Abstract
Purpose
Chemical exchange saturation transfer (CEST) effects at 2 ppm (CEST@2ppm) in brain have previously been interpreted as originating from creatine. However, protein guanidino amine protons may also contribute to CEST@2ppm. This study aims to investigate the molecular origins and specificity of CEST@2ppm in brain.
Methods
Two experiments were performed: (1) samples containing egg white albumin and creatine were dialyzed using a semi-permeable membrane to demonstrate that proteins and creatine can be separated by this method; (2) tissue homogenates of rat brain with and without dialysis to remove creatine were studied to measure the relative contributions of proteins and creatine to CEST@2ppm.
Results
The experiments indicate that dialysis can successfully remove creatine from proteins. Measurements on tissue homogenates show that, with the removal of creatine via dialysis, CEST@2ppm decreases to roughly 34% of its value before dialysis, which indicates that proteins and creatine have comparable contribution to the CEST@2ppm in brain. However, considering the contribution from peptides and amino acids to CEST@2ppm, creatine may have much less contribution to CEST@2ppm.
Conclusion
The contribution of proteins, peptides, and amino acids to CEST@2ppm cannot be neglected. CEST@2ppm measurements of creatine in rat brain should be interpreted with caution.
Keywords: MRI, chemical exchange saturation transfer (CEST), creatine, proteins
INTRODUCTION
Chemical Exchange Saturation Transfer (CEST) has potential for molecular MR imaging of specific resonances with higher sensitivity than conventional proton magnetic resonance spectroscopy (1H MRS) (1–3). In CEST imaging, an irradiation RF pulse is applied at the frequency offsets of exchangeable protons of solute molecules and the subsequent chemical exchange between those saturated protons and bulk water reduces the magnetization of the measured water signal, which provides a way to indirectly detect the solute molecules. Because water is significantly more abundant than the solutes, the detection sensitivity to exchanging protons is magnified especially when long irradiation pulses are used.
Creatine is an essential metabolite for energy production, and a sensitive method for imaging creatine distribution in biological tissues would be of great interest e.g. for studying brain or muscle metabolism. Traditionally, creatine has been measured via 1H MRS, which, however, suffers several limitations such as low spatial resolution and sensitivity for in vivo studies. Recently, CEST@2ppm from water in brain has been suggested as an indicator of creatine distribution (4,5). Studies on samples containing the main brain tissue metabolites at their physiological concentrations and pH showed that creatine dominates CEST@2ppm compared to other compounds (6). This suggests that CEST@2ppm could provide a method to image creatine distributions specifically with high spatial resolution high sensitivity. However, biological tissues also contain a large variety of macromolecules which also have exchangeable protons. Some protons, such as those in proteins with arginine side chains, have similar chemical shifts (2 ppm) and chemical exchange rates (500 to 1000 s−1 (7,8)) as those of creatine, and thus may not be easily distinguished from creatine using CEST. Potentially, such proteins may also contribute to CEST@2ppm and thereby decrease the specificity of CEST@2ppm for detecting creatine. Unfortunately, a comprehensive investigation of the relative contributions from creatine and proteins has not been reported. One challenge is that it is difficult to mimic the arginine residues of proteins at physiological concentration using simple model phantoms because there are many types of proteins which contain different proportions of arginine residues in biological tissues.
Here, we used dialysis to selectively remove creatine and other small molecules from samples of brain homogenates in an attempt to measure the CEST@2ppm of the residual solutes. Dialysis is a classic laboratory technique that relies on selective diffusion of molecules across a semi-permeable membrane to separate molecules based on membrane pore size. A sample and a buffer solution are placed on opposite sides of a dialysis membrane, and sample molecules that are smaller than the pores pass through the membrane, whereas large molecules are retained. Because of the large size difference between creatine and most proteins, this enables the simple separation of creatine and other small molecules from brain tissue samples, and in turn provides us an opportunity to quantitatively evaluate the relative contributions of creatine and proteins to CEST@2ppm.
