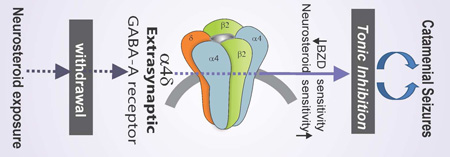
Abstract
Neurosteroids play a key role in catamenial epilepsy, a menstrual cycle-related seizure clustering in women with epilepsy. While neurosteroids act on all GABA-A receptor isoforms, they cause greater effects on extrasynaptic δGABA-A receptors that mediate tonic inhibition in the brain. Previously, we identified a potential GABA-A receptor mechanism for catamenial epilepsy. However, the precise functional role of extrasynaptic δGABA-A receptors in the pathophysiology of catamenial epilepsy remains unclear. In this study, we utilized mice lacking extrasynaptic δGABA-A receptors (δKO) to investigate whether reduction of tonic inhibition affects catamenial seizure susceptibility or intensity. Intact female wildtype (WT) and δKO mice were subjected to hippocampus kindling until they exhibited stage 5 seizures. Elevated gonadal hormone-based neurosteroid levels were induced by standard gonadotropin regimen and neurosteroid withdrawal (NSW) was triggered by finasteride. NSW increased susceptibility to, as well the intensity of evoked catamenial-like seizures in WT and δKO mice. However, fully-kindled δKO mice exhibited an accelerated and augmented response to NSW, with a more rapid increase in seizure susceptibility and intensity than WT mice undergoing the NSW paradigm. Moreover, δKO mice in NSW showed reduced benzodiazepine sensitivity, but in stark contrast to the increased neurosteroid sensitivity observed in WT animals, δKO mice displayed no change in neurosteroid sensitivity in response to NSW. The increased catamenial seizure exacerbation and alterations in antiseizure drug responses are consistent with NSW-induced changes in the abundance of δGABA-A receptors. Collectively, these findings provide evidence of a potential protective role for extrasynaptic δGABA-A receptors in catamenial-like seizures.
Keywords: Allopregnanolone, catamenial epilepsy, neurosteroid withdrawal, δGABA-A receptors, tonic inhibition, kindling
Graphical Abstract
Critical role of extrasynaptic δGABA-A receptors in catamenial epilepsy.
INTRODUCTION
Neurosteroids have been shown to be critically involved in the pathophysiology of catamenial epilepsy, a menstrual cycle-related seizure disorder characterized by seizure clustering at specific times, most often during the perimenstrual or periovulatory periods (Herzog et al., 2004; 2011; Reddy, 2009; Reddy et al., 2012; 2016). Neurosteroids represent a broad class of steroids that rapidly alter neuronal excitability. The three most widely studied neurosteroids are allopregnanolone (AP; 3α-hydroxy-5α-pregnan-20- one), THDOC, and androstanediol (Reddy and Estes, 2016). Progesterone is a major precursor of pregnane neurosteroids, such as AP and pregnanolone in the brain (Reddy et al., 2004; Tuveri et al., 2008). AP and structurally-related neurosteroid inhibit neuronal excitability through direct interaction with GABA-A receptors (Hosie et al., 2007; Carver and Reddy, 2013; 2016; Reddy, 2016b). GABA-A receptors are composed of five subunits from several classes (α1–6, β1–4, γ1–3, δ, θ, ε, and ρ1–3). The major isoforms of the pentameric receptor consist of 2α, 2β, and 1γ or δ subunits. Neurosteroids will bind to all GABA-A-receptor isoforms, but preferentially act on δ-containing extrasynaptic GABA-A receptors that mediate tonic inhibition (Belelli et al., 2002; Stell et al., 2003; Carver et al., 2016). These interactions can exert distinct anticonvulsant properties (Reddy, 2011). Emerging evidence suggests that neurosteroid interactions at GABA-A receptors plays a key role in protecting women against perimenstrual seizure exacerbation, and that neurosteroid fluctuation or withdrawal may be a key factor igniting catamenial seizures (Smith et al., 1998a,b; Reddy et al., 2001, 2012; Reddy, 2009; Gangisetty and Reddy, 2010; Pack et al., 2011; Reddy, 2016).
Presently, there is no approved drug therapy for catamenial epilepsy. Clustered occurrence of seizure exacerbations is a hallmark of catamenial epilepsy (Herzog et al., 2004; Bazan et al., 2005; Quigg et al., 2009). These catamenial cases are further classified into three distinct varieties, based on the timing of the clinical symptoms: perimenstrual and periovulatory in normal cycles, and luteal in women with inadequate luteal-phase cycles (Herzog et al., 1997; 2011). The most prevalent variety is perimenstrual catamenial epilepsy with cyclical seizure exacerbation around menstrual periods. Ovarian cycle-linked fluctuations in progesterone and neurosteroids have been shown to affect the dynamics of GABA-A receptor expression, seizure susceptibility, and pharmacological sensitivity (Carver et al., 2014; Reddy et al., 2001; Maguire et al., 2005; Tuveri et al., 2008). Seizures often decrease in the midluteal phase when serum progesterone levels are high and increase premenstrually when serum progesterone levels drop, igniting the perimenstrual-type catamenial epilepsy. Previous studies including those in progesterone receptor knockout mice have shown that progesterone protects against seizures, elicited by a variety of techniques such as chemoconvulsants and kindling, through its conversion to neurosteroids including AP (Reddy et al., 2004; 2012; Reddy and Mohan, 2011). Thus, perimenstrual seizure exacerbations are ascribed to withdrawal of the protective effects of neurosteroids (Reddy, 2009). Neurosteroid withdrawal (NSW) is associated with a marked up-regulation of the α4-subunit in the hippocampus (Smith et al., 1998a,b), which has been observed in association with an increase in seizure susceptibility and benzodiazepine resistance (Smith and Gong, 2005; Gangisetty and Reddy, 2010). Thus, NSW and the accompanying changes in GABA-A receptor subunit plasticity may be a key triggering factor for catamenial seizure exacerbations (Reddy et al., 2012; Reddy, 2016). However, the functional role of δGABA-A receptors in the pathophysiology of catamenial epilepsy remains unclear. Therefore, we hypothesize that extrasynaptic δGABA-A receptor-mediated tonic inhibition is essential to control seizure susceptibility in perimenstrual catamenial epilepsy.
In this study, we utilized δ-subunit GABA-A receptor knockout (δKO) mice to investigate whether targeted loss of δGABA-A receptor-mediated tonic inhibition within the brain affects catamenial seizure susceptibility. A catamenial-like chronic seizure condition was created by using the hippocampus kindling paradigm as described earlier (Reddy et al., 2012). Our results show a striking increase in catamenial seizure susceptibility in δKO mice following NSW, which further support the role of extrasynaptic δGABA-A receptors in catamenial epilepsy.
MATERIALS AND METHODS
Animals
Female adult (3–5 month-old) female wildtype (WT) and GABA-A receptor δ-subunit knockout (Gabrd−/−; δKO) mice (Mihalek et al., 1999; a generous gift from Dr. Martin Wallner, UCLA, CA) weighing 25–30 g were used in the study (Carver et al., 2014). All strains were maintained on a hybrid C57BL/6–129Sv background and genotyped by a validated method as described previously (Mihalek et al., 1999). Mice were not ovariectomized and maintained normal reproductive physiology prior to induction of NSW. Mice were housed in an environmentally controlled animal facility with a 12 h light/dark cycle with access to food and water ad libitum. The animals were cared for in compliance with the guidelines outlined in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by our university’s Institutional Animal Care and Use Committee.
