Abstract
Objective
This study aimed to assess the effects of single and multiple massage treatments on pressure-pain threshold (PPT) at myofascial trigger points (MTrPs) in people with myofascial pain syndrome expressed as tension-type headache.
Design
Individuals (n=62) with episodic or chronic tension-type headache were randomized to receive twelve twice-weekly 45 minute massage or sham ultrasound sessions, or wait-list control. Massage focused on trigger point release (ischemic compression) of MTrPs in the bilateral upper trapezius and suboccipital muscles. PPT was measured at MTrPs with a pressure algometer pre and post the first and final (12th) treatments.
Results
PPT increased across the study timeframe in all four muscle sites tested for massage, but not sham ultrasound or wait-list groups (p<0.0001 for suboccipital; p<0.004 for upper trapezius). Post hoc analysis within the massage group showed 1) an initial, immediate increase in PPT (all p-values<0.05), 2) a cumulative and sustained increase in PPT over baseline (all p-values<0.05), and 3) an additional immediate increase in PPT at the final (12th) massage treatment (all p-values<0.05, except upper trapezius left, p=0.17).
Conclusions
Single and multiple massage applications increase PPT at MTrPs. The pain threshold of MTrPs have a great capacity to increase; even after multiple massage treatments additional gain in PPT was observed.
Keywords: myofascial pain syndrome, pain threshold, tension-type headache, complementary medicine
Myofascial pain syndrome (MPS) is a common skeletal muscle disorder associated with regional muscle pain and tenderness, characterized by the presence of myofascial trigger points (MTrPs). MTrPs are hyperirritable nodules along a taut band within a skeletal muscle and can be readily identified through palpation by trained therapists.
MTrPs have been identified as latent or active. Both designations cause reduced functional capacity of otherwise healthy muscle and may do so by inducing mechanical inefficiency.1,2 Latent MTrPs have been identified in most adult skeletal muscles.3 In contrast to latent, active MTrPs are spontaneously painful and referral pain originating from these sites reproduce the patient pain complaint.4 Both trigger point classifications may be present in an individual with MPS.5 While specific clinical investigation is needed for confirmation, it is thought that latent MTrPs develop into active MTrPs.6
Clinical trials on MPS with a treatment focus on the MTrP are becoming more common in the scientific literature.7,8 Treatment protocols for TTH have identified MTrPs in cervical musculature as important sites to address to achieve short-term success in headache pain reduction.9 An understanding of changes that occur at the MTrP with treatment is therefore of interest. Few studies have investigated the immediate and short-term effect of massage on MTrPs; these note reliable decreases in local pain and concomitant increase in pain threshold immediately and 24 hours after a single treatment session on the MTrP. However controlled, and especially placebo-controlled, studies on this topic are lacking.10–14 In clinical settings, the MTrP is often acted upon over multiple sessions to address a pain complaint. Insight into changes that occur at the MTrP following multiple treatment sessions is lacking in the research literature, but is needed to identify the magnitude of change per session and to identify when treatment gains plateau.
This study is a placebo-controlled, randomized trial to assess changes in MTrP pressure-pain threshold (PPT) following single and multiple massage interventions at two muscles in patients with MPS as represented by tension-type headache. The hypothesis is that PPT at MTrPs will increase following massage, but no change will occur in PPT at MTrPs in control subjects across the study timeframe.
MATERIALS AND METHODS
Study Population
Participants with MPS as represented by tension-type headache (TTH) were recruited from a large metropolitan area near a University. All participants were screened prior to enrollment for TTH by an anesthesiologist with specialty experience in headache and myofascial pain. A baseline headache diary was maintained and compared to ICHD-II criteria to confirm TTH diagnosis (mild to moderate head pain, 30+ minutes duration, no nausea/vomiting).15 Subjects were further screened by a massage therapist for the presence of at least one MTrP that reproduced the headache pain complaint (active MTrP) in the upper trapezius, suboccipital muscles, or sternocleidomastoid (SCM). Subject recruitment occurred between September 2010 and May 2012. Study procedures were in accordance with the ethical standards of the responsible committee on human experimentation and approved by the Colorado Multiple Institutional Review Board at the University of Colorado at Denver. Written informed consent was obtained by the principal investigator from all participants. The trial is registered at ClinicalTrials.gov, NCT01244555. This study conforms to all CONSORT guidelines and reports the required information accordingly (see Supplementary Checklist). Recruited subjects were obtained from the clinical headache trial portion of the study.16 In that trial, headache frequency was reduced for both massage and placebo-treated groups relative to waitlist control. Statistical power at 80% with 17 subjects per group was found in the initial study for the clinical parameter of headache frequency.
