Abstract
Background
Long non-coding RNAs (lncRNAs) are aberrantly expressed in many types of human cancer including pancreatic cancer (PC) and correlated with tumorigenesis and cancer prognosis, whereas knowledge about regulatory mechanism of lncRNA expression is few known. This study aimed to explore whether polymorphisms in lncRNAs genes are associated with PC susceptibility by affecting its expression.
Methods
We first genotyped three common single-nucleotide polymorphisms (SNPs) of lncRNA genes (HOTTIP rs1859168, HOTAIR rs4759314, and H19 rs217727) in 416 paired PC patients and controls, and then validated the results in another 505 paired PC patients and controls. The genotype-phenotype correlation was examined in 50 PC tissue samples with different genotypes as well as by luciferase reporter assay.
Results
In the discovery set, only the HOTTIP rs1859168 A > C showed to be significantly associated with a reduced PC risk (CC vs AA: odds ratio (OR) = 0.71, 95% confidence interval (95%CI) = 0.57–0.88, P = 0.002; recessive model: adjusted OR = 0.51, 95%CI = 0.38–0.68, P < 0.001; additive model: adjusted OR = 0.67, 95%CI = 0.51–0.82, P < 0.001). The results in validation set and pooled population also indicated that the C allele of HOTTIP rs1859168 could significantly decrease the risk of PC. In addition, the genotype-phenotype association analysis suggested that HOTTIP expression level was significantly lower in PC samples with CC genotype than that in samples with AA and AC genotype. Furthermore, the C allele of HOTTIP rs1859168 could significantly decrease the relative luciferase activity compared to the A allele in three PC cell lines.
Conclusions
The current findings provided evidence that the functional rs1859168 A > C polymorphism may decrease the PC risk by down-regulating the HOTTIP expression.
Keywords: lncRNA, Hottip, Polymorphism, Pancreatic cancer
Background
Pancreatic cancer (PC) is the sixth leading causes of cancer-related deaths in China [1]. According to 2015 Cancer Statistics, the incidence rate of PC was 90.1 per 100,000 and the mortality rate was 79.4 per 100,000 [1]. Moreover, the prognosis for PC patients is ultimately poor due to delay in diagnosis, rapid progress, and conventional treatment resistance [2–4]. Thus, PC had been considered as one of the most serious health threats in China. PC is a complex disease caused by a variety of genetic and environmental factors. Previous epidemiological studies have identified several risk factors for PC morbidity, including high fat and protein diets, smoking, chronic pancreatitis, diabetes mellitus, and family history of cancer [5]. Recently, numerous reports have shown that genomics and epigenetics play risk-modulating roles in the susceptibility, progression, and prognosis of PC.
Long non-coding RNAs (lncRNAs), whose length are more than 200 nucleotides and cannot code protein [6, 7], are getting more and more attention as the important role in the transcriptional, epigenetic, and post-transcriptional regulation of gene expression [7, 8]. A lot of studies have suggested that lncRNAs could deliver function in varieties of cellular processes, including cell cycle regulation, cell differentiation, proliferation, growth, and apoptosis [9–11]. In addition, increasing numbers of studies revealed that the abnormal lncRNA level is closely related to the susceptibility, development, progression, and prognosis of various human cancers including PC [12–15]. Previous studies have showed that three key lncRNAs (HOTTIP, HOTAIR, and H19) might serve as candidate genes for cancerization and therapeutic targets [12, 16–21].
A growing number of evidence has demonstrated that it plays a vital role in the process of carcinogenesis that the expression and function of several key lncRNAs are affected by single-nucleotide polymorphisms (SNPs) [22–24]. To date, a large number of studies have explored the association between the SNPs in lncRNAs and cancer susceptibility. For instance, functional SNP rs4759314 in HOTAIR gene could affect the expression of HOTAIR, and upregulation of HOTAIR was significantly associated with development, progression, and prognosis of various cancers [25–27]. Xia et al. demonstrated the significant association between rs217727 polymorphism and breast cancer risk from 464 breast cancer patients and 467 controls [28]. In addition, functional prediction of SNP revealed that rs1859168 could alter HOTTIP expression by influencing the transcription factor binding sites, and HOTTIP takes part in regulating cancer cell proliferation and survival [29–31]. Furthermore, several meta-analyses about the associations between SNPs in these key lncRNAs genes and various cancers risk have been conducted [32, 33]. However, the results were inconsistent and there were no studies focused on the problem which is the associations between these SNPs and PC risk.
Considering the vital roles of lncRNAs for cancer development, we enforced a two-stage case-control study to evaluate the associations of three common SNPs (HOTTIP rs1859168 A > C, HOTAIR rs4759314 A > G, and H19 rs217727 C > T) in key lncRNAs genes with PC risk in China, and explore the biological mechanism of the SNPs.
