Abstract
Background and Aim:
Lips have a significant role in face aesthetic perception, and lip augmentation is one of the most commonly requested aesthetic procedures. Non-permanent dermal fillers, such as hyaluronic acid (HA), are used for augmenting the lips. This article presents the results of Phase II, before – after designed study, assessing the safety and efficacy of a soft tissue HA filler, for upper lip augmentation.
Materials and Methods:
Investigators treated 10 healthy adult women 28–45 years old, using a single injection of Hyamax Kiss soft tissue HA filler (a product from Hyamed Laboratories, Switzerland) for upper lip augmentation. The primary efficacy endpoint was an increase in lip fullness at least one grade on Medicis Lip Fullness Scale at 2, 12 and 24 weeks post-treatment. Furthermore, the effectiveness and durability of filler were assessed using a 5-point Investigator's Global Assessment (IGA). Adverse events and volunteers' satisfaction were reported using visual analog scale.
Results:
Response to treatment (as defined above) after 2, 12 and 24 weeks were observed in 80%, 70% and 80% of patients, respectively. No statistical difference was found in response to treatment rate between follow-up visits (P = 0.83). The mean value of IGA score in weeks 2, 12 and 24 were 3.4 ± 0.96, 3.3 ± 0.67 and 3.3 ± 0.67, respectively. The study subjects were almost all satisfied with their lip improvement. Reported adverse effects were temporary and mostly mild in severity.
Conclusion:
Soft tissue HA filler tested in this study was well tolerated, efficient and durable when used for upper lip augmentation.
Keywords: Hyaluronic acid, lip augmentation, lip filler
INTRODUCTION
Facial volume loss, resulting from aging, disease or hereditary conditions may lead to dramatic changes in appearance.[1] The use of filling agents for superficial and deep soft tissue augmentation is one of the four main interventions, commonly used for the aging face, which include resurfacing, redraping, relaxing and replacement.[2]
Upper and lower lips have an important role in face aesthetic perception. The 'ideal lip' is a full lip, with a well-defined vermilion border. Furthermore, the correct balance between the upper and lower lips is important from an aesthetic point of view.[3,4] Aesthetic standards vary across cultures and over time, but at present, full, well-defined lips are the ideal in Western cultures.[3,5]
A variety of suitable materials for soft tissue augmentation exists. Natural fillers, such as collagen, hyaluronic acid (HA) and calcium hydroxylapatite, are synthesised or derived from biologic materials. Synthetic fillers may be permanent, such as acrylates and silicone, or biodegradable, such as poly-L-lactic acid.[6]
HA is currently the main active material for lip augmentation; it is used in various procedures including increasing the overall volume of the lip or enhancing the vermilion border and sculpting and accentuating lip.[7,8,9]
HA is a polysaccharide (specifically a glycosaminoglycan that is formed from repeating D-glucuronic acid and D-N-acetylglucosamine disaccharide units) found naturally in the dermis. Its ability to bind water helps in hydration and provides skin turgor.[1]
HA fillers are formed from either bacterial based or animal-based substances. In 2004, these fillers got the Food and Drug Administration (FDA) permission for use in tissue augmentation[10,11] and at this time, there are twelve FDA approved HA fillers used in the USA.[12]
Several open-label reports or randomised, no treatment – controlled studies, have described the effectiveness of HA in lip augmentation.[13,14,15,16] However, there are different HA products on the market, and the process of development in the product is still continued.
This report presents the results of Phase II, before – after designed pilot study, which assesses the safety, efficacy and longevity of a new HA filler with synthetic origin for upper lip augmentation.
MATERIALS AND METHODS
Setting
This study was conducted in the Pharmaceutical, Cosmeceutical and Hygienic Evaluation Lab (DermaLab) of Centre for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences (TUMS). DermaLab is a reference centre for standard evaluation of pharmaceutical, cosmeceuticals and hygienic skin products by the Ministry of Health.
Study subjects
Adult women were eligible as study participants if they were 18 years and older and seeking lip augmentation with scoring 1 or 2 on the 5-point Medicis Lip Fullness Scale (MLFS) (1 = very thin, 5 = very full) for the upper lips.
Participants were required not to have any other facial plastic surgery or cosmetic procedures during the study. Exclusion criteria also included significant abnormalities of the lips, history of severe allergies, especially in case of injectable HA gel or local anaesthetics; occurrence of any disease resulting in edema of the face during the study; history of any tissue augmentation treatment or aesthetic facial surgery below the lower orbital rim and any contraindication to the implant procedures, like use of anticoagulants.