Research Design
Prior to randomization, all subjects maintained a daily headache diary for 4 weeks to confirm TTH diagnosis. Inclusion criteria also included a minimum of two tension-type headaches meeting ICHD-II guidelines and age 18–59 years.15 Exclusion criteria included migraine (>1/mo), headache originating from a secondary cause (e.g., cancer or injury), fibromyalgia, diabetes, major depression, neurological or cardiovascular disease, pregnancy, use of professional massage or ultrasound (US) specifically for headache in the prior 6 months. Participants taking prophylactic medication for headache were also excluded; abortive medication was permitted provided a self-reported use for not >75% of headache episodes. Subjects were block randomized (groups of 6) to receive twice weekly massage or sham ultrasound for six weeks (12 treatment sessions), or to wait-list control. Data are included for all study subjects who completed the headache diary and were randomized to treatment (n=62); 7 subjects were removed from the study prior to randomization (n=5 headache screen failure, n=2 scheduling conflict). Pressure-pain threshold (PPT) of the bilateral upper trapezius and suboccipital muscles was assessed before and after the first (pre1 and post1, respectively) and last (pre12 and post12, respectively) intervention session or at a time-matched period for the wait-list group. The initial PPT assessment occurred immediately prior to subject randomization to groups. Although part of initial subject screening, PPT at the SCM site was not determined in all subjects due to subject reported sensitivity for assessment at this site; hence, these results were not included.
Identification of Latent and Active MTrPs
Massage therapists identified MTrPs using published criteria.3,17–19 Specifically, muscles were palpated for a nodule along a taut band within each muscle. Gentle compression was applied to the nodule and subjects verbally confirmed it as unusually painful. This process identified a tender nodule. Progressive compression was applied to generate referred pain. If referred pain was generated, subjects were asked whether they recognized the pain as “familiar” to their typical headache. If the referral pain was familiar the MTrP was identified as active; if not or no referral pain was generated the MTrP was identified as latent.
Intervention
Each massage or placebo session was 45 minutes in duration, conducted twice per week for six weeks, with treatments separated by at least 48 hours. All interventions and assessments were administered in a clinical setting at the Clinical and Translational Research Center (CTRC) within the University of Colorado Hospital.
Massage treatment
A standardized 45 minute massage protocol was followed at each session. Briefly, 15 minutes of myofascial release applied to warm soft tissues of the upper back, shoulders, chest, and neck was conducted; 20 minutes of trigger point release (TPR) applied bilaterally to MTrPs in the upper trapezius, suboccipital muscles, and SCM; the final 10 minutes consisted of post-isomeric relaxation directed at right and left lateral cervical flexion, circular or cross-fiber friction on the masseter, temporalis, and occipitofrontalis muscles, and ended with gentle effleurage and petrissage to the neck and shoulders.
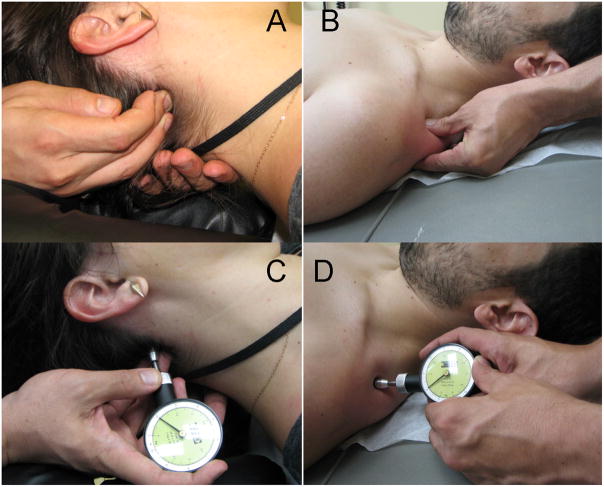
TPR was administered to MTrPs in suboccipital muscle with the fingertips; pressure to MTrPs of the upper trapezius was applied with the MTrP gripped between thumb and first finger (Figure 1A & B). In either case, sufficient force was used to just elicit referred pain or a subjective report of 6 on a 10 point scale. Force on the MTrP was maintained until patient verbally reported dissipation of referred pain or a maximum of 60 seconds had elapsed. End point determination was inability to palpate the trigger point and generate pain referral, or 5 repetitions at each site. A 10 second rest between applications was given to allow for blood reperfusion to the site.
Figure 1.
Trigger point release technique and pressure-pain threshold (PPT) assessment on the suboccipital muscle (A & C) and the upper trapezius muscle (B & D).
Six massage therapists participated in the study. Massage experience, training, and in-study quality assurance for the massage treatment was described previously.16 No technical deviations were detected.
