Abstract
Most regenerative tissues employ transit-amplifying cells (TACs) that are positioned in between stem cells and differentiated progeny. In a classical hierarchical model, stem cells undergo limited divisions to produce TACs, which then proliferate rapidly to expand the system and produce diverse differentiated cell types. Although TACs are indispensable for generating tissues, they have been largely viewed as a transit point between stem cells and downstream lineages. Studies in the past few years however, have revealed some fascinating biology and unanticipated functions of TACs. In the hair follicle, recent findings have placed TACs as key players in tissue regeneration by coordinating tissue production, governing stem cell behaviors, and instructing niche remodeling. In the hematopoietic system, rather than being transient, some TACs may participate in long-term hematopoiesis under steady state. Here, we compare and summarize recent discoveries about TACs in the hair follicle and the hematopoietic system. We also discuss how TACs of these two tissues contribute to the formation of cancer.
Keywords: transit-amplifying cells, progenitors, hair follicle stem cells, hematopoietic stem cells, regeneration, niche, basal cell carcinoma, squamous cell carcinoma, cancer stem cells, leukemia
Introduction
Homeostasis, repair, and regeneration all require tissue production. In a hierarchical system, transit-amplifying cells (TACs, also referred to as the progenitor cells) are directly responsible for the bulk of tissue generation, not the stem cells. TACs are found in many tissues including the hair follicle, the hematopoietic system, the intestine, the nervous system, the corneal epithelium, and the male germline1–7. In contrast to stem cells, which are often dormant and long-lived, TACs are highly proliferative but can only do so for a short time before they undergo terminal differentiation or are eliminated. Despite the indispensable roles of TACs in tissue production, they have received surprisingly little attention and have often been viewed as a passive population whose sole role is to generate differentiated cells transiently.
Several recent findings from multiple tissue regeneration paradigms have begun to change this view. These new studies have uncovered TACs’ critical importance in regulating both stem cells and their surrounding niches1,2. New evidence also suggests that in some cases, TACs may even maintain homeostasis in the long term, arguing against their transient nature8,9. Together, these findings highlight the multifaceted functions of TACs that were not recognized previously, as well as a need to reevaluate TACs’ roles in homeostasis, regeneration, injury repair, and diseases. In this review, we summarize recent progress in TAC biology that has led to these conceptual changes, with a specific focus on the hair follicles and the hematopoietic system. Both systems are organized in a classical stem cells–TACs–differentiated cells hierarchy and are among some of the best-studied regenerating tissues that can serve as examples. We discuss the diverse functions of TACs in each system during regeneration, and compare and contrast the TACs of these two tissues. Lastly, we also discuss whether and how dysregulation of TACs contributes to the formation of skin cancers and leukemia.
Mammalian Hair Follicles
Hair follicles are one of the few tissues that undergo natural regeneration in mammals. Each hair follicle functions as an individual entity, carrying its own stem cells that generate its TACs and differentiated cells at defined positions. Moreover, the stem cells and TACs display stereotypic behaviors during a hair regeneration cycle (see below). These spatially and temporally defined features, together with advances in genetic and imaging tools have allowed precise delineations of cell behavioral changes, cell-cell interactions, and molecular alterations in each population during different phases of regeneration. As such, the hair follicle has become one of the most instrumental paradigms to study the principles of regeneration in mammals.
Hair follicles cycle between destruction (catagen), rest (telogen), and regrowth (anagen). Telogen hair follicles are composed of the bulge and a small cluster of cells beneath the bulge, known as the hair germ. Hair follicle stem cells (HF-SCs) are located in the hair germ and the outer layer of the bulge. TACs of the HFs (HF-TACs), also known as the matrix, are an anagen-specific population: upon anagen entry, HF-TACs are produced by stem cells located in the hair germ, which are a primed stem cell population more sensitive to activation cues2,10,11. HF-TACs are located at the bottom of the growing hair follicles and are in contact with a mesenchymal structure known as the dermal papilla (DP)12,13. Throughout anagen, hair follicles grow downward, while HF-TACs undergo rapid and continuous proliferation to generate the hairs that protrude out from the skin surface. Anagen is the only phase when hairs grow. During catagen, HF-TACs are destroyed, and hair follicles are remodeled back to their telogen morphology. The hairs are then held tightly in place without growth by an inner Keratin 6+ bulge layer, which has lost stem cell potential but maintains the stem cells located at the outer bulge layer in quiescence14 (Figure 1).
Figure 1. The hair cycle and hair follicle structures.
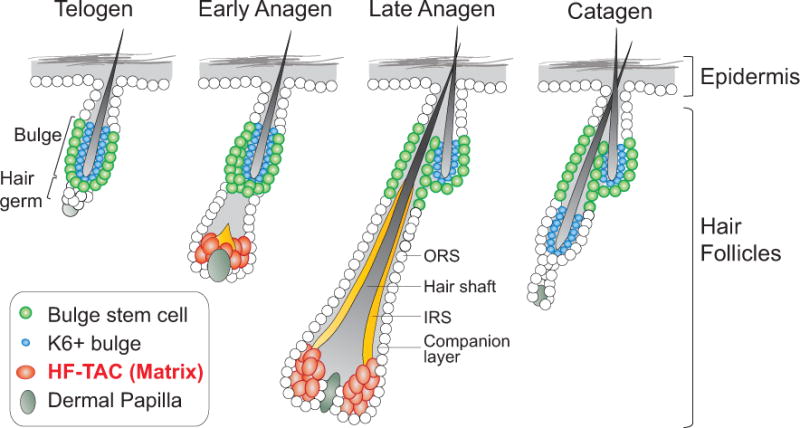
The hair follicle cycles between a regenerative phase (anagen), a destruction phase (catagen), and a resting phase (telogen). Telogen hair follicles contain stem cells located in the outer bulge layer and the hair germ. Proliferation of the hair germ generates the matrix in anagen, which are transit-amplifying cells of the hair follicles (HF-TACs). Proliferation of the bulge generates the outer root sheath (ORS) that wraps around the anagen hair follicles. Differentiated cells, including the hair shaft, the inner root sheath (IRS), and the companion layer, are produced by the HF-TACs. During catagen, HF-TACs are destroyed. The remaining ORS cells form a new bulge responsible for the next round of the hair cycle.
