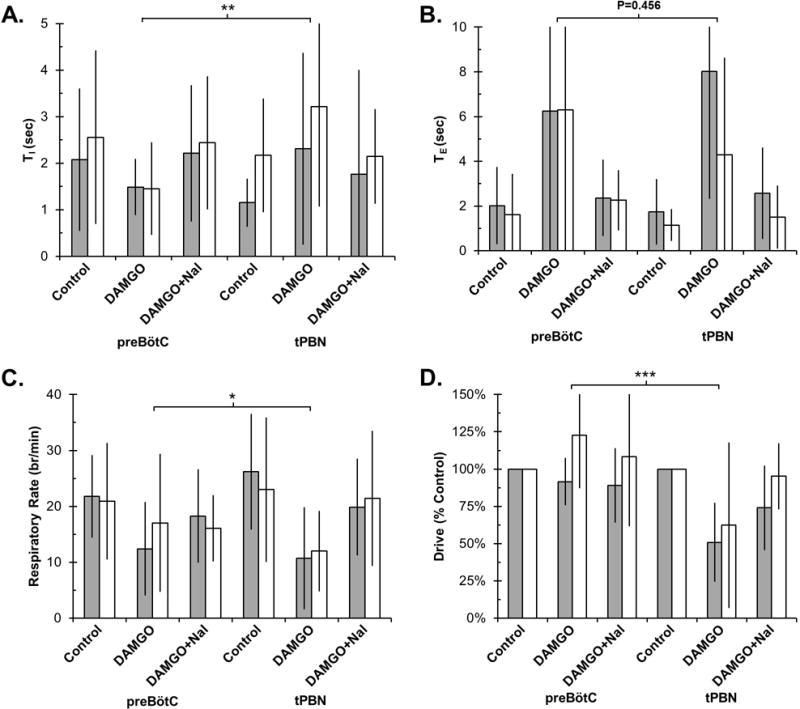
Bilateral DAMGO injection into the tPBN had a significantly different effect on respiratory timing and drive compared to the preBötC. Values are displayed as Mean±SD for TI (A), TE (B), respiratory rate (C), and respiratory drive (D). Note differences in scale for TI and TE. Values on the left are from our previous study in the preBötC and values on the right are from our current tPBN study. PreBötC: Grey bars: young, n=8. White bars: adult, n=16. tPBN: Grey bars: young, n=14. White bars: adult, n=11. * P<0.05, ** P<0.01 and *** P<0.001 indicate significant differences in DAMGO effect between studies (linear mixed model, factors: study, drug application, developmental age).
Clinical concentrations of remifentanil increased TE more in the tPBN than in the preBötC (difference 5.0±1.4sec, P=0.001, Figure 5), and there was an increase in TI in the tPBN but a decrease in the preBötC resulting in a significant difference (1.4±0.4 sec, P=0.002). The depression of respiratory rate was not statistically significant between the tPBN and the preBötC (P=0.491). Remifentanil depressed respiratory drive more in the tPBN than in the preBötC (difference 41±15%, P=0.005).
Figure 5.