All the volunteers signed written informed consent before participating in the trial. The study was approved by the Ethics Committee of TUMS in accordance with the Helsinki Declaration and guidelines of the Iranian Ministry of Health and Medical Education.
Intervention
Subjects who met all of the eligibility criteria were enrolled in the study. After signing the informed consent and baseline assessments, a topical anaesthetic (xyla-P® lidocaine and prilocaine cream, manufactured by Tehran Shimi Company, Iran) was applied, for 45 min to the area to be treated. Then, all the subjects were treated with a single injection of maximum 1 mL of an HA in the upper lip using the retrograde technique, with almost 1 cm distance between entry points (and in the lower one if it was necessary). Since all the subjects did not need to be treated on the lower lip, only the upper lip augmentation was assessed in this study.
The HA filler used in this study was a high viscosity sterile, colourless and transparent aqueous gel of cross-linked HA with the synthetic origin and non-pyrogenic property. It is manufactured from non-animal, medical-grade, bacterial-sourced HA (Streptococcus equi) and each 1 ml of the filler contains 22 mg HA (with particle size of 500 μm).
Immediately, after the injections, to minimise the potential ecchymosis and oedema in the injection site, direct pressure with ice compresses was applied, until there was no sign of bleeding. Massaging of the injected area was also performed when it was considered necessary by the treating investigator.
Assessment
Baseline assessments were obtained before treatment, including pre-treatment photographs and MLFS scoring by the treating investigator.
At weeks 2, 12 and 24 after treatment, following assessments were performed for all the participants:
Re-scoring the MLFS by an independent dermatologist: The MLFS is a validated, 5-point scale of lip fullness (1 = very thin; 2 = thin; 3 = medium; 4 = full; 5 = very full), used in this study to assess effectiveness in the upper lip
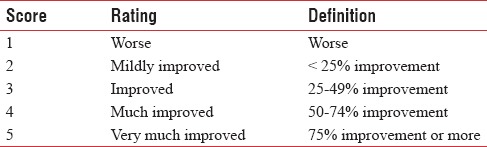
Assessment the efficacy and durability of the filler, by a dermatologist: Dermatologist opinion about the effectiveness and durability of the filler effect assessed, using 5-point Investigator's Global Assessment (IGA) Scale [Table 1].
Table 1.
Investigator's Global Assessment Scale
Volunteers' satisfaction
We used visual analogue scale (VAS) on a 0–10 scale to assess the volunteers' subjective satisfaction with the treatment outcome from an aesthetic point of view.
Assessment of safety
All adverse events reported by the subjects or observed or otherwise identified by the investigator were documented in all follow-up visits. The main safety parameters included: Erythema, pain/tenderness, swelling, lumps/bumps, bruising, discolouration and infection. The severity of them was also assessed according to a 3 point scale described below:
Mild: Awareness of signs or symptom, but easily tolerated
Moderate: Discomfort to a degree that caused interference with normal daily life activities and/or required medication
Severe: Incapacity with regard to work or usual daily life activities which required medical attention/intervention.
Statistical methods
Effectiveness and safety were analysed based on the intention-to-treat population, including all treated volunteers.
A responder was defined as a patient with at least 1-grade improvement on the MLFS for upper lip assessed by the blinded evaluator at weeks 2, 12 and 24 after treatment compared with the treating investigator's baseline MLFS assessment. It was ensured that the independent evaluator had no knowledge of the patient's lip fullness at baseline.
For statistical analysis, we performed descriptive statistics (means, standard deviations and percentages).
Statistical differences in effectiveness were tested between the different visits, using Chi-square statistic SPSS Inc. Released 2011. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc. Significance was set as P < 0.05.
RESULTS
Volunteers were ten healthy adult women 28–45 years old. All of them completed the treatment and were presented at all follow-up visits.
Response to treatment (defined as at least 1-grade improvement on the MLFS for upper lip) 2, 12 and 24 weeks after treatment were 80%, 70% and 80%, respectively. No statistical difference was found in response to treatment rate between follow-up visits. (P = 0.83) [Figure 1].
Figure 1.
