Abstract
BACKGROUND AND OBJECTIVES
Immediately after the 1994 Back-to-Sleep campaign, sudden unexpected infant death (SUID) rates decreased dramatically, but they have remained relatively stable (93.4 per 100 000 live births) since 2000. In this study, we examined trends in SUID rates and disparities by race/ethnicity since the Back-to-Sleep campaign.
METHODS
We used 1995–2013 US period-linked birth-infant death data to evaluate SUID rates per 100 000 live births by non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, American Indian/Alaska Native, and Asian/Pacific Islander racial/ethnic groupings. To examine racial/ethnic disparities, we calculated rate ratios with NHWs as the referent group. Unadjusted linear regression was used to evaluate trends (P < .05) in rates and rate ratios. The distribution and rates of SUID by demographic and birth characteristics were compared for 1995–1997 and 2011–2013, and χ2 tests were used to evaluate significance.
RESULTS
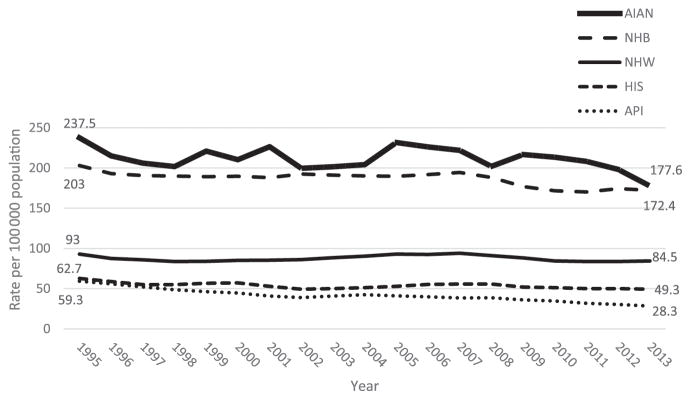
From 1995 to 2013, SUID rates were consistently highest for American Indian/Alaska Natives, followed by NHBs. The rate for NHBs decreased significantly, whereas the rate for NHWs also declined, but not significantly. As a result, the disparity between NHWs and NHBs narrowed slightly. The SUID rates for Hispanics and Asian/Pacific Islanders were lower than the rates for NHWs and showed a significant decrease, resulting in an increase in their advantage over NHWs.
CONCLUSIONS
Each racial/ethnic group showed a unique trend in SUID rates since the Back-to-Sleep campaign. When implementing risk-reduction strategies, it is important to consider these trends in targeting populations for prevention and developing culturally appropriate approaches for racial/ethnic communities.
Disparities in infant mortality between races/ethnicities in the United States are well documented.1 Because the factors that impact infant mortality affect the health of the entire population, infant mortality is often used as an overall indicator of the health and well-being of a nation. Consequently, racial/ethnic disparities in infant mortality are an important aspect of the overall US health status. Although infant mortality rates declined throughout the 20th century, the decrease in infant mortality has slowed in recent years.2 In addition, infant mortality rates have declined differentially by race/ethnicity, causing disparities to shift.3
The leading causes of infant mortality are congenital anomalies, conditions associated with preterm birth, maternal complications of pregnancy, sudden infant deathsyndrome (SIDS), and injuries. In the post–neonatal period (28–364 days), the most prevalent causes shift. SIDS is the leading cause, followed by congenital malformations, deformations, and chromosomal abnormalities; other ill-defined and unspecified causes of mortality; and accidents.4 Among postneonatal deaths due to accidents, most (70%) are attributed to accidental suffocation and strangulation in bed (ASSB).5 SIDS and ASSB, along with other ill-defined and unspecified causes of mortality, comprise what are often referred to as sudden unexpected infant deaths (SUIDs).4, 6 Together, SUID accounts for >35% of postneonatal deaths.5 Although SUID rates decreased dramatically immediately after the 1994 Back-to-Sleep campaign, they have remained relatively stable (93.4 per 100 000 live births) since 2000.
As with infant mortality, racial/ethnic differences in SUID rates exist.7–10 However, it is unknown whether racial/ethnic differences in SUID have changed. In this study, we examined trends in SUID rates and disparities by race/ethnicity from 1995 to 2013, the time after the Back-to-Sleep campaign. In addition, we examined demographic and infant characteristics associated with SUID by race/ethnicity, including infant sex, age at death, season of death, and gestational age at birth.
METHODS
We used 1995–2013 US period-linked birth-infant death data to evaluate SUID rates per 100 000 live births by race/ethnicity. This period facilitated our focus on trends after the 1992 introduction of the Back-to-Sleep campaign. From the available national data, we selected the period-linked data because it included the self-reported measure of maternal race/ethnicity from the birth certificate, which can be applied to both the numerator and denominator of the infant mortality rate and is more complete and reliable than that from other sources.11,12 Period-linked files were not produced by the National Center for Health Statistics from 1992 to 1994.
We defined SUIDs as deaths assigned International Classification of Diseases (ICD), Ninth and Tenth Revisions (ICD-9 and ICD-10, respectively) codes for SIDS (798.0 and R95), other ill-defined and unspecified causes of mortality (799.9 and R99), and ASSB (913.0 and W75). ICD-9 codes were used for 1995–1998 data; ICD-10 codes were used for 1999–2013.6,13 These individual causes of death were grouped to address variations in reporting by death certifiers and allowed for more accurate comparisons in SUID trends.4 The race/ethnicities examined were non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, American Indian/Alaska Native (AIAN), and Asian/Pacific Islander (API).