Generation of diverse progeny
In anagen, HF-TACs proliferate to generate seven morphologically and molecularly distinct downstream cell types: the companion layer that separates the outer root sheath from the inner root sheath (IRS), three layers of IRS (the Henle’s layer, the Huxley’s layer, and the IRS cuticle) that form a channel to guide the growing hair, and three layers of hair shaft (hair shaft cuticle, the cortical layer, and the medulla in the center) that erupt from the skin surface (the organization and molecular markers for each layer are shown in Figure 2). How HF-TACs generate these diverse cell types provides an intriguing model to study cell fate determination. Lineage-tracing studies through labeling HF-TACs at a clonal density reveal that HF-TACs are composed of heterogeneous progenitors that preferentially give rise to one or only a few out of the seven cell types, suggesting that many HF-TACs are lineage-biased15,16.
Figure 2. Marker gene expression in distinct layers of the anagen and telogen hair follicles.
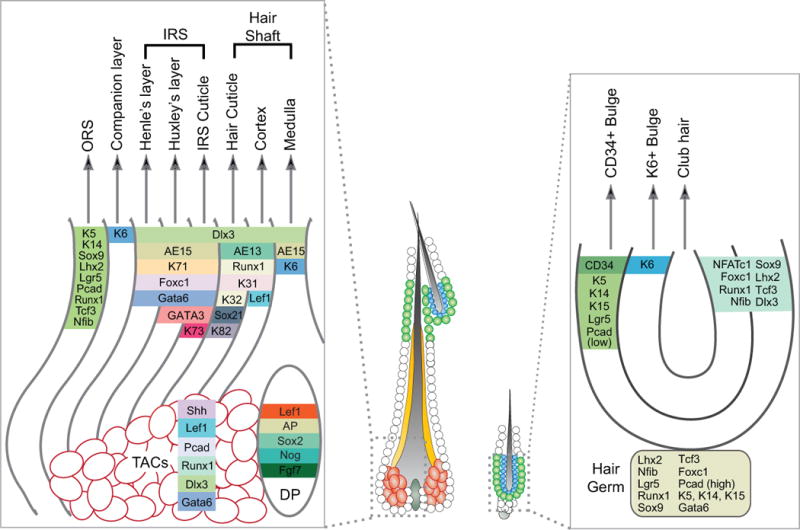
Antibodies or gene markers are shown that are commonly used to distinguish CD34+ bulge, K6+ bulge, transit-amplifying cells (TACs), outer root sheath (ORS), companion layer, inner root sheath (IRS, including Henle’s layer, Huxley’s layer, and the IRS cuticle), hair shaft (including Hair cuticle, Cortex, and Medulla), and dermal papilla (DP). AP: alkaline phosphatase; Pcad: P-Cadherin; Nog: Noggin.
Several transcription factors have been shown to regulate lineage outcomes. Therefore, mice carrying mutations in these transcription factors often appear hairless. For example, loss of Gata3 impairs IRS fate while expanding the hair shaft progenitor pool17,18. By contrast, mutations in Hoxc13, Msx2, Sox21, or Foxn1 (also known as the “nude” gene) lead to severe defects in the hair shaft lineages19–23. Dlx3 is broadly expressed in HF-TACs, IRS, and hair shaft, and Dlx3 mutant displays defects in all of these lineages24. The BMP pathway has been shown to influence these lineage choices. Loss of BMP signaling expands the IRS progenitors at the expense of hair shaft progenitors25–27.
Interestingly, BMP signaling also acts on HF-SCs, but its function is to maintain their quiescence without changing the stem cells’ un-differentiated state28–30. ChIP-seq studies suggest that pSmad1,5,8 (canonical transcriptional factors downstream of BMP) bind to largely distinct targets in HF-SCs and HF-TACs, which may in part explain the distinct functions of the BMP pathway in these two cell types25. What entails pSmads to bind to different target sites within HF-SCs and HF-TACs is currently unknown but likely involves rapid changes of the chromatin environment when HF-TACs are produced from HF-SCs and a different accessibility of the same target sites in these two populations31. It will also be interesting to determine whether cofactors that enable pSmad1,5,8 to bind to a subset of targets may exist in one population but not in another. In this sense, hair follicles provide a valuable model to investigate how closely related SCs and TACs use the same signaling pathways differently to fulfill their distinct roles during regeneration.
Proliferation and destruction of HF-TACs
HF-TACs are one of the most proliferative cell types in adults. A variety of signaling pathways and epigenetic components are involved in the regulation of their proliferation. Sonic Hedgehog (SHH), secreted by the HF-TACs, promotes HF-TAC proliferation through both an autocrine and a paracrine fashion: in addition to directly acting on HF-TACs, SHH signals to DP and enhances the expression of Fgf7 and Noggin (a BMP inhibitor) in DP. These factors together stimulate HF-TACs to proliferate throughout anagen2. In addition to SHH signaling, Wnt signaling has been shown to maintain DP’s potency in stimulating HF-TAC proliferation: knocking out Ctnnb1 (the gene encodes β-Catenin) from DP causes reduction of HF-TAC proliferation32. One potential source of Wnts may be the hair follicle itself, since knocking-out Wntless (a gene required in Wnt-secreting cells) from the hair follicle reduces hair follicle proliferation33. Epigenetic regulators such as components of the PRC2 complex Ezh1, Ezh2, and Eed, also play a critical role in maintaining HF-TAC proliferation by directly repressing cell cycle inhibitors34,35. Lastly, transcriptome analysis has been conducted on multiple skin populations purified by Fluorescence-activated cell sorting (FACS), including HF-TACs and DP36. This study provides a rich resource for uncovering both intrinsic and extrinsic regulation of HF-TACs in the future.
