Abstract
Objective
Differential diagnosis of vascular malformations can be problematic even in specialized interdisciplinary centers. Localized Intravascular Coagulopathy, characterized by elevated D-dimer levels, has been observed in about 40% of patients with venous malformations. We evaluated if this is specific for them, and thus useful for differential diagnosis.
Design
Prospective convenience sample accrued from 2 interdisciplinary sites in Brussels, Belgium and Caen, France.
Participants
The study population comprised 280 patients with clinical data, Doppler ultrasound (for 251 patients) and coagulation parameters.
Main outcome measure
Measurement of D-dimer levels.
Results
A venous malformation was diagnosed in 69,6% (n=195/280) of patients, and 83 of them had elevated D-dimer levels; the sensitivity of D-dimer dosage was 42.6% [95%CI: 35.6%–49.5%]. Among the 85 patients without venous malformation, D-dimer levels were elevated only in 3 patients; the specificity of the dosage was 96.5% [95%CI: 92.5%–100%].
Conclusions
Elevated D-dimer level is highly specific for venous malformations (pure, combined or syndromic), and therefore, this easy and cheap biomarker test should become part of the clinical evaluation of vascular anomalies. It can detect hidden venous malformations and help differentiate glomuvenous malformation (normal D-dimer levels) from other multifocal venous lesions. Elevated D-dimer level also differentiates a venous malformation from a lymphatic malformation. Moreover, slow-flow Klippel-Trenaunay syndrome (capillaro-lymphatico-venous malformation with limb hypertrophy) can be distinguished from fast-flow Parkes Weber syndrome (capillary malformation with underlying multiple microfistulas and limb hypertrophy). For these reasons, D-dimer level measurement is a useful complementary tool for diagnosing vascular anomalies in everyday practice.
Keywords: diagnostic accuracy, sensitivity, specificity, venous anomaly, D-dimer level, localized intravascular coagulopathy (LIC), vascular malformation, venous malformation, arterio-venous malformation, lymphatic malformation, Klippel-Trenaunay syndrome, Maffucci syndrome, Parks Weber syndrome, CM-AVM
Differential diagnosis of vascular malformations can be problematic even in specialized interdisciplinary centers for vascular anomalies, as these lesions can mimic each other and some malignant tumors1, 2. The diagnosis is firstly based on clinical history (presence at birth, growth during life, triggers such as puberty or trauma, and family history) and examination. Important clinical clues are color (variations of pink, red, blue and purple), aspect (flat, raised, homogeneous, patchy, hyperkeratotic and ulcerated), localization, size, distribution (uni- or multifocal), palpation (hard, firm, compressible, and presence of a thrill), temperature (warm or normal), painfulness (spontaneous or provoking factors) and auscultation (bruit). On the basis of these data, an experienced physician can make the diagnosis for most patients3, 4.
The rheological subdivision into fast and slow-flow lesions is best confirmed by Doppler ultrasound (DU)5, 6. In experienced radiological hands, DU can help distinguish the affected vessel type within slow-flow malformations, e.g. venous versus lymphatic. The extensiveness of the lesion on the underlying tissue is visualized by Magnetic Resonance Imaging (MRI), which is mainly used for evaluation of therapeutic options, but can help in diagnosis7, 8. Arteriography, an invasive examination, is rarely needed for diagnosis, but mandatory before treatment of a fast-flow lesion. Conventional radiography detects adjacent skeletal anomalies and overgrowth. Biopsy should be performed whenever diagnosis remains doubtful. Conclusive genetic tests already exist for some rare inherited forms. Biological tests are not available.
In a recent prospective study, Localized Intravascular Coagulopathy (LIC), characterized by elevated D-dimers, was observed in 42 % of patients with venous malformations9. As this activation of coagulation is probably due to blood stagnation in the enlarged venous channels 9, Enjolras(new #17), and current 17, we evaluated if elevated D-dimer levels were specific for venous malformation, and hence a biomarker helpful for diagnosis.
METHODS
We conducted a prospective study from January 2006 to March 2008 in 2 interdisciplinary centers for vascular anomalies (Brussels, Belgium and Caen, France). This study was approved by the ethics committees of Université catholique de Louvain, Brussels, Belgium and Université de Caen, France. All participants signed an informed consent form.
PATIENTS
A total of 280 patients with cutaneous, subcutaneous and mucosal vascular anomalies were evaluated and enrolled to the study by LMB (Brussels) and AD (Caen). Both centers used the biological classification proposed by Mulliken and Glowacki and adopted by ISSVA (International Society for the Study of Vascular Anomalies)10. Clinically and with Doppler ultrasound, all the vascular malformations were subdivided into pure slow-flow, combined slow-flow, syndromic and fast-flow malformations (Table 1).