Infant age at death (in days) was derived by subtracting the birth date from the death date. If the reported age of death on the death certificate was <2 days, the reported age was used. If the exact date of birth and/or death was unknown, age was imputed.12 Season of death was calculated by using the month of death, with deaths in December through February recoded as “winter,” March through May as “spring,” June through August as “summer,” and September through November as “autumn.”
Gestational age was primarily based on mother’s last normal menstrual period (LMP). The obstetric estimate of gestation was used if the LMP date was not reported or was inconsistent with birth weight.12 Although there is increasing evidence that obstetric estimates are more valid than LMP-based measures, the obstetric estimate was not available nationwide until 2007.14 Thus, LMP-based gestational age was grouped as follows: extremely preterm (<28 weeks), very preterm (28 to <32 weeks), moderate preterm (32 to <34 weeks), late preterm (34 to <37 weeks), early term (37 to <39 weeks), full term/late term (39 to <42 weeks), and post-term (≥42 weeks) as well as preterm (<37 weeks) and nonpreterm (≥37 weeks).
Race/ethnicity-specific annual SUID rates per 100 000 live births were calculated. To examine racial/ethnic disparities, we calculated rate ratios with NHWs as the referent group. Unadjusted, race/ethnicity-stratified log-linear regression, with rates and rate ratios regressed on year, was used to evaluate trend significance (P < .05). Annual percentage changes (APCs) in rates and rate ratios were calculated as the multiplicative percentage change from each loglinear model: APC = 100 × (eβ − 1), where β is the coefficient from the log-linear model.15 Regression 2 analyses were conducted by using the SAS system for Windows version 9.3 (SAS Institute, Cary, NC).
In addition to examining SUID rates by race/ethnicity, we assessed racial/ethnic differences in infant sex, age at death, season of death, and gestational age at birth. We compared proportions of SUIDs by age at death and season of death and SUID rates per 100 000 live births for infant sex and gestational age during early (1995–1997) versus late (2011–2013) years. The early and late periods were chosen to reflect the status of SUID as close as possible after the initiation of the Back-to-Sleep campaign and the according to the most recent available data. Three-year periods were used to increase population sizes and stabilize rate estimates for race/ethnicities. χ2 Tests (or Fisher’s exact test when cell sizes were small) were performed to assess significance in comparisons between rates in 1995–1997 and 2011–2013.
RESULTS
Racial/ethnic differences in SUID rates were observed from 1995 to 2013. Rates were consistently highest for AIANs, followed by NHBs. No significant change in NHW and AIAN SUID rates were observed (Table 1, Fig 1). The NHB SUID rate decreased significantly (APC: −0.7; 95% confidence interval: −0.42 to −0.96), whereas the NHW rate decreased, but nonsignificantly. Consequently, a slight narrowing of the disparity between NHWs and NHBs occurred. In 2013, the SUID rates for NHBs and AIANs were double the rates for NHWs. The SUID rates for both Hispanics and APIs were lower than the rates for NHWs throughout the study period, with APIs experiencing the greatest decrease (APC: −3.3; 95% confidence interval: −2.8 to −3.8). This finding resulted in an increase in the Hispanic and API survival advantage over NHWs. In 2013, the NHW SUID rate was almost two- and threefold higher than for Hispanics and APIs, respectively.
TABLE 1.
SUID Rates per 100 000 Live Births by Race/Ethnicity: United States, 1995–2013
| SUID Rates | SUID Rate Ratios Relative to NHWs | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| NHWs | NHBs | Hispanics | AIANs | APIs | NHBs | Hispanics | AIANs | APIs | |
| 1995–2013 | 88.3 | 188.7 | 54.2 | 215.2 | 41.9 | — | — | — | — |
| 1995 | 93.0 | 203.0 | 62.7 | 237.5 | 59.3 | 2.2 | 0.7 | 2.6 | 0.6 |
| 1996 | 87.4 | 192.9 | 58.7 | 215.0 | 55.9 | 2.2 | 0.7 | 2.5 | 0.6 |
| 1997 | 85.8 | 190.7 | 54.9 | 206.0 | 52.1 | 2.2 | 0.6 | 2.4 | 0.6 |
| 1998 | 83.7 | 190.0 | 54.9 | 201.7 | 48.6 | 2.3 | 0.7 | 2.4 | 0.6 |
| 1999 | 83.9 | 189.1 | 56.7 | 221.0 | 46.2 | 2.3 | 0.7 | 2.6 | 0.6 |