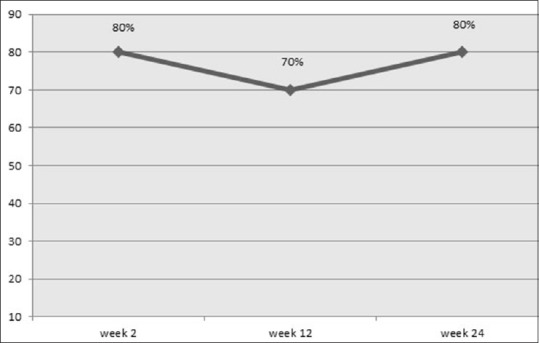
Percent of subjects with improved Medicis Lip Fullness Scale from baseline based on blind evaluator assessments
Representative baseline and post-treatment results are shown in Figure 2.
Figure 2.
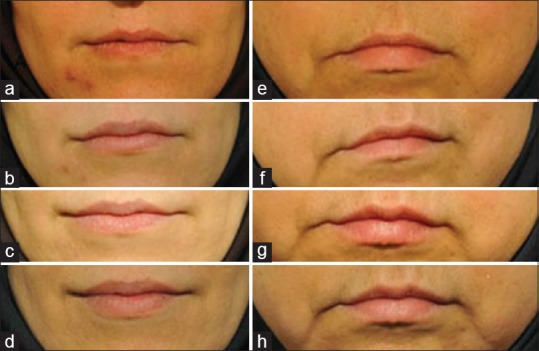
Representative photographs of volunteers' lips (a and e) before treatment, (b and f) 2 weeks after treatment, (c and g) 12 weeks after treatment and (d and h) 24 weeks after treatment
The mean values of IGA scores considering the effectiveness and persistency of injection 2, 12 and 24 weeks after injection were 3.4 ± 0.96, 3.3 ± 0.67 and 3.3 ± 0.67, respectively (P = 0.99) [Figure 3], which means the gel was persistent during the study. No volunteer was rated as score 1 (worst situation) by the dermatologist.
Figure 3.
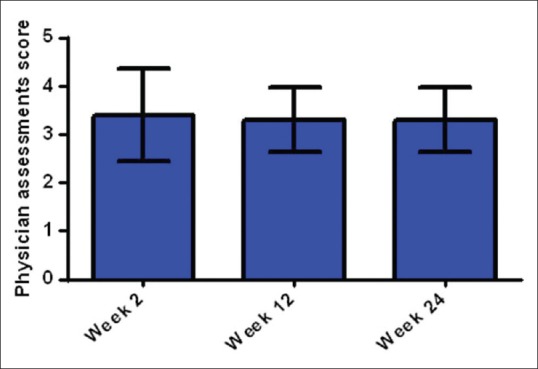
Dermatologist opinion about the effectiveness and durability of filler using 5-point Investigator's Global Assessment Scale. Mean value in 10 volunteers in 2, 12 and 24 weeks after treatment with hyaluronic acid dermal filler
The mean values of VAS scores given by the patients in weeks 2, 12 and 24 after injection were: 7.7 ± 2.05, 6.50 ± 3.29 and 6.88 ± 2.47, respectively (P = 0.60), showing the continued satisfaction of the patients.
In the case of safety assessment, there were 17 reported side effects in volunteers [Table 2]. The majority of them were classified as mild (70%; 12/17) in severity. Whereas only 5 (29%; 5/17) were classified as moderate and no severe event was reported or observed.
Table 2.
Side effects observed in 10 patients after hyaluronic acid injection in upper lip
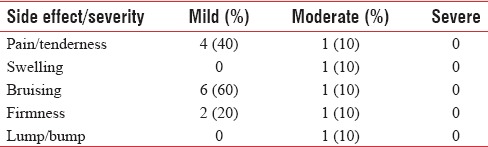
The most commonly reported side effects were pain and bruising which most often were considered mild in severity and generally lasted 1–5 days after the procedure.
Only one patient had lump/bump that lasted till the end of the study. It was probably due to accumulation of too much material in an area as a result of overcorrection or too superficial placement of a filler. The nodule resolved with hyaluronidase treatment.
DISCUSSION
HA filler tested in our study (a cross-linked HA with synthetic origin) showed to be effective for upper lip augmentation, according to the improvement of the MLFS scoring and as well as by the IGA in weeks 2, 12 and 24 after treatment.
Multiple assessment methods were used in this study to confirm the benefit of a HA product injection for lip augmentation. Grading by the investigator, volunteers' satisfaction and independent review of photographs taken during the study by a blinded evaluator, all showed the significant effectiveness of this HA soft tissue filler.
MLFS is 5-point scale that has been validated for measurement of lip fullness and observations suggest that its reported changes are clinically meaningful and aesthetically visible.[17] Additionally using MLFS is very common in lip augmentation assessment studies.[15,18]
Evaluation of MLFS scores, 12 weeks after treatment showed improvement in 70% of volunteers. This result is numerically lower than seen in similar studies performed by Glogau et al.,[16] Eccleston and Murphy[19] and also Solish and Swift;[15] where percentage of responders were between 85% to 95%.