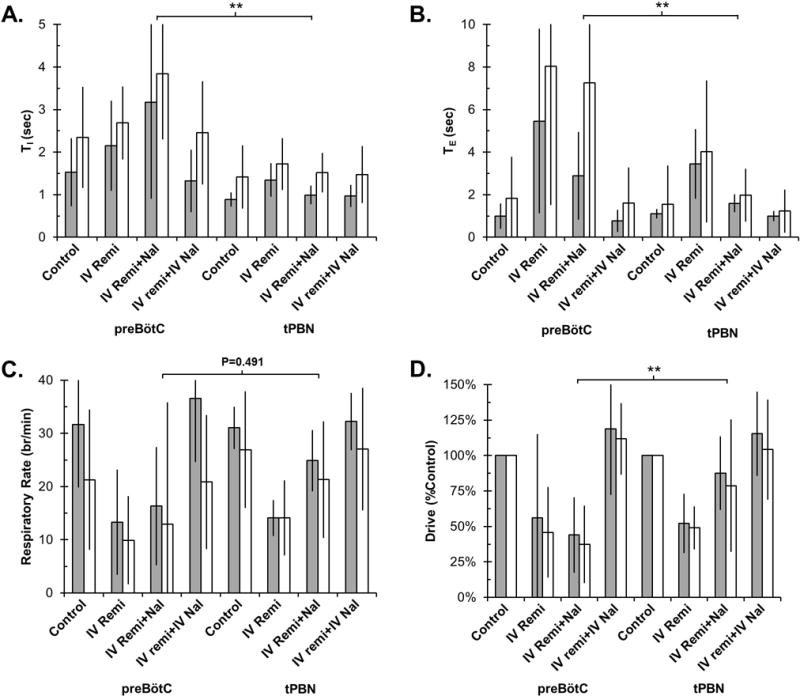
Bilateral naloxone injection into the tPBN during IV remifentanil infusion had a significantly different effect on respiratory timing and drive compared to the preBötC. Values are displayed as Mean±SD for TI (A), TE (B), respiratory rate (C), and respiratory drive (D). Note differences in scale for TI and TE. Values on the left are from our previous study in which naloxone was injected into the preBötC and values on the right are from our current study. PreBötC: Grey bars: young, n=14. White bars: adult, n=16. tPBN: Grey bars: young, n=12. White bars: adult, n=12. ** P<0.01 indicate significant differences between study and drug application conditions (linear mixed model, factors: study, drug application, developmental age).
This experimental protocol allowed us to estimate the contributions of the tPBN versus non-tPBN areas to inspiratory and expiratory phase duration and to compare it with the contributions of the preBötC versus non-preBötC areas obtained in our previous study 23. The calculations are described in detail in Appendix 1, and the resulting input values are summarized in Table 1. Intrinsic activity (Iintrinsic) of inspiratory and expiratory neurons is modulated by opioid-sensitive inputs (F) that originate from the preBötC area (FpreBötC), the tPBN area (FtPBN) and potentially additional areas (Fadditional). Opioid-sensitive input from the preBötC increased TI while input from the tPBN decreased TI. Inputs from the preBötC and the tPBN shortened TE. PreBötC input to TI was significantly different from non-preBötC inputs (P=0.024). Pontine input to TE was significantly larger than medullary input (P=0.0003). The effects are summarized in a hypothetical model (Figure 6).
Table 1.
Pooled Data for Opioid-Sensitive Inputs to Inspiratory and Expiratory Phase Duration
| Study | Input source | Input to TI | Input to TE |
|---|---|---|---|
| preBötC | FpreBötC | *−0.12 (−0.29 to 0.06) | 0.15 (0.04 to 0.34) |
| FNon-preBötC | 0.57 (0.14 to 0.97) | #1.39 (0.73 to 2.27) | |
| tPBN | FPBN | 0.39 (0 to 0.63) | 0.60 (0.33 to 0.72) |
| FNon-PBN | 0.22 (−0.03 to 0.47) | 0.61 (0.26 to 1.01) |
Inputs modulating phase duration (F) were computed separately for inspiratory (TI) and expiratory (TE) duration from our current study in the parabrachial nucleus (tPBN) and our previous study in the preBötzinger Complex (preBötC) 23. There were no differences between young and adult animals (P=0.69 for TI, P=0.385 for TE). Values for both age groups were pooled in the table for readability. Negative inputs increases respiratory phase duration; positive inputs decrease respiratory phase duration.
: preBötC input to TI (FpreBötC) was significantly different from pontine (P=0.024).
: FNon-preBötC inputs to TE were significantly larger than FpreBötC (P=0.0003). Differences in magnitude between FNon-preBötC and FPBN to TI and TE suggest additional opioid-sensitive inputs from other brainstem sites but this was not statistically significant. Median (25–75% range); mixed linear model with fixed effects age, study and input source.
Figure 6.