Methods
Sample selection and design
This study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province (Kunming, China) and written informed consent was also obtained from each participant after a clear explanation about study objective. The study was composed of two independent case-control populations, the discovery set and the validation set, including a total of 921 PC cases and 921 age and gender matched healthy controls. All subjects were genetically unrelated to Han Chinese people. The 416 PC patients were diagnosed and treated at the Department of Hepatobiliary Surgery, First People’s Hospital of Yunnan Province (Kunming, China) from 2010 to 2015; the other 505 PC patients were enrolled from the Department of Hepatobiliary Surgery, First Affiliated Hospital of Kunming Medical University (Kunming, China) from 2011 to 2015. All of PC patients in both populations were consecutively recruited. All patients were newly diagnosed, pathologically confirmed and without treatment of any medications. The diagnosis of PC was confirmed by histopathological or cytological analysis according to the World Health Organization classification. Another total of 921 subjects (416 from discover set and 505 from validation set) without cancer or other serious illness were selected as controls, and they were matched with the PC patients by age (± 5 years), sex, and area of residence (urban and rural).
Information on demographic characteristics and risk factors of participants were collected using structured questionnaires, including smoking status, alcohol intake, pancreatitis, history of hypertension and diabetes mellitus, family history of cancer. Fasting peripheral venous blood samples from patients and controls were collected in sterile tubes with EDTA-Na2 anticoagulant. All blood samples were stored at −80 °C until use. In addition, before receiving any treatment, 50 patients underwent surgery to remove PC tumor tissue and these fresh tissue samples were stored in liquid nitrogen.
Genotyping of lncRNA genes
Genomic DNA was extracted from 200-ul EDTA-Na2 anticoagulant venous blood sample from each participant using genomic DNA purification kit (Tiangen, China) as described in the manufacturer’s instructions. According to the manufacturer’s instructions (Applied Biosystems), TaqMan SNP Genotyping Assay and ABI 7900HT Real-Time PCR System were used to genotype three polymorphisms (HOTTIP rs1859168 A > C, HOTAIR rs4759314 A > G, and H19 rs217727 C > T) in 921 PC patients and 921 controls. The primer sequences for each SNP were obtained from Applied Biosystems and described in Table 1. The quality control was done as follows: 3 duplicates including three negative control samples (water) were performed in each 96 well assay plate; genotypes analysis was performed in a blind fashion; 10% of the samples were randomly selected for repeated genotyping for confirmation, and the results were 100% concordant.
Table 1.
General characteristics of genetic variants in long non-coding RNA genes
| Gene | SNP | Chr: position | MAF | HWE | Primers |
|---|---|---|---|---|---|
| HOTTIP | rs1859168 | Chr7: 27,202,740 | A = 0.131 | 0.099 | Sense: ACGTTGGATGAATGATAGGGACACATCGGG |
| Antisense: ACGTTGGATGAGGTTTGTCTGAGAGGGATG | |||||
| HOTAIR | rs4759314 | Chr12: 53,968,051 | G = 0.095 | 0.166 | Sense: ATGATCTGCTTGGAAGGGATATAAA |
| Antisense: GCCTGGGCTTTTTCAGGTTT | |||||
| H19 | rs217727 | Chr11: 1,995,678 | T = 0.320 | 0.302 | Sense: ACTCAGGAATCGGCTCTGGAAGGTG |
| Antisense: GATGTGGTGGCTGGTGGTCAACGGT |
MAF minor allele frequency, HWE Hardy-Weinberg equilibrium
Real-time PCR analysis
Total RNA was extracted from PC tissue samples using Trizol reagent (Invitrogen, USA) according to instruction. A Roche Light-Cycler (Roche, Basel, Switzerland) and SYBR Green reaction mix (Qiagen, Germany) were used to quantify relative HOTTIP expression, and GAPDH mRNA was chosen as a normalizing control. The primers for HOTTIP or GAPDH mRNA were as follows: HOTTIP forward: 5′-GTGGGGCCCAGACCCGC-3′; HOTTIP reverse: 5′-AATGATAGGGACACATCGGGGAACT-3′; GAPDH mRNA forward: 5′-ACCACAGTCCATGCCATCAC-3′; GAPDH mRNA reverse: 5′-TCCACCACCCTGTTGCTGTA-3′. The relative expression of HOTTIP was evaluated using the delta-delta CT (2−ΔΔCt) method.
Luciferase reporter assay
As rs1859168 was associated with PC risk and HOTTIP expression, we evaluated whether this variant had allele-specific effect on its promoter activity. Luciferase reporter gene plasmid was constructed using the psiCHECK™-2 Vector (Promega). Firstly, we amplified the HOTTIP sequence with rs1859168 AA genotype by Genewiz Company (Suzhou, China) and cloned into pGL3-basic plasmids with Hind III and Bgl II digestion sites (Promega) yielding the wild-type vector. The mutant-type vector including the C allele of rs1859168 was generated using sit-specific mutagenesis (Stratagene). The resulting constructs were verified by direct sequencing. Then, the PC cell lines Panc-1, BxPC-3, and SW1990 was transfected with the constructed reporter plasmid and was cultured in Dulbecco’s modified Eagle’s medium using Attractene Transfection Reagent (QIAGEN). Finally, the cells were harvested after 24 h transfection and were determined for two luciferase activity (Renilla and Firefly) with the Dual-Luciferase Reporter Assay System (Promega).