Maintaining genome integrity in these highly proliferative HF-TACs can be a confounding task because of replication stress. Indeed, when Brca1, an important regulator of DNA damage response is deleted, HF-TACs display the most profound defects among all cells in the epithelial compartment, leading to elevated DNA damage and p53-dependent apoptosis of HF-TACs37. A transcription factor Gata-6 is expressed in the HF-TACs, and is shown to protect the HF-TACs from replication-associated DNA damage38.
HF-TACs are the first population destroyed upon catagen entry. The mechanism of catagen initiation remains largely elusive. However, several mutants have shed light on potential regulators of this interesting process: mice lacking Fgf5 have delayed entry into catagen, while mice lacking a serine-threonine kinase SGK3 (Serum/Glucocorticoid Regulated Kinase Family Member 3) enter catagen precociously39,40. Signals from DP again play an important role in catagen regulation. Inhibition of Wnt signaling by deleting Ctnnb1 from DP or overexpression of Dkk1, a secreted Wnt inhibitor, induces premature catagen entry32,41. On the other hand, eliminating DP through two-photon laser-mediated cell ablation during catagen leads to significantly retarded catagen progression and reduced apoptosis42. It will be intriguing to determine how these signaling pathways and genes are regulated in a precise temporal manner to initiate catagen.
Sending feedback to stem cells
HF-SCs can be separated into two populations: one located in the bulge and another located in the hair germ. In response to proliferation cues secreted from DP, hair germ is the first population to proliferate since the hair germ is closer to DP compared to the bulge10,11. Bulge stem cells (Bu-SCs) remain quiescent until HF-TACs are produced by the hair germ. This delay in Bu-SC activation is mediated by the sensitivity of Bu-SCs to SHH secreted from the HF-TACs. SHH acts directly on Bu-SCs to promote their proliferation (Figure 3). With the progression from early to late anagen, HF-TACs migrate away from Bu-SCs concomitant with hair follicle downgrowth. At late anagen, the distance between the HF-TACs and Bu-SCs has increased beyond the signaling range of SHH, allowing Bu-SCs to resume quiescence2. These findings may explain why Bu-SC activation always occurs in a transient but precise window during anagen.
Figure 3. Hair follicle’s transit-amplifying cells (HF-TACs) regulate both the hair follicle stem cells and the niche.
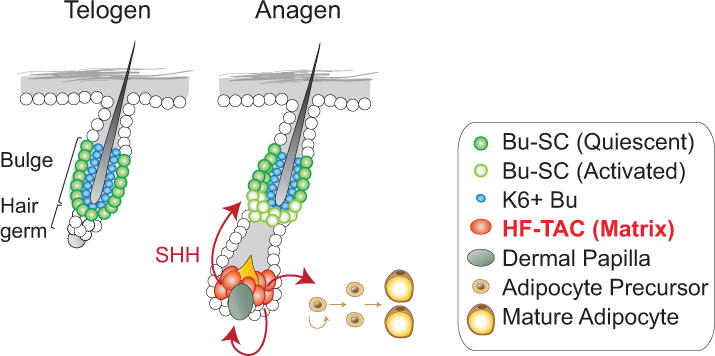
HF-TACs secrete Sonic Hedgehog (SHH) to activate the hair follicle stem cells located in the bulge (Bu-SC). SHH also enhances the expression of Noggin and Fgf7 in the dermal papilla. All three factors together promote HF-TACs’ own proliferation. Lastly, SHH from HF-TACs also promotes adipocyte formation from adipocyte precursors in the dermal niche.
What happens if Bu-SCs cannot be activated? While hair follicle regeneration operates normally in the short term, inhibition of Bu-SC proliferation leads to defects in renewing stem cells in both the bulge and the hair germ, causing regeneration failure in the long term2. Collectively, these findings point toward a novel function of TACs—they send feedback signals to the stem cells to orchestrate stem cell behaviors and promote stem cell self-renewal.
Shaping the surroundings
Recently, HF-TACs have also been shown to regulate the production of surrounding tissues in addition to their own downstream lineages: When hair follicles grow downward rapidly during anagen, they impose a need for the surrounding tissues to expand concurrently. Findings from our group demonstrate that HF-TACs, by secreting SHH, regulate dermal adipogenesis by directly acting on adipocyte precursors1 (Figure 3). This discovery establishes HF-TACs’ key importance in mediating accommodative changes tailored to the need of regenerating hair follicles. SHH secreted from the HF-TACs is also required for the formation of Merkel cells, specialized epidermal cells for touch sensation43,44. The coordination of changes of the surroundings to ensure the newly regenerated tissues are properly supported and integrated is likely a common demand during tissue regeneration. Due to the role of TACs in tissue production, they are well situated to couple tissue production with accommodative changes of the surroundings. In this sense, regulating the niche may be a common function shared among TACs of different tissues.
The Hematopoietic System
The hematopoietic system has a high turnover rate. The number of blood cells produced daily to meet our body’s regular demands exceeds 100 billion cells45. Unlike solid tissues, where stem cells and progeny are often located in fixed positions and form defined structures, the hematopoietic system is highly dynamic—hematopoietic stem cells (HSCs) and immature progeny populations are mostly located in the bone marrow, while differentiated progeny circulate around the entire body through the bloodstream46,47. Bone marrow transplantation has been a standard procedure to treat patients with leukemia since 1970s. The patients’ own hematopoietic system is first eliminated by irradiation or chemotherapy drugs, and then donor bone marrow tissues containing HSCs are transplanted into patients to reconstitute the whole hematopoietic system. This remarkable procedure exemplifies the power of somatic stem cells in treating diseases48,49. Because of this historical reason, together with a lack of ideal tools to track cells in circulation until recently, most of what we know about HSCs and their progeny today is derived from transplantation and reconstitution studies.
