Abstract
This paper reviews our current understanding of age-related meibomian gland dysfunction (MGD) and the role of the nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ), in the regulation of meibomian gland function, meibocyte differentiation and lipid synthesis. The studies suggest that PPARγ is a master regulator of meibocyte differentiation and function, whose expression and nuclear signaling coupled with meibocyte renewal is altered during aging, potentially leading to atrophy of the meibomian gland as seen in clinical MGD. Study of meibomian gland stem cells also suggest that there is a limited number of precursor meibocytes that provide progeny to the acini, that may be susceptible to exhaustion as occurs during aging and other environmental factors. Further study of pathways regulating PPARγ expression and function as well as meibocyte stem cell maintenance may provide clues to establishing cellular and molecular mechanisms underlying MGD and the development of novel therapeutic strategies to treating this disease.
Keywords: Meibomian gland, Dry Eye, Ocular Surface, Meibocyte, PPARγ, Stem Cell
1. Introduction
Meibomian glands are modified, holocrine sebaceous glands located in the upper and lower eyelid (Jester et al., 1981). Similar to sebaceous glands, meibomian glands excrete lipid or meibum onto the ocular surface forming a lipid layer in the uppermost portion of the tear film to provide a smooth optical surface and reduce aqueous tear evaporation (Mishima and Maurice, 1961; Nicolaides et al., 1981). Meibomian gland dysfunction (MGD), evidenced by gland dropout or altered lipid quality, is a common ocular surface disorder with widespread prevalence in the US population that increases with age (Hom et al., 1990; Lemp and Nichols, 2009; Ong, 1996; Ong and Larke, 1990). MGD is a major cause of evaporative dry eye disease (EDED) (Mathers and Lane, 1998), which is characterized by an unstable tear film leading to increased aqueous tear evaporation (Mishima and Maurice, 1961), increased tear film osmolarity (Gilbard et al., 1989), and blepharitis (McCulley and Shine, 2003; Shimazaki et al., 1995).
The proposed pathogenic mechanism underlying the most common form of MGD is thought to involve obstruction of the meibomian gland by hyperkeratinization of the ductal epithelium leading to blockage of the meibomian gland orifice, stasis of the gland, cystic dilatation and then atrophy of the holocrine, excretory acini (Foulks and Bron, 2003; Knop et al., 2011). Recent studies from our laboratory suggest that alternative pathways involving aging and stem cell exhaustion mediated by environmental stress may also play important roles in the development of meibomian gland dropout and altered meibum secretion. In this review article, we will present our latest understanding of the effects of aging, environmental stress and stem cell renewal on meibomian gland function with the goal of rethinking the pathogenesis of MGD and EDED as well as propose new treatments based on these new concepts.
2. Effects of Age on Human Meibomian Glands
Aging has long been recognized as a contributing factor to the development of MGD (Alsuhaibani et al., 2011; Hom et al., 1990; Hykin and Bron, 1992). While past work has primarily focused on the effects of hormones in contributing to these changes (Sullivan et al., 2006; Sullivan et al., 2002), little is known about how aging effects acinar meibocyte differentiation, lipid synthesis and function. In a study using excess surgical eyelid tissue from patients ranging in age from 18–95 years, we identified altered expression of the lipid sensitive, nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ) (Nien et al., 2011). PPARγ is a member of closely homologous genes ubiquitously expressed in various tissues, and is the major subtype expressed in adipocytes and sebocytes, where it regulates the expression of genes involved in lipogenesis (Rosen et al., 1999; Rosen and Spiegelman, 2001). Using immunocytochemistry, PPARγ appears to be highly expressed in meibomian gland acinar cells/meibocytes, showing a distinct cytoplasmic and nuclear localization in tissue samples from youngest patients (ages, 18 and 44 years) (Figure 1A). Interestingly, older individuals (>60 years) showed predominantly nuclear staining, with cytoplasmic staining limited to the basal acinar cells in 17 of 31 subjects (Figure 1B). Using antibodies against the nuclear antigen, Ki67 that labels actively cycling cells (Petroll et al., 1998), the number of positively stained basal acinar cells were significantly higher in meibomian glands from the younger compared with older subjects based on linear regression analysis (r2=0.35; P<.001) (Figure 1C and 1D). Together, these results indicate that aging human meibomian glands show both decreased meibocyte differentiation as identified by decreased and altered PPARγ expression and decreased meibocyte cell renewal as identified by Ki67 staining. These two findings suggest that the development of age-related MGD may involve altered PPARγ signaling and/or loss of stem cell renewal leading to acinar atrophy and development of an age-related MGD.
Figure 1.
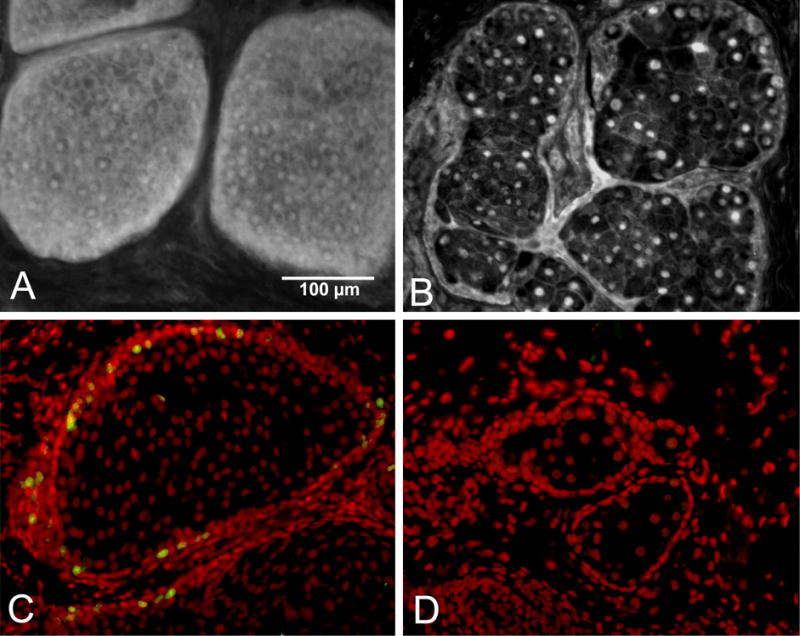
PPARγ localization in tissue obtained from a 44 yr (A) and 72 yr (B) subjects. Note the cytoplasmic acinar cell staining in the tissue from the younger subject that is lost in the older gland. Ki67 staining of tissue from the 44 yr (C) and 72 yr (D) subjects. Note that the younger gland show marked Ki67 labeling that is absent in the older the gland.