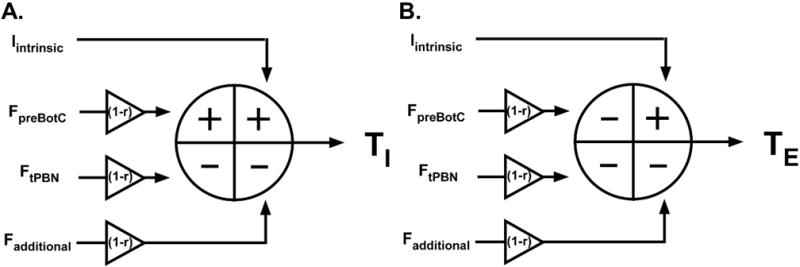
Hypothetical model of inputs determining (A) inspiratory and (B) expiratory phase duration. Intrinsic activity (Iintrinsic) is modified by opioid-sensitive inputs from the preBötzinger Complex (FpreBötC), the tachypneic area of the parabrachial nucleus (FtPBN) and additional brainstem sites (Fadditional). Systemic opioids reduce these inputs by the factor “r”. FpreBötC increases (+) inspiratory duration (TI) while FtPBN and Fadditional decrease (−) TI. Systemic opioids reduce these inputs resulting in a net increase in TI. All opioid-sensitive inputs decrease TE. Systemic opioids reduce these inputs resulting in a large increase in TE.
Discussion
In a developmental rabbit model we identified a subregion of the Parabrachial Nucleus (tPBN) that is involved in respiratory timing and where opioids cause respiratory rate depression at clinical concentrations in vivo. Specifically, we showed that: 1) pharmacological concentrations of the mu-opioid receptor agonist DAMGO decreased respiratory rate in the tPBN; 2) local mu-opioid receptor antagonism in the tPBN substantially reversed respiratory rate depression from systemic opioids; 3) clinical dose-rates of IV remifentanil affected respiratory phase-timing differently in the tPBN and the preBötC; 4) clinical dose-rates of IV remifentanil depressed respiratory drive more in the tPBN than in the preBötC; 5) there was no difference in the opioid effect on the tPBN between young and adult rabbits.
The tPBN plays a major role in respiratory depression by clinically relevant concentrations of systemic opioids
In this study, bilateral microinjection of DAMGO (100 μM) into the tPBN decreased respiratory rate (Figure 2). Similar effects have been observed in the medullary raphe in rats12,13,44 and the preBötC in rats4,10 and in our previous study in rabbits23 (Figure 4). Thus, pharmacological doses (μM-mM) of mu-opioid receptor agonists can affect respiratory rate at multiple sites within the respiratory network. However, the much lower plasma and effect site concentrations (~10 nM in humans41, ~20 nM in dogs41,43) that are achieved with clinically relevant, analgesic doses may not affect these areas. In this study, respiratory rate depression from IV remifentanil could be substantially reversed with localized naloxone injection into the tPBN confirming the clinical relevance of the tPBN in opioid-induced respiratory depression.
We propose a hypothetical model where opioid-sensitive inputs from several brainstem areas modulate intrinsic inspiratory and expiratory phase duration (Figure 6). The anatomical correlate for intrinsically active, opioid-sensitive inspiratory neurons may be type 1, inspiratory preBötC neurons24. The expiratory correlate may be pre-inspiratory neurons with intrinsic phase duration4, although they appear less opioid sensitive than the inspiratory neurons. Our data suggest that opioid-sensitive inputs from the preBötC are different from tPBN in their magnitude as well as effect on phase duration (Table 1). Also, though the differences were not statistically significant, non-preBötC inputs seemed larger than tPBN inputs. We have thus added an additional opioid-sensitive input to our model. This may reflect chemodrive from the retrotrapezoid nucleus45 or the caudal medullary raphe where systemic opioids decrease respiratory rate12.
A limitation of our studies was that we investigated only one dose (“clinical” target ~50% rate depression) of remifentanil. This did not allow us to determine whether inhibition of the tPBN is the main cause for apnea with opioid overdoses. The relative magnitude of opioid-induced inhibition of the different areas may be dose-dependent and may not follow a linear dose-effect relationship. For example, in an in vivo rat model Montandon et al. could completely prevent the ~25% decrease in respiratory rate from 1 μg/kg fentanyl IV with microdialysis of naloxone into the preBötC10. Similarly, when respiratory rate was depressed ~30% with IV DAMGO in in vivo rats, microinjection of the opioid antagonist CTAP into the caudal medullary raphe partially reversed the respiratory rate depression12. In summary, systemic opioid concentrations affect respiratory phase timing in multiple areas of the respiratory network, but specifically the effect on the tPBN leads to marked respiratory rate depression.
