Abstract
Background
Malaria vector control methods involving the use of pyrethroids remain the strategies being used against malaria vectors in Ghana. These methods include the use of long-lasting insecticidal nets and indoor residual spraying in many areas in Ghana. However, there is evidence that pyrethroid resistance is widespread in many areas in Ghana. Synergists have been shown to be useful in inhibiting the enzymes that are responsible for the development of resistance and hence enhance the insecticide susceptibility of Anopheles gambiae sensu lato (s.l.) in many areas. The present study investigated the effect of piperonyl butoxide (PBO) on the susceptibility status of An. gambiae s.l. across some sentinel sites in Ghana.
Methods
Three to five day old An. gambiae s.l. reared from larvae were used in WHO susceptibility tube assays. Batches of 20–25 female adult An. gambiae s.l. were exposed simultaneously to the insecticide alone and to the PBO + insecticide. The knock down rate after 60 min and mortality at 24 h were recorded.
Results
Deltamethrin and permethrin resistance of An. gambiae s.l. was observed in all the sites in 2015 and 2016. The mortality after 24 h post exposure for deltamethrin ranged from 16.3% in Weija to 82.3% in Kade, whereas that for permethrin ranged from 3.8% in Gomoa Obuasi to 91.3% in Prestea. A significant increase in susceptibility to deltamethrin and less to permethrin was observed during both 2015 and 2016 years in most of the sites when An. gambiae s.l. mosquitoes were pre-exposed to PBO.
Conclusion
Findings from this study showed that the use of PBO significantly enhanced the susceptibility of An. gambiae s.l. mosquitoes in most of the sentinel sites. It is recommended that vector control strategies incorporating PBO as a synergist can be effective in killing mosquitoes in the presence of deltamethrin and permethrin resistance.
Keywords: Pyrethroids, Piperonyl butoxide, Anopheles gambiae, Insecticide resistance
Background
The development of resistance by mosquitoes to the major pyrethroid insecticides being used for malaria control still remains one of the major obstacles of the effective control of the disease and threatens to hinder efforts at malaria elimination globally and especially in Africa. The rapid spread of the insecticide resistance of Anopheles gambiae sensu lato (s.l.) throughout sub-Saharan Africa and its impact on the failure of vector control strategies has become the main worry of all the national malaria control programmes. Most of the available control tools are facing a decrease of effectiveness following increasing insecticide resistance of An. gambiae s.l., the main malaria vector [1–3].
In Ghana, the use of insecticides for public health and agriculture remain the main strategy for controlling disease vectors and pests. The distribution and use of insecticide-treated nets (ITNs) as well as indoor residual spraying (IRS) have been scaled up as the main vector control tools in the country. However, resistance to pyrethroids and other classes of insecticides have been reported in parts of Ghana [4–10]. This poses a challenge to the success of most of the insecticide-based vector control tools in controlling disease vectors including those of malaria in Ghana. Therefore, there is the need to monitor the level of insecticide resistance in the country so as to be able to formulate resistance management strategies.
Recent efforts have centered on developing control strategies that can be effective even in the presence of pyrethroid resistance. Studies have shown that the use of synergist in addition to insecticides has revealed to be a good option of controlling resistant mosquitoes [11, 12]. Commonly used synergists include piperonyl butoxide (PBO), which inhibits cytochrome P450 monooxygenase enzyme activity targeting DDT and pyrethroid insecticides [13, 14]. PBO is an organic compound used as a component of pesticide formulations and as synergist. Despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as especially for carbamates, pyrethrins, pyrethroids, and rotenone [14, 15]. PBO acts as an insecticide synergist by inhibiting the natural defenses of the insects. PBO inhibits enzymes present in insects, most important of which is the mixed function oxidase system (MFO) also known as the cytochrome P450 system [16, 17].
Therefore, synergists such as PBO clearly have an important role to play in increasing the efficacy of pyrethroids when used against pyrethroid-resistant mosquitoes. Currently, only pyrethroids are recommended by the World Health Organization (WHO) for use on mosquito nets, and alternatives are urgently required as insecticide resistance is compromising the performance of pyrethroid only treated nets [2, 3]. This study investigated the role of PBO on the insecticide resistance status of An. gambiae s.l. collected across 20 sentinel sites in Ghana.
Methods
Sentinel sites
Ghana is divided into ten regions and two sentinel sites were selected in each region across all the ten regions to cover all the ecological areas in the country (Fig. 1). The selection of the sites was done based on information on agricultural practices and preferentially rice and vegetable growing fields, mining and other activities that can lead to the development of insecticide resistance. The National Malaria Control Programme of Ghana (NMCP) as part of the mass campaign had also distributed pyrethroid-based long-lasting insecticidal nets (LLINs) in all the sentinel sites. For the purposes of the study, the country was divided into southern and northern sectors and also to match with the rainy season in the selected sites. Sites in the southern sector included Akuse (Lat: 6.0868, Long: 0.12139) and Kade (Lat: 6.0000, Long: −0.8332) in the Eastern Region; Nkwanta (Lat: 8.2000, Long: 0.5186) and Afife (Lat: 6.1000, Long: 0.9166) in the Volta Region; Sefwi-Wiaso (Lat: 6.2000, Long: −2.4850) and Prestea (Lat: 5.5000, Long: −1.9166) in the Western Region: Twifo-Praso (Lat: 5.6999, Long: −1.54999) and Gomoa Obuasi (Lat: 5.3000, Long: −0.7397) in the Central Region; Weija (Lat: 5.6000, Long: 0.33333) and Ada-Foah (Lat: 5.7804, Long: 0.618048) in the Greater Accra Region.
