Abstract
CONTEXT
Opioid use and abuse have increased dramatically in recent years, particularly among women.
OBJECTIVES
We conducted a systematic review to evaluate the association between prenatal opioid use and congenital malformations.
DATA SOURCES
We searched Medline and Embase for studies published from 1946 to 2016 and reviewed reference lists to identify additional relevant studies.
STUDY SELECTION
We included studies that were full-text journal articles and reported the results of original epidemiologic research on prenatal opioid exposure and congenital malformations. We assessed study eligibility in multiple phases using a standardized, duplicate review process.
DATA EXTRACTION
Data on study characteristics, opioid exposure, timing of exposure during pregnancy, congenital malformations (collectively or as individual subtypes), length of follow-up, and main findings were extracted from eligible studies.
RESULTS
Of the 68 studies that met our inclusion criteria, 46 had an unexposed comparison group; of those, 30 performed statistical tests to measure associations between maternal opioid use during pregnancy and congenital malformations. Seventeen of these (10 of 12 case-control and 7 of 18 cohort studies) documented statistically significant positive associations. Among the case-control studies, associations with oral clefts and ventricular septal defects/atrial septal defects were the most frequently reported specific malformations. Among the cohort studies, clubfoot was the most frequently reported specific malformation.
LIMITATIONS
Variabilities in study design, poor study quality, and weaknesses with outcome and exposure measurement.
CONCLUSIONS
Uncertainty remains regarding the teratogenicity of opioids; a careful assessment of risks and benefits is warranted when considering opioid treatment for women of reproductive age.
Opioids are powerful substances that bind to opioid receptors in the brain and body and are capable of producing numerous physiologic effects, including reduced perception of pain and euphoria.1 Some prescription opioids (eg, methadone and buprenorphine) are also used to treat opioid use disorder (OUD). The use, misuse, and abuse of prescription and illicit opioids in the United States have increased dramatically in recent years, particularly among women. Between 1999 and 2010, women experienced a >400% increase in prescription opioid overdose deaths, and for every overdose death, there were 30 more opioid misuse/abuse emergency department visits.2
Overprescribing practices appear to be driving the epidemic. In 2012 alone, prescribers wrote an estimated 259 million opioid prescriptions nationwide, which is equivalent to 82.5 opioid prescriptions per 100 persons in the United States.3 Among insured, reproductive-aged women, on average, more than one-quarter filled a prescription for an opioid medication each year during 2008 to 2012.4 Rates of illicit opioid use, including heroin abuse and dependence, are also increasing. From 2002 to 2013, the incidence of women reporting past-year abuse or dependence on heroin increased 100%.5
Opioid use is high among pregnant women in the United States as well, with an estimated 14% to 22% of women receiving an opioid prescription during pregnancy.6,7 From 1998 to 2011, the prevalence of opioid abuse or dependence among pregnant women during hospitalizations for delivery increased 127%.8 The high rates of prescription and illicit opioid use are a significant public health concern, not only for women, but also for their infants. Opioids have the ability to cross placental and blood-brain barriers, thereby posing risks for fetuses and newborns who are exposed to such drugs in utero.9 Spontaneous abortion, premature rupture of membranes, preeclampsia, abruption placentae, and fetal death are all potential obstetric complications of prenatal opioid exposure.10 Adverse neonatal outcomes that have been associated with opioid use during pregnancy include preterm birth,11–19 small for gestational age,15,19–21 lower birth weight,10,13,14,18,19,21,22 reduced head circumference,17,23–25 and sudden infant death.26–28 Neonatal abstinence syndrome (NAS) is another adverse outcome commonly reported in newborns prenatally exposed to opioids. The incidence of NAS diagnoses increased nearly fivefold in the United States during 2000 to 2012, which suggests an increasing number of opioid-exposed pregnancies.29 Neurodevelopmental outcomes of prenatally exposed infants are an additional area of concern, because a recent meta-analysis reported significant impairments in cognitive, psychomotor, and observed behavioral outcomes in infants and preschool-aged children with chronic intrauterine opioid exposure.30,31
The potential teratogenic effects of maternal opioid use during pregnancy are also an area of great public health concern. Congenital malformations are serious, often costly medical conditions that can cause lifelong challenges. They are a leading cause of infant death in the United States, accounting for 20% of all deaths during the first year of life.32 Furthermore, an estimated $2.6 billion was spent in 2004 in total hospital costs for children and adults with congenital malformations, and it is likely that costs have increased since that time.33 Congenital malformations can occur at any time during pregnancy, but the first trimester is typically the most vulnerable period. Some malformations can be prevented by identifying modifiable risk factors, such as exposure to teratogenic substances, during this critical period. Two recent studies funded by the Centers for Disease Control and Prevention have linked opioid use during early pregnancy to congenital malformations.34,35 These studies report a twofold increased risk for some congenital heart defects, neural tube defects, and gastroschisis and highlight the need for a review of the entire body of evidence related to this critical, yet less discussed, public health concern.
The objective of this report was to systematically review the available literature on maternal opioid use during pregnancy and congenital malformations.
METHODS
Data Sources
We identified relevant articles by searching electronic databases, using a combination of opioid- and congenital malformation–related Medical Subject Headings search terms and keywords (Supplemental Materials) for human studies published in the English language. We used the Ovid platform (Ovid Technologies, Inc) to conduct literature searches of Medline (1946 to present) and Embase (1988 to 2016, week 7) for publications indexed through February 19, 2016. We combined and deduplicated the results into a single EndNote X7.5 (Thomson Reuters) library. In addition, we reviewed the reference lists of included publications to identify additional relevant studies.
Study Selection
We included publications in this review if they: (1) were full-text journal articles (we excluded abstracts); (2) reported the results of original epidemiologic research (we excluded case reports, case series, editorials without original data, commentaries without original data, review papers, clinical guidelines, small descriptive studies [<100 participants], and duplicate reports); (3) reported on exposure to opioids during pregnancy (we excluded reports based on exposures during labor/delivery only); and (4) reported the presence or absence of congenital malformations (collectively or as individual subtypes) as an outcome. For simplicity, hereafter, we refer to distinct publications as “studies” and note overlapping data (when known) in Table 1.
TABLE 1.
Characteristics of Studies Included in a Systematic Review of Prenatal Opioid Exposure and Congenital Malformations (n = 68)
| Source | Study Period | Study Type | Country | Population | |
|---|---|---|---|---|---|
|
| |||||
| Pregnancies | Infants | ||||
| Blinick36 (1971) | NS | Cohort | United States | MMT = 188; methadone detoxification = 211 | MMT = 20 |
| Blinick et al37 (1973) | NS | Descriptive | United States | 105 | 61 |
| Blumenthal et al38 (1973) | 1966–1967 and 1970–1971 | Descriptive | United States | By study year 1966 = 153 334 (227 heroin-exposed) 1967 = NS (191 heroin-exposed) 1970 = NS (478 heroin-exposed) 1971 = 131 920 (706 heroin-exposed) |
|
| Bracken and Holford39 (1981)a | 1974–1976 | Case-control | United States | Cases = 1427 Controls = 3001 |
|
| Bracken40 (1986)a | 1974–1976 | Case-control | United States | Cases = 330 Controls = 3002 |
|
| Broussard et al34 (2011)b | 1997–2005 | Case-control | United States | Cases = 17 449 Controls = 6701 |
|
| Brown et al24 (1998) | 1993–1996 | Cohort | United States | Exposed (methadone) = 32 Exposed (cocaine) = 32 Unexposed (drug-free controls) = 32 |
|
| Chasnoff et al41 (1982) | 1976–1980 | Cohort | United States | Exposed (conceived on heroin; switched to low-dose methadone) = 39 Exposed (polydrug-abusing mothers) = 19 Unexposed (drug-free controls) = 27 |
|
| Cleary et al15 (2011) | 2000–2007 | Cohort | Ireland | Exposed (receiving methadone at delivery) = 618 Unexposed = 60 412 |
61 030 |
| Cleary et al42 (2012) | 2009–2010 | Cohort | Ireland | MMT = 117 | Live births = 114 Intrauterine deaths = 2 Delivered elsewhere = 1 |
| Daud et al43 (2015) | 1997–2013 | Case-control | Netherlands | Cases = 4634 Controls = 25 126 |
|
| Davis and Chappel44 (1973) | 1973–NS | Descriptive | United States | Group I (abstinent group/detox treatment) = 14 Group II (regular treatment/methadone/no interventions) = 40 Group III (intensified psychosocial support/addiction treatment) = 11 Group IV (intensified psychosocial support given by paraprofessionals/addiction treatment) = 37 Group V (interagency care/24 h paging service/transportation/addiction treatment) = 38 Group VI (heroin/no treatment) = 15 |
Live births = 113 |
| Ellwood et al45 (1987) | 1983–1985 | Cohort | Australia | Exposed = 174 narcotic abusers (182 pregnancies) Unexposed = 182 |
Exposed = 183 live births (5 stillbirths) Unexposed = 182 |
| Fajemirok-Odudeyi et al46 (2006) | 1997–2003 | Cohort | England | 108 | Exposed (methadone) = 54 Exposed (heroin alone or in combination with other drugs, including methadone) = 47 Unexposed (drug-free or drug usage unknown) = 9 |
| Saleh Gargari et al16 (2012) | 2004–2009 | Cohort | Iran | Exposed (reporting substance abuse) = 439 (293 opioid-exposed) Unexposed = 519 |
|
| Gillogley et al47 (1990) | 1987–1988 | Cohort | United States | Exposed (positive urine results for cocaine, amphetamines, and opioids) = 299 (19 positive for opioids alone) Control group = 293 |
|
| Green et al48 (1988) | 1983–NS | Cohort | United States | Group I (methadone + TCAs) = 17 Group II (MMT) = 18 |
|
| Greig et al17 (2012) | 2005–2008 | Cohort | England | MSP (methadone) group = 44 Non-MSP group = 88 |
|
| Harper et al49 (1974) | 1971–1972 | Descriptive | United States | 104 | Live births = 52 |
| Iosub et al50 (1985) | 1974 | Cohort | United States | Group I (alcohol only) = 92 Group II (alcohol + narcotics) = 36 |
|
| Jicket al51 (1981) | 1977–1979 | Cohort | United States | 6837c | |
| Kahila et al26 (2007) | 2002–2005 | Cohort | Finland | Exposed (buprenorphine) = 67 Reference group/general obstetric population deliveries in Finland 2004 = 57 759 |
