1. Introduction
While exercise is an important component in managing musculoskeletal pain conditions[5, 9, 22, 53], it can enhance pain and hyperalgesia in chronic musculoskeletal pain conditions in humans and animals[14, 55, 57, 59]. An acute bout of exercise enhances hyperalgesia in sedentary mice, while regular physical activity prevents development of hyperalgesia[49, 56]. Hyperalgesia of the muscle, primary hyperalgesia, requires 8 weeks of physical activity to prevent its development, but both 5 days and 8 weeks prevent development of hyperalgesia of the paw, secondary hyperalgesia[4, 49, 56]. These data suggest that 5 days of physical activity produces its effects primarily through modulation of central nervous system pathways. However, the mechanisms for how short-duration physical activity prevents hyperalgesia are unknown.
The nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO), and nucleus raphe pallidus (NRP), part of the rostral ventromedial medulla (RVM), both inhibit and facilitate nociceptive behaviors, but also modulate motor responses[11, 19, 43, 65], and thus may be involved in modulating effects of activity on nociception. In the NRM, NRP and NRO, we previously showed increased phosphorylation of the NR1 subunit of the NMDA receptor (p-NR1) in models of muscle pain, neuron activation by an acute bout of exercise, and increases in the serotonin transporter (SERT) in a neuropathic pain model[6, 12, 57]; the increases in p-NR1 and the increase in SERT are reduced by physical activity or exercise[6, 56]. In nerve injured rats that performed treadmill running, there is an increase in met-enkephalin in the RVM, and supraspinal blockade (i.c.v.) of opioid receptors or depletion of serotonin prevents analgesia[4, 6, 58]. Together these studies suggest that both opioids and serotonin mediate exercise-induced analgesia. However, it is unknown if there are alterations in SERT in models of muscle pain, if increases in SERT mediate hyperalgesia, or if there are interactions between opioid and serotonin systems in activity-induced analgesia.
Classical pharmacological studies show that the RVM uses opioids to produce analgesia and this opioid-induced analgesia is in part mediated by serotonin[1, 28, 43]. Serotonergic neurons receive input from endogenous opioid peptides and both coexist in RVM neurons[3, 21]. Further, systemic depletion of serotonin, or blockade of serotonin receptors in the RVM, prevents the analgesic effects of morphine delivered systemically, or into the RVM[8, 50]. These data provide anatomical and pharmacological evidence for an interaction between opioidergic and serotoninergic systems in the RVM. On the other hand, increased facilitation in the RVM modulates secondary hyperalgesia[42, 43, 61, 63] and ON-cells, facilitatory RVM neurons, express mu-opioid receptors[42, 43]. NMDA receptors play a key role in facilitation from the RVM as blockade of NMDA receptors reduces muscle hyperalgesia, downregulation of NR1 reduces muscle hyperalgesia, and there is increased p-NR1 in models of muscle pain[10–12]. Thus, we propose that activity-induced hyperalgesia and activity-induced analgesia modulate SERT and p-NR1 in the RVM through mu-opioid receptors.
The current study tested if p-NR1 and SERT expression increased in the RVM in a chronic muscle pain model, if blockade of SERT in the RVM reversed the hyperalgesia, and if short-duration physical activity activated mu-opioid receptors to prevent development of analgesia, and reductions in p-NR1 and SERT in the RVM.
2. Methods
2.1. Animals
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Adult, male and female, physically active and sedentary C57BL/6j (WT) and MOR−/− mice were used (Jackson Laboratories, Bar Harbor, ME). Animals were housed in the animal care unit of the University of Iowa, in a 12-h dark/light cycle, with all tests performed during the light cycle. Food and water were available to the animals ad libitum.
2.2. Activity-induced pain model
For the activity-induced pain model, mice were briefly anesthetized with isoflurane (3%). Then a solution of pH 5.0 sterile saline (20 µL) was injected into the left gastrocnemius muscle. Five days later mice performed a bout of 2h of running wheel exercise followed by a second injection of pH 5.0 saline as previously published[64]. This model produces muscle fatigue, a reduction in withdrawal thresholds of the muscle, i.e. primary hyperalgesia, and increased response frequency to mechanical stimulation of the paw, i.e. secondary hyperalgesia[55, 64]. It is generally thought that secondary hyperalgesia reflects changes in the central nervous system while primary hyperalgesia reflects changes in the peripheral nervous system[16, 23]. Two pH 5.0 injections alone or the 2h exercise task alone does not produce tissue damage and does not result in hyperalgesia[64].
2.3. Short-duration physical activity
Physically active mice had free access to running wheels in their home cages for 5 consecutive days to promote a short-duration regular physical activity based on voluntary exercise without the influence of a training effect. This protocol prevents development of hyperalgesia at the paw (secondary hyperalgesia) in chronic muscle pain models[49, 56]. Sedentary control mice were maintained in their regular housing without running wheels. Both physically active and sedentary mice were housed individually for the same 5-day period. Running wheels were removed from the cages prior to induction of the model.
2.4. Behavioral Testing
Mice were acclimated to the behavioral tests two times per day for 2 days. Mice were placed in small clear cubicles on an elevated wire mesh table for 20 min to be acclimated to the testing for paw withdrawal frequency with von Frey filaments (North Coast, CA). Mice were placed in a glove for 5 min to acclimate for the muscle withdrawal thresholds test with tweezers. The investigator performing the tests was blinded to group allocation. Two tests were chosen to examine nociceptive behaviors in mice based on prior studies. The paw withdrawal frequency to noxious mechanical stimuli was used to examine a site outside the site of insult as a measure of secondary hyperalgesia. The muscle withdrawal threshold was used to examine the site of insult as a measure of primary hyperalgesia. The activity-induced hyperalgesia model induces an increased paw withdrawal frequency to mechanical stimulation and a decrease in withdrawal threshold of the muscle[49, 55, 64]. Five days of physical activity reduces the increased withdrawal frequency of the paw, but not the withdrawal threshold of the muscle[49]. These two measures likely reflect different underlying mechanisms.
2.4.1 Paw withdrawal frequency
To assess the effect of exercise on secondary hyperalgesia, paw withdrawal to mechanical stimulation was tested ipsilaterally as previously described[36]. A 0.4 mN von Frey filament was applied to the hind paws 5 times, and the number of withdrawals was assessed; this was repeated 10 times and averaged. Data are presented as number of responses (withdrawals ranging from 0 to 5). An increased number of responses are interpreted as cutaneous secondary hyperalgesia.
2.4.2. Muscle withdrawal threshold
To assess the effect of exercise on primary hyperalgesia, muscle withdrawal thresholds were measured. A pair of calibrated forceps was applied to the gastrocnemius muscle until the minimum pressure required for the animal to withdraw from the stimulus was reached. Three trials on each side were performed at each testing period and averaged[36, 52]. Data are presented as force (in milliNewtons [mN]) and defined as the muscle withdrawal threshold. A decrease in withdrawal threshold was interpreted as primary muscle hyperalgesia.
2.5. Immunohistochemistry
2.5.1. Perfusion, Cryoprotection and Tissue Preparation
After the running wheel and behavioral assessments, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde. Their brainstems were removed and placed in 30% sucrose overnight and then blocked and frozen in cryomolds embedded in optimal cutting temperature compound (OCT, Tissue-Tek; Fisher Scientific, Waltham, MA). Tissue sections were cut on a cryostat at 20 µm and placed on slides.
2.5.2. Immunohistochemistry for p-NR1 expression
All sections were immunohistochemically stained simultaneously for p-NR1 using standard immunofluorescent techniques as previously described[55]. On day 1, sections were washed with background buster and blocked with 5% normal goat serum and then incubated overnight with a primary antibody to p-NR1 (1:1,000, Ser897, catalog no. ABN99; Millipore, Billerica, MA, http://www.millipore.com). On day 2, sections were incubated with a secondary antibody (Biotinylated goat anti-rabbit IgG; 1:1000, Jackson ImmunoResearch, West Grove, PA). Sections were then reacted with streptavidin conjugated to Alexa Fluor 568 (1:1,000; Life Technologies, Eugene, OR) for 1 h at room temperature. Sections were cover slipped with Prolong Diamont anti-fade (Thermo Fisher Scientific, Waltham, MA, https://www.thermofisher.com) and stored at −20° until analysis. We previously established that this antibody is specific for p-NR1 expression in the RVM by showing up-regulation of the NR1 subunit in the RVM increased expression of p-NR1 and downregulation of NR1 in the RVM decreased expression of p-NR1[12].