| 2000 | 85.1 | 189.6 | 57.2 | 210.2 | 44.5 | 2.2 | 0.7 | 2.5 | 0.5 |
| 2001 | 85.4 | 188.0 | 52.9 | 226.4 | 40.7 | 2.2 | 0.6 | 2.7 | 0.5 |
| 2002 | 86.1 | 192.4 | 49.4 | 199.6 | 39.0 | 2.2 | 0.6 | 2.3 | 0.5 |
| 2003 | 88.4 | 191.1 | 50.0 | 201.6 | 40.8 | 2.2 | 0.6 | 2.3 | 0.5 |
| 2004 | 90.4 | 190.2 | 51.3 | 204.1 | 42.4 | 2.1 | 0.6 | 2.3 | 0.5 |
| 2005 | 93.0 | 189.6 | 53.0 | 231.6 | 41.1 | 2.0 | 0.6 | 2.5 | 0.4 |
| 2006 | 92.5 | 191.8 | 55.2 | 226.2 | 39.8 | 2.1 | 0.6 | 2.4 | 0.4 |
| 2007 | 94.0 | 194.5 | 55.8 | 221.8 | 38.5 | 2.1 | 0.6 | 2.4 | 0.4 |
| 2008 | 91.1 | 188.3 | 55.8 | 201.9 | 38.6 | 2.1 | 0.6 | 2.2 | 0.4 |
| 2009 | 88.1 | 176.9 | 52.0 | 216.6 | 36.0 | 2.0 | 0.6 | 2.5 | 0.4 |
| 2010 | 84.4 | 171.6 | 51.2 | 213.3 | 34.6 | 2.0 | 0.6 | 2.5 | 0.4 |
| 2011 | 83.7 | 170.2 | 50.0 | 208.1 | 31.8 | 2.0 | 0.6 | 2.5 | 0.4 |
| 2012 | 83.7 | 174.3 | 50.0 | 198.2 | 30.4 | 2.1 | 0.6 | 2.4 | 0.4 |
| 2013 | 84.5 | 172.4 | 49.3 | 177.6 | 28.3 | 2.0 | 0.6 | 2.1 | 0.3 |
| APC in rates | −0.1 | −0.7 | −0.8 | −0.5 | −3.3 | — | — | — | — |
| P for trend | NS | <.0001 | .001 | NS | <.0001 | — | — | — | — |
| APC in rate ratios | — | — | — | — | — | −0.7 | −0.9 | −0.5 | −3.3 |
| P | — | — | — | — | — | <.005 | <.005 | NS | <.005 |
SUID defined as infant deaths that were assigned ICD-9 and ICD-10 codes for SIDS (798.0 and R95), other ill-defined and unspecified causes of mortality (799.9 and R99), and ASSB (913.0 and W75). NS, not significant; —, not applicable.
FIGURE 1.
Trends in SUID: rates per 100 000 live births by race/ethnicity: United States, 1995–2013. HIS, Hispanic.
Age at Death
For all races/ethnicities and both periods, most deaths occurred at 1 to 2 months of age; the proportion of SUIDs occurring at 0 to 4 months ranged from 76% to 86% (Table 2). The proportion of deaths that occurred at 0 to 4 months decreased over time for NHBs (1995–1997: 83%; 2011–2013: 81%) and Hispanics (1995–1997: 81%; 2011–2013: 76%); the proportion of deaths at 0 to 4 months did not decrease significantly for NHWs, AIANs, and APIs. The proportion of SUIDs occurring at 5 to 11 months increased significantly for all races/ethnicities except for APIs.
TABLE 2.
Distribution of SUID by Race/Ethnicity and Demographic and Birth Characteristics: United States, 1995–1997 Compared With 2011–2013
| Characteristic | SUIDs, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| NHWs | NHBs | Hispanics | AIANs | APIs | ||||||
|
| ||||||||||
| 1995–1997 (n = 6580) | 2011–2013 (n = 5366) | 1995–1997 (n = 3548) | 2011–2013 (n = 2978) | 1995–1997 (n = 1311) | 2011–2013 (n = 1364) | 1995–1997 (n = 254) | 2011–2013 (n = 246) | 1995–1997 (n = 288) | 2011–2013 (n = 242) | |
| Infant age at death, in months | ||||||||||
| 0 | 10.0a | 12.7a | 9.6 | 10.1 | 9.0 | 10.9 | 10.4 | 16.1 | 7.1a | 12.1a |
| 1 | 21.3 | 20.8 | 24.1 | 23.3 | 22.7a | 19.5a | 20.5 | 16.8 | 19.5 | 17.1 |
| 2 | 23.1a | 20.2a | 24.3a | 21.8a | 23.3a | 19.8a | 25.3 | 24.6 | 21.7 | 19.6 |
| 3 | 18.5a | 15.3a | 15.4 | 14.9 | 16.8 | 16.0 | 16.0 | 11.9 | 19.2 | 16.3 |
| 4 | 10.9 | 10.6 | 10.0 | 10.9 | 9.6 | 9.7 | 13.7 | 8.6 | 12.9 | 14.6 |
| 5 | 6.1a | 7.4a | 6.0 | 6.8 | 6.6 | 7.9 | 5.6 | 8.2 | 8.9 | 5.8 |
| 6 | 3.5a | 4.4a | 3.6a | 4.6a | 4.1a | 5.9a | 2.4a | 4.5a | 3.2a | 4.6a |
| 7 | 2.6a | 3.3a | 2.5 | 2.8 | 2.5a | 4.2a | 1.3 | 2.1 | 2.5 | 2.5 |
| 8 | 1.7a | 2.3a | 1.6 | 1.7 | 1.6 | 2.7 | 2.0 | 4.5 | 1.4 | 2.5 |
| 9 | 1.1 | 1.5 | 1.2 | 1.4 | 1.3 | 1.4 | 1.2 | 0.8 | 2.5 | 1.7 |
| 10 | 0.6 | 0.8 | 0.8 | 0.9 | 1.9 | 1.3 | 0.4 | 0.8 | 0.4 | 1.7 |
| 11 | 0.7a | 0.9a | 0.8a | 1.1a | 0.8a | 0.7a | 1.2a | 1.2a | 0.7a | 1.7a |
| Season of death | ||||||||||
| Winter (December–February) | 27.6a | 25.5a | 28.9a | 26.6a | 31.0a | 25.5a | 27.4 | 21.7 | 36.3a | 24.2a |
| Spring (March–May) | 26.0 | 25.6 | 25.6 | 25.4 | 25.0 | 24.3 | 22.8 | 27.5 | 23.5 | 25.4 |
| Summer (June–August) | 20.7a | 23.7a | 19.9a | 23.7a | 19.9a | 24.2a | 21.4 | 26.1 | 20.6 | 23.7 |
| Autumn (September–November) | 25.8 | 25.2 | 25.6 | 24.3 | 24.1 | 25.9 | 28.5 | 24.7 | 19.6 | 26.6 |
SUID is defined as infant deaths that were assigned ICD-9 and ICD-10 codes for SIDS (798.0 and R95), other ill-defined and unspecified causes of mortality (799.9 and R99), and ASSB (913.0 and W75). Due to rounding of weighted percentages, column totals may not add to 100%.