Table 1.
Clinical parameters and D-dimer levels according to vascular anomaly.
| Total Number | Unilateral | Localization | Size >10cm2 | D-dimers >0.5μg/ml | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | % | Head&Neck | Limbs | Trunk | >1region | N | % | N | % | |
| Pure Slow Flow Malformations | 225 | 191 | 84.9 | 78(34.7%) | 63(28.0%) | 20(8.9%) | 64(28.4) | 130 | 57.8 | 71 | 31.6 |
|
| |||||||||||
| Venous Malformations | 172 | 143 | 83.1 | 143(83.1%) | 65(37.8%) | 12(7.0%) | 51(29.7%) | 87 | 50.6 | 69 | 40.1 |
|
| |||||||||||
| • Uni- or multifocal | 154 | 137 | 89.0 | 62(40.3%) | 41(26.6%) | 11(7.1%) | 40(26.0%) | 80 | 52.0 | 67 | 43.5 |
| • Blue rubber bleb naevus syndrome | 2 | 0 | 0 | 0(0%) | 0(0%) | 0(0%) | 2(100%) | 2 | 100 | 2 | 100 |
| • Glomuvenous malformation (1) | 16 | 6 | 37.5 | 3(18.8%) | 3(18.8%) | 1(6.3%) | 9(56.2%) | 5 | 31.3 | 0 | 0 |
|
| |||||||||||
| Capillary Malformations | 33 | 31 | 93.9 | 7(21.7%) | 12(36.4%) | 3(9.1%) | 11(33.3%) | 30 | 90.9 | 2 | 6.0 |
|
| |||||||||||
| • Capillary malformation, unifocal | 22 | 20 | 90.9 | 7(31.8%) | 8(36.4%) | 1(4.6%) | 6(27.3%) | 20 | 90.9 | 2 | 9.1 |
| • Capillary malformation with tissue hypertrophy | 4 | 4 | 100 | 0(0%) | 1(25.0%) | 1(25.0%) | 2(50.0%) | 4 | 100 | 0 | 0 |
| • Capillary malformation with venous dilatation | 7 | 7 | 100 | 0(0%) | 3(42.9%) | 1(14.3%) | 3(42.9%) | 6 | 85.7 | 0 | 0 |
|
| |||||||||||
| Lymphatic Anomalies | 20 | 17 | 85.0 | 6(30.0%) | 7(35.0%) | 5(25.0%) | 2(10.0%) | 13 | 65.0 | 0 | 0 |
|
| |||||||||||
| • Lymphatic malformation | 18 | 16 | 88.9 | 6(23.3%) | 5(27.8%) | 5(27.8%) | 2(11.1%) | 11 | 61.1 | 0 | 0 |
| • Lymphoedema | 2 | 1 | 50.0 | 0(0%) | 2(100%) | 0(0%) | 0(0%) | 2 | 100 | 0 | 0 |
|
| |||||||||||
| Combined slow-flow Malformations | 14 | 14 | 100 | 1(7.0%) | 6(42.9%) | 2(14.3%) | 5(35.7%) | 13 | 92.9 | 6 | 42.9 |
|
| |||||||||||
| • Capillarovenous malformation | 7 | 7 | 100 | 1(14.3%) | 4(57.1%) | 0(0%) | 2(28.6%) | 7 | 100 | 5 | 71.4 |
| • Capillarolymphatic malformation | 6 | 6 | 100 | 0(0%) | 2(33.3%) | 2(33.3%) | 2(33.3%) | 5 | 83.3 | 0 | 0 |
| • Capillary malformation with multifocal venous malformations | 1 | 1 | 100 | 0(0%) | 0(0%) | 0(0%) | 1(100%) | 1 | 100 | 1 | 100 |
|
| |||||||||||
| Syndromic Malformations | 15 | 12 | 80 | 0(0%) | 10(66.7%) | 0(0%) | 5(33.3%) | 13 | 86.7 | 8 | 53.33 |
|
| |||||||||||
| • Klippel-Trenaunay syndrome (2) | 11 | 8 | 72.7 | 0(0%) | 7(63.6%) | 0(0%) | 4(36.4%) | 10 | 90.9 | 8 | 72.7 |
| • Maffucci syndrome (3) | 4 | 4 | 100 | 0(0%) | 3(75.0%) | 0(0%) | 1(25.0%) | 3 | 75.0 | 0 | 0 |
|
| |||||||||||
| Fast Flow Malformations | 26 | 20 | 76.9 | 10(38.5%) | 4(15.4%) | 2(7.7%) | 10(38.5%) | 15 | 57.7 | 1 | 3.8 |
|
| |||||||||||
| • Arteriovenous malformation | 20 | 15 | 75.0 | 9(45.0%) | 2(10.5%) | 2(10.5%) | 7(35.0%) | 7 | 35.0 | 1 | 5.0 |
| • Capillary malformation-arteriovenous malformation (4) | 3 | 2 | 66.7 | 1(33.3%) | 1(33.3%) | 0(0%) | 1(33.3%) | 3 | 100 | 0 | 0 |
| • Parkes Weber syndrome (5) | 3 | 3 | 100 | 0(0%) | 3(100%) | 0(0%) | 0(0%) | 3 | 100 | 0 | 0 |
GVM: histopathological diagnosis and/or glomulin mutation present
KT:capillaro-lymphatico-venous malformation with limb hypertrophy
Maffucci:multiple enchondromas associated with spindle cell hemangioendothelioma.