Images of the stained sections were taken in the Central Microscopy Facility at the University of Iowa on an Olympus BX-61 light microscope equipped with a SPOT camera (RT Slider; Diagnostic Instruments, Meyer Instruments, Houston, TX, http://www.meyerinst.com/html/dgnstc/default.htm). Images were taken with a 20X objective lens, which is sufficient for counting cells expressing p-NR1. Five sections of the nucleus raphe obscurus (NRO), nucleus raphe magnus (NRM) and nucleus raphe pallidus (NRP) were digitally imaged and stored for later analysis. Cells were quantified by manually counting total numbers in a standardized area using ImageJ software (National Institutes of Health, Bethesda, MD) as previously described by us[12, 55, 56]. Areas were determined according to stereotaxic coordinates from Paxinos and Watson Mouse Atlas[41], and were the following: NRO: Bregma −6.96 to −6.24, NRP: bregma −6.64 to −5.68 and NRM: bregma −6.36 to −5.80. Areas for counting were selected by using the “specify” function of Image J software to provide the same area for each section counted as follows: NRO: width 420, height 876, total area: 367920; NRM: width 1030, height 1004, total area: 1034120 for the NRM. For the NRP, counting area was specified manually according to the NRP’s area size in each bregma coordinate and ranged from 300000 to 600000. The sum of the counting from 5 pictures was considered for analysis. The investigator was blinded to group during staining, picture acquisition and counting.
2.5.3. Immunohistochemistry for SERT expression
All sections were immunohistochemically stained simultaneously for SERT using standard protocols[6]. Sections were blocked with 3% normal goat serum followed by a standard Avidin and Biotin blocking protocol (Vector Laboratories, Burlingame, CA). Sections were then incubated overnight with the primary antibody to SERT (Rabbit anti-5-HT transporter; 1:500; ImmunoStar, Hudson, WI). On the second day, sections were incubated in the secondary antibody (Biotinylated Goat anti-Rabbit IgG; 1:1000, 1 h; Invitrogen, Thermo Fisher Scientific, Waltham, MA) followed by Streptavidin-Alexa Fluor 568 conjugate (1:1,000; Life Technologies, Eugene, OR), for 1 h. All antibodies and streptavidin were diluted in 1% normal goat serum with 0.05% Triton-X 100 in 1x PBS. Slides were cover slipped with Vectashield (Vector Laboratories, Burlingame, CA). We performed two separate experiments to test the selectivity of the SERT antibody. First, removal of the primary antibody from the protocol eliminated the SERT immunoreactivity. Second, immunohistochemical staining in tissue from SERT−/− mice (Jackson Laboratories, Bar Harbor, ME) showed a loss of immunoreactivity to the antibody.
Five sections of the NRO, NRM and NRP were digitally imaged and stored for later analysis. Areas were determined as in the p-NR1 analysis. For SERT expression quantification, the density of immunoreactivity was measured using ImageJ 1.24 software (National Institutes of Health, Bethesda, MD). Specifically, each picture was first converted to 8-bit gray scale, and then calibrated independently using the “uncalibrated OD” function with pixel values ranging from 0 to 255. Density values represent pixels per area and are expressed as arbitrary units. The average of density values from 5 pictures was considered for analysis. The investigator was blinded to group during staining, picture acquisition and counting.
2.6. Experimental groups
Physically active (n=5-12, 2-6 males, 3-7 females) wild-type C57BL/6j mice were compared to sedentary mice (n=8, 4-5 males, 3-4 females) to show the effects of the 5-day wheel running protocol on the activity-induced pain model on behavioral and immunohistochemical analysis. Two additional experiments were performed to assess the involvement of the opioidergic system in such effects.
2.6.1. Experiment 1 assessed if systemic blockade of mu-opioid receptors would prevent the analgesia, reduction of RVM expression of p-NR1 and SERT produced by wheel running in the activity-induced hyperalgesia model. Physically active mice were treated with systemic naloxone for 5 days during wheel running (n=7-8, 4 males, 3-4 females) and compared to physically active vehicle-treated controls (n=6-8, 3-4 males, 3-4 females). Osmotic mini-pumps (3 mg/kg/day naloxone, Alzet Osmotic Pumps, Cupertino, CA) were used to deliver naloxone or vehicle continuously over the 5-day wheel running period. The day before wheel running started, mice were anesthetized with isoflurane and the osmotic mini pumps were subcutaneously implanted in the area between their shoulder blades. Mini-pumps were removed after 5 days of wheel running and before the first pH 5.0 injection.
2.6.2. Experiment 2 investigated if genetic deletion of mu-opioid receptors would block the analgesic effect of short-duration physical activity and the reduction of RVM expression of p-NR1 and SERT produced by wheel running in the activity-induced hyperalgesia model. Both physically active (n=5-12, 3-7 males, 2-5 females) and sedentary (n=5-8, 3-4 males, 2-4 female) mu-opioid receptor knockout mice (MOR−/−) were compared to the physically active (n=5-12, 2-6 males, 3-7 females) and sedentary (n=8, 4-5 males, 3-4 females) wild-type C57BL/6j (WT) groups. A group of naive mice (n=6, 3 males, 2-3 females) was added to compare the changes in immunohistochemistry. The number of animals per group in the immunohistochemistry experiments varied because some samples were removed due to poor perfusion or inadequate tissue quality of the stained area. The number of animals per group per measure, and the number of males and females per group per measure are provided in Supplementary Table 1.
2.6.3. Experiment 3 was performed to investigate the role of SERT in the RVM on pain modulation. Sedentary mice had an intracerebral guide cannula stereotaxically implanted in their RVM three day prior to baseline behavior testing and first pH 5.0 injection. The mice were anesthetized with ketamine/xylazine (17.5/2.5 mg/mL i.p.) and positioned in a stereotaxic head holder. The skulls were exposed, and a small hole was drilled for placement of guide cannula. The cannula was placed −5.6 mm caudal from the bregma (intra-aural = −5.6 mm, mediolateral = 0.0 mm, dorsoventral = −5.7 mm from the surface of the skull). Cannulas were secured to the skull with dental cement. A dummy cannula (33 gauge; Plastics One) was inserted into the guide cannula to maintain its patency.
On day six, after post-induction behavioral measurements, one group of mice (n=5, 4 males, 1 female) received Fluoxetine/HBC (10% HBC, 20 nmol/0.2ul) injection through a 33-gauge injection cannula that extended 1 mm below the guide cannula tip. The injection cannula was attached to a 10-µl Hamilton syringe via a length of PE-20 tubing. A control group received saline 10% HBC (n=5, 3 males, 2 females). Behavior testing was performed 15 min and 2h after fluoxetine or saline injection. A third group of mice (n=3, 1 male, 2 females) had cannulas placed outside of the RVM (missed sites) (intra-aural = −5.6 mm caudal from bregma, mediolateral = −1.0, dorsoventral = −1.6 from the surface of the skull) to control for area specificity.
To determine cannula placement in the RVM, an equivalent volume of methylene blue dye was injected at the end of the experiment. Mice were then transcardially perfused with 4% paraformaldehyde and their brains were removed and preserved in 30% sucrose. The brains were cross-sectioned into 30µm sections on a cryostat and analyzed on a light microscope to check for cannula placements.
2.7. Statistical Analysis
Results are presented as mean ± SEM for each group for withdrawal thresholds of the muscle and for immunohistochemistry data. For response frequency of the paw, data are presented as the median with 25th and 75th percentiles. Normality was tested for withdrawal threshold of the muscle and immunohistochemistry data using a Shapiro-Wilk test. These data were all normally distributed. For withdrawal threshold of the muscle, a repeated measures ANOVA tested for differences across time and between groups. Post-hoc tests were not performed as there were no group differences. For immunohistochemistry data, a one-way ANOVA compared differences between groups followed by a post-hoc test with a Tukey’s test, or a t-test for two group comparisons. For response frequency of the paw, non-parametric analysis was chosen since the data is ordinal, and not continuous. A Kruskall Wallis test compared differences between multiple groups, while a Mann-Whitney U test examined for differences between two groups. P values lower than 0.05 were considered statistically significant and all analysis were performed with SPSS Version 23 (IBM Statistics).