Fig. 1.
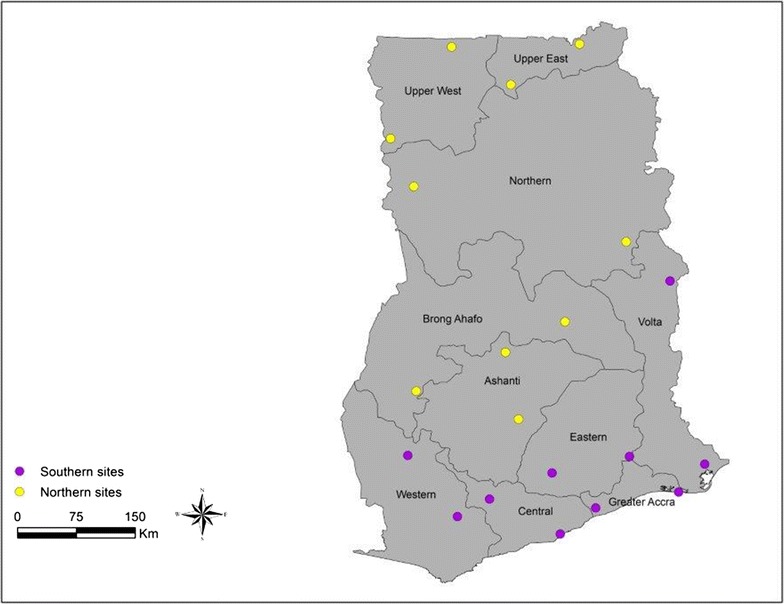
Map showing the location of mosquito collection sites across the different ecological areas in Ghana, (Southern sector sites in purple) (Northern sector sites in yellow)
Sites in the northern sector included Sawla (Lat: 9.2833, Long: −2.4166) and Wulensi (Lat: 8.6500, Long: 0.01664) in the Northern Region; Konongo (Lat: 6.6167, Long: −1.21667) and Ejura (Lat: 7.3833, Long: −1.36667) in the Ashanti Region; Fumbisi (Lat: 10.4511, Long: −1.30595) and Zebilla (Lat: 10.9167, Long: −0.51667) in the Upper East Region; Wechau (Lat: 9.8333, Long: −2.68333) and Tumu (Lat: 10.8833, Long: −1.98335) in the Upper West Region; Kenyasi (Lat: 6.9383, Long: −2.3889) and Kwame-Danso (Lat: 7.7333, Long: −0.68333) in the Brong Ahafo Region (Fig. 1).
WHO insecticide susceptibility bioassays
Larval collections were carried out in all the sentinel sites between the months of May to October of 2015 and 2016. The larvae were reared to adults and used for insecticide susceptibility assays according to the WHO insecticide susceptibility testing procedure [18]. Batches of 20–25 non-blood fed females of An. gambiae s.l. mosquitoes aged 3–5 days were introduced into each of the four tubes (replicates) and exposed to the insecticide-treated papers for an hour. Two tubes were used as controls. The temperature and relative humidity were recorded at the start and end of the testing. Knockdown rates were recorded at 5, 10, 15, 20 30, 40, 50 and 60 min and mortalities recorded 24 h after the holding period.
Synergist assays with PBO
Whatman papers impregnated with piperonyl butoxide (PBO) synergist were used to perform the synergist assay. In total, 20–25 An. gambiae s.l. mosquitoes were exposed to four (4) replicates of 5% piperonyl butoxide (PBO) for 1 h to suppress oxidase enzymes. Mosquitoes were then immediately transferred and exposed to either 0.05% deltamethrin or 0.75% permethrin for an additional hour. Two control tubes were run in parallel at any time of the testing. Knockdown rates were recorded as previously for 60 min, after which the mosquitoes were transferred in holding tubes and held for 24 h after which the mortality recorded.
Data analysis
Xlstat 2010 statistical software was used for the statistical analyses. Values were compared using the χ2 test and the mortalities per insecticide across site were compared using the binomial regression following the formula: f (y) = log (y/(1 − y)). The resistance status of mosquitoes was determined using the WHO criteria [19]; mortality ≤90% is resistant, mortality ≥91–97% is suspected resistant; mortality ≥98% is susceptible. All analyses were carried out at 5% level of significance and 95% confidence level.
Results
The results of the survey indicated very high deltamethrin and permethrin resistance of An. gambiae s.l. in all the sites surveyed both in year 2015 and 2016. Tables 1 and 2 show the 24 h mortality rates of An. gambiae s.l. from the southern and northern sentinel sites exposed to both deltamethrin and permethrin alone or pre-exposed to PBO.
Table 1.
