Abstract
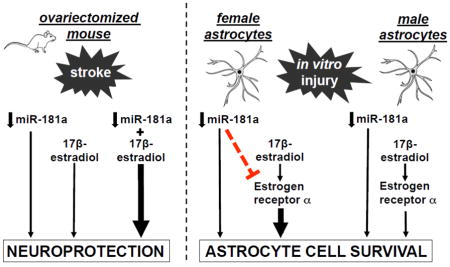
Whether the effect of miR-181a is sexually dimorphic in stroke is unknown. Prior work showed protection of male mice with miR-181a inhibition. Estrogen receptor-α (ERα) is an identified target of miR181 in endometrium. Therefore we investigated the separate and joint effects of miR-181a inhibition and 17β-estradiol (E2) replacement after ovariectomy. Adult female mice were ovariectomized and implanted with an E2- or vehicle-containing capsule for 14d prior to 1h middle cerebral artery occlusion (MCAO). Each group received either miR-181a antagomir or mismatch control by intracerebroventricular injection 24h before MCAO. After MCAO neurologic deficit and infarct volume were assessed. Primary male and female astrocyte cultures were subjected to glucose deprivation with miR-181a inhibitor or transfection control, and E2 or vehicle control, with/without ESRα knockdown with small interfering RNA. Cell death was assessed by propidium iodide staining, and lactate dehydrogenase assay. A miR-181a/ERα target site blocker (TSB), with/without miR-181a mimic, was used to confirm targeting of ERα by miR-181a in astrocytes. Individually, miR-181a inhibition or E2 decreased infarct volume and improved neurologic score in female mice, and protected male and female astrocyte cultures. Combined miR-181a inhibition plus E2 afforded greater protection of female mice and female astrocyte cultures, but not in male astrocyte cultures. MiR-181a inhibition only increased ERα levels in vivo and in female cultures, while ERα knockdown with siRNA increased cell death in both sexes. Treatment with ERα TSB was strongly protective in both sexes. In conclusion, the results of the present study suggest miR-181a inhibition enhances E2-mediated stroke protection in females in part by augmenting ERα production, a mechanism detected in female mice and female astrocytes. Sex differences were observed with combined miR-181a inhibition/E2 treatment, and miR-181a targeting of ERα.
Keywords: microRNA, astrocyte, ischemia-reperfusion injury, MCAO, stroke, estrogen
Graphical Abstract
2. Introduction
Stroke remains a major source of mortality and morbidity worldwide, including in the United States (Mozaffarian et al., 2015). Despite advances in gene therapy techniques and promising results in pre-clinical animal models, treatment for the most common form of stroke (thrombotic/non-hemorrhagic) remains limited to early reperfusion with thrombolytics or clot retrieval. In the human population, sexual dimorphism in thrombotic stroke risk and outcome is well documented. The Framingham Heart Study demonstrated women overall have an lower risk of stroke than men (Petrea et al., 2009), however in old age, women have a higher incidence of stroke and poorer functional outcome (Towfighi et al., 2007; Petrea et al., 2009; Mozaffarian et al., 2015). This age-dependent sexual dimorphism in stroke risk and outcome may hold clues to the translation barrier that persists in the development of new stroke therapies. Importantly, a similar sexual dimorphism has been identified for injury outcome in rodent models of stroke. Overall, adult female rodents have smaller infarct volumes and improved neurobehavioral outcomes following transient cerebral ischemia than their age-matched male counterparts (Alkayed et al., 1998; Selvamani et al., 2014; Xiong et al., 2011), however this effect is reversed in aged female rodents (Manwani et al., 2014). Delineating the mechanisms that regulate the differences between males and females in risk and outcome following stroke may yield new insight and more effective therapeutics.
Although a full understanding of the mechanisms underlying sex differences in stroke remains to be determined, the ovarian hormone estrogen is known to play a role in ischemic protection (Manwani et al., 2015; Simpkins et al., 1997). Lower circulating levels of estrogen that occur with physiologic estrous cycling in female rats and mice are associated with a greater degree of injury from cerebral ischemia (Xiong et al., 2015; Liao et al., 2001). Estrogen exerts a protective effect via several mechanisms including antioxidant and immune modulation, as well as activation of cell survival signaling pathways (for review, Ritzel et al., 2013). The sex difference in infarct volume observed between intact adult males and females is abolished with ovariectomy (OVX), which depletes estrogen, resulting in larger infarcts in adult females (Alkayed et al., 1998). This effect is in part reversed by exogenous replacement of 17β-estradiol (E2, Dubai et al., 1998; Rusa et al., 1999) improving sensorimotor and spatial memory deficits in ovariectomized females (Li et al., 2004; Gulinello et al., 2006).