The functionally defined tPBN is different in location and function from the Kölliker-Fuse Nucleus and the LC
Extensive afferent and efferent projections exist between the Parabrachial Nucleus/Kölliker-Fuse Nucleus (KFN) region, the LC, the retrotrapezoid nucleus and rhythmogenic neurons within the ventral respiratory column in the medulla21,26,27,34,36,37,43,45,46. Much work has focused on KFN contribution to the inspiratory off-switch, which determines inspiratory phase duration47. Glutamatergic excitation of the KFN results in bradypnea from expiratory phase prolongation while inhibition through the N-methyl-D-aspartate antagonist MK-801 severely prolongs inspiratory duration (“apneusis”)39,47–49. Injection of high concentrations of DAMGO (1mM) into this area caused prolongation of TI and TE in spontaneously breathing, anesthetized rats but resulted in apneusis in the decerebrate in situ rat preparation25. Neuronal discharge patterns were described as tonic with inspiratory, expiratory or phase-spanning modulation39,47,49. “Exploratory Study 2” showed that our injection site was distinctly different from the KFN. Postmortem histology (Figure 1B) placed the functionally defined tPBN in the medial PBN region, i.e., rostral, medial and dorsal of the KFN. Injection of the glutamate agonist AMPA into the tPBN resulted in tachypnea with shortening of the expiratory phase (data not shown). This matched Dutschmann et al.’s description of an area rostral to the KFN, where glutamate injection caused transient tachypnea39. Anatomical and functional projections to the medial PBN have been shown from the Nucleus Solitarius and ventral respiratory group50, and neuronal projections have been shown from the PBN to the rostral ventral respiratory group51, the raphe magnus52, and the Bötzinger Complex53. Immunohistology demonstrated intense stain for mu-opioid receptors in this area14,16.
The tPBN injections also did not affect the LC: Numerous enkephalin receptors on LC dendrites37 and the involvement of the LC in multiple regulatory functions including arousal and nociception54 make the LC a theoretical target during our opioid protocols. The importance of awake drive for respiratory rate has recently been shown in pediatric patients55. However, the lack of effect of AMPA injections in “Exploratory Study 2” suggests a lack of direct involvement of the LC in respiratory pattern control in our decerebrate preparation, which may be similar to the lack of hypothalamic drive during NREM sleep56.
In contrast to similar studies in the in vivo dog preparation that used grid-wise drug injections over an area of several millimeters14, we found that the tachypneic response to AMPA injection reliably identified the tPBN and that a single injection of the opioid agonist or antagonist was sufficient to produce the maximal effect. The area contained ~98% non-respiratory modulated neurons, i.e., did not receive pulmonary afferent-mediated inputs or phasic feedback from the medullary preBötzinger/Bötzinger Complex. AMPA and DAMGO injection affected respiratory rate mainly through a change in TE. We propose that the tPBN provides tonic excitatory input primarily to neurons mediating the expiratory off-switch (e.g., pre-I neurons) and that inhibition of this area depresses respiratory rate predominantly through an increase in expiratory duration. Smaller increases in TI may be due to decreased excitation of inspiratory off-switch neurons or to intrinsic network properties where increases in TE cause an increase of the subsequent TI57. While the tPBN does not contain the phasic neurons necessary to generate the respiratory pattern like the preBötzinger/Bötzinger Complex, the significant respiratory slowing that can be achieved through inhibition of tPBN neurons suggests that this area is highly relevant for a mature respiratory pattern.
Opioid-sensitive inputs to respiratory drive
Systemic and local opioid administration in the tPBN also depressed respiratory drive. This suggests that some neurons in this area also provide excitatory drive to inspiratory neurons in the ventral respiratory group or possibly to chemosensitive areas58,59, which provide drive to respiratory neurons in the medulla and pons18. Although respiratory drive appeared completely restored after local naloxone reversal other studies suggest that systemic opioids inhibit respiratory drive in multiple areas including chemoreceptive areas12,13.