The findings of response to treatment rate 24 weeks after treatment support the results reported in Eccleston and Murphy study (80%)[19] as well as Glogau et al.(70%),[16] and seems more impressive than results reported by Fagien et al.; where improvement in MLFS reported in 56% of volunteers.[20]
No statistical difference was found in response to treatment rate between follow-up visits in 2, 12 and 24 weeks after treatment. This is a sign of persistency of the effect of the tested product up to 24 weeks after treatment (P = 0.83). This durability compares favourably with avian HA products which produce results that last between 4 and 6 months and markedly better than collagen products (animal and human) with an average durability of 3 months.[13]
According to the data from the IGA [Figure 3], the efficacy and durability of the tested product were scored between 3 and 4 which represent improved and much improved scores. This assessment matches almost well with the blinded evaluator's assessments, in which 70–80% of subjects were rated as improved at 2, 12 and 24 weeks.
Higher values of effectiveness were reported by the investigators in study conducted by Klein; where 100% improvement reported in week 12, and 84% in week 24.[21] The difference may be due to different scales of measurement in two studies; since Klein used Global Aesthetic Improvement Scale (GAIS) to report the investigator opinion about the effectiveness and persistency of the filler.
Treatment for lip augmentation in this study was almost safe and well tolerated. The safety assessments for lip augmentation are defined based on a set of outcomes, including normal lip texture, firmness and symmetry, preserving the natural movements, function and sensation of the lips and also no mass formation in treated site.[22] Almost all of the mentioned outcomes have been achieved in this study and no severe side effects or significant asymmetries were noted during the trial. Only one subject had lump formation that lasted till the end of the study.
The majority of reported complications were anticipated such as pain and bruising and were mild in severity, and generally resolved within 5 days. The time of resolution of these events in a similar report was almost 2 weeks.[23]
The subjects were almost satisfied with their lip improvement (average VAS = 6.8 in week 24). Although in some trials, GAIS has been used to assess patient's satisfaction from an aesthetic point of view;[16,21] we prefer to use visual analogue scale (VAS) as an alternative tool for this purpose. VAS is reported as a good measurement tool to evaluate volunteers' subjective aesthetic evaluation[24,25,26] besides, it is more easy to understand and less confusing for volunteers in compare with GAIS.
This was an open-label study and one of the most important limitations was the lack of control group. The design of this study did not permit us to omit the placebo effect. It should be considered that this setting is a common design for studies of dermal fillers in the lips, especially in case of initial and pilot studies.[15,17,24] In addition, to compensate this limitation, our assessment methods designed in a way to have the minimum subjective findings (only in case of volunteers' satisfaction scoring).
Although, the small population of study subjects might limit any clinically meaningful conclusion from the data, yet as a pilot study, the results showed that the tested HA filler provided a beneficial, durable treatment for the upper lip augmentation with a good safety profile.
These results should be confirmed with complimentary studies, using larger sample size.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nestor MS. Hyaluronic acid fillers. In: Draelos ZD, editor. Cosmetic Dermatology: Products and Procedures. 2nd ed. Hoboken, New Jersey: Blackwell; 2010. pp. 352–5. [Google Scholar]
- 2.Jorizzo JL, Schaffer JV. Soft tissue augmentation. In: Bolognia J, editor. Text Book of Dermatology. 3rd ed. Philadelphia: Saunders Elsevier; 2012. pp. 2547–60. [Google Scholar]
- 3.Niamtu J., 3rd New lip and wrinkle fillers. Oral Maxillofac Surg Clin North Am. 2005;17:17–28, v. doi: 10.1016/j.coms.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Klein AW. In search of the perfect lip: 2005. Dermatol Surg. 2005;31:1599–603. doi: 10.2310/6350.2005.31247. [DOI] [PubMed] [Google Scholar]