Mortality of Anopheles gambiae s.l. exposed to deltamethrin and permethrin alone as well as PBO + deltamethrin and PBO + permethrin in 2015 and 2016 in the southern sector
| Region | Sentinel site | Insecticide | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total tested | % Mortality | CI 95% Mortality | p value | Total tested | % Mortality | CI 95% mortality | p value | |||
| Greater Accra | Ada | Deltamethrin | 80 | 68.8 | ±2.3 | 1.16E−13 | 98 | 4.1 | ±0.6 | 3.13E−19 |
| PBO + deltamethrin | 80 | 100.0 | ±0.0 | 94 | 93.6 | ±1.4 | ||||
| Permethrin | 80 | 75.0 | ±2.8 | 2.66E−15 | 98 | 19.4 | ±0.4 | 1.00E+00 | ||
| PBO + permethrin | 80 | 97.5 | ±0.6 | 98 | 19.4 | ±0.4 | ||||
| Weija | Deltamethrin | 80 | 16.3 | ±4.4 | 3.22E−05 | 96 | 8.3 | ±1.2 | 2.11E−05 | |
| PBO + deltamethrin | 80 | 43.8 | ±10.5 | 99 | 32.3 | ±1.8 | ||||
| Permethrin | 80 | 12.5 | ±2.6 | 6.79E−02 | 99 | 5.1 | ±0.4 | 3.28E−01 | ||
| PBO + permethrin | 80 | 7.5 | ±1.1 | 93 | 7.5 | ±1.4 | ||||
| Central | Gomoa Obuasi | Deltamethrin | 100 | 19.0 | ±1.7 | 1.16E−14 | 80 | 20.0 | ±1.8 | 1.79E−12 |
| PBO + deltamethrin | 80 | 93.8 | ±1.6 | 86 | 90.7 | ±1.7 | ||||
| Permethrin | 80 | 3.8 | ±1.1 | 1.88E−08 | 85 | 1.2 | ±0.5 | 4.56E−08 | ||
| PBO + permethrin | 80 | 38.8 | ±3.9 | 94 | 33.0 | ±2.4 | ||||
| Twifo Praso | Deltamethrin | 80 | 36.3 | ±1.4 | 3.54E−07 | 73 | 13.7 | ±1.5 | 8.78E−06 | |
| PBO + deltamethrin | 80 | 82.5 | ±4.1 | 71 | 52.1 | ±3.4 | ||||
| Permethrin | 80 | 21.3 | ±1.9 | 3.00E−10 | 90 | 5.6 | ±0.5 | 2.81E−04 | ||
| PBO + permethrin | 80 | 76.3 | ±1.9 | 81 | 25.9 | ±3.4 | ||||
| Eastern | Akuse | Deltamethrin | 80 | 37.5 | ±3.6 | 1.2E−09 | 93 | 8.6 | ±2.1 | 1.1E−16 |
| PBO + deltamethrin | 80 | 97.5 | ±0.6 | 92 | 91.3 | ±1.7 | ||||
| Permethrin | 80 | 51.3 | ±1.4 | 1.8E−06 | 90 | 31.1 | ±1.6 | 1.8E−06 | ||
| PBO + permethrin | 80 | 98.8 | ±0.5 | 93 | 72.0 | ±2.1 | ||||
| Kade | Deltamethrin | 80 | 65.0 | ±8.8 | 4.65E−04 | 87 | 13.5 | ±0.9 | 9.93E−09 | |
| PBO + deltamethrin | 80 | 100.0 | ±0.0 | 83 | 72.8 | ±2.3 | ||||
| Permethrin | 80 | 17.5 | ±0.6 | 2.45E−13 | 86 | 1.2 | ±0.5 | 3.46E−03 | ||
| PBO + permethrin | 80 | 85.0 | ±0.9 | 80 | 12.5 | ±1.3 | ||||
| Volta | Nkwanta | Deltamethrin | 80 | 82.4 | ±3.0 | 7.90E−02 | 87 | 47.1 | ±1.4 | 7.80E−05 |
| PBO + deltamethrin | 80 | 100.0 | ±0.0 | 83 | 90.4 | ±2.6 | ||||
| Permethrin | 80 | 27.5 | ±1.9 | 1.35E−08 | 82 | 17.1 | ±1.5 | 3.77E−04 | ||
| PBO + permethrin | 80 | 77.5 | ±4.9 | 71 | 46.5 | ±3.6 | ||||
| Afife | Deltamethrin | 80 | 76.3 | ±1.1 | 7.07E−02 | 83 | 43.4 | ±6.4 | 1.28E−06 | |
| PBO + deltamethrin | 80 | 93.8 | ±1.1 | 85 | 92.9 | ±0.6 | ||||
| Permethrin | 80 | 10.8 | ±4.2 | 2.18E−07 | 83 | 31.3 | ±2.8 | 3.94E−01 | ||
| PBO + permethrin | 80 | 45.9 | ±2.8 | 83 | 26.5 | ±1.6 | ||||
| Western | Prestea | Deltamethrin | 80 | 75.0 | ±0.9 | 1.24E−02 | 88 | 13.6 | ±0.8 | 1.47E−15 |
| PBO + deltamethrin | 80 | 100.0 | ±0.0 | 88 | 97.7 | ±0.9 | ||||
| Permethrin | 80 | 91.3 | ±0.5 | 3.82E−01 | 81 | 27.2 | ±4.9 | 7.04E−10 | ||
| PBO + Permethrin | 80 | 100.0 | ±0.0 | 88 | 85.2 | ±0.9 | ||||
| Sefwi Wiawso | Deltamethrin | 80 | 25.0 | ±2.4 | 1.16E−13 | 72 | 30.6 | ±3.8 | 6.14E−07 | |
| PBO + deltamethrin | 80 | 98.8 | ±0.5 | 80 | 75.0 | ±1.9 | ||||
| Permethrin | 80 | 15.0 | ±2.0 | 2.66E−15 | 76 | 11.8 | ±2.3 | 5.69E−05 | ||
| PBO + permethrin | 80 | 90.0 | ±2.0 | 83 | 37.3 | ±3.2 | ||||
PBO piperonyl butoxide, CI confidence interval, nd could not be determined, the numbers in italics showed the non-significant p values
Table 2.