CM-AVM: RASA1 mutation present
PW: capillary malformation with underlying multiple microfistulas and limb hypertrophy
The following data points were recorded prospectively from the clinical and radiological evaluations:
Clinical criteria: age, sex, color, unilateral or bilateral location and size of lesions: < or > 10cm2 and corresponding percentages (0.25, 0.5, 0.75, 1) within the affected anatomical unit (AU: head, neck, chest, abdomino-pelvic region, left and right arms, forearms, hands, thighs, and legs and feet) grouped secondarily into 4 anatomic regions (AR: head and neck, limbs, trunk, and more than one region).
Doppler Ultrasound (DU) was performed for 251 patients with Color Doppler equipment: Aloka Alpha 10 machine (Tokyo, Japan) with 4–13 Mhz linear transducer (Caen) and Philips Medical System iU22 machine (Best Netherlands) with 2 linear transducers L 5–17 Mhz and L 5–12 Mhz (Brussels). Color Doppler US was performed using a restricted field and by scanning the entire lesion. The area of higher vascularization identified by color flow was selected and Doppler shifts were ascertained with pulsed Doppler. The same well-trained sonologists (PhC and FH in Brussels, MTB in Caen) belonging to the interdisciplinary centers measured the vascular resistance index, the flow type, and the presence of a nidus, AV fistula and dilated veins. Doppler Ultrasound was performed in all lesions, except on small, pure mucosal venous malformations due to typical clinical presentation (n = 6), or when diagnosis was already confirmed by histopathologic examination (n=19), or genetic analysis (n=4) (1 CM-AVM and 3 GVM).
Magnetic Resonance Imaging (MRI) with T1 and T2-weighted, and fat saturated sequences were performed for 186 patients for therapeutic evaluation. Conventional radiography was used to evaluate syndromic malformations or associated overgrowth in 18 patients. Arteriography was done for 12 patients for therapeutic evaluation of their fast-flow lesion.
PROCEDURES
At the initial examination and at every follow-up every 2–3 months for 1–2 years, blood was drawn from a peripheral vein not involved by the vascular malformation for coagulation tests, outside a symptomatic inflammatory event. Platelets (reference range 150×109/l – 400×109/l) were counted in an EDTA sample using an automated instrument (Sysmex XE-2100 Roche Diagnostics, Basel, Switzerland). Fibrinogen level (reference range 200–450mg/dl, Fibriquick, bioMérieux) were measured in a tube containing 0.129M of trisodium citrate and determined using a coagulation device (MDA 2 bioMérieux). Plasma D-dimers (reference range <0,500μg/ml) were determined using enzyme-linked immunosorbent assay (VIDAS® D-Dimer New DD2 bioMérieux).
STATISTICAL ANALYSIS
Based on an expected specificity of D-dimers in the diagnosis of VM of 90% and a frequency of VM of 60% within patients in specialized interdisciplinary centers, we estimated that a total number of 220 patients would be needed to limit the size of 95% confidence interval to a value of 0.10 (less than 10 percentile unit difference)11, 12. The statistical analysis was performed using SAS software (version 9). Factors associated with high D-dimer levels were analyzed in a univariate analysis using the chi2 test for categorical variables and the non-parametric Kruskal-Wallis test for quantitative variables. A logistic regression model was built for variables significantly associated with positive D-dimer levels with a threshold of 0.20 in univariate analysis. P<0.05 was considered as significant in all the statistical analyses.