3. Results
3.1. Behavioral Testing
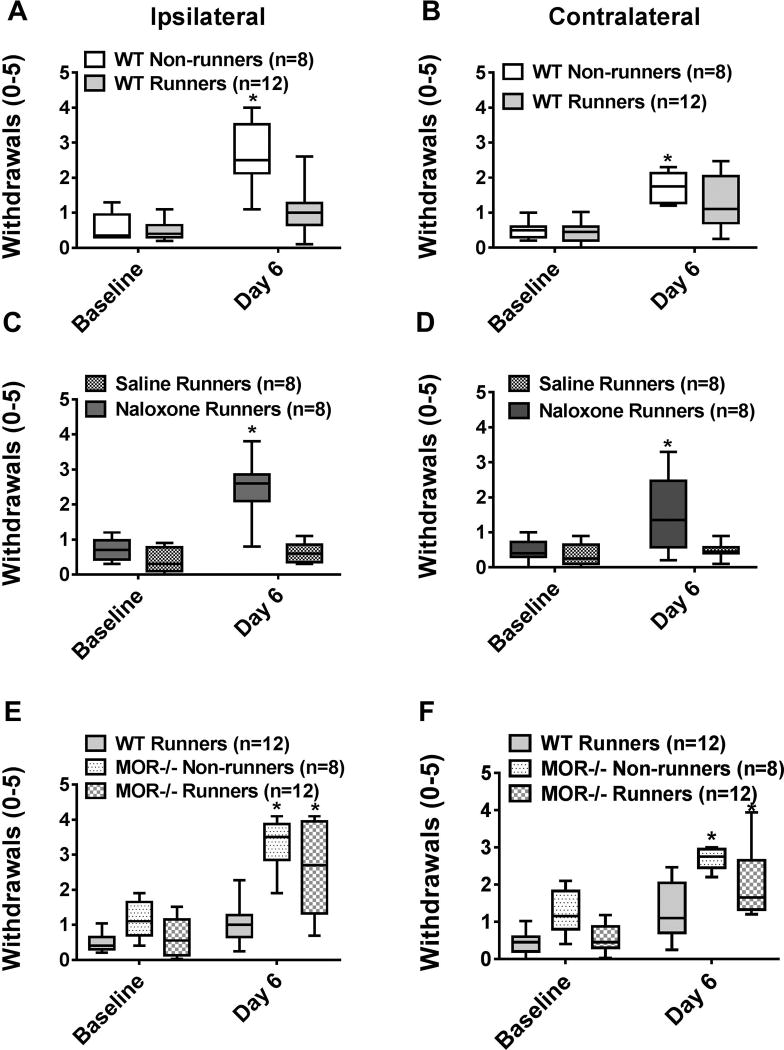
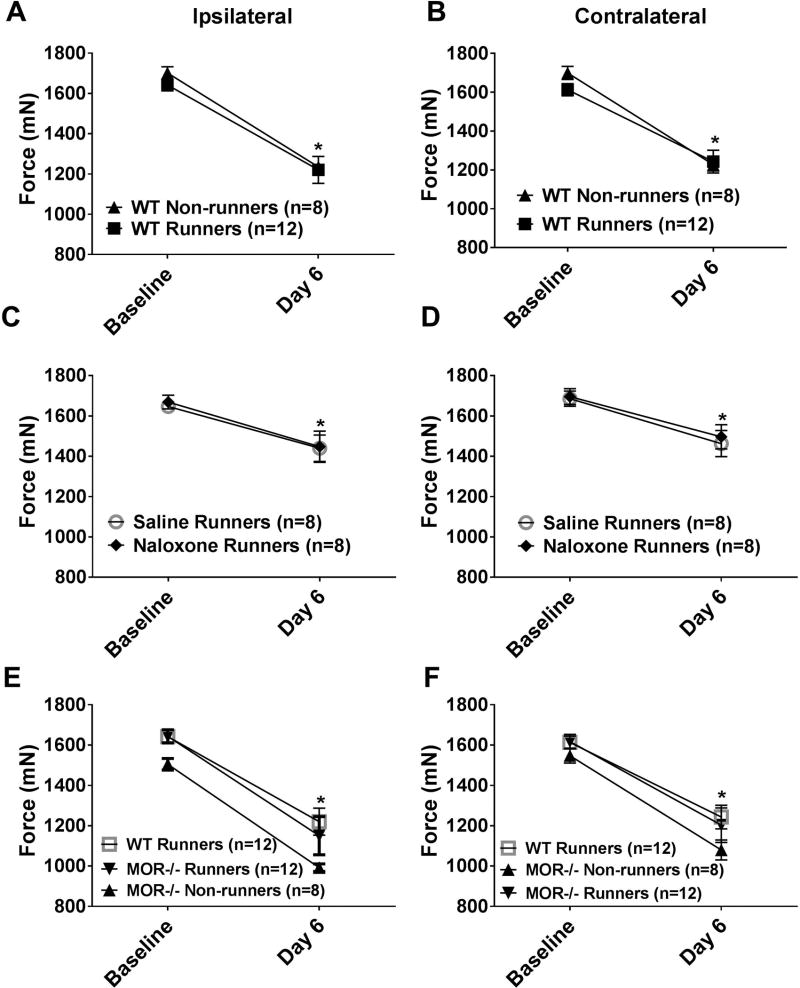
Sedentary WT mice (WT non-runners) showed an increase in the number of withdrawals to noxious mechanical stimulation of the paw (secondary hyperalgesia)(p < 0.05, Mann-Whitney test) and a decrease in withdrawal thresholds of the muscle bilaterally (primary hyperalgesia)(p=0.0001, paired t-test) 24h after induction of the model. Five days of wheel running in WT mice significantly prevented the increased response frequency of the paw bilaterally (p=0.001)(Fig. 1A, 1B), but not the decrease in withdrawal threshold of the muscle (Figure 2A,B), normally observed in sedentary WT mice.
Fig. 1. Mechanical hyperalgesia of the paw.
Graphs represent the number of withdrawals to repeated mechanical stimulation of the hindpaw with an 0.4 mN force for the ipsilateral (A, C, E) and contralateral (B, D, F) sides. A,B. After induction of activity-induced hyperalgesia, the number of responses significantly increases in sedentary WT mice (WT non-runners). 5 days of running wheel activity prevented these increases in the number of responses to mechanical stimuli (WT Runners). *P < 0.05 when compared to WT Non-runners. C,D. The group of mice treated with naloxone during the 5 days of wheel running showed significantly higher responses to mechanical stimulation than saline controls. *P < 0.05 when compared to saline runners. E,F. MOR −/− runners showed significantly greater increases in the number of responses to mechanical stimulation of the paw when compared to WT Runners; responses were similar to MOR−/− non-runners. *P < 0.05 when compared to WT Runners. WT: wild type, MOR−/−: Mu-opioid receptor knockout. Box plots represent the median with the 10th and 90th percentiles.
Fig. 2. Mechanical hyperalgesia of the muscle.
Graphs represent the withdrawal threshold of the muscle for the ipsilateral (A, C, E) and contralateral (B, D, F) sides. A,B. After induction of activity-induced hyperalgesia, the withdrawal threshold of the muscle decreases bilaterally in sedentary WT mice (WT non-runners). 5 days of running wheel activity had no effect on the decreased withdrawal threshold of the muscle (WT Runners). *P < 0.05 when compared to baseline. C,D. Treatment with naloxone during the 5 days of wheel running had no effect on the decreased withdrawal threshold of the muscle when compared to saline treatment. *P < 0.05 when compared to baseline E,F. MOR−/− runners showed significantly greater increases in the number of responses to mechanical stimulation of the paw when compared to WT Runners and MOR−/− non-runners. *P < 0.05 when compared to baseline. WT: wild type, MOR−/−: Mu-opioid receptor knockout. Data are the mean with S.E.M.
Experiment 1 tested if the analgesic effects of wheel running use opioids by treating animals with systemic naloxone during wheel running. Physically active mice treated with systemic naloxone (3 mg/kg/day) showed a significantly greater number of withdrawals to noxious stimulation of the paw when compared to physically active mice treated with vehicle (p=0.001)(Fig. 1C, 1D). When testing if blockade of the mu-opioid receptors with naloxone modulated muscle withdrawal threshold, there was a significant effect for time (F1,12=23.7, p=0.0001), but not for side (F1,12=2.8, p=0.112), treatment (naloxone vs. saline: F1,12=0.002, p=0.96), or sex (F1,12=0.52, p=0.49)(Fig. 2C,D); i.e. there was a decrease in withdrawal threshold bilaterally 24h after the second injection of acidic saline for both groups. Thus, 5 days of running wheel activity prevented the development of activity-induced hyperalgesia of the paw through activation of opioid receptors.