Significant (P < .05) change in SUID rate between 1995–1997 and 2011–2013.
Sex and Seasonality
The SUID rate was consistently higher for boys among all races/ethnicities (Table 3). The male and female SUID rates decreased significantly between the early and late periods for all races/ethnicities, except for AIANs. Significant differences in seasonality of early- and late-period SUID rates by race/ethnicity also occurred. The proportion of deaths during the winter months decreased for all races/ethnicities except for AIANs, whereas the proportion during the summer months increased for NHWs, NHBs, and Hispanics.
TABLE 3.
SUID Rates per 100 000 Live Births by Sex and Gestational Age: United States, 1995–1997 Compared With 2011–2013
| NHWs | NHBs | Hispanics | AIANs | APIs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1995–1997 (n = 6580) | 2011–2013 (n = 5366) | 1995–1997 (n = 3548) | 2011–2013 (n = 2978) | 1995–1997 (n = 1311) | 2011–2013 (n = 1364) | 1995–1997 (n = 254) | 2011–2013 (n = 246) | 1995–1997 (n = 288) | 2011–2013 (n = 242) | |
| Infant sex | ||||||||||
| Female | 74.0a | 69.8a | 177.0a | 152.1a | 53.8a | 42.6a | 214.7 | 188.3 | 47.9a | 27.8a |
| Male | 111.1a | 96.9a | 228.4a | 187.8a | 71.2a | 57.2a | 258.9 | 227.4 | 69.9a | 35.4a |
| Gestational age, completed weeks at birth | ||||||||||
| <28 | 308.7 | 235.7 | 507.3a | 382.3a | 284.0 | 220.8 | — | — | — | 126.6 |
| 28–31 | 307.6a | 206.6a | 488.5a | 367.8a | 223.9 | 162.2 | 788.2 | 292.6 | 118.3 | 113.4 |
| 32–33 | 255.9a | 169.2a | 406.0 | 350.7 | 138.5 | 102.4 | 409.0 | — | 159.9 | 74.9 |
| 34–36 | 171.7a | 148.6a | 291.2a | 250.0a | 105.1a | 84.2a | 405.9 | 403.5 | 78.3 | 53.2 |
| 37–38 | 96.4 | 94.2 | 181.5 | 175.8 | 61.4a | 47.4a | 223.4 | 193.9 | 57.9a | 34.8a |
| 39–41 | 73.2a | 65.7a | 159.7a | 128.4a | 48.6a | 40.4a | 181.0 | 176.6 | 54.0a | 23.5a |
| ≥42 | — | — | —a | —a | —a | —a | — | — | — | — |
SUID is defined as infant deaths that were assigned ICD-9 and ICD-10 codes for SIDS (798.0 and R95), other ill-defined and unspecified causes of mortality (799.9 and R99), and ASSB (913.0 and W75). —, due to the small cell size (n < 20), rates are unstable and have been suppressed.
Significant (P < .05) change in SUID rate between 1995–1997 and 2011–2013.
Preterm Birth
In both the early and late years, the lowest SUID rates for preterm infants (<37 weeks) were among APIs (1995–1997: 92.8 per 100 000; 2011–2013: 65.2 per 100 000) and Hispanics (1995–1997: 130.6 per 100 000; 2011–2013: 101.7 per 100 000). AIANs had the highest SUID rates among preterm infants over the entire study period (1995–1997: 434.4 per 100 000; 2011–2013: 367.4 per 100 000) followed by NHBs (1995–1997: 356.7 per 100 000; 2011–2013: 291.7 per 100 000). Preterm SUID rates dropped significantly between 1995–1997 and 2011–2013 for all races/ethnicities, except for APIs. Variation in the total percentage change for preterm SUID rates by race/ethnicity between periods was minimal: −20% for NHWs, −18% for NHBs, −22% for Hispanics, and −15% for AIANs.
Nonpreterm SUID rates also decreased significantly between 1995–1997 and 2011–2013 for all races/ethnicities. There was more variation among the percentage changes in nonpreterm SUID rates between time periods by race/ethnicity: −8% for NHWs, −14% for NHBs, −21% for Hispanics, −13% for AIANs, and −50% for APIs. For all races/ethnicities with significant changes in both preterm and nonpreterm SUID rates between periods, the declines in preterm SUID rates were greater than declines in nonpreterm SUID rates.