|
| Källén12 (2013)d | 1996–2011 | Cohort | Sweden | 1 552 382 | Exposed (early pregnancy) = 7780 Unexposed (early pregnancy) = 1 568 067 |
| Källén and Reis52 (2015)d | 1997–2013 | Cohort | Sweden | Exposed = 1751 Unexposed = 1 682 846 |
Exposed = 1776 Unexposed = 1 797 678 |
| Kandall et al53 (1977) | 1971–1974 | Cohort | United States | Drug-dependent mothers = 216 Mothers with past histories of drug abuse (but drug-free during current pregnancy) = 33 Control group = 66 |
|
| Kivistö et al54 (2015) | 2000–2007 | Descriptive | Finland | 102 | |
| Lacroix et al55 (2011) | 1998–2006 | Cohort | France | Buprenorphine = 90 Methadone = 45 |
Fetuses = 135 (buprenorphine = 85 live births; methadone = 40 live births) |
| Lam et al56 (1992) | 1983–1990 | Cohort | China | Exposed = 51 Unexposed = 53 |
|
| Lendoiro et al57 (2013) | May 2011– July 2011 | Cohort | Spain | 209c | 212c |
| Little et al18 (1990) | 1987 | Cohort | United States | Exposed (heroin) = 24 Unexposed = 100 |
|
| Ludlow et al13 (2004) | 1997–2000 | Cohort | Australia | Opioid-using group = 91 Amphetamine-using group = 50 HDWA = 25 291 |
Opioid-using group = 91 Amphetamine-using group = 50 HDWA = 25 677 |
| Lund et al58 (2013)e, f | 2004–2010 | Cohort | Norway | Singleton pregnancies = 345 703 (OMT = 159) |
|
| Maas et al59 (1990) | 1983–1989 | Cohort | Germany | Mothers with uncontrolled opioid abuse until delivery = 17 Mothers in methadone detoxification program = 58 |
|
| Metz et al60 (2015) | 1994–2009 | Cohort | Austria | Buprenorphine = 77 Methadone = 184 SROM = 129 |
|
| Meyer et al61 (2015) | 2000–2012 | Cohort | United States | Buprenorphine = 361 Methadone = 248 |
|
| Miles et al62 (2007) | 1991–1994 and 1997–2001 | Descriptive | England | By study period 1991–1994 = 78 1997–2001 = 98 |
|
| Naeye et al63 (1973) | 1954–1972 | Cohort | United States | Mothers used heroin during pregnancy up to delivery = 82 Mothers used heroin only during early pregnancy = 10 Mothers in MMT program = 3 Control group (live births) = 500 Control group (autopsies on infants of non–drug-addicted mothers with clinical features of hepatitis) = 7 Control group (autopsies) = 1044 |
|
| Newman64 (1973) | 1970–1972 | Descriptive | United States | 120 | |
| Nezvalová-Henriksen et al65 (2011)e | 1999–2006 | Cohort | Norway | Exposed (codeine) = 2666 Unexposed (no opioids) = 65 316 |
|
| Nørgaard et al66 (2015) | 1997–2011 | Cohort | Denmark | Exposed (buprenorphine) = 167 Exposed (methadone) = 197 Exposed (heroin) = 28 Exposed (combinations) = 165 Unexposed (no opioids) = 949 615 |
Exposed (any opioids) = 564 |
| Olofsson et al14 (1983) | 1970–1979 | Cross-Sectional | Denmark | 79 | 89 |
| Ostrea and Chavez21 (1979) | 1973–1976 | Cohort | United States | Exposed = 830 (69% methadone and heroin, 31% heroin) Unexposed (drug-free, randomly-selected) = 400 Nursery population = 4811 |
|
| Ramer and Lodge67 (1975) | 1972–1974 | Cohort | United States | 32 | 35 |
| Reddy et al68 (1971) | 1967–1970 | Cohort | United States | Heroin = 40 MMT = 3; additional methadone maintained from other hospitals = 2 |
Heroin = 40 MMT = 3; additional methadone maintained from other hospitals = 2 |
| Rosen and Johnson25 (1982) | 1977–NS | Cohort | United States | Exposed (MMT) = 57 Unexposed (drug-free) = 31 |
Exposed (MMT) = 62 Unexposed (drug-free) = 32 |
| Rothman et al69 (1979) | 1973–1975 | Case-control | United States | Cases = 390 Controls = 1254 |
|
| Saxén70 (1975)g | 1967–1971 | Case-control | Finland | Cases = 599 Controls (matched) = 590 |
|
| Saxén71 (1975)g | 1967–1971 | Case-control | Finland | Cases = 599 Controls (matched) = 590 |
|
| Shaw et al72 (1992) | 1981–1983 | Case-control | United States | Cases = 141 Controls = 176 |
|
| Shaw et al73 (1998) | 1989–1991 | Case-control | United States | Cases = 538 Controls = 539 |
|
| Stimmel and Adamsons74 (1976) | 1968–1974 | Cohort | United States | Exposed (MMT) = 28 Exposed (heroin/methadone) = 57 Unexposed (drug-free) = 30 |
Exposed (MMT) = 31 Exposed (heroin/methadone) = 57 Unexposed (drug-free) = 30 |
| Thaithumyanon et al75 (2005) | 1997–2002 | Cohort | Thailand | Amphetamine = 178 Heroin = 33 (including 5 women who used both drugs) |
211 |
| Thornton et al76 (1990) | 1982–1985 | Cohort | Ireland | Exposed = 38 (29 mothers) Unexposed = 38 |
Exposed = 42 Unexposed = 38 |
| Uebel et al77 (2015) | 2000–2011 | Cohort | Australia | With NAS = 3842 Without NAS = 1 018 421 |
|
| van Baar et al78 (1989) | 1983–1985 | Cohort | Netherlands | Exposed = 35 Unexposed = 37 |
Exposed = 35 Unexposed = 37 |
| van Gelder et al79(2009)b | 1997–2003 | Case-control | United States | Cases = 10 241 Controls = 4967 |
|
| Vucinovic et al19 (2008) | 1997–2007 | Cohort | Croatia | Exposed = 85 Unexposed = 43 096 |
Exposed = 86 Unexposed = 43 529 |
| Walhovd et al80 (2007)h | NS | Cohort | Norway | Exposed (prenatal polysubstance abuse) =14 (10 heroin-exposed) Unexposed = 14 |
|
| Walhovd et al81 (2010)h | NS | Cohort | Norway | Exposed (prenatal polysubstance exposure without fetal alcohol spectrum disorder) = 14 Unexposed = 14 |
|
| Welle-Strand et al82 (2013)f | 1996–2009 | Cohort | Norway | Buprenorphine = 49 Methadone = 90 |
Buprenorphine = 49 Methadone = 90 |
| Werler et al83 (2014) | 2007–2011 | Case-control | United States | Cases = 646 Controls = 2037 |
|
| White et al84 (2006) | Normal birth outcomes: 1999–2000 Illicit drugs database: 1994–NS |
Cohort | England | Exposed (amphetamine without treatment) = 41 Exposed (amphetamine with treatment) = 47 Exposed (heroin without treatment) = 17 Exposed (heroin with treatment) = 64 Unexposed (drug-free) = 7666 |
Normal local population births = 7497 |
| Wilson et al85(1981)i | 1974–1977 | Cohort | United States | Exposed (untreated drug-dependent) = 29 Exposed (methadone-treated) = 39 Unexposed (drug-free) = 57 |
Exposed (untreated drug-dependent) = 30 Exposed (methadone-treated) = 39 Unexposed (drug-free) = 58 |
| Wilson86 (1989)i | 1974–1977 | Cohort | United States | Exposed (untreated drug-dependent) = 29 Exposed (methadone-treated) = 39 Unexposed (drug-free) = 57 |
|
| Wouldes and Woodward87 (2010) | 1996–1999 | Cohort | New Zealand | Exposed = 30 Unexposed = 42 |
Exposed = 32 Unexposed = 42 |
| Yazdy et al35 (2013) | 1998–2010 | Case-control | United States | Cases = 305 Controls (nonmalformed) = 7125 Controls (malformed) = 13 405 |
|
| Zelson et al88 (1971) | 1960–1969 | Cohort | United States | Exposed = 384 Unexposed (hospital population) = 34 886 |
|
| Zierler and Rothman89 (1985) | 1980–1983 | Case-control | United States | Cases = 298 Controls = 738 |
|
HDWA, Health Department of Western Australia; MSP, Methadone Substitution Program; NS, not specified; SROM, slow-release oral morphine; TCA, tricyclic antidepressant.
Overlapping data from selected Connecticut hospitals.
Overlapping data from the National Birth Defects Prevention Study.
Number of exposed and/or unexposed unclear.
Overlapping data from the Swedish Medical Birth Register.
Overlapping data from the Medical Birth Registry of Norway.
Overlapping data from the National OMT Program in Norway.
Overlapping data from the Finnish Register of Congenital Malformations.
Overlapping data from a Norwegian longitudinal project on the development of children born to mothers who used illicit drugs during pregnancy.
Overlapping data from a follow-up study at Houston’s public maternity hospital (institution name not specified).
We assessed study eligibility in 3 phases, title review, abstract review, and full-text review, using standardized, duplicate review by coauthor pairs. If either reviewer specified that the study should be included during any of the review phases, it was flagged to be included in the next phase of review. If both reviewers independently determined that a study should be excluded, it was excluded without additional review. During the review phases, we excluded any duplicate studies that were missed in the EndNote deduplication process.
To systematically extract data, we identified data items of interest and created an electronic data extraction form. We then pilot tested and revised the extraction form as needed. During the data extraction phase, the studies were divided between 2 reviewers. After independently extracting data from their assigned studies, the reviewers exchanged studies and checked the extracted data for completeness. Discrepancies were resolved through discussion and, when necessary, by consulting additional coauthor reviewers.
Study Quality Assessment
We assessed the quality of observational studies included in this review by using modified versions of the (1) Methodological Evaluation of Observational Research–Observational Studies of Risk Factors of Chronic Diseases criteria for studies with comparison groups and (2) Methodological Evaluation of Observational Research–Observational Studies of Population Incidence or Prevalence of Chronic Diseases criteria for large descriptive studies.90 We selected these validated quality assessment checklists because of their ability to distinguish between the external and internal validity of study findings.90 The specific study qualities that we assessed included generalizability, sampling method, sampling frame selection bias, response rate, outcome measurement, exposure measurement, exposure intensity/dose, information bias, differential data collection, differential measurement, and confounding. In the absence of established definitions, we defined “gold standard” methods of assessing outcomes and exposures as outcomes measured in a standard, valid, and reliable way and precise and/or accurate assessment of exposures, respectively.
RESULTS
Our searches of the Medline and Embase databases yielded a total of 20114 potentially relevant publications, whose titles and abstracts were reviewed (Fig 1). Duplicates and studies deemed ineligible were excluded, leaving a total of 890 studies to be examined in detail. Of the 890 studies reviewed, 62 met our inclusion criteria. We identified an additional 6 relevant studies by reviewing the reference lists of these eligible studies. We summarize the characteristics of the 68 studies included in this review in Table 1.
FIGURE 1.
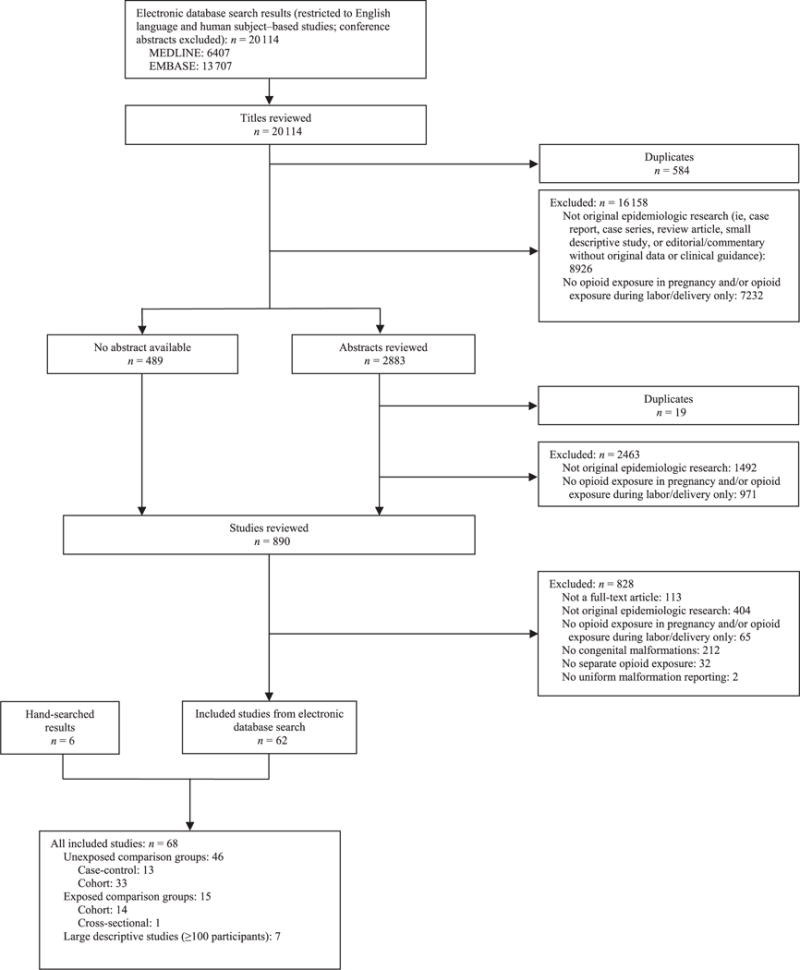
Flowchart for inclusion of studies in a systematic review of prenatal opioid exposure and congenital malformations.
Studies With an Unexposed Comparison Group
We included 46 studies with a comparison group unexposed to opioids during pregnancy that investigated associations between prenatal opioid exposure and congenital malformations; 13 were case-control studies and 33 were cohort studies.
Case-Control Studies
The majority (8 of 13) of the included case-control studies were published from 1975 through 1998 (Table 2), before the current opioid epidemic39,40,69–73,89 Seven opioid exposure34,35,39,70,71,79,83; of these, 2 studies also assessed congenital malformations associated with codeine and/or oxycodone exposure.35,39 Five studies focused specifically on codeine exposures.40, 69,72,73,89 Most (7 of 13) studies did not specify the indications for maternal opioid exposure, and one of the included studies did not present risk estimates of congenital malformations in infants exposed to opioids.79
TABLE 2.