3. Mouse as a Model for Age-Related MGD
Mouse meibomian gland morphogenesis begins after eyelid fusion with the formation of regularly spaced epithelial placodes within the fused epithelium at embryonic day 18.5, which is followed by invasion of the epithelium into the underlying mesenchyme to form the presumptive meibomian gland duct (Nien et al., 2010). The first appearance of PPARγ expression and lipid synthesis begins at postnatal day 3 (PN3), both localized to the nascent lumen of the duct. Nascent acini first appear at PN5, showing both PPARγ expression and lipid synthesis. Fully developed meibomian glands with normal expression patterns for PPARγ are detected by PN15. Western blotting of meibomian gland proteins also detects a 50 kD protein that is the expected molecular weight of PPARγ, as well as a 72 kD band that is presumably a post-translationally modified PPARγ product. Overall, these findings indicate that meibomian gland development has distinct similarities to hair follicle development with the formation of an epithelial placode (Fuchs, 2007), and that PPARγ may play an important role in meibomian gland morphogenesis, function and meibocyte differentiation, with expression of PPARγ preceding formation of meibomian gland acini.
As the adult mouse ages, changes in PPARγ expression and meibocyte renewal in the meibomian gland that parallel changes seen in human meibomian glands are also detected (Nien et al., 2009). In evaluating eyelid tissue obtained from C57Bl/6 mice at 2, 6, 12 and 24 months of age, meibomian glands from younger mice (2 and 6 months) show cytoplasmic and nuclear PPARγ staining with abundant Oil Red O (ORO) staining for presence of neutral lipid (Figure 2, A and B), as contrasted by the loss of cytoplasmic PPARγ staining seen in meibomian glands of older mice (12 and 24 months) that also show less ORO staining (Figure 2, C and D). Interestingly, aging also leads to a significant loss of proliferative potential within the meibomian gland acini that can be detected by Ki67 labeling (P<.005), as well as a significant decrease in the size of the meibomian glands (P<.05) suggesting an age-related gland atrophy.
Figure 2.
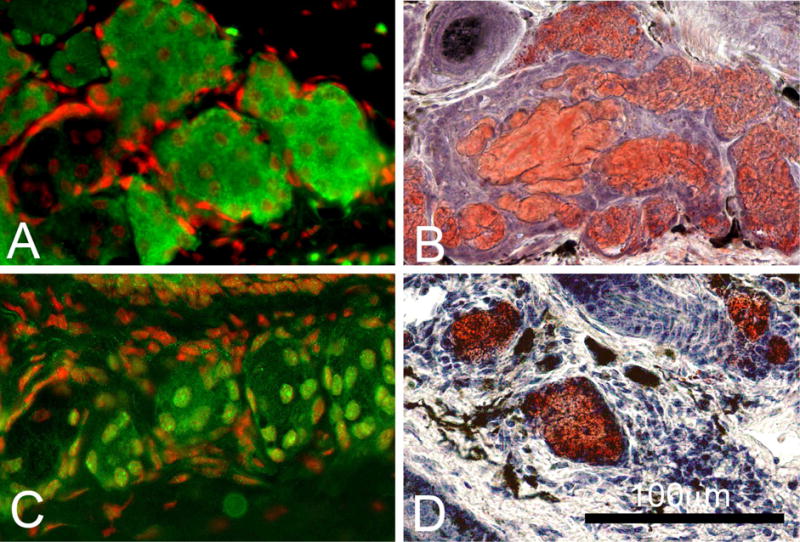
Following anti-PPARγ staining (Green) in adult mice we observed cytoplasmic and nuclear staining in 6 month old glands (A) that change to a nuclear staining in 2 year old mice (C). (Red=Dapi). Following Oil Red O (ORO) staining for neutral lipid in adult mice we observed abundant lipid droplets in 6 months old glands (B) while in 2 years old mice we observed fewer and more condensed ORO stained lipid droplets (D). (Red = ORO).
Age-related changes in PPARγ receptor signaling have also been studied by western blotting (Jester et al., 2016). When meibomian gland tissue extracts from young mice (2 months old) were separated into the cytoplasmic and nuclear fraction and probed for PPARγ expression, the cytoplasmic fraction contained both the 50 kD native protein and 72 kD post translationally modified protein, while the nuclear fraction contained only the 50 kD isoform. By comparison, meibomian gland extracts from old mice (2 years old) showed a loss of the 50 kD, a significant 75% reduction of 72 kD cytoplasmic PPARγ (P<.05), and a significant 40% decrease in the 50 kD nuclear PPARγ (P<.05). Together these finding confirm the immunocytochemical findings and show that the PPARγ receptor is significantly reduced with age, suggesting a significant loss of lipid synthesis.
Overall, these findings indicate that there is altered PPARγ receptor signaling in older mice that parallel changes in meibocyte renewal and lipid synthesis that is associated with age-related MGD in humans. Furthermore, the similarity in the age-related findings for human and mouse suggest that the mouse model may be a valuable tool in understanding the effects of age on meibomian gland function, as well as identifying the underlying mechanism of age-related MGD.