Gonadotropin-Induced Neurosteroid Synthesis and Withdrawal Paradigm
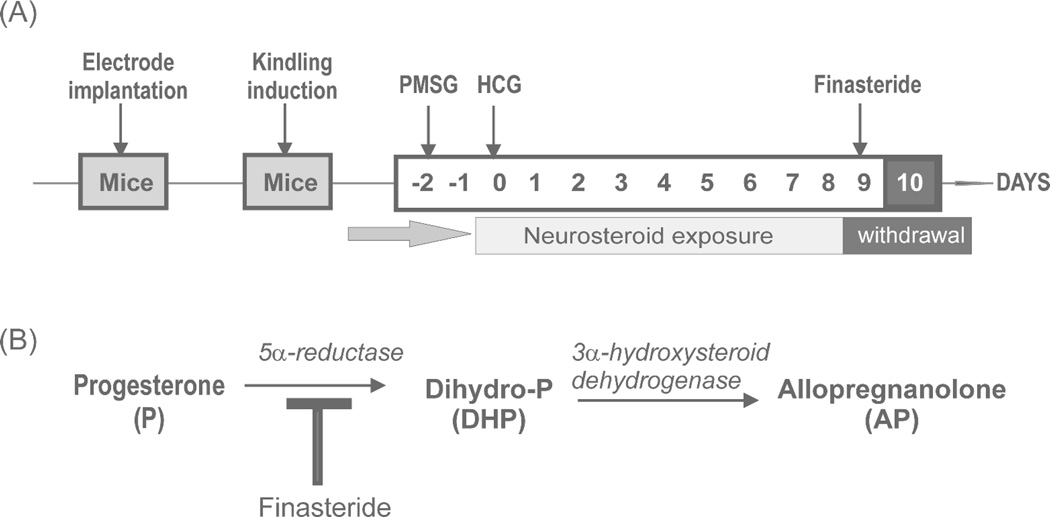
To mimic the physiological condition experienced by women during the luteal phase of the menstrual cycle, a state of elevated progesterone and neurosteroids was induced in mice by a sequential gonadotropin regimen as described previously (Reddy et al., 2012). Sequential gonadotropin treatment produces robust increases in circulating levels of progesterone’s neurosteroid derivative allopregnanolone at 24 hours after the second injection. These elevated levels persist for at least 10 days. Treatment with finasteride induces a sharp drop in circulating levels of allopregnanolone 12 to 24 hours after injection. As shown in Fig. 1, mice were treated with pregnant mare’s serum gonadotropin (PMSG; Sigma G4877; 5 IU s.c.) at 3:00 PM followed 46 h later by human chorionic gonadotropin (HCG; Sigma C0684; 5 IU s.c.) at 1:00 PM. On the ninth day, mice were injected with finasteride (Steraloids Inc. A4370-000; 50 mg/kg i.p.) to produce NSW. AP levels increase following the second gonadotropin injection and remain high through day 10 when uninterrupted. AP levels drop sharply after finasteride injection, falling to lowest levels by 12–24 hr and returning to normal physiological around 48 hr. This protocol was validated previously with neurosteroid measurements and molecular studies (Reddy et al., 2012). Animals were tested at 0, 12, 24, and 48 hours after finasteride administration, with control groups receiving saline injections. While impractical to reproduce the actual endocrine conditions of the menstrual cycle in mice, the physiological state produced here shares similarities with the perimenstrual phase observed in women. Ovariectomy was not utilized to avoid potential confounds associated with a total loss of ovarian hormones and the variable hormone replacement therapy required in such animals (Scharfman et al., 2005).
Fig. 1. Experimental paradigm of mouse catamenial epilepsy model.
(A) Gonadotropin treatment regimen in mice for creating a perimenstrual-like state of elevated neurosteroids followed by neurosteroid withdrawal (Reddy et al., 2012). (B) Abrupt inhibition of the neurosteroid allopregnanolone synthesis was accomplished by pharmacological blockade of 5α-reductase activity with finasteride to produce neurosteroid withdrawal (NSW) as validated previously (Reddy et al., 2012).
Hippocampus Kindling Model of Epilepsy
To determine the role of extrasynaptic δGABA-A receptors on catamenial seizure susceptibility, we used the hippocampus kindling model of temporal lobe epilepsy (Goddard et al., 1969). Kindling is a model of complex partial seizures in which repetition of subconvulsant stimuli that evoke afterdischarges (ADs) leads to the development of a kindled state exhibiting electrographic and behavioral seizures. Once an animal has been kindled, the seizure-inducing response to the stimulus is permanent and seizures occur upon stimulation even after several months (Reddy and Mohan, 2011). Electrode implantation and stimulation procedures for mouse hippocampus kindling were performed as described previously (Gangisetty and Reddy, 2010). Briefly, anesthetized mice (ketamine-100 mg/kg and xylazine-10 mg/kg) were stereotaxically implanted with a twisted bipolar stainless steel electrode (model MS303/1; Plastic One, Roanoke, VA) in the right hippocampus (2.9 mm posterior, 3.0 mm lateral, and 3.0 mm below dura) (Franklin and Paxinos, 1997) and secured to the skull with dental acrylic and small anchor screws. After 7 days of recovery mice were tested or afterdischarge threshold by stimulating at 5 min intervals beginning with an intensity of 25 µA and increasing in increments of 25 µA until an afterdischarge of at least 5 s was obtained. Stimulation on subsequent days used an intensity 125% of the threshold value. Seizure activity following each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; stage 5, falling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on 3 consecutive days. Seizure duration was recorded by a trained observer as the total duration of behavioral seizure expression starting from the first signs of post-stimulus freezing and/or twitching through the cessation of convulsions and/or return to normal activity. Afterdischarge duration was the total duration of hippocampus electrographic spike activity (amplitude > 2 × baseline) occurring in a rhythmic pattern at a frequency > 1Hz. Mice were used for the NSW paradigm when they consistently exhibited stage 5 seizures with stimulation, which is considered the “fully kindled” state.
Test Drug Administration and Stimulation Protocol
To examine the ability of test drugs to suppress kindled seizures, mice were tested 24 h after the induction of NSW. Two different drugs, diazepam (Hospira, 02049-02, Lake Forest, IL) and AP (Steraloids P3800-000, Newport, RI), were selected for mechanistic evaluation in NSW animals. Diazepam is insensitive as an agonist at α4GABA-A receptors (Smith et al., 1998b; Reddy et al., 2015). The neurosteroid AP binds to synaptic and extrasynaptic GABA-A receptors isoforms but has enhanced sensitivity at the extrasynaptic δGABA-A receptors (Carver and Reddy, 2016). In pharmacological studies, animals were injected with diazepam (0.1–1 mg/kg, IP) or AP (1–10 mg/kg, SC) 15 min before kindling stimulations. Behavioral seizure score, seizure duration, and the electrographic AD duration were recorded to evaluate drug responses. Finasteride and allopregnanolone (Steraloids Inc., Newport, RI) were made in 15% β-cyclodextrin (Captisol, Cydex RC-0C7-100, Lawrence, Kansas) solution. Gonadotropins (Sigma, G4877 & C0684 St. Louis) and diazepam were diluted in saline.
Data Analysis
Group data are expressed as the mean ± S.E.M. Comparison of means of the AD threshold, seizure duration, and AD duration between groups and within genotypes was made with two-way analysis of variance, followed by Student’s t-test or Tukey test for multiple comparisons. Differences in median behavioral seizure score between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. Comparison of the percent animals exhibiting seizures and percent difference in seizure duration in various groups was made by Wilcoxon signed ranks test. In all statistical tests, the criterion for statistical significance was p<0.05.