DISCUSSION
This study is the first to examine US SUID trend data stratified by race/ethnicity after the Back-to-Sleep campaign, although an analysis of racial/ethnic trends in SIDS between 1989 and 2001 revealed similar decreasing but disparate rates.16 From 1995 to 2013, the relative ranking of SUID rates by race/ethnicity did not change significantly. AIANs consistently had the highest rates of SUID, followed by NHBs, and Hispanics. APIs consistently had the lowest SUID rates. Although we observed decreases in SUID rates for all races/ethnicities, decreases were only significant in NHBs, Hispanics, and APIs. In addition, the magnitude of the decline varied by racial/ethnic group. When compared with NHWs, the net effect was a broadening of the survival advantage for both Hispanics and APIs and a slight narrowing of the disparity with NHBs.
The reasons for the variations in race/ethnicity-specific SUID rates over the past 2 decades are likely multifactorial and driven by changes in known SIDS risk factors (eg, nonsupine infant sleep position,17 infant bedding use,18 and bedsharing19). Shapiro-Mendoza et al18 showed a significant decrease in the prevalence in soft bedding use across all races/ethnicities from 1993 to 2000. Declines continued from 2001 to 2010 for white and Hispanic infants but not for black infants.18 Longitudinal data have also shown increases in bed-sharing among NHB and Hispanic infants.19 From 1993 to 2000, overall supine sleep prevalence increased, but NHBs consistently had the lowest prevalence. Since 2001, supine infant sleep positioning has plateaued for all races/ethnicities, with persistence of the racial/ethnic disparity.17 Teen maternal age, plurality, low socioeconomic or educational status, maternal smoking, not breastfeeding or decreased breastfeeding duration, lack of prenatal care, and unmarried status are other factors that may influence the observed SUID patterns.2,9,20
Biological factors, such as genetic polymorphisms,21, 22 metabolic disorders, or brainstem abnormalities,23 may also act as unique biological vulnerabilities among the highest-risk groups. Per the Triple Risk Hypothesis, when these factors combine with environmental stressors at developmentally vulnerable periods, SIDS risk increases. Thus, racial/ethnic differences in these biological factors may explain the observed patterns of risk.24,25 For example, metabolic variation might influence endogenous SIDS risk because black mothers metabolize nicotine more slowly than NHW mothers.26 Slower metabolism may lead to higher prenatal exposure to nicotine and its metabolites, which may adversely affect cardiovascular function and arousal.27–31 A review of SIDS genetic factors also revealed a higher prevalence of polymorphisms in a serotonin-transporter gene among NHB SIDS cases.32 The serotonergic system helps regulate arousal and autonomic functions, including breathing, and these abnormalities may be associated with SIDS.33–36 In contrast, cultural or behavioral factors may also contribute to SIDS risk. For example, a 2001 study found that infants of US-born Hispanic mothers had a 50% greater rate of SIDS than infants of foreign-born Hispanic mothers after controlling for birth weight, maternal age, education, marital status, prenatal care, and socioeconomic status.37 The possibility of an interplay between behavioral factors and biological vulnerabilities related to factors such as metabolic or genetic abnormalities should be considered in further exploration of the persistent racial/ethnic disparities in SIDS/SUID.
Age at Death
Our observed peak SUID incidence at ages 1 to 2 months and that ~90% of deaths occurred in the first 4 months after birth for all races/ethnicities correspond to previous findings.6,15,38,39 However, the consistent decline between the early and late periods in the proportion of deaths occurring between 5 and 11 months of age for all races/ethnicities, except for APIs, and between 0 to 4 months of age for NHBs and Hispanics is novel. This finding might suggest that changes in infant sleep practices after the Back-to-Sleep campaign40 affected older infants more broadly, but had a more directed effect in younger, NHB, and Hispanic infants.
Sex and Seasonality
The predominance of SUID in males is consistent with other studies.10,38,39,41–44 Also, as in previous studies, we found a higher incidence of SUID during winter months in the earlier years, but not in the later years.10,20,45 Our finding of a decreasing proportion of winter deaths from 1995–1997 to 2011–2013 among all races/ethnicities, except for AIANs, and an increasing proportion of summer deaths from 1995–1997 to 2011–2013 among NHWs, NHBs, and Hispanics has not been previously documented. One explanation may be that safe-sleep recommendations against overbundling and overheating infants, particularly during winter months, have been successful, resulting in a more even distribution of SUID throughout the year.
Preterm Birth
Preterm birth increases the risk of infant mortality, including SIDS and SUID.2,4,20,24,38 This risk is particularly pronounced in the most premature infants. One study showed that being born at 22 to 28 or 28 to 32 weeks’ gestation doubled the SIDS risk compared with infants born at term, even after adjustment for maternal and pregnancy factors.9 Similarly, we found large variations in SUID occurrence by gestational age. Across all races/ethnicities, the SUID rate among infants born before 32 weeks gestation was >2.5 times the overall SUID rate.