RESULTS
Within the 280 patients, 184 were females and 96 were males with a mean age of 26.8 years (SD=16.18) and a medium age of 23 years (Table 1). The medium size of the vascular malformation was 1.04 AU (SD 1.48) with a median size of 0.50 AU. Sixty-one percent (n=171/280) were >10cm2. The lesions in the 280 patients were localized in the following anatomical regions: head and neck (n=89), limbs (n=83), trunk (n=25) and in more than one region (n=83). Eighty-five percent of them were unilateral (n=237/280). Slow-flow vascular malformations were present in 239 patients (85,7%): 172 venous lesions (154 VM, 16 GVM, 2 BRBN), 33 capillary lesions (22 CM, 7 CMVD, 4 CM with tissue hypertrophy), 20 lymphatic anomalies (18 LM, 2 lymphoedema) and 14 combined slow-flow lesions (7 CVM, 6 CLM, 1 CM+VM). Fifteen patients had a syndromic malformation (11 KT and 4 Maffucci syndrome). Fast-flow lesions were present in 26 patients (20 AVM, 3 CM-AVM and 3 Parkes Weber syndrome) (Table 1).
Eighty-six patients with vascular malformations (30.7%) had repeatedly elevated D-dimers (> 0.500μg/ml) (Table 1 and Table 2). The frequency of elevated D-dimers decreased according to the diagnosis:
Table 2.
Mean and Median D-dimer levels per localization
| Mean and Median D-dimer levels per localization (of those > 0,5 mg/ml) | D-dimers >0.5μg/ml | ||||
|---|---|---|---|---|---|
| N | % | mean | median | ||
| Malformations with venous component | Blue rubber bleb naevus syndrome | 2/2 | 100 | 7.824 | NA |
| Capillary malformation with multifocal venous malformations | 1/1 | 100 | 3.598 | NA | |
| Klippel-Trenaunay syndrome | 8/11 | 72.7 | 6.021 | 6.710 | |
| Limbs | 4/7 | 57.1 | 5.301 | NA | |
| > 1 region | 4/4 | 100 | 6.742 | NA | |
| Capillarovenous malformation | 5/7 | 71.4 | 1.600 | 0.989 | |
| Head & Neck | 1/1 | 100 | 0.638 | NA | |
| Limbs | 2/4 | 50 | 0.973 | NA | |
| > 1 region | 2/2 | 100 | 2.709 | NA | |
| Venous malformation, uni- or multifocal | 67/154 | 43.5 | 2.439 | 1.500 | |
| Head & Neck | 21/62 | 33.9 | 1.827 | 1.000 | |
| Limbs | 17/41 | 41.5 | 2.444 | 1.411 | |
| Trunk | 8/11 | 72.7 | 2.735 | 2.009 | |
| > 1 region | 21/40 | 52.5 | 2.934 | 2.909 | |
|
| |||||
| Malformations without venous component | Capillary malformation with venous ulcer | 1/33 | 3.0 | 0.504 | NA |
| Capillary malformation, thrombophilia & diverticulosis | 1/33 | 3.0 | 2.289 | NA | |
| Arteriovenous malformation | 1/20 | 5.0 | 0.501 | NA | |
N = D-dimer positive lesions per total in category
NA= median statistically not applicable if <5 patients
Syndromic malformations: 8/15 patients (53.3%), all with KT (8/11). All 8 had large, deep venous lesions. Among the 3 remaining KT patients, one had extensive venous anomalies, but was under oral vitamin K antagonist to prevent deep venous thrombosis and pulmonary embolism; the other two patients had limited, superficial venous lesions, but important lymphatic ones with frequent infections of the limb. All 4 patients with Maffucci syndrome had normal D-dimer levels.
Combined malformations: 6/14 patients (42.85%); 1/1 CM+VM, 5/7 with CVM, and 0/6 CLM.
Venous malformations: 69/172 patients (40.1%); 5/5 with multifocal sporadic VM, 2/2 with BRBN, 62/149 with a solitary VM, and 0/16 with GVM.
Capillary malformations: 2/33 patients with CM (6.06%); 2/22 with unifocal CM. One had repeatedly D-dimer levels very close to normal range (0.504μg/ml) and the second had elevated D-dimers (2.289μg/ml) associated with various pathologies, such as hereditary thrombophilic defect (G 20210A prothrombin gene mutation) and colic diverticulosis. All patients with CMVD (n=7) or CM with tissue hypertrophy (n=4) had normal D-dimer levels.