RESULTS
Progression of Hippocampus Kindling Development in δKO Mice
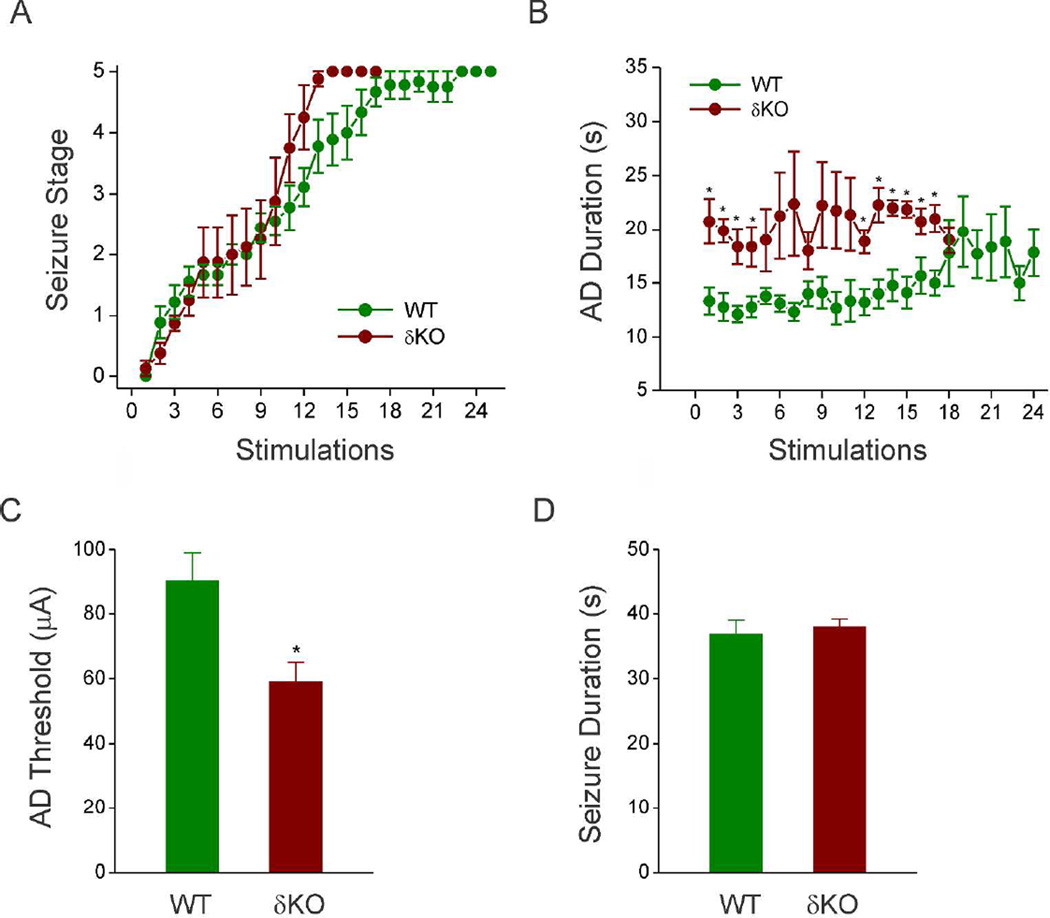
To create a model for developing catamenial epilepsy in female δKO mice, we used the hippocampus kindling model of complex partial seizures. As shown in Fig. 2, daily kindling stimulation of δKO mice was associated with a steady progression of behavioral seizures (Fig. 2A) and AD duration (Fig. 2B). Mice were subjected to once-daily kindling via an implanted electrode in the dentate gyrus region until they exhibited stage 5 seizures for 3 consecutive days, which is considered the fully kindled state. WT mice reached the fully-kindled state with consistent stage 5 seizures after 15 stimulations (Fig. 2A). Compared to WT mice, δKO mice exhibited accelerated kindling epileptogenesis, but once at fully-kindled state, did not significantly differ from WT animals in measures of seizure severity (AD duration, behavioral seizure intensity, seizure duration). δKO mice did exhibit a significantly lower seizure threshold (ADT- Fig. 2C) and reduced number of stimulations to reach fully kindled state (2A) compared to WT mice. Afterdischarge threshold does not change for individual WT or δKO mice during kindling, and AD thresholds were confirmed immediately prior to induction of NSW. δKO mice also exhibited longer early-stage AD’s than WT counterparts; however, this difference was diminished by the time animals reached fully-kindled state. Within fully-kindled animals, average seizure and AD duration did not differ between WT and δKO mice (Fig. 2D). Overall, fully kindled δKO mice do not display significant differences in evoked-seizures when compared to WT mice.
Fig. 2. Hippocampus kindling epilepsy development in δKO mice.
Mice were stimulated daily until they exhibit a steady-state, fully-kindled stage 5 (generalized) seizures. δKO mice exhibit a lower AD threshold and faster rate of kindling than WT mice, but AD and behavioral seizure duration do not differ between fully-kindled WT or δKO mice. (A) Behavioral seizure stage during 24-days period associated with kindling stimulations. (B) AD duration during the 24-days study period. (C) Average AD threshold used for hippocampal kindling stimulation in WT and δKO mice. (D) Average duration of generalized seizures in individual fully-kindled WT and δKO mice immediately prior to the induction of NSW. Data was presented as mean ± SEM. *p < 0.05 vs. WT group (N=12 DKO, N= 8 WT mice). Abrogation of δ-subunit gene in δKO mouse was confirmed by a validated genotyping method as described previously (Mihalek et al., 1999).
NSW Exacerbates Seizure Susceptibility and Evoked-Seizures in Fully-Kindled δKO Mice
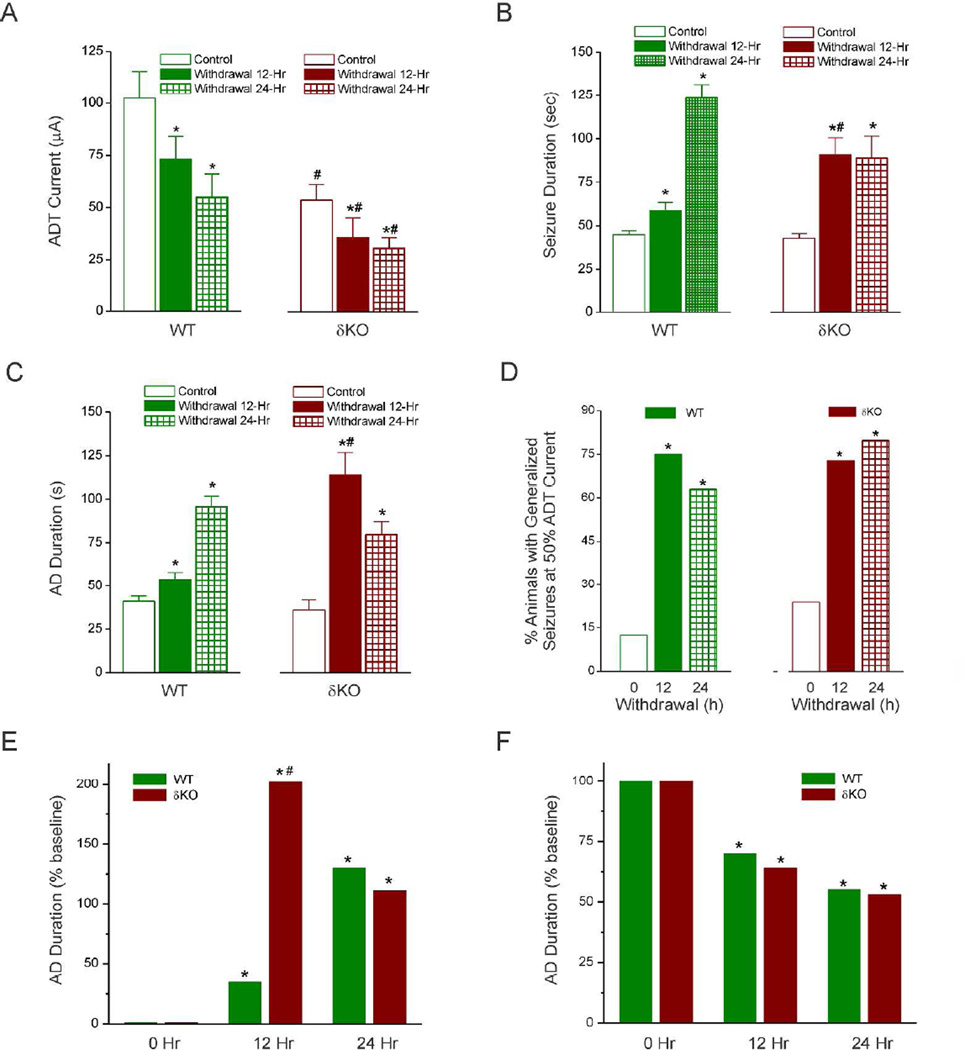