4. Absence of ductal hyper-keratinization in Mouse age-related MGD
Because age-related MGD in the mouse and human both lead to acinar atrophy and loss of the meibomian glands, we have investigated the role of hyperkeratinization in the development of age-related MGD in mouse as hyperkeratinization is putatively the most common pathway leading to acinar atrophy and meibomian gland dropout (Foulks and Bron, 2003; Knop et al., 2011). For a part of these studies we have developed a novel imaging approach, Immunofluorescent Tomography (IT), to localize and quantify multiple biomarkers of cell differentiation and function in large tissue volumes at high resolution (Parfitt et al., 2012). IT uses computer reconstruction of serial sectioned and sequentially immunostained butyl-methyl methacrylate (BMMA) embedded tissue (Figure 3). Using IT we have 3-dimensionally reconstructed the mouse lower eyelid that contains the meibomian gland and localized cell nuclei (DAPI), Ki67 and keratin proteins (Krt) 1, 5 and 6 to assess cell density, cell proliferation, gland keratinisation and gland volume.
Figure 3.
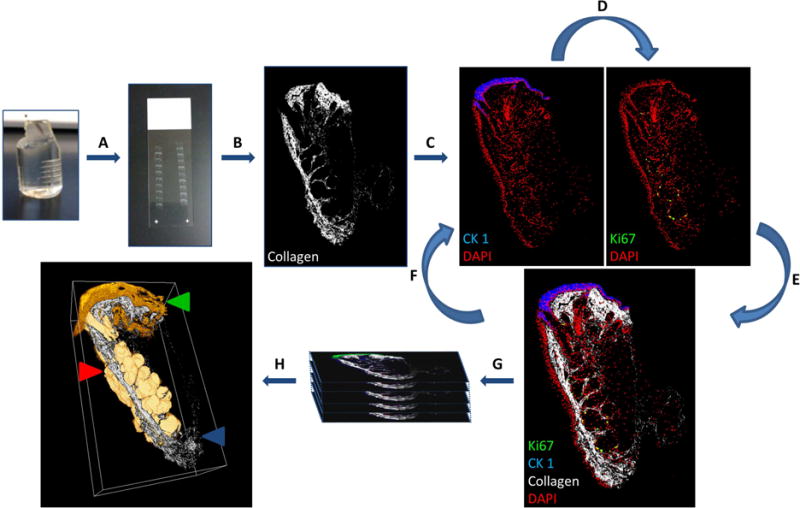
Stepwise IT protocol coupled with non-linear optical microscopy. (A) Serial section tissue embedded in plastic (2μm). (B) Second harmonic imaging visualises fibrillar collagen. (C) Immunofluorescent labelling and subsequent imaging. (D) Strip antibodies and re-stain to visualise multiple antigens. (E and F) Overlay images or repeat iterative antibody labelling. (G) Align and reconstruct. (H) Quantitative and qualitative analysis of cell populations may then be performed from 3-D reconstructions. (Red arrow) Meibomian gland segmented volume (Green arrow) CK 1 (Blue arrow) collagen SHG signal orthoslice.
In reconstructions of 5-month and 2-year old mouse eyelids, we detected a dramatic decrease in gland size, combined with a loss of ductal, acinar and lipid volume in old meibomian glands (Jester et al., 2011; Parfitt et al., 2013). Using immunocytochemistry to localize keratin biomarkers for keratinization (Krt1) and non-keratinized stratified epithelium (Krt6) (Figure 4), we detected extension of Krt1 from the skin epidermis into the orifice of the meibomian gland in young mice, with continued immunostaining posterior to the orifice where it abruptly stopped at the mucocutaneous junction (MCJ) and was replaced by Krt6 stained conjunctival epithelium. Krt1 staining also abruptly stopped at the orifice of the gland, where it was replaced again by Krt6 stained meibomian gland ductal epithelium. In old mouse meibomian glands, Krt1 staining from the skin epidermis extended up to the anterior border of the orifice, but did not extend posteriorly, indicating that the MCJ had been displaced anteriorly, and that the lid margin was less, not more, keratinized. Krt1 staining also did not appear to extend any deeper into the duct of the gland. Interestingly, epithelial plugs were detected in old meibomian glands, but these plugs were comprised of Krt5 and Krt6 immunostained epithelial cells, not Krt1.
Figure 4.
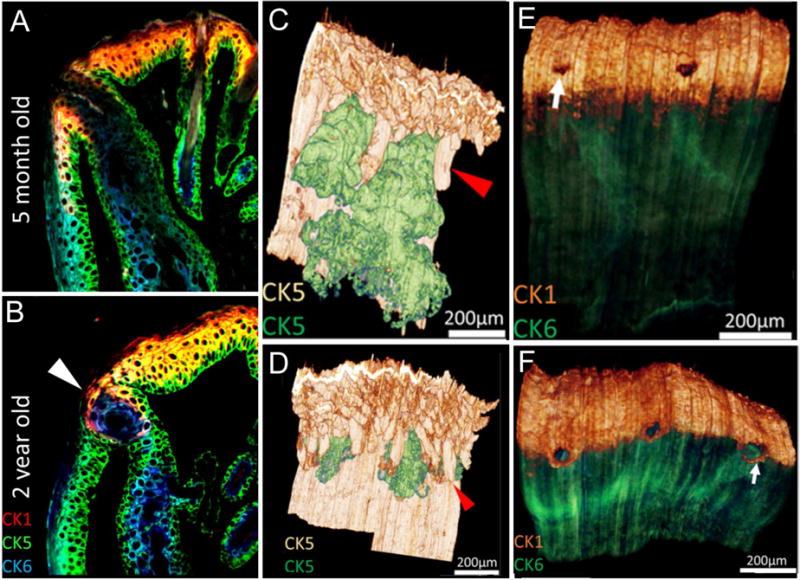
IT of eyelids from 5 month-old (A, C, E) and 2 year-old (B, D, F) mice. In single sections (A and B) sequentially stained for cytokeratin 1 (CK1, red), 5 (CK5, green) and 6 (CK6, blue) the mucocutaneous junction is detected by the transition from fully keratinized skin (CK1+) to non-keratinized conjunctiva (CK6+). Note the presence of ductal plugging with CK6+ epithelial cells in the 2 year-old eyelid (arrowhead). Volume reconstruction of anti-CK5 staining of eyelids (C and D) with segmentation of the meibomian glands (green) from skin epithelium (gold) shows marked atrophy of the meibomian glands in the 2 year-old compared to the 5 month-old eyelid. Reconstruction of young and old eyelids (E and F) stained for CK1 epidermis (gold) and CK6 conjunctiva (green) show the anterior migration of the mucocutaneous junction (E and F, arrow).