Case-Control Studies With an Unexposed Comparison Group That Investigated Associations Between Prenatal Opioid Exposure and Congenital Malformations (n = 13)
| Source | Opioid Exposures/Reasons for Opioid Exposure | Congenital Malformations | Main Findings |
|---|---|---|---|
| Bracken and Holford39 (1981) | Narcotic analgesics; codeine; thebaine (oxycodone) | Any and specific major congenital malformations | First exposure to narcotic analgesics in first trimester Major congenital malformations: OR, 3.6; 95% CI, 1.8–7.2 Specific malformations (P < .01): cleft lip/palate; VSD + ASD; and other heart and circulatory defects Specific malformations (P < .05): dislocated hip/musculoskeletal defects; inguinal hernia with/without obstruction Specific malformations (P > .05): alimentary tract; CNS anomalies/spina bifida; heart valve defect; polydactyly/syndactyly; down syndrome; hemangioma; pyloric stenosis; skanomalies; talipes; TGV; and other congenital malformations First exposure to specific opioids in first trimester Codeine: P = .004 Thebaine (oxycodone): P = .07 |
| Reasons: medical (prescribed) | First exposure to narcotic analgesics in second trimester Major congenital malformations: P > .05 Specific malformations (P < .05): alimentary tract Specific malformations (P > .05): CNS anomalies/spina bifida; cleft lip/palate; dislocated hip/musculoskeletal defects; down syndrome; heart valve defect; hemangioma; inguinal hernia with/without obstruction; polydactyly/syndactyly, pyloric stenosis; skanomalies; talipes, TGV; VSD + ASD; other heart and circulatory defects; and other congenital malformations First exposure to narcotic analgesics in third trimester Major congenital malformations: P > .05 |
||
| Brack40 (1986) | Codeine Reasons: medical (prescribed) | CHDs | Controls (without any congenital malformations): PR, 2.4; 95% CI, 1.1–5.2 Controls (including infants with other malformations): PR, 1.3; 95% CI, 0.7–3.9 |
| Broussard et al34 (2011) | Opioid analgesic treatment (ie, codeine; hydrocodone; meperidine; oxycodone; propoxyphene; morphine; tramadol; methadone; hydromorphone; fentanyl; pentazocine) | Specific major congenital malformations | Non-heart defects Anencephaly/craniorachischisis: aOR 1.7; 95% CI 0.84–3.4 Spina bifida: aOR, 2.0; 95% CI, 1.3–3.2 Cleft palate: aOR, 1.3; 95% CI, 0.84–2.0 Cleft lip with cleft palate: aOR, 1.4; 95% CI, 0.96–2.1 Cleft lip without cleft palate: aOR, 0.68; 95% CI, 0.34–1.3 CHDs Any of included CHDs: aOR, 1.4; 95% CI, 1.1–1.7 Anomalous pulmonary venous return: aOR, 0.71; 95% CI, 0.22–2.3 Aortic stenosis: aOR, 1.3; 95% CI, 0.61–2.9 ASD secundum: aOR, 1.3; 95% CI, 0.94–1.9 ASD not otherwise specified: aOR, 2.0; 95% CI, 1.2–3.6 AVSD: aOR, 2.4; 95% CI, 1.2–4.8 Coarctation of aorta: aOR, 0.88; 95% CI, 0.47–1.6 |
| Reasons: medical (not maintenance treatment) | Conotruncal defects: aOR 1.5; 95% CI, 1.0–2.1 d-TGA: aOR, 1.1; 95% CI, 0.56–2.1 HLHS: aOR, 2.4; 95% CI, 1.4–4.1 Laterality defects with CHD: aOR, 1.2; 95% CI, 0.42–3.2 Left ventricular outflow tract obstruction defects: aOR, 1.5; 95% CI, 1.0–2.2 PVS: aOR, 1.7; 95% CI, 1.2–2.6 Right ventricular outflow tract obstruction defects: aOR, 1.6; 95% CI, 1.1–2.3 Septal defects: aOR, 1.2; 95% CI, 0.93–1.6 Single ventricle/complex: aOR, 1.1; 95% CI, 0.42–3.2 Tetralogy of Fallot: aOR, 1.7; 95% CI, 1.1–2.8 VSD conoventricular: aOR, 2.7; 95% CI, 1.1–6.3 VSD perimembranous: aOR, 0.99; 95% CI, 0.65–1.5 VSD + ASD: aOR, 1.7; 95% CI, 1.0–2.9 VSD + PVS: aOR, 1.3; 95% CI, 0.46–3.7 |
||
| Daud et al43 (2015) | Morphine Reasons: medical (prescribed) | Specific congenital malformations (ie, CHDs; musculosk; digestive; urinary; oral clefts; genital; CNS; limb; eye, ear, face, neck; respiratory) | Respiratory: OR, 100.9; 95% CI, 10.39–979.94 |
| Rothman et al69 (1979) | Codeine Reasons: not specified | CHDs | CHDs: PR, 4.1; 90% CI, 1.3–13 |
| Saxén70 (1975) | Opioids Reasons: not specified | Oral clefts | Matched-pair analysis: RR, 3.42a Random-sample study: RR, 3.40a Yule’s Q coefficient analysis (describes the degree of association between two 2-category variables) Oral clefts crude association: P < .025 |
| Saxén71 (1975) | Opioids (mainly codeine) Reasons: not specified | Specific congenital malformations | Exposure in first trimester Entire study group: P < .001 Specific malformations (P < .01): cleft palate with no additional defects; cleft lip with or without cleft palate with no additional defects Specific malformations (P > .05): cases with additional defects Exposure in second trimester Entire study group: P > .05 Specific malformations (P > .05): cleft palate with no additional defects; cleft lip with or without cleft palate with no additional defects; cases with additional defects Exposure in third trimester Entire study group: P > .05 Specific malformations (P > .05): cleft palate with no additional defects; cleft lip with or without cleft palate with no additional defects; cases with additional defects |
| Shaw et al72 (1992) | Codeine Reasons: not specified | CHDs | CHDs: OR, 0.70; 95% CI, 0.20–2.4 |
| Shaw et al73 (1998) | Codeine Reasons: not specified | NTDs | NTDs: OR, 0.89; 95% CI, 0.35–2.24 |
| van Gelder et al79 (2009) | Opioids (ie, diacetylmorphine/heroin; oxycodone hydrochloride; hydrocodone bitartrate; methadone) Reasons: illicit; medical (not maintenance treatment) | Specific congenital malformations (ie, NTDs; several CHDs; oral clefts; certain gastrointestinal defects) | Too few infants exposed to estimate risks of congenital malformations |
| Werler et al83 (2014) | Opioids (ie,hydrocodone; codeine; oxycodone; morphine; methadone; buprenorphine; fentanyl; proxyphene; meperidine) Reasons: not specified | Isolated clubfoot | Any length of opioid exposure Isolated cases: aOR, 1.56; 95% CI, 0.92–2.66 Isolated cases among those with first degree clubfoot relatives: aOR, 1.77; 95% CI, 1.03–3.03 ≤ 14 d of opioid exposure: aOR, 1.44; 95% CI, 0.67–3.12 >14 d of opioid exposure: aOR, 1.65; 95% CI, 0.81–−3.35 |
| Yazdy et al35 (2013) | Opioids (ie, codeine; oxycodone; hydrocodone; morphine; propoxyphene; meperidine; methadone; tramadol; hydromorphone; butorphanol; heroin; fentanyl; buprenorphine; nalbuphine; diphenoxylate); codeine-containing products; non–codeine- containing products Reasons: illicit; medical (not maintenance treatment) |
NTDs (ie, anencephaly; encephalocele; spina bifida); spina bifida separately | Controls (without congenital malformations) Any opioids: all NTDs: aOR, 2.2; 95% CI, 1.2–4.2 Any opioids: spina bifida: aOR, 2.5; 95% CI, 1.3–5.0 Codeine-all NTDs: aOR, 2.5; 95% CI, 1.0–6.3 Codeine: spina bifida: aOR, 2.5; 95% CI, 0.9–7.4 Noncodeine: all NTDs: aOR, 2.2; 95% CI, 1.0–4.9 Noncodeine: spina bifida: aOR, 2.8; 95% CI, 1.3–6.3 Controls (with congenital malformations) Any opioids: all NTDs: aOR, 1.9; 95% CI, 1.0–3.4 Any opioids: spina bifida: aOR, 2.2; 95% CI, 1.1–4.1 Codeine: all NTDs: aOR, 2.0; 95% CI, 0.9–4.7 Codeine: spina bifida: aOR, 2.0; 95% CI, 0.7–5.5 Noncodeine: all NTDs: aOR, 1.9; 95% CI, 0.9–4.1 Noncodeine: spina bifida: aOR, 2.5; 95% CI, 1.1–5.4 |
| Zierler and Rothman89 (1985) | Codeine | Any and specific CHDs | Controls (no congenital malformations) Any CHD: cPOR, 2.0; 90% CI 1.1–3.6 Controls (population) Any CHD (exposure from maternal report): cPOR, 1.9; 90% CI, 0.78–4.4 Any CHD (exposure from obstetric record): cPOR, 2.4; 90% CI, 0.55–10.3 VSD: cPOR, 2.5; 90% CI 1.2–5.2 |
| Reasons: not specified | DORV: cPOR, 5.7; 90% CI 1.2–19.7 Controls (other CHDs) VSD: cPOR, 1.5; 90% CI, 0.60–3.9 DORV: cPOR, 3.2; 90% CI, 0.66–11.6 Controls (other congenital malformations) DORV: aPOR, 5.0; 90% CI, 1.2–21.7 |
aOR: Adjusted odds ratio; aPOR: Adjusted prevalence odds ratio; ASD: Atrial septal defect; AVSD: Atrioventricular septal defect; CHD: Congenital heart defect; CI: Confidence interval; CNS: Central nervous system; cPOR: Crude prevalence odds ratio; DORV: Double-outlet right ventricle; d-TGA: dextro-transposition of the great arteries; HLHS: Hypoplastic left heart syndrome; NTD: Neural tube defect; OR: Odds ratio; PR: Prevalence ratio; PVS: Pulmonary valve stenosis; RR: Riskratio; TGV: Transposition of the great vessels.
Confidence limits and/or P values not specified.
Ten case-control studies reported statistically significant positive associations between opioid exposure during pregnancy and congenital malformations.34,35,39,40,43,69–71,83,89 Studies evaluating opioid exposure in aggregate found that use during early pregnancy was associated with an increased risk of congenital malformations overall,39 as well as heart malformations overall,34 inguinal hernia with/without obstruction,39 ventricular septal defects (VSD)/atrial septal defects,34,39 oral clefts,39,70,71 dislocated hip/musculoskeletal defects,39 spina bifida,34,35 tetralogy of Fallot,34 hypoplastic left heart syndrome,34 right ventricular outflow tract obstruction defects,34 pulmonary valve stenosis,34 atrioventricular septal defects,34 isolated clubfoot,83 neural tube defects,35 and other heart and circulatory defects.39 Bracken and Holford39 also reported that exposure to opioids for the first time during the second trimester was associated with alimentary tract defects.
Eight case-control studies evaluated exposures to specific types of opioids.35,39,40,43,69,72,73,89 Of these, 4 studies found codeine to be associated with an increased risk of: congenital malformations overall,39 heart malformations overall,40,69,89 VSD,89 and double-outlet right ventricle defects.89 In 2 studies by Shaw et al,72,73 codeine use in pregnancy was not significantly associated with congenital cardiac malformations or neural tube defects. Bracken40 initially reported an increased prevalence of heart malformations in codeine-exposed infants compared with unexposed infants; however, when Bracken40 recomputed the prevalence ratios to include infants with other malformations as the controls, the association was no longer statistically significant. Yazdy et al35 reported an increased risk of spina bifida with noncodeine opioid exposures. And Daud et al43 found an increased risk for respiratory malformations associated with prenatal exposure to morphine. However, in a study that evaluated first-trimester exposure to oxycodone, no increased risk of congenital malformations were reported.39
Cohort Studies
The 33 cohort studies with an unexposed comparison group included in our review were published from 1971 through 2015 (Table 3). Similar to the case-control studies, many (17 of 33) of the cohort studies were published before 199918,21,24,25,41,45,47,51,53,56,63,74,76,78,85,86,88 Methadone and heroin were the most common opioid exposures evaluated, with methadone maintenance treatment (MMT) as the most common indication for methadone exposure. Ten studies did not calculate risk estimates of congenital malformations in infants exposed to opioids,13,24,41,45,51,74,80,81,85,85 and in 5 studies, no congenital malformations were reported in any infant.25,25,57,78,84 Of the remaining 18 cohort studies that performed statistical tests to measure associations,12,15–,21,47,52,53,56,63,65,66,76,77,87,88 7 reported statistically significant increased risks of congenital malformations as a result of prenatal opioid exposure.12,15,19,21,52,55,87 Four of the 7 studies assessed associations with opioid exposure in aggregate,12,19,21,55 reporting a statistically significant increased risk of congenital malformations overall in 3 studies19,21,55 and clubfoot (pes equinovarus) in 1 study.12
TABLE 3.