Methodological Considerations
Statistical power
We have discussed limitations of our experimental technique in a previous publication23. Our studies use a complex in vivo setup with multiple drug injections over several hours. Despite a stable preparation respiratory parameters at baseline as well as the response to systemic and locally applied drugs vary between animals. This limits the power of our analysis to identify small drug effects within each study and also smaller differences in respiratory parameters between this study and our previous study23. Using the linear mixed model allowed us to directly compare our current data on the tPBN with our historical data on the preBötC while accounting for random variation between studies. This approach makes our analysis the largest in vivo study to date to directly compare opioid effects with the same protocols on two different brainstem sites.
Choice of age
Developmental studies suggest that respiratory rhythm originates within one or two neuronal oscillators located in the preBötC and parafacial respiratory group30. The age where pontine inputs begin to shape respiratory pattern remains poorly defined. Dutschmann et al. showed that the role of the KFN in the pulmonary stretch-receptor mediated component of the inspiratory off-switch (Hering-Breuer reflex) was mature by postnatal day 15 in rats32. Technical difficulty currently prevents us from expanding our experiments to neonatal rabbits (<7 days). However, the similar results in rabbits age 2–3 weeks (i.e., pre-weaning) and adults suggests that the role of the tPBN in respiratory pattern generation is already established at a relatively early stage of development32.
Conclusions
Mu-opioid receptors within a functionally identified PBN subregion play a major role in mediating respiratory rate depression during the administration of systemic opioids at clinically relevant dose-rates in young and adult rabbits. Pharmacological manipulation of this area mainly affects expiratory duration suggesting that this area provides excitatory drive to neurons of the expiratory off-switch. This is consistent with the observation that systemic opioids depress respiratory rate predominantly by increasing expiratory duration.
Acknowledgments
The authors thank Jack Tomlinson (Biological Laboratory Technician) and Jennifer Callison (B.S.) for excellent technical assistance.
Funding Sources
This work was supported by the Foundation for Anesthesia Education and Research, Schaumburg, Illinois (FAERMRTG-BS-02-15-2010 to Dr. Stucke), the National Institutes of Health (R01GM112960-02 to Dr. Stucke) and by the Department of Veterans Affairs, Washington, D.C. (VA Merit Review BLRD Award # 2 I01 BX000721-05 to Dr. Zuperku). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Appendix 1
Model to assess tPBN, preBötC and additional contributions to respiratory phase timing.
The timing of respiratory phase-switch can be described with a physical timer model where the sum of the inputs (Σ) determines the time (T) until the threshold (Vthreshold) is reached and the phase is terminated.
| Eq. 1 |
The values for inspiratory (TI) and expiratory (TE) phase duration obtained with protocol 2 allow us to estimate the magnitude and polarity of inputs from the naloxone injection site (Fi) as compared to all other brainstem sites (Fo). Intravenous remifentanil reduces all inputs by the factor “r”.
| Eq. 2 |
Inputs under control conditions result in control phase duration TC. Systemic remifentanil inhibits opioid-sensitive inputs to all areas of the brainstem resulting in TR. Local naloxone injection into the study area, i.e., the tPBN or preBötC, restores the input at the injection site (Fi) to control values while all other sites (Fo) are still inhibited by remifentanil, resulting in phase duration TRN. These calculations apply for inspiratory and expiratory duration.
When Vthr = 1,
| Eq. 3 |
| Eq. 4 |
| Eq. 5 |
We used the actual values for TIC, TIR and TIRN, or TE resp., for each individual animal from protocol 2 in the current and our previous study 23 to determine I, Fi, and Fo. We assumed r=0.5 for the reduction in respiratory rate of ~50%. For the current study Fi described input from the tPBN and Fo all inputs outside the tPBN, while in our previous study Fi described input from the preBötC and Fo all inputs from outside the preBötC 23. Differences between Fi (tPBN) and Fo (preBötC) or Fi (preBötC) and Fo (tPBN), resp., suggest additional inputs to phase duration from outside these two areas.