Through transplantation assays, an elaborate hierarchical relationship has been established for HSCs and their downstream progeny: HSCs divide infrequently to generate multipotent progenitors (MPPs) that cycle more rapidly to expand the system and generate lineage-restricted common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs). CMPs and CLPs further produce downstream cell types of the myeloid and lymphoid lineages3,50–53.
Over the years, refinement of FACS strategies using additional cell surface markers has uncovered interesting heterogeneity in both the HSC and the MPP populations50,54–56. Functional assays have revealed that only a small fraction among the originally enriched HSC population has the ability to sustain long-term, multi-lineage reconstitution upon transplantation. This special subset of HSCs is sometimes referred to as the long-term HSCs (LT-HSCs). LT-HSCs are also particularly dormant in nature57. Other cells defined by the original “HSC” or “MPP” surface markers behave as TACs upon transplantation— they act downstream of LT-HSCs to amplify the system through proliferation and generate downstream cells of both myeloid and lymphoid lineages but can only do so for the short term58.
Since different research groups adopt slightly different sorting strategies and nomenclatures, we will use “HSCs” to refer to only “LT-HSCs” hereafter to facilitate the conceptual discussion for a broad readership and to unite the terminology used in this review. In addition, we will use “MPPs” to indicate all the other populations that display different degrees of multipotency and short-term reconstitution abilities upon transplantation.
MPPs contribute to steady-state hematopoiesis in the long term
HSCs are efficient in sustaining long-term, multi-lineage reconstitution upon transplantation. However, under steady-state hematopoiesis, HSCs remain mostly dormant and are estimated to divide about 5 times within the lifetime of a mouse57,59,60. This extreme quiescent nature raises the question of whether HSCs participate in normal hematopoiesis. Recent advances in lineage-tracing tools have made it possible to study hematopoiesis under physiological conditions without transplantation, and have revealed some unexpected surprises. Two studies using independent strategies— one relies on a barcoding approach and the other uses an inducible Cre line expressed preferentially in HSCs to drive a Rosa-reporter— reveal that steady-state hematopoiesis in adult mice is sustained by large numbers of diverse MPP clones, with minimum input from HSCs8,9. In addition, under steady state, MPPs display self-renewal features on the time scale from months to a lifetime of the animals, arguing against their transient nature8,9. Interestingly, a third study using a different CreER line (Pdzk1ip1-CreER) that preferentially labels HSCs led to a different conclusion: the Pdzk1ip1-labled HSCs seem to be the major contributor of downstream progeny at steady state61. The exact reasons underlying these different conclusions remain to be clarified. It is likely that HSCs are heterogeneous, and different methodologies preferentially label distinct subsets of HSCs. In light of this possibility, a recent study using multicolor-based clonal analysis demonstrates that individual HSCs display diverse proliferation capacities and lineage contributions under steady state. Despite the heterogeneity among HSCs, the behavior of HSCs originated from the same clone is fixed even upon transplantation62.
Functional studies also suggest that MPPs are able to sustain long-term hematopoiesis in mice under steady state: depletion of HSCs through diphtheria toxin–mediated HSC ablation results in no change in hematopoiesis for more than a year. The residual HSCs in these animals also remain quiescent and display no signs of compensatory proliferation. Interestingly, depletion of differentiated cell types including platelets and erythrocytes is well tolerated upon HSC ablation, consistent with the notion that HSCs contribute minimally to these differentiated cells. By contrast, when these HSC-depleted animals are subjected to Fluorouracil (5-FU), which eliminates rapidly proliferating cells, including most of the MPPs, hematopoiesis is severely compromised63. Consistent with these findings, mice carrying a deletion in a gene named monocytic leukemia zinc finger gene (Moz) lose HSCs, but do not display noticeable defects in endogenous hematopoiesis for 1.5 years64. Collectively, these findings reveal the differential requirements of HSCs and MPPs in transplantation-induced versus steady-state hematopoiesis.
Molecular and functional heterogeneity among MPPs
Although all MPPs are capable of generating cells of both the myeloid and the lymphoid lineages to some degree, some researchers have further divided MPPs into MPP1–MPP4, characterized by increasing proliferation rate but decreasing self-renewal potential coupled with more pronounced lineage bias65,66 (Figure 4). How MPPs produce diverse downstream cell types of the blood system remains poorly understood. However, recent advances in profiling approaches combined with functional reconstitution assays have provided some clues65–69. MPP1 behaves most similarly to HSCs upon transplantation and is therefore sometimes referred to as “short-term HSCs” since they also display multilineage reconstitutions as well as self-renewal ability, albeit only for a short term. By contrast, MPP3 shows strong myeloid bias and MPP4 shows strong lymphoid bias upon transplantation56,65,66. Individual MPP subsets express genes indicative of their distinct lineage priming, suggesting that different MPP populations are intrinsically primed to differentiate towards specific lineages even under steady-state. Interestingly, a cytokine Interleukin 6 (IL6) acts on MPP4 to skew their lymphoid bias towards myeloid differentiation70, suggesting that the lineage-priming state of MPPs is not fixed. Together, these analyses of distinct MPP subsets provide a framework for future identification of molecular mechanisms controlling MPP differentiation.
Figure 4. FACS strategies for purification of hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs) and functional differences among different subtypes of MPPs.