Statistical analysis
All data was analyzed using SPSS 21.0 software (Statistical Package for the Social Sciences, Chicago, USA) and when P < 0.05 was considered as significant (two-tailed). Firstly, the Pearson chi-square test and goodness-of-fit chi-square test were used for assessing differences in characteristics between two groups and for detecting Hardy-Weinberg equilibrium among the controls and patients for SNPs, respectively. Secondly, the multiple logistic regression analysis was performed to assess the relationship of each SNP and the risk of PC, with adjustment of age, gender, smoking status, alcohol intake, body mass index, hypertension, and history of pancreatitis, diabetes mellitus, and cancer. Lastly, comparing the relative expression levels of HOTTIP in PC tissues among different genotypic groups and luciferase activities in PC cell lines among different rs1859168 allele groups were carried out using Non-parametric Mann-Whitney U test and Student’s t test, respectively.
Results
Characteristics of the participants
A total of 921 PC patients and 921 unrelated gender and age-matched healthy controls were recruited in our study, including the discovery set (416 patients and 416 controls) and validation set (505 patients and 505 controls). The demographic characteristics for the PC patients and controls of both discovery and validation sets were showed in Table 2. The distributions of age, gender, body mass index, smoking status, alcohol intake, and hypertension status were not different between the PC patients and controls in both discovery and validation sets (P > 0.05). However, there were more individuals with history of pancreatitis and diabetes mellitus and family history of cancer in the PC patients than in the controls for both sets (P < 0.05).
Table 2.
Characteristics of the study subjects
| Variable | Discovery set | Validation set | ||||
|---|---|---|---|---|---|---|
| patients | Controls | P | Patients | Controls | P | |
| (N = 416) | (N = 416) | (N = 505) | (N = 505) | |||
| Age | 0.532 | 0.184 | ||||
| < 60 | 191 (45.9) | 200 (48.1) | 217 (43.0) | 238 (47.1) | ||
| ≥ 60 | 225 (54.1) | 216 (51.9) | 288 (57.0) | 267 (52.9) | ||
| Gender | 0.357 | 0.477 | ||||
| Male | 245 (58.9) | 258 (62.0) | 316 (62.6) | 305 (60.4) | ||
| Female | 171 (41.1) | 158 (38.0) | 189 (37.4) | 200 (39.6) | ||
| Body mass index | 0.329 | 0.613 | ||||
| < 23 | 178 (42.8) | 192 (46.2) | 227 (45.0) | 235 (46.5) | ||
| ≥ 23 | 238 (57.2) | 224 (53.8) | 278 (55.0) | 270 (53.5) | ||
| Smoking status | 0.363 | 0.334 | ||||
| No | 229 (55.0) | 242 (58.2) | 298 (59.0) | 313 (62.0) | ||
| Yes | 187 (45.0) | 174 (41.8) | 207 (41.0) | 192 (38.0) | ||
| Alcohol consumer | 0.360 | 0.339 | ||||
| No | 237 (57.0) | 250 (60.1) | 300 (59.4) | 285 (56.4) | ||
| Yes | 179 (43.0) | 166 (39.9) | 205 (40.6) | 220 (43.6) | ||
| Hypertension | 0.082 | 0.067 | ||||
| No | 297 (71.4) | 319 (76.7) | 353 (69.9) | 379 (75.0) | ||
| Yes | 119 (28.6) | 97 (23.3) | 152 (30.1) | 126 (25.0) | ||
| History of pancreatitis | 0.003 | 0.001 | ||||
| No | 400 (96.2) | 413 (99.3) | 481 (95.2) | 499 (98.8) | ||
| Yes | 16 (3.8) | 3 (0.7) | 24 (4.8) | 6 (1.2) | ||
| History of diabetes mellitus | < 0.001 | <0.001 | ||||
| No | 332 (79.8) | 383 (92.1) | 409 (81.0) | 460 (91.1) | ||
| Yes | 84 (20.2) | 33 (7.9) | 96 (19.0) | 45 (8.9) | ||
| Family history of cancer | 0.002 | 0.003 | ||||
| No | 394 (94.7) | 410 (98.6) | 480 (95.0) | 497 (98.4) | ||
| Yes | 22 (5.3) | 6 (1.4) | 25 (5.0) | 8 (1.6) | ||
Associations between SNPs in lncRNA genes and PC risk
The observed genotypic frequencies of HOTTIP rs1859168, HOTAIR rs4759314, and H19 rs217727 in controls are all consistent with the Hardy-Weinberg equilibrium (P > 0.05). The genotype distributions of the three polymorphisms between PC patients and controls in the discovery population were shown in Table 3. Compared with HOTTIP rs1859168 AA genotype, subjects with the CC genotype obviously cut down the risk of PC (adjusted OR = 0.71, 95%CI = 0.57–0.88, P = 0.002). A significant association was found between rs1859168 and PC risk in recessive model (adjusted OR = 0.51, 95%CI = 0.38–0.68, P < 0.001) and additive model (adjusted OR = 0.67, 95%CI = 0.51–0.82, P < 0.001). However, HOTAIR rs4759314 A>G and H19 rs217727 C>T showed no significant associations with the risk of PC. HOTTIP rs1859168 was further replicated in validation set and was found to be significantly associated with decreased risk of PC (CC vs AA: adjusted OR = 0.70, 95%CI = 0.58–0.85, P < 0.001; dominant model: adjusted OR = 0.71, 95%CI = 0.51–0.99, P = 0.042; recessive model: OR = 0.56, 95%CI = 0.43–0.75, P < 0.001; additive model: OR = 0.69, 95%CI = 0.57–0.84, P < 0.001). When the two populations are pooled together, the results also indicated that the C allele of rs1859168 could meaningfully decrease PC incidence (CC vs AA: adjusted OR = 0.71, 95%CI = 0.61–0.81, P < 0.001; dominant model: adjusted OR = 0.72, 95%CI = 0.56–0.93, P = 0.010; recessive model: OR = 0.54, 95%CI = 0.44–0.66, P < 0.001; additive model: OR = 0.68, 95%CI = 0.59–0.78, P < 0.001)
Table 3.