Placebo treatment
Detuned ultrasound (US) meets important parameters for a body-oriented placebo treatment: it is a believable intervention for MTrPs, controls for therapist-patient interaction, involves tactile contact, provides no potentially effective treatment, and is indistinguishable from live US.20 Detuned US was administered with a Dynatron 150plusa using a non-functional soundhead to 4 regions (10 minutes each) of the upper back and neck to approximate location of left and right upper trapezius and suboccipital muscle groups. The sham ultrasound procedure followed a standardized protocol and has been described.16 Water-soluble coupling agentb was used and the soundhead moved in small overlapping circles at a rate of 2 cm/s, with display settings at 1 W/cm2 intensity, 1.0 Mhz frequency, and 20% duty cycle. Each US session lasted 45 minutes; in accordance with the massage duration.
Application of ultrasound by a nurse trained in its use was considered more plausible as within their scope of practice than US application from massage therapists. Eight nurses or nurse’s aides conducted US application; each received a 1-hour training in its application and was observed by a certified US technician on 1 unannounced visit to assess US application and confirm treatment protocol compliance. Subjects were aware of the credentials and training of the nurses. No technical deviations were observed.
Wait-list
Subjects randomized to the wait-list control group rested quietly on a massage table for 45 minutes at the first assessment and again six weeks later time-matching the treatment groups final visit, but did not attend intervening sessions.
Primary Outcome Measures
Algometric assessment at an MTrP provides a simple, reliable method to assess change in pain sensitivity at the MTrP.21 The algometerc plunger consists of a flat, circular rubber disk with 1.0 cm2 surface with recording pressure in 0.5 increments from 5.0 to 50N/cm2. The algometer tip was placed over the MTrP with force slowly applied until the patient verbally indicated sensation changed from pressure to pain (Figure 1C & D). Massage therapists identified MTrPs using procedures described above; they also received a 1 hour training on algometer use and conducted the PPT assessments. Three measurements were conducted at each site and averaged. Approximately 30 seconds separated each measurement.
PPT was assessed at 4 time points: Baseline (“pre1”, just prior to subject randomization), immediately following the first treatment (“post1”), immediately pre- and post- final (12th) treatment “pre12” and “post 12”, respectively). PPT determination at the pre-12th time point occurred at least 48 hours after the previous treatment.
Measurement of PPT at a flat portion of the tibia was conducted in all subjects at each time point to provide reference PPT levels at a non-treated site to account for change in pain sensitivity across the study time frame.
Blinding
Subjects, massage therapists, and nurses were aware of all treatment groups, but were unaware of the sham nature of the ultrasound apparatus. Subjects were aware that they were receiving treatment from either a nurse or massage therapist. Statistical analysis was conducted with the statistician blinded to group identification. Assessors were blind to subject group allocation at the initial PPT assessment (Pre 1), but may have been aware of group assignment thereafter.
Statistical analysis
PPT between MTrPs identified as active or latent was examined on the baseline scores (pre1 visit) for the upper trapezius and suboccipital muscles (left and right sides examined separately). No significant differences between active and latent MTrP PPT were observed (p-values 0.29–0.50); active and latent trigger points were therefore combined for all subsequent analyses. Furthermore, no change was observed across the three PPT replicates when stratified by location or time point (p=0.65–0.99).
Change over time of PPT at the five sites was examined in a multilevel framework using SAS Proc Mixed to address time points nested within individual and to make use of all available data. The group main effect, time main effect, and group by time interaction were examined as predictors of each of the five outcomes, using relevant covariates discussed below. A significant group by time interaction indicates group differences in changes in scores over time and were interpreted using post hoc contrasts.
Potential covariates examined at baseline were: Age, gender, marital status, ethnicity, employment status, income, years with TTH, professional massage in past six months, massage expectation, US expectation, physical activity, daily stress (Daily Stress Inventory), chronic stress (Perceived Stress Scale), headache diagnosis, depression (Beck Depression Inventory), and anxiety (State-Trait Anxiety Inventory). Of the large number of tests examining the relationship of covariates to massage group and PPT scores only a small percentage were significant. Covariates were not related to group assignment, but were related to some PPT scores at visit 1 only. Therefore, years with TTH was used as a covariate for models for upper trapezius-left, suboccipital-left and suboccipital-right. Age was used as a covariate for models for suboccipital left and right. Gender was used for any models for upper trapezius-right and left and lower leg measurement. Inclusion of covariates not did not influence the observed conclusions. Unless otherwise noted, data are presented as mean ± standard deviation.
RESULTS
Subjects
Sixty-two individuals completed data collection; seven subjects were removed prior to randomization due to headache screening failure (n=5) or time/scheduling (n=2). Table 1 presents group demographic and headache variables. No group differences were detected for age, percent female, percent white, activity level, headache classification, years with headache, or number of treatment sessions attended (p-values>0.05).
Table 1.
