Abstract
Background
Childhood blood lead levels (BLL) have been associated with growth impairment.
Objectives
We assessed associations of peripubertal BLL with adolescent growth and near adult height in a longitudinal cohort of Russian boys.
Methods
481 boys were enrolled at ages 8–9 years and followed annually to age 18. At enrollment, BLL was measured, and height, weight, and pubertal staging were obtained annually during 10 years of follow-up. Mixed effects models were used to assess the associations of BLL with longitudinal age-adjusted World Health Organization Z-scores for height (HT-Z) and body mass index (BMI-Z), and annual height velocity (HV). Interactions between boys’ age and BLL on growth outcomes were evaluated.
RESULTS
The median (range) BLL was 3.0 (0.5–31.0) μg/dL. At age 18 years, 79% of boys had achieved near adult height (HV <1.0 cm/year), and means (SD) for HT-Z and BMI-Z were 0.15 (0.92) and −0.32 (1.24). Over 10 years of follow-up, after covariate adjustment, boys with higher (≥ 5 μg/dL) BLL compared with lower BLL were shorter (adjusted mean difference in HT-Z = −0.43, 95% CI −0.60, −0.25, p-value <0.001), translating to a 2.5 cm lower height at age 18 years. The decrement in height for boys with higher BLL was most pronounced at 12 to 15 years of age (interaction p=0.03). Boys with higher BLL were leaner (adjusted mean difference in BMI-Z = −0.22, 95% CI: −0.45, 0.01, p=0.06).
Conclusions
Higher peripubertal BLLs were associated with shorter height through age 18 years, suggesting a persistent effect of lead on linear growth.
Keywords: lead, metals, children, childhood growth, height, body mass index
1. Introduction
Exposure to lead during childhood has been associated with a broad spectrum of deleterious health effects (Bellinger 2011). Although childhood lead exposure in the U.S. has been dramatically reduced over the past fifty years, primarily through elimination of leaded gasoline and paint and lead abatement in housing stock, blood lead levels (BLLs) above the CDC’s current reference level of 5 μg/dL continue to be seen, particularly in lower socioeconomic communities (Raymond and Brown 2015) and areas contaminated from industrial sources (Brink et al. 2016; Laidlaw et al. 2016). There is renewed interest in the effects of lead on children’s health in the U.S due to recent incidents of increased lead exposure via contaminated drinking water from lead-containing water distribution and plumbing infrastructure (Hanna-Attisha et al. 2016).
High BLL in childhood has been associated with neurological and behavioral effects (Rauh and Margolis 2016) and later pubertal onset (Hauser et al. 2008; Selevan et al. 2003; Williams et al. 2010; Wu et al. 2003). Evidence also links high BLL during early childhood with lower height and weight (Ballew et al. 1999; Cantoral et al. 2015; Cassidy-Bushrow et al. 2016; Ignasiak et al. 2006; Kafourou et al. 1997; Little et al. 2009; Min et al. 2008; Schwartz et al. 1986). However, no longitudinal studies have examined whether the negative effects of lead on height and body mass index (BMI; kg/m2) in childhood persist and ultimately result in reduced adult height.
Previously, we reported that higher peripubertal BLLs (≥5 μg/dL) measured at age 8 to 9 years in our cohort were associated with lower height (Burns et al. 2012), delayed pubertal onset (Hauser et al. 2008; Williams et al. 2010) and reduced insulin-like growth factor 1 (IGF-1) (Fleisch et al. 2013) at ages 12–13 years. In the current analysis, we assess the longitudinal relationship of peripubertal BLL with height and BMI over 10 years of follow-up, through age 18, when most young men have achieved sexual maturity (Burns et al. 2016) and near adult height.
2. Methods
2.1. Study population
The Russian Children’s Study is a prospective cohort of 499 boys residing in Chapaevsk, Russia, enrolled in 2003–2005 at ages 8–9 years (Hauser et al. 2005) and followed annually through 2012–2015 to age 18 years. For this analysis, 10 boys in the original cohort were excluded due to chronic illnesses that could affect growth and/or pubertal development. Of the remaining 489 subjects, 481 (98%) with baseline BLL measurements were included for this analysis. The study was approved by the Human Studies Institutional Review Boards of the Chapaevsk Medical Association, Harvard T.H. Chan School of Public Health, University of Massachusetts Medical School, and Brigham and Women’s Hospital. Before participation, the parent/guardian provided informed consent and the boys signed assent forms; at age 18 years the young men signed informed consent forms.
At study entry, each boy’s parent or guardian completed nurse-administered health and lifestyle questionnaires (Lee et al. 2003), ascertaining birth and medical history, and demographic and socioeconomic status (SES) information. A validated Russian Institute of Nutrition semi-quantitative food frequency questionnaire was used to characterize each child’s diet (Martinchik et al. 1998; Rockett et al. 1997).
2.2. Physical examination
At study entry and annual follow-up visits, a standardized anthropometric examination (http://www.cdc.gov/nchs/products/elec_prods/subject/video.htm) was performed by a trained research nurse and pubertal staging was performed by a single physician-investigator (O.S.) according to a written protocol. Height was measured to the nearest 0.1 cm using a stadiometer. Weight was measured to the nearest 100 grams with a metric scale. Age-adjusted z-scores were calculated for height (HT-Z) and BMI (BMI-Z) using the World Health Organization standards (WHO) (WHO 2011). Annual height velocity (HV) was calculated by computing the difference in height between visits adjusted to one year increments (cm/year).