METHODS
Sample preparation
The major metabolites in the brain that have CEST signals at physiological concentrations are creatine (Cre), glutamate (Glu), glucose (Glc), myo-inositol (MI), and phosphocreatine (PCr) (9). To evaluate their contributions to CEST@2ppm, 5 control samples containing these metabolites at their physiological concentrations were prepared in 1 × phosphate buffered saline (PBS) and titrated to pH of 7.0; specifically, 6 mM Cre, 10 mM Glu, 3 mM Glc, 10 mM MI, and 5 mM PCr were prepared. pH values were adjusted using NaOH and HCl. Experiments on egg white albumin (EWA) were also performed to evaluate the contribution from the guanidino amine in arginine side chains of this protein (9). Samples were prepared by adding 10% (w/w) EWA to 1 × PBS and titrated to pH of 7.0.
To evaluate the ability of a dialysis membrane to separate creatine from proteins, four samples with EWA were prepared (sample #1: 10% (w/w) EWA + 20 mM Cre; sample #2: 10% (w/w) EWA; sample #3: dialyzed EWA + Cre; sample #4: diluted EWA). Sample #1 and #2 were used to study the CEST and 1H MRS signals from only EWA or Cre, respectively. Sample #3 was prepared by putting 10% (w/w) EWA + 20 mM Cre solution in a dialysis bag (with a membrane with 1 kDa molecular weight cutoff (WMCO)) and then dialyzed against PBS solution (pH = 7.0) at 4 °C for 3 days with constant gentle stirring. The dialyzing solution was changed 3 times every 24 hours. Sample #4 was prepared using 10% (w/w) EWA solution with the same volume as that in sample #3 before dialysis, and was used as a control for sample #3. Water diffuses across the membrane of the dialysis bag causing the sample to expand, so an appropriate amount of PBS was added to sample #4 at the end so that the volume of the control was subject to the same dilution as that inside the dialysis bag. The four samples were titrated to pH 7.0. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dialysis tubing was purchased from Fisher Scientific (Hampton, NH, USA).
Tissue sample preparation
Tissue homogenates were prepared by removing whole rat brains from freshly sacrificed rats. The intact brain tissue was weighted and washed quickly in PBS to remove residual blood. After addition of 4 times PBS (w/w), the tissues were homogenized with a motor-driven blade-type homogenizer (Brinkmann Polytron PT3000, Kinematica AG, CH) at max speed ~23,000 rpm for 2 × 30 s bursts. The homogenates were then divided into two aliquots. One sample was put inside a dialysis bag to dialyze against PBS solution (pH = 7.0) at 4 °C for 3 days as described above. The other sample was also stored at 4 °C for 3 days, but without dialysis, and was used as a control (control #1). An appropriate amount of PBS was added to the control at the end so that the volume of the control was subject to the same dilution as caused by dialysis. Another sample containing brain tissue homogenates was also prepared and stored at 4 °C for 3 days. This sample was measured on the 1st day (immediately after homogenization) and the 3rd day and was used as a second control (control #2) to assess any variations of CEST and 1H MRS signals potentially caused by degradation during the 3-day period. All samples were titrated to pH of 7.0 before MRI measurements.