Subject demographic and headache information by treatment group
| Demographic Variable | Massage Mean±SD or % |
Placebo Mean±SD or % |
Wait-list Mean±SD or % |
p-value |
|---|---|---|---|---|
| n | 20 | 21 | 21 | |
| Age | 31.2±11.3 | 34.3±10.7 | 33.0±9.0 | 0.50 |
| % Female | 95% | 90.5% | 80.9% | 0.67 |
| % White | 90% | 81.0% | 85.7% | 0.91 |
| Activity Level* | 2.0±0.7 | 1.8±0.6 | 2.0±0.7 | 0.43 |
| Headache | ||||
| Chronic TTH | 65.0% | 52.4% | 47.6% | 0.36 |
| Episodic TTH | 35.0% | 47.6% | 52.4% | |
| Years with TTH | 8.2±6.4 | 10.4±8.2 | 8.8±7.4 | 0.84 |
| Treatment Sessions Attended | 11.6 ± 1.0 | 11.5 ± 0.84 | - | 0.72 |
Activity level determined by self-report where sedentary/inactive =0, light =1, moderate = 2, heavy = 3 (HOWLEY, E. T. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med. Sci. Sports Exerc, 33(6, Suppl), 2001, S364–S369).
A myofascial trigger point (active or latent) was identified in at least 88.7% of all subjects for each muscle site assessed (Table 2). In the suboccipital muscles, 61.3% (left) to 64.5% (right) of subjects had active MTrPs. A slightly lower percentage of subjects had active MTrPs in the upper trapezius muscles (50.8% right, 54.8% left).
Table 2.
Percentage of subjects with active or latent MTrPs at baseline
| Muscle | Sites Assessed at Baseline | Identified as Active | Identified as Latent | Percent with an Active or Latent MTrP |
|---|---|---|---|---|
|
| ||||
| Suboccipital-Right (All) | 62 | 64.5% | 25.8% | 90.3% |
| Massage | 20 | 70.0% | 25.0% | 95.0% |
| Placebo | 21 | 57.1% | 33.3% | 90.5% |
| Wait-list | 21 | 66.7% | 19.0% | 85.7% |
|
| ||||
| Suboccipital-Left (All) | 62 | 61.3% | 27.4% | 88.7% |
| Massage | 20 | 70.0% | 25.0% | 90.0% |
| Placebo | 21 | 47.6% | 42.9% | 95.5% |
| Wait-list | 21 | 66.7% | 14.3% | 80.9% |
|
| ||||
| UT-Right (All) | 61 | 50.8% | 41.0% | 91.8% |
| Massage | 19 | 42.1% | 47.4% | 89.5% |
| Placebo | 21 | 61.9% | 28.6% | 90.5% |
| Wait-list | 21 | 47.6% | 47.6% | 95.2% |
|
| ||||
| UT-Left (All) | 62 | 54.8% | 33.9% | 88.7% |
| Massage | 20 | 65.0% | 25.0% | 90.0% |
| Placebo | 21 | 47.6% | 42.9% | 90.9% |
| Wait-list | 21 | 52.4% | 33.3% | 85.7% |
Two adverse events were reported during the study; only one of which was considered reasonably probable it was related to the study. In that case, thirty minutes after the initial PPT assessment and sham US treatment the study subject reported onset of headache and neck pain with difficulty turning her head. Neck pain was relieved with Aleve pain medication; headache resolved within 48 hours.
PPT across study timeframe
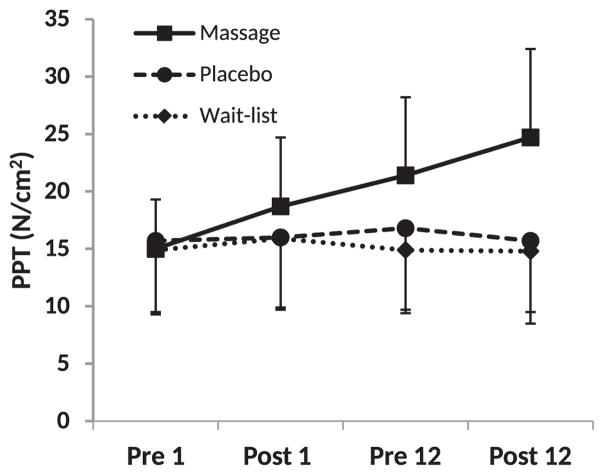
PPT data for all muscles and the tibia is presented in Table 3. Figure 2 presents a graphical representation of the PPT for MTrPs on the right side suboccipital muscle; data for the other muscles followed this trend and are detailed in Table 3 and below.
Table 3.
