Abstract
Context
While the prevalence of vitamin D deficiency is well described in various populations, limited data are available regarding longitudinal variation in serum 25-hydroxyvitamin D concentrations.
Objectives
To evaluate the temporal trends in serum 25(OH)D, prevalence of vitamin D deficiency, and factors influencing these trends.
Participants, Design and Setting
Adults enrolled in the Dallas Heart Study, a longitudinal, probability-based, multiethnic, population study in Dallas, Texas, USA.
Main Outcome Measures
Prevalence of vitamin D deficiency and predictors of change in serum 25(OH)D.
Results
2045 participants had serum 25(OH)D measured on two occasions (2000–2002 and 2007–2009) at a median interval of seven years. Serum 25(OH)D decreased (42.7 to 39.4 nmol/l, p<0.001) and the prevalence of vitamin D deficiency [25(OH)D < 50 nmol/l] increased significantly (60.6% to 66.4%, p<0.0001) despite vitamin D supplementation increasing over the interval (7.2% to 23.0%; p<0.0001). In a multivariable model adjusting for sex, race, BMI, age, season of blood draw, smoking, and exercise, a greater decline in serum 25(OH)D was noted in men compared with women (−8.0 vs. −3.5 nmol/l, p < 0.0001), in participants of Hispanic ethnicity vs. White and Black ethnicity (p<0.0001), in non-obese vs. obese participants (−7.2 vs. −4.0 nmol/l, p=0.005), and in non-users vs. users of vitamin D supplements (−5.7 vs. −1.7 nmol/l, p=0.032).
Conclusions
Despite increased vitamin D supplementation, serum 25(OH)D decreased in an ethnically diverse cohort of Dallas County residents between 2000–2002 and 2007–2009. Features most predictive of a decline in serum 25(OH)D include male sex, Hispanic ethnicity, and weight gain.
Keywords: Vitamin D, Vitamin D deficiency, 25-hydroxyvitamin D, Longitudinal
Introduction
While the calciotropic effects of vitamin D are well characterized, the extra-skeletal benefits of vitamin D represent an evolving area of active interest1. Vitamin D deficiency has been associated with impaired innate immunity and increased incidence and progression of cancer, autoimmune diseases, cardiovascular disease, and diabetes mellitus2.
Presently, vitamin D status is most accurately assessed by measuring serum 25-hydroxyvitamin D [25(OH)D], which has a circulating half-life of approximately three weeks. In 2010, the Institute of Medicine characterized vitamin D deficiency using a threshold 25(OH)D concentration of 50 nmol/L (20 ng/mL)3. The following year, guidelines published by the Endocrine Society defined vitamin D deficiency as a level of 25(OH)D below 50 nmol/L (20 ng/ml) and insufficiency as a level of 50–74 nmol/L (20–29 ng/ml)4. In the United States, approximately 4 out of 10 adolescents5 and young adults6 are vitamin D deficient.
Several cross-sectional studies have assessed the prevalence of vitamin D deficiency in various populations7. The available data that analyze longitudinal variation in the measures within individuals and overall in the population8–13 tend to be limited in their scope of racial/ethnic heterogeneity10,11,14–16. Using data from the Dallas Heart Study, a longitudinal, probability-based, multiethnic, population study, we sought to 1) evaluate the longitudinal change in the prevalence of vitamin D deficiency and insufficiency using measurements made between 2000–2002 (DHS1) and repeated between 2007–2009 (DHS2); and 2) identify independent predictors of change in participants’ vitamin D status during the study interval with regard to baseline characteristics, including analyses of anthropometric, metabolic, and physical activity parameters. A better understanding of the epidemiology of 25(OH)D deficiency, secular trends in its prevalence, and their predictors are of paramount importance to comprehending its correlation with disease and to identify targets for preventive interventions at the population level.