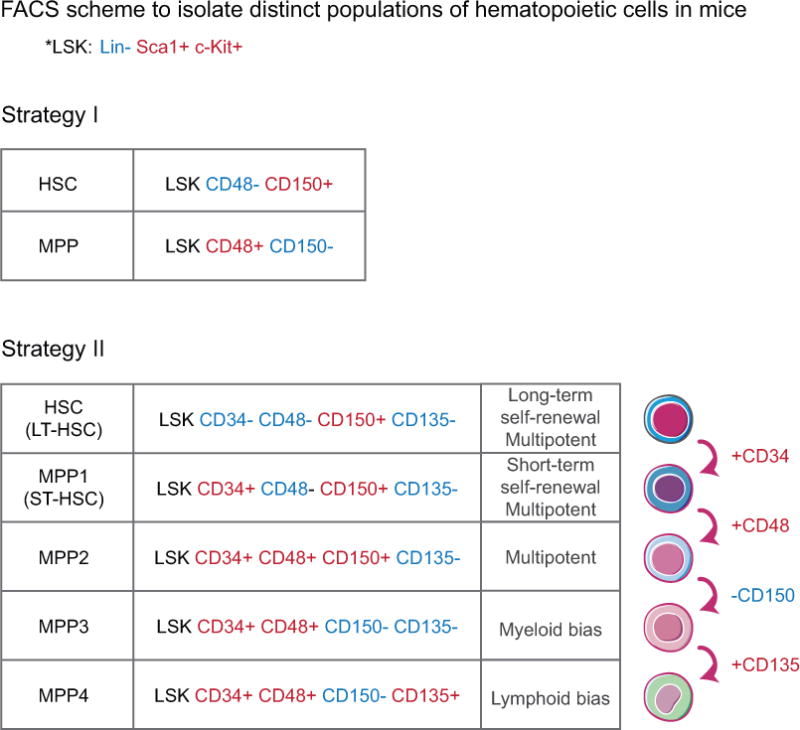
Cell surface markers used to purify HSCs and MPPs. Different strategies have been employed to enrich HSCs and MPPs. Two common strategies are listed here: In strategy I, Lineage-negative (Lin−) cells are bone marrow cells that do not express mature hematopoietic cell lineage markers. MPPs are purified as cells within the LSK (Lin−, Sca1+, cKit+) cells that do not display long-term lineage reconstitution ability upon transplantation. In strategy II, distinct MPP subsets are further separated by additional surface markers. MPP1–MPP4 display different lineage biases upon transplantations.
Intrinsic vs. extrinsic factors regulating the distinct potential of HSCs and MPPs
Although incompletely understood, HSC’s ability to sustain long-term, multilineage reconstitution seems to be intimately linked to their proliferation history. A recent study suggests that after four divisions, HSCs have lost their long-term reconstitution potential and behave like MPPs upon transplantation60. In line with this finding, extrinsic niche factors such as interleukin-18 and angiogenin that enforce HSC quiescence often enhance their regenerative potential in transplantation71–73. It will be intriguing to determine the molecular mechanisms by which the division history of an HSC controls its long-term reconstitution ability.
The tight coupling of division history and HSC’s functionality also implies that MPPs could be HSCs that have gone through more than 4 rounds of division. Supporting this notion, genomic and proteomic analyses have revealed that HSCs and MPP1 are not substantially different: only 47 proteins and about 500 transcripts are differentially expressed between HSCs and MPP1, and more than half are related to cell cycle regulation which is consistent with the proliferation differences between the two. Increase in cell cycle activity in MPPs is accompanied by up-regulation of DNA repair machinery in MPPs compared to HSCs65,66. It is possible that DNA replication in proliferating MPPs creates a need to enhance DNA repair and maintain genome integrity. It will be intriguing to determine whether MPPs may be more sensitive to the loss of DNA repair proteins such as Brca1, a situation analogous to what was observed in the hair follicle.
When Things Go Awry: Do TACs Contribute to Cancer?
In the past decade, advances in tissue biology have also brought new insights into how cancer forms and propagates. We now learn that mutations alone are not sufficient: the same oncogenic mutations occurring in different cells often lead to distinct outcomes, and not all cells can be transformed to form tumors. Defining the cellular origins of cancers reveals how cancer arises in the first place and informs treatment designs. Can TACs be the origin of tumors? Here, the hair follicle and the hematopoietic system have some interesting differences.
HF-TACs cannot be transformed by oncogenic mutations
Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC) are common skin cancers and are two of the most prevalent cancers in the world. In the United States alone, more than 5 million cases are diagnosed each year, which is higher than the incidence of breast, prostate, lung, and colon cancer combined74. Both BCCs and SCCs appear mostly in sun-exposed areas. BCC is caused by mutations that lead to elevation of the SHH pathway, while mutations that cause oncogenic transformation of Ras together with loss-of-function in the tumor suppressor p53 are commonly found in SCC75–80. Manipulations of these deleterious mutations using cell-type specific inducible Cre lines offer a direct comparison of tumorigenic potential among different populations within the skin, including the hair follicle stem cells and HF-TACs. The results suggest that while epidermal cells and hair follicle stem cells can be readily transformed to form BCC or SCC upon introduction of tumorigenic mutations, HF-TACs are resistant. In fact, tumorigenic mutations that specifically occur in HF-TACs are well tolerated, with minimum perturbations of hair follicle growth, morphology, or hair cycle81–84.
To counterbalance TACs’ highly proliferative potential, it is possible that some TACs might carry a built-in protective mechanism to prevent them from transforming, while stem cells lack this mechanism and are more vulnerable. For example, committed progenitors of the epidermis (which behave like TACs) are shown to elevate p53-mediated cell death upon abnormal elevation of the SHH pathway, lowering their tendency to generate BCC85. It will be interesting to determine whether HF-TACs employ a similar mechanism to inhibit transformation.
While HF-TACs cannot initiate tumors in these animal models, they may facilitate tumor formation through their regulatory roles on the HF-SCs or the microenvironment: the skin is significantly more susceptible to SCC transformation in the anagen stage than in the telogen stage, and quiescent HFSCs are particularly refractory to tumorigenic mutations for SCCs86. Since HF-TACs are anagen specific and central to many changes in the anagen skin, it will be important to determine if specific signals and tissue remodeling orchestrated by HF-TACs facilitate tumor formation even though they are not tumorigenic per se.