MicroRNAs (miRs) are short (19–22 nucleotide), non-coding RNAs that modulate endogenous gene expression in all tissues during both normal physiological functioning and in response to stress. miR expression patterns are organ- and cell type-specific, and in the brain miRs play a central role in the response to cerebral ischemia (for review(Ouyang et al., 2013). MiR expression patterns have also been observed to exhibit both sex- and age-related dysregulation in response to cerebral ischemia (Selvamani et al., 2014). MiRs most commonly function by binding the 3′ untranslated region (UTR) of target messenger RNAs (mRNAs) to inhibit protein translation. We have previously demonstrated a protective effect of miR-181a-5p inhibition in male rodent models of stroke (Ouyang et al., 2012) and forebrain ischemia (Moon et al., 2013) by targeting a stress protein and regulators of apoptosis. However, whether sex differences exist in the role of miR-181a-5p (hereafter miR-181a) in stroke has not been previously investigated. In endometrial cells, the miR-181 family has been shown to target estrogen receptor-α (ERα, Su et al., 2014), a transcription factor that activates pro-survival pathways (Yu et al., 2012), suggesting that sex differences in downstream targets of miR-181a may exist. Therefore, in the present study we examined the roles of miR-181a, E2, and ERα in female mice subjected to OVX with or without replacement of E2, and subjected to cerebral ischemia two weeks later.
Astrocytes are principle regulators of neuronal homeostasis and survival following injury and express greater levels of miR-181a than neurons (Hutchison et al., 2013). We have previously demonstrated that local astrocyte dysfunction precedes and contributes to neuronal cell death following forebrain ischemia (Ouyang et al., 2007), and that targeting astrocytes can be an effective therapeutic approach for protection (Xu et al., 2010). In focal ischemia as used here, astrocyte cell death is part of the core injury. We previously demonstrated that reducing levels of miR-181a in astrocytes helps preserve mitochondrial function and protect against astrocyte cell death from in vitro ischemia (Ouyang et al., 2012). Substantial previous work supports a role for E2 and estrogen receptors in astrocytes (see Ritzel et al., 2013 for review). Therefore, in the present study we followed up stroke studies in female mice with studies in female and male astrocyte cultures evaluating differences in the effect of miR-181a, E2 and ERα in the response to cell stress.
3. Materials and Methods
3.1 In vivo experimental protocols
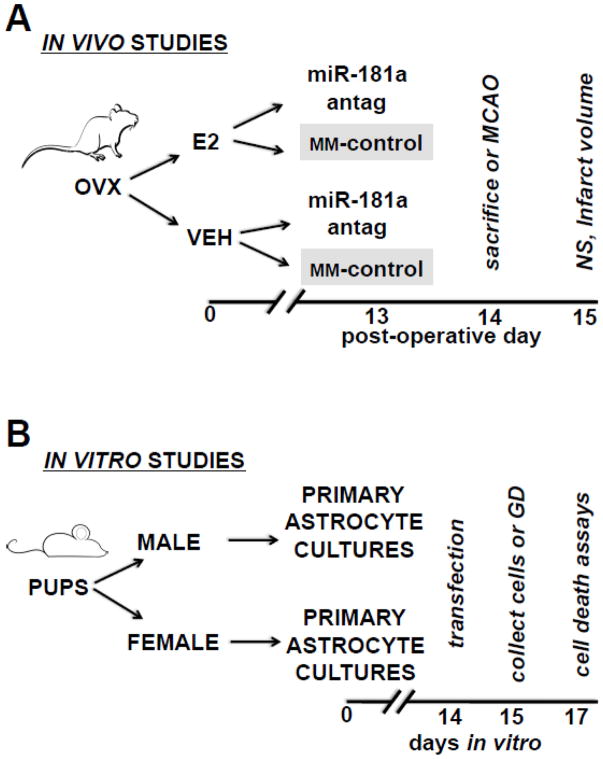
All experimental protocols using animals were approved by the Stanford University Animal Care and Use Committee, and performed in accordance with NIH guidelines. Adult female C57/B6 mice (age 8–10 weeks, Charles River, Wilmington, MA) were surgically ovariectomized (OVX, described below) and randomly assigned by coin flip to subcutaneous placement of a capsule with or without E2. These two groups were further randomly subdivided (Figure 1A) into groups receiving intracerebroventricular injection (ICV) of either miR-181a antagomir, or mismatch-control sequence, on post-OVX day 13. Animals were either sacrificed for assessment of miR-181a levels and protein expression, or subjected to 1 hr middle cerebral artery occlusion (MCAO) 24 hr following ICV treatment. A total of 140 mice underwent OVX, of those 78 also underwent MCAO.
Figure 1.
Experimental protocols for in vivo (A) and primary astrocyte (B) studies. Antag = antagomir; E2 = 17β-estradiol; GD = glucose deprivation; MCAO = 1 hr middle cerebral artery occlusion; MM-control = mismatch control; NS = neurological deficit score; OVX = ovariectomy; V = control vehicle.