Cohort Studies With an Unexposed Comparison Group That Investigated Associations Between Prenatal Opioid Exposure and Congenital Malformations (n = 33)
| Source | Opioid Exposures/Reasons for Opioid Exposure | Congenital Malformations | Main Findings |
|---|---|---|---|
| Brown et al24 (1998) | Methadone; other opioids Reasons: illicit; maintenance treatment |
Major congenital malformations | Methadone group: 9.3% prevalence of congenital malformations Unexposed group: none of the infants had a congenital malformation |
| Chasnoff et al41 (1982) | Polydrug abuse (no methadone); heroin to methadone Reasons: illicit; maintenance treatment |
Specific congenital malformations | Polydrug-abuse group: 2 infants with hand deformities (exposed to pentazocine and pyribenzamine) Heroin to methadone group: 5 infants with inguinal hernia (2 also had second-degree hypospadias) |
| Cleary et al15 (2011) | Methadone Reasons: maintenance treatment |
Congenital malformations (major; minor; chromosomal) | Any congenital malformation: aOR, 2.20; 95% CI, 1.54–3.14 Major congenital malformation: aOR, 1.94; 95% CI, 1.10–3.43 Minor congenital malformation: aOR, 2.12; 95% CI, 1.26–3.56 Chromosomal malformation: aOR, 1.48; 95% CI, 0.19–11.4 Unclassified congenital malformation: aOR, 7.26; 95% CI, 2.58–20.4 |
| Ellwood et al45 (1987) | Heroin; methadone Reasons: illicit; maintenance treatment | Any and specific congenital malformations | Exposed group: 1 infant with anencephaly Unexposed group: 1 infant with severe spina bifida |
| Saleh Gargari et al16 (2012) | Opium; heroin; methadone Reasons: illicit |
Any and specific congenital malformations (ie, clubfoot; micropenis; macrocephaly; cardiac anomaly; anomalies of limbs; hypospadias; polydactyly) | Opioid-exposed group: there was no statistically significant difference in congenital malformations between exposed and unexposed groups All drugs (not limited to opioids) group: RR, 2.66; 95% CI, 1.16–6.05 |
| Gillogley et al47 (1990) | Opioids Reasons: illicit | Any congenital malformations | Opiates-only group: none of the infants had a congenital malformation Multichemical (cocaine, amphetamine, and/or opiates) group: 2.9% prevalence of congenital malformations but there was no statistically significant difference in congenital malformations between exposed and unexposed groups |
| Greig et al17 (2012) | Heroin; methadone Reasons: illicit; maintenance treatment |
Any congenital malformations | There was no statistically significant difference in congenital malformations between exposed and unexposed groups |
| Jick et al51 (1981) | Codeine; propoxyphene N; meperidine; propoxyphene hydrochloride and acetaminophen (Darvocet N) Reasons: medical (prescribed) | Any and specific congenital malformations | Terpin hydrate and codeine group: 1 infant with congenital malformations Propoxyphene N group: 1 infant with congenital malformations Meperidine group: 1 infant with congenital malformations Aspirin + phenacetin + caffeine + codeine phosphate (APC with codeine) group: 3 infants with congenital malformations |
| Kahila et al26 (2007) | Buprenorphine Reasons: maintenance treatment |
Any congenital malformations | Buprenorphine group: none of the infants had a congenital malformation Controls (population): no mention of prevalence of congenital malformations |
| Källén12 (2013) | Opioids (ie, morphine; morphine + spasmolytics; hydromorphone; hydromorphone + spasmaolytics; oxycodone; codeine + paracetamol; ketobemidone; ketobemidone + spasmolytica; pethidine; fentanyl; methadone; dextropropoxyphene; dextropropoxyphene + paracetamol/aspirin; pentazocine; buprenorphine; tramadol; unspecified opioid; naltrexone; buprenorphine; methadone; buprenorphine combination) Reasons: not specified |
Any and specific congenital malformations | Any opioids in early pregnancy Any congenital malformations: OR, 1.02; 95% CI, 0.92–1.12 Chromosomal malformations: OR, 0.83; 95% CI, 0.50–1.37 Relatively severe congenital malformations: OR, 1.03; 95% CI, 0.91–1.15 NTDs: RR, 1.22; 95% CI, 0.36–2.60 Other CNS malformations: RR, 1.40; 95% CI, 0.60–2.76 Orofacial clefts: OR, 0.49; 95% CI, 0.25–0.96 Any cardiovascular malformations: OR, 1.04; 95% CI, 0.85–1.27 Septal cardiac defect: OR, 1.04; 95% CI, 0.82–1.32 Pyloric stenosis: RR, 0.92; 95% CI, 0.34–2.01 Abdominal wall defect: RR, 1.44; 95% CI, 0.30–4.19 Diaphragmatic hernia: RR, 1.36; 95% CI, 0.28–3.99 Hypospadias: OR, 0.97; 95% CI, 0.65–1.44 Major renal malformations: RR, 0.58; 95% CI, 0.12–1.71 Pes equinovarus: OR, 1.68; 95% CI, 1.10–2.55 Poly- or syndactyly: OR, 0.95; 95% CI, 0.58–1.56 Limb reduction defects: RR, 1.73; 95% CI, 0.75–3.41 Craniostenosis: RR, 0.60; 95% CI, 0.12–1.76 Codeine + paracetamol in early pregnancy: there was no statistically significant difference in congenital malformations Dextropropoxyphene in early pregnancy: there was no statistically significant difference in congenital malformations Tramadol in early pregnancy Any tramadol: pes equinovarus: RR, 3.60; 95% CI, 1.72–6.62 Excluding anticonvulsant: pes equinovarus: RR, 3.88; 95% CI, 1.86–7.13 Excluding women with previous miscarriages and/or born outside Sweden: pes equinovarus: RR, 4.17; 95% CI, 1.35–9.72 Any opioids + anticonvulsants in early pregnancy Relatively severe malformations: RR, 1.37; 95% CI, 0.44–3.19 Any opioids + sedative/hypnotics in early pregnancy Relatively severe malformations: OR, 0.75; 95% CI, 0.44–1.29 Any cardiovascular malformations: RR, 0.49; 95% CI, 0.10–1.44 Any opioids + antidepressants in early pregnancy Relatively severe malformations: RR, 1.09; 95% CI, 0.71–1.68 Any cardiovascular malformations: RR, 1.23; 95% CI, 0.53–2.43 |
| Källén and Reis52 (2015) | Opioids (ie, tramadol; other opioids not used for MMT; codeine + paracetamol/aspirin; other natural opiates (not codeine); dextropropoxyphene ± paracetamol/aspirin; other synthetic opioids (not tramadol/dextropropoxyphene) Reasons: medical (prescribed) |
Any and specific congenital malformations | Tramadol in early pregnancy Any malformations: aOR, 1.30; 95% CI, 1.06–1.69 Relatively severe malformations: aOR, 1.33; 95% CI, 1.05–1.70 Any cardiovascular malformations: aOR, 1.56; 95% CI, 1.04–2.29 Isolated cardiac septum malformation: aRR, 1.78; 95% CI, 1.02–2.90 Pes equinovarus: aRR, 3.63; 95% CI, 1.61–6.89 Hypospadias: aRR, 0.95; 95% CI, 0.31–2.21 Polydactyly: aRR, 1.77; 95% CI, 0.48–4.33 Codeine in early pregnancy Any malformations: aOR, 1.42; 95% CI, 1.19–1.69 Relatively severe malformations: aOR, 1.42; 95% CI, 1.15–1.76 Any cardiovascular malformations: aOR, 1.38; 95% CI, 0.97–1.96 Isolated cardiac septum malformation: aOR, 1.31; 95% CI, 0.80–2.14 Pes equinovarus: aRR, 1.24; 95% CI, 0.34–3.18 Other natural opiates in early pregnancy Any malformations: aOR, 1.20; 95% CI, 0.80–1.81 Relatively severe malformations: aOR, 1.17; 95% CI, 0.71–1.93 Any cardiovascular malformations: aRR, 0.86; 95% CI, 0.23–2.19 Dextropropoxyphene in early pregnancy Any malformations: aOR, 1.07; 95% CI, 0.91–1.26 Relatively severe malformations: aOR, 1.06; 95% CI, 0.87–1.28 Any cardiovascular malformations: aOR, 0.97; 95% CI, 0.68–1.32 Isolated cardiac septum malformation: aOR, 1.01; 95% CI, 0.62–1.66 Pes equinovarus: aRR, 1.68; 95% CI, 0.72–3.30 Other synthetic opioids in early pregnancy Any malformations: aOR, 1.25; 95% CI, 0.75–2.08 Relatively severe malformations: aOR, 1.30; 95% CI, 0.71–2.38 Any cardiovascular malformations: aRR, 2.94; 95% CI, 1.18–6.06 Isolated cardiac septum malformation: aRR, 1.59; 95% CI, 0.52–3.72 |
| Kandall et al53 (1977) | Heroin; methadone; heroin + methadone Reasons: illicit; maintenance treatment |
Any and specific congenital malformations | Heroin group: 1 infant with stigmata of Down syndrome and 1 infant with isolated microcephaly Methadone group: 1 infant with stigmata of Down syndrome Heroin + methadone group: 1 infant with isolated microcephaly Past history of drug abuse (but drug-free during current pregnancy) group: 1 infant with isolated microcephaly Frequencies of “recognizable” malformations across groups were not statistically significantly different |
| Lam et al56 (1992) | Heroin; methadone Reasons: illicit; maintenance treatment |
Any congenital malformations | There was no statistically significant difference in congenital malformations between exposed and unexposed groups |
| Lendoiro et al57 (2013) | Opioids; methadone; fentanyl Reasons: illicit; medical (prescribed) |
Any congenital malformations | None of the infants in either the exposed or the unexposed groups had a congenital malformation |
| Little et al18 (1990) | Heroin; methadone Reasons: illicit |
Any, major, and specific congenital malformations (ie, hip dislocation; natal teeth; polydactyly; skin tag; supernumerary nipple; umbilical hernia; undescended testes; vaginal tag) | There was no statistically significant difference in congenital malformations between exposed and unexposed groups |
| Ludlow et al13 (2004) | Heroin alone or with other drugs; heroin with methadone; methadone only Reasons: illicit; maintenance treatment |
Specific congenital malformations (ie, talipes; cleft palate and low set ears; coarctation of the aorta, laevocardia, and cerebral anomalies; renal anomaly) | Opioid-exposed group: 3 infants with talipes and 1 infant with cleft palate and low-set ears |
| Naeye et al63 (1973) | Heroin; methadone Reasons: illicit; maintenance treatment |
Specific congenital malformations (ie, cardiac malformations; tracheoesophageal fistula; diaphragmatic hernia; clubfeet) | Any opioid exposure: there was no statistically significant difference in congenital malformations between exposed and unexposed groups in infants who were stillborn/died within the first 72 h after birth Heroin until delivery group: 4% of infants with cardiac malformations, 4% with tracheoesophageal fistula, and 4% with clubfeet Methadone until delivery group: none of the infants had a congenital malformation Heroin during early pregnancy only group: 10% of infants with diaphragmatic hernia Non-drug-addicted group: 8% of infants with cardiac malformations, 1% with tracheoesophageal fistula, 1% with diaphragmatic hernia, and 1% with clubfeet Non-drug-addicted + hepatitis group: 14% of infants had cardiac malformations |
| Nezvalova- Henriksen et al65 (201 1) | Codeine (alone or in fixed combination with paracetamol) | Any and major congenital malformations | Any exposure in pregnancy Any congenital malformations: aOR, 0.9; 95% CI, 0.8–1.1 Major congenital malformations: aOR, 0.9; 95% CI, 0.7–1.2 Exposure in first trimester Any congenital malformations: aOR, 0.9; 95% CI, 0.7–1.1 Major congenital malformations: aOR, 0.8; 95% CI, 0.5–1.1 |
| Reasons: not specified | Exposure in second trimester Any congenital malformations: aOR, 0.9; 95% CI, 0.7–1.1 Major congenital malformations: aOR, 0.8; 95% CI, 0.6–1.1 Exposure in third trimester Any congenital malformations: aOR, 1.0; 95% CI, 0.7–1.3 Major congenital malformations: aOR, 1.1; 95% CI, 0.8–1.6 |
||
| Nørgaard et al66 (2015) | Any opioids; methadone only; buprenorphine only; heroin only; combinations Reasons: illicit; maintenance treatment; medical (prescribed) |
Any congenital malformations | Any opioids group: PR, 2.0; 95% CI, 1.5–2.6 Buprenorphine group: PR, 2.0; 95% CI, 1.2–3.2 Methadone group: PR, 2.4; 95% CI, 1.6 -3.7 Heroin group: PR, 0.9; 95% CI, 0.1–57 Combination group: PR, 1.6; 95% CI, 0.9–2.8 |
| Ostrea and Chavez21 (1979) | Heroin; heroin and methadone Reasons: illicit | Any and specific congenital malformations | Opioid-exposed group 17 infants with minor congenital malformations 20 infants with significant congenital malformations (2 with hydrocephalus, 2 with interrupted aortic arch, 4 with patent ductus arteriosus, 1 with VSD, 1 with malrotation of the intestines, 2 with posterior urethral valves, 1 with multicystic kidney, 3 with hypospadias, 1 with hypoplastic lung, 1 with cleft lip, and 2 with inguinal hernias) Controls (unexposed) Significant congenital malformations: P < .01 Controls (population) Significant congenital malformations: P < .01 |