Animal preparation
A healthy rat was immobilized and anesthetized before MR imaging. Respiration and rectal temperature were continuously monitored to be stable, and a rectal temperature of 37 °C was maintained throughout the experiments using a warm-air feedback system (SA Instruments, Stony Brook, NY, USA) (10). Animals were anesthetized with 2–3% isoflurane for induction and 2% for maintenance during the experiments. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
MRI measurements
All sample and animal measurements were performed on a Varian DirectDrive™ horizontal 9.4 T magnet with a 38-mm Litz RF coil (Doty Scientific Inc. Columbia, SC, USA). A continuous wave (CW)-CEST sequence with a 5 s hard irradiation pulse followed by single-shot spin-echo echo planar imaging (SE-EPI) acquisitions with TR of 7 s was used to study the anesthetized animal. Images were acquired with matrix size 64 × 64, field of view 30 mm × 30 mm, and one acquisition. A CW-CEST sequence with an 8 s hard irradiation pulse followed by free induction decay (FID) readout with TR of 10 s was used to study the samples and tissue homogenates. Z-spectra were acquired with RF offsets from −2000 Hz to 2000 Hz (−5 to 5 ppm at 9.4 T) with a step size of 50 Hz (0.125 ppm at 9.4 T). Control images were acquired with RF offsets of 100000 Hz (250 ppm at 9.4 T). All CEST measurements were performed with irradiation power of 1 μT at 37°C. Water longitudinal relaxation time (T1w = 1/R1w) was also measured at 37°C using an inversion recovery method. 1H MR spectra were obtained using a point-resolved spectroscopy (PRESS) sequence with variable pulse power and optimized relaxation delays (VAPOR) water suppression and the following parameters: spectral width = 4 kHz, number of points = 4096, number of acquisitions averaged = 500, TE1 = 8 ms, TE2 = 7 ms, and TR = 2 s. The corresponding water reference spectra were also acquired for 1H MRS signal normalization with the same parameters, but without water suppression and 4 acquisitions were averaged. The 1H MRS measurements were performed at 26°C.
Data analysis
CEST@2ppm was quantified by an apparent exchange-dependent relaxation method (11–13) using a fitting approach (AREXfit), which is insensitive to T1w and semi-solid magnetization transfer (MT) effects and thus allow quantitative comparisons of CEST@2ppm from different samples and rat brain.
where Slab, Sref, and S0 are the label, reference, and control signals. Sref was obtained from a spline interpolation of the CEST data obtained with RF offsets from 1 ppm to 1.5 ppm and 2.5 ppm to 3 ppm.
RESULTS
Animal and sample experiments
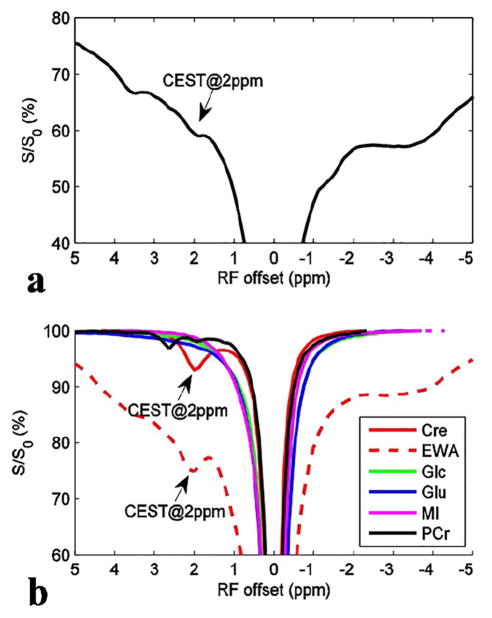
Figure 1a shows a CEST Z-spectrum from a healthy rat brain, in which a CEST dip at 2 ppm can be observed. Figure 1b shows CEST Z-spectra from the control samples. Note that both creatine and proteins may contribute to the CEST@2ppm.
Figure 1.
CEST Z-spectra from a healthy rat brain (a) and control samples (b). Note that the CEST@2 ppm observed in rat brain may originate from both creatine and proteins. The region of interest (ROI) was chosen from the whole brain. The control samples contain creatine (Cre), glutamate (Glu), glucose (Glc), myo-inositol (MI), and phosphocreatine (PCr), respectively.