To investigate the role of δGABA-A receptors in NSW-associated increase in seizure susceptibility, we analyzed the stimulation-evoked seizure activity in animals undergoing NSW. Fully-kindled adult female WT and δKO mice were subjected to the NSW protocol as described in Fig. 1A. Four key parameters were assessed as indices of catamenial-like seizure expression: (i) ADT current, (ii) AD duration, (iii) seizure intensity as per the Racine scale, and (iv) duration of seizures. Consistent with theorized heightened excitability due to sudden reduction of inhibition-augmenting neurosteroids, there was a marked decrease in the ADT current to induce generalized seizures in WT and δKO mice at 12 and 24 h after NSW (mean ADT value, 105 and 60 µA for WT control and withdrawal, respectively, and 62 and 30 µA for δKO control and withdrawal, respectively) (Fig. 3A). The reduction in thresholds for evoked seizures was similar between genotypes (Fig. 3F). The mean duration of the individual generalized seizures was similarly increased and longer in withdrawal than in control animals of either genotype (Fig. 3B). Although increases in AD duration were observed in both WT and δKO mice at 12 hr and 24 hr after NSW, δKO mice displayed a significantly greater peak increase (at 12 hr) in AD duration than WT counterparts (Fig. 3C). This response was significantly higher 12 h and 24 h after NSW and returned to control level by 48 h after withdrawal in both WT and δKO animals (Fig. 3C and Fig. 4), indicating a transitory period for seizure exacerbation after NSW. However, as evident from Fig. 3E, the NSW-induced seizure-exacerbation effect in δKO mice occurred an accelerated pace (12 hr peak in δKO vs 24 hr in WTs), and to a significantly greater degree at peak effect as reflected by the percent increase from baseline, reflecting an accelerated rate of the NSW-induced seizure exacerbation in δKO mice. The number of animals exhibiting generalized seizures at 50% of normal kindling stimulation ADT current (a separate but valuable measure of seizure threshold) was significantly higher after NSW than in the control group (Fig. 3D).
Fig. 3. Enhanced catamenial-like evoked seizures during neurosteroid withdrawal in δKO mice.
(A) The time-course of changes in the afterdischarge threshold (ADT) current for eliciting generalized (stage 4/5) behavioral seizures after NSW. (B) The time-course of changes in the duration of behavioral (stage 4/5) seizures after NSW. (C) The time-course of changes in the duration of AD-based electrographic seizures after NSW. (D) The time-course of percent of animals exhibiting generalized seizures at ≤50% ADT current. (E) The normalized percent reduction of ADT current for eliciting generalized behavioral seizures after NSW. (F) The normalized percent increase in the duration of AD-based electrographic seizures after NSW. Data presents the mean ± SEM. *p < 0.05 vs. non-withdrawal control group; #p <0.05 vs. WT withdrawal group (N=9 DKO, N= 8 WT mice).
Fig. 4. Neurosteroid-withdrawal induced exacerbation of electrographic seizure activity in fully-kindled in δKO mice.
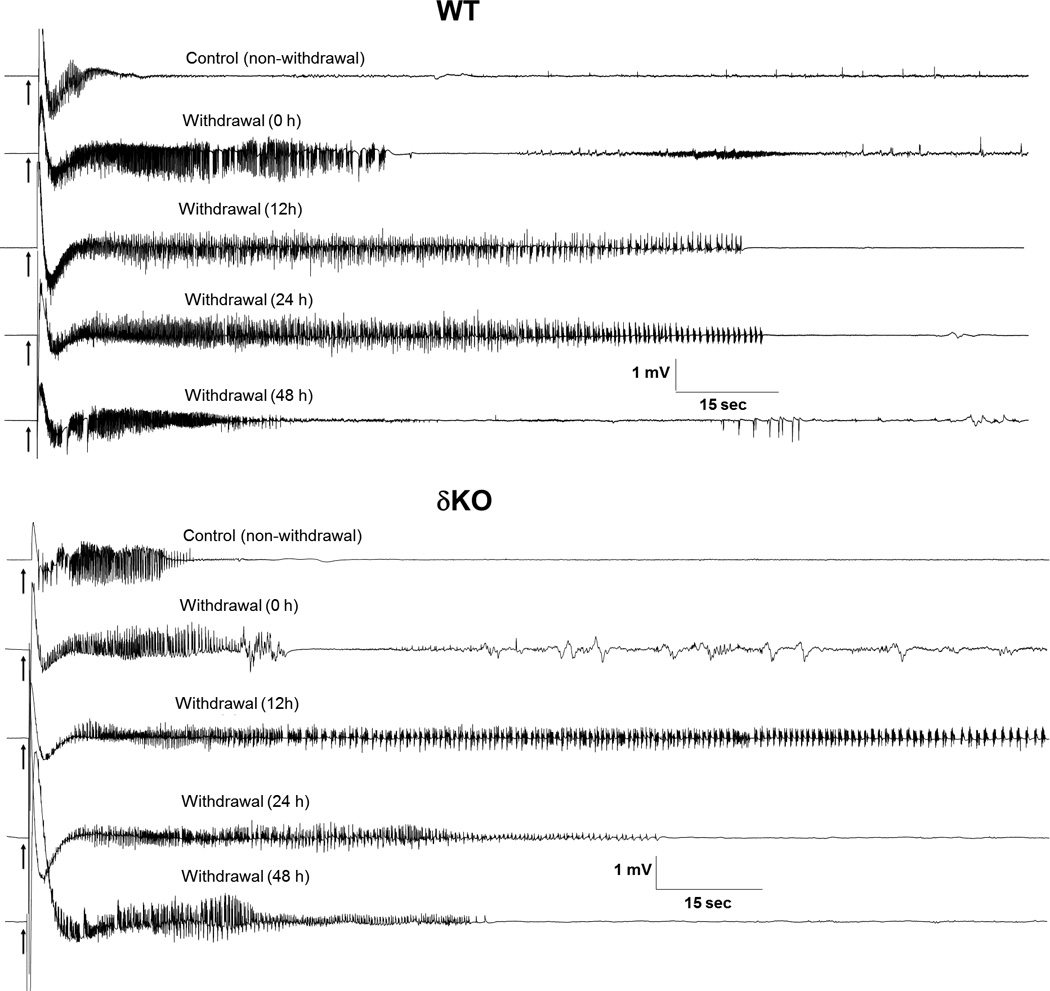
Representative traces illustrating stimulation-induced exacerbation of electrographic seizure activity in a fully-kindled WT (top panel) and δKO mouse (bottom panel) during neurosteroid-withdrawal period of 0 to 48 hours. Compared to WT mice, δKO mice exhibited a more rapid increase in AD duration, and well as a greater increase in AD duration at peak effect (12 hr). Average AD durations are similar at all other time points (see Fig. 3E).