Overall, these findings support the premise that hyperkeratinization leading to gland obstruction does not play a role in the development of age-related MGD in the mouse. Additionally, the anterior displacement of the MCJ in old mouse eyelids appears to mimic the anterior displacement of the MCJ, clinically identified as Marx line, observed in aging humans (Bron et al., 2011a, b; Yamaguchi et al., 2006). Since there is a strong correlation between the anterior displacement of the MCJ and the development of clinical MGD (Yamaguchi et al., 2006), these findings also bring into question the role of hyperkeratinization in the development of human MGD (Jester et al., 2015).
To further evaluate the role of keratinisation in meibomian gland function we have identified the gene expression patterns of young and old mouse meibomian glands by performing a transcriptome analysis using RNA sequencing (Parfitt et al., 2016a). The results of differential gene expression data indicated that 18 genes were 2 fold enriched in meibomian glands of young male and female mice while 151 genes were enriched in old meibomian glands. Of genes showing significant differences, a number of pathways were identified by GO ontology involving genes known to be associated with PPARγ function that were decreased in old tissue. Of particular interest was the finding that there was no significant difference in the expression of keratinisatio-related genes, including Krt 1 and Krt10, as well as cornified envelope proteins, Sprr1a and Sprr2a, that are necessary for keratinization. Together, these findings support our immunocytochemical and biochemical findings indicating that PPARγ signaling is significantly altered in age-related MGD, and that obstruction due to hyperkeratinization plays little, if any, role.
5. PPARγ Control of Mouse Meibocyte Differentiation
To begin to understand the role of PPARγ in regulating meibomian gland function, we developed an immortalized mouse meibocyte cell line using a lentiviral vector containing the SV40 T-antigen (Jester et al., 2016). Immortalized, lipid synthesizing clones were tested for effects of PPARγ agonist, rosiglitazone, on lipid synthesis and PPARγ localization, post translational modification and induction of PPARγ response genes. Cultured meibocytes produced neutral lipid containing equal amounts of wax and cholesterol esters, similar to mouse meibum. Addition of rosiglitazone (10–50 μM) produced a significant 8–9 fold increase in neutral lipid accumulation by meibocytes in culture (P<.05). Increased lipid synthesis was also associated with a significant accumulation of cytoplasmic 50 kD and 72 kD PPARγ. Furthermore, immunoprecipitation and western blotting detected sumoylation of PPARγ by SUMO1 indicating that the 72 kD PPARγ was indeed a post translational modification of PPARγ that was associated with receptor signaling and lipid synthesis in meibocytes. Rosiglitazone also up-regulated mRNA for PPARγ, adiponectin and adipocyte differentiation related protein, all genes that are important in adipocyte differentiation and lipid synthesis. Overall these findings support the premise that PPARγ signaling regulates lipid synthesis of meibocytes. Furthermore, the loss of cytoplasmic/vesicular PPARγ localization in older human and mouse meibomian glands indicates that there is a loss in the ability of meibomian glands to synthesize and secrete meibum, suggesting a loss in meibocyte differentiation.
6. Effect of Desiccating Stress on Mouse Meibomian Gland Function
The only available animal model to study the underlying mechanisms of dry eye is a desiccating stress, mouse model shown to induce ocular surface inflammation (Stern et al., 1998a, b). Using this model, we have recently evaluated the effects of desiccating stress on meibomian gland function (Suhalim et al., 2014). In our study, low humidity stress caused a 3-fold increase in basal acinar cell proliferation from 18.3 ±11.1% in untreated mice to 64.4 ± 19.9% and 66.6 ± 13.4% after 5 and 10 days exposure, respectively (P < .001) (Figure 5). Since the meibomian gland is a holocrine gland, in which cells undergo differentiation and disintegration to release lipid, the increase proliferation suggests an increase in meibocyte renewal compensatory to the increase disintegration and release of lipid into the meibomian gland duct. This increased release was also suggested by dilation of the duct as shown in Figure 5.
Figure 5.
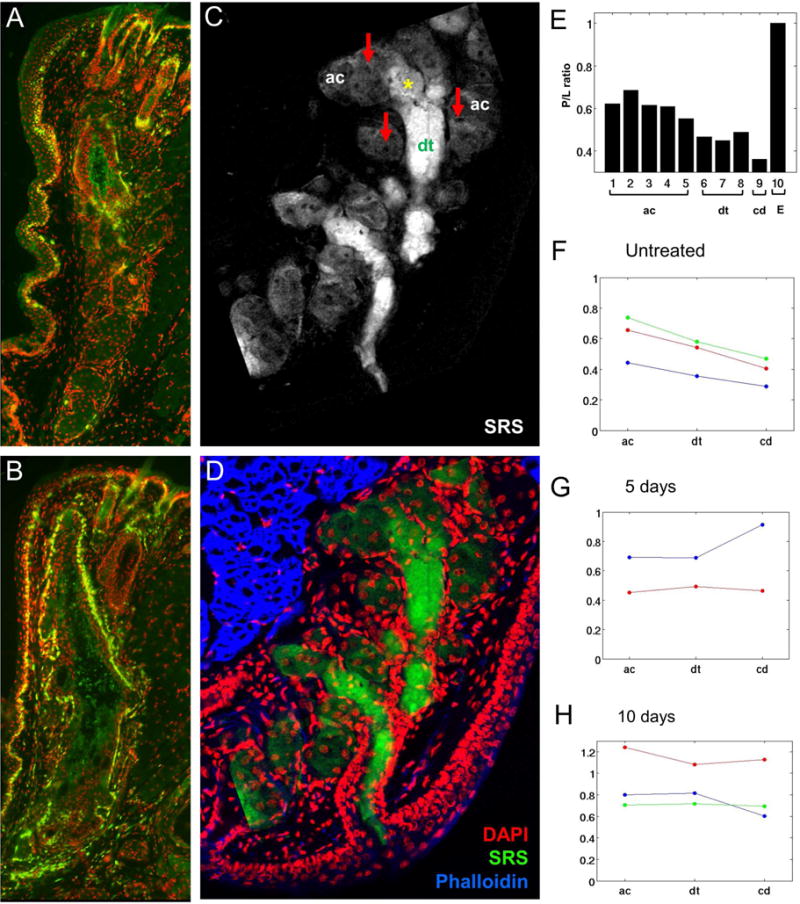
Effects of environmental stress on meibomian gland cell proliferation (A and B) and lipid quality (C–H). Cryosections of eyelids from normal mice (A) and mice exposed to 10 days of desiccating stress (B) stained for the cell cycling marker, Ki67 (green). SRS microscopy of lipid within cryosections from eyelids of normal mice (C) and the overlay (D) with immunostaining for nuclei (DAPI, red), and actin (Phalloidin, blue) to identify acini (ac, arrows) and ductules (dt). E, Graph of protein to lipid ratio from different regions of the gland, including acini (ac), ductule (dt), central duct (cd) and ECM (E). F–H, graph of protein to lipid ratio for control mice (F) and mice exposed to 5 days (G) and 10 days (H) desiccating stress. Note that in the normal gland there is low glandular proliferation (A) and a gradual decreasing protein to lipid ratio moving from the acini to the central duct (E and F). By comparison meibomian glands from mice exposed to desiccating stress show up-regulation of cell cycling (B) and no change in the protein to lipid ratio (G and H).