2.3 Blood sample analysis for lead measurement
At study entry, a 3.0-mL venous blood sample was collected in a trace metal-free vacutainer tube (Becton-Dickinson, Franklin Lakes, NJ) after alcohol cleansing of the skin. Whole-blood samples were diluted with a matrix modifier solution and analyzed for lead by Zeeman background-corrected, flameless graphite furnace, atomic absorption spectrometry (ESA Laboratories, Chelmsford, MA). The detection limit was 1.0 μg/dL; 14 (2.9%) of the 481 boys had BLLs below the limit of detection.
2.4. Statistical analysis
We used mixed effects linear regression models to evaluate the associations of high BLL (≥5 versus <5 μg/dL) at age 8–9 years with age-adjusted BMI-Z, HT-Z, and annual HV measured over ten years of follow-up (through age 18 years). An autoregressive covariance structure was utilized to account for within-boy correlation in growth measures over time. Initially we evaluated unadjusted associations of higher versus lower BLL with each growth outcome. We then fit a full multivariable model including all covariates with univariate p≤0.20; these included maternal prenatal exposure to tobacco smoke (either active or passive), birth weight, gestational age, breastfeeding duration, and covariates at baseline including boys’ nutritional intake (total caloric intake and proportions of calories from protein, fat, and carbohydrates) and beer consumption, parental education, household income, residence of biological father in the home, number of siblings, and age. Final models for each outcome were reduced, retaining covariates with p<0.10 in the multivariable analysis, or which changed the high BLL effect estimate by more than 10%. The final models for both HT-Z and BMI-Z included age and birth weight; in addition, the model for HT-Z included preterm vs. term birth and percent calories from protein whereas the model for BMI-Z included no biological father in the home and percent calories from fat. We also evaluated associations of growth outcomes with loge-transformed BLL. We evaluated interactions between age (closest integer year) and high BLL (≥5 μg/dL) for each outcome using an overall Wald chi-square test for cross-product terms (10df for HT-Z and BMI-Z, 9df for HV). In sensitivity analyses conducted to assess the dose-response relationship at lower BLLs, we restricted the data to BLL ≤5 μg/dL and evaluated the associations of categories of BLL (≤2, 3, 4, and 5 μg/dL) with growth. Parameter estimates were obtained using restricted maximum likelihood methods implemented via PROC MIXED in SAS Version 9.4 (SAS Institute, Cary NC). We defined near final adult height as the first height at which HV was <1 cm/yr. The mean age and near adult height were estimated using PROC LIFEREG with right censoring for boys who had not yet attained adult height. Statistical significance was defined as two-sided p-values ≤0.05.
3. Results
3.1 Study Population
Table 1 summarizes perinatal history and baseline characteristics overall and by BLL; anthropometric measurements at entry and age 18 are also provided.
Table 1.
Descriptive characteristics of 481 boys with blood lead (BLL) measurements in the Russian Children’s Study
| Characteristica | Overall (N = 481) | Blood Lead Level (BLL)
|
|
|---|---|---|---|
| Low (<5 μg/dL) (N = 347) | High (≥5 μg/dL) (N = 134) | ||
| Blood lead levels at study entry, median (range) | 3.0 (0.5–31.0) | 3.0 (0.5–4.0) | 6.0 (5.0–31.0) |
|
| |||
| Growth measures at study entry (ages 8–9 years)b | |||
| Height z-scorec,† | 0.13 ± 1.00 | 0.27 ± 0.95 | −0.25 ± 1.04 |
| BMI z-scorec | −0.18 ± 1.27 | −0.10 ± 1.28 | −0.39 ± 1.21 |
| Height (cm)† | 130.2 ± 6.3 | 130.8 ± 5.9 | 128.5 ± 6.9 |
| BMI (kg/m2) | 15.9 ± 2.3 | 16.1 ± 2.3 | 15.6 ± 2.1 |
| Growth measures at age 18 years | (N = 305) | (N = 221) | (N = 84) |
| Height z-scorec,† | 0.15 ± 0.92 | 0.27 ± 0.89 | −0.18 ± 0.91 |
| BMI z-scorec | −0.33 ± 1.24 | −0.25 ± 1.26 | −0.56 ± 1.16 |
| Height (cm)† | 177.3 ± 6.8 | 178.2 ± 6.6 | 174.9 ± 6.7 |
| BMI (kg/m2) | 21.4 ± 3.8 | 21.7 ± 4.0 | 20.8 ± 3.2 |
| Birth and neonatal history | |||
| Birthweight (kg)† | 3.35 ± 0.52 | 3.38 ± 0.51 | 3.25 ± 0.55 |
| Gestational age (wks) | 39.1 ± 1.7 | 39.1 ± 1.6 | 38.9 ± 2.0 |
| Breastfeeding duration (wks)† | 27.7 ± 34.5 | 24.6 ± 28.9 | 35.4 ± 45.1 |
| Preterm (<37 wk gestation), n (%) | 41 (9) | 27 (8) | 14 (10) |
| Maternal characteristics and exposures during pregnancy | |||
| Mother’s age at son’s birth | 23.9 ± 5.1 | 23.79 ± 5.0 | 24.3 ± 5.2 |
| Mother’s alcohol consumption, n (%)† | 59 (13) | 35 (10) | 24 (18) |
| Mother’s tobacco smoking, n (%)† | 36 (8) | 21 (6) | 15 (12) |
| Any smoking in household, n (%)† | 228 (49) | 155 (46) | 73 (56) |
| Boys daily dietary intake at study entry | |||
| Total calories (calories)† | 2825 ± 987 | 2719 ± 956 | 3100 ± 1015 |
| Percent calories from carbohydrates | 54.4 ± 6.6 | 54.1 ± 6.5 | 55.1 ± 6.8 |
| Percent calories from fat | 34.0 ± 5.9 | 34.3 ± 5.8 | 33.4 ± 6.1 |
| Percent calories from protein | 11.6 ± 1.6 | 11.6 ± 1.6 | 11.5 ± 1.6 |
| Household characteristics at study entry, n (%) | |||
| Low parental education (≤secondary school)† | 38 (8) | 20 (6) | 18 (14) |
| Low income (<250 U.S. dollars/month)† | 293 (61) | 194 (56) | 99 (74) |
| Biological father resides in home† | 315 (65) | 237 (68) | 78 (58) |
Summary statistics, including proportions, were calculated among those with non-missing data for that characteristic. Means ± SD given unless otherwise noted.