Accelerated and Augmented NSW-Induced Exacerbation of Electrographic Seizures in δKO Mice
Representative electrographic events for each group are illustrated in Fig. 4. Neurosteroid-withdrawn WT and δKO animals showed continuous bursts of spikes that progressively increased in amplitude and duration, indicating heightened epileptiform activity (Fig. 4). In WT mice, average electrographic seizure (AD) duration was increased by 30% over baseline values 12 h after withdrawal, reached maximal levels of 200% over baseline 24 h after withdrawal, and declined to near-control level by 48 h after NSW (Fig. 3E and Fig. 4). Compared to WT animals, neurosteroid-withdrawn δKO mice displayed an accelerated timeline and augmentation of electrographic seizure exacerbation, with average AD duration peaking at nearly 300% of baseline value at 12h after withdrawal (Fig. 3E). At 24h after withdrawal, δKO mice continued to exhibit elevated AD duration, which returned to near-control levels at 48 h after withdrawal. Finasteride without gonadotropin pretreatment did not cause exacerbation of evoked-seizures in fully-kindled WT and αKO animals (data not shown), indicating the specificity of NSW on the exacerbation of seizure activity in fully-kindled WT and δKO mice. In summary, fully-kindled δKO mice undergoing the NSW paradigm experience a similar, but significantly more rapid and robust exacerbation of evoked-seizures than WT counterparts.
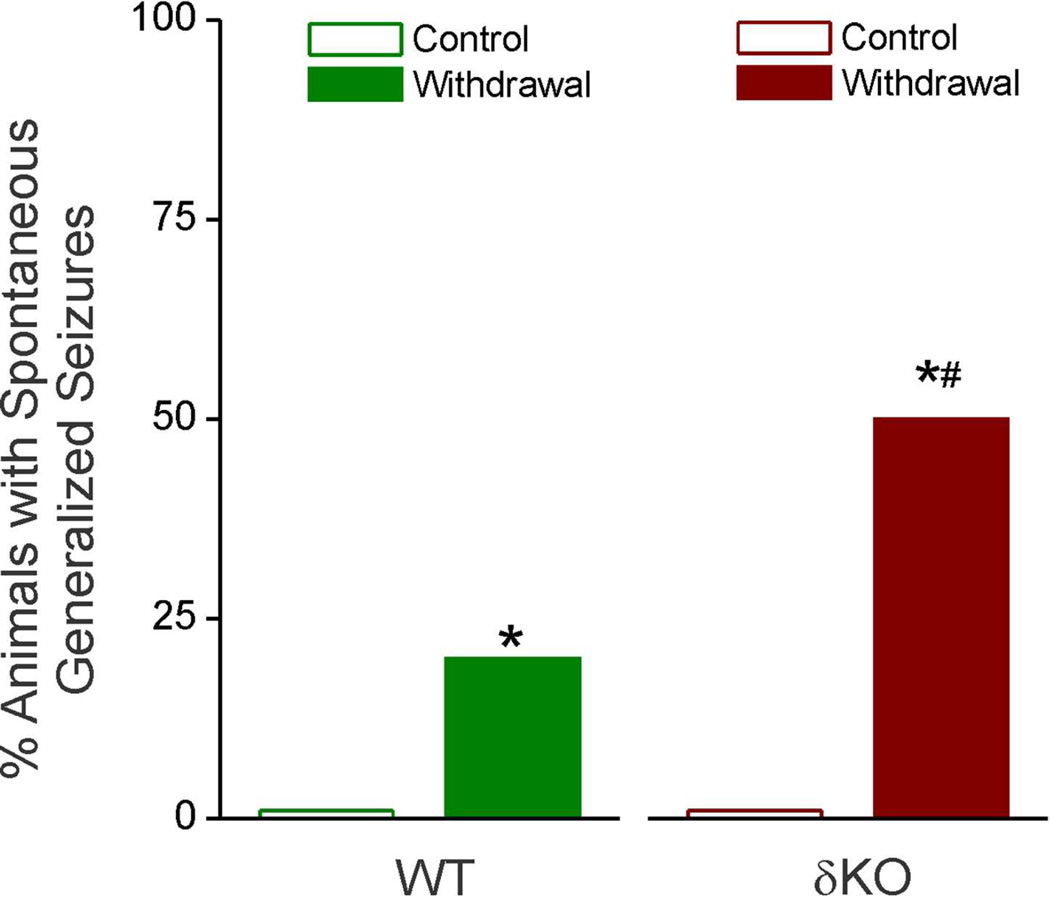
Neurosteroid Withdrawal Induces Spontaneous Generalized Seizures in δKO Mice
To further characterize the possible effects of NSW, animals were placed under continuous video surveillance to monitor for spontaneous generalized seizures and related behavior during the 24 hours following induction of NSW (Fig. 5). This period corresponds with the greatest increase in excitability in both WT and δKO mice, as observed in the exacerbated severity of stimulation-evoked electrographic and behavioral seizures (Fig. 3 & 4). Spontaneous (unprovoked) generalized seizures are not normally observed in fully-kindled, non-NSW animals of either genotype. Videos were analyzed for any mice displaying spontaneous generalized behavioral seizures of Racine level 5 by a trained observer. As shown in Fig. 5, the δKO cohort exhibited significantly increased incidence of spontaneous seizures during 24 h after NSW as compared to their WT counterparts (50% of δKO animals, compared to 20% of WT), indicating the striking increase in seizure exacerbation in δKO mice.
Fig. 5. Neurosteroid withdrawal-induced increase in spontaneous behavioral seizure activity in δKO mice.
Data represents percent of mice showing spontaneous seizures (without stimulation) during the first 24 h following induction of NSW. Wilcoxon Signed Rank Test, *p < 0.05 vs. non-withdrawal control group; #p <0.05 vs. WT withdrawal group (N=8 DKO, N= 10 WT mice).