Fast-flow malformations: 1/26 patient (3.84%); 1/20 AVM. He had a chronically ulcerated scrotal lesion with repeatedly a borderline D-dimer level at 0.501 μg/ml. All patients with CM-AVM (n=3) or Parkes Weber syndrome (n=3) had normal D-dimer levels.
Lymphatic anomalies: 0/20. No patient with LM (n=18) or lymphedema (n=2) had elevated D–dimer levels.
Among the patients with elevated D-dimers (n=86), 83 had malformations with a venous component: KT (n=8/11), CM+VM (n=1/1), CVM (n=5/7) and VM (n=69/172) (Table 2). Thus, the sensitivity of D-dimer dosage to detect a venous anomaly was 43.5% [95%CI : 36.4–50.5].
Among the 85 patients with lesions without a venous component, D-dimer levels were elevated only in 3 patients (2 with unifocal CM and 1 with AVM). They all had an explicable reason. The specificity of the dosage was 96.5% [95%CI: 92.5%–100%]. In the multivariate analysis, the results confirmed that the size of the venous malformation and the presence of palpable phleboliths were statistically associated with elevated D-dimers, as previously reported 9. This was underscored by higher mean/median D-dimer levels observed for lesions involving more than one anatomic region (Table 2).
COMMENTS
In this prospective study, we measured D-dimer levels among patients with vascular malformations seen in 2 interdisciplinary centers for vascular anomalies (Table 1). Among them, a coagulation abnormality was frequent (n=86/280, 30.7%). This was almost exclusively due to venous anomalies with a high specificity (96.5%, [95%CI: 92.5%–100%]): the patients had pure venous malformation (VM), capillarovenous malformation (CVM), diffuse CM with multifocal VM (CM+VM), or Klippel-Trenaunay syndrome (KT). The test had a low sensitivity (43.5% [95%CI: 36.4%–50.5%]) as expected, because only 42% of venous malformations had repeatedly elevated D-dimer levels in our previous study9.
All patients with multifocal venous malformations (5 with sporadic multifocal VM and 2 with BRBN) had very high D-dimer levels (≥5.649μg/ml). In one of the BRBN patients, the high D-dimers were associated with low fibrinogen level (95mg/dl), similar to two reported BRBN patients, who had acute or chronic disseminated intravascular coagulopathy13, 14. We suggested earlier that the high D-dimer levels could be due to the combined lesional volume in multifocal VM patients9. Interestingly, hereditary multifocal VM (VMCM) is not always associated with elevated D-dimer levels15. This might be due to etiology. Thus, the identified somatic Tie2 mutations in 50% of sporadic VMs may play a role16.
Most of the CVM patients (n=5/7) had elevated D-dimer levels. All these lesions had an important venous component, and they were located in the limbs, two of which with a truncal extension. These extensive lesions had D-dimer levels over 1μg/ml reinforcing the observation that severe LIC is associated with large lesions that often affect an extremity9, 17, 18. In contrast, the venous component of the 2 CVM patients with normal D-dimer levels (n=2/7) was not important: one had a small CM (<10cm2) of the left thigh associated with a small VM, which was surgically removed, and the other one had a large CM of the left lower limb associated with a superficial and limited venous malformation.
Klippel-Trenaunay patients (capillaro-lymphatico-venous malformation with limb hypertrophy) with deep, extensive venous malformations had elevated D-dimer levels (n=8/11). This underscores the specificity of D-dimer levels for venous malformations, an important criterion for KT diagnosis19–21. This also helps differentiate fast-flow Parkes Weber syndrome, with or without a RASA1 mutation, from the Klippel-Trenaunay syndrome, a frequent diagnostic dilemma.
Among all the vascular anomaly patients, only three without a venous component had elevated D-dimer levels. Two of them had D-dimers only slightly above the normal limit (0.501μg/ml and 0.504μg/ml, respectively). One of them had an ulcerated AVM of the scrotum, and the other one a CM with a venous ulcer on the ankle. The latter was also using oral hormonal contraceptives. Venous insufficiency regardless of accompanying ulceration, mildly enhances D-dimer levels22, 23. Moreover, slight elevation of D-dimer levels occurs in patients taking hormonal contraceptives, with mean level increasing from 0.172μg/ml to 0.351μg/ml, which is still within the normal limits (< 0.5μg/ml)24–26. The third patient had a CM and very high D-dimer levels (2.200μg/ml) associated with hereditary thrombotic defect and an inflammatory bowel disease. These two disorders are known to lead to increase in D-dimer levels27, 28.