In this study we also assessed changes in meibum content using stimulated Raman scattering (SRS) microscopy, a multidimensional imaging method detecting chemical vibrational signals, to measure the content of protein (amide I) and lipid (CH2) (Lin et al., 2011). Using SRS to evaluate tissue sections taken from the mouse eyelid, we have established for the first time that acinar/ductule regions contain meibum that has a high protein content that decreases moving from the ductule to the central duct to the orifice of the gland. This finding indicates that meibum undergoes a maturation process with removal of protein prior to excretion onto the ocular surface. Interestingly, meibum in meibomian glands of mice exposed to desiccating stress showed little or no decrease in protein throughout the ductal system, and at times increased protein, suggesting that desiccating stress leads to alterations in lipid/protein content. How this effects meibum quality is not known; however, the failure to adequately remove protein may effect lipid fluidity based on recent studies assessing the effects of adding purified keratin proteins to meibum lipid that show an increased surface pressure above meibum in Lagmuir trough experiments (Palaniappan et al., 2013). Based on these findings, it is likely that retention of proteins in meibum may lead to a more rigid lipid tear film layer that is subject to fracture and instability, potentially explaining the effects of MGD on tear film stability.
Overall, these data are consistent with the model that environmental stress directly effects meibomian gland function, leading to increased basal acinar cell proliferation, abnormal meibocyte differentiation, and altered lipid production. Not only may environmental stress lead to changes in lipid quality, but continual or repeated exposure may potentially effect meibocyte renewal by depleting meibocyte stem cells, leading to early aging and gland atrophy.
7. Meibomian Gland Stem cells
Until recently, the location and characterization of meibomian gland stem cells was controversial with Lavker et al suggesting that stem cells were located along the central duct, similar to the location of hair follicle stem cells along the hair follicle ‘bulge’ region (Lavker et al., 2003), and Olami et al suggesting that stem cells were located at the interface between the acinar and ductal basal cells (Olami et al., 2001). To address this question, we used the H2B-GFP/K5tTA ‘tet-off’ transgenic mouse to initially label proliferating cells with nuclear GFP and then follow labeled cells after tetracycline treatment to turn-off nuclear GFP expression (Parfitt et al., 2015). IT was then used to identify, localize and quantify the label retaining cells (LRCs) at different intervals after tetracycline treatment. In our study, there was a rapid loss of GFP labeled cells due to either dilution (continued proliferation to dilute nuclear label) or differentiation and disintegration. By 28 days after tetracycline treatment, there were on average only 25±4 GFP labeled cells within individual meibomian glands, located at the interface between the acini and the ductal epithelium. The number of GFP labeled cells continued to decline over time with all LRCs observed at the interface between Krt6+ ductal epithelium and Krt6−/PPARγ+ acinar basal epithelium of the meibomian gland. Additionally, LRCs in the meibomian gland were exclusively SOX9+ and BLIMP1−, putative sebocyte progenitor markers, however both SOX9+ and BLIMP1+ differentiated ductal and acinar epithelial cells could also be detected. Overall, these data suggest that meibomian gland stem cells are located at the interface between the ductal and acinar basal epithelium.
Since meibocytes rapidly transit through the acinus, migrating from the basal compartment to the disintegrating compartment within 9 days (Olami et al., 2001), continuous basal meibocyte renewal is critical to normal meibomian gland function. To begin to understand this process we have used lineage tracing to better assess the origin and number of stem cell that participate in this process (Parfitt et al., 2016b). For these studies we have used Krt14CreERT2-Confetti mouse, which express the conditional Brainbow 2.1 allele which incorporates open reading frames for membrane-bound mCerulean (CFP), nuclear GFPII (GFP), cytoplasmic monomeric enhanced yellow fluorescent protein (YFP) and tdimer2(12) (RFP) (Amitai-Lange et al., 2015; Di Girolamo et al., 2015). When Confetti mice are crossed with tamoxifen-activable K14CreER mice, ten possible recombination’s of the four fluorescent proteins can occur within a single dividing cell that is Krt14+. Since progeny of these cells are uniquely marked, lineage tracing can be performed to determine the fate of progenitor cells/stem cells that are responsible for meibocyte renewal. As shown in Figure 6, meibomian glands from Confetti mice show unique cell populations that can be identified by the differential expression of fluorescent tags controlled by the brainbow cassette. Interestingly, the meibomian gland duct appears to be comprised of cells from multiple origins, while cells within a single acinus appear to be derived from the same cell. It was also interesting to note that there was no labeling of ductal epithelium adjacent to labeled acini, indicating that the cells giving rise to meibocytes were uni-potential. These finding suggests that acini are derived from a single stem cell, that once depleted could result in acinar atrophy and dropout. By comparison, the duct is renewed by multiple progenitor cells of different origin. These new findings, coupled with our earlier observations suggest the hypothesis that meibomian gland LRC/stem cells may have two different stem cell populations that give rise independently to both ductal epithelium and acinar meibocytes. Since acinar stem cells are localized to sites of individual acini, it is possible that exhaustion of these stem cells lead to loss of individual acini, and meibomian gland dropout as seen in clinical MGD.