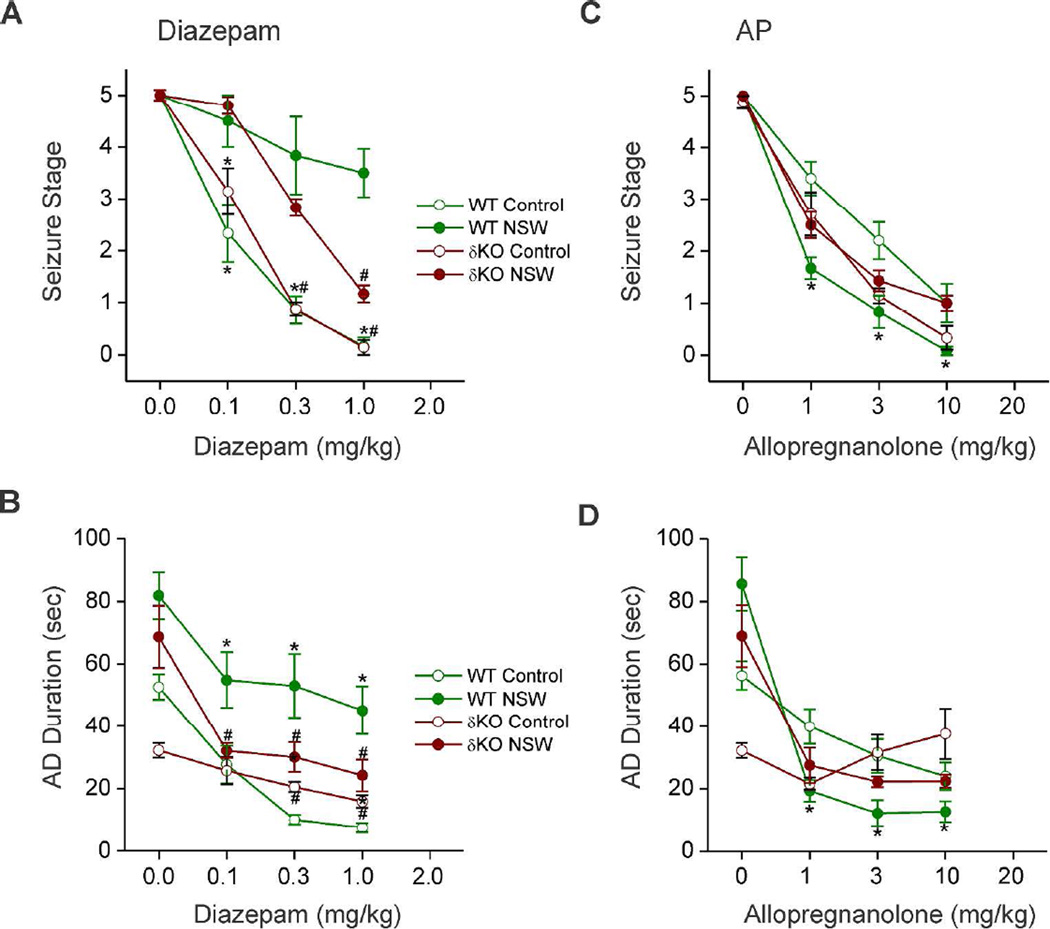
Neurosteroid Withdrawal Induces Diazepam Insensitivity in Fully-Kindled δKO Mice
To further investigate the role of δGABA-A receptors in NSW-associated alterations in the antiseizure profile of benzodiazepines, diazepam was characterized pharmacologically in fully-kindled δKO mice, as well as 24 h after NSW (Fig. 6AB). In control (non-withdrawal) WT and δKO animals, diazepam produced a dose-dependent suppression of behavioral seizure activity (Fig. 6A) and AD duration (Fig. 6B) with significant effects at 0.1, 0.3, and 1 mg/kg, confirming diazepam protection against hippocampus kindling-induced seizures. In contrast, δKO mice undergoing NSW had significantly decreased seizure protection by diazepam (Fig. 6AB). In control WT and δKO mice, doses of 0.1, 0.3, and 1 mg/kg diazepam produced 40%, 90%, and 95% seizure suppression, respectively (Reddy et al., 2012). However, doses of 0.1, 0.3, and 1 mg/kg produced 5%, 40%, and 85% seizure suppression in neurosteroid-withdrawn δKO mice. These results suggest δKO mice experience similar but reduced shunting effects on diazepam efficacy during NSW compared to WT mice, with greater differentiation of effects at higher doses.
Fig. 6. Pharmacological evaluation of antiseizure sensitivity of diazepam and allopregnanolone in δKO mice.
(AB) NSW diminishes the antiseizure efficacy of diazepam in fully-kindled WT and δKO mice. Dose-response curve for diazepam (0.1–1 mg/kg, i.p.)-induced suppression of behavioral seizure stage (A) and AD duration (B) in WT and δKO groups. (CD) NSW diminishes the antiseizure efficacy of allopregnanolone in δKO mice. Dose-response curve for allopregnanolone (1–10 mg/kg, s.c.)-induced suppression of behavioral seizure stage (A) and AD duration (B) in WT and δKO groups. Test drugs were given 15 min before kindling stimulations. Data represents the mean ± SEM (N=6–9 mice group). *p<0.05 vs. control (non-withdrawn) group; p<0.05 vs. WT (genotype) group (WT data adapted from Reddy et al., 2012).
Unaltered Neurosteroid Anticonvulsant Sensitivity in Fully-Kindled δKO Mice during NSW
In addition to diazepam, we investigated the efficacy of the prototype neurosteroid AP in δKO mice 24 h after NSW (Fig. 6CD). Fully-kindled control and neurosteroid-withdrawn δKO mice were tested in the hippocampal kindling model with three doses of AP (1, 5, and 10 mg/kg s.c.). At these doses, AP exerted dose-dependent suppression of the behavioral seizures (Fig. 6C) and AD duration (Fig. 6D) in fully-kindled control WT and δKO mice. However, in contrast to the enhanced neurosteroid sensitivity observed in fully-kindled WT mice after NSW (Reddy, et al. 2012), no change in AP’s suppression of behavioral seizures was observed between control and neurosteroid-withdrawn δKO mice (Fig. 6C), and suppression of AD in neurosteroid-withdrawn δKO mice was not significantly different beyond the 1 mg/kg dose (Fig 6D). Moreover, previous studies showed plasma levels of AP achieved at various doses of AP treatment were similar between control and withdrawn WT mice, especially without significant drug accumulation in withdrawn animals (Reddy et al, 2012), indicating that AP sensitivity was not caused by pharmacokinetic factors. The synthetic neurosteroid ganaxolone (1, 3, and 10 mg/kg) also produced enhanced (60%) efficacy in fully kindled neurosteroid-withdrawn WT animals (data not shown), confirming the enhanced sensitivity to neurosteroids in the NSW model of catamenial epilepsy (Reddy and Rogawski, 2001). Overall, these data provide powerful evidence for potential therapeutic viability of δGABA-A receptor targeting drugs for catamenial epilepsy patients suffering from intractable seizures around menses.
DISCUSSION
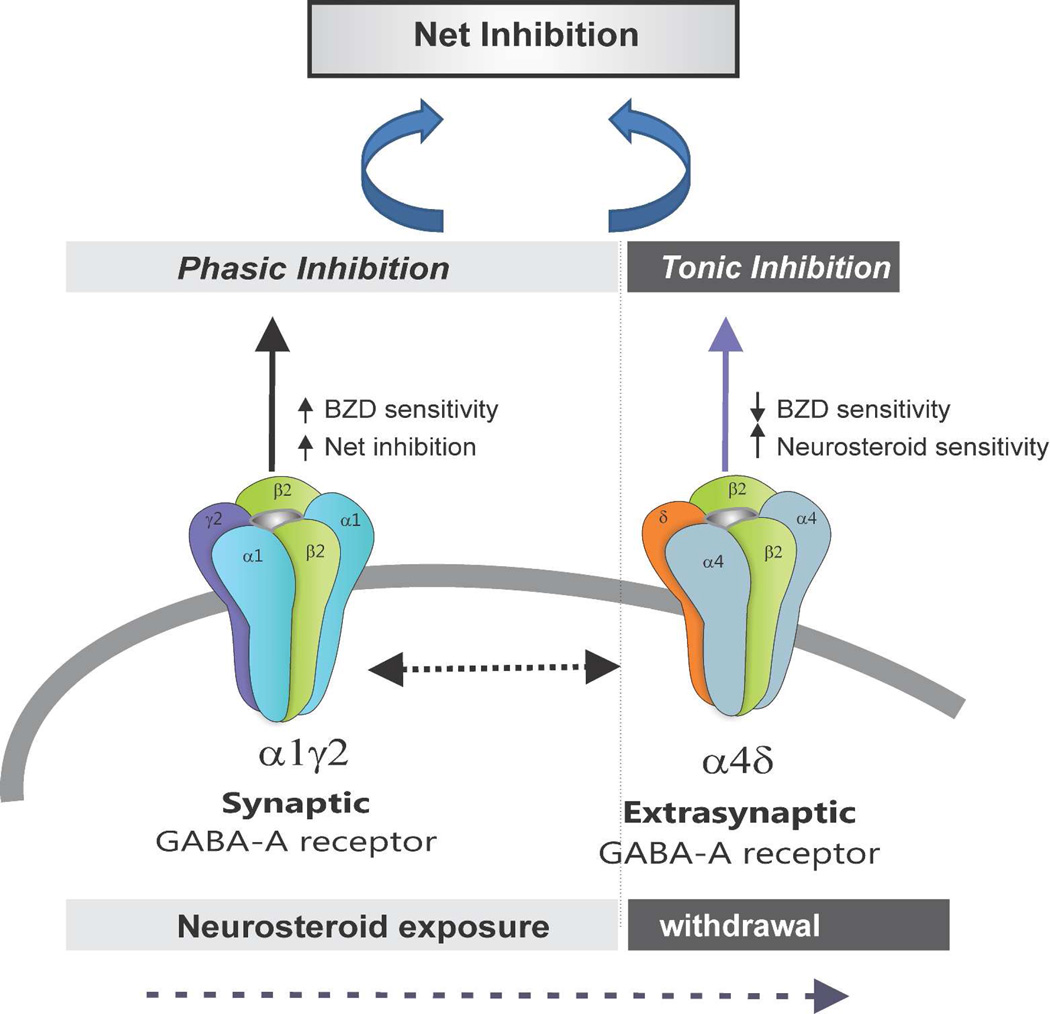
The principal findings of the study are that loss of extrasynaptic δGABA-A receptors results in striking amplification of NSW-induced hyperexcitability and seizure exacerbation in the mouse model of catamenial epilepsy. In fully-kindled female mice, NSW has been shown previously to trigger a heighted exacerbation of seizure activity as evident by an increase in seizure severity, AD duration, generalized seizure duration (Reddy et al., 2012). In δKO mice, the seizure threshold for generalized seizures was markedly decreased 12−24 h after NSW resulting in manifestation of spontaneous generalized seizures, suggesting enhanced vulnerability of δKO mice to catamenial-like seizures. NSW is known to be associated with increased GABA-A receptor α4- and δ-subunit expression, reduced antiseizure sensitivity to diazepam, and enhanced antiseizure sensitivity to AP (Reddy et al., 2012). This potential pathophysiological profile is consistent with clinical catamenial seizure features. When exposed to the NSW paradigm, mice lacking the δGABA-A receptor-mediated tonic inhibition displayed conspicuous measures of increased hyperexcitability to a greater degree than WT counterparts. Moreover, δKO mice in NSW displayed marked differences in NSW-associated changes in pharmacological response to diazepam and AP, including reduced diazepam insensitivity and lack of enhanced neurosteroid-sensitivity. Overall, these results reinforce the notion that extrasynaptic δGABA-A receptors and their endogenous neurosteroid ligands play a critical role in protecting against perimenstrual catamenial seizures in women, and that loss of this signaling through natural fluctuations in neurosteroids, such as during menstruation, may exacerbate seizure activity (Fig. 7).
