Abstract
Traumatic brain injury (TBI) is the leading cause of injury related death in children, with boys and children under 4 years having particularly poor outcomes. Activation of ATP and Calcium sensitive (Katp and Kca) channels produce cerebrovasodilation and contribute to autoregulation, both impaired after TBI, contributory to poor outcome. Upregulation of the c-Jun-terminal kinase (JNK) isoform of mitogen activated protein kinase produces K channel function impairment after CNS injury. Vasoactive agents can be used to normalize cerebral perfusion pressure. Epinephrine (EPI) prevents impairment of cerebral autoregulation and hippocampal neuronal cell necrosis after TBI in female and male newborn and female juvenile but not male juvenile pigs via differential modulation of JNK. The present study used anesthetized pigs equipped with a closed cranial window to address the hypothesis that differential K channel impairment contributes to age and sex differences in EPI-mediated outcomes after brain injury. Results show that pial artery dilation in response to the Katp and Kcan channel agonists cromakalim and NS 1619 was impaired after TBI and that such impairment was prevented by EPI in female and male newborn and female juvenile but not male juvenile pigs. Using vasodilation as an index of function, these data indicate that EPI protects cerebral autoregulation and limits histopathology after TBI through protection of K channel function via blockade of JNK in an age and sex dependent manner.
Keywords: Katp, Kca channels, autoregulation, traumatic brain injury, pressor support
Graphical abstract
Cerebral autoregulation is impaired more in young and male compared to older and female pigs after fluid percussion brain injury (FPI). Cerebrovasodilation to the K channel agonist cromakalim contributes to autoregulation and is blunted after FPI more in the male compared to the female. Epinephrine (EPI) administered after FPI to normalize cerebral perfusion pressure prevents impairment of cromakalim induced vaosodilation in young male and female, older female but not young male safter FPI via blockade of the JNK isoform of mitogen activated protein kinase. Using vasodilation as an index of function, these data indicate that EPI protects cerebral autoregulation after FPI through protection of K channel function via blockade of JNK in an age and sex dependent manner.
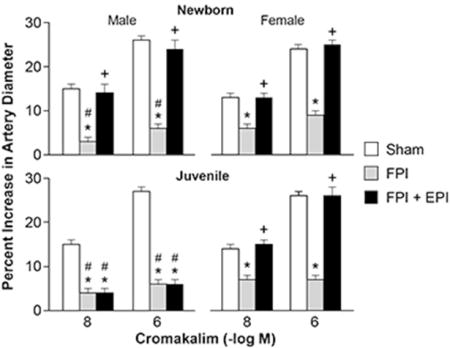
Legend for Figure: Influence of cromakalim on pial artery diameter in newborn male and female pigs and in juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI.
Introduction
Traumatic brain injury (TBI) is the primary contributor to injury related death in children (Langlois et al 2005), with boys and those less than the age of 4 having particularly poor outcomes (Newacheck et al 2004). By definition mathematically, cerebral perfusion pressure (CPP) is the difference between mean arterial pressure [MAP] and intracranial pressure [ICP]). CPP is low after TBI, causing cerebral ischemia and impaired cerebral autoregulation (Langlois et al 2005; Newacheck et al 2004; Freeman et al 2008). The 2012 Pediatric Guidelines direct clinicians to keep CPP above 40 mm Hg in children after TBI (Kochanek et al 2012). However, strategies that use employment of vasoactive agents to increase MAP and thereby augment CPP following TBI, such as norepinephrine (NE), phenylephrine (Phe), epinephrine (EPI) and dopamine (DA) (Ishikawa et al 2009; Sookplung et al 2011; Steiner et al 2004) have not been rigorously conducted so as to compare their relative effects on protection of cerebral autoregulation and improvement of ultimate outcome post insult. Clinical use of vasoactive agents in treatment of TBI is quite variable. The cerebral effects of these commonly used drugs in clinical care of TBI patients are not known.
Many basic science animal studies have been conducted in rodents. However, pigs are more human-like and have a gyrencephalic brain containing a white/grey ratio more similar to the human. The latter is important because white matter is more vulnerable to injury following TBI. (Dobbing 1981).
Cerebral autoregulation is a homeostatic mechanism to regulate CBF over a range of blood pressures. Previous studies showed that cerebral autoregulation is more impaired in young and male compared to female and older pigs after TBI, which parallels the clinical experience (Armstead 2000; Dobbing 1981; Digennaro et al 2011). From a mechanistic standpoint, our earlier studies have noted a more augmented increase in the phosphorylated form of the the c-Jun-terminal kinase (JNK) isoform of mitogen activated protein kinase (MAPK) in males compared to females following FPI, which contributed to the equally noted greater impairment of cerebral autoregulation in the former compared to the latter (Armstead et al 2012. Recent studies have shown that epinephrine (EPI) protects cerebral autoregulation and limits hippocampal neuronal cell necrosis after TBI in both female and male newborn and female juvenile but not male juvenile pigs via differential modulation of JNK (Armstead et al, in press).