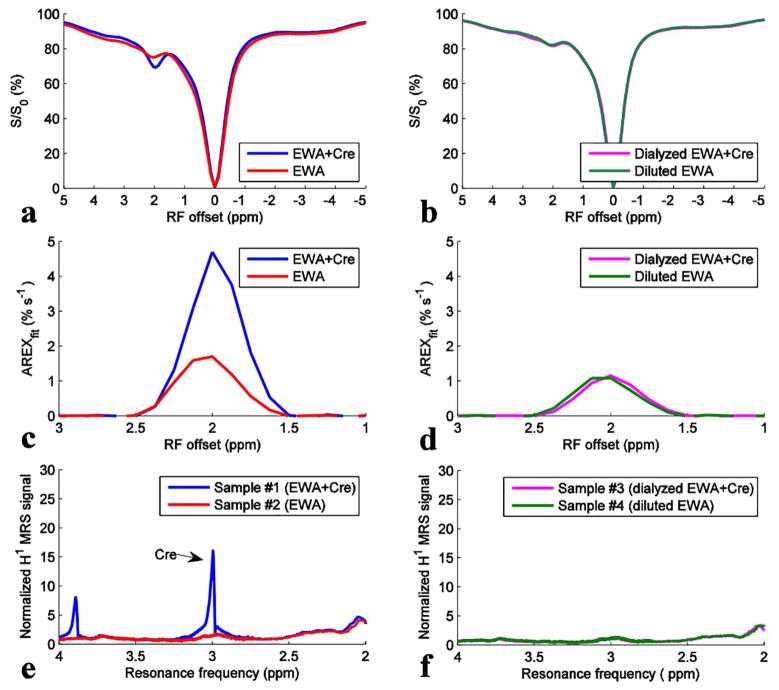
Figures 2a, 2c, and 2e show CEST Z-spectra, AREXfit spectra, and 1H MRS spectra from sample #1 (EWA + Cre) and sample #2 (EWA), respectively. It was found that although 1H MRS shows no distinct resonances in EWA alone, the CEST@2ppm peak is significant in this sample. In contrast, EWA+creatine gives both a clear 1H MRS peak at approximately 3 ppm from Tetramethylsilane (TMS)) and a strong CEST@2ppm effect. Thus MRS confirmed that creatine could be detected in mixtures of EWA + Cre before dialysis. Figures 2b, 2d, and 2f show CEST Z-spectra, AREXfit spectra, and 1H MRS spectra from sample #3 (dialyzed EWA + Cre) and sample #4 (diluted EWA), respectively. It was found (Figure 2f) that both the dialyzed EWA + Cre and the diluted EWA show very similar 1H MRS spectra to EWA alone, confirming that the creatine in sample #3 was successfully removed via dialysis. It was also found in Figures 2b and 2d that the dialyzed EWA + Cre and the diluted EWA give similar CEST signals at 2 ppm, indicating that the EWA is retained in sample #4 and is not affected by dialysis. These results indicate that dialysis is a selective means to separate creatine from protein.
Figure 2.
CEST Z-spectra (a, b), AREXfit spectra (c, d), and 1H MRS spectra (e, f) from sample #1 (EWA + Cre) (blue), sample #2 (EWA) (red), sample #3 (dialyzed EWA + Cre) (magenta), and sample #4 (diluted EWA) (green). T1w are ~3.2 s for sample #1 and #2, and ~3.4 s for sample #3 and #4.
Tissue homogenates
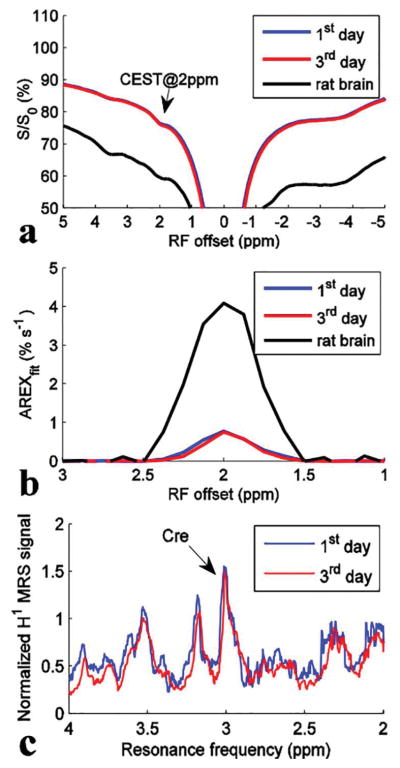
Figure 3 shows CEST Z-spectra, AREXfit spectra, and 1H MRS spectra measured immediately after homogenization and on the 3rd day for homogenized brain (control #2). It was found that both the CEST@2ppm signal and the 1H MRS resonance at 3 ppm from TMS did not significantly change during the 3-day period. This experiment indicates that our results are unaffected by potential degeneration of constituent molecules in vitro. To compare with the in vivo results, the Z-spectrum and AREXfit spectrum on rat brain were also plotted in Figure 3. Note that the AREXfit at 2 ppm in rat brain (~4.09 % s−1) is roughly 5 times of that in these tissue homogenates (~0.77 % s−1 and ~0.75 % s−1), which is in agreement with the 1/5 dilution of tissue homogenates, indicating that our results are not significantly affected by the homogenization process. This is consistent with a previous report that CEST effects are independent on the homogenization process (14).