3.2 Ovariectomy (OVX) and E2 replacement
Surgery was performed as previously described (Sisk & Meek, 2001; McCullough et al., 2003). Mice were anesthetized with isoflurane, and an incision bisecting the angle formed by the ribs and spinal column was made. The ovary, fat pad, and uterine horn were exposed, exteriorized, the uterine horn ligated with surgical silk and the ovary excised. The ligated uterine horn was fed back into the body cavity, the wound closed and the procedure repeated on the opposite side. Prior to closure and dressing of the second incision, a capsule containing 30 μg E2 (E8875, Sigma-Aldrich, St. Louis, MO) in sesame oil, or a capsule with sesame oil alone, was placed subcutaneously. Concentration of serum E2 was assessed prior to OVX and in OVX mice with and without E2 replacement at post-OVX days 7 and 14 by enzyme-linked immunosorbent assay (ELISA, ES180S-100, Calbiochem, San Diego, CA).
3.3 Intracerebroventricular (ICV) injection
Mice were anesthetized with 2% isoflurane, placed in a stereotaxic frame, and a 26-gauge brain infusion needle was placed stereotaxically into the left lateral ventricle (bregma: – 0.58 mm; dorsoventral: 2.1 mm; lateral: 1.2 mm) as previously described (Ouyang et al., 2012). MiR-181a antagomir, 3 pmol/g body weight, or mismatch-control (details in Supplemental Table SI) was mixed with the cationic lipid DOTAP (6μl total volume; Roche, Basel, Switzerland) and infused over 20 minutes as previously (Ouyang et al., 2012).
3.4 Transient focal cerebral ischemia, infarct and neurologic assessment
Mice were anesthetized with isoflurane in oxygen and 1h transient ischemia was produced by occluding the middle cerebral artery (MCAO) with a monofilament followed by 24 hr of reperfusion as described previously (Xiong et al., 2015; Ouyang et al., 2012). The neuroscore was assessed 24 hr after MCAO: a score of 0 (no observable neurological deficit), 1 (failure to extend right forepaw), 2 (circling to the right), 3 (falling to the right) or 4 (unable to walk spontaneously) was assigned (Yang et al., 1994). Brains were harvested to assess infarct volume after transcardiac perfusion with saline, then 4% paraformaldehyde. Infarct volume was quantified by a blinded observer on 4 sections/brain using 50 μm coronal sections stained with Cresyl Violet (EMD-Millipore Chemicals, Hayward, CA) and corrected for edema using Image J software (v1.46, NIH, Bethesda, MD) as described (Ouyang et al., 2012). Mice with no evidence of acute neurological deficit (n=2), that died < 24 hr following surgery (n=3), or with evidence of significant bleeding (n=1) were excluded from analysis. Treatment groups did not differ in numbers of excluded animals.
3.5 Cell cultures
Primary astrocyte cultures were prepared from postnatal day 1–3 mice as previously described (Dugan et al., 1995). Experimental treatment protocol is outlined in Figure 1B. Briefly, following euthanasia pups were sexed according to visual inspection of genitalia (Greenham & Greenham, 1977). Correct sex of pups was confirmed using RT-qPCR on tail biopsies for SRY, the male sex determining region on the Y chromosome. C6 glioma cells (originally from a male rat) were purchased from American Type Culture Collection (CCL-107) and grown in DMEM (ThermoFisher Scientific, Waltham, MA) supplemented with 10% FBS and 100 μg/ml penicillin/streptomycin. Cell cultures were transfected when near-confluent (days-in-vitro 14–17) with 30 pmol miR-181a inhibitor (see Supplemental Table S2 for details) or mismatch-control, and treated with either 20 nM E2 (in 1:5000 DMSO) or 1:5000 DMSO alone (Figure IB). Primary astrocyte cultures were treated with 30 pmol miR-181a mimic, a dose previously (Xiong et al., 2015; Ouyang et al., 2012) shown to increase miR-181a levels by > 50 fold, and/or a custom target site blocker (Exiqon, Vedbaek, Denmark) to competitively inhibit the mmu-miR-181a-5p binding site on the mmu-ER1 mRNA 3′UTR (2000 5′-UUGAAUGU-3′). To verify transfection, cultures were assessed for 6-FAM™ fluorescence (the reporter on the TSB) 24 hr after transfection, following fixation as previously described (Stary et al., 2016).
3.6 Cell Injury and Assessment
Cells were subjected to injury 24 hr following transfection (Figure IB). Injury was induced by glucose deprivation (GD) for 48 hr, this longer duration was needed to induce injury in these relatively young cultures (Papadopoulos et al., 1998a). We chose GD as a cell stress because it reliably induces mitochondrial dysfunction as seen in ischemia (both substrate deprivation and oxidative stress) that progresses to cell death. We quantified cell death because it can be reliably quantified by parallel measures, and is the common final end-point for cellular dysfunction. Cell death was assessed by staining with Hoechst 33342 (5 μM, Sigma-Aldrich) and propidium iodide (PI, 5 μM, Sigma-Aldrich) and quantitated by measuring lactate dehydrogenase (LDH) released from dead cells, as previously described (Ouyang et al., 2012). In all cell culture experiments non-injury wash control resulted in <5% cell death.