Missing information: any prenatal tobacco exposure (n=11), maternal tobacco smoke (n=13), maternal alcohol consumption (n=16), mother’s age at son’s birth (n=5), birth weight (n=3), gestational age (n=4), dietary information (n=3), parental education (n=4), household income (n=1), breastfeeding (n=11), z-scores at age 18 years (n=1).
A total of 301 boys were enrolled at age 8 and 180 were enrolled at age 9.
WHO age-adjusted z-scores: http://www.who.int/childgrowth/en/.
Two-sided Wilcoxon rank-sum test or Mantel-Haenszel chi-square with P-value≤0.05
Among the 481 boys, the median (interquartile range) of BLL was 3.0 (2.0–5.0) μg/dL; the distribution was right skewed, 10 (2.1%) boys had BLL ≥10 μg/dL at entry (Hauser et al. 2009). On average, boys with BLL ≥5 μg/dL had lower birthweight, breastfed longer, more often had mothers who drank alcohol and smoked tobacco during pregnancy, were from households with lower socioeconomic status (SES), and were significantly shorter. At study entry, 14% of boys had signs of pubertal onset. Of the original cohort, 305 (61%) boys completed follow-up through age 18 years; those remaining in the study were more often full term at birth (94% vs 87%), with younger mothers (23.5 vs. 24.6 years), and a higher percentage of parents with post-secondary education (95% vs. 87%) than those who discontinued. There were no substantial differences for other anthropometric or demographic characteristics.
3.2. Growth measures at entry and during follow-up
Most boys were within the normal range for height and BMI both at entry and at age 18 (Table 1) (de Onis et al. 2007). Annual HV was greatest at 14 years (mean HV (SD) = 7.5 (2.3) cm/yr). By age 18 years, 79% had achieved near adult height. The overall mean height and BMI at age 18 were 177.3 cm and 21.4, respectively (Table 1).
3.3. Blood lead and growth associations
Boys with higher compared to lower BLL were significantly shorter over the 10 years of follow-up (Table 2).
Table 2.
Estimated differences in mean growth z-scores by peripubertal blood lead levels (BLL) among Russian boys followed for up to 10 years.
| Exposure | Unadjusted Model (N=481) | Adjusted Model (N=475) | ||
|---|---|---|---|---|
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | |
| Annual WHO age-adjusted height z-scoresa | ||||
|
| ||||
| Higher vs. lower BLL (≥5 vs. <5 μg/dL) | −0.49 (−0.67, −0.31) | <0.001 | −0.43 (−0.60, −0.25) | <0.001 |
| Loge-transformed BLL | −0.30 (−0.44, −0.16) | <0.001 | −0.26 (−0.40, −0.13) | <0.001 |
|
| ||||
| Annual WHO age-adjusted BMI z-scoresb | ||||
|
| ||||
| Higher vs. lower BLL (≥5 vs. <5 μg/dL) | −0.34 (−0.57, −0.10) | 0.005 | −0.22 (−0.45, 0.006) | 0.06 |
| Loge-transformed BLL | −0.19 (−0.37, −0.01) | 0.04 | −0.14 (−0.31, 0.04) | 0.12 |
WHO=World Health organization; CI=confidence interval; BLL=blood lead level; BMI=body mass index
Adjusted repeated measures linear regression model, including birthweight, preterm birth, percent calories from protein at baseline, and age (years).
Adjusted repeated measures linear regression model, including birthweight, no biological father in home, percent calories from fat at baseline, and age (years).
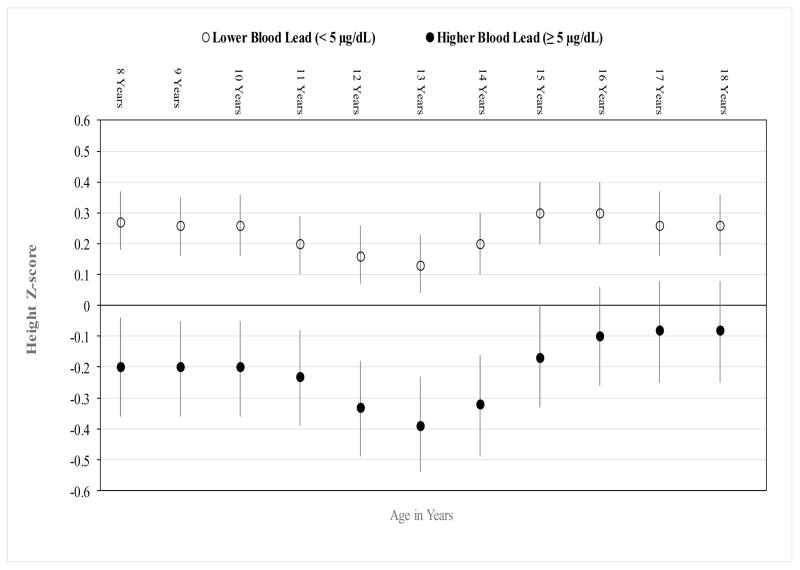
On a continuous scale, each unit increase in log-transformed BLL was associated with an adjusted mean decrease of 0.26 in HT-Z (95% CI −0.40, −0.13, p<0.001). There was an overall significant interaction between BLL (≥5 μg/dL vs <5 μg/dL) and age in years on HT-Z (p=0.03); with the most pronounced lead-associated decrements in HT-Z between ages 12 to 15 years (Figure 1). At age 18 years, young men with high BLL had an adjusted mean HT-Z (95% CI) of −0.08 (−0.25, 0.08) compared to 0.26 (0.16, 0.36) among those with low BLL, equivalent to mean heights of 175.6 and 178.1cm, respectively; a 2.5 cm difference. Using censored survival methods, the estimated means for near adult height in those with higher versus lower BLL were 177.9 and 180.5 cm, respectively; a decrease of 2.6 cm (95% CI: −4.4, −0.8). There was no significant difference in age at attaining near adult height between those with higher (17.9 years) versus lower (17.8 years) BLL (p=0.25).