Figure 6.
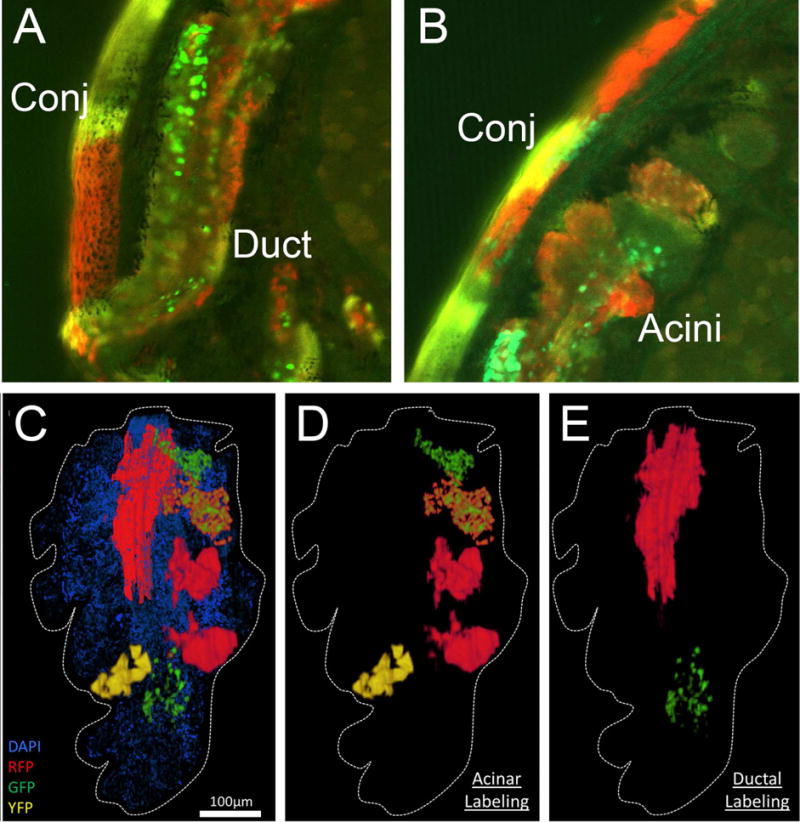
Confocal imaging of vibratome section taken from the eyelid of Confetti mouse showing the orifice and duct (A) and acini (B) of one meibomian gland. C) IT, high-resolution 3-D reconstruction of lineage-tracing in the K14CreERT2-Confetti mouse shown in (C) illustrates that acini were independently labeled (D) and the duct was comprised of more than a single lineage (E).
8. New pathogenesis of meibomian gland dysfunction
The most commonly recognized mechanism for the development of MGD has been a ‘ductal centric’ hypothesis involving epithelial hyperkeratinization causing obstruction of meibomian orifice, stasis of meibum, and cystic dilation of the duct that leads to a secondary, disuse acinar atrophy and gland dropout (Knop et al., 2011). By contrast, our studies suggest a ‘meibocyte centric’ hypothesis involving mechanisms that regulate differentiation and renewal of meibocytes that may directly impact meibum quality, lipid synthesis and acinar atrophy without confounding changes in the ductal epithelium. A key finding in support of this alternative hypothesis has been the observation of altered PPARγ receptor localization and expression in older individuals and animal models. Our studies have shown that PPARγ is expressed as early as PN3 during meibomian gland development, and is perhaps responsible for both the formation of the ductal lumen as well as the differentiation of epithelia to a meibocyte phenotype at PN5. Furthermore, the changes in PPARγ expression detected in aging individuals suggest a loss of differentiation and ability to synthesize lipid, as evidenced by both the decreased 50 kD PPARγ in the nucleus and cytoplasm, and the loss of the 72 kD PPARγ that is a post translational modification associated with active lipid synthesis. Since these changes appear to occur in the absence of hyperkeratinization and ductal dilation, it is not likely that the observed changes in PPARγ signaling are the result of a ‘disuse atrophy’ as proposed for obstructive MGD. Rather, it is more likely that factors regulating PPARγ receptor expression and function play the central role.
Unfortunately, little is known regarding the molecular pathways controlling PPARγ-regulated meibocyte differentiation. Some possible factors that may influence this pathway are presented in Figure 7. In particular aging and undefined age-related factors may clearly play a role, since our experimental findings are common in older individuals and animals. Additionally, increased tear evaporation associated with low humidity and increased airflow also leads to increased meibocyte proliferation, differentiation and altered lipid synthesis, suggesting that a range of environmental stress responses may effect meibocyte differentiation, including contact lens wear, long known to cause MGD (Henriquez and Korb, 1981; Korb and Henriquez, 1980), prolonged blinking intervals associated with video display terminal usage and reading (Fenga et al., 2008), and low humidity environments (McCulley et al., 2006). Hormonal factors are also known to be associated with ocular surface disease and thought to be involved in the development of MGD (Sullivan et al., 2006; Sullivan et al., 2002). Dietary factors also influence meibomian gland differentiation, as recently shown in the Hairless mouse fed on a limited lipid diet containing low amounts of lipids known to be PPARγ agonists (Miyake et al., 2016). Neurogenic factors, while little studied, are likely to play an important role since the meibomian gland is highly innervated, unlike their counterpart, the sebaceous gland (Kam and Sullivan, 2011; Kirch et al., 1996). Finally, recent studies indicate that inflammation and allergy may have marked effects on meibomian gland function leading to plugging, ductal dilation and gland hypertrophy (Reyes and Saban, 2016).