Three important mechanisms for blood vessel dilation involve either cAMP, cGMP, and/or K+ channels (Faraci and Heistad 1998). Vascular tone is highly dependent on the level of the resting membrane potential and K+ channels are a major regulator of membrane potential (Faraci and Heistad 1998). Membrane hyperpolarization results from increased K+ flux, resulting in relaxation of vascular smooth muscle. ATP sensitive (KATP) and calcium sensitive (Kca) channels are important contributors to the regulation of cerebral blood vessels (Faraci and Heistad 1998) and vasodilation to K channel agonists can be used as an index of the intactness of K channel function after TBI (Armstead 1997, Salvucci and Armstead 2000). Katp and Kca channel activation are important contributors to autoregulation (Faraci and Heistad 1998; Armstead 1999).
This study addressed the hypothesis that differential K channel impairment contributes to age and sex differences in EPI-mediated outcomes after brain injury.
Materials and Methods
Anesthetic regimen, closed cranial window technique, and fluid percussion brain injury
All animal protocols were approved by the University of Pennsylvania Animal Care and Use Committee. Newborn and juvenile Yorkshire pigs obtained from Meck Swine LLC (Lancaster PA) (1–5 days old, 1.0–1.3 kg, and 4 weeks old, 6.0–7.1 Kg) of either sex were studied. The pigs as provided by the vendor did not necessarily all come from the same litter. The University of Pennsylvania Department of Laboratory Animal Services provides husbandry service for fee for all animals used in research. The type of housing is a plastic enclosure that can be sanitized containing an over head heat lamp and non movable bowls for water and pig milk replacer food (Advance Birthright replacer, Ralco Animal Health in Marshall MN). The pigs are not housed with the dams, having been weaned at 1 day of age prior to transport by the vendor to the University of Pennsylvania. The anesthetic regimen consisted of: pre-medication with dexmedetomidine (20 μg/kg im; Orion Finland), induction with isoflurane (2–3%; Abbott North Chicago IL), isoflurane taper to 0% after start of total intravenous anesthesia (TIVA) with fentanyl (200 ug/kg/hr in newborns but 100 ug/kg/hr in juveniles; Hospira Lake Forest, IL), dexmedetomidine (2 μg/kg/hr;), propofol (2–10 mg/kg/hr; Zoetis Kalamazoo MI), midazolam (1mg/kg/hr; Hospira), and saline (2ml/kg/hr in newborns and 10 ml/kg/hr in juveniles) and maintenance of TIVA for the balance of the surgical and experimental portions of the pig preparation. A catheter was inserted into a femoral artery to monitor blood pressure and femoral veins for drug administration. The trachea was cannulated, the animals ventilated with room air, and temperature maintained in the normothermic range (37° – 39° C), monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring, and a circular glass coverslip. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 1.5 MgCl2, 3.0 KCl, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3 (all from Sigma St Louis Missouri). This artificial CSF was warmed to 37° C and had the following chemistry: pH 7.35, pCO2 48 mm Hg, and pO2 45 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter (resting diameter, 120–160 μm) was measured with a microscope, a camera, a video output screen and a video microscaler. For sample collection, 300 μl of the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side. An Integra Camino monitor (San Diego, CA) was used to measure ICP.
A device designed by the Medical College of Virginia was used to produce fluid percussion brain injury (FPI). A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura. This shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9 % saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9–2.3 atm. with a constant duration of 19–23 ms) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Pigs were randomized to one of each experimental intervention group: (1) sham control, (2) FPI, (3) FPI post-treated with EPI. We used an n of 5 for each sex and age. Calculations of the total n therefore are: 3 groups with n=5 each or 15 × 2 sexes = 30 × 2 ages = 60 pigs. CPP was targeted (55–60 and 65–70 mm Hg for newborns and juveniles, respectively, per 2012 Pediatric Guidelines) to determine the dose of the iv infusion (in μg/kg/min) of EPI and EPI treatment is started when CPP decreases below 45 mm Hg. From the equation CPP = MAP – ICP, when CPP decreased below 45 mm Hg the EPI infusion (0.8–1.4 ug/kg/min iv; IMS So El Monte CA) was then started and the dose was increased until the target CPP was reached; this approach is typically used in the clinical setting. The FPI alone group received saline. Investigators were blinded as to whether EPI or saline was infused post FPI.