3.7 Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA from tissue and cell culture was isolated with TRIzol® (ThermoFisher Scientific). Reverse transcription was performed as previously described (Ouyang et al., 2012). PCR was performed with predesigned primer/probes for mmu-miR-181a-5p, sex-determining region Y (SRY), GAPDH, and U6 (see Supplemental Table S3 for reagent details). qPCR reactions were conducted as previously (Ouyang et al., 2012). Ct values for SRY we normalized to GAPDH, miR-181a was normalized to U6 (ΔCt). Comparisons were calculated as the inverse log of the ΔΔCT (Livak & Schmittgen, 2001).
3.8 Immunoblotting
Immunoblotting was performed using 30 μg of protein/sample as previously described (Ouyang et al., 2012), using primary antibodies to ERα, GRP78, XIAP, Bcl2 and β-actin. Briefly, cells were harvested 24 hr after transfection or brain protein was isolated after perfusion with iced saline, then 30 μg of protein/sample was separated on a 4–10% Bis-Tris mini-gel (NP0304BOX, ThermoFisher Scientific), and electro-transferred to Immobilon polyvinylidene fluoride membrane (IPVH00010, Millipore EMD Corp.). Membranes were blocked and incubated with the appropriate primary antibody and dilution (see Supplemental Table S4) overnight at 4°C. Membranes were then washed (and incubated with 1:15,000 the appropriate conjugated secondary antibody (see Table S4). Immunoreactive bands were visualized using the LICOR Odyssey infrared imaging system. Densitometric analysis was performed using Image J software (vl.46, National Institutes of Health) by an observer blinded to treatment group. Target band intensity was normalized to β-actin. See Table S4 for reagent details and antibody information.
3.9 Fluorescence detection for 6-FAM™
To verify transfection, cultures were assessed for 6-FAM™ fluorescence (the reporter on the TSB) 24 hr after transfection, following fixation in 24-well plates as previously described (Stary et al., 2016). Primary astrocyte cell cultures were fixed in 4% paraformaldehyde for 30 min at room temperature. Nonspecific binding was blocked with 5% normal goat serum (S-1000, Vector) and 0.3% Triton X-100 (X-100, Sigma Aldrich) in PBS for 1 hr. Cells were counterstained with the nuclear dye DAPI (4′,6′-diamidino-2-phenylindole, 0.5 μg/ml; D9542, Sigma-Aldrich). To verify transfection of ERα TSB, cells were assessed for 6-FAM™ fluorescence with a Zeiss (Carl Zeiss AG, Jena, Germany) Axiovert 200M epifluorescence microscope and AxioVision software (v4.8.1) by an observer blinded to treatment groups.
3.10 Statistical Analysis
All cell culture data represent experiments repeated 3X with cells from 3 different dissections. All data shown are mean±SE. Statistical analysis was two-way analysis of variance with repeated measures for assessment of serum E2 levels, and one-way analysis of variance with Bonferroni post-hoc test for all other comparisons. A p-value of <0.05 was considered significant.
4. Results
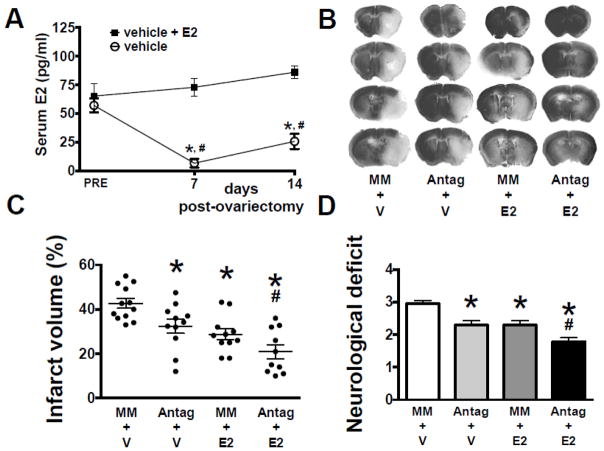
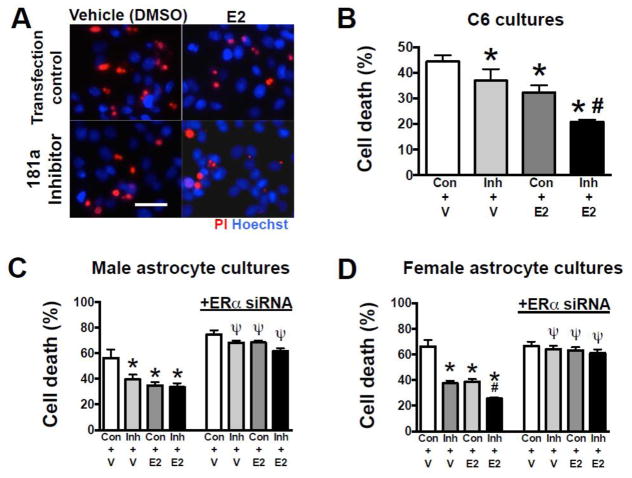
When measured before OVX, serum E2 levels were equivalent, while 7 and 13 days after OVX, E2 was significantly lower in animals replaced with vehicle, versus E2 replacement (Figure 2A). Treatment with either miR-181a antagomir or E2 decreased MCAO infarct volume (Figures 2B,C), while combined treatment resulted in significantly greater protection than either treatment alone. Similarly, miR-181a antagomir or E2 significantly improved neurological deficit score (Figures 2D), with combined treatment resulting in better neurological outcome than either treatment alone. Treatment with either miR-181a inhibitor or E2 alone resulted in significant protection of C6 glioma cells from GD (Figures 3A,B). However, in contrast to in vivo observations in female mice (Figure 2C), combined treatment did not induce additional protection. To further investigate, primary cultures of astrocytes isolated from male or female pups were compared. Treatment with either miR-181a inhibitor or E2 resulted in significant protection (Figures 3C,D). Male cultures, like C6 cells (from a male rat), did not show greater protection with combined treatment, while female cultures did, matching the female in vivo observations. Co-treatment with ERα siRNA abolished the protective effects of both miR-181a inhibitor and E2 in both male (Figure 3C) and female (Figure 3D) primary astrocyte cultures, suggesting that ERα is important for protection by both miR-181a inhibition and E2 treatment in cultures of both sexes.