The racial/ethnic differences we observed in SUID by gestational age mirror overall infant mortality and SIDS rates. In previous studies, gestational age–specific infant mortality rates for NHBs and AIANs are typically higher than those of NHWs, Hispanics, and APIs at all gestational ages. Similarly, the highest rates of SIDS are observed among AIAN and NHB infants, among whom the rates are typically about double those of NHWs,2 and the SIDS rates for Hispanic and API infants are significantly lower, as we observed for SUID.2
Differential patterns in sleep position, infant bedding use, and bed-sharing practices are commonly proposed explanations for the persistence of, and in some cases increases in, racial/ethnic disparities in SIDS and other sleep-related infant deaths. After the Back-to-Sleep campaign in the early 1990s, there was a substantial decrease in the US SIDS rate.44,46–48 Although large decreases in SIDS for AIANs were observed after the Back-to-Sleep campaign, substantial disparities compared with NHWs remain, even after adjusting for socioeconomic status, maternal age, birth weight, and prenatalcare.49–51 This finding suggests that the relationship between SIDS/SUID and sleep position among AIANs is more complex than previously acknowledged.51 Among NHBs, although a substantial decrease in prone sleep positioning was observed in the early 1990s, the decline appears to be smaller than in other races/ethnicities and is largely accounted for by an increase in side rather than supine sleep positioning.8,46 This high prevalence of nonsupine sleep position persists, with NHB caregivers twice as likely to place infants to sleep in the prone position compared with whites.52 Asian and Hispanic infants are placed in the supine position in higher proportions compared with NHWs and blacks.46,53
Temporal trends in infant bedding use and bed-sharing also provide insight into the persistence of racial/ethnic disparities. From 1993 to 2003, the use of bedding over or under sleeping infants declined overall, including a statistically significant decrease in bedding use for all races/ethnicities. The declines in bedding use slowed from 2001 to 2010, with modest declines among white and Hispanic infants and no significant decline in bedding use for NHB infants.18 Why Hispanic infants are more likely than NHWs to be placed to sleep with infant bedding, but have lower SUID rates, is unknown. A further paradox is the significant increase in bed-sharing among Hispanics and NHWs and NHBs from 1993 to 2010, the same time during which SUID/SIDS rates decreased significantly.19
Our study had a few limitations. First, the linked birth-death data, like all administrative data sources, are limited in scope and lack data on environmental risk and protective factors for SUID. Second, our estimation of gestational age may be subject to error due to reliance on the mother’s reported LMP rather than an obstetric estimate of gestation.14 Finally, our examination of race/ethnicity was limited by the inherent heterogeneity of cultural practices and biological factors within the available racial/ethnic groupings.
Our study also had several strengths. First, it examined nearly 2 decades of national data. The use of vital statistics data allowed examination of all reported US SUID cases. Our focus on racial/ethnic trends was especially suited to the linked birth-death file in which the self-reported race/ethnicity of the mother from the birth certficate is used in both the numerator and denominator of the mortality rate. Second, the availability of a variety of maternal and infant characteristics in the data set allowed for more detailed analyses of SUID patterns. Third, combining 3 ICD-10 codes (R95, R99, and W75) to represent SUID, rather than using a single SIDS code alone, lessened the effect of the wide variation in reporting across jurisdictions.15,54
Conclusions
In sum, we found that significant racial/ethnic disparities in SUID have persisted into the 21st century. In addition, trends in known risk factors from this and previous studies provide evidence of the multifactorial explanations for the observed trends. Together, these data suggest that, although prevention efforts dating to the early to mid-1990s have positively affected SUID rates, further effort is needed to continue decreasing SUIDs, a major cause of infant mortality in the United States. To further decrease SUID rates, attention is needed to address how current prevention messaging can be improved to reduce the racial/ethnic disparities. It is clear that each racial/ethnic group has experienced differential changes in known risk behaviors. What remains to be explored are the reasons for the disparities and how best to eliminate them. Perhaps public health campaigns to reduce SUID are not reaching certain races/ethnicities, not addressing the most important risk factors for these groups, or not being framed in the most effective way to ensure uptake among diverse populations. Sufficient data exist on the epidemiologic profile of SUID in the wake of major public health prevention events, such as the Back-to-Sleep (Safe-to-Sleep) campaigns and the American Academy of Pediatrics Safe Sleep Recommendations.40,55 We must use these detailed data to delve deeper into reasons for the persistence of SUID and SUID disparities.
What’s Known on This Subject
Sudden unexpected infant death (SUID) rates decreased dramatically after the 1994 Back-to-Sleep campaign and have remained relatively stable since 2000. The changing epidemiology of SUID risk factors, including preterm birth and infant sleep practices, may have influenced race/ethnicity-specific SUID trends.
What This Study Adds
This is the first study to examine race/ethnicity-stratified trends in SUID rates from the United States. From 1995 through 2013, there were unique SUID trends for each racial/ethnic group, but the relative ranking of SUID rates did not significantly change.
Acknowledgments
FUNDING: Ms Lambert was supported by a contract between DB Consulting Group and the Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (200-2010-37208).
Abbreviations
- AIAN
American Indian/Alaska Native
- APC
annual percentage change
- API
Asian/Pacific Islander
- ASSB
accidental suffocation and strangulation in bed
- ICD
International Classification of Diseases
- LMP
last normal menstrual period
- NHB
non-Hispanic black
- NHW
non-Hispanic white
- SUID
sudden unexpected infant death
- SIDS
sudden infant death syndrome
Footnotes
Dr Parks conceptualized and designed the study, carried out all analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Erck Lambert assisted with data analyses and critically reviewed and revised the manuscript; Dr Shapiro-Mendoza conceptualized and designed the study and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2017-0898.