In sham control animals, responses to cromakalim and NS 1619, Katp and Kca agonists, the NO releaser sodium nitroprusside (SNP) (all 10−8, 10−6 M; Sigma), and PGE2 (1, 10 ng/ml; Upjohn Kalamazoo MI) were obtained initially and then again 4h later in the presence of the agent vehicle. The vehicle for all agents was 0.9% saline. In drug post-treated animals, drugs were administered after FPI and responses to cromakliam, NS1619, SNP, and PGE2 were obtained initially and at 4h post insult. The order of agonist administration was randomized within animal groups. A wait period of 20 min occurred between each set of stimuli in order to allow CBF and pial artery diameter to return to control value.
ELISA
Commercially available enzyme-linked immunosorbent assay (ELISA) Kits were used to quantify CSF phosphorylated JNK-MAPK (Assay Designs, Farmingdale, NY) concentration.
Statistical analysis
Sample size was determined by using power analysis to calculate the size necessary to achieve a reliable measurement of the effect. Power analysis from prior studies shows that a sample size of 5 for hemodynamic data sets will yield statistical significance at the p<0.05 level with power of 0.84. Similar analysis for biochemical the index of outcome (JNK MAPK) has a power of 0.82. This number was not altered during the course of the study. Data collection was stopped, however, if the animal’s health was deemed to be not within physiological norms, eg normocapnia, normoxia. In this study, no animals were excluded for data analysis. Mortality was not a factor; all animals reached study endpoint. The order of vascular stimulus administration was randomized. the investigator was blinded to treatment group.
Pial artery diameter and CSF phosphorylated JNK values were analyzed using ANOVA for repeated measures and consideration given towards parametric analysis. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An a level of p<0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value. Vasodilator responses are presented as a percentage change from baseline in order to normalize the pial artery diameter values.
Results
FPI impairs pial artery dilation in response to Katp and Kca channel agonists more in the male compared to the female.