Materials and Methods
Design and Study Population
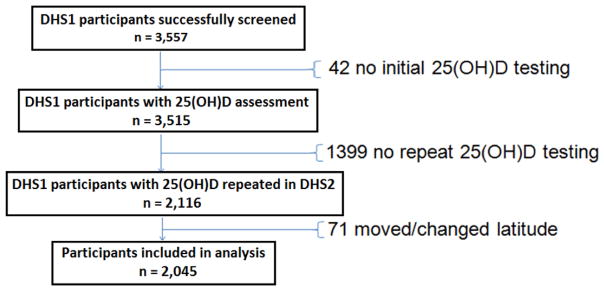
The Dallas Heart Study (DHS) is a single-site, multiethnic, probability-based population sample of Dallas County adult residents aged 18–65 at study initiation, with full details of study design and participant enrollment previously published.17 African Americans were intentionally oversampled to comprise 50% of the DHS cohort. All participants provided written informed consent, and the study was approved by the institutional review board of the University of Texas Southwestern Medical Center. Participants underwent extensive health survey data collection, blood testing, and multi-modality cardiovascular and anthropometric imaging, both at baseline (2000–2002) and at follow up (2007–2009). The dataset for the present analyses comprises all participants who had serum 25(OH)D levels measured at both the baseline and the follow up visit. Since 25(OH)D levels are correlated with latitude18, we excluded individuals who moved to an area of different latitude following data collection for the DHS1 (n=71). Participants who moved from Dallas to a region of comparable latitude (i.e., Oklahoma, Louisiana, Mississippi, New Mexico, Arkansas, Georgia, Florida, and Arizona) were included in the analysis. The selection process for participants included in this analysis is summarized in Figure 1.
Figure 1.
Flow chart of the study selection process
Health Survey
Baseline demographics, medical and family history, anthropometric measurements, and laboratory data were obtained from the initial clinical encounter of the Dallas Heart Study cohort. Body mass index was calculated by dividing weight in kilograms by height in square metres. Participant race and ethnicity were determined by self-report. Physical activity was self-reported and quantitatively estimated as metabolic equivalents by minutes per week ((MET-min)/week). Smoking status was self-reported, with current smokers defined as those who had smoked more than 100 cigarettes during their lifetime and who had smoked within the previous 30 days. Participants were asked about vitamin D supplementation [combined calcium/vitamin D supplements, vitamin D supplements, and multivitamins]. Dietary consumption of vitamin D and sunlight exposure were not assessed. Of note, study participants were not counselled on their serum 25(OH)D concentration or on vitamin D supplementation between DHS1 and DHS2, as serum 25(OH)D levels were not available until after the end of the DHS2 evaluation.
Laboratory Analysis
Fasting venous blood samples were collected into ethylenediaminetetraacetic acid (EDTA) tubes stored at 4°C and centrifuged for 15 minutes at this temperature within 4 hours of collection. The serum was stored in aliquots at −80°C. The baseline blood samples were stored for a median (± SD) of 7.1 (± 0.73) years and analyzed for serum 25(OH)D as a single batch simultaneous with the follow-up DHS2 samples. Serum samples of 25(OH)D were assayed using immunoextraction followed by enzyme immunoassay quantitation (Immunodiagnostics Systems, Scottsdale, AZ) with a sensitivity of 5 nmol/l, inter-assay CV of < 10%, and intra-assay CV of < 8%, respectively.
Statistical Analysis
25(OH)D values between DHS1 and DHS2 were compared via a paired t-test, and the categorical prevalence of vitamin D deficiency/insufficiency was compared by using the McNemar’s test. Repeated-measures linear regression mixed modelling with random effects was used to determine longitudinal change in 25(OH)D, adjusted for age, sex, race, BMI, vitamin D supplement use, and season of blood collection. Given the longitudinal nature of the data and that all risk factors were re-assessed at DHS2, all variables in the models were time updated, resulting in time-dependent covariables. Two-sided p-values ≤ 0.05 were considered statistically significant. All subgroup analyses used unadjusted linear regression mixed modelling, and appropriate linear contrast tests were used to assess significance between mean DHS1 and DHS2 serum 25(OH)D values. Change in 25(OH)D in multivariable linear mixed modelling was assessed with a time (dichotomous) by subgroup interaction term. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
Participant Characteristics
Baseline characteristics of the sampled population are listed in Table 1. A total of 2,045 participants had levels of serum 25(OH)D measured in the DHS1 (2000–2002) and subsequently in the DHS2 (2007–2009). The mean age of these participants was 44 years in the DHS1 and 51 years in the DHS2. The sample population included 50% African Americans by design (n = 1029). The participants were 41.6% male (n = 851) and 58.4% female (n = 1194). Notably, participants had a significantly greater body mass index in the DHS2 versus DHS1 (30.1 vs 28.4 kg/m2). The level of 25(OH)D decreased from the DHS1 to the DHS2 (42.7 nmol/l vs 39.4 nmol/l, p < 0.001) despite an increase in vitamin D supplementation among study participants (7.2% vs 23.0%, p=<0.0001). The prevalence of current smoking was higher in DHS1 compared with DHS2 (25.9% vs 22.1%; p < 0.0001).