Distribution of long non-coding RNA genes in case and control subjects and their associations with risk of pancreatic cancer
| Gene | SNPs | Patients | Controls | ORa (95%CI) | P |
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Discovery set | N = 416 | N = 416 | |||
| HOTTIP | rs1859168 | ||||
| AA | 80 (19.2) | 60 (14.4) | 1 | ||
| AC | 220 (52.9) | 175 (42.1) | 0.99 (0.66–1.49) | 0.973 | |
| CC | 116 (27.9) | 181 (43.5) | 0.71 (0.57–0.88) | 0.002 | |
| Dominant model | 0.74 (0.51–1.08) | 0.122 | |||
| Recessive model | 0.51 (0.38–0.68) | < 0.001 | |||
| Additive model | 0.67 (0.51–0.82) | < 0.001 | |||
| HOTAIR | rs4759314 | ||||
| AA | 333 (80.0) | 325 (78.1) | 1 | ||
| AG | 75 (18.0) | 82 (19.7) | 0.91 (0.64–1.31) | 0.621 | |
| GG | 8 (2.0) | 9 (2.2) | 0.99 (0.60–1.64) | 0.957 | |
| Dominant model | 0.92 (0.65–1.30) | 0.621 | |||
| Recessive model | 0.96 (0.35–2.61) | 0.928 | |||
| Additive model | 0.93 (0.69–1.26) | 0.646 | |||
| H19 | rs217727 | ||||
| CC | 133 (32.0) | 128 (30.8) | 1 | ||
| CT | 200 (48.0) | 196 (47.1) | 1.04 (0.75–1.44) | 0.821 | |
| TT | 83 (20.0) | 92 (22.1) | 0.93 (0.76–1.13) | 0.462 | |
| Dominant model | 0.98 (0.72–1.32) | 0.877 | |||
| Recessive model | 0.84 (0.59–1.19) | 0.325 | |||
| Additive model | 0.94 (0.78–1.14) | 0.509 | |||
| Validation set | N = 505 | N = 505 | |||
| HOTTIP | rs1859168 | ||||
| AA | 105 (20.8) | 76 (15.0) | 1 | ||
| AC | 277 (54.9) | 246 (48.7) | 0.86 (0.60–1.22) | 0.387 | |
| CC | 123 (24.4) | 183 (36.2) | 0.70 (0.58–0.85) | < 0.001 | |
| Dominant model | 0.71 (0.51–0.99) | 0.042 | |||
| Recessive model | 0.56 (0.43–0.75) | < 0.001 | |||
| Additive model | 0.69 (0.57–0.84) | < 0.001 | |||
| Pooled analysis | N = 921 | N = 921 | |||
| HOTTIP | rs1859168 | ||||
| AA | 185 (20.1) | 136 (14.8) | 1 | ||
| AC | 497 (54.0) | 421 (45.7) | 0.92 (0.70–1.20) | 0.522 | |
| CC | 239 (26.0) | 364 (39.5) | 0.71 (0.61–0.81) | < 0.001 | |
| Dominant model | 0.72 (0.56–0.93) | 0.010 | |||
| Recessive model | 0.54 (0.44–0.66) | < 0.001 | |||
| Additive model | 0.68 (0.59–0.78) | < 0.001 | |||
ORs and P values were obtained from logistic regression models with adjustment for age, gender, body mass index, smoking status, alcohol intake, hypertension, history of pancreatitis, diabetes mellitus, and family history of cancer
Stratification analysis for associations between HOTTIP rs1859168 and PC risk
The association between HOTTIP rs1859168 polymorphism and PC risk in the pooled populations was further evaluated by the stratified analysis. The associations between rs1859168 AC + CC genotype and decreased risk of PC were more obvious in populations with age longer than 60, males, non-smokers, drinkers, and populations with hypertension, no history of pancreatitis, no history of diabetes mellitus, and no family history of cancer than in their counterparts (Table 4).