Figure 2. Effect of miR-181a antagomir and E2 on injury from middle cerebral artery occlusion (MCAO).
(A) Serum E2 levels in female mice prior to and following ovariectomy (OVX), with and without 17β-estradiol (E2) replacement. (B) Coronal brain sections stained with Cresyl Violet to assess infarct volume in OVX mice treated with mismatch control (MM) or miR-181a antagomir (Antag), and E2 or vehicle (V), 24 hr following 1 hr MCAO; infarcted areas are lighter in color. Quantification of infarct volume (C) and neurological deficit (D) 24 hr following MCAO. All graphs show mean±SE, * = p<0.05 versus pre or control injury; # = p<0.05 versus Antag or E2 alone. N = 8–9 OVX animals per group. E2 = 17β-estradiol.
Figure 3. Effect of miR-181a inhibitor and E2 on in vitro glucose deprivation (GD) astrocyte injury.
(A) Representative micrographs of cell death assessed by propidium iodide (PI, red) staining following GD injury in C6 cells treated with control scramble sequence (Con) or miR-181a inhibitor, and E2 or vehicle (V). All cell nuclei are stained blue with Hoechst. Quantification of cell death by LDH release from C6 cells (B), male primary astrocyte cultures (C), and female primary astrocyte cultures (D) treated with miR-181a inhibitor, E2, or both. Blocking ERα using small interfering RNA (siRNA) abolished the protection seen with miR-181a inhibitor and E2 in both male and female cultures (C, D). N = 8 cultures per experiment. All graphs = mean±SE, * =p<0.05 versus control; # =p<0.05 versus E2 or inhibitor alone; Ψ= p<0.05 versus same condition without siRNA. E2 = 17β-estradiol Inh = inhibitor. Bar =15 μm.
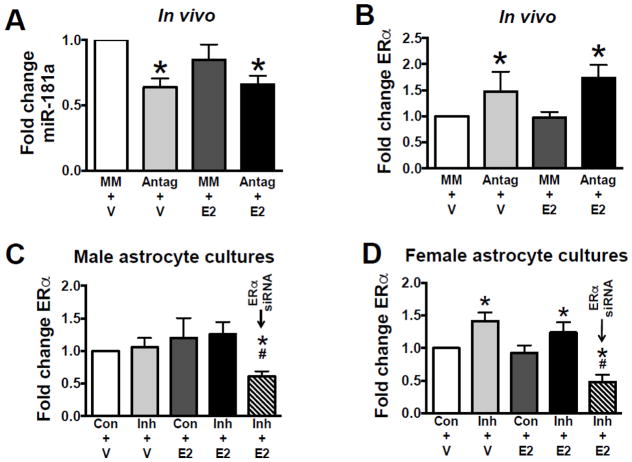
Brain levels of miR-181a and ERα (example of ERα immunoblot is shown in Supplemental Figure S1) were assessed 24 hr after ICV injection of miR-181a antagomir in OVX mice (Figures 4A,B). ICV injection with miR-181a antagomir significantly decreased miR-181a levels to 64%±7 (relative to ICV injection with MM-control oligonucleotide) and significantly increased ERα protein expression by 48±13%. In contrast, no change in ERα protein expression was observed following miR-181a inhibition in male primary astrocyte cultures (Figure 4C). However, in female primary astrocyte cultures, an increase in ERα was observed in miR-181a inhibitor treated groups (Figure 4D), similar to observations in vivo. Male and female cultures without treatment had similar levels of ERα. Three other targets previously identified in male rodents, GRP78, Bcl2 and XIAP were also assessed. Protein levels of Bcl2 and XIAP were not altered in female brain (Table S5). Grp78 levels were increased about 30% both by E2 and miR-181a antagomir, as well as combined treatment (Table S5).
Figure 4. In vivo and in vitro ERα protein expression.