Mortality of Anopheles gambiae s.l. exposed to deltamethrin and permethrin alone as well as PBO + deltamethrin and PBO + permethrin in 2015 and 2016 in the northern sector
| Regions | Sentinel site | Insecticide | 2015 | 2016 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total tested | % Mortality | CI 95% mortality | p value | Total tested | % Mortality | CI 95% mortality | p value | |||
| Nothern | Sawla | Deltamethrin | 77 | 18.2 | ±2.1 | 9.24E−11 | 96 | 7.3 | ±0.4 | 3.29E−16 |
| PBO + deltamethrin | 79 | 84.8 | ±2.1 | 95 | 84.2 | ±2.4 | ||||
| Permethrin | 79 | 15.2 | ±0.9 | 1.58E−03 | 94 | 5.3 | ±1.1 | 1.61E−02 | ||
| PBO + permethrin | 80 | 36.3 | ±1.0 | 95 | 14.7 | ±0.9 | ||||
| Wulensi | Deltamethrin | 80 | 27.5 | ±1.4 | 2.29E−10 | 83 | 0.0 | ±0.0 | 5.55E−13 | |
| PBO + deltamethrin | 78 | 100.0 | ±0.0 | 83 | 62.7 | ±2.5 | ||||
| Permethrin | 82 | 42.7 | ±2.7 | 9.25E−05 | 89 | 3.4 | ±1.0 | 1.76E−07 | ||
| PBO + permethrin | 80 | 83.8 | ±2.4 | 88 | 37.5 | ±2.0 | ||||
| Ashanti | Konongo | Deltamethrin | 78 | 12.8 | ±1.5 | 2.92E−10 | 89 | 1.1 | ±0.5 | 4.87E−15 |
| PBO + deltamethrin | 79 | 73.4 | ±1.0 | 84 | 75.0 | ±2.2 | ||||
| Permethrin | nd | nd | nd | nd | 83 | 13.3 | ±0.6 | 1.11E−09 | ||
| PBO + permethrin | nd | nd | nd | 83 | 68.7 | ±1.0 | ||||
| Ejura | Deltamethrin | 79 | 24.1 | ±1.9 | 1.62E−10 | 80 | 41.3 | ±0.9 | 5.18E−07 | |
| PBO + deltamethrin | 80 | 92.5 | ±2.1 | 78 | 98.7 | ±0.5 | ||||
| Permethrin | 77 | 15.6 | ±2.4 | 1.61E−04 | 80 | 95.0 | ±1.3 | 7.49E−01 | ||
| PBO + permethrin | 78 | 43.6 | ±3.8 | 78 | 100.0 | ±0.0 | ||||
| Upper East | Zebilla | Deltamethrin | 79 | 89.9 | ±1.5 | 3.68E−01 | 84 | 21.4 | ±1.3 | 1.35E−11 |
| PBO + deltamethrin | 80 | 98.8 | ±0.5 | 91 | 84.6 | ±0.9 | ||||
| Permethrin | nd | nd | nd | nd | 82 | 8.5 | ±0.9 | 1.44E−02 | ||
| PBO + permethrin | nd | nd | nd | 85 | 20.0 | ±1.3 | ||||
| Fumbisi | Deltamethrin | 95 | 20.0 | ±1.2 | 2.14E−12 | 81 | 49.4 | ±3.2 | 3.19E−06 | |
| PBO + deltamethrin | 88 | 94.3 | ±0.9 | 85 | 96.5 | ±0.9 | ||||
| Permethrin | 84 | 42.9 | ±1.7 | 1.31E−06 | 90 | 17.8 | ±1.2 | 1.29E−04 | ||
| PBO + permethrin | 84 | 94.0 | ±0.5 | 95 | 42.1 | ±1.8 | ||||
| Upper West | Wecheau | Deltamethrin | 84 | 20.2 | ±1.6 | 1.62E−09 | 84 | 34.5 | ±1.6 | 4.06E−08 |
| PBO + deltamethrin | 87 | 75.9 | ±3.2 | 80 | 96.3 | ±0.5 | ||||
| Permethrin | 82 | 10.4 | ±1.3 | 5.81E−07 | 87 | 10.3 | ±0.9 | 3.08E−13 | ||
| PBO + permethrin | 83 | 49.4 | ±3.9 | 87 | 80.5 | ±1.4 | ||||
| Tumu | Deltamethrin | 86 | 38.4 | ±0.5 | 5.32E−07 | 87 | 4.6 | ±1.3 | 1.05E−15 | |
| PBO + deltamethrin | 77 | 100.0 | ±0.0 | 84 | 85.7 | ±2.9 | ||||
| Permethrin | 78 | 43.6 | ±4.0 | 5.46E−04 | 98 | 12.2 | ±2.1 | 1.16E−05 | ||
| PBO + permethrin | 81 | 75.3 | ±0.6 | 91 | 42.9 | ±3.2 | ||||
| Brong Ahafo | Kenyasi | Deltamethrin | 84 | 17.9 | ±1.0 | 1.23E−05 | 91 | 70.3 | ±4.8 | 6.49E−02 |
| PBO + deltamethrin | 84 | 52.4 | ±2.8 | 80 | 95.0 | ±1.3 | ||||
| Permethrin | 84 | 7.1 | ±2.2 | 7.63E−03 | 87 | 63.2 | ±3.9 | 2.04E−02 | ||
| PBO + permethrin | 83 | 20.5 | ±3.5 | 85 | 88.2 | ±0.7 | ||||
| Kwame Danso | Deltamethrin | 78 | 12.8 | ±1.9 | 1.66E−13 | 84 | 36.9 | ±4.3 | 1.12E−08 | |
| PBO + deltamethrin | 83 | 88.0 | ±1.0 | 86 | 96.5 | ±1.0 | ||||
| Permethrin | 89 | 21.3 | ±0.7 | 7.64E−07 | 82 | 13.4 | ±1.1 | 2.73E−06 | ||
| PBO + permethrin | 88 | 63.6 | ±1.6 | 84 | 48.8 | ±5.3 | ||||
PBO piperonyl butoxide, CI confidence interval, nd could not be determined, the numbers in italics showed the non-significant p values
In 2015, the average mortality of An. gambiae s.l. against deltamethrin alone in the southern sector was 50.2% for the 10 sites with the lowest mortality recorded at Weija in the coastal area in the Greater Accra region and the highest rate recorded at Nkwanta in the Volta region (82.4%). When the mosquitoes were pre-exposed to PBO before deltamethrin, there was a 45% increase in mortality with an average of 91% in all the sites (Fig. 2). The resistance status of mosquitoes tested was completely reversed to full susceptibility in more than five sites in the southern sector. Hundred percent (100%) mortality was observed at Ada, Kade, Nkwanta and Prestea whilst 99% was recorded at Sefwi Wiawso. The lowest mortality of mosquitoes to PBO + deltamethrin exposure was recorded in Weija (43.8%).