Figure 7.
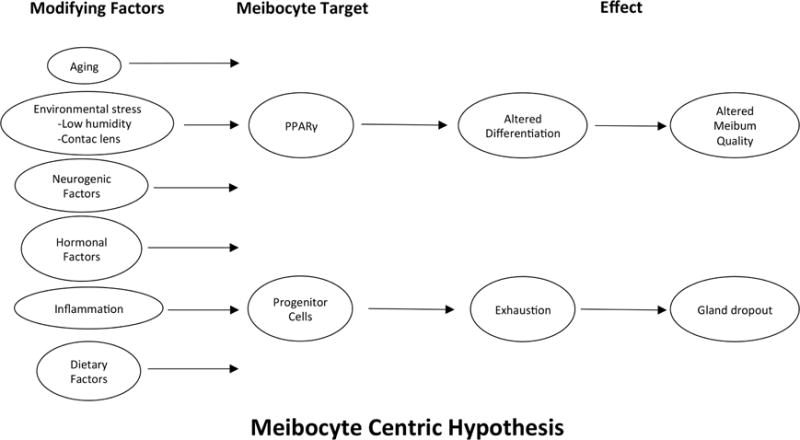
‘Meibocyte Centric’ hypothesis. Possible factors that may be involved in the development of MGD.
A second pathway leading to altered meibocyte function is suggested by the finding of a limited number of meibomian gland LRC, suggesting a limited stem cell population. The additional finding that acini are renewed by a single stem cell adjacent to individual acini, further suggest that stem cell exhaustion or depletion may play an important role in the loss of acini due to age. Our finding that environmental factors may influence the proliferative rate of meibocytes, also suggest that early stem cell depletion may underlie meibomian gland dropout and acinar atrophy in younger individuals. There are only a handful of studies that have evaluated meibomian gland stem cells, and little is known about control of meibomian gland stem cell fate and survival. Nevertheless, similar factors influencing meibocyte differentiation as presented in Figure 7, may also play a role in stem cell maintenance as discussed above.
9. Future Direction and Therapeutic Strategies
Clearly, a better understanding of the cellular and molecular pathways regulating meibomian gland function and meibocyte differentiation are needed to help establish a clearer mechanistic foundation for the development and progression of MGD. The recent establishment of a telomerized human meibomian gland epithelial cell line will undoubtedly help toward the discovery of some of these pathways (Liu et al., 2010). Additionally, better models of MGD in mice are needed to validate putative pathways and understand the relationship between meibomian gland function, meibocyte differentiation and ocular surface integrity and disease. Such models, both in vitro and in vivo, will also help in the discovery of new therapies that may restore meibomian gland function and/or reverse pathologic changes leading to ocular surface disease. Some potential targets are suggested by our studies of PPARγ, and include agonist, such as Rosiglitazone or other drugs currently used in diabetes, that stimulate PPARγ receptor signaling and induce lipid synthesis and potentially meibocyte differentiation. Understanding the effects of upstream regulators of PPARγ and how they influence PPARγ signaling may also lead to the development of new approaches to treating MGD. Finally, a fuller understanding of meibocyte renewal and the maintenance of meibocyte stem cells may have a dramatic impact on our ability to maintain meibomian gland function in patients that show marked loss of meibomian glands as well as regenerate meibomian glands and acini to replace lost tissue.
Highlights.
This review discusses our current understanding of age-related meibomian gland dysfunction (MGD) and the role of the nuclear receptor, peroxisome proliferator-activated receptor gamma (PPARγ). Our finding suggest that PPARγ is a master regulator of meibocyte differentiation and function, and that altered receptor signaling and progenitor cell depletion may underlie the development of MGD.
Acknowledgments
Support in part by: NEI EY021510, the Skirball Program in Molecular Ophthalmology, Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsuhaibani AH, Carter KD, Abramoff MD, Nerad JA. Utility of meibography in the evaluation of meibomian glands morphology in normal and diseased eyelids. Saudi J Ophthalmol. 2011;25:61–66. doi: 10.1016/j.sjopt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem cells. 2015;33:230–239. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Yokoi N, Gaffney EA, Tiffany JM. A solute gradient in the tear meniscus. I. A hypothesis to explain Marx’s line. Ocul Surf. 2011a;9:70–91. doi: 10.1016/s1542-0124(11)70014-3. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Yokoi N, Gaffney EA, Tiffany JM. A solute gradient in the tear meniscus. II. Implications for lid margin disease, including meibomian gland dysfunction. Ocul Surf. 2011b;9:92–97. doi: 10.1016/s1542-0124(11)70015-5. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Bobba S, Raviraj V, Delic NC, Slapetova I, Nicovich PR, Halliday GM, Wakefield D, Whan R, Lyons JG. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem cells. 2015;33:157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]
- Fenga C, Aragona P, Cacciola A, Spinella R, Di Nola C, Ferreri F, Rania L. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye (Lond) 2008;22:91–95. doi: 10.1038/sj.eye.6703025. [DOI] [PubMed] [Google Scholar]
- Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- Henriquez AS, Korb DR. Meibomian glands and contact lens wear. Br J Ophthalmol. 1981;65:108–111. doi: 10.1136/bjo.65.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci. 1990;67:710–712. doi: 10.1097/00006324-199009000-00010. [DOI] [PubMed] [Google Scholar]
- Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- Jester BE, Nien CJ, Winkler M, Brown DJ, Jester JV. Volumetric reconstruction of the mouse meibomian gland using high-resolution nonlinear optical imaging. Anat Rec (Hoboken) 2011;294:185–192. doi: 10.1002/ar.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20:537–547. [PubMed] [Google Scholar]
- Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015;15(Suppl 1):156. doi: 10.1186/s12886-015-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Potma E, Brown DJ. PPARgamma Regulates Mouse Meibocyte Differentiation and Lipid Synthesis. Ocul Surf. 2016;14:484–494. doi: 10.1016/j.jtos.2016.08.001. doi: 410.1016/j.jtos.2016.1008.1001. Epub 2016 Aug 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam WR, Sullivan DA. Neurotransmitter influence on human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:8543–8548. doi: 10.1167/iovs.11-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch W, Horneber M, Tamm ER. Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis) Anat Embryol (Berl) 1996;193:365–375. doi: 10.1007/BF00186693. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51:243–251. [PubMed] [Google Scholar]