Table 1.
Baseline characteristics of Dallas Heart Study participants with repeat 25(OH)D assessment in 2000–2002 (DHS1) and 2007–2009 (DHS2) (n = 2045)
| DHS1(2000–2002) | DHS2(2007–2009) | |
|---|---|---|
| Men | ||
| Black | 378 (18%) | |
| White | 319 (16%) | |
| Hispanic | 128 (6%) | |
| Women | ||
| Black | 651 (32%) | |
| White | 363 (18%) | |
| Hispanic | 165 (8%) | |
| Age (years) | 44 (37, 52)a | 51 (44, 59)a |
| BMI (kg/m2) | 28.4 (24.7, 33.5)a | 30.1 (26.2, 35.6)a |
| 25(OH)D (nmol/l) | 42.7 (30.0, 60.0)a | 39.4 (27.2, 56.4)a |
| Supplemental Vitamin D Use | 147 (7%) | 471 (23%) |
| Season of Blood Draw | ||
| Fall | 519 (25%) | 540 (26%) |
| Winter | 464 (23%) | 432 (21%) |
| Spring | 365 (18%) | 524 (26%) |
| Summer | 697 (34%) | 549 (27%) |
| Current Smoking | 530 (26%) | 452 (23%) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index;
Median (25, 75 percentiles)
Prevalence of 25(OH)D Deficiency and Insufficiency
The demographic and clinical characteristics of DHS1 and DHS2 participants with regards to 25-hydroxyvitamin D < 50 nmol/l (vitamin D deficiency) and < 75 nmol/l (vitamin D deficiency + insufficiency) are listed in Table 2. The prevalence of vitamin D deficiency increased significantly between DHS1 and DHS2 (60.6% to 66.4%; p < 0.0001).
Table 2.
Prevalence of 25(OH)D Deficiency: < 50 nmol/L (20 ng/mL); and Deficiency + Insufficiency: < 75 nmol/L (30 ng/mL) in the DHS1 and DHS2, unadjusted data
| DHS1 | DHS2 | |||
|---|---|---|---|---|
|
|
|
|||
| < 50 nmol/l | < 75 nmol/l | < 50 nmol/l | < 75 nmol/l | |
|
|
|
|||
| Overall | 1240 (60.6%) | 1791 (87.6%) | 1358 (66.4%) | 1845 (90.2%) |
|
| ||||
| Black | 838 (81.4%) | 1000 (97.2%) | 834 (69.8%) | 997 (96.9%) |
|
| ||||
| White | 204 (29.9%) | 485 (71.1%) | 297 (43.5%) | 533 (78.2%) |
|
| ||||
| Hispanic | 175 (59.7%) | 273 (93.2%) | 200 (68.3%) | 278 (94.9%) |
|
| ||||
| Men | 426 (50.1%) | 716 (84.1%) | 524 (61.6%) | 759 (89.2%) |