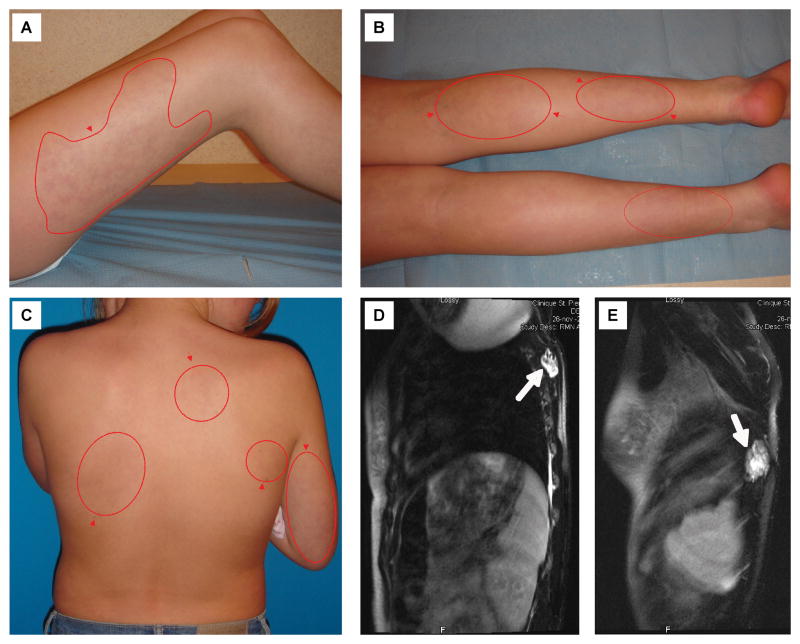
One of our patients was initially diagnosed with an extensive patchy capillary malformation of the body. However, D-dimer levels were very high (3.589 μg/ml) and she had unexplained pain. Subsequent Doppler ultrasound and MRI detected multiple deep venous malformations. This illustrates how D-dimer level measurement can help in clinical examination. (Figure 1)
Figure 1.
11-year-old girl with extensive patchy capillary malformation (red contours and arrows, A–C) and multiple deep venous malformations (white arrows) identified with T2-weighted MRI (D–E).
All other patients had normal D-dimer levels. They had glomuvenous, lymphatic, capillary or fast-flow malformations, or Maffucci syndrome. For GVMs, this had been noted by Boon and co-workers29. The lack of coagulation abnormality in GVM may be due to the more cellular architecture and therefore less compressible texture of these lesions. GVMs are also more superficially located, probably accounting for the absence of coagulation abnormality even in extensive plaque-like GVM30. As differential diagnosis between multifocal VM and GVM may be difficult, D-dimer level measurement is an interesting novel biological tool. All the 20 LM and 6 CLM patients had normal D-dimer levels too, when measured outside an infectious period. This seems logic as lymphatic stagnation should not generate fibrin thrombi. Similarly, all except three pure capillary malformations and fast-flow lesions had normal D-dimer levels, likely due to the absence of blood stagnation. The three with elevated D-dimer levels, had explicable reasons. D-dimer levels were also normal for all 4 Maffucci patients. They had spindle cell hemangioendotheliomas, specific slow-flow histopathological lesions31, 32.
In conclusion, D-dimer test is a useful tool for diagnosing a venous component of a vascular malformation. In our interdisciplinary centers, slow-flow malformations (n=225, 83.4%), and especially venous malformations (VMs) (n=172, 61.4%) account for the majority of consultations. When D-dimer levels are elevated in a vascular anomaly patient with no other associated pathology, a venous malformation is present in 96.5% of patients. However, when D-dimer levels are normal, a small VM cannot be ruled out. D-dimer level can help evaluate and thus diagnose the presence of a venous component in combined and syndromic malformations. This is especially interesting for KT. Furthermore, fast and slow-flow lesions may be more easily separated. Finally, this tool helps in differentiating GVMs from other multifocal venous lesions. Thus, this easy and cheap biomarker test is useful and highly specific for VMs, and should be used as a routine test in clinical evaluation of vascular anomaly patients. However, it does not replace any imaging needed for evaluation of management.