References
- 1.Singh GK, Yu SM. Infant mortality in the United States: trends, differentials, and projections, 1950 through 2010. Am J Public Health. 1995;85(7):957–964. doi: 10.2105/ajph.85.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDorman MF. Race and ethnic disparities in fetal mortality, preterm birth, and infant mortality in the United States: an overview. Semin Perinatol. 2011;35(4):200–208. doi: 10.1053/j.semperi.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 3.MacDorman M, Hoyert D, Mathews T. Recent declines in infant mortality in the United States, 2005–2011. NCHS Data Brief. 2013;(120):1–8. [PubMed] [Google Scholar]
- 4.Mathews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. 2015;64(9):1–30. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Accessed June 13, 2016];Compressed mortality file 1999–2014 on CDC WONDER online database. released December 2015. Available at: http://wonder.cdc.gov/cmf-icd10.html.
- 6.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163(8):762–769. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 7.Unger B, Kemp JS, Wilkins D, et al. Racial disparity and modifiable risk factors among infants dying suddenly and unexpectedly. Pediatrics. 2003;111(2) doi: 10.1542/peds.111.2.e127. Available at: www.pediatrics.org/cgi/content/full/111/2/e127. [DOI] [PubMed] [Google Scholar]
- 8.Malloy MH, Eschbach K. Association of poverty with sudden infant death syndrome in metropolitan counties of the United States in the years 1990 and 2000. South Med J. 2007;100(11):1107–1113. doi: 10.1097/SMJ.0b013e318158b9de. [DOI] [PubMed] [Google Scholar]
- 9.Hakeem GF, Oddy L, Holcroft CA, Abenhaim HA. Incidence and determinants of sudden infant death syndrome: a population-based study on 37 million births. World J Pediatr. 2015;11(1):41–47. doi: 10.1007/s12519-014-0530-9. [DOI] [PubMed] [Google Scholar]
- 10.Adams EJ, Chavez GF, Steen D, Shah R, Iyasu S, Krous HF. Changes in the epidemiologic profile of sudden infant death syndrome as rates decline among California infants: 1990–1995. Pediatrics. 1998;102(6):1445–1451. doi: 10.1542/peds.102.6.1445. [DOI] [PubMed] [Google Scholar]
- 11.Prager K. Infant mortality by birthweight and other characteristics: United States, 1985 birth cohort. Vital Health Stat. 1994;20(24):1–14. [PubMed] [Google Scholar]
- 12.National Center for Health Statistics, Centers for Disease Control and Prevention. Public Use Data File Documentation: 1995–2013 Period Linked Birth/Infant Death Data Sets. Hyattsville, MD: Department of Health and Human Services; 1997–2015. [Google Scholar]
- 13.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. 2. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 14.Martin J, Osterman M, Kirmeyer S, Gregory E. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep. 2015;64(5):1–20. [PubMed] [Google Scholar]
- 15.Malloy MH, MacDorman M. Changes in the classification of sudden unexpected infant deaths: United States, 1992–2001. Pediatrics. 2005;115(5):1247–1253. doi: 10.1542/peds.2004-2188. [DOI] [PubMed] [Google Scholar]
- 16.Frisbie WP, Hummer RA, Powers DA, Song S-E, Pullum SG. Race/ethnicity/nativity differentials and changes in cause-specific infant deaths in the context of declining infant mortality in the U.S.: 1989–2001. Popul Res Policy Rev. 2010;29(3):395–422. [Google Scholar]
- 17.Colson ER, Rybin D, Smith LA, Colton T, Lister G, Corwin MJ. Trends and factors associated with infant sleeping position: the National Infant Sleep Position Study, 1993–2007. Arch Pediatr Adolesc Med. 2009;163(12):1122–1128. doi: 10.1001/archpediatrics.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro-Mendoza CK, Colson ER, Willinger M, Rybin DV, Camperlengo L, Corwin MJ. Trends in infant bedding use: National Infant Sleep Position Study, 1993–2010. Pediatrics. 2015;135(1):10–17. doi: 10.1542/peds.2014-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colson ER, Willinger M, Rybin D, et al. Trends and factors associated with infant bed sharing, 1993–2010: the National Infant Sleep Position Study. JAMA Pediatr. 2013;167(11):1032–1037. doi: 10.1001/jamapediatrics.2013.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming PJ, Blair PS, Bacon C, et al. Sudden Unexpected Death in Infancy: The CESDI SUDI Studies 1993–1996. London: The Stationary Office; 2000. [Google Scholar]
- 21.Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden infant death syndrome: review of implicated genetic factors. Am J Med Genet A. 2007;143A(8):771–788. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- 22.Hunt CE, Hauck FR. Gene and gene–environment risk factors in sudden unexpected death in infants. Curr Pediatr Rev. 2010;6(1):56–62. [Google Scholar]
- 23.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauck FR, Tanabe KO, Moon RY. Racial and ethnic disparities in infant mortality. Semin Perinatol. 2011;35(4):209–220. doi: 10.1053/j.semperi.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65(3–4):194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 27.Chang AB, Wilson SJ, Masters IB, et al. Altered arousal response in infants exposed to cigarette smoke. Arch Dis Child. 2003;88(1):30–33. doi: 10.1136/adc.88.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen G, Vella S, Jeffery H, Lagercrantz H, Katz-Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118(18):1848–1853. doi: 10.1161/CIRCULATIONAHA.108.783902. [DOI] [PubMed] [Google Scholar]