| Rosen and Johnson25 (1982) | Heroin; methadone; opioids Reasons: illicit; maintenance treatment |
Any congenital malformations | None of the infants had a congenital malformation |
| Stimmel and Adamsons74 (1976) | Heroin; methadone Reasons: illicit; maintenance treatment |
Specific congenital malformations | Opioid exposed group: 1 infant with microcephaly, 1 infant with polydactyly, and 1 infants with hydrocele |
| Thornton et al76 (1990) | Heroin; methadone Reasons: illicit; maintenance treatment |
Any and specific congenital malformations | Opioid exposed group 1 infant with gastrointestinal atresia and 1 infant with dislocatable hip in a twin breech Incidence: 4.8%; 95% CI, 0.58%-16.16% Controls (unexposed) 1 infant with CHD Incidence: 2.63%; 95% CI, 0.07%-13.81% Controls (population) Incidence: 2.8% |
| Uebel et al77 (2015) | Opioids (assumed based on diagnosis of NAS) Reasons: not specified |
Any congenital malformations | NAS-diagnosed group: 3 infants admitted to hospital for congenital malformations No-NAS group: 1359 admitted to hospital for congenital malformations NAS versus no-NAS comparison: P = .35 |
| van Baar et al78 (1989) | Methadone with or without heroin and other drugs Reasons: illicit; maintenance treatment |
Any congenital malformations | None of the infants had a congenital malformation |
| Vucinovic et al19 (2008) | Heroin and/or methadone with or without other drugs Reasons: illicit |
Any and specific congenital malformations | Opioid-exposed group Any congenital malformation: RR 4; 95% CI, 1.9–9.2 Specific congenital malformations: 5 infants with CHDs (3 with VSD, 1 with TGV, and 1 with HLHS), 1 with small intestine malrotation, 1 with polydactyly, and 1 with single umbilical artery |
| Walhovd et al80 (2007) | Heroin with or without other substance abuse Reasons: illicit |
Myelomeningocele | Heroin-exposed group: 1 infant with myelomeningocele |
| Walhovd et al81 (2010) | Opioids; heroin Reasons: illicit |
Myelomeningocele | Opioid-exposed group: 1 infant with myelomeningocele |
| White et al84 (2006) | Heroin with dihydrocodeine; methadone Reasons: illicit; maintenance treatment |
Any and specific congenital malformations | None of the infants had a congenital malformation |
| Wilson et al85 (1981) | Heroin; methadone Reasons: illicit; maintenance treatment |
Specific congenital malformations (ie, hydrocephalus; flexion contractures; cystic fibrosis) | Heroin-exposed group: 1 infant with hydrocephalus Methadone-exposed group: 1 infant with flexion contractures Unexposed group: 1 infant with cystic fibrosis |
| Wilson86 (19 89) | Heroin; methadone Reasons: illicit; maintenance treatment |
Any and specific congenital malformations | Heroin-exposed group: 1 infant with spastic diplegia and 1 infant with hydrocephalus |
| Wouldes and Woodward87 (2010) | Methadone Reasons: maintenance treatment |
Any and specific congenital malformations | High-dose methadone group: 1 infant with periventricular leukomalacia, 1 infant with CHD and left vocal palsy, and 1 infant with cleft palate None versus low-dose versus high-dose methadone comparison: P = .003 |
| Zelson et al88 (1971) | Heroin | Major congenital malformations | Heroin-exposed group: 1 infant with congenital heart lesion, 1 infant with multiple anomalies including a tracheoesophageal fistula, 1 infant with arthrogryposis multiplex, 1 infant with incontinenti pigmenti, and 7 infants developed inguinal hernias in the immediate newborn period |
| Reasons: illicit | Unexposed population (hospital): congenital malformations not reported Congenital malformations did not occur with any more frequency as a result of ingestion of heroin and the many other drugs taken than in the general population |
aOR, adjusted odds ratio; CHD, congenital heart defect; CI, confidence interval; CNS, central nervous system; HLHS, hypoplastic left heart syndrome; NTD, neural tube defect; OR, odds ratio; RR, risk ratio; TGV, transposition of the great vessels.
Five of the 7 cohort studies that reported statistically significant increased risks evaluated associations between exposure to specific types of opioids and congenital malformations.12,15,52,55,87 In 2 studies by Källén et al,12,52 tramadol exposure in early pregnancy was associated with a statistically significant increased risk of clubfoot. Källén and Reis52 also reported an increased risk of congenital malformations overall, “relatively severe malformations” (authors excluded preauricular appendix, tongue tie, patent ductus arteriosus in preterm infants, single umbilical artery, undescended testicle, unstable hip or hip (sub) luxation, and nevus), heart malformations overall, and isolated cardiac septum malformations with tramadol exposure in early pregnancy, as well as congenital malformations overall, and “relatively severe malformations” with codeine exposure and an increased risk of heart malformations overall with the use of synthetic opioids in early pregnancy. The remaining 3 studies evaluated associations with methadone exposure; all studies reported an increased risk of malformations overall.15,55,87 Nørgaard et al55 also reported an increased risk of malformations associated with prenatal exposure to buprenorphine.
Studies With an Exposed Comparison Group
We identified 15 eligible studies with an exposed comparison group, of which 14 were cohort studies36, 42, 46, 48, 50, 55, 58–61, 67, 68, 75, 82 and 1 was a cross-sectional study (Table 1).14 Eleven studies compared methadone exposure to other opioid exposures, including methadone detoxification,36 methadone with additional drugs,42 illicit opioids, such as heroin,14,46,67,68 MMT with tricyclic antidepressant exposure,48 slow-release oral morphine,60 and buprenorphine (Table 4).55,60,61,82 Other studies compared polydrug abuse (including opioids) to alcohol abuse alone,50 uncontrolled opioid abuse to methadone detoxification,59 opioid maintenance treatment (OMT) alone to OMT with other prescription medications,58 and heroin exposure to amphetamine exposure.75 Five studies did not specify which exposure groups the congenital malformations were observed in, making their findings difficult to interpret.14,42,59,60,67 No congenital malformations were reported in the main opioid-exposed groups in 4 other studies.35,48,68,75
TABLE 4.
Studies With an Exposed Comparison Group That Investigated Associations Between Different Prenatal Opioid-Related Exposures and Congenital Malformations (n = 15)
| Source | Main Comparison Groups/Reasons for Opioid Exposure | Congenital Malformations | Main Findings |
|---|---|---|---|
| Blinick36 (1971) | Methadone detoxification versus MMT Reasons: detoxification; maintenance treatment |
Any congenital malformation | Methadone detoxification group: 2 infants born with congenital malformations MMT group: none of the infants had a congenital malformation |
| Cleary et al42 (2012) | Methadone only versus methadone + additional drugs Reasons: maintenance treatment; illicit |
Any and specific congenital malformations | Congenital malformations (exposure group not specified): 1 each of trigonocephaly, VSD, and congenital melanocytic naevus |
| Fajemirokun-Odudeyi et al46 (2 0 0 6) | Methadone versus heroin Reasons: maintenance treatment; illicit |
Any congenital malformation | χ2 comparison of congenital malformations between groups: not significant |
| Green et al48 (1988) | MMT + TCA exposure versus MMT (no TCA exposure) Reasons: maintenance treatment; illicit (other opioids) |
Specific congenital malformations (ie, palate deformity) | MMT + TCA exposed group: 1 palate deformity |
| losub et al50 (1985) | Alcohol abuser only versus polydrug abusers (alcohol and narcotics) Reasons: illicit | Major congenital malformations | Prevalence of major congenital malformations: alcohol-only group (group I) = 33% compared with polydrug-abuse group (group II) = 14% (P = .05; authors considered this to be statistically significant) Prevalence of major congenital malformations (excluding severe microcephaly): group I = 31.5% compared with group II = 14% |
| Lacroix et al55 (2011) | Buprenorphine versus methadone Reasons: maintenance treatment |
Any and specific congenital malformations | Malformation rates: similar in the 2 groups of pregnant women (note: higher than the general French population) Buprenorphine group: 1 each of tragus appendix; nasal septum deviation plus short neck; laproschisis; facial abnormalities plus microcephaly; and a therapeutic abortion due to malformation of legs, arms, and genitourinary system Methadone group: 1 polymalformation with facial malformations plus short thorax, short legs, and arms plus syndactyly plus micropenis plus multicystic kidneys; and 1 stillbirth due to achondroplasia |
| Lund et al58 (2013) | OMT without other prescribed medications versus OMT with other prescribed medications Reasons: maintenance treatment |
Major congenital malformations (ie, hydrocephalus, VSD, clubfoot, hypospadias torticollis, muscle macrocephaly, gastroschisis, trisomy 21, pulmonary infundibular stenosis) | Prevalence of major malformations: significantly higher in children whose mothers were comedicated with opioids, benzodiazepines, or z-hypnotics (P > .05 according to table footnote) |
| Maas et al59 (1990) | Uncontrolled opioid abuse versus methadone detoxification program Reasons: illicit; detoxification |
Any and specific congenital malformations | Congenital malformations (exposure group not specified): 1 pyeloureteral stenosis with vesicoureteral reflux and 1 VSD |
| Metz et al60 (2015) | Methadone; buprenorphine; SROM; other opioids Reasons: illicit; maintenance treatment |
Any congenital malformations | Congenital malformations (exposure group not specified): 2 infants with cleft lip and palate and 1 infant with trisomy 18 |
| Meyer et al61 (2015) | Methadone; buprenorphine Reasons: maintenance treatment |
Any congenital malformations | Methadone group: 1 infant with absent hand Buprenorphine group: 1 infant with isolated cleft palate |
| Olofsson et al14 (1983) | Mainly illicit opioids (intravenous heroin and morphine) versus mainly methadone Reasons: illicit; maintenance treatment |
Severe congenital malformations; specific congenital malformations | Congenital malformations (exposure group not specified): 1 infant with gastroschisis and 2 infants with intracranial hemorrhage |
| Ramer and Lodge67 (1975) | Methadone versus heroin at conception (subanalysis) Reasons: maintenance treatment; illicit |
Any congenital malformations | Congenital malformations (exposure group not specified): there were no congenital malformations noted in any infant except for bilateral rudimentary extra digits on 1 infant |
| Reddy et al68 (1971) | Methadone versus heroin Reasons: maintenance treatment; illicit |
Serious congenital malformations | Serious congenital malformations: none Heroin group: 3 infants developed inguinal hernias |
| Thaithumyanon et al75 (2005) | Heroin exposure versus amphetamine exposure Reasons: illicit; not specified (2 heroin users also received methadone) |
Any and specific congenital malformations | Heroin group: none of the infants had a congenital malformation Amphetamine group: 5 infants with congenital malformations; 1 each of large nevus flammeus; pigmented nevus; genu recurvatum (vertex presentation infant); down syndrome; and congenital heart disease (hypoplastic right ventricle) |
| Welle-Strand et al82 (20 1 3) | Buprenorphine versus methadone Reasons: maintenance treatment |
Any and specific congenital malformations | Buprenorphine group: 1 each of spina bifida and gastroschisis Methadone group: none of the infants had a congenital malformation |
SROM, slow-release oral morphine; TCA, tricyclic antidepressant.