Fig. 2.
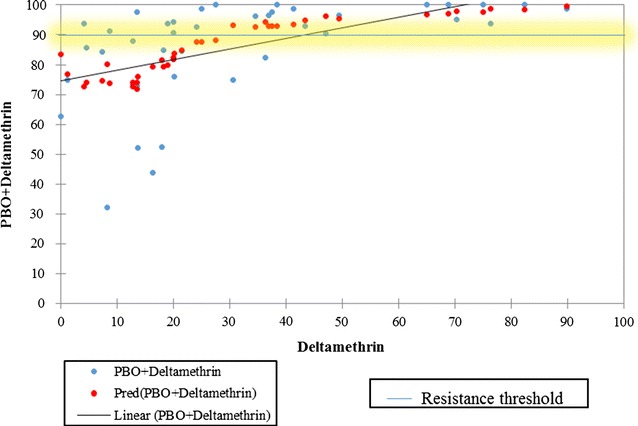
Trends and prediction of the percentage increment in susceptibility of Anopheles gambiae s,l. against PBO + deltamethrin in relation to deltamethrin alone during 2015 and 2016 for both Southern and Northern sectors. Significant increment in susceptibility was observed with the addition of PBO (p = 0.001)
The average mortality of An. gambiae s.l. when exposed to permethrin alone during the same year was 32.6% within the 10 sites in the southern sector. Half of the sites recorded less than 20% mortality indicating high permethrin resistance in this part of the country. Only Ada and Prestea showed a much higher mortality of 75 and 91.3% respectively. The lowest mortality of An. gambiae s.l. against permethrin was recorded at Gomoa Obuasi in the Central region (3.8%). Similarly to the deltamethrin in the same part of the country, pre-exposure of to PBO increased the mortality of the mosquitoes against permethrin to an average of 71% (Fig. 3). Moreover, Weija still recorded a low non-significant mortality of 7.5% after pre-exposure to PBO compared to the permethrin alone.
Fig. 3.
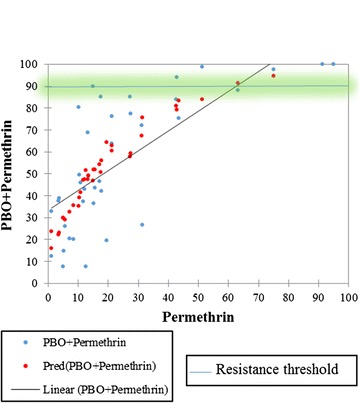
Trends and prediction of the percentage increment in susceptibility of Anopheles gambiae s.l. against PBO + permethrin in relation to permethrin alone during 2015 and 2016 for both Southern and Northern sectors. Significant increment in susceptibility was observed with the addition of PBO (p < 0.001)
In 2016, there was an increase in deltamethrin resistance compared to the previous year. An average of 20.3% mortality was recorded across the 10 southern sites with a lowest rate of 4.1% at Ada and the highest at Nkwanta (47.1%). A significant enhancement of susceptibility to deltamethrin was observed during both years after pre-exposure to PBO. An average of 79% mortality was recorded in 2016. However, the lowest mortality was recorded at Weija (32.3%).
Permethrin resistance was also high across all the sites in 2016 with an average mortality of 15%. Mosquitoes in 80% of the sites in the southern sector recorded less than 20% mortality against permethrin alone, with a low mortality observed at Gomoa Obuasi and Kade (1.2%). The average mortality was enhanced two times for permethrin after pre-exposure of mosquitoes to PBO. The average mortality recorded for all the sites was 36.6% with the highest of 85.2% occurring at Prestea (Table 1).
In the northern sector, the mortality of An. gambiae s.l. against deltamethrin in 2015 ranged from 12% at Konongo in the Ashanti region to 89% at Zebilla in the Upper East region (Table 2). The average mortality of mosquitoes against deltamethrin was 28.2% and susceptibility was enhanced three times to 86% averagely after pre-exposure to PBO. The lowest rate was recorded at Kenyasi (52.4%) while the highest rate of 100% was recorded at Wulensi and Tumu in the Northern and Upper West regions respectively. Similar trends were observed with permethrin and PBO + permethrin with 24.9 and 58.3% as average mortalities, respectively.