Table 4.
Stratification analysis of the association between rs1859168 genotypes and pancreatic cancer risk
| Variables | rs1859168 (AA/AC + CC) | |||
|---|---|---|---|---|
| Patients | Control | OR (95%CI) | P | |
| Age | ||||
| < 60 | 81/327 | 68/370 | 0.78 (0.54–1.13) | 0.184 |
| ≥ 60 | 104/409 | 68/415 | 0.67 (0.48–0.95) | 0.025 |
| Sex | ||||
| Male | 118/443 | 82/481 | 0.64 (0.47–0.88) | 0.006 |
| Female | 67/293 | 54/304 | 0.86 (0.57–1.30) | 0.482 |
| Body mass index | ||||
| < 23 | 81/324 | 64/363 | 0.73 (0.50–1.05) | 0.092 |
| ≥ 23 | 104/412 | 72/422 | 0.72 (0.51–1.01) | 0.058 |
| Smoking status | ||||
| No | 109/418 | 74/481 | 0.64 (0.46–0.89) | 0.008 |
| Yes | 76/318 | 62/304 | 0.84 (0.57–1.23) | 0.364 |
| Alcohol consumer | ||||
| No | 103/434 | 85/450 | 0.82 (0.59–1.13) | 0.220 |
| Yes | 82/302 | 51/335 | 0.59 (0.40–0.87) | 0.008 |
| Hypertension | ||||
| No | 136/514 | 97/601 | 0.64 (0.47–0.85) | 0.003 |
| Yes | 49/222 | 39/184 | 0.95 (0.59–1.54) | 0.829 |
| History of pancreatitis | ||||
| No | 177/704 | 135/777 | 0.72 (0.56–0.93) | 0.012 |
| Yes | 8/32 | 1/8 | 0.90 (0.07–10.97) | 0.937 |
| History of diabetes mellitus | ||||
| No | 148/593 | 123/720 | 0.72 (0.55–0.94) | 0.015 |
| Yes | 37/143 | 13/65 | 0.72 (0.35–1.48) | 0.372 |
| Family history of cancer | ||||
| No | 170/704 | 132/775 | 0.72 (0.56–0.93) | 0.011 |
| Yes | 15/32 | 4/10 | 0.42 (0.10–1.87) | 0.258 |
ORs and P values were obtained from logistic regression models with adjustment for age, gender, body mass index, smoking status, alcohol intake, hypertension, history of pancreatitis, diabetes mellitus, and family history of cancer
The genotype-phenotype correlation between rs1859168 and HOTTIP expression
In order to assess the genotype-phenotype of rs1859168 in PC, we then calculated the HOTTIP expression in PC tissue samples with different genotypes. As shown in Fig. 1a, HOTTIP expression level was significantly lower in samples with CC genotype than that in samples with AA and AC genotype (P < 0.001).
Fig. 1.
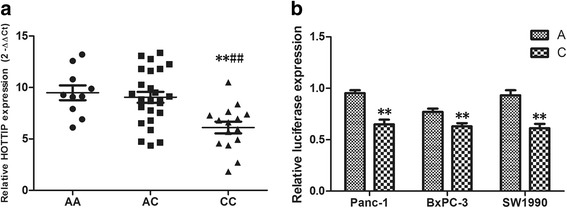
The genotype-phenotype association analysis and luciferase reporter assay. a The relative expression of HOTTIP with different genotypes (compared with AA genotype, ** P < 0.001; compared with AC genotype, ## P < 0.001). AA: N = 10, AC: N = 25, CC: N = 15. b The effect of rs1859168 on HOTTIP transcriptional activity as determined by luciferase reporter assay (compared with A allele, **P < 0.001)
The influence of rs1859168 and HOTTIP transcription activity
To further understand the mechanism of genotype-phenotype correlation, the luciferase transfection system was performed to assess the effects of rs1859168 on the promoter activity. As illustrated in Fig. 1b, the luciferase activity of the cells containing rs1859168 C allele was significantly lower than that of the cells containing rs1859168 A allele in three cell lines tested, which indicate that rs1859168 C allele may associate with a lower HOTTIP level than the rs1859168 A allele.
Discussion
In the study, the association between important SNPs in three key lncRNA genes (HOTTIP, HOTAIR, and H19) and the risk of PC was analyzed using discovery and validation approach, and the function of HOTTIP rs1859168 A > C was also evaluated. This is the first assessment about the association between three common SNPs in critical lncRNAs (HOTTIP rs1859168 A > C, HOTAIR A > G, and H19 rs217727 C > T) and PC risk. Consistent with the results of validation set and pooled analysis, the results of discovery set showed rs1859168 carrying C allele in HOTTIP gene was significantly correlated with reduced PC risk, suggesting that the polymorphism of rs1859168 locus was significantly correlated with the incidence of PC. To explore the association between rs1859168 locus and PC, all samples in the discovery set and validation set were classified based on general characteristics. Analysis results showed a significant association under recessive model (AA/AC + CC) between rs1859168 and the risk of PC in subgroup populations with age longer than 60, males, non-smokers, drinkers, and populations with hypertension, no history of pancreatitis, no history of diabetes mellitus, and no family history of cancer. To explore the function of rs1859168, the expression of HOTTIP in PC tissues with different genotypes was measured using RT-PCR and luciferase reporter gene assay, and the results showed C allele was meaningfully correlated to the decreased HOTTIP expression. Taken together, these data suggest that the rs1859168 locus is an important factor in assessing the risk of PC and the expression of HOTTIP.
