Abstract
Overexpression of the transcriptional coregulators C-terminal binding proteins 1 and 2 (CtBP) occurs in many human solid tumors and is associated with poor prognosis. CtBP modulates oncogenic gene expression programs and is an emerging drug target, but its oncogenic role is unclear. Consistent with oncogenic potential, exogenous CtBP2 transformed primary mouse and human cells to anchorage independence similarly to mutant H-Ras. To investigate CtBP’s contribution to in vivo tumorigenesis, Apcmin/+ mice, which succumb to massive intestinal polyposis, were bred to Ctbp2+/− mice. CtBP interacts with Adenomatous Polyposis Coli (APC) protein, and is stabilized in both APC-mutated human colon cancers and Apcmin/+ intestinal polyps. Ctbp2 heterozygosity increased the median survival of Apcmin/+ mice from 21 to 48 weeks, and reduced polyp formation by 90%, with Ctbp2+/− polyps exhibiting reduced levels of β-catenin and its oncogenic transcriptional target, cyclin D1. Ctbp’s potential as a therapeutic target was studied by treating Apcmin/+ mice with the CtBP small molecule inhibitors 4-methlythio-2-oxobutyric acid and 2-hydroxy-imino phenylpyruvic acid, both of which reduced polyposis by more than half compared with vehicle treatment. Phenocopying Ctbp2 deletion, both Ctbp inhibitors caused substantial decreases in the protein level of Ctbp2, as well its oncogenic partner β-catenin, and the effects of the inhibitors on CtBP and β-catenin levels could be modeled in an APC mutated human colon cancer cell line. CtBP2 is thus a druggable transforming oncoprotein critical for the evolution of neoplasia driven by Apc mutation.
Keywords: CtBP, transformation, APC, FAP, dehydrogenase, colon cancer
Introduction
C-terminal binding proteins (CtBPs) 1 and 2 are evolutionarily conserved transcriptional coregulators that interact with both DNA binding transcription factors and chromatin remodelers, such as histone methyl-transferases and histone deacetylases, to activate or repress gene expression 1. Both CtBP1 and 2 are found overexpressed in a number of solid tumors, including colorectal, prostate, ovarian, and breast cancer, with overexpression correlating with lower overall median survival, leading to a proposed role of CtBP as a driver oncogene in solid tumors 2–6. CtBP’s potential tumorigenic capabilities arise from its oncogenic transcriptional reprogramming of cells that includes 1) repression of pro-apoptotic genes such as Bik, Puma, Noxa, and PERP; 2) repression of tumor suppressors including PTEN, p16Ink4a, p15Ink4b, and p21waf1/cip1; and 3) activation of the metastasis associated gene TIAM-1 that promotes migration and invasion 2,7–10. CtBP’s activation of migration/invasion combined with repression of epithelial genes such as E-cadherin and keratin-8 additionally promotes EMT, which may be linked to metastasis and poor outcomes in CtBP overexpressing malignancies 2,8–10. CtBP is also an emerging target in cancer as it encodes a druggable dehydrogenase domain for which 1st and 2nd generation inhibitors have already been identified 6,11.
Although multiple indirect lines of evidence suggest CtBP plays a role in tumorigenesis, its designation as a driver of cellular transformation and oncogenesis in vivo has yet to be established, aside from one report demonstrating lower efficiency of Ras transformation of MEFs doubly homozygous for Ctbp1 and 2 8. For this reason, we initiated a set of experiments designed to determine the oncogenic potential of CtBP in vitro using both murine and human fibroblasts, and in vivo using the Apcmin/+ mouse intestinal polyposis model. We demonstrate that CtBP2 can transform primary murine embryonic fibroblasts (MEFs) by cooperating with large T-antigen (LT) of simian virus 40 (SV40), and can cooperate with h-TERT, LT and SV40 small T-antigen (ST) to induce migration/invasion and anchorage-independent growth in BJ human foreskin fibroblasts. Haploinsufficiency of Ctbp2 in Apcmin/+ mice resulted in a dramatic decrease in intestinal polyp number and a marked increase in survival. Furthermore, treatment of Apcmin/+ mice with the Ctbp2 small molecule inhibitors 4-methlythio-2-oxobutyric acid (MTOB) and 2-hydroxy-imino phenylpyruvic acid (HIPP) resulted in a significant decrease in polyp number. Thus, Ctbp2 plays a critical role in driving the min phenotype, and moreover, is a novel drug target in neoplasia resulting from Apc loss.