In 2016, the average mortality of mosquitoes against deltamethrin was 26.7% for all the sites. No mortality was recorded at Wulensi in the Northern region and as low as 1.1% was recorded in Konongo in the Ashanti Region (Table 2). The highest mortality of 70.3% was recorded at Kenyasi. When the mosquitoes were first pre-exposed to PBO, the mortality against deltamethrin was increased more than three times with an average rate of 87.5% in all the sentinel sites. The trends observed were almost the same as in 2015. There was also a significant (p < 0.001) increase in mortality of An. gambiae s.l. to permethrin during the same year after pre-exposure to PBO (average mortality 23.2% for permethrin alone vrs 54.3% for PBO + permethrin).
Furthermore, no significant difference was observed in mortality between both years for deltamethrin insecticide (p = 0.909 for deltamethrin alone and p = 0.061 for PBO + deltamethrin). Similarly, no difference was observed between permethrin alone and PBO + permethrin during the 2 years (p = 0.141 permethrin alone and p = 0.236 for PBO + permethrin).
In contrast, there is a significant difference between insecticides. Deltamethrin alone is significantly more effective than permethrin alone (p = 0.007) and PBO + Deltamethrin was more effective than PBO + permethrin (p < 0.001) (Figs. 2 and 3).
Discussion
Pyrethroid resistance has in recent years become widespread among anopheline mosquitoes in Western, Eastern, Central and South Africa [5, 6, 8, 20–32]. In spite of that, most of the malaria vector control programme of countries is still relying on pyrethroid treated tools to control the vectors [1, 33–37]. The results of this study are in line with other studies carried out in some areas in Ghana [5, 8–10, 20]. This is a cause for concern because the use of ITNs and indoor residual spraying (IRS) form the main vector control tools in the country. Pyrethroids are the insecticide of choice for ITNs/LLINs. It is known that cross resistance between permethrin and DDT does occur, so although DDT is not used in the country for public health, permethrin resistance is particularly high and that may explain the trend that was observed at all the sites [38, 39]. These observations raise the possibility that mass use of insecticide-treated bed nets for malaria control could be rendered ineffective if the vectors are already resistant to pyrethroids [3, 40, 41]. However, the impact of the insecticide resistance of An. gambiae s.l. on the vector control tools such as LLINs need to be well described to delimit the real effects of pyrethroid resistance on the effectiveness of all the mass distribution of long-lasting insecticidal nets across countries [42, 43].
The study also found that when the mosquitoes were pre-exposed to PBO before exposure to deltamethrin or permethrin, there was a significant enhancement of susceptibility of An. gambiae s.l. from almost all the sentinel sites. Weija in the southern region and Kenyasi in the northern sector showed a non-significant difference of 24 h mortality both in year 2015 and 2016 after pre-exposure to PBO. The other sites encountering a non-significant effect of the PBO were due to the high susceptibility level of the mosquitoes in those sites. Among those sites are Nkwanta, Afife and Prestea in the southern regions and Zebilla in the northern region. PBO is one of many synergist which when added to insecticides can increase their lethality, or more generally their effectiveness against insect pests [15, 44–46]. PBO is known to enhance the effects of several insecticides, including the pyrethroid deltamethrin and this is achieved by inhibiting metabolic enzyme defense systems, such as P450 s within the insect [16, 17]. The results of these synergist assays carried out in 2015 and 2016 indicate that metabolic enzyme activities such as oxidases could be involved in the development of resistance to the pyrethroids and other insecticides tested. This means that vector control products that incorporate this synergist may be useful in killing An. gambiae s.l. even in the presence of pyrethroid resistance. However, the ability of PBO to synergize pyrethroid insecticides seems to diminish at higher levels of resistance as observed at Weija in the southern region. A similar trend was observed in an initial meta-analyses reported by Churcher et al. using bioassay data collected from different experimental hut trials of PBO-LLINs [47]. Such trends need to be more described in order to understand the implications on vector control strategies. The study also recommends that the involvement of other enzymes apart from P450s in the development of resistance across the sentinel sites needs to be investigated as to understand the full influence of biochemical mechanisms.
Conclusion
Pyrethroid resistance was high in all the sites surveyed and therefore a rational use of insecticides especially pyrethroids should be encouraged in the country whilst promoting strategies that can help reduce the insecticide pressure. The enhancement of deltamethrin and permethrin susceptibility with PBO indicates that vector control products such as LLINs that have the synergist incorporated can be effective in killing mosquitoes even in the presence of pyrethroid resistance.
Authors’ contributions
SKD, JC conceptualised the study and drafted the manuscript; OOA, SKD, ABW, KM, CBP, MAA, DAB proof read the manuscript; SKD, AAA, SC coordinated the study and analysed the data. All authors read and approved the final manuscript.
Acknowledgements
Our sincere thanks go to residents of the different regional heath directors and collaborators for their support during the sample collections. We also acknowledge the contributions from Melinda Hadi, students and staff of Parasitology department, Noguchi Memorial Institute for Medical Research (NMIMR), VESTERGAARD-NMIMR Vector Labs and Africa IRS program in Ghana. Our gratitude also goes to the fieldworkers for their hard work. This study was financially supported by Global Fund and the President Malaria Initiative (PMI/USAID).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samuel K. Dadzie, Email: sdadzie@noguchi.edu.ug.gh
Joseph Chabi, Email: chabijoseph@yahoo.fr.
Andy Asafu-Adjaye, Email: akelva@yahoo.com.
Otubea Owusu-Akrofi, Email: o.ansah@hotmail.com.
Aba Baffoe-Wilmot, Email: ababaffoe@hotmail.com.
Keziah Malm, Email: keziah.malm@ghsmail.org.
Constance Bart-Plange, Email: constance.bartplange@ghsmail.org.
Sylvester Coleman, Email: sylvester_coleman@africairs.net.