Pressure-pain threshold for the upper trapezius, sub occipital and lower leg (tibia) across study time frame
| Site | Pre 1st visit | Post 1st visit | Pre 12th visit | Post 12th visit | Group X Time interaction |
|---|---|---|---|---|---|
| Sub-Occipital-Right | |||||
| Massage | 15.0±4.3 | 18.7±6.0 | 21.4±6.8 | 24.7±7.7 | p<0.0001 |
| Placebo | 15.7±6.2 | 16.0±6.1 | 16.8±7.1 | 15.7±6.2 | |
| Wait-list | 14.9±5.6 | 15.9±6.2 | 14.9±5.5 | 14.8±6.3 | |
|
| |||||
| Sub-Occipital-Left | |||||
| Massage | 15.1±5.2 | 19.2±6.6 | 20.4±5.4 | 24.7±8.4 | p<0.0001 |
| Placebo | 15.4±5.1 | 15.8±6.3 | 17.0±6.2 | 16.2±5.6 | |
| Wait-list | 14.8±5.3 | 15.5±6.1 | 14.1±4.8 | 14.0±5.1 | |
|
| |||||
| UT-Right | |||||
| Massage | 17.8±5.2 | 19.8±7.5 | 23.7±9.2 | 25.9±10.2 | p=0.004 |
| Placebo | 19.0±6.6 | 18.0±6.2 | 18.5±7.1 | 19.2±6.9 | |
| Wait-list | 19.4±7.4 | 18.5±7.8 | 18.8±7.7 | 18.2±8.0 | |
|
| |||||
| UT-Left | |||||
| Massage | 18.3±8.0 | 20.9±8.3 | 23.5±9.2 | 25.4±10.5 | p=0.004 |
| Placebo | 17.9±5.2 | 17.7±6.0 | 17.8±5.6 | 18.0±7.2 | |
| Wait-list | 19.6±6.9 | 18.8±7.8 | 18.6±6.8 | 17.3±6.4 | |
|
| |||||
| Lower Leg | |||||
| Massage | 33.2±10.5 | 32.9±11.3 | 35.5±8.9 | 36.1±10.3 | p=0.41 |
| Placebo | 31.3±10.0 | 30.6±11.0 | 30.3±9.5 | 30.0±9.8 | |
| Wait-list | 31.1±11.3 | 31.7±12.3 | 31.1±8.6 | 32.8±9.1 | |
Data are presented as mean±SD in newtons/cm2.
Figure 2.
Representative depiction of pressure-pain threshold (PPT) across study time frame. Data are presented for right side suboccipital muscles. Pre- and Post- designation indicates assessment conducted relative to the first or twelfth intervention session, or time-matched for wait-list group. Data are presented as mean ± standard deviation.
Suboccipital muscle
Mean baseline PPT for MTrPs in the right suboccipital muscle was 15.2 ± 5.3 N/cm2 and 15.1 ± 5.0 N/cm2 for the left suboccipital. There was a significant group by time interaction for PPT in both the suboccipital-right and -left (both p-values<0.0001). Simple effects tests examining time effects within group showed change over time for those in the massage group (both p-values<0.0001) but not for those in the placebo (p=0.28, right; p = 0.30, left) or waitlist (p=0.42, right; p=0.59, left) groups. Post hoc analysis of PPT in the right suboccipital muscle within the massage group showed an immediate increase after the first massage with mean difference gains from pre1-post1 of 3.7N/cm2 (95%CI 1.9, 5.4). PPT gains from multiple massage treatments yielded short-term gains of 6.3N/cm2 from pre1 to pre 12 (95%CI 3.9, 8.8). Finally, and an additional immediate gain of 3.4N/cm2 on the final massage was detected. (pre12-post 12, 95%CI 1.8, 5.0). P-values for all post hoc comparisons were <0.05.
For the left side, a similar profile emerged with statistically significant differences between pre1-post1 (mean difference 4.1N/cm2, 95%CI 2.9, 5.3), pre1-pre12 (5.2N/cm2, 95%CI 3.0, 7.5), and pre12-post 12 (4.2N/cm2, 95%CI 1.7, 6.7) (all comparisons p<0.05).
Upper Trapezius muscle
Mean baseline PPT in MTrPs in the right upper trapezius muscle was 18.7 ± 6.3 N/cm2 and was 18.6 ± 6.7 N/cm2 in the left upper trapezius. There was a significant group by time interaction for both sides (both p-values<0.004). Simple effects tests again showed change over time for those in the massage group (p=0.0002, right; p=0.0004, left) but not for those in the placebo (p=0.30, right; p=0.93, left) or waitlist (p=0.68, right; p=0.42, left) groups. Post hoc analysis of MTrP on the right side upper trapezius showed significant mean increases in PPT between pre1-post 1 (1.9N/cm2, 95%CI 0.1, 3.9), pre1-pre12 (5.7N/cm2, 95%CI 2.3, 9.1), and pre12-post12 (2.2N/cm2, 95%CI 0.2, 4.1) (all p values <0.05).
For the left side upper trapezius trigger point, there were significant differences mean increases in PPT (p-values<0.05) between pre 1st visit and post 1st visit (2.6N/cm2 95%CI 0.7, 4.5) as well as pre1-pre12 (4.7N/cm2 95%CI 0.5, 8.9), but not at the final massage (pre12-post 12, 1.9N/cm2 95%CI −0.8, 4.5, p=0.17).