Figure 3.
CEST Z-spectra (a), AREXfit spectra (b), and 1H MRS spectra (c) from control #2 measured at day 1 (immediately after homogenization) (blue) and on the 3rd day (red) after the preparation of tissue homogenates, respectively. To compare with the in vivo result, the Z-spectrum and AREXfit spectrum on rat brain were also plotted in (a) and (b), respectively. T1w are ~3.6 s for control #2 measured at day 1, ~3.5 s for control #2 measured at the 3rd day, and ~1.9 s for the rat brain, respectively.
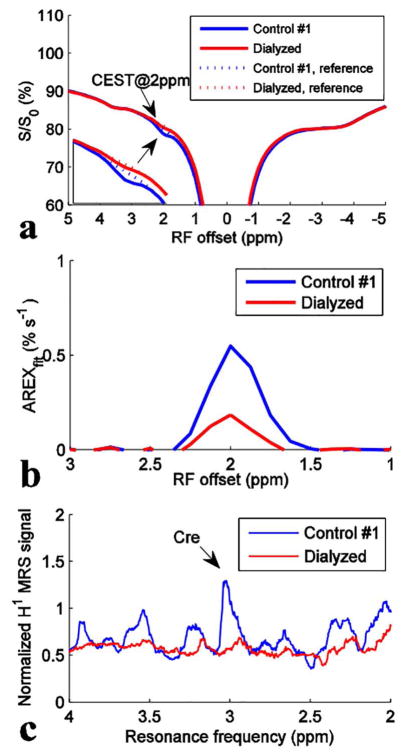
Figure 4 shows CEST Z-spectra with spline interpolated references, AREXfit spectra, and 1H MRS spectra from the tissue homogenates and control #1. Figure 4c shows that the 1H MRS signal at approximately 3 ppm from TMS in the dialyzed tissue homogenates significantly decreases compared with that in the control, indicating that mobile creatine was mostly removed. However, the CEST@2ppm effect from the dialyzed tissue homogenates decreases only to roughly 34% of the signal from the control, suggesting that the residual contribution is provided by other molecular constituents that are not affected by the dialysis. Thus proteins and creatine may provide comparable contributions to the CEST@2ppm.
Figure 4.
CEST Z-spectra with spline interpolated references (a), AREXfit spectra (b), and 1H MRS spectra (c) from control #1 (blue) and the dialyzed tissue homogenates (red), respectively. T1w are ~3.6 s for control #1 and ~3.4 s for the dialyzed tissue homogenates, respectively.
DISCUSSION
Proteins are relatively large biomolecules constructed from amino acids. The synthetic precursors of macromolecules may also exist in their unlinked free forms, and some of them function as important regulatory metabolites, such as glutamate. In addition, many proteins contain similar chemical structural features as simpler compounds in tissues (e.g. creatine). The amine groups of free metabolites and proteins also may have similar chemical exchange rates. Our sample experiments show that both albumin and creatine produce CEST effects at 2 ppm. In vitro experiments on brain tissue homogenates suggest that proteins and creatine may make comparable contributions to CEST@2ppm in rat brain, which casts doubt on whether CEST@2ppm is a specific indicator of creatine distribution.