In the discovery set of this study, three locus (HOTTIP rs1859168 A > C, HOTAIR rs4759314 A > G, and H19 rs217727 C > T) on three lncRNAs referred to the initiation and progress of PC was explored. Our results indicated that neither HOTAIR rs4759314 A > G nor H19 rs217727 C > T was significantly correlated to the risk of PC. Numerous researchers have explored the relationship between these two sites and the risk of a variety of tumors. However, the results were not consistent. Our study explored the association of two loci with the risk of PC for the first time. Although they cannot be used as screening markers for PC in Chinese Han population, what surprised us was that HOTTIP rs1859168 A > C is strongly associated with the occurrence of PC.
HOTTIP, located at the 5′ end of the HOXA gene cluster, is an antisense non-coding transcript. To date, a great number of studies have reported the role of HOTTIP in pancreatic cancers. For example, a study by Cheng observed that knockdown of HOTTIP had decreased proliferation, induced apoptosis and decreased migration of PC cells [20]. Moreover, there is increasing evidence about upregulated HOTTIP in various types of cancer tissues and that was associated with cancer progression and poor prognosis for PC [34–39]. These evidences showed a tumor-promoting role of HOTTIP.
rs1859168, which is located at HOTTIP, was suggested to affect transcription factor binding sites and later the centroid secondary structure and minimum free energy, which might influence the HOTTIP expression and function [29]. In the present study, we first evaluated the association of HOTTIP rs1859168 A > C with PC risk, the study is a two-stage case-control study with large sample size provided plenty statistical power and decreased the false-positive report probability. The study showed that the C in rs1859168 had a significantly decreased risk of PC in all genetic models, suggesting this site has the potential to be a marker for PC screening. There were only two studies that have evaluated the associations of HOTTIP rs1859168 A > C with cancer risk. Gong and his colleagues performed a case-control study with a total of 498 lung cancer patients and 213 controls, and found patients carried HOTTIP rs1859168 A allele had higher lung cancer risk [29]. Similarly, our work indicated that patients carrying AA + AC have more hazards developing PC compared with individuals carrying CC.
However, a study by Richards showed that HOTTIP rs1859168 was not related to epithelial ovarian cancer risk in allele model in 1201 epithelial ovarian cancer patients and 2009 controls [40]. Extensive research has proved the expression of HOTTIP is closely related to the occurrence and development of lung cancer and pancreatic cancer, whereas no data exist concerning the relationship between the expression of HOTTIP and epithelial ovarian cancer. Thus, tumor type may be the main factor affecting the HOTTIP rs1859168 as a molecular marker for tumor screening, so as the study population. The study by Gong et al. [29] and the present work are both based on the Chinese Han population, while the research by Edward J. Richards is not, therefore, we conclude that HOTTIP rs1859168 can be used as a marker for PC in Chinese Han population, but it is not a broad-spectrum tumor marker. To further investigate the relationship between this site and the risk of pancreatic cancer, the case-control was classified based on general characteristics and analysis results showed the recessive model of rs1859168 (AA/AC + CC) was a well diagnosis biomarker of risk of PC in populations with age longer than 60, males, non-smokers, drinkers, and populations with hypertension, no history of pancreatitis, no history of diabetes mellitus, and no family history of cancer, which were not consistent with that of research in lung cancer by Gong. Therefore, attention should be paid to the population difference when using HOTTIP rs1859168 as the screening marker of PC. The work laid a good foundation for the genetic counseling of PC in future.
As the association of HOTTIP rs1859168 with the risk of PC has been demonstrated in this work, combined with the fact that HOTTIP expression was significantly related to the occurrence and development of PC, we hypothesize that HOTTIP rs1859168 polymorphism may affect the HOTTIP expression by altering its transcript activity and consequently correlate with the PC risk. Our results from the genotype-phenotype association analysis and luciferase activity confirmed this hypothesis.
There were several strengths and limitations in our present study. First, this is the first study to evaluate the association between three common SNPs in key lncRNAs genes and PC risk, and found positive association between HOTTIP rs1859168 polymorphism and PC risk. Second, the two-stage case-control study with large sample size provided plenty statistical power and decreased the false-positive report probability. Third, the functional studies of genotype-phenotype analysis and luciferase activity reporter assay mad a plausible biologic explanation of how HOTTIP rs1859168 variant affected PC risk. However, the study was hospital-based case-control study and all subjects were ethnic Han Chinese. Thus, well-designed population-based prospective studies with different ethnicities are needed to validate these results.