Lower Leg
No significant group by time interaction for PPT at the flat tibial bone portion was detected (p = 0.41), nor were there a significant main effect for group (p = 0.19) or time (p = 0.92). Effects for this outcome were therefore not interpreted further.
DISCUSSION
In a placebo and wait-list controlled study, we report an increase in PPT at MTrPs of the suboccipital and upper trapezius muscles following TPR massage in individuals with TTH. Our study found three results of note: 1) An immediate (pre1-post 1) increase in PPT was recorded at the initial massage. 2) Relative to baseline, PPT was elevated when measured just prior to the final massage session (pre 1-pre 12); a measurement time point assessed at least 48 hours after the previous (11th) massage. This finding highlights the sustainability of prior gain in PPT from massage treatments. 3) An additional immediate gain in MTrP PPT was observed at the final session (pre12-post 12; bilaterally for suboccipital muscle and right side-only for upper trapezius). This third finding identifies that additional change in MTrP tenderness is attainable even after multiple massage treatments. No change in PPT at the MTrP in the placebo or wait-list control groups or at flat portion of the tibia in all three groups was found.
MTrPs are believed to be important elements in MPS.22 Reduced PPT at the MTrP is an indicator of increased sensitivity and treatments directed at MTrPs have shown promise at improving clinical outcomes, including improved muscle strength and range of motion, and reduced shoulder pain.11,22,23 Only a few studies have investigated change in PPT at MTrP following massage intervention, with a single intervention and assessment immediately pre-post serving as a common design.12,13,24 Our observation of an immediate effect confirms, in a placebo-controlled trial, this prior research and forwards the notion of an underlying change in physiology at the MTrP.
Longer lasting effects represent a sustained change in the MTrP and may confer prolonged gain to clinical parameters. We observed an elevation in PPT at least 48 hours following the 11th massage. In some cases this measurement was conducted up to 7 days following the previous treatment. These short-term gains are in agreement with Oliveira-Campelo et al (2013) who found a single IC treatment of a latent MTrP elevated PPT at 24 hours with statistically significant effects a week later.22 While assessment at intervening time points was not conducted in the present study, a significant increase in PPT was detected between post1 and pre12 for the right-sided muscles. Thus, not only was an immediate gain in PPT detected at the initial treatment, but intervening sessions further increased PPT above the initial amount gained. Massage therapists also subjectively reported increased difficulty locating the MTrP nodule across the study treatment time frame for subjects receiving massage.
The third finding in this study detected a further immediate increase in PPT at the 12th session. Interestingly, the magnitude of this change was comparable to that observed during the 1st session even though the pre-12 PPT was raised above values reported at the first treatment. The ultimate PPT “top end” was not identified with any certainty although the failure to find a statistically significant change in the left side upper trapezius at visit 12 may indicate the MTrP is approaching resolution. An understanding of that value could be important in the determination of the characteristics of a fully-resolved MTrP. Incorporation of a non-MTrP site in the muscle for healthy tissue comparison might provide insight into typical or maximal pain sensitivity.
The mechanism for generation of the MTrP and subsequent pain reduction following treatment remains speculative. Simons proposed an integrated hypothesis whereby repetitive muscle use leading to overload increases spontaneous acetylcholine leakage resulting in local muscle sarcomeres contraction and formation of the contraction nodule or trigger point.19 Subsequently, blood vessels are compressed and local hypoxia ensues. The poor nutrient and waste exchange leads to a distress response, production of pain-invoking noxious chemicals and subsequently autonomic modulation that perpetuates the cycle through positive feedback. Intervening factors that interrupt this cycle may elicit recovery. Trigger point release massage (or ischemic compression) provides an external force that could separate sarcomeres and reduce compression on blood vessels. Several pain recovery hypotheses have been proposed25 and it is possible that multiple effects occur simultaneously at the MTrP following the trigger point release: first is a counter irritant effect that results in a rapid reduction in local pain. The second effect could be a more gradual reactive hyperemia whereby nutritive blood flow needed for cellular repair and removal of pain-invoking chemicals require multiple treatments over time to enact clinical improvement. Repeated treatments may continually interrupt the cycle preventing regression and allowing for long-term repair and recovery.
We found no group difference in baseline PPT between active and latent MTrPs in either the upper trapezius or suboccipital muscles. Only a few studies have compared the two MTrP designations on PPT; both statistical differences26 and no differences are reported,12,27,28 although there are biochemical differences.29 While it is thought that latent MTrPs are precursors to active MTrPs,6 factors that facilitate the transformation remains difficult to identify. Prolonged postural activities, such as 30 minutes of prolonged computer typing, may contribute to the formation or propagation of a MTrP.30 Also, in the present study, comparison of the change in PPT between active and latent MTrPs following massage did not find a statistical difference in any of the groups.