Dialysis tubing is made of a semi-permeable membrane which facilitates the removal of small molecules from mixtures in solution based on differential diffusion. Dialysis tubing with different WMCO ranging from 1 – 1,000,000 kDa can be used to remove specific sizes of molecules selectively. In this study, dialysis tubing with WMCO of 1 kDa was used, which filters out small molecules (including free metabolites and short peptides with less than 9 amino acids), but retains large molecules (including proteins, membrane lipids, nucleic acids, and many polysaccharides). This separation technique allows us to distinguish CEST signals from macromolecules from smaller molecules in tissue homogenates. Instead of using a simple protein sample made with extracted proteins (e.g. EWA), tissue homogenates provide a relatively more accurate simulation of the CEST effects from proteins in biological tissues. As shown by our measurements, dialysis effectively removed most of the creatine (based on 1H MRS metrics), but significant CEST signals at 2 ppm were still detected in dialyzed samples. This result unambiguously suggests that protein is another major contribution to the CEST@2ppm in brain.
Protease inhibitors are typically used in the preparation of tissue homogenates to avoid potential protein degeneration. However, the most potent and widely used protease inhibitor is ethylenediaminetetraacetic acid (EDTA), a cation (Ca++/Mg++) chelator. Ca++ and Mg++ play an essential role for not only protease inhibitors but also many other proteins’ structure. So the addition of this inhibitor may affect protein structure as well as CEST signals, which is undesirable in the current work. In addition, brain tissue has low protease activity and the whole process was performed at 4°C to minimize protease activities. Figure 3 shows that CEST and 1H MRS signals did not change significantly during a 3-day delay, indicating that our results are not significantly influenced by protein degeneration, even without the use of protease inhibitors. Even if some proteins may degenerate to peptides during the sacrifice of the rats, the peptides would be removed via dialyses, and our study would indicate more contribution of proteins to CEST@2ppm. Figure 4b shows a reduction of 0.36 % s−1 of AREXfit contrast at 2 ppm due to the removal of creatine via dialysis. Considering the ~1/5 dilution of tissue homogenates, this value would be ~1.8 % s−1 in rat brain. However, the AREXfit contrast at 2 ppm of 6 mM creatine sample was 0.88 % s−1 (T1w of the creatine sample is ~4 s). This indicates a creatine concentration of ~12 mM in brain, which, however, is greater than the reported physiological concentration of creatine (< 10 mM) in brain (15). This may suggest that other small molecules, such as peptides and amino acids, may also contribute to CEST@2ppm. Thus, the contribution to CEST@2ppm from creatine may be even smaller. The creatine to phosphocreatine ratio tends to increase after death because of dephosphorylation of phosphocreatine (16). Thus, the creatine levels in the homogenates are likely greater than those found in live animals. This also implies that proteins would make greater contributions to CEST@2ppm in vivo. Restricted creatine, which may be bound to enzymes (17), could be retained in the dialysis bag. However, this immobile creatine has very short proton transverse relaxation time and thus is largely MRS invisible. Moreover, a previous study has shown that restricted creatine accounts for only a few percent of all forms of creatine (18). Thus, immobile creatine is not expected to influence our experimental results significantly.
Previously, CEST@2ppm was quantified using a multiple-pool Lorentzian fit (4) that may be contaminated by the presence of a broad glutamate CEST signal (Figure 1b). In addition, coalescence effect between fast exchanging pools (19) and water pool may cause inaccurate fitting of CEST@2ppm using the multiple-pool Lorentzian fit (20). Here, a spline interpolation of the CEST Z-spectrum was performed to isolate the narrow CEST@2ppm signal from the broad glutamate CEST signal as well as the effects of background direct water saturation and semi-solid MT. To fit the reference signals, sampling points in the frequency range from 1 ppm to 1.5 ppm and 2.5 ppm to 3 ppm, in which there were no significant CEST signals with narrow peaks, were used. This method is believed to be more accurate than the three-point method (21,22) which may isolate only the tip of CEST peaks. 1H MRS measures total creatine including free creatine and phosphocreatine. In contrast, CEST measures only the free creatine (Figure 1b). Figure 4c indicates a significant decrease of free creatine after dialysis. Therefore, comparisons of CEST and 1H MRS measurements of dialyzed and control tissue homogenates can be used to evaluate the relative contributions of creatine and proteins to CEST@2ppm.