Maxwell A. Appawu, Email: mappawu@noguchi.ug.edu.gh
Daniel A. Boakye, Email: dboakye@noguchi.ug.edu.gh
References
- 1.Dabire RK, Diabate A, Baldet T, Pare-Toe L, Guiguemde RT, Ouedraogo JB, et al. Personal protection of long-lasting insecticide-treated nets in areas of Anopheles gambiae s.s. resistance to pyrethroids. Malar J. 2006;5:12. doi: 10.1186/1475-2875-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toe KH, Jones CM, N’Fale S, Ismail HM, Dabire RK, Ranson H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis. 2014;20:1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adasi K, Hemingway J. Susceptibility to three pyrethroids and detection of knockdown resistance mutation in Ghanaian Anopheles gambiae sensu stricto. J Vector Ecol. 2008;33:255–262. doi: 10.3376/1081-1710-33.2.255. [DOI] [PubMed] [Google Scholar]
- 5.Chabi J, Baidoo PK, Datsomor AK, Okyere D, Ablorde A, Iddrisu A, et al. Insecticide susceptibility of natural populations of Anopheles coluzzii and Anopheles gambiae (sensu stricto) from Okyereko irrigation site, Ghana, West Africa. Parasit Vectors. 2016;9:182. doi: 10.1186/s13071-016-1462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coetzee M, van Wyk P, Booman M, Koekemoer LL, Hunt RH. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bull Soc Pathol Exot. 2006;99:400–403. [PubMed] [Google Scholar]
- 7.Dery DB, Segbaya S, Asoalla V, Amoyaw F, Amoako N, Agyeman-Budu A, et al. Anopheles gambiae (Diptera: Culicidae) susceptibility to insecticides and knockdown resistance genes prior to introduction of indoor residual spraying in 11 districts in Ghana. J Med Entomol. 2016;53:1403–1409. doi: 10.1093/jme/tjw098. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RH, Fuseini G, Knowles S, Stiles-Ocran J, Verster R, Kaiser ML, et al. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasit Vectors. 2011;4:107. doi: 10.1186/1756-3305-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okoye PN, Brooke BD, Koekemoer LL, Hunt RH, Coetzee M. Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Trans R Soc Trop Med Hyg. 2008;102:591–598. doi: 10.1016/j.trstmh.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Yawson AE, McCall PJ, Wilson MD, Donnelly MJ. Species abundance and insecticide resistance of Anopheles gambiae in selected areas of Ghana and Burkina Faso. Med Vet Entomol. 2004;18:372–377. doi: 10.1111/j.0269-283X.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 11.Corbel V, Chabi J, Dabire RK, Etang J, Nwane P, Pigeon O, et al. Field efficacy of a new mosaic long-lasting mosquito net (PermaNet 3.0) against pyrethroid-resistant malaria vectors: a multi centre study in Western and Central Africa. Malar J. 2010;9:113. doi: 10.1186/1475-2875-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennetier C, Bouraima A, Chandre F, Piameu M, Etang J, Rossignol M, et al. Efficacy of Olyset(R) Plus, a new long-lasting insecticidal net incorporating permethrin and piperonyl-butoxide against multi-resistant malaria vectors. PLoS ONE. 2013;8:e75134. doi: 10.1371/journal.pone.0075134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingham G, Gunning RV, Gorman K, Field LM, Moores GD. Temporal synergism by microencapsulation of piperonyl butoxide and alpha-cypermethrin overcomes insecticide resistance in crop pests. Pest Manag Sci. 2007;63:276–281. doi: 10.1002/ps.1336. [DOI] [PubMed] [Google Scholar]
- 14.Moores GD, Philippou D, Borzatta V, Trincia P, Jewess P, Gunning R, Bingham G. An analogue of piperonyl butoxide facilitates the characterisation of metabolic resistance. Pest Manag Sci. 2009;65:150–154. doi: 10.1002/ps.1661. [DOI] [PubMed] [Google Scholar]
- 15.Bingham G, Strode C, Tran L, Khoa PT, Jamet HP. Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti? Trop Med Int Health. 2011;16:492–500. doi: 10.1111/j.1365-3156.2010.02717.x. [DOI] [PubMed] [Google Scholar]
- 16.Young SJ, Gunning RV, Moores GD. The effect of piperonyl butoxide on pyrethroid-resistance-associated esterases in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) Pest Manag Sci. 2005;61:397–401. doi: 10.1002/ps.996. [DOI] [PubMed] [Google Scholar]
- 17.Young SJ, Gunning RV, Moores GD. Effect of pretreatment with piperonyl butoxide on pyrethroid efficacy against insecticide-resistant Helicoverpa armigera (Lepidoptera: Noctuidae) and Bemisia tabaci (Sternorrhyncha: Aleyrodidae) Pest Manag Sci. 2006;62:114–119. doi: 10.1002/ps.1127. [DOI] [PubMed] [Google Scholar]
- 18.WHO . Test procedures for insecticide resistance monitoring in malaria vectors mosquitoess. Geneva: World Health Organization; 2013. [Google Scholar]
- 19.WHO. Guidelines for laboratory and field testing of long-lasting insecticidal nets. WHO Pesticide Evaluation Scheme (WHOPES); 2013, WHO/HTM/NTD/WHOPES/2013.1.