Results and Discussion
CtBP2 in combination with large T-antigen transforms primary MEFs
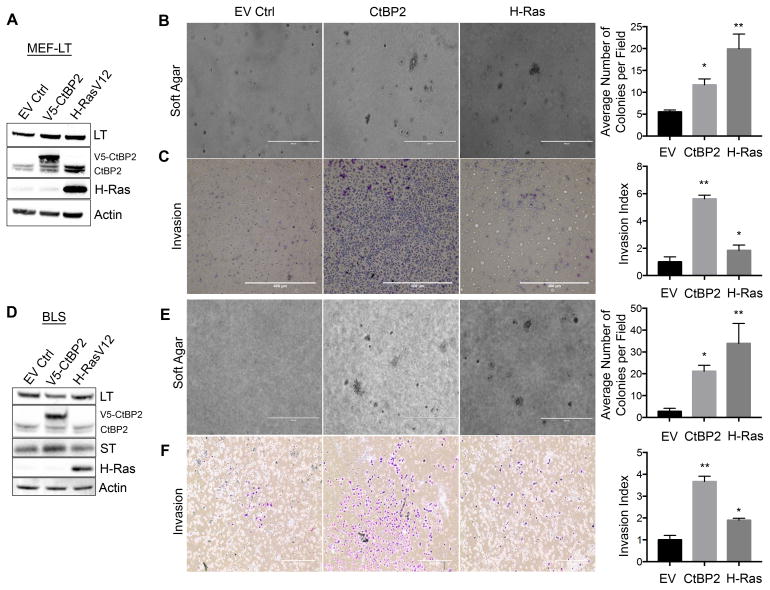
Given CtBP’s proposed role as an oncogene, we explored its ability to transform primary MEFs, which require introduction of cooperating oncogenes that can inactivate the p53/Rb tumor suppressor pathways (such as SV40 LT or human papillomavirus [HPV] E6/E7) and drive proliferation (such as activated Ras) 12. We hypothesized that CtBP2 could act as an activating oncogene that when combined with LT, could induce transformation. Early passage MEFs stably expressing LT (MEF-LT) (Supplemental Figure 1A) were therefore infected with empty vector control (pBABEpuro-EV), V5-CtBP2 (pBABEpuro-V5-CtBP2), or positive control H-RasV12 (pBABEpuro-HRasV12) retroviruses (Figure 1A), and expression of V5-CtBP2 (~2-fold over endogenous Ctbp2) and H-Ras confirmed by immunoblot (Fig. 1A, Supplemental Figure 1B). Each cell line was then plated in soft agar, and analyzed for colony formation after 3 weeks. Both H-RasV12 and CtBP2 cooperated with LT to induce significantly more colonies than control cells (p<0.05) (Figure 1B), consistent with a rodent cell transforming ability for CtBP2.
Figure 1. CtBP2 transforms primary mouse and human cells.
(A–C) CtBP2 cooperates with SV40 Large T-antigen to induce transformation of primary MEFs. (A) Immunoblot with indicated antibodies of LT-expressing MEFs infected with indicated retroviruses. Endogenous CtBP2 and V5-CtBP2 bands are indicated. (B) Soft-agar colony formation assay of LT-expressing MEFs infected with the indicated retroviruses (*p<0.05). (C) Invasion assay of LT-expressing MEFs infected with indicated retroviruses (*p<0.01). (D–F) CtBP2 cooperates with both SV40 Large T and Small T-antigens to transform human fibroblasts. (D) Immunoblot with indicated antibodies of LT/ST-expressing BJ-hTERT cells infected with indicated retroviruses. Endogenous CtBP2 and V5-CtBP2 bands are indicated. (E) Soft-agar colony formation assay of LT/ST-expressing BJ-hTERT cells infected with the indicated retroviruses (*p<0.05) (F) Invasion assay of LT/ST-expressing BJ-hTERT cells infected with indicated retroviruses (*p<0.01). Scale bars represent 400um. Statistical analyses were performed by t-test. Error bars represent ±SD of three independent experiments performed in triplicate.
Methods: BJ-hTERT-Blast cells (tested and confirmed mycoplasma free) were kindly provided by L. Litovchick and were maintained in EMEM (GIBCO) supplemented with 10% FBS and incubated at 37ºC in a humidified, 5% CO2 environment. Primary MEFs were obtained from mouse embryos at age E10.5. After harvest, cells were plated onto gelatin-coated plates and left to attach for 24 hours before use in assays. MEFs were maintained in DMEM supplemented with 10% FBS, 5% MEM non-essential amino acids (Life Technologies), and 5% L-glutamine (Life Technologies). MEF cells infected with CtBP2 or H-RasV12-expressing retroviruses were selected for one week using 1μg/ml puromycin. MEF cells infected with LT (cDNA)-expressing retrovirus were selected for two weeks using 200 μg/ml zeocin. BJ-hTERT cells were serially infected with specified plasmids and selected with puromycin (CtBP2, H-Ras) and/or zeocin (LT/ST genomic construct). For invasion assays, cells were plated into 6-well Matrigel-coated invasion chambers (Corning). After 24 hours, chambers were washed in PBS, fixed in methanol for 20 minutes and stained in Giemsa solution overnight. For soft agar assays, cells expressing specified plasmids were mixed into 0.3% noble agar and poured onto 60 mm plates containing a solidified bottom layer of 0.6% noble agar. Plates were left to form colonies for 21 days. For western blotting, cells were washed in cold PBS and lysed in RIPA buffer (150mM NaCl, 50mM Tris HCL, pH 8.0, 1.7% NP-40, 0.17% sodium dodecyl sulfate (SDS), 0.5% Na-deoxycholate monohydrate, 5mM EDTA) containing 1 tablet of complete Mini Protease Inhibitor Cocktail/10ml (Roche). Lysates were cleared of insoluble material by centrifugation at 15,000 RPM. Proteins (30 μg) were loaded onto a Bis-Tris 4–12% gel containing NuPAGE MES buffer. pBABE-zeo-LargeTgenomic (expressing LT and ST), pBABE-zeo-LargeTcDNA, and pBABE-puro-H-Ras were obtained from Addgene. CtBP2 cDNA was cloned into pBABE-puro-V5 to produce pBABE-puro-CtBP2V5. Pc-CtBP2V5 has been previously described 20. Antibodies used were as follows: anti-CtBP2 E-16 (Santa Cruz Biotechnologies, Santa Cruz, CA); anti-SV40-LT Pab 101 (Santa Cruz); anti-SV40-ST Pab 419 (Santa Cruz); anti-Actin (Sigma Aldrich); anti-Ras C-20 (Santa Cruz).