In addition to brain, CEST@2ppm has been used in muscle and heart (6,23,24). Because the creatine content in muscle and heart is more abundant than that in brain, it is possible that CEST signals from creatine overwhelm signals from proteins in these organs. Phosphocreatine has CEST signal at 2.7 ppm. In our experiments, the CEST signal at 2.7 ppm is very weak and thus it is difficult to see its change after dialysis. In muscle and heart, this CEST signal at 2.7 ppm may be also significant due to its increased content. Glutamate is another major brain metabolite which shows significant CEST effect centered at 3 ppm at high irradiation powers (9,25–27). However, protein lysine amine protons (7,8) also have CEST effects close to glutamate’s resonance frequency offset. Further evaluation of their relative contributions is of considerable interest. Different from 1H MRS which has high specificity to specific metabolites, CEST@2ppm has much lower specificity due to contributions from both free metabolites and proteins. It will be prudent to show caution in the interpretation of CEST@2ppm, which in turn may limit its application in preclinical and clinical studies as a specific molecular imaging tool.
CONCLUSION
CEST@2ppm has been suggested as an indicator of creatine, an important energetic metabolite. However, our experiments on brain tissue homogenates with and without dialysis indicate that the contribution of proteins to CEST@2ppm cannot be neglected. The results of this study provide insights into the interpretation of the molecular origins of CEST signals at 2 ppm in brain.
Acknowledgments
Grant Sponsor: R21 EB17873, R01CA109106, R01CA184693, R01EB017767.
References
- 1.Ward K, Aletras A, Balaban R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST) J Magn Reson [Internet] 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med [Internet] 2011;65:927–48. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc [Internet] 2006;48:109–36. doi: 10.1016/j.pnmrs.2006.01.001. [DOI] [Google Scholar]
- 4.Cai K, Singh A, Poptani H, Li W, Yang S, Lu Y, Hariharan H, Zhou XJ, Reddy R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed [Internet] 2015;28:1–8. doi: 10.1002/nbm.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai K, Tain R-W, Zhou XJ, Damen FC, Scotti AM, Hariharan H, Poptani H, Reddy R. Creatine CEST MRI for Differentiating Gliomas with Different Degrees of Aggressiveness. Mol Imaging Biol [Internet] 2017;19:225–232. doi: 10.1007/s11307-016-0995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kogan F, Haris M, Singh A, Cai K, Debrosse C, Nanga RPR, Hariharan H, Reddy R. Method for high-resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med [Internet] 2014;71:164–72. doi: 10.1002/mrm.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin T, Kim S-G. Quantitative chemical exchange sensitive MRI using irradiation with toggling inversion preparation. Magn Reson Med [Internet] 2012;68:1056–64. doi: 10.1002/mrm.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zong X, Wang P, Kim S-G, Jin T. Sensitivity and source of amine-proton exchange and amide-proton transfer magnetic resonance imaging in cerebral ischemia. Magn Reson Med [Internet] 2014;71:118–32. doi: 10.1002/mrm.24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nat Med [Internet] 2012;18:302–306. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X-Y, Robledo BN, Harris SS, Hu XP. A bacterial gene, mms6, as a new reporter gene for magnetic resonance imaging of mammalian cells. Mol Imaging [Internet] 2014;13:1–12. doi: 10.2310/7290.2014.00046. [DOI] [PubMed] [Google Scholar]