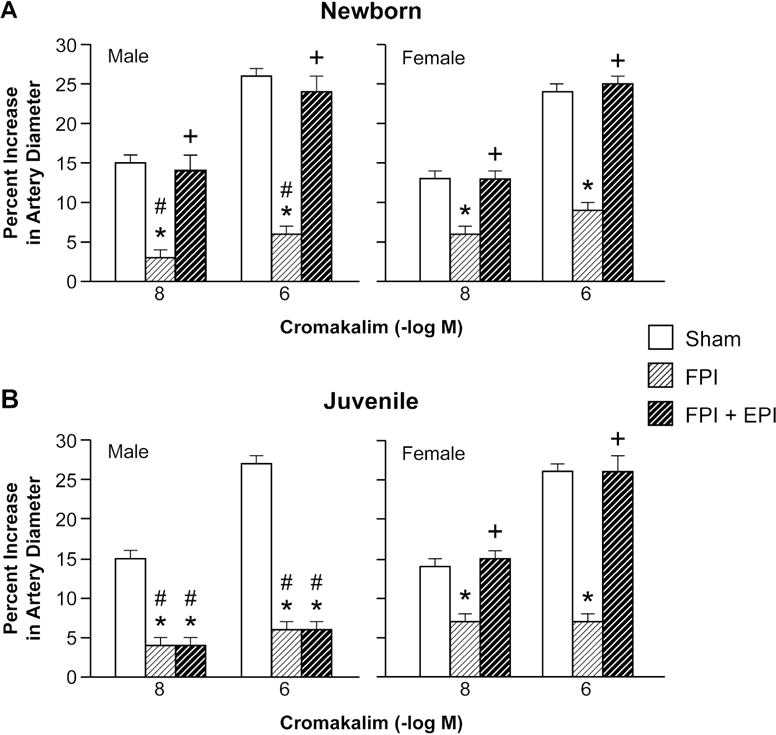
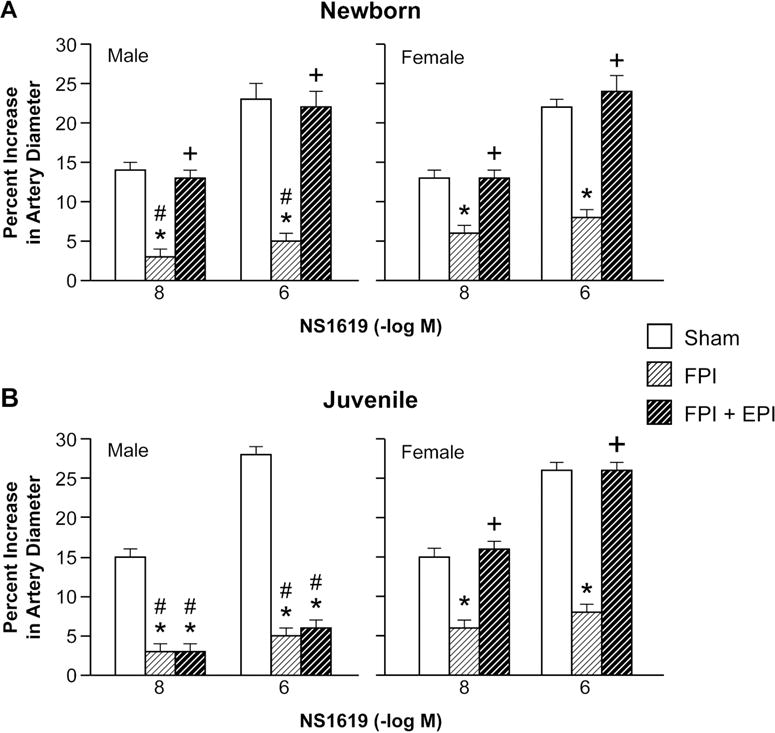
Cromakalim and NS 1619 (10−8, 10−6) elicited reproducible pial small artery dilation prior to injury. FPI produced injury of equivalent intensity in male and female newborn and juvenile pigs. Vasodilation in response to both K channel agonists was blunted after FPI (ANOVA, n=5, p = 0.01) more in the male than the female in both newborn and juvenile age pigs (Fig 1,2) (ANOVA, n=5, p = 0.01). SNP (10−8, 10−6) and PGE2 (1, 10 ng/ml) elicited reproducible pial small artery dilation prior to injury, similar to cromakalim and NS 1619. Vasodilation to SNP and PGE2 was blunted after FPI (ANOVA, n=5, p = 0.02), but, in contrast to the observations with the K channel agonists, impairment of such vasodilator responses was not greater in males compared to females after FPI (ANOVA, n = 5, p = 0.09) (Fig 3, 4).
Figure 1.
Influence of cromakalim (10−8, 10−6 M) on pial artery diameter in (A) newborn male and female pigs and in (B) juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI, n=5. *p<0.05 compared to corresponding sham value, +p<0.05 compared to corresponding FPI alone value, #p<0.05 compared to corresponding female value.
Figure 2.
Influence of NS 1619 (10−8, 10−6 M) on pial artery diameter in (A) newborn male and female pigs and in (B) juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI, n=5. *p<0.05 compared to corresponding sham value, +p<0.05 compared to corresponding FPI alone value, #p<0.05 compared to corresponding female value.
Figure 3.

Influence of SNP (10−8, 10−6 M) on pial artery diameter in (A) newborn male and female pigs and in (B) juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI, n=5. *p<0.05 compared to corresponding sham value, +p<0.05 compared to corresponding FPI alone value.
Figure 4.
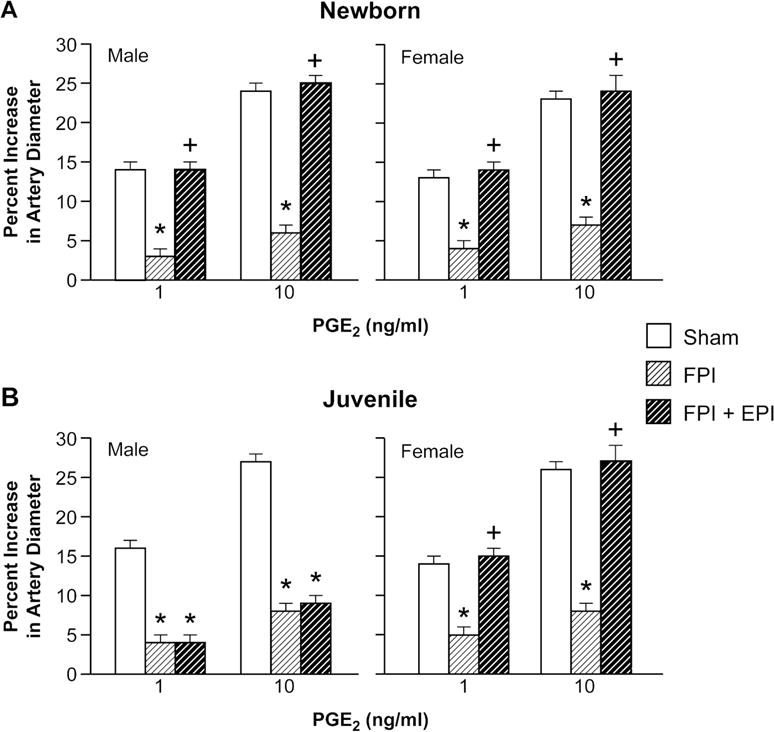
Influence of PGE2 (1, 10 ng/ml) on pial artery diameter in (A) newborn male and female pigs and in (B) juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI, n=5. *p<0.05 compared to corresponding sham value, +p<0.05 compared to corresponding FPI alone value.