|
| ||||
| Black | 267 (70.6%) | 357 (94.4%) | 293 (77.5%) | 368 (97.4%) |
|
| ||||
| White | 82 (25.7%) | 222 (69.6%) | 132 (41.4%) | 247 (77.4%) |
|
| ||||
| Hispanic | 61 (47.7%) | 116 (90.6%) | 79 (61.7%) | 120 (93.8%) |
|
| ||||
| Women | 814 (68.2%) | 1075 (90.0%) | 834 (69.8%) | 1086 (91.0%) |
|
| ||||
| Black | 571 (87.7%) | 643 (98.8%) | 541 (83.1%) | 629 (96.6%) |
|
| ||||
| White | 122 (33.6%) | 263 (72.5%) | 165 (45.5%) | 286 (78.8%) |
|
| ||||
| Hispanic | 114 (69.1%) | 157 (95.2%) | 121 (73.3%) | 158 (95.8%) |
|
| ||||
| Age (years) | ||||
|
| ||||
| < 40 | 486 (61.5%) | 698 (88.4%) | 225 (70.3%) | 293 (91.6%) |
|
| ||||
| 41–50 | 411 (61.4%) | 577 (86.2%) | 450 (70.8%) | 587 (92.3%) |
|
| ||||
| 51–60 | 283 (58.7%) | 421 (87.3%) | 422 (63.5%) | 594 (89.3%) |
|
| ||||
| > 60 | 60 (57.7%) | 95 (91.3%) | 261 (61.6%) | 371 (87.5%) |
|
| ||||
| Body Mass Index (kg/m2) | ||||
|
| ||||
| < 25 | 254 (46.1%) | 430 (78.0%) | 214 (56.9%) | 304 (80.9%) |
|
| ||||
| 25–30 | 373 (56.2%) | 576 (86.7%) | 389 (61.3%) | 565 (89.0%) |
|
| ||||
| ≥ 30 | 602 (73.7%) | 772 (94.5%) | 750 (73%) | 970 (94.5%) |
25(OH)D Subgroups: DHS1 to DHS2
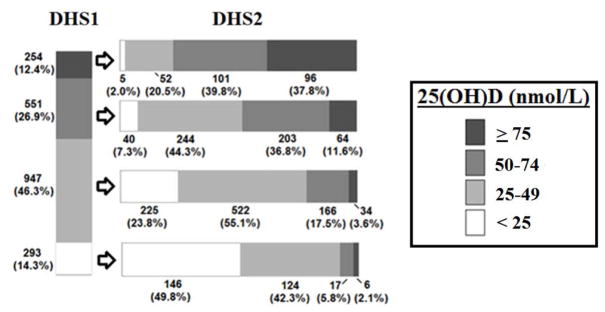
We examined whether serum 25(OH)D concentration declined similarly along the entire spectrum of 25(OH)D status or if certain subgroups were more affected than others. Figure 2 depicts the distribution in serum 25(OH)D in DHS participants as divided into four 25(OH)D levels based on baseline (DHS1) values: < 25 nmol/l, 25–49 nmol/l, 50–74 nmol/l, and ≥ 75 nmol/l. The vast majority of participants with 25(OH)D deficiency in DHS1 continued to exhibit vitamin D deficiency in DHS2. 51.6% of participants with 25(OH) insufficiency in the DHS1 developed 25(OH)D deficiency in the DHS2. 39.8% of participants of DHS1 with adequate 25(OH)D (i.e., ≥ 75nmol/l) developed 25(OH)D insufficiency in DHS2 and 22.5% developed 25(OH)D deficiency.
Figure 2.