Figure 1.
Age-Specific Adjusted Mean Height Z-score (95% CI) by Baseline Blood Lead Levels.
Adjusted repeated measures linear regression model, including birthweight, preterm birth, percent calories from protein at baseline, and age (years), and interaction between age and BLL.
P for interaction = 0.03
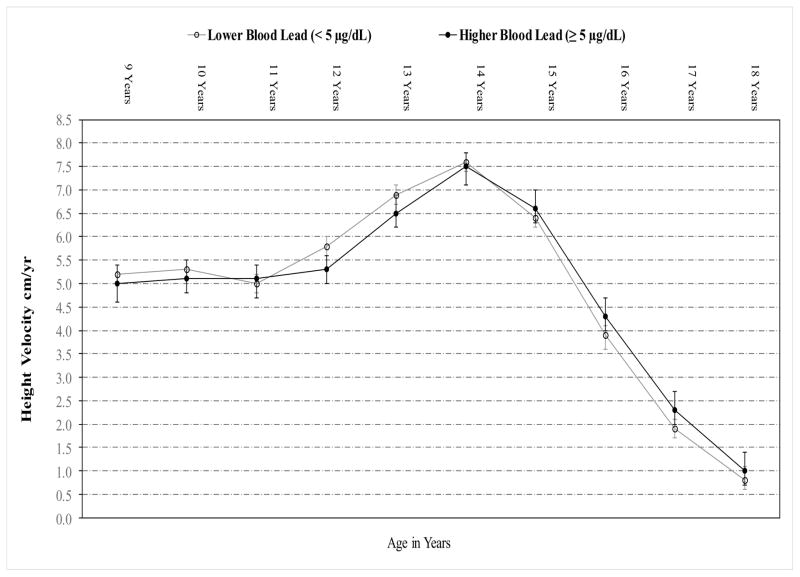
Although we observed no overall association between BLL and HV (p=0.74), we did observe a significant overall interaction between BLL and age (in years) on HV (p=0.03), indicating that the effect of BLL on HV varied by age. Compared to boys with lower BLL, those with higher BLL had lower HV at ages 12 and 13 years but greater HV at ages 15 to 18 years (Figure 2).
Figure 2.
Age-Specific Adjusted Mean Height Velocity (95% CI) by Baseline Blood Lead Levels
Repeated measures linear regression model accounting for age, BLL, and interaction between age and BLL.
P for interaction = 0.03
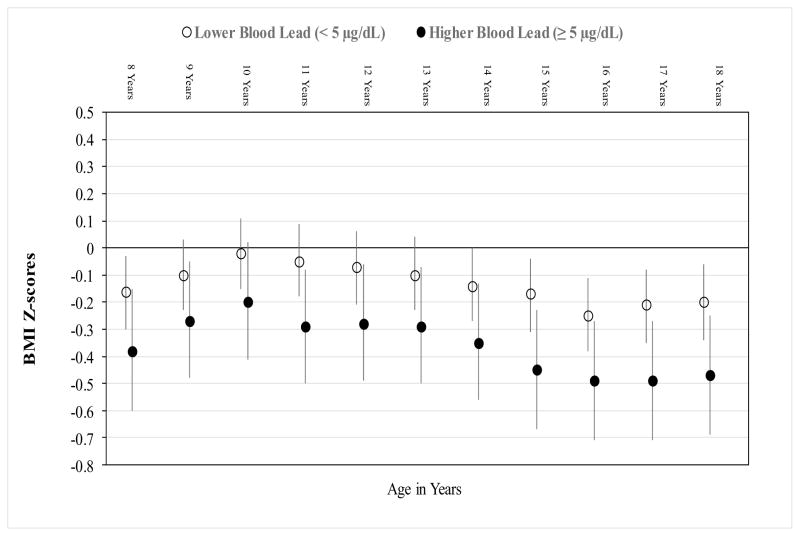
Over 10 years of follow-up, boys with higher compared to lower BLL had consistently lower BMI-Z (adjusted mean difference in BMI-Z= −0.22, 95% CI: −0.47, 0.01), with a mean BMI at age 18 years of 20.4 versus 21.1, although the association did not attain statistical significance (p=0.06) (Table 2, Figure 3). There was no significant interaction between BLL and age on BMI-Z (p=0.53).
Figure 3.
Age-Specific Adjusted Mean (95% CI) BMI Z-scores by Baseline Blood Lead Levels
Adjusted repeated measures linear regression model, including birthweight, no biological father in home, percent calories from fat at baseline, age (years), and interaction between BLL and age.