As part of the characterization of its oncogenic properties, CtBP is known to positively regulate migration/invasion in human cancer cell lines, which is a correlate of its presumed pro-metastatic functions 2,9–10. However, it is not known whether CtBP can induce migration/invasion in primarily transformed cells. To understand if the combination of CtBP2 and LT could cooperate to induce invasion/migration of MEFs, control, CtBP2, or H-RasV12 expressing MEF-LT cells were seeded onto Matrigel-coated well inserts. CtBP2 expressing MEF-LT cells displayed a robust invasive phenotype, with a near six-fold increase over empty-vector (EV) control (p<0.01), and a three-fold increase over the activated H-Ras positive control (Figure 1C), consistent with prior observations in cell lines, but also highlighting the distinct role for CtBP in driving invasion/migration in cells apart from the various transforming properties of LT related to p53 and Rb inactivation.
CtBP2 cooperatively transforms human fibroblasts
We next determined whether CtBP2 also possessed human primary cell transformation potential. Unlike murine embryonic fibroblasts, transformation of human fibroblasts requires expression of the catalytic subunit of telomerase (hTERT), inactivation of tumor suppressors p53 and Rb (such as by LT), inactivation of PP2A signaling, and the addition of an activating oncogene such as mutant Ras 12. The viral onco-products of the SV40 early region, LT and ST, facilitate human cell transformation through binding and inactivation of p53/Rb and PP2A, respectively. With this in mind, we designed experiments to determine whether CtBP2 could transform BJ human foreskin fibroblasts and serve as an activating oncogene when combined with hTERT, LT, and ST.
BJ-hTERT cells were first infected with an SV40-early region genomic retrovirus (expressing both LT and ST) and selected with Zeocin for two weeks (Supplemental Figure 1C). Following selection, BJ-hTERT-LT-ST (hereafter known as BLS) cells were subsequently transduced with either empty vector control, V5-CtBP2, or H-RasV12 retroviruses and expression of V5-CtBP2 (~2.5-fold over endogenous CtBP2) and H-Ras confirmed by immunoblot (Figure 1D; Supplemental Figure 1B). Each cell line was subsequently assayed for anchorage independent growth and induction of migration/invasion (Figs. 1E–F). When assayed for growth in soft agar, BLS-CtBP2 cells formed significantly more colonies than vector transduced BLS cells (2-fold; p<0.05), similar to BLS-H-Ras cells (Figure 1E). As with the MEFs, BLS-CtBP2 cells exhibited a 2-fold higher invasion/migration index than BLS-H-Ras cells and a ~4-fold higher index versus control cells (p<0.01; Figure 1F). This data suggests, that like H-RasV12, CtBP2 acts as an activating oncogene to transform telomerase-expressing human cells following loss of cell-cycle regulation and p53 function via LT/ST.
Ctbp2 haploinsufficiency in Apcmin/+ mice leads to increased survival and decreased polyposis
Mutations in the APC tumor suppressor gene occur in roughly 70% of human colorectal cancers 13 and inheritance of a single truncated allele of APC is the cause of the human syndrome familial adenomatous polyposis (FAP), characterized by massive colonic polyposis and early onset colon cancer 14. A similarly mutated Apc gene in the min mouse model of FAP results in the rapid onset of intestinal polyposis and premature death due to obstruction 15. The Apc min model was especially well-suited to study CtBP-driven tumorigenesis due to known targeting of CtBP for proteasomal degradation by the APC complex 16,17. Indeed, multiple studies have linked increased levels of CtBP to loss or mutation of APC in human cancers, including one study that reported elevated levels of CtBP in polyps obtained from FAP patients harboring mutant APC 16,17. Having shown that CtBP2 can drive transformation of murine and human fibroblasts in vitro, we sought to understand the role of CtBP2 in a tumor model that incorporated its overexpression, and where genetic knockout could be used to endogenously modulate the cellular level of Ctbp2.