Acknowledgments
These studies were partially supported by the Interuniversity Attraction Poles initiated by the Belgian Federal Science Policy network 6/05; concerted Research Actions (A.R.C) - Convention N° 07/12-005 of the Belgian French Community Ministry ; the National Institute of Health Program Project P01 AR048564; EU FW6 Integrated project LYMPHANGIOGENOMICS, LSHG-CT-2004-503573 ; and the F.R.S.-FNRS (Fonds de Recherche scientifique) (to M.V., a “Maitre de recherche honoraire du F.R.S.-FNRS”). The authors thank Ms Liliana Niculescu for secretarial work.
Footnotes
Role of the Sponsors : The sponsors had no role in the design and conduct of the study ; in the collection, analysis, and interpretation of data ; or in the preparation, review, or approval of the manuscript.
Financial Disclosure : None reported
Author Contributions: Prof. Boon had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dompmartin, Vikkula, and Boon.
Acquisition of data: Dompmartin, Bailleux, Lequerrec, Hermans, Clapuyt, Barrellier, Hammer, Labbé, and Boon.
Analysis and interpretation of data: Dompmartin, Bailleux, Thibon, Vikkula, and Boon.
Drafting of the manuscript: Dompmartin, Vikkula, and Boon.
Critical revision of the manuscript for important intellectual content: Dompmartin, Bailleux, Thibon, Hermans, Lequerrec, Labbé, Barrellier, Vikkula, and Boon.
Statistical analysis: Thibon.
Obtained funding: Vikkula.
Administrative, technical, and material support: Vikkula.
Study supervision: Dompmartin, Vikkula, and Boon.
Financial support and conflict of interest: I certify that all my affiliations with or financial involvement, from the conception of the study until the publication of the manuscript with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript are completely disclosed.
References
- 1.Boon LM, Fishman SJ, Lund DP, Mulliken JB. Congenital fibrosarcoma masquerading as congenital hemangioma: report of two cases. J Pediatr Surg. 1995 Sep;30(9):1378–1381. doi: 10.1016/0022-3468(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 2.Dompmartin A, Boon LM, Labbe D. Infantile hemangiomas: differential diagnosis and associated anomalies. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4–5):300–309. doi: 10.1016/j.anplas.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Enjolras O, Mulliken JB. The current management of vascular birthmarks. Pediatr Dermatol. 1993 Dec;10(4):311–313. doi: 10.1111/j.1525-1470.1993.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 4.Buckmiller LM. Update on hemangiomas and vascular malformations. Curr Opin Otolaryngol Head Neck Surg. 2004 Dec;12(6):476–487. doi: 10.1097/01.moo.0000145946.67222.01. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Yusa H, Ueno E. Use of Doppler color flow imaging for differential diagnosis of vascular malformations: a preliminary report. J Oral Maxillofac Surg. 1995 Apr;53(4):369–374. doi: 10.1016/0278-2391(95)90706-8. [DOI] [PubMed] [Google Scholar]
- 6.Gold L, Nazarian LN, Johar AS, Rao VM. Characterization of maxillofacial soft tissue vascular anomalies by ultrasound and color Doppler imaging: an adjuvant to computed tomography and magnetic resonance imaging. J Oral Maxillofac Surg. 2003 Jan;61(1):19–31. doi: 10.1053/joms.2003.50003. [DOI] [PubMed] [Google Scholar]
- 7.Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, Therasse E. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics. 2001 Nov-Dec;21(6):1519–1531. doi: 10.1148/radiographics.21.6.g01nv031519. [DOI] [PubMed] [Google Scholar]
- 8.Hyodoh H, Hori M, Akiba H, Tamakawa M, Hyodoh K, Hareyama M. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics. 2005 Oct;25( Suppl 1):S159–171. doi: 10.1148/rg.25si055509. [DOI] [PubMed] [Google Scholar]
- 9.Dompmartin A, Acher A, Thibon P, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008 Jul;144(7):873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982 Mar;69(3):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Arkin CF, Wachtel MS. How many patients are necessary to assess test performance? JAMA. 1990 Jan 12;263(2):275–278. [PubMed] [Google Scholar]
- 12.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003 Jan 7;138(1):W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 13.Apak H, Celkan T, Ozkan A, et al. Blue rubber bleb nevus syndrome associated with consumption coagulopathy: treatment with interferon. Dermatology. 2004;208(4):345–348. doi: 10.1159/000077846. [DOI] [PubMed] [Google Scholar]