P for interaction = 0.53
In sensitivity analyses considering only boys with BLL ≤5 μg/dL, we observed lower HT-Z with increasing BLL category, with mean [95% CI] z-scores of −0.07 [−0.27, 0.13], −0.18 [−0.41, 0.04], and −0.38 [−0.64, −0.12], respectively, for 3, 4, and 5 μg/dL compared to ≤2 μg/dL. Although the test for trend was significant (p=0.003) when including those with BLL of 5 μg/dL, it was no longer significant after considering only those with BLL<5 μg/dL. In this analysis, only the pairwise comparison of BLL=5 μg/dL versus ≤2 μg/dL was significant. For the analogous evaluation of BMI-Z with BLL categories ≤5 μg/dL, there was no significant association with or without inclusion of the 5 μg/dL BLL category (data not shown).
4. Discussion
Higher lead exposure during childhood has been associated with reduced growth (Ballew et al. 1999; Cantoral et al. 2015; Cassidy-Bushrow et al. 2016; Ignasiak et al. 2006; Kafourou et al. 1997; Little et al. 2009; Min et al. 2008; Schwartz et al. 1986), and later pubertal onset (Hauser et al. 2008; Selevan et al. 2003; Williams et al. 2010; Wu et al. 2003). In our current analysis of annual growth measurements to age 18 years, we found that higher peripubertal BLL was associated consistently with lower height from ages 8 to 18 years among our cohort. The height difference was most pronounced between ages 12 to 15. Near adult height was significantly shorter by 2.6 cm for those with higher compared with lower BLL.
Most cross-sectional studies have found that elevated BLLs were associated with lower height (Ballew et al. 1999; Cantoral et al. 2015; Ignasiak et al. 2006; Kafourou et al. 1997; Little et al. 2009; Min et al. 2008; Schwartz et al. 1986; Yang et al. 2013). One of the earliest assessments of BLL and children’s height used data from the 1976–1980 NHANES cohort (ages 1–7 years), with much higher BLLs than in the current study (range: 5.0–35 μg/dL), and found a linear dose-response relationship (Schwartz et al. 1986). Even with substantially lower BLLs, analyses using 1988–1994 NHANES data (mean male BLL 3.79 μg/dL) also observed a cross-sectional association of higher BLLs with lower height in children 1–7 years old (Ballew et al. 1999). Similarly, Little and colleagues (2009) combined two separate cross-sectional cohorts (ages 1–12 years) in Dallas, Texas, from 1980–1989 (mean BLL 23.6 μg/dL) and 2002 (mean BLL 1.6 μg/dL) in a pooled analysis, and found that higher BLL was associated with lower age- and sex-specific height z-scores, after adjustment for socio-demographic factors. A number of other environmental lead exposure studies have also reported cross-sectional associations of higher BLL with lower height in children ranging in age from 3 to 15 years (Ignasiak et al. 2006; Kafourou et al. 1997; Min et al. 2008; Yang et al. 2013).
In contrast to the above studies (which primarily examined younger children), studies in Inuit and Flemish cohorts have not found cross-sectional associations between childhood BLL and height (Dallaire et al. 2014; Dhooge et al. 2010). The Inuit and Flemish cohorts included children who were peripubertal and older (8–14 years and 14–15 years, respectively). However, the Inuit cohort reported a significant negative association between prenatal BLL (as measured by cord blood) and height at ages 8–14 years (Dallaire et al. 2014). Previously, we reported a negative cross-sectional association between BLL and height at age 8–9 years when 86% of the boys were prepubertal (Hauser et al. 2008). These results suggest that lead’s association with stature may vary depending on exposure timing and pubertal status, with younger ages being potentially more susceptible.
In our Russian cohort, we evaluated the association of peripubertal (ages 8–9 years) BLL with subsequent height; in contrast, most prior prospective epidemiologic studies have examined prenatal lead exposures (Afeiche et al. 2011; Dallaire et al. 2014; Hong et al. 2014; Jedrychowski et al. 2015; Renzetti et al. 2017; Shukla et al. 1991) or exposures during early childhood (e.g., up to age 2 years) (Afeiche et al. 2012; Cassidy-Bushrow et al. 2016; Shukla et al. 1991). Moreover, with few exceptions (Dallaire et al. 2014; Jedrychowski et al. 2015; Renzetti et al. 2017), children were not followed beyond age 5 years. Among prospective studies with measures of perinatal lead exposure, the timing of exposure and nutritional deficiencies impacted associations of lead with height. In Mexican (Renzetti et al. 2017) and Korean (Hong et al. 2014) cohorts, reduced childhood growth was associated with higher BLLs measured during the third trimester of pregnancy but not during the first or second trimesters, or with cord blood levels. An earlier U.S. study found prenatal BLLs associated with lower growth only in the presence of continued lead exposure during early childhood (Shukla et al. 1991). Hong and colleagues (2014) observed a stronger association of higher third-trimester BLLs with reduced height at age 2 years among children with low compared to high maternal serum calcium levels. Prospective studies of early life BLL in young children in the U.S. (geometric mean BLL of 15.5 μg/dL) (Shukla et al. 1991) and in Mexico (median BLL of 4.5 μg/dL) (Afeiche et al. 2012) showed associations with lower height, in contrast to a more recent U.S. study with lower exposures (Cassidy-Bushrow et al. 2016) (mean BLL of 2.45 μg/dL) that did not find an association. None of the above longitudinal studies extended follow-up through puberty or to adulthood to assess associations with pubertal HV or adult height. Consequently, these earlier studies were unable to assess whether childhood BLL disrupted the pubertal growth spurt or affected adult stature.
We also assessed the role of diet as an important determinant of near adult height and therefore a potential confounder of lead associations. As expected, in our longitudinal analyses, higher protein intake at age 8–9 years was associated with greater height from ages 8–18 years. The association of higher BLL with lower height was essentially unchanged after adjustment for baseline protein intake. However, we did not have data on diet available throughout adolescence, therefore we could not assess whether protein intake at other time points had an additional effect on height.