EPI preserves vasodilation to Katp, Kca channel agonists, SNP, and PGE2 in newborn male and female and juvenile female pigs, but not juvenile males after FPI.
After FPI, MAP slowly decreases while ICP is increased, thereby resulting in lower CPP. Clinically, administration of a vasoactive agent to normalize CPP is begun when CPP drops below a given threshold. Following this clinical paradigm, CPP was targeted (55–60 and 65–70 mm Hg for newborn and juvenile pigs, per 2012 Pediatric Guidelines) to determine the dose of EPI. CPP was equivalent in males and females. EPI preserved vasodilation to Katp, Kca channel agonists, SNP, and PGE2 in newborn male and female and juvenile female pigs (ANOVA, n = 5, p = 0.01), but not juvenile males after FPI (ANOVA, n = 5, p = 0.12) (Figs 1–4).
EPI blocked elevation of CSF JNK MAPK in newborn male and female and female juvenile but not in male juvenile pigs after FPI.
Phosphorylated JNK MAPK concentration in CSF was elevated more in males compared to females within each age group and more in newborn males compared to juvenile males after FPI (ANOVA, n = 5, p = 0.01) (Fig 5). EPI blocked elevation of CSF phospho JNK MAPK concentration in newborn males and females along with juvenile females after FPI (ANOVA, n = 5, p = 0.01) (Fig 5). However, CSF phospho JNK MAPK concentration was further elevated when EPI was administered after FPI in juvenile male pigs (ANOVA, n =5, p = 0. 02) (Fig 5). The JNK MAPK antagonist SP 600125 (1 mg/kg iv) co-administered with EPI prevented reductions in pial artery dilation to cromakalim, NS 1619, SNP, and PGE2 after FPI. SP 600125 (1 mg/kg iv) blocked elevation of CSF phospho JNK MAPK after FPI, without effect on other MAPK isoforms (Armstead et al 2011a).
Figure 5.

Phosphorylated JNK MAPK (pg/ml) before (0 time) and 4h after FPI in in A) newborn male and female pigs and in (B) juvenile male and female pigs before (sham), after FPI, and after FPI treated with EPI, n=5. *p<0.05 compared to corresponding 0 time value, +p<0.05 compared to corresponding FPI alone value, #p<0.05 compared to corresponding female value.
Blood Chemistry
Blood chemistry values served as an internal index of uniformity of animal health to limit conclusions being drawn owing to differences in variable health state instead of therapeutic treatment with EPI. Values were no different at the end of the experiment as compared to those observed at the start of the experiment, indicating a healthy preparation throughout (Table 1).
Table 1.
Blood Chemistry
| Beginning of Experiment | End of Experiment | |||||
|---|---|---|---|---|---|---|
| pH | pCO2 | pO2 | pH | pCO2 | ||
| pO2 | ||||||
| Newborn Male | Newborn Male | |||||
| Sham | 7.45 ± 0.03 | 35 ± 4 | 93 ± 10 | 7.43 ± 0.04 | 37 ± 6 | 90 ± 11 |
| FPI | 7.46 ± 0.02 | 38 ± 5 | 90 ± 11 | 7.42 ± 0.05 | 32 ± 6 | 88 ± 10 |
| FPI + EPI | 7.47 ± 0.04 | 36 ± 4 | 99 ± 10 | 7.43 ± 0.03 | 38 ± 5 | 86 ± 10 |
| Newborn Female | Newborn Female | |||||
| Sham | 7.47 ± 0.06 | 31 ± 6 | 99 ± 11 | 7.40 ± 0.05 | 40 ± 5 | 84 ± 11 |
| FPI | 7.45 ± 0.03 | 33 ± 5 | 97 ± 9 | 7.40 ± 0.05 | 42 ± 7 | 90 ± 12 |
| FPI + EPI | 7.46 ± 0.03 | 38 ± 6 | 90 ± 10 | 7.41 ± 0.04 | 36 ± 4 | 92 ± 11 |
| Juvenile Male | Juvenile Male | |||||
| Sham | 7.45 ± 0.05 | 36 ± 5 | 92 ± 13 | 7.42 ± 0.06 | 40 ± 6 | 91 ± 10 |
| FPI | 7.47 ± 0.04 | 32 ± 6 | 82 ± 10 | 7.43 ± 0.04 | 36 ± 4 | 97 ± 12 |
| FPI + EPI | 7.46 ± 0.03 | 35 ± 6 | 99 ± 14 | 7.40 ± 0.06 | 38 ± 4 | 97 ± 10 |
| Juvenile Female | Juvenile Female | |||||
| Sham | 7.46 ± 0.05 | 37 ± 4 | 93 ± 10 | 7.42 ± 0.05 | 36 ± 7 | 97 ± 10 |
| FPI | 7.45 ± 0.06 | 37 ± 7 | 98 ± 12 | 7.42 ± 0.04 | 37 ± 4 | 98 ± 10 |
| FPI + EPI | 7.45 ± 0.04 | 37 ± 8 | 99 ± 13 | 7.44 ± 0.06 | 37 ± 8 | 95 ± 10 |
Blood chemistry values for pH, pCO2 and pO2 for animals allocated to sham, FPI, or FPI + EPI groups, n=5, obtained at the beginning and at the end of all experiments.