Serum concentration of 25-hydroxyvitamin D [25(OH)D] among 25(OH)D subgroups (< 25, 25–49, 50–74, and ≥ 75 nmol/l) in the DHS 1 and DHS2, unadjusted data
Change in Serum 25(OH)D by Selected Variables in the DHS1 and DHS2
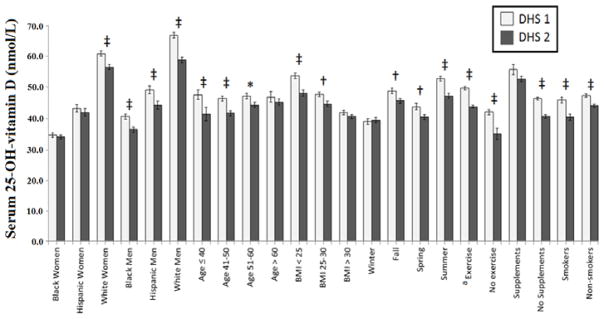
The mean serum 25(OH)D levels stratified by selected participant characteristics from the DHS1 compared with DHS2 are shown in Figure 3. With regards to race/ethnicity, a significant decline in 25(OH)D concentration was found in men of each race/ethnicity group (i.e., Black, White, Hispanic); however, only in white women was the decrease in 25(OH)D noted to be statistically significant. A significant decrease in 25(OH)D was found in participants in all age categories except for the subgroup age ≥ 60 years at baseline. We examined whether cohort aging predicted a decline in 25(OH)D levels between the DHS1 and DHS2 (Supplemental Figure 1). Serum 25(OH)D was persistently lower in DHS2 than DHS1 across the wide age range, although the decrement diminishes at older ages.
Figure 3.
Mean serum concentration of 25-hydroxyvitamin D [25(OH)D] stratified by selected characteristics in the DHS 1 and DHS 2.
* p < 0.05, † p < 0.01, ‡ p < 0.001
a Exercise is positive if (METS x minutes of exercise/week) > 0
A significant decline in serum 25(OH)D from the DHS1 to the DHS2 was noted in participants with BMI < 25 and 25–29.9, but no significant change in 25(OH)D was seen in obese participants. We examined the association between 25(OH)D change and the season during which the DHS evaluation occurred, and found a significant decrease for all seasons except winter. Participants who reported taking vitamin D supplements did not exhibit a significant change in 25(OH)D between the DHS1 and the DHS2, whereas those not taking supplements experienced a significant decline in 25(OH)D levels. Finally, we noted a significant decrease in 25(OH)D both in exercisers and non-exercisers, as well as smokers and nonsmokers.
Predictors of 25(OH)D Change by Selected Variables in the DHS1 and DHS2
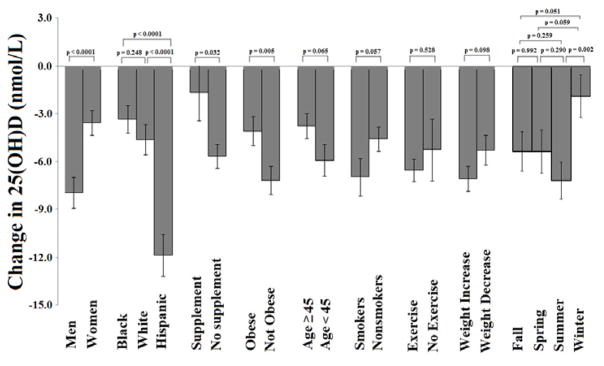
To assess the impact of independent predictors on change in 25(OH)D level over time, we used multivariable linear mixed regression modelling, and calculated the interaction term between time and each of the following predictors: sex, race/ethnicity, BMI, age, season of evaluation, smoking status, exercise, use of vitamin D supplementation, and weight gain (Figure 4). A significantly greater decline in serum 25(OH)D was noted in men compared with women (−8.0 vs. −3.5 nmol/l, ptime × sex interaction < 0.0001), in Hispanic participants vs. White and Black race (p time × race interaction < 0.0001 for both), in nonusers vs. users of vitamin D supplements (−5.7 vs −1.7 nmol/l, ptime × supplement interaction = 0.032), in non-obese vs. obese participants (−7.2 vs. −4.0 nmol/l, ptime × obesity interaction = 0.005), and in summer vs. winter study participants (ptime × season interaction = 0.002). In the multivariable model, there was no significant difference in the decline in 25(OH)D between DHS1 and DHS2 in participants below or above median age of 45 years, in smokers vs. non-smokers, in participants who exercise vs. non-exercisers, in participants who gained weight vs. did not gain weight (pinteraction > 0.05 for each), and in assessments done during different seasons aside from summer vs. winter.
Figure 4.
Predictors of change in serum 25-hydroxyvitamin D [25(OH)D] adjusted for age, sex, race, BMI, vitamin D supplement use, smoking, exercise, change in weight, and season in the DHS 1 and DHS 2.