- 5.Bisson M, Grobbelaar A. The esthetic properties of lips: A comparison of models and nonmodels. Angle Orthod. 2004;74:162–6. doi: 10.1043/0003-3219(2004)074<0162:TEPOLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Mandy SH. Fillers that work by fibroplasia: Poly-L-lactic acid. Philadelphia. USA: Saunders Elsevier; 2008. [Google Scholar]
- 7.Beer KR. Rejuvenation of the lip with injectables. Skin Therapy Lett. 2007;12:5–7. [PubMed] [Google Scholar]
- 8.Glavas IP. Filling agents. Ophthalmol Clin North Am. 2005;18:249. doi: 10.1016/j.ohc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Buck DW, 2nd, Alam M, Kim JY. Injectable fillers for facial rejuvenation: A review. J Plast Reconstr Aesthet Surg. 2009;62:11–8. doi: 10.1016/j.bjps.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 10.Maloney BP. Cosmetic surgery of the lips. Facial Plast Surg. 1996;12:265–78. doi: 10.1055/s-0028-1082417. [DOI] [PubMed] [Google Scholar]
- 11.Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–95. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- 12.FDA. Soft Tissue Fillers Approved by the Center for Devices and Radiological Health. Hampton, Virginia: US Food and Drug Administration, Inc; c2015. [Last cited on 2016 Jan 26]. Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm . [Google Scholar]
- 13.Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556–63. doi: 10.1016/j.asj.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Bosniak S, Cantisano-Zilkha M, Glavas IP. Nonanimal stabilized hyaluronic acid for lip augmentation and facial rhytid ablation. Arch Facial Plast Surg. 2004;6:379–83. doi: 10.1001/archfaci.6.6.379. [DOI] [PubMed] [Google Scholar]
- 15.Solish N, Swift A. An open-label, pilot study to assess the effectiveness and safety of hyaluronic acid gel in the restoration of soft tissue fullness of the lips. J Drugs Dermatol. 2011;10:145–9. [PubMed] [Google Scholar]
- 16.Glogau RG, Bank D, Brandt F, Cox SE, Donofrio L, Dover J, et al. Arandomized, evaluator-blinded, controlled study of the effectiveness and safety of small gel particle hyaluronic acid for lip augmentation. Dermatol Surg. 2012;38:1180–92. doi: 10.1111/j.1524-4725.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 17.Kane MA, Lorenc ZP, Lin X, Smith SR. Validation of a lip fullness scale for assessment of lip augmentation. Plast Reconstr Surg. 2012;129:822e–8e. doi: 10.1097/PRS.0b013e31824a2df0. [DOI] [PubMed] [Google Scholar]
- 18.Beer K, Glogau RG, Dover JS, Shamban A, Handiwala L, Olin JT, et al. Arandomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(Suppl 1):S127–36. doi: 10.1097/DSS.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 19.Eccleston D, Murphy DK. Juvéderm® volbella™ in the perioral area: A 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167–72. doi: 10.2147/CCID.S35800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagien S, Maas C, Murphy DK, Thomas JA, Beddingfield FC., 3rd Juvéderm Lips Study Group. Juvederm ultra for lip enhancement: An open-label, multicenter study. Aesthet Surg J. 2013;33:414–20. doi: 10.1177/1090820X13478609. [DOI] [PubMed] [Google Scholar]
- 21.Klein A. The efficacy of hyaluronic acid in the restoration of soft tissue volume of the lips and lower 1/3 of the face: The evolution of the injection technique. J Cosmet Dermatol Sci Appl. 2011;1:147–15. [Google Scholar]
- 22.Smith SR, Vander Ploeg HM, Sanstead M, Albright CD, Theisen MJ, Lin X. Functional safety assessments used in a randomized controlled study of small gel particle hyaluronic acid for lip augmentation. Dermatol Surg. 2015;41(Suppl 1):S137–42. doi: 10.1097/DSS.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 23.Fardal O, Johannessen AC, Linden GJ. Patient perceptions of periodontal therapy completed in a periodontal practice. J Periodontol. 2002;73:1060–6. doi: 10.1902/jop.2002.73.9.1060. [DOI] [PubMed] [Google Scholar]
- 24.Czochrowska EM, Stenvik A, Bjercke B, Zachrisson BU. Outcome of tooth transplantation: Survival and success rates 17-41 years posttreatment. Am J Orthod Dentofacial Orthop. 2002;121:110–9. doi: 10.1067/mod.2002.119979. [DOI] [PubMed] [Google Scholar]
- 25.Wolfart S, Thormann H, Freitag S, Kern M. Assessment of dental appearance following changes in incisor proportions. Eur J Oral Sci. 2005;113:159–65. doi: 10.1111/j.1600-0722.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 26.Cho HL, Lee JK, Um HS, Chang BS. Esthetic evaluation of maxillary single-tooth implants in the esthetic zone. J Periodontal Implant Sci. 2010;40:188–93. doi: 10.5051/jpis.2010.40.4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]