Conclusions
In summary, our study provided the first evidence that the functional rs1859168 A > C polymorphism may decrease the PC risk by downregulating the HOTTIP expression levels. Extensive functional researches and additional well-designed population-based prospective studies with different ethnic groups are warranted to confirm and extend our findings.
Acknowledgements
Not applicable.
Funding
The study was supported by no funding.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CI
Confidence interval
- lncRNAs
Long non-coding RNAs
- PC
Pancreatic cancer
- SNPs
Single-nucleotide polymorphisms
Authors’ contributions
PHH and YJ designed and conducted the experiments. OQ, JW, and JL surveyed the literature and developed the text of the manuscript. HJ and ZLL helped for the statistical analysis. PHH and YJ refined the write-up. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province (Kunming, China) and written informed consent was also obtained from each participant after a clear explanation of study objective.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pinghai Hu, Email: hupinghai75@sina.com.
Ou Qiao, Email: qiaoou1122@163.com.
Jun Wang, Email: wangjun882233@sohu.com.
Jiao Li, Email: lijiao246@21cn.com.
Hao Jin, Email: jinhao135@2980.com.
Zhaolian Li, Email: lizhaolian135@tom.com.
Yan Jin, Phone: +86-13700676730, Email: jinyan63@aliyun.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Useros J, Garcia-Foncillas J. Can molecular biomarkers change the paradigm of pancreatic cancer prognosis? Biomed Res Int. 2016;2016:4873089. doi: 10.1155/2016/4873089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viterbo D, Gausman V, Gonda T. Diagnostic and therapeutic biomarkers in pancreaticobiliary malignancy. World J Gastrointest Endosc. 2016;8:128–142. doi: 10.4251/wjgo.v8.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Marechal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10–22. doi: 10.1016/j.ejca.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Wada K, Takaori K, Traverso LW. Screening for pancreatic cancer. Surg Clin North Am. 2015;95:1041–1052. doi: 10.1016/j.suc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciuti B, Mencaroni C, Paglialunga L, Paciullo F, Crino L, Chiari R, Metro G. Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33:18. doi: 10.1007/s12032-016-0731-2. [DOI] [PubMed] [Google Scholar]
- 10.Evans JR, Feng FY, Chinnaiyan AM. The bright side of dark matter: lncRNAs in cancer. J Clin Invest. 2016;126:2775–2782. doi: 10.1172/JCI84421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Gesualdo F, Capaccioli S, Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget. 2014;5:10976–10996. doi: 10.18632/oncotarget.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taucher V, Mangge H, Haybaeck J. Non-coding RNAs in pancreatic cancer: challenges and opportunities for clinical application. Cell Oncol (Dordr) 2016;39:295–318. doi: 10.1007/s13402-016-0275-7. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Tian X, Wang F, Zhang Z, Du C, Xie X, Kornmann M, Yang Y. The long noncoding RNA H19 promotes cell proliferation via E2F-1 in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2016;17:1051-61. [DOI] [PMC free article] [PubMed]
- 14.Lian Y, Cai Z, Gong H, Xue S, Wu D, Wang K. HOTTIP: a critical oncogenic long non-coding RNA in human cancers. Mol BioSyst. 2016;12:3247-53. [DOI] [PubMed]
- 15.Saus E, Brunet-Vega A, Iraola-Guzman S, Pegueroles C, Gabaldon T, Pericay C. Long non-coding RNAs as potential novel prognostic biomarkers in colorectal cancer. Front Genet. 2016;7:54. doi: 10.3389/fgene.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai H, An Y, Chen X, Sun D, Chen T, Peng Y, Zhu F, Jiang Y, He X. Epigenetic inhibition of miR-663b by long non-coding RNA HOTAIR promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget. 2016;7:86857–86870. doi: 10.18632/oncotarget.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z, Chen X, Li J, Guo Y, Li H, Pan X, Jiang J, Liu H, Wu B. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7:25408–25419. doi: 10.18632/oncotarget.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H, Wang Y, Zeng J, Song Y, Gao W, et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Transl Med. 2015;13:84. doi: 10.1186/s12967-015-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6:10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaiewicz V, Sorin V, Fellig Y, Birman T, Mizrahi A, Galula J, Abu-Lail R, Shneider T, Ohana P, Buscail L, et al. Use of H19 gene regulatory sequences in DNA-based therapy for pancreatic cancer. J Oncol. 2010;2010:178174. doi: 10.1155/2010/178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao R, Hu S, Wang S, Zhou X, Zhang Q, Wang C, Zhao X, Zhou W, Zhang S, Li C, et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis. 2015;36:1136–1143. doi: 10.1093/carcin/bgv099. [DOI] [PubMed] [Google Scholar]