Discussion
Several key findings emerged from this study. First, vasodilation in response to activators of the Katp and Kca channels was observed to be impaired more in the male than the female in both newborn and juvenile age pigs after TBI. These data extend prior studies in which only the younger age pig was investigated (Armstead et al 2011b). Additionally, vasodilation in response to the NO releaser SNP and the prostaglandin PGE2 were also impaired after TBI, though no gender or age dependent differences in the magnitude of impairment was observed. Because the degree of injury was equivalent in younger and older male and female pigs, these data suggest intrinsic gender differences in outcome after pediatric TBI for some but not all signaling pathways for cerebrovasodilation.
Second, the results of this study show that EPI prevents impairment of K channel function after FPI in both male and female newborn and female juvenile but not male juvenile pigs. Recent studies (Armstead et al in press) have shown that EPI also profoundly protects cerebral autoregulation and prevents injury induced reductions in cerebral blood flow (CBF) in the setting of FPI in male and female newborns and female juveniles, but not in juvenile males. In earlier studies, cerebrovasodilation during hypotension was observed to be dependent on the activation of both Katp and Kca channels as well as NO and prostaglandins such as PGE2 (Armstead 1999, 2000). Together, these studies provide a mechanistic interpretation of data wherein EPI produced age and sex dependent differences in cerebrohemodynamic outcome after TBI resulting from age and sex dependent protection of K channel function. This mechanism was similarly observed to be operative for age and sex dependent protection of cerebral autoregulation with use of Phe as the vasoactive agent (Armstead et al 2011b). However, this has yet to be explicitly investigated for use of NE or DA as the vasoactive agent after TBI. Future studies will investigate this as a potential unifying mechanism for differences in outcome as a function of sex and age with choice of vasoactive agent after TBI. Since newborn and juvenile pigs may approximate the human neonate (6 months- 2yrs old) and child (8–10 yrs old) respectively (Dobbing 1981), these observations suggest the consideration of individualized medical approaches boys and girls and for younger and older children.
In the context of the neurovascular unit concept, CBF is thought to contribute to neuronal cell integrity and health. In cases where EPI protected cerebral hemodynamics, there was limitation of neuronal cell necrosis in CA1 and CA3 hippocampus (Armstead et al in press). Impairment of autoregulation following TBI appears linked to Glasgow Coma Scale (GCS), with greater autoregulatory impairment associated with worse GCS (Freeman et al 2008). We suggest that EPI may affect cognitive outcome differently in older males and females and younger vs older males. However, histology was done at an early time point (4h post injury). Differences between treatment groups and sex may disappear after more neurons die after 4h.
The role of the JNK in outcome in CNS pathology has been prior investigated. FPI increases phosphorylated JNK MAPK in cortical tissue of the rat and the CSF of the pig (Armstead et al 2011a, in press; Otani et al 2002). Treatment with the JNK inhibitor DJNKI1 improved outcome after TBI (Ortolano et al 2009). Another JNK inhibitor, SP 600125, prevented impairment of PGE2 cerebrovasodilation, while inhibition of JNK prevented impairment of cerebral autoregulation after FPI (Ross and Armstead 2005; Armstead et al 2012). Our data indicate that inhibition of JNK MAPK upregulation by EPI prevents impairment of Katp, Kca channel, NO, and PGE2 mediated cerebrovasodilation, thereby limiting impairment of cerebral autoregulation. Nonetheless, early activation of JNK may also be protective since it mediates hypothermic prevention of cell death after FPI in the rat (Lotocki et al 2006).
Study Design Limitations
Female piglets, like most mammalian species, undergo puberty earlier than males, and although puberty is not fully in place until 3–5 months, hormonal surges begin well ahead of puberty. Further, in piglets, sex-specific behaviors are observed as early as 4 weeks demonstrating an influence of sex steroids at this early time point. It is therefore possible that although the juvenile groups were the same age between males and females in the current study that the pubertal stage may not have been equivalent. While the present study did not collect blood samples for determination of sex steroid concentrations, it is acknowledged that lack of such data is a design limitation.