(A) Quantification of miR-181a levels from brains of OVX mice. Quantification of ERα protein from brains of OVX mice (B), male (C) and female (D) primary astrocyte cultures. ERα levels were significantly (p<0.05) lower in cultures co-treated with ERα siRNA (C, D). N = 8 cultures per experiment. All graphs = mean±SE, * = p<0.05 versus control; # = p<0.05 versus all other conditions. Antag = miR-181a antagomir, Con = transfection control (scrambled sequence), E2 = 17β-estradiol, Inh = miR-181a inhibitor, V = vehicle.
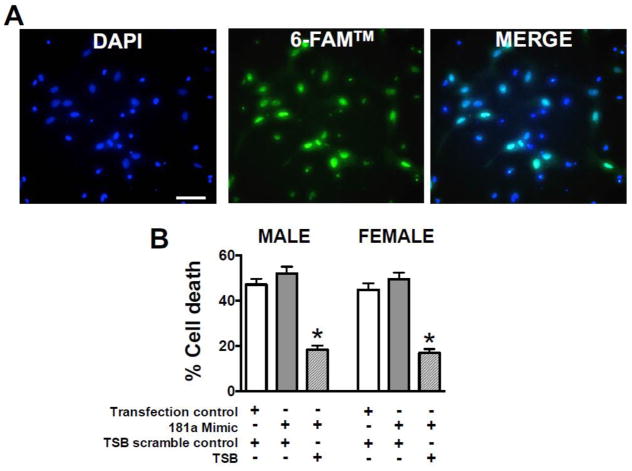
To confirm that targeting of ERα by mir-181a contributes to cell death, we co-transfected primary astrocyte cultures with miR-181a mimic or miR-181a mimic/ERα TSB. Transfection efficiency of ERα TSB assessed with a covalent 6-FAM™ fluorescent reporter (Figure 5A), was 85±10%. In both female and male cultures, transfection with miR-181a mimic, which increases levels of miR-181a, caused a trend to increased cell death from glucose deprivation (Figure 5B). In astrocyte cultures of both sexes co-transfection with mir-181a mimic and ERα TSB led to marked reduction in GD injury to levels below control injury, suggesting endogenous levels of miR-181a were already increasing cell death.
Figure 5. Cell death following miR-181a/ERα binding inhibition in astrocytes.
(A) Primary astrocytes stained with the nuclear dye DAPI (4′,6′-diamidino-2-phenylindole, blue, left panel) display 6-FAM™ reporter fluorescence (green, middle panel) when transfected with miR-181a/ERα target site blocker (TSB). Transfection efficiency is quantified by blue/green co-localization (right panel). Bars = 15 μm. (B) Quantification of cell death from GD injury of primary male and female astrocytes treated with control transfection or miR-181a mimic and TSB scramble control sequence or miR-181a/ESRα TSB. N = 8 cultures per experiment, mean±SE, * = p<0.05 versus control.
5. Discussion
The present study examined the efficacy of miR-181a antagomir in female stroke, with and without E2. We observed significant protection, similar in magnitude to the protection seen in male mice (about 48% reduction in infarct volume, (Ouyang et al., 2012) only with combined E2 and miR-181a antagomir (53% reduction), while antagomir alone provided a significant reduction of infarct volume in females of 24%. We identified ERα as a target for miR-181a in female brain and astrocyte culture, but not in male cultures. Other targets investigated included Grp78, Bcl2 and XIAP. Of these only Grp78 showed a trend to increase with antagomir in female brain of about 30%, while prior observations in males showed a greater than 50% increase (Ouyang et al., 2012). Here, E2 treatment also led to increased Grp78 levels, consistent with the reported ability of E2 to reduce levels of miR-181a, previously observed in breast cancer cells (Maillot et al., 2009; Kastrati et al., 2015). However, we did not observe a decrease in miR-181a levels with E2 alone, which suggests there are additional mechanisms by which E2 can influence Grp78 levels. Thus targets and magnitudes of change differed between male and female brains, as well as the magnitude of protection.
E2 has been shown to rapidly activate pro-survival signaling pathways in astrocytes, including MAPK (Ivanova et al., 2001; Zhang et al., 2002) and phosphatidylinositol 3-kinase (PI3K)/Akt (Dhandapani et al., 2005). E2 also regulates the expression of neuroprotective proteins in astrocytes, including heat shock proteins (Lu et al., 2002), glutamate transporters (Cimarosti et al., 2005; Lee et al., 2009; Pawlak et al., 2005), and growth factors (Galbiati et al., 2002; Melcangi et al., 2001). E2 signaling occurs via ligand-mediated activation of the two known estrogen receptors, ERα and ERβ, the non-canonical G-protein coupled estrogen receptor (GPER/GPR30), as well as by non-genomic effects. In the present study, we identified ERα as a physiological target of miR-181a in female mice and astrocytes. This is consistent with the presence of a complementary sequence in the 3′ UTR of ERα, but not in the 3′ UTR of ERβ or GPER (Targetscan.org 7.1). Despite parallel signaling pathways, substantial differences between ERα and ERβ exist. The importance of each receptor in ischemic protection is controversial with different findings reported. Dubal et al. (Dubal et al., 2001) demonstrated that genetic deletion of ERα but not ERβ abolished E2 protection in a model of distal MCAO. Antisense knockdown also suggested a more critical role for ERα versus ERβ in E2 protection from cerebral ischemia (Zhang et al., 2009). Selective ERα activation but not ERβ was neuroprotective (Dai et al., 2007). However, a role for ERβ in neuroprotection was seen in some injury models, including no change in infarct in ERα knockout mice, protection seen with an ERβ agonist, or protection via both receptors (Miller et al., 2005; Sampei et al., 2000; Carswell et al., 2004).