Blood-TACs are an important cellular source for AML
In contrast to the skin system, MPPs can be transformed to form acute myeloid lymphoma (AML), an aggressive form of blood cancer characterized by rapid expansion of abnormal white blood cells that fill up the bone marrow. AML contains many morphologically and molecularly distinct subtypes. The cause of AML is also complex, but chromosomal translocations of the MLL (mixed-lineage leukemia) gene are commonly involved87,88. Mouse models mimicking these translocations recapitulate the pathological hallmarks of AML and have been instrumental in discovering disease mechanisms89.
AML is the model in which cancer stem cells were first established. In 1994, John Dick’s group demonstrated that a small population of leukemic cells isolated from AML patients was able to initiate and propagate tumors when transplanted into nude mice. This special group of leukemia cells is named “leukemia stem cells (LSCs)”, since they reside at the apex of a hierarchy, retain remarkable self-renewal ability, and generate a whole spectrum of downstream leukemia cells upon transplantation similar to HSCs90,91. Since these LSCs express surface markers similar to those used in enriching human HSCs, it was postulated that LSCs originate from HSCs.
Later studies using various mouse models of AMLs, however, led to a different conclusion. Since the entire hematopoietic hierarchy and isolation strategy is clearly defined in mice, mouse models offer opportunities to isolate and test each population’s ability to form tumors to a precision that is difficult to achieve using human samples. These studies suggest that although with various efficiencies, MPPs or even downstream myeloid-restricted progenitors can all be transformed to become LSCs and propagate leukemia upon transplantation89,92–95. HSCs and MPPs in general display the highest transformation efficiency compared to lineage-restricted progenitors96. However, since normal MPPs or lineage-restricted progenitors do not have the ability to reconstitute the blood system, their ability to initiate and propagate tumors upon serial transplantations is a newly acquired property driven by oncogenic transformations. Consistent with this change, genome-wide profiling approaches also suggest that upon oncogenic transformation, several self-renewal genes normally only expressed in HSCs, including Bmi1, Sox4, Hoxa9, and Meis1, are now up-regulated in MPPs and myeloid progenitors94,97,98. These changes in gene expression might enable these transit-amplifying populations to acquire long-term self-renewal potential. Alternatively, it is also possible that HSCs and LSCs rely on distinct self-renewal mechanisms, as their different sensitivities to PTEN-dependent self-renewal have been demonstrated99. To determine which possibility is correct, genes shared by HSCs and LSCs as well as genes unique to LSCs will have to be functionally tested for their requirement in mediating the transformation of MPPs to LSCs.
A revisit of cells collected from human AML patients further substantiates the idea that transit-amplifying population of the blood can be transformed to form a tumor. Using large number of primary AML samples from patients paired with newly developed surface markers, Goardon et al. identified two predominant populations of LSCs that are most similar to two progenitor populations rather than HSCs: One is the granulocyte-monocyte progenitors (GMPs). The other is a lymphoid-biased MPP population (functionally similar to MPP4 in mice). These discoveries suggest that MPPs and GMPs might actually be the cells of origins of most AML100.
Currently, studies of LSCs still heavily rely on transplantation approaches, which are defined functional tests for LSCs. Although informative, the transplantation process selects and amplifies the best-fit clones, which may not recapitulate all aspects of AML. The recently developed bar-coding approach paired with appropriate inducible models should provide an unparalleled opportunity to identify the cell of origin for AML without the need of transplantation8. In addition, this approach provides a possibility to monitor the transformation processes over time in the same animal. It is tempting to speculate that since MPPs have long-term self-renewal ability while HSCs are mostly dormant, MPPs might be particularly vulnerable to tumor initiation under endogenous conditions.
Conclusions and Perspectives
In this review, we provide an overview illustrating the diverse roles TACs play in tissue regeneration, turnover, and tumor formation (Figure 5). In the hair follicle, HF-TACs not only generate the differentiated structures of the hairs, but also remodel the niche and promote stem cell renewal. Together, these actions not only allow successful tissue production in an optimum environment in the short term but also ensure that the system can operate in the long term. In the blood, TACs are responsible for generating and sustaining hematopoiesis under steady state in the long term, with minimum contributions from HSCs. In this sense, HSCs are a rare and special reservoir reserved for catastrophic insults, such as transplantation into an irradiated host, where the system is forced to start anew.
Figure 5. Comparison between the hair follicle system and the hematopoietic system.