Experiment 2 tested if mu-opioid receptors mediate the analgesia produced by wheel running by examining effects in MOR−/− mice. Physically active MOR−/− mice had a significantly greater number of withdrawals when compared to physically active WT mice (p=0.004), and were similar to sedentary MOR−/− mice (p=0.27) and WT non-runners (p=0.79)(Fig. 1E, 1F). When testing if MOR−/− mice modulated muscle withdrawal thresholds, there was a significant effect for time (F1,32=194, p=0.0001), but not for side (F1,32=1.9, p=0.19), genotype (WT vs. MOR−/−, F1,32=0.48, p=0.49), runner status (runner vs. sedentary, F1,32=0.25, p=0.62), or sex (F1,32=0.60, p=0.45); i.e. there was a decrease in threshold bilaterally for all groups 24h after the second acidic saline injection(Fig. 2E,F). An analysis of wheel-running activity with 8 mice (4 MOR−/−, 4 WT) showed that mice ran an average of 2.29 km after 5 days (range: 1.3 km-4.24 km). There was no significant difference in the distance ran by MOR−/− and WT mice. Thus, 5 days of running wheel activity prevented the development of activity-induced hyperalgesia of the paw through activation of mu-opioid receptors.
3.2. p-NR1 expression in the RVM
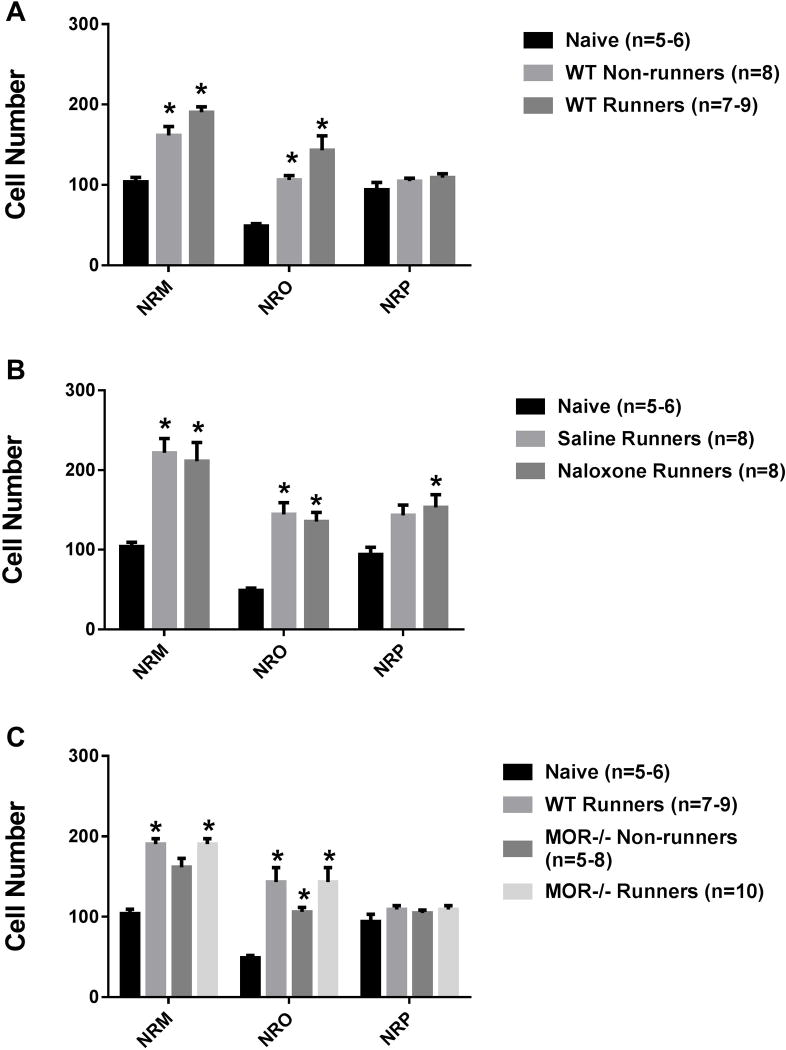
Figure 3 shows photomicrographs with representative images of p-NR1-positive cells in the NRO, NRP and NRM. When comparing all groups, there was an overall significant difference in the number of pNR1 positively stained cells in the NRM (F6,53=4.2, p=0.002), NRO (F6,55=5.4, p=0.0001), and the NRP (F6,50=4.6, p=0.001). We initially tested if there was an increase in pNR1-postitive cells after induction of the activity-induced pain model and if this was modulated by wheel running. Sedentary WT mice showed a significant increase in the number of p-NR1 positive cells in NRM (p=0.02) and NRO (p=0.0001), but not the NRP (p=0.28) 24h after induction of the model when compared to naive mice. Similarly, there was a significant increase in p-NR1 positive cells in the NRM (p=0.04) and NRO (p=0.001) in animals with 5 days of wheel running prior to induction of the model; there were no significant differences between wheel running and sedentary animals (NRM p=0.87; NRO p=0.12, NRP p=0.43)(Fig. 4A).
Fig. 3. Photomicrographs of p-NR1 immunohistochemistry.
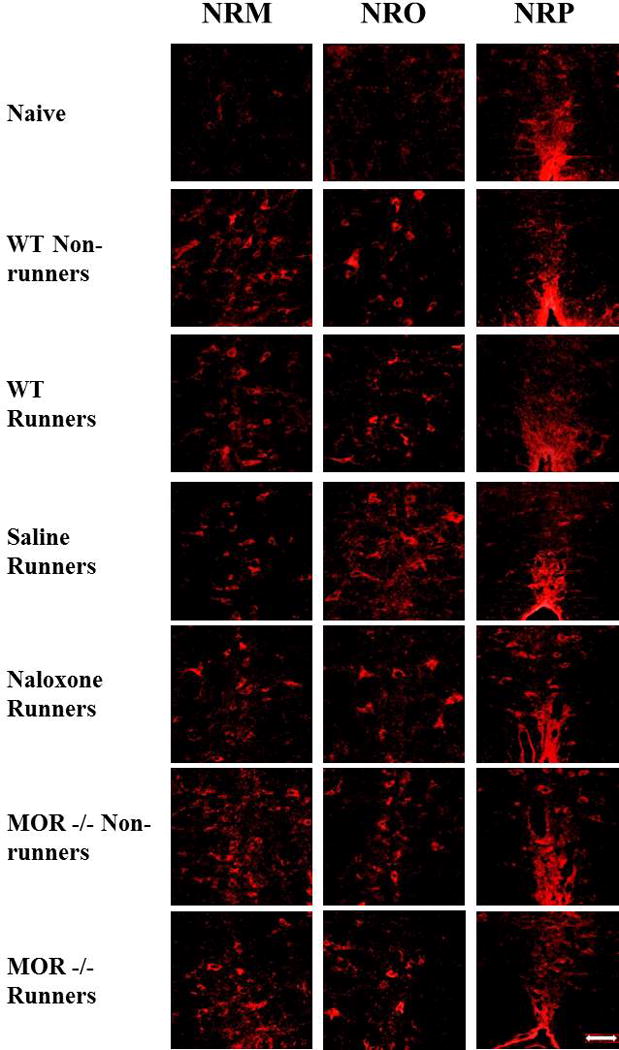
Immunohistochemical staining of p-NR1 in the nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO), and nucleus raphe pallidus (NRP) are represented for each group: Naive, WT-Non-runners, WT Runners, Saline Runners, Naloxone Runners, MOR−/− Non-runners and MOR−/− Runners. Bar represents 50 µm. WT: wild type, MOR−/−: Mu-opioid receptor knockout.
Fig. 4. P-NR1 staining.
Graphs represent the number of immunohistochemically stained p-NR1 in the RVM. A. Increases in the number of p-NR1 positive cells occurred in the NRM and NRO 24h after induction of the pain model in sedentary mice (WT Non-runners). WT Runners showed a similar increase in the number of p-NR1 positive cells after induction of the model. *p < 0.05 when compared to naive. B. Administration of naloxone during the 5 days of running wheel had no effect on the number of p-NR1 positive cells in the RVM. Significant increases in p-NR1 cells occurred in the NRM and the NRO in both the naloxone runners and the saline runners when compared to naive mice (*p < 0.05). C. MOR−/− runner mice had significantly greater number of p-NR1 positive cells when compared to WT runners in the NRM and NRO (*p < 0.05). WT: wild type, MOR−/−: Mu-opioid receptor knockout. Data are the mean with S.E.M.