Discussion
Using data from a longitudinal probability-based cohort study comprising 2,045 adult participants living in Dallas County, Texas, who participated in 2 study assessments over a span of 6–9 years (DHS1-2000–2002; and DHS2-2007–2009) we a) characterized the temporal trends in serum 25(OH)D levels and changing prevalence of vitamin D deficiency and insufficiency; and b) examined factors associated with changes in serum 25(OH)D. We found that the overwhelming majority of participants in Dallas County were vitamin D deficient in both the DHS1 and DHS2, a lesser number were vitamin D insufficient, and only a small minority had sufficient levels of 25(OH)D. Furthermore, the level of 25(OH)D decreased from a mean of 42.7 nmol/l to 39.4 nmol/l (p < 0.001) over the 5–10 years between the DHS1 and DHS2 evaluations. The decline in 25(OH)D from the DHS1 to DHS2 occurred in spite of an increase in vitamin D supplementation and smoking cessation19, factors that are protective against vitamin D deficiency. In multivariable analysis, features most significantly associated with a decrease in 25(OH)D levels included male sex, Hispanic race, absence of vitamin D supplementation, and obesity.
Based on past studies, we suspected cohort aging20,21, increased BMI22, and fewer 25(OH)D measurements in the summer months when levels tend to be highest23 would be significant predictors of change in serum 25(OH)D and adjusted for these factors in our multivariable model. While men had a higher baseline 25(OH)D concentration than women, after multivariable adjustment, men had a greater reduction in 25(OH)D compared with women over the study interval, consistent with prior observations.8,9 Given the impact of increasing skin pigmentation on reduced cutaneous production of vitamin D, it is not surprising that African American participants had the highest rates of 25(OH)D deficiency and insufficiency in both the DHS1 and DHS2, as has been shown previously.12,24 However, African American participants had the smallest change in 25(OH)D levels among any of the studied racial groups between the DHS1 and DHS2. Interestingly, the Hispanic subset of participants had the greatest decline in 25(OH)D levels. Genetic contributions to 25(OH)D levels have been implicated in studies of single-nucleotide polymorphisms affecting the vitamin D binding protein gene in African Americans and Hispanics25. It is possible that genetic variation in vitamin D-binding protein levels could also impact assessment of 25(OH)D status for diverse populations26, although this was not measured in our cohort.
The inverse correlation between body mass index and 25(OH)D levels has been well established in adults27 and African American and Hispanic cohorts28. In the present analyses, a greater proportion of nonobese (versus obese) participants had a decline in 25(OH)D level from the DHS1 to the DHS2; however, this is not surprising, since nearly 95% of obese participants had a 25(OH)D level less than 75 nmol/l at baseline. The potential mechanisms for low 25(OH)D levels in obese participants have been reviewed.29 Reduced sun exposure habits among obese versus lean individuals has been implicated in one study30 but not in another.31 Wortsman, et al. found that serum vitamin D3 concentrations were lower after whole-body ultraviolet radiation and peak serum vitamin D2 levels were lower after a dose of ergocalciferol 50,000 IU in obese participants than in matched lean control participants, attributing this to sequestration of vitamin D within body fat.32 More recently, Drincic, et al. have suggested that changes in 25(OH)D concentrations between normal and obese individuals can be completely explained by dilution of vitamin D within the fat mass (coining this “volumetric dilution”), advocating that vitamin D replacement therapy be dosed according to body size.33 Aside from vitamin D supplementation, 25(OH)D levels in obese participants have been shown to improve with weight loss, with levels increasing 6.7 nmol/l (with 5–10% weight loss), and 14.0 nmol/l (with > 15% weight loss) (p = 0.005).34