Only 3 of the 15 studies with an exposed comparison group performed statistical tests to compare findings between exposure groups, with mixed results.45,50,58 Fajemirokun-Odudeyi et al46 did not report significant differences in the percentage of congenital malformations between infants exposed to methadone and those exposed to heroin. Lund et al58 reported a significantly higher prevalence of major malformations in children exposed to OMT with other prescribed medications compared with those exposed to OMT alone, but the documented P value was > .05. Similarly, Iosub et al50 stated that there was a statistically significant lower percentage of infants with malformations in the polydrug-exposed group (14%) compared with the alcohol-only-exposed infants (33%). However, the documented P value was equal to .05.
The remaining 3 studies compared buprenorphine and methadone exposures.55,61,82 Lacroix et al55 described similar malformation rates in buprenorphine-exposed and methadone-exposed infants, and the rates among both prenatally exposed groups were reported to be higher than the general French population. Welle-Strand et al82 compared infants prenatally exposed to buprenorphine to those prenatally exposed to methadone and reported 2 cases with malformations (spina bifida and gastroschisis) in the buprenorphine group, but no malformations in the methadone group. Meyer et al61 also reported 2 cases with malformations; 1 infant with an absent hand in the methadone-exposed group and 1 infant with isolated cleft palate in the buprenorphine-exposed group.
Descriptive Studies
We included 7 large studies (≥100 participants) that described prenatal opioid exposure and congenital malformations, but did not include any comparison group (Table 5).37,38,44,49,54,62,64 Three of the 7 studies described congenital malformations collectively.37,38,44 Blumenthal et al38 reported a higher prevalence of congenital malformations in the heroin-exposed group (12.7 per 1000 live births) than among all live births in New York City (10 per 1000 live births). Blinick et al37 did not observe any congenital malformations among 61 live births prenatally exposed to methadone and/or heroin. Davis and Chappel44 reported 4 congenital malformations among the 113 live births included in their study, 2 of which were exposed to methadone at conception; however, the authors stated that their findings of teratogenic and toxigenic effects of opioids were inconclusive.
TABLE 5.
Large Descriptive Studies (≥100 Participants) on Prenatal Opioid Exposure and Congenital Malformations (n = 7)
| Source | Opioid Exposures/Reasons for Opioid Exposure | Congenital Malformations | Main Findings |
|---|---|---|---|
| Blinick et al37 (1973) | Methadone; heroin Reasons: illicit; maintenance treatment |
Any congenital malformation | None of the infants had a congenital malformation |
| Blumenthal et al38 (1973) | Heroin Reasons: illicit |
Any congenital malformation | Heroin-exposed: the prevalence of congenital malformations was 12.7 per 1000 live births All live births (New York City): the prevalence of congenital malformations was 10 per 1000 live births |
| Davis and Chappel44 (1973) | Methadone; heroin Reasons: illicit; maintenance treatment |
Any congenital malformation | 4 congenital malformations were noted overall; 2 of which were exposed to methadone at conception. The authors noted that the findings are inconclusive in regards to teratogenic and toxigenic effects |
| Harper et al49 (1974) | Methadone; heroin Reasons: illicit; maintenance treatment | Any and specific congenital malformations | Congenital malformations (specific exposure not specified): 1 each of diaphragmatic hernia, bifid thoracic vertebrae, and polydactyly |
| Kivisto et al54 (2015) | Buprenorphine Reasons: illicit, maintenance treatment |
Any and major congenital malformations | Congenital malformations noted: 1 each of pulmonary artery stenosis, VSDs, multiple VSD, primary vesicoureteral reflux grade III, primary vesicoureteral reflux grade III–IV + hydronephrosis, duplex thumb + left-sided duplex urinary collecting system, palatal cleft and ankyloglossia, Pierre Robin syndrome + undescended testicle, microtia + stenotic external ear canal, tetralogy of Fallot + bilateral inguinal hernias + multiple skeletal anomalies + thymic aplasia (additionally 1 boy had mild hypospadias) Major congenital malformations: 5 of the 10 infants noted above had a major anomaly with functional or cosmetic significance Study infants had slightly more major anomalies than newborns on average in the general population (3.4%) |
| Miles et al62 (2007) | Methadone only; methadone + illicit substances (ie, cannabis, heroin, benzodiazepines, crack/cocaine, amphetamines, codeine, and dihydrocodeine) Reasons: illicit; maintenance treatment |
Any and specific congenital malformations | Congenital malformations (specific exposure not specified): 2 children were diagnosed with cleft palates; there were no cases of microcephaly |
| Newman64 (1973) | Methadone Reasons: maintenance treatment |
Specific congenital malformations | Congenital malformations noted: 3 infants had heart murmursa; 1 hernia; 1 bilateral foot deformity; 1 imperforate anus; and 1 esophageal malformation There was no predominance of complications in any ethnic or methadone dosage group |
Heart murmurs are not generally considered a congenital malformation.
The remaining 4 studies reported on specific malformations observed with prenatal opioid exposure.49,54,62,64 Of the infants prenatally exposed to methadone and/or heroin described by Harper et al,49 congenital malformations were observed in 3 (ie, diaphragmatic hernia, bifid thoracic vertebrae, and polydactyly). Kivistö et al54 observed malformations in 10 out of 102 infants (ie, pulmonary artery stenosis; VSDs; primary vesicoureteral reflux grade III; primary vesicoureteral reflux grade III–IV with hydronephrosis; duplex thumb with left-sided duplex urinary collecting system; palatal cleft with ankyloglossia; Pierre Robin syndrome with undescended testicle; microtia with stenotic external ear canal; tetralogy of Fallot with bilateral inguinal hernias, multiple skeletal anomalies, and thymic aplasia; and mild hypospadias) prenatally exposed to buprenorphine. Of these, 5 infants had a major anomaly with functional or cosmetic significance, which was reported to be slightly higher than what is observed on average in the general population (3.4%). Miles et al62 reported 2 cases of cleft palate among infants exposed to methadone during pregnancy (either alone or in combination with illicit substances). Lastly, Newman64 reported malformations in 7 infants exposed to methadone (ie, heart murmurs [not generally considered a congenital malformation], hernia, bilateral foot deformity, imperforate anus, and esophageal defect).
Study Quality
We used 2 validated checklists to assess the quality of the 68 studies included in this review (Supplemental Figures 3-1, 3-2, 4, and 5).90 We also presented the distribution of the included studies with respect to their bias characteristics (Fig 2). Among the 46 studies with an unexposed comparison group, 76% were not generalizable, 61% had a high risk of bias based on their sampling frame, and 57% did not report response rates. Additionally, less than half of the studies assessed outcomes and exposures using gold standards (48% and 28%, respectively). However, 61% of the studies evaluated associations after adjusting for potential confounders.
FIGURE 2.
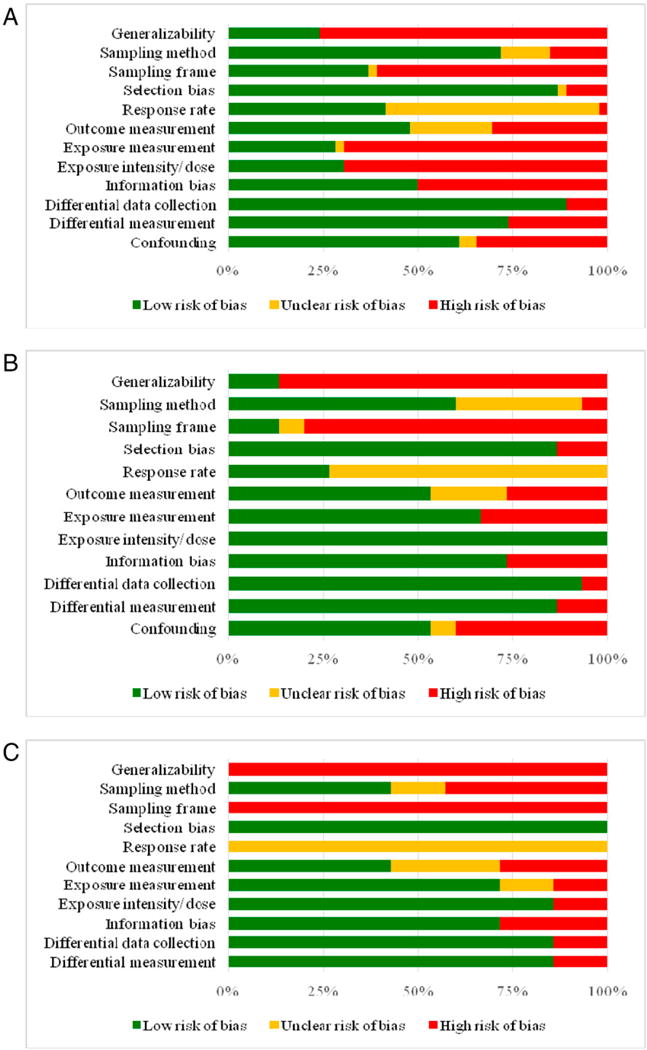
Risk of bias across studies included in a systematic review of prenatal opioid exposure and congenital malformations. (A) Studies with an unexposed comparison group (n = 46). (B) Studies with an exposed comparison group (n = 15). (C) Descriptive studies (n = 7).
Among the 15 studies with an exposed comparison group, 87% were not generalizable, 80% had a high risk of bias based on their sampling frame, and 73% did not report response rates. Approximately half of these studies used gold standard assessments for the outcome and addressed confounding. However, because many of these studies used data collected from opioid treatment facilities, a much larger proportion (67%) of studies used gold standard measurements for exposure assessment than studies with an unexposed comparison group. Among the 7 descriptive studies, none were generalizable, all had a high risk of bias based on their sampling frame, and none reported response rates. Although only 43% of the descriptive studies had a low risk of bias in outcome assessment, 71% used gold standards to assess exposures.
DISCUSSION
We included 68 studies in this systematic review, of which 30 (12 case-control and 18 cohort studies with an unexposed comparison group) performed statistical tests to measure associations between opioid exposure during pregnancy and congenital malformations. Of those 30 studies, 17 demonstrated statistically significant positive associations between prenatal opioid exposure and at least 1 congenital malformation (Supplemental Table 6); 10 were case-control studies and 7 were cohort studies. Among the 10 case-control studies, oral clefts and VSDs/atrial septal defects were the most frequently reported specific malformations (reported in 3 studies each; Supplemental Table 7), followed by spina bifida, which was reported in 2 studies. Four of these studies also reported statistically significant positive associations with codeine exposure, where heart malformations were the most frequently reported (3 of 4) congenital malformations mentioned. Among the 7 cohort studies, 6 reported increased risks of congenital malformations overall with prenatal opioid exposure, and the most frequently reported specific malformation was clubfoot (reported in 2 studies).
We have considerable concerns regarding the quality of the studies included in this review. There were no randomized controlled trials and few high-quality observational studies that evaluated the association between prenatal opioid use and congenital malformations. However, we acknowledge that this is a limitation of most medication-related studies in the pregnancy literature. The majority of the included studies lacked generalizability, failed to report response rates, and were older publications (published before 1999), which is a concern given the dramatic increases in opioid use since 1999.91 Although most of the case-control studies with an unexposed comparison group used appropriate sampling frames and methods, almost all of the other studies had flaws in their sampling frame. Many of the studies also had limitations with outcome and/or exposure measurement, which might have resulted in misclassification. Although the studies with an unexposed comparison group would be considered the highest quality of those included in this review, potential information biases were identified in half of them, and confounding was not properly addressed in many. Additionally, over half of the 68 studies included in this review were cohort studies. In general, population-based cohort studies are not ideal for assessing rare outcomes because most have insufficient power to assess specific congenital malformations. Thus, many of the included studies assessed congenital malformations as 1 homogenous, aggregate group. However, congenital malformations are etiologically heterogeneous, and examining all congenital malformations combined is unlikely to identify potentially teratogenic effects.92 This underpowering of cohort studies for rare outcomes likely explains why a much higher proportion of the case-control studies (10 of 12) documented statistically significant positive associations between prenatal opioid use and congenital malformations when compared with the cohort studies (7 of 18) included in this review. Furthermore, the majority of the studies included in this review had relatively small numbers of participants, which additionally limits their ability to assess the risk for congenital malformations due to insufficient power.