In our cohort, there was no significant difference in annual HV by BLL among boys with BLL≥5 μg/dL compared to those with BLL<5 ug/dL; however, we observed a significant interaction of BLL with age in determining HV. In boys with higher BLL compared to those with lower BLL, HV was lower at ages 12 and 13, but higher from ages 15 to 18 years. This pattern of growth would be consistent with a delay in the timing of the pubertal growth spurt consistent with the delayed pubertal onset associated with higher BLLs in our cohort (Hauser et al. 2008; Williams et al. 2010).
Findings from cross-sectional studies assessing the association of BLL with weight and BMI have been inconsistent (Ballew et al. 1999; Dallaire et al. 2014; Dhooge et al. 2010; Ignasiak et al. 2006; Little et al. 2009; Min et al. 2008; Schwartz et al. 1986; Scinicariello et al. 2013). One of the first assessments of BLL and weight used NHANES data (1976–1980), and found higher BLL associated with lower weight (Schwartz et al. 1986). However, subsequent analyses evaluating BLL associations with BMI using NHANES 1988–1994 (Ballew et al. 1999) and 1999–2006 (Scinicariello et al. 2013) data reported discrepant results; whereas analysis of 1988–1994 data observed no association, and analysis of 1999–2006 NHANES data reported a negative association. Scinicariello and colleagues (2013) compared the highest quartile of BLL (≥1.61 μg/dL) to the lowest (≤0.7 μg/dL), using age and sex standardized BMI z-scores as endpoints, and included children ages 3–19 years; whereas Ballew and colleagues (1999) used continuous BLL, BMI without standardization, and restricted children’s ages to 1–7 years. Thus, the inconsistent findings between these two later studies may be due to differences in analytic approaches and age distributions. A combined analysis of two cross-sectional Texan cohorts separated by more than 20 years (1980–1989 vs. 2002), with a substantial decrease in mean BLL over this time period (23.6 μg/dl to 1.6 μg/dl), found a significant negative association between BLL and BMI z-scores, even after adjustment for cohort and other covariates (Little et al. 2009).
In our longitudinal Russian cohort, boys with higher BLL had consistently lower BMI (though not attaining significance). The few longitudinal studies which have evaluated the association between BLL and BMI have yielded mixed findings, with few having follow-up past early childhood. Maternal third-trimester BLL was associated with lower age-adjusted weight at age 2 years in a Korean cohort (Hong et al. 2014), and U.S. children with measurable BLL at age 2 years had significantly lower BMI (Cassidy-Bushrow et al. 2016). In one Mexican cohort, maternal bone lead measurements were significantly associated with lower weight among girls at age 5 years (Afeiche et al. 2011), while another Mexican cohort found higher maternal third-trimester BLLs associated with lower age-adjusted weight at ages 4–6 years (Renzetti et al. 2017). However, cord BLL in an Inuit cohort followed to age 14 years reported no association with BMI (Dallaire et al. 2014). Given the inconsistent findings in the literature, and our inconclusive (although suggestive) results, the impact of higher BLL on BMI remains to be fully established.
Both clinical and experimental evidence support the potential of lead to impair bone growth via toxicity to both cellular and non-cellular components of bone (Hicks et al. 1996; Pounds et al. 1991). High BLLs among children have been associated with lower osteocalcin, a biomarker of osteoblastic (bone forming) activity (Markowitz et al. 1988). In rodent models, the direct effects of lead on the epiphyseal growth plate (Pounds et al 1991) are consistent with findings in cross-sectional cohorts of Polish (ages 7–15 years) (Ignasiak et al. 2006) and Korean children (ages 5–13 years) (Min et al. 2008) of negative associations of BLL with height and a marked reduction in arm and leg length suggesting disruption of long bone epiphyses. Lead has also been associated with suppression of pituitary growth hormone release (Camoratto et al. 1993) and lower serum IGF-1 (Ronis et at. 1998) in rodent models, as well as with lower serum IGF-1 in our study cohort (Fleisch et al. 2013), suggesting two other mechanisms for disrupting linear growth.
This is one of the few prospective cohorts to measure childhood BLL in the peripubertal period and to follow growth through adolescence to young adulthood. This long-term follow-up allowed evaluation of whether lead’s association with height persists at completion of linear growth. With this unique design, we found higher BLLs consistently associated with lower height throughout adolescence, with greater decrements in height z-score at ages 12–15 years, lower HV at ages 12–13 years, and a significantly lower near adult height. We collected extensive data on diet and SES indicators, allowing for adjustment for these factors. Additional strengths of our study include annual measurements of height, weight, and pubertal stage, and relatively high retention rate. In our study we had a single measurement of BLL, reflecting recent exposure, therefore we cannot directly evaluate the effects of prenatal or later adolescent BLLs on growth. However, in prospective cohort studies in Nunavik, Canada (Dallaire et al. 2014) and Krakow, Poland (Jedrychowski et al. 2015), positive associations have been observed between cord and childhood BLLs up to ages 5 and 14 years, respectively. Moreover, in the prospective Cincinnati (Ohio) Lead Study, average early childhood BLLs (from ages 3–78 months) were strongly correlated with BLLs at age 6 years (Wright et al. 2008), suggesting that a single childhood BLL may be a reasonable proxy for measures in earlier childhood. Therefore, BLLs measured in our study at age 8 to 9 years may also correlate with prenatal and earlier childhood BLLs.