For Experiment 1, naloxone-treated and vehicle-treated physically active mice showed a similar number of p-NR1 positive cells in the NRM (p=1.0), NRO (p=1.0), and NRP (p=.93). When compared to naïve mice, saline-treated physically active mice showed significant increases in the number of p-NR1 positive cells in the NRM (p=0.001) and NRO (p=0.001), but not the NRP (p=0.06). Similarly naloxone-treated mice also showed significant increases in the number of p-NR1 positive cells in the NRM (p=0.002), NR) (p=0.001), as well as the NRP (p=0.004). For experiment 2, we tested if mu-opioid receptors modulated p-NR1 immunoreactivity by examining MOR−/− mice. Physically active MOR−/− mice showed significant increases in the number of p-NR1 positive cells in the NRM (p=0.02) and NRO (p=0.0001) when compared to naive mice, but not when compared to physically active WT mice (NRM p=0.87, NRO p=0.38)(Fig. 4B, 4C). No sex differences were observed.
3.3. SERT expression in the RVM
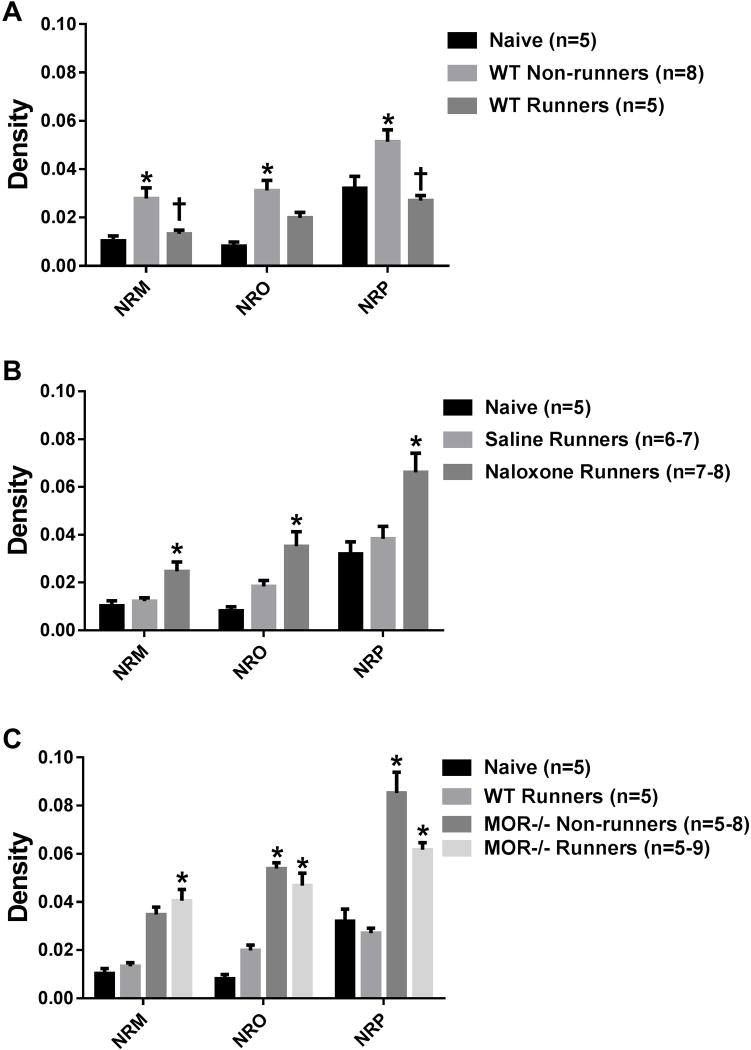
Figure 5 shows photomicrographs representing SERT expression in the NRO, NRP and NRM from all groups. The inset in the photomicrographs from naive mice shows a lack of immunoreactivity in the SERT−/− mice demonstrating specificity of the antibody to SERT. When comparing all groups, there were significant overall differences in staining density for SERT in the NRM (F6,44=9.5, p=0.0001), NRO (F6,47=12.1, p=0.0001), and NRP (F6,43=11.5, p=0.0001). We initially tested if there was an increase in SERT after induction of the activity-induced pain model and if this was modulated by wheel running. Sedentary WT mice showed a significant increase in SERT density in the NRM (p=0.009), NRO (p=0.001), and NRP (p=0.02) 24h after induction of the model when compared to naive mice. When compared to sedentary mice, the increase in SERT in the activity-induced pain model was prevented by 5 days of wheel running in the NRM (p=0.03) and NRP (p=0.006), but not the NRO (p=0.09)(Fig. 6A). The staining density for SERT in the physically active WT mice was similar to that observed in naive mice (NRM: p=0.85; NRO: p=0.12; NRP: p=0.77).
Fig. 5. Photomicrographs of immunohistochemistry for the serotonin transporter (SERT).
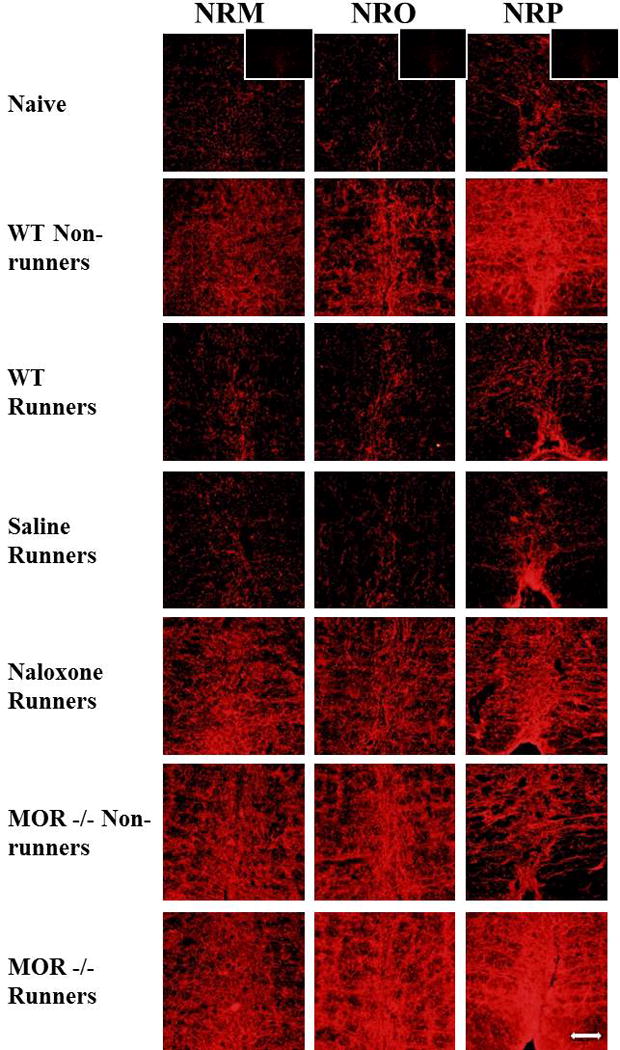
Immunohistochemical staining for the nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO), and nucleus raphe pallidus (NRP) are represented for each group: Naive, WT-Non-runners, WT Runners, Saline Runners, Naloxone Runners, MOR−/− Non-runners and MOR−/− Runners. Bar represents 50 µm. WT: wild type, MOR−/−: Mu-opioid receptor knockout. *Inset shows photomicrograph from SERT−/− mouse incubated with the antibody to SERT. There was no immunoreactivity for SERT in SERT−/− mice.
Fig. 6. SERT staining.
Graphs represent the quantification of SERT immunoreactivity in the RVM. A. In sedentary mice (WT Non-runners), there was a significant increase in SERT immunoreactivity in the nucleus raphe magnus (NRM), nucleus raphe obscurus (NRO), and nucleus raphe pallidus (NRP) when compared to naive mice (*p < 0.05). This increase did not occur in mice who had done 5 days of wheel running prior to induction of the model in the NRM and the NRP (†p < 0.05). B. Naloxone-treated physically active mice (Naloxone Runners) had a significant increase in SERT immunoreactivity in all three areas when compared to both saline-treated and naive mice (*p < 0.05). C. Physically active MOR−/− mice (MOR−/− Non-runners) had significantly higher SERT immunoreactivity when compared to both physically active WT (WT Runners) and naive mice (*p < 0.05). Sedentary MOR−/− mice had a similar increase in SERT when compared to WT Runners. WT: wild type, MOR−/−: Mu-opioid receptor knockout.