Limitations and Strengths
It is important to acknowledge some additional limitations of this review. Restricting our literature search to the English language may have led to a lack of heterogeneity among the reported settings and populations. Additionally, restricting to full-text journal articles may have introduced publication bias by excluding any reports of negative findings that did not become full-text publications. Moreover, in instances of substance use, it is rare for only 1 substance to be misused or abused, making it difficult to evaluate and understand the effects of individual substances on birth outcomes.10 This challenge is compounded by the often absent or insufficient prenatal care observed in pregnant women with OUD, significantly higher rates of tobacco use among pregnant women with substance use disorders,93 and lifestyle issues associated with illicit drug use that expose pregnant women to sexually transmitted infections and other risks,94 all of which increase the risk for poor birth outcomes,94,95 additionally limiting our ability to draw conclusions from study findings. Finally, due to exposure measurement limitations and the overall poor quality of many of the studies included in this systematic review, we were unable to incorporate information on exposure intensity/dose or additionally group the studies by reasons for exposure (eg, illicit, maintenance treatment, or prescribed). Because several factors play a role in substance use among women, including ethnicity, culture, sexual orientation, and socioeconomic status, it is likely that the study populations varied based on the reasons for prenatal opioid exposure10; yet, many of the studies we included failed to properly address confounding, which additionally prevents the generalizing of study findings.
Our review has a number of strengths. We attempted to address the potential for retrieval bias that is inherent in most reviews by using well-defined search terms in multiple electronic databases and by hand-searching the reference lists of eligible studies. Another strength was our use of a systematic, standardized, duplicate review process to identify eligible studies and ensure a relatively thorough retrieval of published literature on opioid use during pregnancy and congenital malformations. Finally, we used validated checklists to assess study quality, which allowed for more objective assessments.
CONCLUSIONS
Our findings in this systematic review have implications for future research and clinical practice. Well-designed studies with unexposed comparison groups that estimate measures of association are needed. Ideally, these studies should also have enough power to assess associations between specific opioids used during pregnancy and specific congenital malformations, rather than malformations and/or opioids as aggregate groups, and to adequately control for potential confounding factors, including polysubstance use and tobacco use. Given the uncertainty that remains regarding the teratogenicity of opioids, a careful evaluation of the potential risks and benefits is warranted when making clinical decisions regarding the use of opioid therapy in reproductive-aged and pregnant women. According to the recent Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain, when opioids are being considered for reproductive-aged women to manage chronic pain, health care providers are encouraged to discuss (1) family planning and (2) how long-term opioid use might affect any future pregnancy.96 For health care providers caring for pregnant women taking opioid medications, the guidelines recommend that they (1) access appropriate expertise if considering tapering opioids, (2) offer medication-assisted therapy with buprenorphine or methadone to pregnant women with OUD, and (3) arrange for delivery at a facility prepared to monitor, evaluate for, and treat NAS.
Supplementary Material
Acknowledgments
This work was supported in part by an appointment to the Research Participation Program at the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Centers for Disease Control and Prevention.
Dr Lind conceptualized and designed the review, coordinated and supervised the study appraisal phases, screened publications, assessed studies for eligibility, extracted data, and drafted the initial manuscript; Mrs Interrante designed the data collection instruments, led study quality assessments, screened publications, assessed studies for eligibility, extracted data, and reviewed and revised the manuscript; Drs Ailes and Gilboa conceptualized the review, screened publications, assessed studies for eligibility, and reviewed and revised the manuscript; Ms Khan reviewed reference lists for eligible studies and reviewed and revised the manuscript; Drs Honein, Dowling, Razzaghi, Creanga, and Broussard and Ms Frey and Ms Dawson screened publications, assessed studies for eligibility, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING: No external funding.
Abbreviations
- MMT
methadone maintenance treatment
- NAS
neonatal abstinence syndrome
- OMT
opioid maintenance treatment
- OUD
opioid use disorder
- VSD
ventricular septal defect
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.National Institute on Drug Abuse; US Department of Health and Human Services. Research Report Series: Prescription Drug Abuse. Bethesda, MD: National Institute on Drug Abuse, US Department of Health and Human Services; 2014. (NIH Publication Number 15-4881). [Google Scholar]
- 2.Mack KA, Jones CM, Paulozzi LJ, Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers and other drugs among women–United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(26):537–542. [PMC free article] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Mack KA, Hockenberry JM, Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–568. [PMC free article] [PubMed] [Google Scholar]
- 4.Ailes EC, Dawson AL, Lind JN, et al. Centers for Disease Control and Prevention (CDC) Opioid prescription claims among women of reproductive age United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64(2):37–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users - United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–725. [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman BT, Hernandez-Diaz S, Rathmell JP, et al. Patterns of opioid utilization in pregnancy in a large cohort of commercial insurance beneficiaries in the United States. Anesthesiology. 2014;120(5):1216–1224. doi: 10.1097/ALN.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda A, Bateman BT, Clancy CR, Creanga AA, Leffert LR. Opioid abuse and dependence during pregnancy: temporal trends and obstetrical outcomes. Anesthesiology. 2014;121(6):1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 9.Hudak ML, Tan RC, Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics Neonatal drug withdrawal. Pediatrics. 2012;129(2) Available at: www.pediatrics.org/cgi/content/full/129/2/e540. [Google Scholar]
- 10.Center for Substance Abuse Treatment. Substance Abuse Treatment: Addressing the Specific Needs of Women. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services; 2009. (Treatment Improvement Protocol (TIP) Series, No. 51. HHS Publication No. (SMA) 14-4426). [PubMed] [Google Scholar]
- 11.Kelly L, Dooley J, Cromarty H, et al. Narcotic-exposed neonates in a First Nations population in northwestern Ontario: incidence and implications. Can Fam Physician. 2011;57(11):e441–e447. [PMC free article] [PubMed] [Google Scholar]
- 12.Källén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel) 2013;6(10):1221–1286. doi: 10.3390/ph6101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Aust N Z J Obstet Gynaecol. 2004;44(4):302–306. doi: 10.1111/j.1479-828X.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 14.Olofsson M, Buck W, Andersen GE, Friis-Hansen B. Investigation of 89 children born by drug-dependent mothers. I. Neonatal course. Acta Paediatr Scand. 1983;72(3):403–406. doi: 10.1111/j.1651-2227.1983.tb09736.x. [DOI] [PubMed] [Google Scholar]
- 15.Cleary BJ, Donnelly JM, Strawbridge JD, et al. Methadone and perinatal outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2011;204(2):139.e1–139.e9. doi: 10.1016/j.ajog.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Saleh Gargari S, Fallahian M, Haghighi L, Hosseinnezhad-Yazdi M, Dashti E, Dolan K. Maternal and neonatal complications of substance abuse in Iranian pregnant women. Acta Med Iran. 2012;50(6):411–416. [PubMed] [Google Scholar]
- 17.Greig E, Ash A, Douiri A. Maternal and neonatal outcomes following methadone substitution during pregnancy. Arch Gynecol Obstet. 2012;286(4):843–851. doi: 10.1007/s00404-012-2372-9. [DOI] [PubMed] [Google Scholar]
- 18.Little BB, Snell LM, Klein VR, Gilstrap LC, III, Knoll KA, Breckenridge JD. Maternal and fetal effects of heroin addiction during pregnancy. J Reprod Med. 1990;35(2):159–162. [PubMed] [Google Scholar]
- 19.Vucinovic M, Roje D, Vucinovic Z, Capkun V, Bucat M, Banovic I. Maternal and neonatal effects of substance abuse during pregnancy: our ten-year experience. Yonsei Med J. 2008;49(5):705–713. doi: 10.3349/ymj.2008.49.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MV, Costello D, Yonkers KA. Clinical correlates of prescription opioid analgesic use in pregnancy. Matern Child Health J. 2015;19(3):548–556. doi: 10.1007/s10995-014-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrea EM, Chavez CJ. Perinatal problems (excluding neonatal withdrawal) in maternal drug addiction: a study of 830 cases. J Pediatr. 1979;94(2):292–295. doi: 10.1016/s0022-3476(79)80847-1. [DOI] [PubMed] [Google Scholar]
- 22.Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction. 1997;92(11):1571–1579. [PubMed] [Google Scholar]
- 23.Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. J Pediatr. 1994;124(5 pt 1):757–764. doi: 10.1016/s0022-3476(05)81371-x. [DOI] [PubMed] [Google Scholar]
- 24.Brown HL, Britton KA, Mahaffey D, Brizendine E, Hiett AK, Turnquest MA. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459–463. doi: 10.1016/s0002-9378(98)70379-5. [DOI] [PubMed] [Google Scholar]
- 25.Rosen TS, Johnson HL. Children of methadone-maintained mothers: follow-up to 18 months of age. J Pediatr. 1982;101(2):192–196. doi: 10.1016/s0022-3476(82)80115-7. [DOI] [PubMed] [Google Scholar]
- 26.Kahila H, Saisto T, Kivitie-Kallio S, Haukkamaa M, Halmesmäki E. A prospective study on buprenorphine use during pregnancy: effects on maternal and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86(2):185–190. doi: 10.1080/00016340601110770. [DOI] [PubMed] [Google Scholar]
- 27.Burns L, Conroy E, Mattick RP. Infant mortality among women on a methadone program during pregnancy. Drug Alcohol Rev. 2010;29(5):551–556. doi: 10.1111/j.1465-3362.2010.00176.x. [DOI] [PubMed] [Google Scholar]
- 28.Habel L, Kaye K, Lee J. Trends in reporting of maternal drug abuse and infant mortality among drug-exposed infants in New York City. Women Health. 1990;16(2):41–58. doi: 10.1300/J013v16n02_04. [DOI] [PubMed] [Google Scholar]
- 29.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):650–655. doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:104. doi: 10.1186/1471-244X-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Erratum: neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:134. doi: 10.1186/s12888-015-0438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linkbirth/infant death data set. Natl Vital Stat Rep. 2015;64(9):1–30. [PubMed] [Google Scholar]
- 33.Russo CA, Elixhauser A. Hospitalizations for Birth Defects, 2004. Rockville, MD: Agency for Healthcare Research and Quality. US Department of Health and Human Services; 2006. (Statistical Brief #24. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs). [Google Scholar]
- 34.Broussard CS, Rasmussen SA, Reefhuis J, et al. National Birth Defects Prevention Study Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204(4):314.e1–314.e11. doi: 10.1016/j.ajog.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 35.Yazdy MM, Mitchell AA, Tinker SC, Parker SE, Werler MM. Periconceptional use of opioids and the risk of neural tube defects. Obstet Gynecol. 2013;122(4):838–844. doi: 10.1097/AOG.0b013e3182a6643c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blinick G. Fertility of narcotics addicts and effects of addiction on the offspring. Soc Biol. 1971;18:S34–S39. [PubMed] [Google Scholar]
- 37.Blinick G, Jerez E, Wallach RC. Methadone maintenance, pregnancy, and progeny. JAMA. 1973;225(5):477–479. [PubMed] [Google Scholar]
- 38.Blumenthal S, Bergner L, Nelson F. Low birth weight of infants associated with maternal heroin use: New York City, 1966–67 and 1970–71. Health Serv Rep. 1973;88(5):416–418. [PMC free article] [PubMed] [Google Scholar]