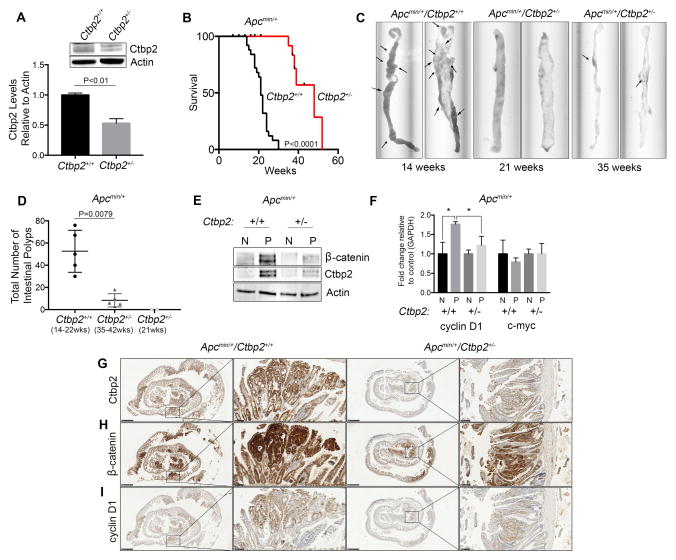
Since homozygous deletion of Ctbp2 is embryonic lethal 18, phenotypically normal Ctbp2 heterozygous mice (Ctbp2+/−) were crossed with Apcmin/+ mice to produce Apcmin/+/Ctbp2+/− compound heterozygotes. To confirm the reduction of Ctbp2 levels in Ctbp2+/− mice, small intestinal tissue from each genotype was immunoblotted with Ctbp2 antibody. Compared to WT mice, Ctbp2 protein levels in normal small intestine were reduced by 50% in Ctbp2+/− mice (Figure 2A). This decrease in Ctbp2 protein level was confirmed additionally in the colon, lung, and spleen (Supplemental Figure 2A). Having confirmed the decrease in expression of Ctbp2 protein in Ctbp2+/− mice, we next determined the survival to a humane endpoint of Apcmin/+ mice in either the Ctbp2+/+ or Ctbp2+/− backgrounds (Figure 2B). Apcmin/+ mice generally succumb to polyposis at 6–8 months of life 15, and as expected, the Apcmin/+/Ctbp2+/+ mice were all deceased by 30 weeks. Surprisingly however, all Apcmin/+/Ctbp2+/− mice were still alive at 30 weeks, and continued to live for many weeks beyond (Figure 2B). Median survival for Apcmin/+/Ctbp2+/+ mice was 21 weeks (n=25), while Apcmin/+/Ctbp2+/− mice exhibited a significantly longer (more than doubled) median survival of 48 weeks (n=20) (p=<0.0001).
Figure 2. Ctbp2 haploinsuffciency in Apcmin/+ mice reduces polyp formation and increases survival.
(A) Intestinal tissue from Ctbp2+/+ and Ctbp2+/− mice was immunoblotted using anti-Ctbp2 antibody and Ctbp2 abundance determined by densitometry. Representative immunoblot shown of 3 independent biologic replicates. (B) Overall survival of Apcmin/+/Ctbp2+/+ (n=25) vs Apcmin/+/Ctbp2+/− mice (n=20). Median survival for Ctbp+/+ mice was 21 weeks while median survival for Ctbp2+/− was 39 weeks (log-rank test, p<0.0001). (C) Representative small intestines from Apcmin/+/Ctbp2+/+ or Apcmin/+/Ctbp2+/−mice sacrificed at indicated times (arrows represent polyps). (D) Box plot comparing total intestinal polyp numbers from Apcmin/+/Ctbp2+/+ or Apcmin/+/Ctbp2+/−mice at the indicated ages. Median intestinal polyp numbers found in mice at time of death, sacrifice at humane endpoint, or censoring were Ctbp2+/+ = 56 and Ctbp2+/− = 5 (n=5 per group). *** Apcmin/+/Ctbp2+/− mice at 21 weeks (n=2) had no visible polyps. Statistical analysis employed the Mann-Whitney test. (E) Immunoblot (representative of 3 biologic replicates) demonstrating Ctbp2 and β-catenin protein levels from normal (N) and polyp (P) intestinal tissue obtained from Apcmin/+/Ctbp2+/+ or Apcmin/+/Ctbp2+/−mice obtained at 21 weeks of age and 37 weeks of age. (F) Q-PCR analysis on mRNA derived from normal (N) and polyp (P) tissue from both groups of mice, probing for mouse c-myc and cyclin-D1 (t-test, *p<0.05). Error bars for Q-PCR represent ± SEM of two independent experiments performed in triplicate. (G–I) Immunohistochemical staining of intestinal tissue for (G) Ctbp2, (H) β-catenin, (I) cyclin D1. Representative sections shown from polyps analyzed from at least 3 different animals. Scale bars represent 1mm and 100μm.