Experiment 1 tested if systemic naloxone altered p-NR1 staining when compared to vehicle controls in physically active mice. Physically active mice treated with systemic naloxone showed greater SERT immunoreactivity in the NRM (p=0.02), NRO (p=0.04) and NRP (p=0.02) when compared to physically active vehicle treated mice 24h after induction of the model (Fig. 6B). Experiment 2 tested if mu-opioid receptors mediated the alterations in SERT immunoreactivity by examining MOR−/− mice. Physically active MOR−/− mice showed significant increases in SERT density in the NRM (p=0.002), NRO (p=0.004), and NRP (p=0.0001) when compared to physically active WT mice. However, it should be noted that in sedentary MOR−/− mice there was a greater increase in SERT immunoreactivity 24h after induction of the model when compared to sedentary WT mice for the NRO (p=0.006) and NRP (p=0.001), but not for the NRM (p=0.68)(Fig. 6C). No sex differences were observed.
3.4. Experiment 3
Since there were increases in SERT immunoreactivity in the RVM after induction of the activity-induced pain model, we tested if blockade of SERT in the RVM would reverse the muscle and paw hyperalgesia. All groups of mice showed an increase in the paw withdrawal frequency to mechanical stimulation and a decrease in muscle withdrawal thresholds (Fig 7). Microinjection of fluoxetine (20nmol/0.2ul) into the RVM decreased the paw withdrawal frequency to mechanical stimulation (15 min, p=0.008 ipsilateral and contralateral) and increased the muscle withdrawal threshold 15 min (15 min, p=0.005 ipsilateral and contralateral) after injection when compared to vehicle controls. By 2h the fluoxetine effects were reversed and there was an increase in paw withdrawal frequency to mechanical stimulation and a decrease in muscle withdrawal threshold similar to that observed 24h after induction of the activity-induced pain model. Mice injected with fluoxetine outside of the RVM into the medial vestibular nucleus (MVe) and spinal vestibular nucleus (SpVe)(missed sites, Figure 7) showed no change in the number of responses of the paw to mechanical stimulation (n=3; ipsilateral: median 3.2, 25th percentile 3.0; 75th percentile 3.4; contralateral: median 2, 25th percentile 1.9, 75th percentile, 2.6) or the muscle (n=3; mean±SD, ipsilateral 1,188±91; contralateral 1,191±30) 15 minutes after injection and were similar to vehicle controls. When injections were placed in the lateral paragigantocellularis or the gigantocellularis on the contralateral side there was a significant decrease in the number of responses to mechanical stimulation of the paw 15 minutes after injection bilaterally (n=5; median with 25th and 75th percentiles; ipsilateral: p=0.04 1.2, 1.2-1.2; contralateral: p=0.02; 0.8, 0.6-1) when compared to vehicle controls. Similarly, injections into the lateral paragigantocellularis or the gigantocellularis on the contralateral side increased the withdrawal thresholds of the muscle bilaterally (n=5; mean±SD; ipsilateral 1,365±74, p=0.01; contralateral 1,330±62 p=0.02; contralateral) when compared to vehicle controls.
Fig. 7. Mechanical hyperalgesia of the paw and muscle after microinjection of fluoxetine.
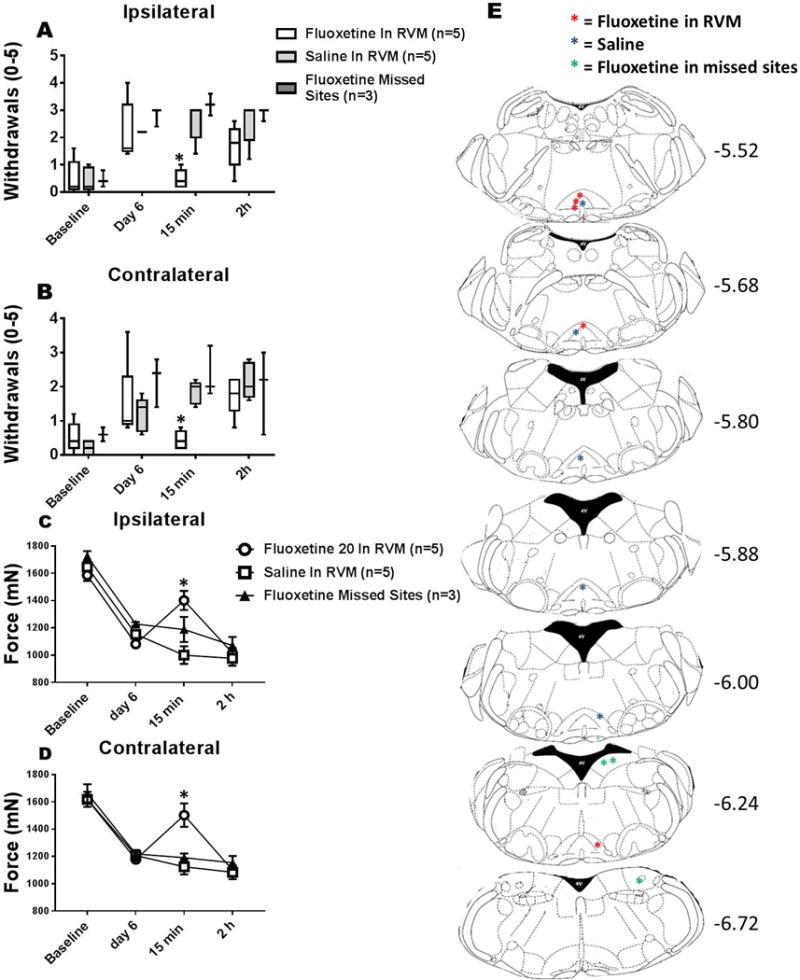
Graphs represent the number of withdrawals to repeated mechanical stimulation of the hindpaw with an 0.4 mN force for the ipsilateral (A) and contralateral (B) sides and the withdrawal threshold of the muscle for the ipsilateral (C) and contralateral (D) sides. All groups showed an increase in the paw withdrawal frequency to mechanical stimulation and a decrease in muscle withdrawal thresholds on day 6. Microinjection of fluoxetine (20nmol/0.2ul) into the RVM decreased the paw withdrawal frequency to mechanical stimulation (*p=0.008) and increased the muscle withdrawal threshold (*p=0.005) 15 min after injection when compared to vehicle controls, but not after 2h (p > 0.005). Mice injected with saline in the RVM or fluoxetine outside of the RVM (missed sites) had no reversal of hyperalgesia (p > 0.05). E. Maps showing location of injection sites in the RVM for fluoxetine (red) and saline (blue), and for sites outside the RVM (green) for each individual animal at Bregma levels −5.52 to −6.72.
4. Discussion
The current study showed that short-duration physical activity prevented development of secondary hyperalgesia of the paw, but not primary muscle hyperalgesia, in an activity-induced pain model that was mediated by activation of mu-opioid receptors. We further show, in sedentary mice, there is an increase in SERT expression in the RVM, and blockade of SERT in the RVM after induction of the activity-induced pain model, reverses the hyperalgesia showing a functional role for the increases in SERT. Regular physical activity prevents the increases in SERT expression in the RVM, and alterations in SERT by wheel running do not occur after treatment of animals with naloxone or genetic deletion of MOR. Together these data suggest an interaction between the serotonergic and opioidergic systems in the analgesic effects of regular physical activity.
Consistent with prior studies[49, 56], short-duration wheel running prevents secondary, but not primary, hyperalgesia, suggesting that the analgesia involves central nervous system mechanisms[62]. On the other hand, prior studies show that 8 weeks of running wheel activity reduces both primary muscle hyperalgesia and secondary paw hyperalgesia[49, 56], suggesting additional mechanisms underlie the reduction in primary hyperalgesia with longer duration physical activity. In support, we show that 8 weeks of prior running wheel activity alters macrophage phenotype in the muscle and the analgesia produced is reversed by blockade of IL-10 receptors in the muscle[36]. Thus, short-duration exercise primarily has an effect on modulating central nervous system excitability and inhibition.
4. 1. Interactions between opioid and serotonin systems mediate analgesia from short-duration exercise
Data from the current study show that mu-opioid receptors mediate the analgesia produced by short-duration wheel running which is consistent prior work showing systemic blockade of opioid receptors prevents exercise-induced analgesia in healthy human subjects, normal uninjured animals, and animal models of pain, and increased endogenous opioid peptides and altered MOR expression occurs in central nervous system sites including the RVM[4, 15, 18, 20, 27, 30, 34, 39, 58]. The current study also shows that short-duration running wheel activity reduces SERT expression in the RVM, which is consistent with prior studies showing regular exercise increases 5-HT in central nervous system sites, and reduces SERT in RVM, and increases 5-HT-receptor expression in RVM, in normal uninjured animals and animals with nerve injury[4, 6, 7, 17, 39, 58]. Although previous studies have shown a role of opioidergic and serotoninergic systems in exercise-induced analgesia, separately, we show, for the first time, an interaction between the two systems mediates the analgesia produced by regular exercise.