Footnotes
Author Contributions:
J.R.M. performed experiments, analyzed data and wrote the manuscript; E.J.Z. contributed to study design, data analysis, technical support, manuscript editing; E.A.E.S. contributed to study design, manuscript writing and editing; A.B. contributed to statistical analysis and manuscript writing and editing; F.A.H. contributed to software/technical support and manuscript editing; A.G.S contributed to study design, experiments, data analysis and manuscript writing and editing
Disclosures
The authors state no conflicts of interest.
References
- 1.Dahan A, Aarts L, Smith TW. Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology. 2010;112:226–38. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 2.Hanna MH, Elliott KM, Fung M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology. 2005;102:815–21. doi: 10.1097/00000542-200504000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Lalley PM. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1387–96. doi: 10.1152/ajpregu.00530.2005. [DOI] [PubMed] [Google Scholar]
- 4.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–6. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, Olofsen E. Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology. 2004;101:1201–9. doi: 10.1097/00000542-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Dahan A, Sarton E, Teppema L, Cees O, Niewenhuijs D, Matthes HWD, Kieffer BL. Anesthetic potency and influence of morphine and sevoflurane on respiration in μ-opioid receptor knockout mice. Anesthesiology. 2001;94:824–32. doi: 10.1097/00000542-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Romberg R, Sarton E, Teppema L, Matthes HW, Kieffer BL, Dahan A. Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and mu-opioid receptor deficient mice. Br J Anaesth. 2003;91:862–70. doi: 10.1093/bja/aeg279. [DOI] [PubMed] [Google Scholar]
- 8.Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett. 2003;351:37–40. doi: 10.1016/s0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- 9.Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Botzinger complex region. J Neurophysiol. 2010;103:409–18. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole SL, Deuchars J, Lewis DI, Deuchars SA. Subdivision-specific responses of neurons in the nucleus of the tractus solitarius to activation of mu-opioid receptors in the rat. J Neurophysiol. 2007;98:3060–71. doi: 10.1152/jn.00755.2007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid mu receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology. 2007;107:288–97. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Xu F, Zhang C, Liang X. Opioid mu-receptors in medullary raphe region affect the hypoxic ventilation in anesthetized rats. Respir Physiol Neurobiol. 2009;168:281–8. doi: 10.1016/j.resp.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine mu-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol. 2012;108:2430–41. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlin NL, Mansour A, Watson SJ, Saper CB. Localization of mu-opioid receptors on amygdaloid projection neurons in the parabrachial nucleus of the rat. Brain Res. 1999;827:198–204. doi: 10.1016/s0006-8993(99)01168-3. [DOI] [PubMed] [Google Scholar]
- 16.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–87. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–62. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009 doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denavit-Saubié M, Champagnat J, Zieglgänsberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 1978;155:55–67. doi: 10.1016/0006-8993(78)90305-0. [DOI] [PubMed] [Google Scholar]
- 22.Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol. 2008;100:2878–88. doi: 10.1152/jn.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, Stuth EA. Opioid-induced Respiratory Depression Is Only Partially Mediated by the preBotzinger Complex in Young and Adult Rabbits In Vivo. Anesthesiology. 2015;122:1288–98. doi: 10.1097/ALN.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–8. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitt ES, Abdala AP, Paton JF, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol. 2015;593:4453–69. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montandon G, Horner R. Rebuttal from Gaspard Montandon and Richard Horner. J Physiol. 2014;592:1167. doi: 10.1113/jphysiol.2013.268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montandon G, Horner R. CrossTalk proposal: The preBotzinger complex is essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014;592:1159–62. doi: 10.1113/jphysiol.2013.261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. CrossTalk opposing view: The pre-Botzinger complex is not essential for respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014;592:1163–6. doi: 10.1113/jphysiol.2013.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. Rebuttal from Peter M. Lalley, Paul M. Pilowsky, Hubert V. Forster and Edward J. Zuperku. J Physiol. 