Anesthesia can influence CBF, metabolism and potentially autoregulation. Equally, choice of anesthetic may also influence outcome, particularly in that dexmedetomidine and propofol are less often used clinically in children. Nonetheless, while anesthetics may influence the magnitude of the effect, we feel that the direction of the change should remain the same. We feel that such interference with data interpretation is minimized by the fact that we have performed these experiments with two very different anesthetic regimens but the effects of TBI were no different in the one versus the second. Earlier studies used alpha chloralose as the anesthetic in studies wherein CPP was managed by Phe or DA administration. In those studies, autoregulation was impaired after FPI to a similar extent as those given TIVA and more impaired in male than female newborn pigs (Armstead et al 2010a,b). Alpha chloralose is an old line anesthetic not used clinically so more recent studies used to TIVA to make the results more translationally relevant. We are unaware of anyone who has documented that chloralose is either neuroprotective or injurious to the brain. Since CPP management improved hemodyanamic outcome in both chloralose and TIVA animals after FPI, it is doubtful that TIVA synergizes with CPP management to improve outcome.
Conclusions
There are no evidence based guidelines or recommendations regarding choice of vasoactive agent after adult or pediatric TBI. Choice of vasoactive agent across medical centers is variable, and may be related to outcome. Our previous studies in pigs have observed that NE protects autoregulation in newborn female, juvenile male, and juvenile female but not newborn males pigs after TBI (Armstead et al 2016a,b). Phe augments impairment of autoregulation in newborn male pigs but protects autoregulation in newborn females after TBI (Armstead et al 2011b). However, DA protects autoregulation after TBI in both male and female newborn pigs (Armstead et al 2013). Taken together, the latter studies support the consideration of age and sex in vasoactive agent choice to improve outcome after TBI. The ongoing multiple medical therapies (MMT) project (Bell et al 2013) will provide 3,6, and 12 month outcome for patients given various pressors for CPP support. However, this project will not be able to answer cerebral autoregulation or mechanistic questions. Therefore, results of this study inform the mechanistic downstream interpretation of cerebral hemodynamic findings observed in the MMT project.
Results of this study show that pial artery dilation in response to the Katp and Kcan channel agonists cromakalim and NS 1619 was impaired after TBI and that such impairment was prevented by EPI in both male and female newborn and female juvenile but not male juvenile pigs. Using vasodilation as an index of function, these data indicate that EPI protects cerebral autoregulation and limits histopathology after TBI through protection of K channel function via blockade of JNK in an age and sex dependent manner.
Significance Statement.
Traumatic brain injury (TBI) is the leading cause of injury related death in children, with boys and children under 4 years having particularly poor outcomes. Low cerebral perfusion pressure (CPP) after TBI is associated with poor outcome. Vasoactive agents such as epinephrine are used to normalize CPP after TBI. Yet, cerebral effects of this commonly used vasoactive agent are not known. Mechanistically driven questions that cannot be investigated in humans for ethical reasons can be conducted in pigs, which ultimately will inform and lead to improvements of clinical care of pediatric TBI patients.
Acknowledgments
Sources of Funding: This study was supported by RO1 NS090998 from the NIH.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: WMA, MSV. Acquisition of data: WMA, JR. Analysis and interpretation of data: WMA, MSV. Drafting of the manuscript: WMA, MSV. Critical revision of the manuscript for important intellectual content: WMA, JR, MSV. Statistical analysis: WMA, MSV. Obtained funding: WMA. Administrative, technical, and material support: WMA, JR. Study supervision: WMA.
Footnotes
Conflict of Interest Statement
The authors have nothing to declare.