The CDC revised the reference level for childhood BLL from 10 to 5 μg/dL in 2012 because of the impact of lower level lead exposure on several important child health measures including neurological and behavioral development. Childhood somatic growth is also an important indicator of overall health, but few longitudinal studies have followed lead-exposed children through the pubertal growth spurt to determine whether associations of lower height with higher BLL persist into adulthood. Our results clearly demonstrate that in our cohort of Russian boys, those with higher peripubertal BLL had significantly lower height every year through attainment of adult height. In sensitivity analyses restricted to boys with BLL ≤ 5 μg/dL, we observed a significant decreasing trend in HT-Z with increasing BLLs. Although analyses restricted to BLLs below 5 μg/dL did not demonstrate significant associations with HT-Z, there was a suggestive trend consistent with the association observed between log BLL and HT-Z in the entire cohort. We found that boys with higher peripubertal BLL also had lower BMI at every age, although this difference was not statistically significant. These results suggest that lead exposure during the sensitive peripubertal window may have long-term impacts on growth that persist into adulthood. Research that elucidates the biological mechanism underlying these associations may provide new insight into the potential long-term health impacts of childhood lead exposure.
Supplementary Material
Highlights.
Median (range) blood lead level was 3.0 (0.5–31.0) μg/dL in Russian boys at 8–9 yrs.
Boys with childhood blood lead ≥5 vs. <5 μg/dL had reduced height up to age 18 yrs.
Boys with higher childhood blood lead also tended to be leaner up to age 18 yrs.
Acknowledgments
We would like to thank the study participants, Chapaevsk government (Dmitry Blynsky and Nikolay Malakhov), and the Chapaevsk Medical Association and Chapaevsk Central Hospital staff as well as chiefs (Vladimir Zeilert, Svetlana Nikolaeva, Anatoly Kochkaryov).
Funding: This work was supported by the U.S. EPA (grant R82943701); the National Institute of Environmental Health Sciences (grants R01 ES014370 and P30 ES000002); and Russian Science Foundation grant #14-45-00065 (O.S. and R. H. effort).
Abbreviations
- BLL
blood lead level
- BMI-Z
body mass index z-score
- CI
confidence interval
- HT-Z
height z-score
- HV
height velocity
- SD
standard deviation
- SES
socio-economic status
- WHO
World Health Organization
Footnotes
Conflicts of interest: The authors report that they have no conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afeiche M, Peterson KE, Sánchez BN, Cantonwine D, Lamadrid-Figueroa H, Schnaas L, et al. Prenatal lead exposure and weight of 0- to 5-Year-Old Children in Mexico City. Environ Health Perspect. 2011;119(10):1436–1441. doi: 10.1289/ehp.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche M, Peterson KE, Sánchez BN, Schnaas L, Cantonwine D, Ettinger AS, et al. Windows of lead exposure sensitivity, attained height, and body mass index at 48 months. J Pediatr. 2012;160(6):1044–1049. doi: 10.1016/j.jpeds.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew C, Khan LK, Kaufmann R, Mokdad A, Miller DT, Gunter EW. Blood lead concentration and children’s anthropometric dimensions in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. J Pediatr. 1999;134(5):623–630. doi: 10.1016/s0022-3476(99)70250-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. The protean toxicities of lead: new chapters in a familiar story. Int J Environ Res Public Health. 2011;8(7):2593–628. doi: 10.3390/ijerph8072593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink LA, Talbott EO, Marsh GM, Sharma R, Benson S, Wu WC, et al. Revisiting nonresidential environmental exposures and childhood lead poisoning in the US: findings from Kansas, 2000–2005. J Environ Public Health. 2016;2016:8791686. doi: 10.1155/2016/8791686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Lee MM, Williams PL, Korrick SA, Sergeyev O, Lam T, et al. Associations of peripubertal serum dioxin and polychlorinated biphenyl concentrations with pubertal timing among Russian boys. Environ Health Perspect. 2016;124(11):1801–1807. doi: 10.1289/EHP154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick SA, Lee MM, Revich B, et al. Serum concentrations of organochlorine pesticides and growth among Russian boys. Environ Health Perspect. 2012;120(2):303–308. doi: 10.1289/ehp.1103743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camoratto AM, White LM, Lau YS, Ware GO, Berry WD, Moriarty CM. Effect of exposure to low level lead on growth and growth hormone release in rats. Toxicology. 1993;83(1–3):101–114. doi: 10.1016/0300-483x(93)90095-a. [DOI] [PubMed] [Google Scholar]
- Cantoral A, Téllez-Rojo MM, Levy TS, Hernández-Ávila M, Schnaas L, Hu H, et al. Differential association of lead on length by zinc status in two-year old Mexican children. Environ Health. 2015;14:95. doi: 10.1186/s12940-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Havstad S, Basu N, Ownby DR, Park SK, Ownby DR. Detectable blood lead level and body size in early childhood. Biol Trace Elem Res. 2016;171(1):41–47. doi: 10.1007/s12011-015-0500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, Dewailly É, Ayotte P, Forget-Dubois N, Jacobson SW, Jacobson JL, et al. Growth in Inuit children exposed to polychlorinated biphenyls and lead during fetal development and childhood. Environ Res. 2014;134:17–23. doi: 10.1016/j.envres.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhooge W, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De Mieroop E, et al. Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int. 2010;36(4):330–337. doi: 10.1016/j.envint.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Fleisch AF, Burns JS, Williams PL, Lee MM, Sergeyev O, Korrick SA, et al. Blood lead levels and insulin-like growth factor 1 concentrations in peripubertal boys. Environ Health Perspect. 2013;121(7):854–858. doi: 10.1289/ehp.1206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283–290. doi: 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Sergeyev O, Korrick S, Lee MM, Revich B, Gitin E, et al. Association of blood lead levels with onset of puberty in Russian boys. Environ Health Perspect. 2008;116(7):976–980. doi: 10.1289/ehp.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Williams P, Altshul L, Korrick S, Peeples L, Patterson DG, Jr, et al. Predictors of serum dioxin levels among adolescent boys in Chapaevsk, Russia: a cross-sectional pilot study. Environ Health. 2005;4(1):8. doi: 10.1186/1476-069X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks DG, O’Keefe RJ, Reynolds KJ, Cory-Slechta DA, Puzas JE, Judkins A, et al. Effects of lead on growth plate chondrocyte phenotype. Toxicol Appl Pharmacol. 1996;140(1):164–172. doi: 10.1006/taap.1996.0209. [DOI] [PubMed] [Google Scholar]