2014;592:1169. doi: 10.1113/jphysiol.2013.268318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–52. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurlé MA, Mediavilla A, Flórez J. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology. 1985;24:597–606. doi: 10.1016/0028-3908(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 32.Dutschmann M, Mörschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–48. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond JC. MAC for halothane, enflurane, and isoflurane in the New Zealand white rabbit: and a test for the validity of MAC determinations. Anesthesiology. 1985;62:336–8. doi: 10.1097/00000542-198503000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol. 1998;80:2368–2377. doi: 10.1152/jn.1998.80.5.2368. [DOI] [PubMed] [Google Scholar]
- 35.Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, McCrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol. 1999;82:60–68. doi: 10.1152/jn.1999.82.1.60. [DOI] [PubMed] [Google Scholar]
- 36.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–69. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drolet G, Van Bockstaele EJ, Aston-Jones G. Robust enkephalin innervation of the locus coeruleus from the rostral medulla. J Neurosci. 1992;12:3162–74. doi: 10.1523/JNEUROSCI.12-08-03162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattie E, Li A. Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–84. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 40.Ma D, Chakrabarti MK, Whitwam JG. Effects of propofol and remifentanil on phrenic nerve activity and nociceptive cardiovascular responses in rabbits. Anesthesiology. 1999;91:1470–80. doi: 10.1097/00000542-199911000-00041. [DOI] [PubMed] [Google Scholar]
- 41.Burkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. 1996;83:646–51. doi: 10.1097/00000539-199609000-00038. [DOI] [PubMed] [Google Scholar]
- 42.Ma D, Chakrabarti MK, Whitwam JG. The combined effects of sevoflurane and remifentanil on central respiratory activity and nociceptive cardiovascular responses in anesthetized rabbits. Anesth Analg. 1999;89:453–61. [PubMed] [Google Scholar]
- 43.Michelsen LG, Salmenpera M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology. 1996;84:865–72. doi: 10.1097/00000542-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Dias MB, Nucci TB, Branco LG, Gargaglioni LH. Opioid mu-receptors in the rostral medullary raphe modulate hypoxia-induced hyperpnea in unanesthetized rats. Acta Physiol (Oxf) 2012;204:435–42. doi: 10.1111/j.1748-1716.2011.02345.x. [DOI] [PubMed] [Google Scholar]
- 45.Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci. 2011;31:16410–22. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol. 2008;586:4265–82. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol. 2004;143:127–40. doi: 10.1016/j.resp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Dhingra RR, Jacono FJ, Fishman M, Loparo KA, Rybak IA, Dick TE. Vagal-dependent nonlinear variability in the respiratory pattern of anesthetized, spontaneously breathing rats. J Appl Physiol (1985) 2011;111:272–84. doi: 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–75. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–80. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 51.Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59:341–6. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Gang S, Mizuguchi A, Aoki M. Axonal projections from the pontine pneumotaxic region to the nucleus raphe magnus in cats. Respir Physiol. 1991;85:329–39. doi: 10.1016/0034-5687(91)90072-q. [DOI] [PubMed] [Google Scholar]
- 53.Gang S, Watanabe A, Aoki M. Axonal projections from the pontine parabrachial-Kolliker-Fuse nuclei to the Botzinger complex as revealed by antidromic stimulation in cats. Adv Exp Med Biol. 1998;450:67–72. doi: 10.1007/978-1-4757-9077-1_13. [DOI] [PubMed] [Google Scholar]
- 54.Zitnik GA. Control of arousal through neuropeptide afferents of the locus coeruleus. Brain Res. 2016;1641:338–50. doi: 10.1016/j.brainres.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Montandon G, Cushing SL, Campbell F, Propst EJ, Horner RL, Narang I. Distinct Cortical Signatures Associated with Sedation and Respiratory Rate Depression by Morphine in a Pediatric Population. Anesthesiology. 2016;125:889–903. doi: 10.1097/ALN.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 56.Nattie E, Li A. Central chemoreceptors: locations and functions. Compr Physiol. 2012;2:221–54. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuperku EJ, Hopp FA. On the relation between expiratory duration and subsequent inspiratory duration. J Appl Physiol. 1985;58(2):419–430. doi: 10.1152/jappl.1985.58.2.419. [DOI] [PubMed] [Google Scholar]
- 58.Song G, Wang H, Xu H, Poon CS. Kolliker-Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct Funct. 2012 doi: 10.1007/s00429-012-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]