References
- Armstead WM. Brain injury impairs ATP-sensitive K+ channel function in piglet cerebral arteries. Stroke. 1997;28:2273–2280. doi: 10.1161/01.str.28.11.2273. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Hypotension dilates pial arteries by Katp and Kca channel activation. Brain Res. 1999;816:158–164. doi: 10.1016/s0006-8993(98)01146-9. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7:225–235. [PubMed] [Google Scholar]
- Armstead WM, Kiessling JW, Bdeir K, Kofke WA, Vavilala MS. Adrenomedullin prevents sex dependent impairment of cerebal autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J Neurotrauma. 2010a;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Impaired cerebral blood flow autoregulation during post traumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit Care Med. 2010b;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Kiessling JW, Riley J, Cines DB, Higazi AAR. tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK. Neurological Research. 2011a;33:726–733. doi: 10.1179/016164110X12807570509853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Kiessling JW, Riley J, Kofke WA, Vavilala Phenylephrine infusion prevents impairment of ATP- and calcium sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male piglets through modulation of ERK MAPK upregulation. J Neurotrauma. 2011b;28:105–111. doi: 10.1089/neu.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Riley J, Cines EB, Higazi AAR. Combination therapy with glucagon and a novel plasminogen activator inhibitor-1 derived peptide enhances protection against cerebrovasodilation during hypotension after traumatic brain injury through inhibition of ERK and JNK MAPK. Neurological Research. 2012;34:530–537. doi: 10.1179/1743132812Y.0000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Riley J, Vavilala MS. Dopamine prevents impairment of autoregulation after TBI in the newborn pig through inhibition of upregulation of ET-1 and ERK MAPK. Ped Crit Care Med. 2013;14:e103–e111. doi: 10.1097/PCC.0b013e3182712b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead WM, Riley J, Vavilala M. Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr Crit Care Med. 2016a Mar;17(3):e130–137. doi: 10.1097/PCC.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Armstead WM, Riley J, Vavilala MS. Norepinephrine protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury via block of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma. 2016b;33:1761–1767. doi: 10.1089/neu.2015.4290. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Armstead WM, Riley J, Vavilala MS. Sex and age differences in epinephrine mechanisms and outcomes after brain injury. J Neurotrauma. doi: 10.1089/neu.2016.4770. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Armstead WM, Vavilala MS. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J Cereb Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Adelson PD, Hutchison JS, Kochanek PM, Tasker RC, Vavilala MS, Beers SR, Fabio A, Kelsey SF. Multiple medical therapies for pediatric brain injury workgroup. Differences in medical therapy goals for children with severe traumatic brain injury – an International Study. Pediatr Crit Care Med. 2013;14:811–818. doi: 10.1097/PCC.0b013e3182975e2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digennaro JL, Mack CD, Malakouti A, Zimmerman JJ, Chesnut R, Armstead W, Vavilala MS. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 2011;32:420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J. The later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Pediatrics. London: Heineman Medical; 1981. pp. 744–759. [Google Scholar]
- Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ito H, Yokoyama K, Makita K. Phenylephrine ameliorates cerebral cytotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid occlusion with severe hypotension. Anesth Analg. 2009;108:1631–1637. doi: 10.1213/ane.0b013e31819d94e3. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Carney N, Adelson PD, Aswhal SM, Bell MJ, Bratton S, Carson S, Chesnut RM, Goldstein B, Grant GA, Kisson N, Peterson K, Selden NR, Tasker RC, Tong A, Vavilala MS, Wainwright MS, Warden CR. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. (Second) 2012;13(Suppl 1):S24–S29. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20(22):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Lotocki G, De Reviro Vaccari JP, Perez ER, Alonso OF, Curbelo K, Keane R, Dietrich WD. Therapeutic hypothermia modulates TNFR1 signaling in the traumatized brain via early transient activation of the JNK pathway and suppression of XIAP cleavage. European J of Neurosci. 2006;24:2283–2290. doi: 10.1111/j.1460-9568.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Newacheck PW, Inkelas M, Kim SE. Health services use and health care expenditures for children with disabilities. Pediatrics. 2004;114:79–85. doi: 10.1542/peds.114.1.79. [DOI] [PubMed] [Google Scholar]
- Otani N, Nawashiro H, Fukui S, Nomura N, Shima K. Temporal and spatial profile of phosphorylated mitogen activated protein kinase pathways after lateral fluid percussion injury in the cortex of the rat brain. J Neurotrauma. 2002;19:1587–1596. doi: 10.1089/089771502762300247. [DOI] [PubMed] [Google Scholar]
- Ortolano F, Colombo A, Zanier ER, Sclip A, Longhi L, Perego C, Stocchetti N, Borsello T, De Simoni MG. c-Jun N-terminal kinase pathway activation in human and experimental cerebral contusion. J Neuropathol Exp Neurol. 2009;68:964–971. doi: 10.1097/NEN.0b013e3181b20670. [DOI] [PubMed] [Google Scholar]
- Ross J, Armstead WM. NOC/oFQ activates ERK and JNK but not p38 MAPK to impair prostaglandin cerebrovasodilation after brain injury. Brain Res. 2005;1054:95–102. doi: 10.1016/j.brainres.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Salvucci A, Armstead WM. Vasopressin impairs KATP and Kca channel function after brain injury. Brain Res. 2000;887:406–412. doi: 10.1016/s0006-8993(00)03079-1. [DOI] [PubMed] [Google Scholar]
- Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang MJ, Souter MJ, Chesnut RM, Vavilala MS. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. NeuroCrit Care. 2011;15:46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner LA, Johnston AJ, Czosnyka M, Chatfield DA, Salvador R, Coles JP, Gupta AK, Pickard JD, Menon DK. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head injured patients. Crit Care Med. 2004;32:1049–1054. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]