Methods: Apcmin/+ mice (C57BL/6J, Jackson Labs) were crossed with Ctbp2+/− mice 18 (B6;129S4 X C57BL/6J backcrossed > 6 generations, Jackson Labs) to generate mice harboring the min mutation and either WT Ctbp2 (Apcmin/+/Ctbp2+/+) or hemizygous Ctbp2 (Apcmin/+/Ctbp2+/−). Sample sizes for each cohort were estimated to ensure 80% power. All mouse experiments were performed with approval of the VCU IACUC. To confirm desired genotypes of study mice, the following primers were used for endpoint PCR: Apcmin/+ F: GGGAAGTTTAGACAGTTCTCGT, R: TGTTGGATGGTAAGCACTGAG, Mut: AGACAGAAGTTAGGAGAGAGAGC, WT: AGACAGAAGTTTGGAGAGAGAGC. Ctbp2+/− Internal Con F: 5′-CAAATGTTGCTTGTCTGGTG-3′, Internal Con R: 5′-GTCAGTCGAGTGCACAGTTT-3′, Mut F: 5′-CCAGTGGGGATCGACGGTATC-3′, Mut R: CACTCCAACGCAGCACCATC. Survival curves were generated from mice that were monitored daily and euthanized when moribund. In the case of Apcmin/+/Ctbp2+/− mice in the survival study, six mice were sacrificed before humane endpoint (while still healthy, counted as censored) to obtain intestinal tissues (2 at 21 weeks, 3 at 37 weeks, and 1 at 38 weeks). Ctbp2+/+ intestinal tissues, used for polyp number, protein, mRNA, and histopathological analysis, were obtained at time of death (or humane endpoint). Whole intestines were surgically removed, perfused with PBS, and analyzed for polyp number. For H&E staining, intestines were cut into 4–5cm strips and perfused with PBS followed by 10% formalin solution. Once clean, strips were cut longitudinally on blotting paper and Swiss-roll preparations were stained with H&E. For protein and mRNA analysis, polyps (and adjacent normal tissue) were cut into roughly 1 cm3 pieces and ground by mortar and pestle followed by resuspension in either RIPA supplemented with protease inhibitors for protein analysis, or RNAeasy buffer (Qiagen) to obtain mRNA. To examine changes of protein level and localization of Cyclin D, CtBP2, and β-catenin, immunohistochemical analysis was performed using mouse anti-Cyclin D (72-13G, Santa Cruz), β-catenin and CtBP2 (Clone 14 and 16, respectively; BD BioSciences); each were used at a 1:50 dilution. The paraffin-embedded tissue was de-paraffinized in xylene and rehydrated through graded ethanol, followed by antigen retrieval using 10 mM sodium citrate buffer (pH 6.0) for 15 min. The slides were allowed to cool to room temperature for 30 min. After the slides were washed, exogenous peroxidase activity was blocked by incubating the slides in 3% peroxidase for 20 min. The slides were then washed, blocked in 5% NGS-PBS for 60 min at room temperature, and incubated overnight at 4°C in primary antibody diluted in 5% NGS-PBS. Following primary antibody incubation and washing with PBS, the slides were incubated in secondary HRP anti-mouse IgG reagent (Dako Agilent Technologies) at room temperature for 1 hour, washed with PBS and then detected using EnVision+ kit (Dako Agilent Technologies). Counterstaining with hematoxylin was performed and the slides were dehydrated through graded ethanol and then xylene and mounted with Entelen (Electron Microscopy, Inc.). Total cell RNA was purified using RNeasy (Qiagen) according to manufacturer’s suggested protocol. cDNA was generated using Affinity Script RT (Agilent Technologies). One μL cDNA was amplified in triplicate using SYBRgreen and the specified primers (see Supplemental Information). Q-PCR primers: c-myc (F5′-AACAGGAACTATGACCTCG-3′, R5′-AGCAGCTCGAATTTCTTC-3′) cyclin-d1 (F5′-CAGACGTTCAGAACCAGATTC-3′, R5′-CCCTCCAATAGCAGCGAAAAC-3′). GAPDH (F5′-AAGGTCATCCCAGAGCTGAA-3′, R5′-CTGCTTCACCACCTTCTT-3′).
Analysis of intestines obtained from both groups of mice revealed a stark contrast in polyp number between the two genotypes, which likely explained the improved survival of Apcmin/+/Ctbp2+/− mice. The intestines of the Apcmin/+/Ctbp2+/− mice at their humane endpoint between 35–52 weeks appeared grossly normal with a few scattered polyps, compared to the polyp-ridden intestines of the Apcmin/+/Ctbp2+/+ mice observed at the humane endpoint or death between 14–30 weeks (Figure 2C). The median total number of intestinal polyps/mouse for 14–30 week old Apcmin/+/Ctbp2 +/+ mice was 50, while 35–52 week old Apcmin/+/Ctbp2+/− mice demonstrated a median of only 6 polyps/mouse (n=5 per group; p=0.0079) (Figure 2D). Since Apcmin/+/Ctbp2+/− mice live much longer than Apcmin/+/Ctbp2+/+ mice, the intestines from two outwardly healthy Apcmin/+/Ctbp2+/− mice sacrificed at 21 weeks of age (the median survival of Apcmin/+/Ctbp2+/+ mice) were analyzed for polyps to fairly compare the rate of polyp formation at an age where at least half the Apcmin/+/Ctbp2+/+ were still alive. Strikingly, no polyps were visible upon inspection of the intestines of Apcmin/+/Ctbp2+/− mice sacrificed at 21 weeks, suggesting that the onset for polyp formation in these mice, when it occurs, is delayed till later than 21 weeks (Figure 2C–D).