- 14.Hofhuis WJ, Oranje AP, Bouquet J, Sinaasappel M. Blue rubber-bleb naevus syndrome: report of a case with consumption coagulopathy complicated by manifest thrombosis. Eur J Pediatr. 1990 May;149(8):526–528. doi: 10.1007/BF01957684. [DOI] [PubMed] [Google Scholar]
- 15.Wouters VL, Uebelhoer M, Irrhum A, Boon LM, Mulliken JB, Enjolras O, Baselga E, berg J, Dompmartin A, Ivarsson SA, Kangesu L, Lacassie Y, Murphy J, Teebi AS, Pennington A, Rieu P, Vikkula M. Hereditary Cutaneomucosal Venous Malformations are caused by Tie2 mutations with widely variable hyper-phosporylating effects. doi: 10.1038/ejhg.2009.193. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009 Jan;41(1):118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997 Feb;36(2 Pt 1):219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- 18.Mazoyer E, Enjolras O, Bisdorff A, Perdu J, Wassef M, Drouet L. Coagulation disorders in patients with venous malformation of the limbs and trunk: a case series of 118 patients. Arch Dermatol. 2008 Jul;144(7):861–867. doi: 10.1001/archderm.144.7.861. [DOI] [PubMed] [Google Scholar]
- 19.Oduber CE, van der Horst CM, Hennekam RC. Klippel-Trenaunay syndrome: diagnostic criteria and hypothesis on etiology. Ann Plast Surg. 2008 Feb;60(2):217–223. doi: 10.1097/SAP.0b013e318062abc1. [DOI] [PubMed] [Google Scholar]
- 20.Huiras EE, Barnes CJ, Eichenfield LF, Pelech AN, Drolet BA. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics. 2005 Oct;116(4):e596–600. doi: 10.1542/peds.2004-1607. [DOI] [PubMed] [Google Scholar]
- 21.Howlett DC, Roebuck DJ, Frazer CK, Ayers B. The use of ultrasound in the venous assessment of lower limb Klippel-Trenaunay syndrome. Eur J Radiol. 1994 Aug;18(3):224–226. doi: 10.1016/0720-048x(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 22.Falanga V, Kruskal J, Franks JJ. Fibrin- and fibrinogen-related antigens in patients with venous disease and venous ulceration. Arch Dermatol. 1991 Jan;127(1):75–78. [PubMed] [Google Scholar]
- 23.Cuderman TV, Bozic M, Peternel P, Stegnar M. Hemostasis activation in thrombophilic subjects with or without a history of venous thrombosis. Clin Appl Thromb Hemost. 2008 Jan;14(1):55–62. doi: 10.1177/1076029607304408. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JV, Lowell J, Badger GJ, Rosing J, Tchaikovski S, Cushman M. Effects of oral and transdermal hormonal contraception on vascular risk markers: a randomized controlled trial. Obstet Gynecol. 2008 Feb;111(2 Pt 1):278–284. doi: 10.1097/AOG.0b013e3181626d1b. [DOI] [PubMed] [Google Scholar]
- 25.Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception. 2008 Feb;77(2):77–83. doi: 10.1016/j.contraception.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Quehenberger P, Kapiotis S, Partan C, et al. Studies on oral contraceptive-induced changes in blood coagulation and fibrinolysis and the estrogen effect on endothelial cells. Ann Hematol. 1993 Jul;67(1):33–36. doi: 10.1007/BF01709663. [DOI] [PubMed] [Google Scholar]
- 27.Gouin-Thibault I, Arkam R, Nassiri S, et al. Markers of activated coagulation in patients with factor V Leiden and/or G20210A prothrombin gene mutation. Thromb Res. 2002 Jul 15;107(1–2):7–11. doi: 10.1016/s0049-3848(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 28.Xu G, Tian KL, Liu GP, Zhong XJ, Tang SL, Sun YP. Clinical significance of plasma D-dimer and von Willebrand factor levels in patients with ulcer colitis. World J Gastroenterol. 2002 Jun;8(3):575–576. doi: 10.3748/wjg.v8.i3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004 Aug;140(8):971–976. doi: 10.1001/archderm.140.8.971. [DOI] [PubMed] [Google Scholar]
- 30.Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006 Jul;142(7):892–896. doi: 10.1001/archderm.142.7.892. [DOI] [PubMed] [Google Scholar]
- 31.Couvineau A, Wouters V, Bertrand G, et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008 Sep 15;17(18):2766–2775. doi: 10.1093/hmg/ddn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enjolras O, Wassef M, Merland JJ. Maffucci syndrome: a false venous malformation? A case with hemangioendothelioma with fusiform cells. Ann Dermatol Venereol. 1998 Aug;125(8):512–515. [PubMed] [Google Scholar]