- 29.Parslow PM, Cranage SM, Adamson TM, Harding R, Horne RS. Arousal and ventilatory responses to hypoxia in sleeping infants: effects of maternal smoking. Respir Physiol Neurobiol. 2004;140(1):77–87. doi: 10.1016/j.resp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Richardson HL, Walker AM, Horne RS. Maternal smoking impairs arousal patterns in sleeping infants. Sleep. 2009;32(4):515–521. doi: 10.1093/sleep/32.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawnani H, Jackson T, Murphy T, Beckerman R, Simakajornboon N. The effect of maternal smoking on respiratory and arousal patterns in preterm infants during sleep. Am J Respir Crit Care Med. 2004;169(6):733–738. doi: 10.1164/rccm.200305-692OC. [DOI] [PubMed] [Google Scholar]
- 32.Weese-Mayer DE, Corwin MJ, Peucker MR, et al. CHIME Study Group. Comparison of apnea identified by respiratory inductance plethysmography with that detected by end-tidal CO(2) or thermistor. Am J Respir Crit Care Med. 2000;162(2 pt 1):471–480. doi: 10.1164/ajrccm.162.2.9904029. [DOI] [PubMed] [Google Scholar]
- 33.Panigrahy A, Filiano J, Sleeper LA, et al. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59(5):377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 34.Ozawa Y, Takashima S. Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic Sci Int. 2002;130(suppl):S53–S59. doi: 10.1016/s0379-0738(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 35.Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2009;117(3):257–265. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- 36.Kinney HC, Randall LL, Sleeper LA, et al. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62(11):1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- 37.Collins JW, Jr, Papacek E, Schulte NF, Drolet A. Differing postneonatal mortality rates of Mexican-American infants with United-States-born and Mexico-born mothers in Chicago. Ethn Dis. 2001;11(4):606–613. [PubMed] [Google Scholar]
- 38.Fleming PJ, Blair PS, Pease A. Sudden unexpected death in infancy: aetiology, pathophysiology, epidemiology and prevention in 2015. Arch Dis Child. 2015;100(10):984–988. doi: 10.1136/archdischild-2014-306424. [DOI] [PubMed] [Google Scholar]
- 39.Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year populationbased study in the UK. Lancet. 2006;367(9507):314–319. doi: 10.1016/S0140-6736(06)67968-3. [DOI] [PubMed] [Google Scholar]
- 40.National Institute of Child Health and Human Development. [Accessed January 29, 2014];Safe to Sleep public education campaign. Available at: www.nichd.nih.gov/sts/Pages/default.aspx.
- 41.Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: report of meeting held January 13 and 14, 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93(5):814–819. [PubMed] [Google Scholar]
- 42.Peterson DR, Sabotta EE, Strickland D. Sudden infant death syndrome in epidemiologic perspective: etiologic implications of variation with season of the year. Ann N Y Acad Sci. 1988;533(1):6–12. doi: 10.1111/j.1749-6632.1988.tb37229.x. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman HJ, Hillman LS. Epidemiology of the sudden infant death syndrome: maternal, neonatal, and postneonatal risk factors. Clin Perinatol. 1992;19(4):717–737. [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Sudden infant death syndrome: United States, 1983–1994. MMWR Morb Mortal Wkly Rep. 1996;45(40):859–863. [PubMed] [Google Scholar]
- 45.Blair PS, Platt MW, Smith IJ, Fleming PJ CESDI SUDI Research Group. Sudden infant death syndrome and sleeping position in pre-term and low birth weight infants: an opportunity for targeted intervention. Arch Dis Child. 2006;91(2):101–106. doi: 10.1136/adc.2004.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willinger M, Hoffman HJ, Wu KT, et al. Factors associated with the transition to nonprone sleep positions of infants in the United States: the National Infant Sleep Position Study. JAMA. 1998;280(4):329–335. doi: 10.1001/jama.280.4.329. [DOI] [PubMed] [Google Scholar]
- 47.Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics. 2012;129(4):630–638. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minino AM, Murphy SL, Xu JQ, Kochanek KD. Deaths: Final Data for 2008. Hyattsville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Decrease in infant mortality and sudden infant death syndrome among Northwest American Indians and Alaskan Natives–Pacific Northwest, 1985–1996. MMWR Morb Mortal Wkly Rep. 1999;48(9):181–184. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Postneonatal mortality among Alaska Native infants—Alaska, 1989–2009. MMWR Morb Mortal Wkly Rep. 2012;61(1):1–5. [PubMed] [Google Scholar]
- 51.Wong CA, Gachupin FC, Holman RC, et al. American Indian and Alaska Native infant and pediatric mortality, United States, 1999–2009. Am J Public Health. 2014;104(suppl 3):S320–S328. doi: 10.2105/AJPH.2013.301598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauck FR, Moore CM, Herman SM, et al. The contribution of prone sleeping position to the racial disparity in sudden infant death syndrome: the Chicago Infant Mortality Study. Pediatrics. 2002;110(4):772–780. doi: 10.1542/peds.110.4.772. [DOI] [PubMed] [Google Scholar]
- 53.Hauck FR, Signore C, Fein SB, Raju TN. Infant sleeping arrangements and practices during the first year of life. Pediatrics. 2008;122(suppl 2):S113–S120. doi: 10.1542/peds.2008-1315o. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro-Mendoza CK, Kim SY, Chu SY, Kahn E, Anderson RN. Using death certificates to characterize sudden infant death syndrome (SIDS): opportunities and limitations. J Pediatr. 2010;156(1):38–43. doi: 10.1016/j.jpeds.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Moon RY Task Force on Sudden Infant Death Syndrome. SIDS and other sleeprelated infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128(5):1030–1039. doi: 10.1542/peds.2011-2284. [DOI] [PubMed] [Google Scholar]