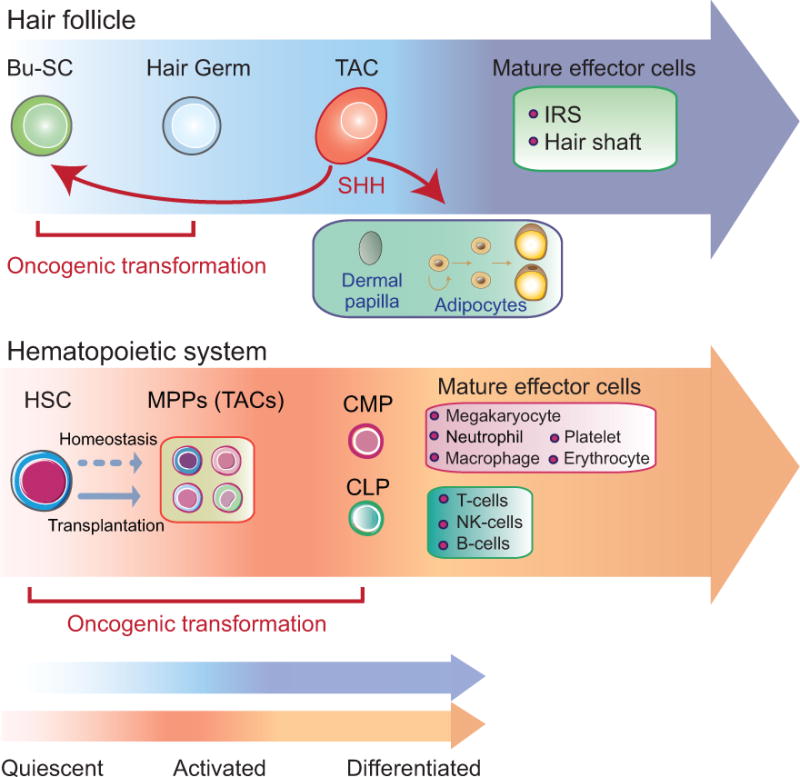
The diagram summarizes the lineage hierarchy in the hair follicle system and the hematopoietic system. In the hair follicle, bulge stem cells (Bu-SCs) are more quiescent than the hair germ. Transit-amplifying cells (TACs) are produced by the hair germ. Through Sonic Hedgehog (SHH), TACs send feedback signals to the Bu-SCs and at the same time promote critical changes of the surrounding tissues including the dermal papilla and adipocytes. Hematopoietic stem cells (HSCs) generate Multipotent progenitors (MPPs) upon transplantation. MPPs further produce Common myeloid progenitors (CMPs) and Common lymphoid progenitors (CLPs) to generate downstream effector cells of the lymphoid or myeloid lineages, albeit in the short term. During homeostasis, HSCs are mostly quiescent and MPPs display long-term multilineage contributions. TACs of the hair follicles cannot be transformed by oncogenic mutations. However, MPPs and their committed progenitors downstream can be transformed readily to initiate leukemia.
In the hair follicle, TACs partake in a wide range of regulatory roles. The advantages of employing a TAC-governed regulatory circuitry are multiple: First, communication between TACs and stem cells might allow a means for direct feedback regulation. Second, communication between TACs and the neighboring cell types allows tissue production to occur in a favorable environment and might also facilitate the integration of newly regenerated tissues with the rest. It will be interesting to investigate whether TACs in other systems might share a similar regulatory role.
The field of regenerative biology has been “stem cell-centric”. With the examples presented in this review, one can begin to appreciate the importance of TACs and their diverse functions that go beyond producing tissues. Defects in regeneration or injury repair are also likely to be caused by problems in TACs, not just in stem cells. In addition, TACs’ impact on both the stem cells and the niche may represent an effective therapeutic entry point for halting or reversing degenerative diseases. The transient nature of TACs in many tissues also makes them a safer and more ideal target than stem cells for manipulation, particularly when transient rather than permanent changes in tissue production are desired (such as wound healing or during infection). Empowering tissue biology for regenerative medicine relies on effective manipulations of tissues tailored to various physiological and pathological conditions. In this sense, understanding the biology of TACs is timely and important not only for elucidating the fundamental principles of tissue development and regeneration, but also for expanding our current toolbox for disease treatments.
Acknowledgments
We thank David Scadden, Fernando Camargo, Yick W. Fong, Lev Silberstein, Eva Fast and members of the Hsu laboratory in particular Meryem Gonzalez-Celeiro, Pai-Chi Tsai, and Yulia Shwartz for discussions and critical feedback of the manuscript. This work was supported by grants from the NIH/NIAMS (R00-AR063127, R01-AR070825), the Smith Family Awards Program, Basil O’Connor Starter Scholar award, and the HSCI seed grant.
References
- 1.Zhang B, et al. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev. 2016 doi: 10.1101/gad.285429.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 4.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 5.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 6.Lavker RM, Sun TT. Epithelial stem cells: the eye provides a vision. Eye. 2003;17:937–942. doi: 10.1038/sj.eye.6700575. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty P, et al. LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. STEM CELLS. 2014;32:860–873. doi: 10.1002/stem.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch K, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 10.Greco V, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4:a015180. doi: 10.1101/cshperspect.a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sennett R, Rendl M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legué E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- 16.Sequeira I, Nicolas JF. Redefining the structure of the hair follicle by 3D clonal analysis. Development. 2012;139:3741–3751. doi: 10.1242/dev.081091. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman CK, et al. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003;17:2108–2122. doi: 10.1101/gad.1115203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurek D, Garinis GA, Doorninck JH, Wees van, van der J, Grosveld FG. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134:261–272. doi: 10.1242/dev.02721. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, et al. Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter CS, et al. The nude mutant gene Foxn1 is a HOXC13 regulatory target during hair follicle and nail differentiation. J Invest Dermatol. 2011;131:828–837. doi: 10.1038/jid.2010.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns SA, Soullier S, Rashbass P, Cunliffe VT. Foxn1 is required for tissue assembly and desmosomal cadherin expression in the hair shaft. Dev Dyn Off Publ Am Assoc Anat. 2005;232:1062–1068. doi: 10.1002/dvdy.20278. [DOI] [PubMed] [Google Scholar]
- 22.Kiso M, et al. The disruption of Sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proc Natl Acad Sci. 2009;106:9292–9297. doi: 10.1073/pnas.0808324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ar G, Mr C. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang J, Mehrani T, Millar SE, Morasso MI. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135:3149–3159. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genander M, et al. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15:619–633. doi: 10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulessa H, Turk G, Hogan BLM. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. STEM CELLS. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 29.Kobielak K, Stokes N, Cruz J, de la Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lien WH, et al. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–232. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31–41. doi: 10.1038/jid.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezhkova E, et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dauber KL, et al. Dissecting the roles of polycomb repressive complex 2 subunits in the control of skin development. J Invest Dermatol. 2016;136:1647–1655. doi: 10.1016/j.jid.2016.02.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezza A, et al. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep. 2016;14:3001–3018. doi: 10.1016/j.celrep.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sotiropoulou PA, et al. BRCA1 deficiency in skin epidermis leads to selective loss of hair follicle stem cells and their progeny. Genes Dev. 2013;27:39–51. doi: 10.1101/gad.206573.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang AB, Zhang YV, Tumbar T. Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J. 2017;36:61–78. doi: 10.15252/embj.201694572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hébert JM, Rosenquist T, Götz J, Martin GR. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 40.Alonso L, et al. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170:559–570. doi: 10.1083/jcb.200504131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi YS, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13:720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesa KR, et al. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perdigoto CN, et al. Polycomb-mediated repression and sonic hedgehog signaling interact to regulate merkel cell specification during skin development. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao Y, et al. A cascade of Wnt, Eda, and Shh signaling is essential for touch dome merkel cell development. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon MY, Lewis JL, Marley SB. Of mice and men … and elephants. Blood. 2002;100:4679–4679. doi: 10.1182/blood-2002-08-2517. [DOI] [PubMed] [Google Scholar]