Two populations of neurons in the RVM, ON-cells and OFF-cells, modulate nociception, with increased activation of ON-cells promoting nociception, and increased activity of OFF-cells promoting analgesia [19, 24, 26, 29, 34]. MOR are expressed on ON-cells, MOR agonists directly inhibit ON-cells, and removal of ON-cells prevents secondary hyperalgesia[42, 43]. A third subset of neurons in the RVM, neutral cells, do not change their firing in response to noxious stimuli and are thought to be serotonergic[44]. Recent evidence, however, shows 5-HT connections with all cells types in the RVM with greatest number of 5-HT connections are on neutral cells, slightly less on OFF-cells, and the least on ON-cells[44]. Further, 5-HT and opioids coexist in RVM neurons, and opioids enhance serotonin activity in the RVM by increasing the rate of serotonin turnover[3, 21]. Our data show that the reduced SERT expression in the RVM by wheel running does not occur after blockade of opioid receptors, or in MOR−/− mice, suggesting MOR activation modulates SERT expression in the RVM. MOR regulation of SERT could be a result of direct interactions between endogenous agonists and their intracellular pathways within the same cell, indirect modulation between different pain sites, or by direct gene interactions. MOR activation increases activation of intracellular signaling pathways, such as protein kinase C which can directly phosphorylate and downregulate SERT[45]. Alternatively, systemic morphine induces release of serotonin in the RVM, and microinjection of a mu-opioid agonist into the PAG produces analgesia through activation of serotonin receptors in the RVM[33, 60] showing an interaction between different analgesic pain sites. In people with fibromyalgia exercise-induced analgesia is associated with significant gene-to-gene interactions between the mu-opioid receptor and the serotonin transporter[31]. Specifically, stronger exercise-induced analgesia to a single isometric contraction is associated strong opioid signaling (OPRM1-G) in combination with low serotonin transporter expression[31]. Thus, interactions between opioid receptors and the serotonin transporter could occur through multiple different mechanisms. Future studies should determine which mechanisms contribute to the analgesic effects of exercise in animals and human subjects with pain.
4.2. Increases in SERT expression in the RVM underlie development of activity-induced hyperalgesia
The activity-induced pain model produces widespread hyperalgesia with decreases in muscle withdrawal thresholds and increases in paw withdrawal frequency to mechanical stimulation bilaterally. These data are consistent with prior studies using this model, as well as those in other animal models of muscle pain[55–57]. These models mimic clinical conditions with chronic widespread pain, like fibromyalgia[54]. These widespread pain conditions are associated with alterations in central excitability in both animal models and human subjects with fibromyalgia, show responsiveness to centrally directed pharmaceutical agents, and are responsive to exercise (for review see [54])[4, 51, 56, 64].
The current study shows that blockade of SERT in the RVM of sedentary mice reverses the activity-induced hyperalgesia, showing for the first time that alterations in the serotonin transporter, an inhibitory neurotransmitter system, plays a function role in hyperalgesia. Consistent with this hypothesis, classical pharmacological studies in uninjured animals show microinjection of 5-HT or a SERT inhibitor into the RVM increases the tail flick latency, blockade of serotonin receptors in the RVM prevents analgesia produced by stimulation of the PAG, systemic morphine increases 5-HT in the RVM, and application of 5-HT to RVM neurons excites 66% of RVM cells[33, 37, 38, 60]. Thus, we propose that increases in serotonin in the RVM mediate analgesia while decreases in serotonin mediate hyperalgesia.
The current data are also consistent with human studies in people with chronic widespread pain which show clinical efficacy of non-selective reuptake inhibitors in people with chronic widespread pain such as fibromyalgia[54], and reduced endogenous pain inhibition as measured by a conditioned pain modulation (CPM) test[32, 35, 54]. This altered inhibition may be related to altered serotonin levels since people with fibromyalgia have reduced serum and cerebrospinal levels of serotonin, its precursor, tryptophan, and its metabolite 5-HIAA[47, 48]. Simultaneously, there is reduced mu-opioid receptor binding in brain modulating regions in people with fibromyalgia, despite higher CSF levels of enkephalins[2, 25]. It is possible, therefore, that the loss of inhibition in those with chronic widespread pain is due to alterations in serotonin and opioid system.
4.3 Short-duration exercise does not alter p-NR1 expression in the RVM
We hypothesized that increases in p-NR1 in the RVM, induced in chronic muscle pain models, would be reduced by short-duration physical activity through activation of MOR in the RVM. MOR are expressed on and decreased activation of the ON-cells, pain facilitation neurons in the RVM[42, 43]. In models of chronic muscle pain, in the RVM, prior studies show increases in glutamate and p-NR1, blockade of NMDA receptors reduces hyperalgesia, and downregulation of NR1 prevents hyperalgesia, while over-expression of NR1 in uninjured animals produces mechanical hyperalgesia[11, 12, 46, 55, 56]. The current study shows increases in p-NR1 in the RVM after induction of activity-induced pain model, which is consistent with prior studies in chronic muscle pain models[11, 12, 55, 56]. Prior studies also show that wheel running reduced p-NR1 in both an activity-induced and a chronic muscle pain model [56]. This is in direct contrast to the current study, which shows that wheel running had no effect on the increased p-NR1 in a activity-induced pain model. The reasons for these differences are unclear but could be related to the duration of the wheel running prior to induction of the model, and/or the muscle insult. Our prior study showing 5-days of wheel running prevented p-NR1 expression in a activity-induced model using the same acute bout of 2h running wheel activity, but used a different muscle insult with a single-injection of a low-dose of carrageenan (0.03%)[56]. The inflammatory pain model could be easier to modulate with prior exercise than a non-inflammatory pain model like the one used in the current study. In support, we previously show that 8 weeks of wheel running was necessary to reduce hyperalgesia and p-NR1 in a non-inflammatory pain model induced by repeated acidic saline injection[56]. We propose that regular physical activity activates multiple, parallel mechanisms, and activation of these mechanisms occurs in a time-dependent fashion. For example, prior studies show an initial increase in MOR expression in hippocampus with short-duration exercise (7 days), which is followed by a decrease in MOR expression with longer-duration exercise (9 weeks)[15]. Similarly, neurogenesis and altered synaptic plasticity in the hippocampus occur after long-duration exercise (2-6 weeks), but not after short-duration exercise (3-7 days)[40]. Thus, alternate mechanisms that are activated with longer-term exercise may be necessary to modulate the p-NR1 increases in the RVM in the non-inflammatory activity-induced pain model.
4.4 Conclusion
In individuals with chronic musculoskeletal pain, increased fatiguing exercise exacerbates their pain not only during the activity but this effect can outlast the task for days[13, 35]. The current study suggests that activity-induced hyperalgesia activates multiple parallel mechanisms in the brainstem that result in increases in excitatory neurotransmitter activity (increased p-NR1), and decreased availability of inhibitory neurotransmitters (increased SERT). As little as 5 days of physical activity modulates the alterations in SERT and reduces secondary hyperalgesia through activation of MOR. Understanding the underlying mechanisms for how exercise increases pain, as well as the mechanisms for how exercise decreases pain could lead to new strategies to reduce the enhanced pain to unaccustomed exercise, and provide a time-course and scientific rationale to people with chronic pain to engage in regular physical activity.
Supplementary Material
Acknowledgments
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). This project was supported by NIH AR061371.
Footnotes
The authors declare no conflict of interest.