Other studies have evaluated longitudinal changes in vitamin D status on a population level. Looker, et al. used data from the National Health and Nutrition Examination Surveys (NHANES) to compare 25(OH)D levels between 20,289 participants in the NHANES III (1988–1994) and 18,158 participants in the NHANES 2000–20048. Different participants were sampled during each time period, and so intra-individual differences could not be assessed. While a significant decline in mean 25(OH)D levels was noted over time (18.3 nmol/L decrease in men; 10.3 nmol/L decrease in women), after adjustment for changes in assay methodology between NHANES III and NHANES 2000–2004, the difference in age-standardized 25(OH)D means between surveys was reduced by approximately 10 nmol/L. Ganji, et al. revisited the NHANES data over a lengthier period (i.e., 1988–1994, 2001–2006) and found that the mean, assay-adjusted 25(OH)D levels declined over to a lesser magnitude (60.7 nmol/L in 1988–1994 to 55.2 nmol/L in 2001–2006) than reported by Looker, et al.9 Berger, et al. assessed changes in 25(OH)D among 2,725 participants in the Canadian Multicentre Osteoporosis Study over a ten-year period starting in 1995–1997.11 The same participants were followed at study initiation and follow up, and 25(OH)D levels were shown to increase by 4.7 nmol/L in women and 2.7 nmol/L in men after adjustment for the increase in vitamin D supplementation in this osteoporosis study. Jorde, et al. tracked 25(OH)D levels using the same 25(OH)D immunometric assay for a single group of 2,668 Norwegian participants in the 1994 and 2008 Tromsø surveys and found that mean serum 25(OH)D level increased from 53.7 nmol/L to 55.3 nmol/L (p < 0.01).10 More recently, McKibben, et al. assessed 25(OH)D levels among a group of U.S. white and black participants in 1990–1992 and 1993–1994; a portion of black participants returned a median of 11 years later for a third assessment of 25(OH)D12. While the black participants had a significantly higher proportion of vitamin D deficiency compared with whites (69.6% versus 25.1% during the second visit), this number decreased to 46.6% 25(OH)D deficiency upon the 11-year follow-up testing, attributed to the sizeable increase in vitamin D supplementation among this group.
While ours is not the first longitudinal study assessing changes in 25(OH)D status across a population, it is notable for several reasons. First, the sampling process of the Dallas Heart Study intentionally incorporated a large number of minority participants (namely African American), enabling the analysis of a group of participants who have been frequently underexamined.35 Second, since the same individuals were assessed in the DHS1 and DHS2, we were able to determine predictors of change in the 25(OH)D concentration of our study population. This is in contrast to some previous longitudinal studies that have assessed different groups of people over two separate time periods.8,9 Third, the samples on which 25(OH)D was assayed were frozen at the time of collection and analyzed as a single batch after DHS2 recruitment, minimizing the assay variations that have affected other longitudinal studies.8,9 Because 25(OH)D levels were not analyzed immediately after the DHS1, the participants remained blinded to this information until the completion of the study and would therefore not intentionally have treated a subnormal 25(OH)D value. The increased prevalence of 25(OH)D supplementation in the DHS2 versus the DHS1 was likely attributable to a heightened perception of the benefits of vitamin D supplementation by individual participants and/or their health care providers. A similar significant increase in the use of vitamin D supplements was noted among US adults using data from the National Health and Nutrition Examination Survey from 1999 through 2012.36
Some limitations of this study are notable. Since recruitment incorporated an analysis of Dallas County postal addresses, this would have selected against a certain subset of the population, namely institutionalized or incarcerated participants or those without a place of residence. During the household interview, vitamin D supplementation was assessed in study participants, while sunlight exposure and dietary intake of vitamin D (e.g., dairy products, fatty fish) were not. More precise details regarding consumption of vitamin D containing foods and liquids, time spent in direct sunlight, sun tanning habits and frequency of sunblock use may have explained some of the temporal variation in 25(OH)D levels. Also, smoking habits and physical activity were assessed as qualitative variables. If, instead, they had been quantified, the relationship of these variables with changes in 25(OH)D levels could have been characterized more robustly. Finally, given the cross-sectional nature of this study, causal inferences cannot be assumed.