- 46.Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31 Has our knowledge matured? Ann N Y Acad Sci. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Little MT, Storb R. History of haematopoietic stem-cell transplantation. Nat Rev Cancer. 2002;2:231–238. doi: 10.1038/nrc748. [DOI] [PubMed] [Google Scholar]
- 49.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 50.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 51.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 52.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 53.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegué E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 54.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 55.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 58.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foudi A, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernitz JM, Kim HS, MacArthur B, Sieburg H, Moore K. Hematopoietic stem cells count and remember self-renewal divisions. Cell. 2016;167:1296–1309.e10. doi: 10.1016/j.cell.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawai CM, et al. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu VWC, et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell. 2016;167:1310–1322.e17. doi: 10.1016/j.cell.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 63.Schoedel KB, et al. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood. 2016;128:2285–2296. doi: 10.1182/blood-2016-03-706010. [DOI] [PubMed] [Google Scholar]
- 64.Sheikh BN, et al. MOZ (KAT6A) is essential for the maintenance of classically defined adult hematopoietic stem cells. Blood. 2016;128:2307–2318. doi: 10.1182/blood-2015-10-676072. [DOI] [PubMed] [Google Scholar]
- 65.Cabezas-Wallscheid N, et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and dna methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Pietras EM, et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gazit R, et al. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Rep. 2013;1:266–280. doi: 10.1016/j.stemcr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kowalczyk MS, et al. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 2015;25:1860–1872. doi: 10.1101/gr.192237.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo M, et al. Long non-coding RNAs control hematopoietic stem cell function. Cell Stem Cell. 2015;16:426–438. doi: 10.1016/j.stem.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynaud D, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silberstein L, et al. Proximity-based single cell analysis of the bone marrow niche identifies interleukin-18 as a quiescence regulator of early hematopoietic progenitors. Blood. 2014;124:773–773. [Google Scholar]
- 72.Goncalves KA, et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell. 2016;166:894–906. doi: 10.1016/j.cell.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silberstein L, et al. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell. 2016;19:530–543. doi: 10.1016/j.stem.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 75.Dahmane N, Lee J, Robins P, Heller P, Altaba A. R.i. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 76.van der Schroeff JG, Evers LM, Boot AJM, Bos JL. Ras oncogene mutations in basal cell carcinomas and squamous cell carcinomas of human skin. J Invest Dermatol. 1990;94:423–425. doi: 10.1111/1523-1747.ep12874504. [DOI] [PubMed] [Google Scholar]
- 77.Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 78.Wakabayashi Y, Mao JH, Brown K, Girardi M, Balmain A. Promotion of Hras-induced squamous carcinomas by a polymorphic variant of the Patched gene in FVB mice. Nature. 2007;445:761–765. doi: 10.1038/nature05489. [DOI] [PubMed] [Google Scholar]
- 79.Pierceall WE, Mukhopadhyay T, Goldberg LH, Ananthaswamy HN. Mutations in the p53 tumor suppressor gene in human cutaneous squamous cell carcinomas. Mol Carcinog. 1991;4:445–449. doi: 10.1002/mc.2940040606. [DOI] [PubMed] [Google Scholar]
- 80.Ziegler A, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 81.Youssef KK, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 82.Wang GY, Wang J, Mancianti ML, Epstein EH. Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/−) mice. Cancer Cell. 2011;19:114–124. doi: 10.1016/j.ccr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lapouge G, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White AC, et al. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci. 2011;108:7425–7430. doi: 10.1073/pnas.1012670108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sánchez-Danés A, et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature. 2016;536:298–303. doi: 10.1038/nature19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White A, et al. Stem cell quiescence acts as a tumor suppressor in squamous tumors. Nat Cell Biol. 2014;16:99–107. doi: 10.1038/ncb2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox MC, et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation. Am J Clin Pathol. 2004;122:298–306. doi: 10.1309/RX27-R8GJ-QM33-0C22. [DOI] [PubMed] [Google Scholar]
- 88.Satake N, et al. Chromosome abnormalities and MLL rearrangements in acute myeloid leukemia of infants. Leukemia. 1999;13:1013–1017. doi: 10.1038/sj.leu.2401439. [DOI] [PubMed] [Google Scholar]
- 89.Lavau C, Luo RT, Du C, Thirman MJ. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 91.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 92.Cozzio A, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.So CW, et al. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3:161–171. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 94.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 95.Somervaille TCP, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 96.George J, et al. Leukaemia cell of origin identified by chromatin landscape of bulk tumour cells. Nat Commun. 2016;7:12166. doi: 10.1038/ncomms12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, et al. The wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kvinlaug BT, et al. Common and overlapping oncogenic pathways contribute to the evolution of acute myeloid leukemias. Cancer Res. 2011;71:4117–4129. doi: 10.1158/0008-5472.CAN-11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yilmaz ÖH, et al., editors. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 100.Goardon N, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19:138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]