Ctbp2 drives β-catenin accumulation and cyclin D1 expression in Apcmin/+ polyps
Apc truncation mutations such as the one harbored by the min mouse result in loss of β-catenin regulation, leading to increased β-catenin levels in the nucleus and activation of downstream transcription of target genes such as c-myc and cyclin D1 19. Analysis of β-catenin protein levels in polyps of each genotype revealed higher levels, as expected, in polyp tissue compared to normal tissue (Figure 2E). However, β–catenin levels also varied with the expression levels of Ctbp2, with lower β-catenin levels (though still higher than normal tissue) found in polyps from the Apcmin/+/Ctbp2+/− mice vs. Apcmin/+/Ctbp2+/+ backgrounds (Figure 2E). Notably, β-catenin mRNA levels showed no difference between Apcmin/+/Ctbp2+/+ and Apcmin/+/Ctbp2+/− polyps (Supplemental Figure 2B), suggesting that Ctbp2, or a transcriptional target of Ctbp2, post-transcriptionally regulates β-catenin protein abundance in intestinal polyps.
To understand what effect lowered Ctbp2 levels could have on downstream pathways of known importance in APC-defective tumors, we performed Q-PCR analysis on mRNA derived from normal and polyp tissue from each genotype, using primers against targets known to be active in Wnt-signaling, including c-myc and cyclin-D1 (Figure 2F). Although mRNA levels of c-myc did not show a significant change in expression levels between normal or polyp tissue, or between Ctbp2 wt or +/− strains, levels of cyclin-D1 were significantly elevated in polyp tissue compared to normal tissue in Apcmin/+/Ctbp2+/+ mice (p<0.05), and furthermore, were significantly elevated over levels found in polyps from Apc+/−/Ctbp2+/− mice (p<0.05; Figure 2F). The level of cyclin D1 in Apc+/−/Ctbp2+/− polyps trended toward an increase over normal tissue, but the difference did not achieve statistical significance.
To further analyze the relationship between Ctbp2, β-catenin, and cyclin D1, immunohistochemical staining was performed. Ctbp2 staining levels in polyps correlated with gene dosage, with Apcmin/+/Ctbp2+/+ polyps displaying a strong staining intensity, while Apcmin/+/Ctbp2+/− polyp tissue displayed a moderate staining intensity (Allred scores of 8 and 4, respectively; Figure 2G). The intensity of β-catenin staining was strong in both groups of polyps, though Apcmin/+/Ctbp2+/+ polyps displayed a far greater proportion of positive cells versus Apcmin/+/Ctbp2+/− polyps (Allred scores of 8 and 6 respectively; Figure 2H). Finally, levels of cyclin D1 also correlated with Ctbp2 gene dosage with Apcmin/+/Ctbp2+/+ polyps displaying a strong staining intensity compared to moderate intensity for Apcmin/+/Ctbp2+/− polyps (Allred scores of 8 and 4, respectively; Figure 2I). These data suggest that levels of Ctbp2 regulate induced β-catenin levels in polyps, and furthermore, suggest that Ctbp2 directly, or indirectly through β-catenin, regulates the expression of cyclin D1, a known oncogenic driver of polyposis in APC mutant phenotypes 19.
Pharmacological inhibition of Ctbp2 rescues polyposis in Apcmin/+ mice
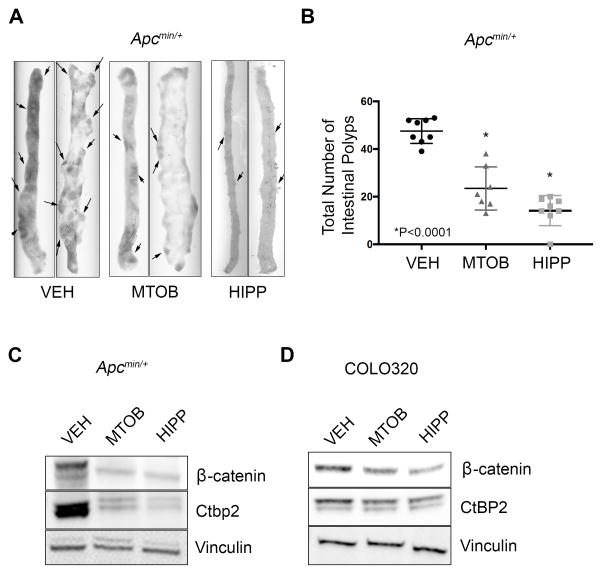
Having shown that Ctbp2 haploinsufficiency dramatically reduces polyposis in Apcmin/+ mice, we next investigated whether polyposis could be rescued by treatment with the 1st or 2nd generation Ctbp2 inhibitors MTOB and HIPP 6,11. 6-week old Apcmin/+ mice received tri-weekly intraperitoneal (IP) injections of either vehicle (VEH-control), MTOB (750mg/kg), or HIPP (250mg/kg) for 8 weeks. Following treatment, mice were humanely sacrificed and analyzed for polyposis. Control mice that received vehicle injections displayed polyposis similar to that of untreated Apcmin/+/Ctbp2+/+ mice (median 45 polyps/mouse; Figure 3A–B). Surprisingly, mice that received injections of MTOB and HIPP retained a more normal intestinal morphology and exhibited a 56% reduction (21/mouse, p<0.0001) and a 69% reduction (15/mice p<0.0001) in polyp number, respectively (Figure 3A–B).