In conclusion, we found that 25(OH)D levels decreased by a small but significant amount in a diverse cohort of Dallas County participants from the DHS1 (2000–2002) to the DHS2 (2007–2009). In multivariable analyses, the features most predictive of a decline in 25(OH)D value include male sex, Hispanic ethnicity, and obesity. Continued evaluation of secular trends in serum 25-OH-vitamin D, the health consequences of vitamin D deficiency, its modifiable causes, and the clinical efficacy of supplementation remain of public health importance.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Maalouf NM. The noncalciotropic actions of vitamin D: recent clinical developments. Curr Opin Nephrol Hypertens. 2008;17(4):408–415. doi: 10.1097/MNH.0b013e3283040c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of clinical endocrinology and metabolism. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Archives of pediatrics & adolescent medicine. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 6.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic proceedings. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 8.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. The American journal of clinical nutrition. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142(3):498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 10.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. American journal of epidemiology. 2010;171(8):903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 11.Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(6):1381–1389. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKibben RA, Zhao D, Lutsey PL, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal followup in the ARIC study. The Journal of clinical endocrinology and metabolism. 2015:jc20151711. doi: 10.1210/jc.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroll MH, Bi C, Garber CC, et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS One. 2015;10(3):e0118108. doi: 10.1371/journal.pone.0118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Schoor NM, Knol DL, Deeg DJ, Peters FP, Heijboer AC, Lips P. Longitudinal changes and seasonal variations in serum 25-hydroxyvitamin D levels in different age groups: results of the Longitudinal Aging Study Amsterdam. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25(5):1483–1491. doi: 10.1007/s00198-014-2651-3. [DOI] [PubMed] [Google Scholar]
- 15.Skaaby T, Husemoen LL, Thuesen BH, et al. Longitudinal associations between lifestyle and vitamin D: A general population study with repeated vitamin D measurements. Endocrine. 2015:342–350. doi: 10.1007/s12020-015-0641-7. [DOI] [PubMed] [Google Scholar]
- 16.Gill TK, Hill CL, Shanahan EM, et al. Vitamin D levels in an Australian population. BMC public health. 2014;14:1001. doi: 10.1186/1471-2458-14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 18.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. The Journal of clinical endocrinology and metabolism. 1988;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 19.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. European journal of clinical nutrition. 1999;53(12):920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 20.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. The Journal of clinical investigation. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry HM, 3rd, Horowitz M, Morley JE, et al. Longitudinal changes in serum 25-hydroxyvitamin D in older people. Metabolism: clinical and experimental. 1999;48(8):1028–1032. doi: 10.1016/s0026-0495(99)90201-9. [DOI] [PubMed] [Google Scholar]
- 22.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2013;14(5):393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell JD. Seasonal variation in vitamin D. The Proceedings of the Nutrition Society. 1994;53(3):533–543. doi: 10.1079/pns19940063. [DOI] [PubMed] [Google Scholar]
- 24.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 25.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. The Journal of clinical endocrinology and metabolism. 2008;93(9):3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Hormone research. 2006;66(5):211–215. doi: 10.1159/000094932. [DOI] [PubMed] [Google Scholar]
- 28.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. The Journal of clinical endocrinology and metabolism. 2009;94(9):3306–3313. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanlint S. Vitamin D and obesity. Nutrients. 2013;5(3):949–956. doi: 10.3390/nu5030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kull M, Kallikorm R, Lember M. Body mass index determines sunbathing habits: implications on vitamin D levels. Internal medicine journal. 2009;39(4):256–258. doi: 10.1111/j.1445-5994.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 31.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. The Journal of clinical endocrinology and metabolism. 2007;92(8):3155–3157. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 32.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. The American journal of clinical nutrition. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 33.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 34.Rock CL, Emond JA, Flatt SW, et al. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity. 2012;20(11):2296–2301. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd GS, Edwards CL, Kelkar VA, et al. Recruiting intergenerational African American males for biomedical research Studies: a major research challenge. Journal of the National Medical Association. 2011;103(6):480–487. doi: 10.1016/s0027-9684(15)30361-8. [DOI] [PubMed] [Google Scholar]
- 36.Kantor EDRC, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. Journal of the American Medical Association. 2016;316(14):1464–1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.