Interestingly, in breast tissue (Kastrati et al., 2015) and endometrium (Gao et al., 2016) E2 downregulates expression of miR-181a, providing evidence for a positive feedback loop for ERα activation. E2-mediated activation of ERs also regulates pro-survival gene expression via non-classical/ER-independent co-activation of critical transcription factors, most notably cAMP-response element binding protein (CREB, Coleman et al., 2003) and c-fos/jun (Kalaitzidis & Gilmore, 2005). Interestingly, c-fos (Gao et al., 2016) and CREB (Liu et al., 2013) are both negatively regulated by miR-181a in other tissues, which may have also contributed to our observations in the present study of a combined neuroprotective effect of E2 plus miR-181a inhibition.
The present study is the first to demonstrate sex differences in the effect of miR-181a on astrocyte survival. Although astrocytes from both males and females express ERα (Garcia-Ovejero et al., 2002; Bondar et al., 2009) sex differences in the effect of E2 in astrocytes have been described. Zhang et al. demonstrated sexual dimorphism in activation and downstream signaling of MAPK (Zhang et al., 2002). Kuo et al. (Kuo et al., 2010) demonstrated in female but not male astrocytes that E2 treatment increased insertion of ERα into the cell membrane and enhanced progesterone synthesis. Astrocyte estrogen receptors appear to be altered by the hormone loss that occurs during aging (Arimoto et al., 2013), and an estrous-related response in the number of tyrosine kinase A receptor (TrkA)-immunoreactive astrocytes has been described (McCarthy et al., 2002).
One caveat to the present study is that astrocytes cultured from neonatal mice may differ substantially from astrocytes in the adult brain. In the present study we did not observe any differences in baseline levels of ERα expression between male and female cultures, or in susceptibility to injury in vehicle-treated (control) cultures. We previously observed in sex-mixed astrocyte cultures that susceptibility to oxidative stress increases with age (Papadopoulos et al., 1998b), but this effect has not yet been investigated in single sex cultures. A future approach to further study sexual dimorphism in the effects of miR-181a and E2 on astrocytes in the injured adult brain would be to investigate the effects of pre-treatment in astrocytes isolated from the adult brain following injury.
A final important consideration is that other cell types in the brain express ERs and likely play a role in the effect of E2 and miR-181a on injury outcomes in the brain. Resident microglia, central to the inflammatory response to injury and relevant to neuronal survival, express ERs and respond to E2 (Ritzel et al., 2013). Other resident brain cell types that express ERs include neurons, dendritic cells and oligodendrocytes, as well as migrating ER-expressing immune cells (monocytes, macrophages and neutrophils) all of which may participate in the response to E2 and miR-181a levels. Future studies investigating the effects of E2 and miR-181a in other cell types will further define the relevance of miR-181a inhibition as a potential therapy for stroke.
6. Summary
This report is the first to demonstrate that decreasing brain levels of miR-181a in female mice is protective, and enhances the neuroprotective effects of E2. This combined effect was recapitulated in female primary astrocyte cultures but not male cultures. We identified ERα as a novel target of miR-181a in female cortical astrocytes.
Supplementary Material
Highlights.
Inhibition of microRNA-181a in the brain is protective against middle cerebral artery occlusion in female mice.
Estrogen receptor-α is identified as a novel target of microRNA-181a in female cortical astrocytes.
Sex differences are observed in primary cortical astrocyte cultures in the effects of estradiol and microRNA-181a.