A potential limitation to the study is that PPT was assessed at a non-treated reference site (tibia) to control for changes in pain sensitivity that an individual may experience across a multi-week study. Assessment of PPT at an anatomically matched (muscular) site from a healthy subject might provide insight into typical or maximal pain sensitivity in the suboccipital and upper trapezius muscles. The measurement of PPT, while determined through instrumentation, remains largely a subjective measurement. Therefore bias from assessor or subject can influence final outcome. We believe this to be the first study in which massage therapists conducted PPT assessment. Massage therapists were generally aware of subject group assignment, which could influence findings. Bias was minimized by having a third disinterested party record algometer readings with the massage therapist blind to the measurement. While MTrPs were directly addressed through trigger point release (ischemic compression), the massage treatment also incorporated additional techniques that could potentially contribute to improvement in PPT.31 Teasing apart components of a massage to identify those most effective for improving PPT at latent MTrPs was recently done with stand-alone interventions including muscle energy techniques (MET’s), passive stretching, and ischemic compression (IC).22 In that study, the authors note gains in PPT following MET, stretching, or IC immediately following intervention, but only the IC group retained a significant elevation at 24 hours and 1 week.
In a randomized, placebo-controlled trial we observed immediate and continued improvement in PPT at MTrPs in individuals with myofascial pain expressed as tension-type headache. Gains in PPT of similar magnitude to the first session were also observed after the 12th massage treatment even though a significantly higher baseline was established. The present study underscores MTrP responsiveness to treatment, yet also shows that attaining full resolution of trigger point pain may require multiple treatment sessions.
Supplementary Material
Acknowledgments
The authors thank the massage therapists Heather Gunnerson, Darci Gau, Lynne Jordan, Michelle Stevens-Hogue, Jennifer Leete, Ann Mathews, Jonathan Hebert, Crystal Escartega, as well as Sharon Jordan, Lea Stenerson, and the nursing and support staff at the Clinical and Translational Research Center at the University of Colorado Hospital for their assistance on this study.
Abbreviations
- MPS
myofascial pain syndrome
- MTrP
myofascial trigger point
- PPT
pressure-pain threshold
- TTH
tension-type headache
- SCM
sternocleidomastoid
- TPR
trigger point release
- US
ultrasound
- MET
muscle energy techniques
- IC
ischemic compression
Footnotes
Dynatron 150plus ultrasound from Ultrasound Dynatronics Corporation, Salt Lack City, UT.
Water-soluble coupling agent from ScripHessco Ultrasound Gel; Bolingbrook, IL.
Algometer from Wagner Instruments, Inc., Greenwich, CT.
Author Disclosures.
This study was supported by the NIH/NCCAM Grant number R21 AT00469 and by NIH/CATS Colorado CTSI Grant number UL1 TR000154, Bethesda, MD. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
The authors declare no conflicts of interest or competing financial interests. The trial is registered at ClinicalTrials.gov, number NCT01244555.
References
- 1.Lucas KR. The impact of latent trigger points on regional muscle function. Curr Pain Headache Rep. 2008 Oct;12(5):344–349. doi: 10.1007/s11916-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 2.Lucas KR, Rich PA, Polus BI. Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: the effects of Latent Myofascial Trigger Points. Clin Biomech (Bristol, Avon) 2010 Oct;25(8):765–770. doi: 10.1016/j.clinbiomech.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Simons DG, Travell JG, Simons LS. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual. 2. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 4.Fernandez-de-Las-Penas C, Ge HY, Alonso-Blanco C, Gonzalez-Iglesias J, Arendt-Nielsen L. Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. J Bodyw Mov Ther. 2010 Oct;14(4):391–396. doi: 10.1016/j.jbmt.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo-Lozano A, Fernandez-de-las-Penas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res. 2010 May;202(4):915–925. doi: 10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 6.Celik D, Mutlu EK. Clinical implication of latent myofascial trigger point. Curr Pain Headache Rep. 2013 Aug;17(8):353. doi: 10.1007/s11916-013-0353-8. [DOI] [PubMed] [Google Scholar]