Fig. 7.
Overview of potential molecular changes in synaptic and extrasynaptic GABA-A receptor subunit plasticity in the NSW model of perimenstrual catamenial epilepsy. A working model is devised to illustrate the alterations in pharmacology due to NSW-induced changes in extrasynaptic GABA-A receptor subunit composition within the hippocampus. NSW-induced upregulation of extrasynaptic δ-subunit-containing receptors may regulate tonic inhibition and neurosteroid sensitivity. Loss of such tonic inhibition in δKO mice leads to catamenial-like seizure exacerbation following NSW paradigm.
Previously we reported the development of a novel mouse model of catamenial epilepsy for use in mechanistic and therapeutic studies (Reddy et al., 2012). Our data on δ- and α4-subunit expression guided us towards a potential molecular mechanism for the enhanced seizure susceptibility and drug sensitivity in this epilepsy model. Alterations in ovarian hormones and modification of GABAergic inhibition are intensely investigated as potential pathophysiological mechanisms underlying catamenial epilepsy (Scharfman and MacLusky, 2006; Tuveri et al., 2008; Reddy, 2009, Carver et al, 2014). Until recently, an overarching theme in the investigation into catamenial epilepsy revolved around the lack of a suitable mouse model to investigate the pathophysiological mechanisms by which seizure activity is exacerbated. Using the NSW paradigm, we developed first a rat model, followed by a mouse model, both of which were successfully used for pharmacological testing of agents to reduce catamenial seizures (Reddy et al., 2001; Reddy and Rogawski, 2001, Reddy et al, 2012). A series of follow up studies have led to the identification of an extrasynaptic molecular mechanism of catamenial epilepsy (Reddy, 2016). The importance of δGABA-A receptors in protecting against hyperexcitability in catamenial epilepsy is highlighted by neurosteroid’s increased activity at δGABA-A receptors, and the lack of change observed in pharmacological efficacy of neurosteroids between NSW and control δKO mice. Therefore, the findings from the present study demonstrate an important step in the investigation of mechanisms driving hyperexcitability and seizure activity in catamenial epilepsy.
Seizure exacerbation observed in the NSW mouse model is caused by reduced neurosteroid levels in the brain. Measurements of plasma AP levels are consistent with the NSW induction effect of finasteride (Reddy et al., 2012). Finasteride blocks the synthesis of neurosteroids such as AP and related 5α-reduced pregnane analogs that modulate GABA-A receptor function (Mukai et al., 2008), and accordingly, we observed a latent period of 12–24 h after finasteride treatment before the start of NSW-related hyperexcitability. Exposing mice lacking δGABA-A receptors to NSW revealed seizure exacerbation beyond the catamenial-mimicking effects of NSW observed in fully-kindled WT mice. Compared to WT mice, δKO mice undergoing NSW displayed a striking increase in excitability, with electrographic and behavioral seizure exacerbation, including increased incidence of spontaneous generalized seizures. Such spontaneous catamenial-like seizures were not widespread in WT mice subjected to similar NSW. We have previously published similar results obtained after NSW paradigm in rats (Reddy et al., 2001) and fully-kindled mice (Reddy et al, 2012). Patients treated with finasteride had significantly reduced levels of neurosteroids (Duskova et al., 2009) and finasteride may result in seizure exacerbation in women with epilepsy (Herzog and Frye, 2003). Therefore, these reports suggest that endogenous neurosteroids and δGABA-A receptors are regulated within the ovarian cycle and serve as compelling mediators of extrasynaptic inhibition in the hippocampus and overall seizure propensity (Reddy et al., 2012; Wu et al., 2013).
The potential molecular basis for catamenial-like seizures in δKO is illustrated in Fig. 7. The enhanced seizure susceptibility following NSW is likely due, at least in part, to increased hippocampal expression of synaptic α4GABA-A receptors (Smith et al., 1998a, 2007; Reddy, 2009; Gulinello et al., 2001). The receptors exhibit faster decay kinetics than α1GABA-A receptors and confer transient benzodiazepine insensitivity. Prior studies have confirmed increases in the α4-subunit and its pharmacological properties after withdrawal from progesterone or neurosteroids (Smith et al., 1998a,b, Reddy et al, 2012, Carver et al, 2014). In the hippocampus dentate neurons, the δ-subunit preferentially coassembles with the α4 subunit to form perisynaptic/extrasynaptic GABA-A receptors (Sur et al., 1999). Tonic inhibition from dentate gyrus neurons is critically mediated by δGABA-A receptors (Stell et al., 2003). Interestingly, both δ and α4-subunits are increased in the hippocampus of NSW animals (Gangisetty and Reddy, 2010; Reddy et al., 2012; Carver et al., 2014). Therefore, when neurosteroids are withdrawn at the time of menstruation, the α4, and in-turn, δ subunit, is up-regulated, diminishing synaptic inhibition, resulting in enhanced excitability and a predisposition to catamenial seizures. However, compensatory changes such as increased expression of α4δ-subunit-containing receptors appear to preserve GABAergic inhibition in the dentate gyrus, particularly around the perimenstrual period which is associated with heightened seizure symptoms in patients with catamenial epilepsy.