Acknowledgments
Support
This study was funded in part by NIH grants NS084396 and NS080177 to RGG, American Heart Association grant FTF-19970029 to CMS.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Arimoto JM, Wong A, Rozovsky I, Lin SW, Morgan TE, Finch CE. Age increase of estrogen receptor-alpha (ERalpha) in cortical astrocytes impairs neurotrophic support in male and female rats. Endocrinology. 2013;154:2101–2113. doi: 10.1210/en.2012-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–4. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O’Shea RD, Pow DV, Salbego C, Beart PM. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett. 2005;385:52–57. doi: 10.1016/j.neulet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-alpha (ER alpha)- and ER beta-dependent gene expression by cAMP signaling pathway(s) J Biol Chem. 2003;278:12834–12845. doi: 10.1074/jbc.M212312200. [DOI] [PubMed] [Google Scholar]
- Dai X, Chen L, Sokabe M. Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacology. 2007;52:1124–1138. doi: 10.1016/j.neuropharm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2759. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci. 1995;15:4545–4555. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati M, Martini L, Melcangi RC. Oestrogens, via transforming growth factor alpha, modulate basic fibroblast growth factor synthesis in hypothalamic astrocytes: in vitro observations. J Neuroendocrinol. 2002;14:829–835. doi: 10.1046/j.1365-2826.2002.00852.x. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang G, Liu W, Kinser H, Franco HL, Mendelson CR. Reciprocal Feedback between miR-181a and E2/ERalpha in Myometrium Enhances Inflammation Leading to Labor. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-2078. jc20162078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- Greenham LW, Greenham V. Sexing mouse pups. Lab Anim. 1977;11:181–184. doi: 10.1258/002367777780936620. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, Wood WH, Lehrmann E, Camandola S, Becker KG, Gorospe M, Mattson MP. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61:1018–1028. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Karolczak M, Beyer C. Estrogen stimulates the mitogen-activated protein kinase pathway in midbrain astroglia. Brain Res. 2001;889:264–269. doi: 10.1016/s0006-8993(00)03149-8. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kastrati I, Canestrari E, Frasor J. PHLDA1 expression is controlled by an estrogen receptor-NFkappaB-miR-181 regulatory loop and is essential for formation of ER+ mammospheres. Oncogene. 2015;34:2309–2316. doi: 10.1038/onc.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M. Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes. J Neurochem. 2009;110:530–544. doi: 10.1111/j.1471-4159.2009.06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Z, Yang F, Gao Y, Song J, Wan Y. microRNA-181a is involved in insulin-like growth factor-1-mediated regulation of the transcription factor CREB1. J Neurochem. 2013;126:771–780. doi: 10.1111/jnc.12370. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu A, Ran RQ, Clark J, Reilly M, Nee A, Sharp FR. 17-beta-estradiol induces heat shock proteins in brain arteries and potentiates ischemic heat shock protein induction in glia and neurons. J Cereb Blood Flow Metab. 2002;22:183–195. doi: 10.1097/00004647-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Bénès V, Roché H, Dalenc F, Auboeuf D, Millevoi S, Vagner S. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35:221–229. doi: 10.1038/jcbfm.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Friedler B, Verma R, Venna VR, McCullough LD, Liu F. Perfusion of ischemic brain in young and aged animals: a laser speckle flowmetry study. Stroke. 2014;45:571–578. doi: 10.1161/STROKEAHA.113.002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JB, Barker-Gibb AL, Alves SE, Milner TA. TrkA immunoreactive astrocytes in dendritic fields of the hippocampal formation across estrous. Glia. 2002;38:36–44. doi: 10.1002/glia.10060. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Galbiati M, Martini L. Formation and effects of neuroactive steroids in the central and peripheral nervous system. Int Rev Neurobiol. 2001;46:145–176. doi: 10.1016/s0074-7742(01)46062-4. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab. 2013;33:1976–1982. doi: 10.1038/jcbfm.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis. 2012;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Stary CM, Yang GY, Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Yuan TY, Giffard RG. Increasing vulnerability of astrocytes to oxidative injury with age despite constant antioxidant defenses. Neuroscience. 1998a;82:915–925. doi: 10.1016/s0306-4522(97)00320-5. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Yuan TY, Giffard RG. Increasing vulnerability of astrocytes to oxidative injury with age despite constant antioxidant defenses. Neuroscience. 1998b;82:915–925. doi: 10.1016/s0306-4522(97)00320-5. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000;31:738–43. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci (Lond) 2014;127:77–89. doi: 10.1042/CS20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Meek LR. Sexual and reproductive behaviors. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8.2) doi: 10.1002/0471142301.ns0802s00. [DOI] [PubMed] [Google Scholar]
- Stary CM, Sun X, Ouyang Y, Li L, Giffard RG. miR-29a differentially regulates cell survival in astrocytes from cornu ammonis 1 and dentate gyrus by targeting VDAC1. Mitochondrion. 2016;30:248–254. doi: 10.1016/j.mito.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Liu R, Cheng W, Zhu M, Li X, Zhao S, Yu M. Expression patterns of microRNAs in porcine endometrium and their potential roles in embryo implantation and placentation. PLoS One. 2014;9:e87867. doi: 10.1371/journal.pone.0087867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026–2032. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG. IL-4 Is Required for Sex Differences in Vulnerability to Focal Ischemia in Mice. Stroke. 2015;46:2271–2276. doi: 10.1161/STROKEAHA.115.008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- Yu L, Moore AB, Castro L, Gao X, Huynh HL, Klippel M, Flagler ND, Lu Y, Kissling GE, Dixon D. Estrogen Regulates MAPK-Related Genes through Genomic and Nongenomic Interactions between IGF-I Receptor Tyrosine Kinase and Estrogen Receptor-Alpha Signaling Pathways in Human Uterine Leiomyoma Cells. J Signal Transduct. 2012;2012:204236. doi: 10.1155/2012/204236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li B, Zhao W, Chang YH, Ma W, Dragan M, Barker JL, Hu Q, Rubinow DR. Sex-related differences in MAPKs activation in rat astrocytes: effects of estrogen on cell death. Brain Res Mol Brain Res. 2002;103:1–11. [PubMed] [Google Scholar]
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.