- 23.Bayram S, Ulger Y, Sumbul AT, Kaya BY, Rencuzogullari A, Genc A, Sevgiler Y, Bozkurt O, Rencuzogullari E. A functional HOTAIR rs920778 polymorphism does not contributes to gastric cancer in a Turkish population: a case-control study. Familial Cancer. 2015;14:561–567. doi: 10.1007/s10689-015-9813-0. [DOI] [PubMed] [Google Scholar]
- 24.Pan W, Liu L, Wei J, Ge Y, Zhang J, Chen H, Zhou L, Yuan Q, Zhou C, Yang M. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog. 2016;55:90–96. doi: 10.1002/mc.22261. [DOI] [PubMed] [Google Scholar]
- 25.Miao Z, Ding J, Chen B, Yang Y, Chen Y. HOTAIR overexpression correlated with worse survival in patients with solid tumors. Minerva Med. 2016;107:392–400. [PubMed] [Google Scholar]
- 26.Lee M, Kim HJ, Kim SW, Park SA, Chun KH, Cho NH, Song YS, Kim YT. The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the notch pathway. Oncotarget. 2016;7:44558-71. [DOI] [PMC free article] [PubMed]
- 27.Sun W, Yang Y, Xu C, Xie Y, Guo J. Roles of long noncoding RNAs in gastric cancer and their clinical applications. J Cancer Res Clin Oncol. 2016;142:2231–2237. doi: 10.1007/s00432-016-2183-7. [DOI] [PubMed] [Google Scholar]
- 28.Xia Z, Yan R, Duan F, Song C, Wang P, Wang K. Genetic polymorphisms in long noncoding RNA H19 are associated with susceptibility to breast cancer in Chinese population. Medicine (Baltimore) 2016;95:e2771. doi: 10.1097/MD.0000000000002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong WJ, Yin JY, Li XP, Fang C, Xiao D, Zhang W, Zhou HH, Li X, Liu ZQ. Association of well-characterized lung cancer lncRNA polymorphisms with lung cancer susceptibility and platinum-based chemotherapy response. Tumour Biol. 2016;37:8349–8358. doi: 10.1007/s13277-015-4497-5. [DOI] [PubMed] [Google Scholar]
- 30.Sang Y, Zhou F, Wang D, Bi X, Liu X, Hao Z, Li Q, Zhang W. Up-regulation of long non-coding HOTTIP functions as an oncogene by regulating HOXA13 in non-small cell lung cancer. Am J Transl Res. 2016;8:2022–2032. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SR, Yang JK, Xie JK, Zhao LC. Long noncoding RNA HOTTIP contributes to the progression of prostate cancer by regulating HOXA13. Cell Mol Biol (Noisy-le-grand) 2016;62:84–88. [PubMed] [Google Scholar]
- 32.Chu M, Yuan W, Wu S, Wang Z, Mao L, Tian T, Lu Y, Zhu B, Yang Y, Wang B, et al. Quantitative assessment of polymorphisms in H19 lncRNA and cancer risk: a meta-analysis of 13,392 cases and 18,893 controls. Oncotarget. 2016;7:78631-9. [DOI] [PMC free article] [PubMed]
- 33.Qi Q, Wang J, Huang B, Chen A, Li G, Li X, Wang J. Association of HOTAIR polymorphisms rs4759314 and rs920778 with cancer susceptibility on the basis of ethnicity and cancer type. Oncotarget. 2016;7:38775-84. [DOI] [PMC free article] [PubMed]
- 34.Wang Y, Li Z, Zheng S, Zhou Y, Zhao L, Ye H, Zhao X, Gao W, Fu Z, Zhou Q, et al. Expression profile of long non-coding RNAs in pancreatic cancer and their clinical significance as biomarkers. Oncotarget. 2015;6:35684–35698. doi: 10.18632/oncotarget.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren YK, Xiao Y, Wan XB, Zhao YZ, Li J, Li Y, Han GS, Chen XB, Zou QY, Wang GC, et al. Association of long non-coding RNA HOTTIP with progression and prognosis in colorectal cancer. Int J Clin Exp Pathol. 2015;8:11458–11463. [PMC free article] [PubMed] [Google Scholar]
- 36.Quagliata L, Matter MS, Piscuoglio S, Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z, Boldanova T, et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology. 2014;59:911–923. doi: 10.1002/hep.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Cao L, Hang D, Wang F, Wang Q. Long non-coding RNA HOTTIP is up-regulated and associated with poor prognosis in patients with osteosarcoma. Int J Clin Exp Pathol. 2015;8:11414–11420. [PMC free article] [PubMed] [Google Scholar]
- 38.Ye H, Liu K, Qian K. Overexpression of long noncoding RNA HOTTIP promotes tumor invasion and predicts poor prognosis in gastric cancer. Onco Targets Ther. 2016;9:2081–2088. doi: 10.2147/OTT.S95414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Zhao L, Wang YX, Xi M, Liu SL, Luo LL. Long non-coding RNA HOTTIP is correlated with progression and prognosis in tongue squamous cell carcinoma. Tumour Biol. 2015;36:8805–8809. doi: 10.1007/s13277-015-3645-2. [DOI] [PubMed] [Google Scholar]
- 40.Richards EJ, Permuth-Wey J, Li Y, Chen YA, Coppola D, Reid BM, Lin HY, Teer JK, Berchuck A, Birrer MJ, et al. A functional variant in HOXA11-AS, a novel long non-coding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget. 2015;6:34745–34757. doi: 10.18632/oncotarget.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