In the present study, the differential changes in antiseizure activity of AP and of diazepam observed between WT and δKO mice are consistent with the premise that up-regulation of α4/δ-subunit-containing GABA-A receptors following NSW is the major contributing factor to these pharmacological changes (Gangisetty and Reddy, 2010; Reddy et al., 2012). Moreover, the increased NSW-associated seizure exacerbation observed in δKO mice supports previous studies which have pointed to δGABA-A receptors as important mediators of tonic inhibition and protection against catamenial seizures. A more defined picture of the molecular mechanisms underlying enhanced neurosteroid sensitivity in the catamenial model is now coming into view, and recent work has provided meaningful insights into the role of GABA-A receptors in the catamenial epilepsy (Carver et al., 2014; Reddy, 2016). As such, enhanced neurosteroid sensitivity in catamenial epilepsy has powerful therapeutic implications. Synthetic neurosteroids may provide a practical approach for suppressing catamenial seizures at low doses with little GABAergic side effects, and provide a unique opportunity for conjunctive therapies that work through related but divergent mechanisms. Such therapy could significantly reduce or prevent seizure occurrence in women with epilepsy (Reddy and Rogawski, 2009).
There are certain caveats of using constitutional knockout mice for epilepsy research. While receptor expression data from control knockout mice show signs of compensatory adaptation in many regions of the brain, the dynamics of NSW on the regulatory processes involved in subunit assembly and incorporation of receptors into the membrane remain unclear. Furthermore, genetic ablation of δ-GABA-A receptors signaling resulted in accelerated kindling epileptogenesis when compared to WT mice. Subunit expression analysis from δKO control mice indicates expression of γ2-subunit levels increase while α4-subunit expression levels decrease in the forebrain, implicating functional compensation in response to the loss of δ-subunit (Korpi et al., 2002; Peng et al., 200; Spigelman et al., 2003). The compensational changes to other subunits have been a challenge in understanding the selective role of δ-subunit receptors in the net inhibition of the brain.
In conclusion, these results show a striking increase in seizure exacerbation in δKO mice in the NSW model of perimenstrual catamenial epilepsy, which strongly supports the role of extrasynaptic δGABA-A receptor-mediated tonic inhibition in catamenial epilepsy. As expected, δKO mice exhibited reduced antiseizure activity to benzodiazepines but not to neurosteroids. The heightened seizure susceptibility and altered antiseizure drug responses are consistent with the relative deficiency of δGABA-A receptors in the brain. These studies provide further support for the tonic inhibition-based neurosteroid replacement therapy for catamenial epilepsy.
SIGNIFICANCE.
Catamenial epilepsy is a complex neuroendocrine condition characterized by seizure clusters during particular phases of the menstrual cycle in women with epilepsy. Currently, there is no effective drug therapy for catamenial epilepsy because the mechanism is not clearly understood. This study shows a striking role of extrasynaptic δGABA-A receptors in a perimenstrual catamenial-like epilepsy model. These findings further reinforce the potential of tonic inhibition-based neurosteroid replacement therapy for catamenial epilepsy.
Acknowledgments
This work was partly supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke grants NS051398 and NS083460 (to DSR). We would like to thank the Reddy lab staff especially Chase Carver for technical support. We thank Dr. Martin Wallner (UCLA) for the δKO transgenic mice.
ABBREVIATIONS
- AD
afterdischarge
- ADT
afterdischarge threshold
- AP
allopregnanolone
- δKO
δ-subunit knockout
- GABA
γ-aminobutyric acid
- NSW
neurosteroid withdrawal
Footnotes
The authors have no competing financial interests.
ROLE OF ATUHORS
All authors had full access to all the data in the study and take responsibility for the study and data analysis. Study concept and design: BLC, DSR. Acquisition of data: BLC, DSR. Analysis and interpretation of data: BLC, DSR. Drafting of the manuscript and critical revision of the manuscript: BLC, DSR. Statistical analysis: BLC, DSR. Obtained funding: DSR.
REFERENCES
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arq Neuropsiquiatr. 2005;63:751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid interactions with synaptic and extrasynaptic GABA-A receptors: Regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology. 2013;230:151–188. doi: 10.1007/s00213-013-3276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Wu X, Gangisetty O, Reddy DS. Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014;34:14181–14197. doi: 10.1523/JNEUROSCI.0596-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CM, Reddy DS. Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABA-A receptors in the hippocampus. J Pharmacol Exp Therap. 2016;357:188–204. doi: 10.1124/jpet.115.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duskova M, Hill M, Hanus M, Matouskova M, Starka L. Finasteride treatment and neuroactive steroid formation. Prague Med Rep. 2009;110:222–230. [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABAA receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 1st. New York: Academic Press; 1997. [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases 4 GABA-A receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Liporace JD, Kalayjian LA, Heck CN, Krauss GL, Dworetzky BA, Pennell PB Progesterone Trial Study Group. Variation of seizure frequency with ovulatory status of menstrual cycles. Epilepsia. 2011;52:1843–1848. doi: 10.1111/j.1528-1167.2011.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA-A receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA-A receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA-A receptor plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Biggio F, Mancuso L, Cabras S, Cocco PL, Gorini G, Manca A, Marra C, Purdy RH, Follesa P, et al. Changes in GABAA receptor gene expression induced by withdrawal of but not by long-term exposure to, ganaxolone in cultured rat cerebellar granule cells. J Pharmacol Exp Ther. 2002;303:1014–1020. doi: 10.1124/jpet.102.040063. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Higashi T, Nagura Y, Shimada K. Studies on neurosteroids XXV. Influence of a 5α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol Pharm Bull. 2008;31:1646–1650. doi: 10.1248/bpb.31.1646. [DOI] [PubMed] [Google Scholar]
- Pack AM, Reddy DS, Duncan S, Herzog A. Neuroendocrinological aspects of epilepsy: important issues and trends in future research. Epilepsy Behav. 2011;22:94–102. doi: 10.1016/j.yebeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG and NIH Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology. 2009;73:223–227. doi: 10.1212/WNL.0b013e3181ae7adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Front Endocrinol. 2011;2:1–11. doi: 10.3389/fendo.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy SD, Younus I, Clossen B, Reddy DS. Antiseizure activity of midazolam in mice lacking delta-subunit extrasynaptic GABA-A receptors. J Pharmacol Exp Ther. 2015;353:517–528. doi: 10.1124/jpet.114.222075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. J Neurosci. 2011;31:650–658. doi: 10.1523/JNEUROSCI.4488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–344. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Zeng YC. Effect of neurosteroid withdrawal on spontaneous recurrent seizures in a rat model of catamenial epilepsy. FASEB J. 2007;21:885.14. [Google Scholar]
- Reddy DS, Gould J, Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. J Pharmacol Exp Ther. 2012;341:784–793. doi: 10.1124/jpet.112.192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Catamenial epilepsy: discovery of an extrasynaptic molecular mechanism for targeted therapy. Front Cell Neurosci. 2016;10(101):1–14. doi: 10.3389/fncel.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Estes W. Clinical potential of neurosteroids for CNS disorders. Trends Pharmacol Sci. 2016;37:543–561. doi: 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Neurosteroid administration and withdrawal alter GABA-A receptor kinetics in CA1 hippocampus of female rats. J Physiol. 2005;564:421–436. doi: 10.1113/jphysiol.2004.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA-A receptor 4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3-OH-5 -pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABA-A receptor 4-subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ-subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J Neurophysiol. 2003;90:903–910. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ-subunit-containing GABA-A receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the 4-subunit of GABA-A receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ-subunits of the γ-aminobutyric acid A receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, et al. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA-A acid A receptors containing the 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance δsubunit containing GABA-A receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Whiting P, Wafford KA, McKernan RM. Pharmacologic subtypes of GABA-A receptors based on subunit composition, in GABA in the Nervous System: The View at Fifty Years. In: Martin DL, Olsen RW, editors. 2000. pp. 113–126. [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA-A receptors containing the δsubunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABA-A receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]