- 20.Anto F, Asoala V, Anyorigiya T, Oduro A, Adjuik M, Owusu-Agyei S, et al. Insecticide resistance profiles for malaria vectors in the Kassena-Nankana district of Ghana. Malar J. 2009;8:81. doi: 10.1186/1475-2875-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chouaibou M, Etang J, Brevault T, Nwane P, Hinzoumbe CK, Mimpfoundi R, et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in Northern Cameroon. Trop Med Int Health. 2008;13:476–486. doi: 10.1111/j.1365-3156.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 22.Cisse MB, Keita C, Dicko A, Dengela D, Coleman J, Lucas B, et al. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar J. 2015;14:327. doi: 10.1186/s12936-015-0847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbel V, Hougard JM, N’Guessan R, Chandre F. Evidence for selection of insecticide resistance due to insensitive acetylcholinesterase by carbamate-treated nets in Anopheles gambiae s.s. (Diptera: Culicidae) from Cote d’Ivoire. J Med Entomol. 2003;40:985–988. doi: 10.1603/0022-2585-40.6.985. [DOI] [PubMed] [Google Scholar]
- 24.Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Dabire KR, Diabate A, Djogbenou L, Ouari A, N’Guessan R, Ouedraogo JB, et al. Dynamics of multiple insecticide resistance in the malaria vector Anopheles gambiae in a rice growing area in South-Western Burkina Faso. Malar J. 2008;7:188. doi: 10.1186/1475-2875-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djenontin A, Chabi J, Baldet T, Irish S, Pennetier C, Hougard JM, et al. Managing insecticide resistance in malaria vectors by combining carbamate-treated plastic wall sheeting and pyrethroid-treated bed nets. Malar J. 2009;8:233. doi: 10.1186/1475-2875-8-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edi CV, Koudou BG, Jones CM, Weetman D, Ranson H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Cote d’Ivoire. Emerg Infect Dis. 2012;18:1508–1511. doi: 10.3201/eid1809.120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 29.Ngufor C, N’Guessan R, Fagbohoun J, Subramaniam K, Odjo A, Fongnikin A, et al. Insecticide resistance profile of Anopheles gambiae from a phase II field station in Cove, southern Benin: implications for the evaluation of novel vector control products. Malar J. 2015;14:464. doi: 10.1186/s12936-015-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perera MD, Hemingway J, Karunaratne SP. Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malar J. 2008;7:168. doi: 10.1186/1475-2875-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranson H, Abdallah H, Badolo A, Guelbeogo WM, Kerah-Hinzoumbe C, Yangalbe-Kalnone E, et al. Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J. 2009;8:299. doi: 10.1186/1475-2875-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, Corbel V, et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83. doi: 10.1186/1475-2875-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corbel V, Akogbeto M, Damien GB, Djenontin A, Chandre F, Rogier C, et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis. 2012;12:617–626. doi: 10.1016/S1473-3099(12)70081-6. [DOI] [PubMed] [Google Scholar]
- 34.Curtis CF, Jana-Kara B, Maxwell CA. Insecticide treated nets: impact on vector populations and relevance of initial intensity of transmission and pyrethroid resistance. J Vector Borne Dis. 2003;40:1–8. [PubMed] [Google Scholar]
- 35.Okumu FO, Moore SJ. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J. 2011;10:208. doi: 10.1186/1475-2875-10-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens ER, Aldridge A, Degbey Y, Pignandi A, Dorkenoo MA, Hugelen-Padin J. Evaluation of the 2011 long-lasting, insecticide-treated net distribution for universal coverage in Togo. Malar J. 2013;12:162. doi: 10.1186/1475-2875-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tungu P, Magesa S, Maxwell C, Malima R, Masue D, Sudi W, et al. Evaluation of PermaNet 3.0 a deltamethrin-PBO combination net against Anopheles gambiae and pyrethroid resistant Culex quinquefasciatus mosquitoes: an experimental hut trial in Tanzania. Malar J. 2010;9:21. doi: 10.1186/1475-2875-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 39.Ranson H, Jensen B, Wang X, Prapanthadara L, Hemingway J, Collins FH. Genetic mapping of two loci affecting DDT resistance in the malaria vector Anopheles gambiae. Insect Mol Biol. 2000;9:499–507. doi: 10.1046/j.1365-2583.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 40.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387:1785–1788. doi: 10.1016/S0140-6736(15)00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lengeler C, Snow RW. From efficacy to effectiveness: insecticide-treated bednets in Africa. Bull World Health Organ. 1996;74:325–332. [PMC free article] [PubMed] [Google Scholar]
- 42.Briet OJ, Penny MA, Hardy D, Awolola TS, Van Bortel W, Corbel V, et al. Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study. Malar J. 2013;12:77. doi: 10.1186/1475-2875-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, Bigoga J, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:282. doi: 10.1186/s12936-015-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darriet F, Chandre F. Combining piperonyl butoxide and dinotefuran restores the efficacy of deltamethrin mosquito nets against resistant Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 2011;48:952–955. doi: 10.1603/ME11022. [DOI] [PubMed] [Google Scholar]
- 45.Fakoorziba MR, Eghbal F, Vijayan VA. Synergist efficacy of piperonyl butoxide with deltamethrin as pyrethroid insecticide on Culex tritaeniorhynchus (Diptera: Culicidae) and other mosquitoe species. Environ Toxicol. 2009;24:19–24. doi: 10.1002/tox.20386. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Thomas A, Sahgal A, Verma A, Samuel T, Pillai MK. Effect of the synergist, piperonyl butoxide, on the development of deltamethrin resistance in yellow fever mosquito, Aedes aegypti L. (Diptera: Culicidae) Arch Insect Biochem Physiol. 2002;50:1–8. doi: 10.1002/arch.10021. [DOI] [PubMed] [Google Scholar]
- 47.Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5:e16090. doi: 10.7554/eLife.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]