Figure 3. Effect of the Ctbp2 inhibitors MTOB and HIPP on Apcmin/+ polyposis.
(A) Representative small intestine tissue from Apcmin/+ mice treated with VEH-control, MTOB, or HIPP. (B) Box plot comparing total intestinal polyp numbers found in mice at time of sacrifice. Median numbers were VEH = 45, MTOB = 21, and HIPP = 15 (n=7 per group; *p<0.0001, Mann-Whitney test). (C) Immunoblot of Ctbp2 and β-catenin protein levels in polyps recovered from Apcmin/+ mice treated with VEH-control, MTOB, or HIPP. (D) Immunoblot demonstrating CtBP2 and β-catenin levels from COLO320 cells treated with VEH-control, MOTB, or HIPP. Immunoblots in C and D are representative of 3 biologic replicates.
Methods: For MTOB/HIPP studies, Apcmin/+ mice received IP injections (3X weekly for 8 weeks) of VEH-control (0.2M NaHO3 solution in water), 750mg/kg MTOB (dissolved in 0.2M NaHO3/water solution; Sigma-Aldrich), or 250mg/kg HIPP 11 (dissolved in 0.2M NaHO3/water solution). Sample sizes for each cohort were estimated to ensure 80% power. Mice with the correct genotype were randomly assigned to the vehicle or drug group. COLO320 cells were obtained from ATCC (confirmed mycoplasma free) and maintained in RPMI-1640 supplemented with 10% FBS and incubated at 37ºC in a humidified, 5% CO2 environment.
Since Ctbp2 haploinsufficiency resulted in a decrease in β-catenin protein levels in the few polyps detected in Apcmin/+/Ctbp2+/− mice, polyps were harvested from Apcmin/+ mice treated with MTOB or HIPP to determine if pharmacological inhibition of Ctbp2 also resulted in β-catenin depletion. Compared to polyps arising in vehicle-treated Apcmin/+ mice, MTOB and HIPP treated polyps indeed did display dramatically reduced levels of Ctbp2 and β-catenin protein similar to that seen in Apcmin/+/Ctbp2+/− mice (Figure 3C). Finally, to determine if CtBP inhibitor actions toward CtBP2 and β-catenin in Apcmin/+ polyps were mirrored in human colon cancer cells harboring mutant APC, we treated COLO320 cells (which have an APC truncation that closely mirrors the min truncation) with MTOB or HIPP. Similar to Apcmin/+ polyps, treatment of COLO320 cells with MTOB or HIPP resulted in modest but reproducible decreases of both CtBP2 and β-catenin protein levels (Figure 3D). Thus, CtBP inhibitors may reduce polyposis by driving reductions in CtBP2 and β -catenin expression, consistent with the effects observed by reducing Ctbp2 gene dosage by 50%, and can induce similar effects in an APC mutated human colon cancer cell line.
Our data firmly establish Ctbp2 as a gatekeeper in Apc-related tumorigenesis, where even modest 50% reductions in Ctbp2 abundance, as in Apcmin/+/Ctbp2+/− mice, or its partial chemical inhibition by MTOB or HIPP, reveal a sharp threshold effect for sufficiency of Ctbp2 levels for polyp formation. β-catenin protein levels tracked closely with Ctbp2 expression levels in both normal and tumor tissues of Apc min mice, suggesting a direct relationship between Ctbp2 and β-catenin abundances. This phenomenon is almost certainly related to recent findings that CtBP induces mutant APC oligomerization in human colon cancer cells, which contributes to β-catenin stabilization by blocking APC/β-catenin interaction 20. Indeed, selection for the length of retained APC alleles in FAP, the syndrome modeled in Apcmin/+, invariably results in retention of CtBP interaction domains by the mutant APC protein 20.
Our work also establishes CtBP inhibition with dehydrogenase substrate competitive inhibitors as safe and effective for the reduction of Apc min-mediated polyposis. CtBP inhibitors reduce cellular CtBP2 abundance, at least in Apc mutated mouse intestine or human APC mutated colon cancer cells, with the likely result of reduced β-catenin levels that phenocopies β-catenin reductions in Apcmin/+/Ctbp2+/− polyps. The mechanism for CtBP2 depletion by inhibitor is unknown, but inhibitor disruption of CtBP2 transcriptional complexes as seen with MTOB 6, and likely with HIPP 11, might expose uncomplexed CtBP2 to ubiquitination and proteasome degradation 2,16–17,21.
We have defined a novel role for CtBP2 as an oncogene that drives transformation of primary fibroblasts in vitro and is required for tumorigenesis in the Apcmin/+ mouse model of intestinal polyposis. Demonstration of CtBP’s transforming and tumorigenic properties underscores its potential importance in human cancer, where it is overexpressed and frequently associated with drug resistance and poor prognosis. Our finding that CtBP inhibitors safely and potently reduce polyposis in the APC min mouse model also reinforces the critical importance of further anti-CtBP therapeutic development, especially for settings such as APC-mutated cancers and cancer predisposition syndromes such as FAP, which may be especially sensitized to CtBP inhibition.