- Hong YC, Kulkarni SS, Lim YH, Kim E, Ha M, Park H, et al. Postnatal growth following prenatal lead exposure and calcium intake. Pediatrics. 2014;134(6):1151–1159. doi: 10.1542/peds.2014-1658. [DOI] [PubMed] [Google Scholar]
- Ignasiak Z, Sławińska T, Rozek K, Little BB, Malina RM. Lead and growth status of school children living in the copper basin of south-western Poland: differential effects on bone growth. Ann Hum Biol. 2006;33(4):401–414. doi: 10.1080/03014460600730752. [DOI] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Majewska R, Mrozek-Budzyn D, Mroz E, Roen EL, et al. Depressed height gain of children associated with intrauterine exposure to polycyclic aromatic hydrocarbons (PAH) and heavy metals: the cohort prospective study. Environ Res. 2015;136:141–147. doi: 10.1016/j.envres.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafourou A, Touloumi G, Makropoulos V, Loutradi A, Papanagiotou A, Hatzakis A. Effects of lead on the somatic growth of children. Arch Environ Health. 1997;52(5):377–383. doi: 10.1080/00039899709602214. [DOI] [PubMed] [Google Scholar]
- Laidlaw MA, Filippelli GM, Sadler RC, Gonzales CR, Ball AS, Mielke HW. Children’s blood lead seasonality in Flint, Michigan (USA), and soil-sourced lead hazard risks. Int J Environ Res Public Health. 2016;13(4) doi: 10.3390/ijerph13040358. pii: E358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Sergeyev O, Williams P, Korrick S, Zeilert V, Revich B, et al. Physical growth and sexual maturation of boys in Chapaevsk, Russia. J Pediatr Endocrinol Metab. 2003;16(2):169–178. doi: 10.1515/jpem.2003.16.2.169. [DOI] [PubMed] [Google Scholar]
- Little BB, Spalding S, Walsh B, Keyes DC, Wainer J, Pickens S, et al. Blood lead levels and growth status among African-American and Hispanic children in Dallas, Texas--1980 and 2002: Dallas Lead Project II. Ann Hum Biol. 2009;36(3):331–341. doi: 10.1080/03014460902806615. [DOI] [PubMed] [Google Scholar]
- Markowitz ME, Gundberg CM, Rosen JF. Sequential osteocalcin (Oc) sampling as a biochemical marker of the success of treatment in moderately lead (Pb) poisoned children. Pediatr Res. 1988;23:393A. [Google Scholar]
- Martinchik AN, Baturin AK, Baeva VS, Feoktistova AI, Piatnitskaia IN, Azizbekian GA, et al. Development of a method of studying actual nutrition according to analysis of the frequency of consumption of food products: creation of a questionnaire and general evaluation of the reliability of the method [in Russian] Vopr Pitan. 1998;3:8–13. [PubMed] [Google Scholar]
- Min KB, Min JY, Cho SI, Kim R, Kim H, Paek D. Relationship between low blood lead levels and growth in children of white-collar civil servants in Korea. Int J Hyg Environ Health. 2008;211(1–2):82–87. doi: 10.1016/j.ijheh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Pounds JG, Long GJ, Rosen JF. Cellular and molecular toxicity of lead in bone. Environ Health Perspect. 1991;91:17–32. doi: 10.1289/ehp.919117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE. Research review: environmental exposures, neurodevelopment, and child mental health - new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry. 2016;57(7):775–793. doi: 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J, Brown MJ. Summary of notifiable noninfectious conditions and disease outbreaks: childhood blood lead levels - United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2015;62(54):76–80. doi: 10.15585/mmwr.mm6254a5. [DOI] [PubMed] [Google Scholar]
- Renzetti S, Just AC, Burris HH, Oken E, Ararasiriwarden C, Svensson K, et al. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environ Int. 2017;152:226–232. doi: 10.1016/j.envres.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26(6):808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, Ringer D, et al. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. J Toxicol Environ Health A. 1998;54(2):101–20. doi: 10.1080/009841098158944. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Angle C, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77:281–288. [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, Mevissen M, Portier CJ. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicol Appl Pharmacol. 2013;273(3):516–523. doi: 10.1016/j.taap.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348(16):1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Shukla R, Dietrich KN, Bornschein RL, Berger O, Hammond PB. Lead exposure and growth in the early preschool child: A follow-up report from the Cincinnati lead study. Pediatrics. 1991;88:886–889. [PubMed] [Google Scholar]
- Williams PL, Sergeyev O, Lee MM, Korrick SA, Burns JS, Humblet O, et al. Blood lead levels and delayed onset of puberty in a longitudinal study of Russian boys. Pediatrics. 2010;125(5):e1088–1096. doi: 10.1542/peds.2009-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) [accessed 26 June 2016];The WHO Child Growth Standards. 2016 Available: http://www.who.int/childgrowth/en/
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5(5):e10. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Environ Health Perspect. 2003;111(5):737–741. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Huo X, Yekeen TA, Zheng Q, Zheng M, Xu X. Effects of lead and cadmium exposure from electronic waste on child physical growth. Environ Sci Pollut Res Int. 2013;20(7):4441–4447. doi: 10.1007/s11356-012-1366-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.