- Lavker RM, Treet J, Sun T. Label-retaining cells (LRCs) are preferentially located in the ductal epithelium of the meibomian gland: Implications on the mucocutaneous junctional (MCJ) epithelium of the eyelid. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 3781. [Google Scholar]
- Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7:S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- Lin CY, Suhalim JL, Nien CL, Miljkovic MD, Diem M, Jester JV, Potma EO. Picosecond spectral coherent anti-Stokes Raman scattering imaging with principal component analysis of meibomian glands. J Biomed Opt. 2011;16:021104. doi: 10.1117/1.3533716. doi: 021110.021117/021101.3533716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, Immortalization and Characterization of Human Meibomian Gland Epithelial Cells. Invest Ophthalmol Vis Sci. 2010;51:3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Uchiyama E, Aronowicz JD, Butovich IA. Impact of evaporation on aqueous tear loss. Trans Am Ophthalmol Soc. 2006;104:121–128. [PMC free article] [PubMed] [Google Scholar]
- Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- Miyake H, Oda T, Katsuta O, Seno M, Nakamura M. Meibomian Gland Dysfunction Model in Hairless Mice Fed a Special Diet With Limited Lipid Content. Invest Ophthalmol Vis Sci. 2016;57:3268–3275. doi: 10.1167/iovs.16-19227. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- Nien CJ, Massei S, Lin G, Liu H, Paugh JR, Liu CY, Kao WW, Brown DJ, Jester JV. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–1140. [PMC free article] [PubMed] [Google Scholar]
- Nien CJ, Massei S, Lin G, Nabavi C, Tao J, Brown DJ, Paugh JR, Jester JV. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol. 2011;129:462–469. doi: 10.1001/archophthalmol.2011.69. doi: 410.1001/archophthalmol.2011.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien CJ, Paugh JR, Massei S, Wahlert AJ, Kao WW, Jester JV. Age-related changes in the meibomian gland. Exp Eye Res. 2009;89:1021–1027. doi: 10.1016/j.exer.2009.08.013. doi: 1010.1016/j.exer.2009.1008.1013. Epub 2009 Sep 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olami Y, Zajicek G, Cogan M, Gnessin H, Pe’er J. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Res. 2001;33:170–175. doi: 10.1159/000055665. [DOI] [PubMed] [Google Scholar]
- Ong BL. Relation between contact lens wear and Meibomian gland dysfunction. Optom Vis Sci. 1996;73:208–210. doi: 10.1097/00006324-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10:144–148. doi: 10.1111/j.1475-1313.1990.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Palaniappan CK, Schutt BS, Brauer L, Schicht M, Millar TJ. Effects of keratin and lung surfactant proteins on the surface activity of meibomian lipids. Invest Ophthalmol Vis Sci. 2013;54:2571–2581. doi: 10.1167/iovs.12-11084. [DOI] [PubMed] [Google Scholar]
- Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Mol Vis. 2016a;22:518–527. eCollection 2016. [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Geyfman M, Xie Y, Jester JV. Characterization of quiescent epithelial cells in mouse meibomian glands and hair follicle/sebaceous glands by immunofluorescence tomography. J Invest Dermatol. 2015;135:1175–1177. doi: 10.1038/jid.2014.484. doi: 1110.1038/jid.2014.1484. Epub 2014 Nov 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Lewis PN, Young RD, Richardson A, Lyons JG, Di Girolamo N, Jester JV. Renewal of the Holocrine Meibomian Glands by Label-Retaining, Unipotent Epithelial Progenitors. Stem Cell Reports. 2016b;7:399–410. doi: 10.1016/j.stemcr.2016.07.010. doi: 310.1016/j.stemcr.2016.1007.1010. Epub 2016 Aug 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Xie Y, Geyfman M, Brown DJ, Jester JV. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD) Aging (Albany NY) 2013;5:825–834. doi: 10.18632/aging.100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Xie Y, Reid KM, Dervillez X, Brown DJ, Jester JV. A novel immunofluorescent computed tomography (ICT) method to localise and quantify multiple antigens in large tissue volumes at high resolution. PloS one. 2012;7:e53245. doi: 10.1371/journal.pone.0053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Jester JV, Bean JJ, Cavanagh HD. Myofibroblast transformation of cat corneal endothelium by transforming growth factor-beta1, -beta2, and -beta3. Invest Ophthalmol Vis Sci. 1998;39:2018–2032. [PubMed] [Google Scholar]
- Reyes N, Saban DR. Pathogenesis of meibomian gland dysfunction (MGD) requires the T cell-neutrophil axis, in the allergy setting. Invest Ophthalmol Vis Sci. 2016;57(12):1431. [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998a;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. A unified theory of the role of the ocular surface in dry eye. Adv Exp Med Biol. 1998b;438:643–651. doi: 10.1007/978-1-4615-5359-5_91. [DOI] [PubMed] [Google Scholar]
- Suhalim JL, Parfitt GJ, Xie Y, De Paiva CS, Pflugfelder SC, Shah TN, Potma EO, Brown DJ, Jester JV. Effect of desiccating stress on mouse meibomian gland function. Ocul Surf. 2014;12:59–68. doi: 10.1016/j.jtos.2013.1008.1002. Epub 2013 Oct 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Sullivan BD, Evans JE, Schirra F, Yamagami H, Liu M, Richards SM, Suzuki T, Schaumberg DA, Sullivan RM, Dana MR. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Kutsuna M, Uno T, Zheng X, Kodama T, Ohashi Y. Marx line: fluorescein staining line on the inner lid as indicator of meibomian gland function. Am J Ophthalmol. 2006;141:669–675. doi: 10.1016/j.ajo.2005.11.004. [DOI] [PubMed] [Google Scholar]