- 7.Bodes-Pardo G, Pecos-Martin D, Gallego-Izquierdo T, Salom-Moreno J, Fernandez-de-Las-Penas C, Ortega-Santiago R. Manual treatment for cervicogenic headache and active trigger point in the sternocleidomastoid muscle: a pilot randomized clinical trial. J Manipulative Physiol Ther. 2013 Sep;36(7):403–411. doi: 10.1016/j.jmpt.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Bron C, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. High prevalence of shoulder girdle muscles with myofascial trigger points in patients with shoulder pain. BMC Musculoskelet Disord. 2011;12:139. doi: 10.1186/1471-2474-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-de-las-Penas C, Cleland JA, Palomeque-del-Cerro L, Caminero AB, Guillem-Mesado A, Jimenez-Garcia R. Development of a clinical prediction rule for identifying women with tension-type headache who are likely to achieve short-term success with joint mobilization and muscle trigger point therapy. Headache. 2011 Feb;51(2):246–261. doi: 10.1111/j.1526-4610.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 10.Cagnie B, Castelein B, Pollie F, Steelant L, Verhoeyen H, Cools A. Evidence for the Use of Ischemic Compression and Dry Needling in the Management of Trigger Points of the Upper Trapezius in Patients with Neck Pain: A Systematic Review. Am J Phys Med Rehabil. 2015 Jul;94(7):573–583. doi: 10.1097/PHM.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 11.Cagnie B, Dewitte V, Coppieters I, Van Oosterwijck J, Cools A, Danneels L. Effect of ischemic compression on trigger points in the neck and shoulder muscles in office workers: a cohort study. J Manipulative Physiol Ther. 2013 Oct;36(8):482–489. doi: 10.1016/j.jmpt.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-de-las-Penas C, Alonso-Blanco C, Fernandez-Carnero J, Miangolarra JC. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial trigger points: a pilot study. J Bodyw Mov Ther. 2006;10:3–9. [Google Scholar]
- 13.Fryer G, Hodgson L. The effect of manual pressure release on myofascial trigger points in the upper trapezius muscle. J Bodyw Mov Ther. 2005;9:248–255. [Google Scholar]
- 14.Gulick DT, Palombaro K, Lattanzi JB. Effect of ischemic pressure using a Backnobber II device on discomfort associated with myofascial trigger points. J Bodyw Mov Ther. 2011 Jul;15(3):319–325. doi: 10.1016/j.jbmt.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 16.Moraska AF, Stenerson L, Butryn N, Krutsch JP, Schmiege SJ, Mann JD. Myofascial trigger point-focused head and neck massage for recurrent tension-type headache: a randomized, placebo-controlled clinical trial. Clin J Pain. 2015 Feb;31(2):159–168. doi: 10.1097/AJP.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbero M, Bertoli P, Cescon C, Macmillan F, Coutts F, Gatti R. Intra-rater reliability of an experienced physiotherapist in locating myofascial trigger points in upper trapezius muscle. J Man Manip Ther. 2012 Nov;20(4):171–177. doi: 10.1179/2042618612Y.0000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mense S. How do muscle lesions such as latent and active trigger points influence central and nociceptive neurons. J Musculoskelet Pain. 2010;18(4):348–353. [Google Scholar]
- 19.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004 Feb;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Ilter L, Dilek B, Batmaz I, et al. Efficacy of Pulsed and Continuous Therapeutic Ultrasound in Myofascial Pain Syndrome: A Randomized Controlled Study. Am J Phys Med Rehabil. 2015 Jul;94(7):547–554. doi: 10.1097/PHM.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 21.Sciotti VM, Mittak VL, DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001 Sep;93(3):259–266. doi: 10.1016/S0304-3959(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira-Campelo NM, de Melo CA, Alburquerque-Sendin F, Machado JP. Short- and medium-term effects of manual therapy on cervical active range of motion and pressure pain sensitivity in latent myofascial pain of the upper trapezius muscle: a randomized controlled trial. J Manipulative Physiol Ther. 2013 Jun;36(5):300–309. doi: 10.1016/j.jmpt.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Hains G, Descarreaux M, Hains F. Chronic shoulder pain of myofascial origin: a randomized clinical trial using ischemic compression therapy. J Manipulative Physiol Ther. 2010 Jun;33(5):362–369. doi: 10.1016/j.jmpt.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Takamoto K, Bito I, Urakawa S, et al. Effects of compression at myofascial trigger points in patients with acute low back pain: A randomized controlled trial. Eur J Pain. 2015 Sep;19(8):1186–1196. doi: 10.1002/ejp.694. [DOI] [PubMed] [Google Scholar]
- 25.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil. 2002 Oct;83(10):1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 26.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associated with cervical pain. J Ultrasound Med. 2011 Oct;30(10):1331–1340. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-de-Las-Penas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. Referred pain from trapezius muscle trigger points shares similar characteristics with chronic tension type headache. Eur J Pain. 2007 May;11(4):475–482. doi: 10.1016/j.ejpain.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009 Nov;90(11):1829–1838. doi: 10.1016/j.apmr.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008 Jan;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Treaster D, Marras WS, Burr D, Sheedy JE, Hart D. Myofascial trigger point development from visual and postural stressors during computer work. J Electromyogr Kinesiol. 2006 Apr;16(2):115–124. doi: 10.1016/j.jelekin.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Trampas A, Kitsios A, Sykaras E, Symeonidis S, Lazarou L. Clinical massage and modified Proprioceptive Neuromuscular Facilitation stretching in males with latent myofascial trigger points. Phys Ther Sport. 2010 Aug;11(3):91–98. doi: 10.1016/j.ptsp.2010.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.