- 11.Zaiss M, Bachert P. Exchange-dependent relaxation in the rotating frame for slow and intermediate exchange -- modeling off-resonant spin-lock and chemical exchange saturation transfer. NMR Biomed [Internet] 2013;26:507–18. doi: 10.1002/nbm.2887. [DOI] [PubMed] [Google Scholar]
- 12.Zaiss M, Zu Z, Xu J, Schuenke P, Gochberg DF, Gore JC, Ladd ME, Bachert P. A combined analytical solution for chemical exchange saturation transfer and semi-solid magnetization transfer. NMR Biomed [Internet] 2015;28:217–30. doi: 10.1002/nbm.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaiss M, Xu J, Goerke S, Khan IS, Singer RJ, Gore JC, Gochberg DF, Bachert P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed [Internet] 2014;27:240–52. doi: 10.1002/nbm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaiss M, Windschuh J, Goerke S, et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med [Internet] 2017;77:196–208. doi: 10.1002/mrm.26100. [DOI] [PubMed] [Google Scholar]
- 15.Petroff OA, Pleban LA, Spencer DD. Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn Reson Imaging [Internet] 1995;13:1197–211. doi: 10.1016/0730-725x(95)02033-p. [DOI] [PubMed] [Google Scholar]
- 16.Hackett MJ, Lee J, El-Assaad F, McQuillan JA, Carter EA, Grau GE, Hunt NH, Lay PA. FTIR imaging of brain tissue reveals crystalline creatine deposits are an ex vivo marker of localized ischemia during murine cerebral malaria: general implications for disease neurochemistry. ACS Chem Neurosci [Internet] 2012;3:1017–24. doi: 10.1021/cn300093g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leibfritz D, Dreher W. Magnetization transfer MRS. NMR Biomed [Internet] 2001;14:65–76. doi: 10.1002/nbm.681. [DOI] [PubMed] [Google Scholar]
- 18.Roell SA, Dreher W, Leibfritz D. Combining CW and pulsed saturation allows in vivo quantitation of magnetization transfer observed for total creatine by (1)H-NMR-spectroscopy of rat brain. Magn Reson Med [Internet] 1999;42:222–7. doi: 10.1002/(sici)1522-2594(199908)42:2<222::aid-mrm2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X-Y, Wang F, Li H, Xu J, Gochberg DF, Gore JC, Zu Z. CEST imaging of fast exchanging amine pools with corrections for competing effects at 9.4 T. NMR Biomed. 2017 doi: 10.1002/nbm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X-Y, Wang F, Li H, Xu J, Gochberg DF, Gore JC, Zu Z. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed [Internet] 2017 doi: 10.1002/nbm.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin T, Wang P, Zong X, Kim S-G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med [Internet] 2013;69:760–70. doi: 10.1002/mrm.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Zaiss M, Zu Z, Li H, Xie J, Gochberg DF, Bachert P, Gore JC. On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed [Internet] 2014;27:406–16. doi: 10.1002/nbm.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haris M, Singh A, Cai K, et al. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med [Internet] 2014;20:209–14. doi: 10.1038/nm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage [Internet] 2015;112:180–8. doi: 10.1016/j.neuroimage.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 25.Wermter FC, Bock C, Dreher W. Investigating GluCEST and its specificity for pH mapping at low temperatures. NMR Biomed [Internet] 2015;28:1507–17. doi: 10.1002/nbm.3416. [DOI] [PubMed] [Google Scholar]
- 26.Haris M, Nath K, Cai K, et al. Imaging of glutamate neurotransmitter alterations in Alzheimer’s disease. NMR Biomed [Internet] 2013;26:386–91. doi: 10.1002/nbm.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai K, Singh A, Roalf DR, Nanga RPR, Haris M, Hariharan H, Gur R, Reddy R. Mapping glutamate in subcortical brain structures using high-resolution GluCEST MRI. NMR Biomed [Internet] 2013;26:1278–84. doi: 10.1002/nbm.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]