References
- 1.Arvidsson U, Cullheim S, Ulfhake B, Ramírez V, Dagerlind Å, Luppi PH, Kitahama K, Jouvet M, Terenius L, Åman K. Distribution of enkephalin and its relation to serotonin in cat and monkey spinal cord and brain stem. Synapse. 1992;11(2):85–104. doi: 10.1002/syn.890110202. [DOI] [PubMed] [Google Scholar]
- 2.Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48. doi: 10.1186/1471-2474-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7(1):309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 4.Bement MKH, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86(9):1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Bidonde J, Jean Busch A, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Current rheumatology reviews. 2014;10(1):45–79. doi: 10.2174/1573403x10666140914155304. [DOI] [PubMed] [Google Scholar]
- 6.Bobinski F, Ferreira TA, Córdova MM, Dombrowski PA, da Cunha C, do Espírito Santo CC, Poli A, Pires RG, Martins-Silva C, Sluka KA. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain. 2015;156(12):2595–2606. doi: 10.1097/j.pain.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown BS, Payne T, Kim C, Moore G, Krebs P, Martin W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol. 1979;46(1):19–23. doi: 10.1152/jappl.1979.46.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Carruba MO, Nisoli E, Garosi V, Sacerdote P, Panerai AE, Da Prada M. Catecholamine and serotonin depletion from rat spinal cord: effects on morphine and footshock induced analgesia. Pharmacol Res. 1992;25(2):187–194. doi: 10.1016/1043-6618(92)91387-v. [DOI] [PubMed] [Google Scholar]
- 9.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J. Noninvasive Treatments for Low Back Pain. 2016 [PubMed] [Google Scholar]
- 10.Da Silva L, DeSantana J, Sluka K. Activation of NMDA receptors in the brainstem, RVM and NGC, mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. The journal of pain: official journal of the American Pain Society. 2010;11(4):378. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Silva LF, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. The journal of pain. 2010;11(4):378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain. 2010;151(1):155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dailey DL, Keffala VJ, Sluka KA. Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res (Hoboken) 2015;67(2):288–296. doi: 10.1002/acr.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damsgard E, Thrane G, Anke A, Fors T, Røe C. Activity-related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil. 2010;32(17):1428–1437. doi: 10.3109/09638280903567877. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira MSR, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull. 2010;83(5):278–283. doi: 10.1016/j.brainresbull.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 16.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Current pain and headache reports. 2008;12(5):338–343. doi: 10.1007/s11916-008-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey S, Singh R, Dey P. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992;52(6):1095–1099. doi: 10.1016/0031-9384(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 18.Farrell PA, Gustafson AB, Garthwaite TL, Kalkhoff RK, Cowley A, Morgan W. Influence of endogenous opioids on the response of selected hormones to exercise in humans. J Appl Physiol. 1986;61(3):1051–1057. doi: 10.1152/jappl.1986.61.3.1051. [DOI] [PubMed] [Google Scholar]
- 19.Fields H, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74(4):1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 20.Galdino G, Duarte I, Perez A. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res. 2010;43(9):906–909. doi: 10.1590/s0100-879x2010007500086. [DOI] [PubMed] [Google Scholar]
- 21.Görlitz B-D, Frey H-H. Central monoamines and antinociceptive drug action. Eur J Pharmacol. 1972;20(2):171–180. doi: 10.1016/0014-2999(72)90146-x. [DOI] [PubMed] [Google Scholar]
- 22.Gowans SE. Effectiveness of exercise in management of fibromyalgia. Curr Opin Rheumatol. 2004;16(2):138–142. doi: 10.1097/00002281-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. The Journal of Pain. 2013;14(11):1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harasawa I, Johansen JP, Fields HL, Porreca F, Meng ID. Alterations in the rostral ventromedial medulla after the selective ablation of µ-opioid receptor expressing neurons. Pain. 2016;157(1):166–173. doi: 10.1097/j.pain.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinricher M, Morgan M, Tortorici V, Fields H. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63(1):279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann P, Terenius L, Thorén P. Cerebrospinal fluid immunoreactive β-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat. Regulatory Peptides. 1990;28(2):233–239. doi: 10.1016/0167-0115(90)90021-n. [DOI] [PubMed] [Google Scholar]
- 28.Hunt S, Lovick T. The distribution of serotonin, met-enkephalin and β-lipotropin-like immunoreactivity in neuronal perikarya of the cat brainstem. Neurosci Lett. 1982;30(2):139–145. doi: 10.1016/0304-3940(82)90286-5. [DOI] [PubMed] [Google Scholar]
- 29.Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011;1381:187–201. doi: 10.1016/j.brainres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19(1):13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 31.Jeanette T, Monika L, Kaisa M, Björn G, Anette L, Annie P, Indre B-L, Jan B, Martin I, Malin E. Gene-to-gene interactions regulate endogenous pain modulation in fibromyalgia patients and healthy controls-antagonistic effects between opioid and serotonin related genes. Pain. 2017 doi: 10.1097/j.pain.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1-2):295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Kiefel JM, Cooper ML, Bodnar RJ. Serotonin receptor subtype antagonists in the medial ventral medulla inhibit mesencephalic opiate analgesia. Brain Res. 1992;597(2):331–338. doi: 10.1016/0006-8993(92)91490-6. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y-J, Byun J-H, Choi I-S. Effect of Exercise on µ-Opioid Receptor Expression in the Rostral Ventromedial Medulla in Neuropathic Pain Rat Model. Annals of rehabilitation medicine. 2015;39(3):331–339. doi: 10.5535/arm.2015.39.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151(1):77–86. doi: 10.1016/j.pain.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Leung A, Gregory NS, Allen L-AH, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157(1):70–79. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llewelyn M, Azami J, Roberts M. The effect of modification of 5-hydroxytryptamine function in nucleus raphe magnus on nociceptive threshold. Brain Res. 1984;306(1-2):165–170. doi: 10.1016/0006-8993(84)90365-2. [DOI] [PubMed] [Google Scholar]
- 38.Llewelyn MB, Azami J, Roberts MH. Effects of 5-hydroxytryptamine applied into nucleus raphe magnus on nociceptive thresholds and neuronal firing rate. Brain Res. 1983;258(1):59–68. doi: 10.1016/0006-8993(83)91226-x. [DOI] [PubMed] [Google Scholar]
- 39.Mazzardo-Martins L, Martins DF, Marcon R, dos Santos UD, Speckhann B, Gadotti VM, Sigwalt AR, Guglielmo LGA, Santos ARS. High-intensity extended swimming exercise reduces pain-related behavior in mice: involvement of endogenous opioids and the serotonergic system. The Journal of Pain. 2010;11(12):1384–1393. doi: 10.1016/j.jpain.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Patten AR, Sickmann H, Hryciw BN, Kucharsky T, Parton R, Kernick A, Christie BR. Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn Memory. 2013;20(11):642–647. doi: 10.1101/lm.030635.113. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates: Gulf Professional Publishing. 2004 [Google Scholar]
- 42.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the µ-opioid receptor. The Journal of neuroscience. 2001;21(14):5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porreca F, Ossipov MH, Gebhart G. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 44.Potrebic SB, Mason P, Fields HL. The density and distribution of serotonergic appositions onto identified neurons in the rat rostral ventromedial medulla. The Journal of neuroscience. 1995;15(5):3273–3283. doi: 10.1523/JNEUROSCI.15-05-03273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. The Journal of neuroscience. 1997;17(1):45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett. 2009;457(3):141–145. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell IJ, Michalek JE, Vipraio GA, Fletcher EM, Javors MA, Bowden CA. Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome. J Rheumatol. 1992;19(1):104–109. [PubMed] [Google Scholar]
- 48.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35(5):550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 49.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157(2):387–398. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schul R, Frenk H. The role of serotonin in analgesia elicited by morphine in the periaqueductal gray matter (PAG) Brain Res. 1991;553(2):353–357. doi: 10.1016/0006-8993(91)90849-q. [DOI] [PubMed] [Google Scholar]
- 51.Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Phys Ther. 2010;90(5):714. doi: 10.2522/ptj.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. The Journal of Pain. 2005;6(1):41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Sluka KA. Mechanisms and management of pain for the physical therapist: Lippincott Williams & Wilkins. 2016. [Google Scholar]
- 54.Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sluka KA, Danielson J, Rasmussen L, Dasilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44(3):420. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sluka KA, O'Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 2013;114(6):725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148(2):188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Malan TP. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain ModelRole of Endogenous Opioids. The Journal of the American Society of Anesthesiologists. 2011;114(4):940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118(1):176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Taylor BK, Basbaum AI. Systemic morphine-induced release of serotonin in the rostroventral medulla is not mimicked by morphine microinjection into the periaqueductal gray. J Neurochem. 2003;86(5):1129–1141. doi: 10.1046/j.1471-4159.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- 61.Tillu D, Gebhart G, Sluka K. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136(3):331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treede R-D, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 63.Urban M, Gebhart G. Supraspinal contributions to hyperalgesia. Proceedings of the National Academy of Sciences. 1999;96(14):7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. The Journal of Pain. 2007;8(9):692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuo M, Sengupta J, Gebhart G. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87(5):2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.