- 39.Bracken MB, Holford TR. Exposure to prescribed drugs in pregnancy and association with congenital malformations. Obstet Gynecol. 1981;58(3):336–344. [PubMed] [Google Scholar]
- 40.Bracken MB. Drug use in pregnancy and congenital heart disease in offspring. N Engl J Med. 1986;314(17):1120. doi: 10.1056/NEJM198604243141717. [DOI] [PubMed] [Google Scholar]
- 41.Chasnoff IJ, Hatcher R, Burns WJ. Polydrug- and methadone-addicted newborns: a continuum of impairment? Pediatrics. 1982;70(2):210–213. [PubMed] [Google Scholar]
- 42.Cleary BJ, Eogan M, O’Connell MP, et al. Methadone and perinatal outcomes: a prospective cohort study. Addiction. 2012;107(8):1482–1492. doi: 10.1111/j.1360-0443.2012.03844.x. [DOI] [PubMed] [Google Scholar]
- 43.Daud AN, Bergman JE, Bakker MK, et al. P-glycoprotein-mediated drug interactions in pregnancy and changes in the risk of congenital anomalies: a case-reference study. Drug Saf. 2015;38(7):651–659. doi: 10.1007/s40264-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis RC, Chappel JN. Pregnancy in the context of narcotic addiction and methadone maintenance. Proc Natl Conf Methadone Treat. 1973;2:1146–1152. [PubMed] [Google Scholar]
- 45.Ellwood DA, Sutherland P, Kent C, O’Connor M. Maternal narcotic addiction: pregnancy outcome in patients managed by a specialized drug-dependency antenatal clinic. Aust N Z J Obstet Gynaecol. 1987;27(2):92–98. doi: 10.1111/j.1479-828x.1987.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 46.Fajemirok-Odudeyi O, Sinha C, Tutty S, et al. Pregnancy outcome in women who use opiates. Eur J Obstet Gynecol Reprod Biol. 2006;126(2):170–175. doi: 10.1016/j.ejogrb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Gillogley KM, Evans AT, Hansen RL, Samuels SJ, Batra KK. The perinatal impact of cocaine, amphetamine, and opiate use detected by universal intrapartum screening. Am J Obstet Gynecol. 1990;163(5 pt 1):1535–1542. doi: 10.1016/0002-9378(90)90621-d. [DOI] [PubMed] [Google Scholar]
- 48.Green L, Ehrlich S, Finnegan L. The use of tricyclic antidepressants in methadone maintained pregnant women and infant outcome. NIDA Res Monogr. 1988;81:266–272. [PubMed] [Google Scholar]
- 49.Harper RG, Solish GI, Purow HM, Sang E, Panepinto WC. The effect of a methadone treatment program upon pregnant heroin addicts and their newborn infants. Pediatrics. 1974;54(3):300–305. [PubMed] [Google Scholar]
- 50.Iosub S, Fuchs M, Bingol N, Stone RK, Gromisch DS, Wasserman E. Incidence of major congenital malformations in offspring of alcoholics and polydrug abusers. Alcohol. 1985;2(3):521–523. doi: 10.1016/0741-8329(85)90127-2. [DOI] [PubMed] [Google Scholar]
- 51.Jick H, Holmes LB, Hunter JR, Madsen S, Stergachis A. First-trimester drug use and congenital disorders. JAMA. 1981;246(4):343–346. [PubMed] [Google Scholar]
- 52.Källén B, Reis M. Use of tramadol in early pregnancy and congenital malformation risk. Reprod Toxicol. 2015;58:246–251. doi: 10.1016/j.reprotox.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Kandall SR, Albin S, Gartner LM, Lee KS, Eidelman A, Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Hum Dev. 1977;1(2):159–169. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- 54.Kivistö K, Tupola S, Kivitie-Kallio S. Prenatally buprenorphine-exposed children: health to 3 years of age. Eur J Pediatr. 2015;174(11):1525–1533. doi: 10.1007/s00431-015-2562-0. [DOI] [PubMed] [Google Scholar]
- 55.Lacroix I, Berrebi A, Garipuy D, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur J Clin Pharmacol. 2011;67(10):1053–1059. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- 56.Lam SK, To WK, Duthie SJ, Ma HK. Narcotic addiction in pregnancy with adverse maternal and perinatal outcome. Aust N Z J Obstet Gynaecol. 1992;32(3):216–221. doi: 10.1111/j.1479-828x.1992.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 57.Lendoiro E, González-Colmenero E, Concheiro-Guisán A, et al. Maternal hair analysis for the detection of illicit drugs, medicines, and alcohol exposure during pregnancy. Ther Drug Monit. 2013;35(3):296–304. doi: 10.1097/FTD.0b013e318288453f. [DOI] [PubMed] [Google Scholar]
- 58.Lund IO, Skurtveit S, Engeland A, Furu K, Ravndal E, Handal M. Prescription drug use among pregnant women in opioid maintenance treatment. Addiction. 2013;108(2):367–376. doi: 10.1111/j.1360-0443.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 59.Maas U, Kattner E, Weingart-Jesse B, Schäfer A, Obladen M. Infrequent neonatal opiate withdrawal following maternal methadone detoxification during pregnancy. J Perinat Med. 1990;18(2):111–118. doi: 10.1515/jpme.1990.18.2.111. [DOI] [PubMed] [Google Scholar]
- 60.Metz VE, Comer SD, Pribasnig A, Wuerzl J, Fischer G. Observational study in an outpatient clinic specializing in treating opioid-dependent pregnant women: neonatal abstinence syndrome in infants exposed to methadone-, buprenorphine- and slow-release oral morphine. Heroin Addict Relat Clin Probl. 2015;17(1):5–16. [Google Scholar]
- 61.Meyer MC, Johnston AM, Crocker AM, Heil SH. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J Addict Med. 2015;9(2):81–86. doi: 10.1097/ADM.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miles J, Sugumar K, Macrory F, Sims DG, D’Souza SW. Methadone-exposed newborn infants: outcome after alterations to a service for mothers and infants. Child Care Health Dev. 2007;33(2):206–212. doi: 10.1111/j.1365-2214.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 63.Naeye RL, Blanc W, Leblanc W, Khatamee MA. Fetal complications of maternal heroin addiction: abnormal growth, infections, and episodes of stress. J Pediatr. 1973;83(6):1055–1061. doi: 10.1016/s0022-3476(73)80550-5. [DOI] [PubMed] [Google Scholar]
- 64.Newman RG. Results of 120 deliveries of patients in the New York City methadone maintenance treatment program. Proc Natl Conf Methadone Treat. 1973;2:1114–1121. [PubMed] [Google Scholar]
- 65.Nezvalová-Henriksen K, Spigset O, Nordeng H. Effects of codeine on pregnancy outcome: results from a large population-based cohort study. Eur J Clin Pharmacol. 2011;67(12):1253–1261. doi: 10.1007/s00228-011-1069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nørgaard M, Nielsson MS, Heide-Jørgensen U. Birth and neonatal outcomes following opioid use in pregnancy: a Danish population-based study. Subst Abuse. 2015;9(suppl 2):5–11. doi: 10.4137/SART.S23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramer CM, Lodge A. Neonatal addiction: a two-year study. Part I. Clinical and developmental characteristics of infants of mothers on methadone maintenance. Addict Dis. 1975;2(1–2):227–234. [PubMed] [Google Scholar]
- 68.Reddy AM, Harper RG, Stern G. Observations on heroin and methadone withdrawal in the newborn. Pediatrics. 1971;48(3):353–358. [PubMed] [Google Scholar]
- 69.Rothman KJ, Fyler DC, Goldblatt A, Kreidberg MB. Exogenous hormones and other drug exposures of children with congenital heart disease. Am J Epidemiol. 1979;109(4):433–439. doi: 10.1093/oxfordjournals.aje.a112701. [DOI] [PubMed] [Google Scholar]
- 70.Saxén I. The association between maternal influenza, drug consumption and oral clefts. Acta Odontol Scand. 1975;33(5):259–267. doi: 10.3109/00016357509004631. [DOI] [PubMed] [Google Scholar]
- 71.Saxén I. Associations between oral clefts and drugs takduring pregnancy. Int J Epidemiol. 1975;4(1):37–44. doi: 10.1093/ije/4.1.37. [DOI] [PubMed] [Google Scholar]
- 72.Shaw GM, Malcoe LH, Swan SH, Cummins SK, Schulman J. Congenital cardiac anomalies relative to selected maternal exposures and conditions during early pregnancy. Eur J Epidemiol. 1992;8(5):757–760. doi: 10.1007/BF00145398. [DOI] [PubMed] [Google Scholar]
- 73.Shaw GM, Todoroff K, Velie EM, Lammer EJ. Maternal illness, including fever and medication use as risk factors for neural tube defects. Teratology. 1998;57(1):1–7. doi: 10.1002/(SICI)1096-9926(199801)57:1<1::AID-TERA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 74.Stimmel B, Adamsons K. Narcotic dependency in pregnancy. Methadone maintenance compared to use of street drugs. JAMA. 1976;235(11):1121–1124. doi: 10.1001/jama.235.11.1121. [DOI] [PubMed] [Google Scholar]
- 75.Thaithumyanon P, Limpongsanurak S, Praisuwanna P, Punnahitanon S. Perinatal effects of amphetamine and heroin use during pregnancy on the mother and infant. J Med Assoc Thai. 2005;88(11):1506–1513. [PubMed] [Google Scholar]
- 76.Thornton L, Clune M, Maguire R, Griffin E, O’Connor J. Narcotic addiction: the expectant mother and her baby. Ir Med J. 1990;83(4):139–142. [PubMed] [Google Scholar]
- 77.Uebel H, Wright IM, Burns L, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics. 2015;136(4) doi: 10.1542/peds.2014-2767. Available at: www.pediatrics.org/cgi/content/full/136/4/e811. [DOI] [PubMed] [Google Scholar]
- 78.van Baar AL, Fleury P, Soepatmi S, Ultee CA, Wesselman PJ. Neonatal behavior after drug dependent pregnancy. Arch Dis Child. 1989;64(2):235–240. doi: 10.1136/adc.64.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N, National Birth Defects Prevention Study Maternal periconceptional illicit drug use and the riskof congenital malformations. Epidemiology. 2009;20(1):60–66. doi: 10.1097/EDE.0b013e31818e5930. [DOI] [PubMed] [Google Scholar]
- 80.Walhovd KB, Moe V, Slinning K, et al. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007;36(4):1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walhovd KB, Westlye LT, Moe V, et al. White matter characteristics and cognition in prenatally opiate- and polysubstance-exposed children: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2010;31(5):894–900. doi: 10.3174/ajnr.A1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welle-Strand GK, Skurtveit S, Jones HE, et al. Neonatal outcomes following in utero exposure to methadone or buprenorphine: a National Cohort Study of opioid-agonist treatment of Pregnant Women in Norway from 1996 to 2009. Drug Alcohol Depend. 2013;127(1–3):200–206. doi: 10.1016/j.drugalcdep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Werler MM, Yazdy MM, Kasser JR, et al. Medication use in pregnancy in relation to the risk of isolated clubfoot in offspring. Am J Epidemiol. 2014;180(1):86–93. doi: 10.1093/aje/kwu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White R, Thompson M, Windsor D, Walsh M, Cox D, Charnaud B. Dexamphetamine substitute-prescribing in pregnancy: a 10-year retrospective audit. J Subst Use. 2006;11(3):205–216. [Google Scholar]
- 85.Wilson GS, Desmond MM, Wait RB. Follow-up of methadone-treated and untreated narcotic-dependent women and their infants: health, developmental, and social implications. J Pediatr. 1981;98(5):716–722. doi: 10.1016/s0022-3476(81)80830-x. [DOI] [PubMed] [Google Scholar]
- 86.Wilson GS. Clinical studies of infants and children exposed prenatally to heroin. Ann N Y Acad Sci. 1989;562:183–194. doi: 10.1111/j.1749-6632.1989.tb21017.x. [DOI] [PubMed] [Google Scholar]
- 87.Wouldes TA, Woodward LJ. Maternal methadone dose during pregnancy and infant clinical outcome. Neurotoxicol Teratol. 2010;32(3):406–413. doi: 10.1016/j.ntt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Zelson C, Rubio E, Wasserman E. Neonatal narcotic addiction: 10 year observation. Pediatrics. 1971;48(2):178–189. [PubMed] [Google Scholar]
- 89.Zierler S, Rothman KJ. Congenital heart disease in relation to maternal use of Bendectin and other drugs in early pregnancy. N Engl J Med. 1985;313(6):347–352. doi: 10.1056/NEJM198508083130603. [DOI] [PubMed] [Google Scholar]
- 90.Shamliyan TA, Kane RL, Ansari MT, et al. Development quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new checklists. J Clin Epidemiol. 2011;64(6):637–657. doi: 10.1016/j.jclinepi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Paulozzi LJ, Jones CM, Mack KA, Rudd RA, Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 92.Khoury MJ, James LM, Flanders WD, Erickson JD. Interpretation of recurring weak associations obtained from epidemiologic studies of suspected human teratogens. Teratology. 1992;46(1):69–77. doi: 10.1002/tera.1420460110. [DOI] [PubMed] [Google Scholar]
- 93.Jones HE, Heil SH, O’Grady KE, et al. Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant patient samples. Am J Drug Alcohol Abuse. 2009;35(5):375–380. doi: 10.1080/00952990903125235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ACOG Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG committee opinion no. 524: opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 95.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17(5):589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