Supplementary Material
Acknowledgments
The authors wish to thank L. Litovchick for kindly donating BJ-blast cells and VSV-g/gag-pol retroviral expression vectors. We also would like to thank S. Jones for his insight and thoughtful suggestions. SRG was funded by P30CA016059 from NCI and a Research Scholar Grant from ACS.
Footnotes
Conflict of Interest
The authors have declared that no conflict of interest exists.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- 1.Turner J, Crossley M. The CtBP family: enigmatic and enzymatic transcriptional co-repressors. Bio Essays News Rev Mol Cell Dev Biol. 2001;23:683–690. doi: 10.1002/bies.1097. [DOI] [PubMed] [Google Scholar]
- 2.Chinnadurai G. The transcriptional corepressor CtBP: a foe of multiple tumor suppressors. Cancer Res. 2009;69:731–734. doi: 10.1158/0008-5472.CAN-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di L-J, Byun JS, Wong MM, Wakano C, Taylor T, Bilke S, et al. Genome-wide profiles of CtBP link metabolism with genome stability and epithelial reprogramming in breast cancer. Nat Commun. 2013;4:1449. doi: 10.1038/ncomms2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Gao C, Xu Y, Zhang Z. CtBP2 could promote prostate cancer cell proliferation through c-Myc signaling. Gene. 2014;546:73–79. doi: 10.1016/j.gene.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Barroilhet L, Yang J, Hasselblatt K, Paranal RM, Ng S-K, Rauh-Hain JA, et al. C-terminal binding protein-2 regulates response of epithelial ovarian cancer cells to histone deacetylase inhibitors. Oncogene. 2013;32:3896–3903. doi: 10.1038/onc.2012.380. [DOI] [PubMed] [Google Scholar]
- 6.Straza MW, Paliwal S, Kovi RC, Rajeshkumar B, Trenh P, Parker D, et al. Therapeutic targeting of C-terminal binding protein in human cancer. Cell Cycle. 2010;9:3740–3750. doi: 10.4161/cc.9.18.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovi RC, Paliwal S, Pande S, Grossman SR. An ARF/CtBP2 complex regulates BH3-only gene expression and p53-independent apoptosis. Cell Death Differ. 2010;17:513–521. doi: 10.1038/cdd.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grooteclaes M, Deveraux Q, Hildebrand J, Zhang Q, Goodman RH, Frisch SM. C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci U S A. 2003;100:4568–4573. doi: 10.1073/pnas.0830998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paliwal S, Kovi RC, Nath B, Chen Y-W, Lewis BC, Grossman SR. The alternative reading frame tumor suppressor antagonizes hypoxia-induced cancer cell migration via interaction with the COOH-terminal binding protein corepressor. Cancer Res. 2007;67:9322–9329. doi: 10.1158/0008-5472.CAN-07-1743. [DOI] [PubMed] [Google Scholar]
- 10.Paliwal S, Ho N, Parker D, Grossman SR. CtBP2 Promotes Human Cancer Cell Migration by Transcriptional Activation of Tiam1. Genes Cancer. 2012;3:481–490. doi: 10.1177/1947601912463695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korwar S, Morris BL, Parikh HI, Coover RA, Doughty TW, Love IM, et al. Design, synthesis, and biological evaluation of substrate-competitive inhibitors of C-terminal Binding Protein (CtBP) Bioorg Med Chem. 2016;24:2707–2715. doi: 10.1016/j.bmc.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 13.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, et al. APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc Natl Acad Sci. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groden J, Gelbert L, Thliveris A, Nelson L, Robertson M, Joslyn G, Samowitz W, Spirio L, Carlson M, Burt R, et al. Mutational analysis of patients with adenomatous polyposis: identical inactivating mutations in unrelated individuals. Am J Hum Genet. 1993;52:263–272. [PMC free article] [PubMed] [Google Scholar]
- 15.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 16.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, et al. A two step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadauld LD, Phelps R, Moore BC, Eisinger A, Sandoval IT, Chidester S, et al. Adenomatous polyposis coli control of C-terminal binding protein-1 stability regulates expression of intestinal retinol dehydrogenases. J Biol Chem. 2006;281:37828–37835. doi: 10.1074/jbc.M602119200. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrand JD, Soriano P. Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol. 2002;22:5296–5307. doi: 10.1128/MCB.22.15.5296-5307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yekkala K, Baudino TA. Inhibition of intestinal polyposis with reduced angiogenesis in ApcMin/+ mice due to decreases in c-Myc expression. Mol Cancer Res. 2007;5:1296–1303. doi: 10.1158/1541-7786.MCR-07-0232. [DOI] [PubMed] [Google Scholar]
- 20.Schneikert J, Brauburger K, Behrens J. APC mutations in colorectal tumours from FAP patients are selected for CtBP-mediated oligomerization of truncated APC. Hum Mol Genet. 2011;20:3554–3564. doi: 10.1093/hmg/ddr273. [DOI] [PubMed] [Google Scholar]
- 21.Paliwal S, Pande S, Kovi RC, Sharpless NE, Bardeesy N, Grossman SR. Targeting of C-Terminal Binding Protein (CtBP) by ARF Results in p53-Independent Apoptosis. Mol Cell Biol. 2006;26:2360–2372. doi: 10.1128/MCB.26.6.2360-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.