Abstract
Cannabis and alcohol are believed to have widespread effects on the brain. Although adolescents are at increased risk for substance use, the adolescent brain may also be particularly vulnerable to the effects of drug exposure due to its rapid maturation. Here, we examined the association between cannabis and alcohol use duration and resting-state functional connectivity in a large sample of male juvenile delinquents.
The present sample was drawn from the Southwest Advanced Neuroimaging Cohort, Youth sample, and from a youth detention facility in Wisconsin. All participants were scanned at the maximum-security facilities using The Mind Research Network’s 1.5T Avanto SQ Mobile MRI scanner. Information on cannabis and alcohol regular use duration was collected using self-report. Resting-state networks were computed using group independent component analysis in 201 participants. Associations with cannabis and alcohol use were assessed using Mancova analyses controlling for age, IQ, smoking and psychopathy scores in the complete case sample of 180 male juvenile delinquents.
No associations between alcohol or cannabis use and network spatial maps were found. Longer cannabis use was associated with decreased low frequency power of the default mode network, the executive control networks (ECNs), and several sensory networks, and with decreased functional network connectivity. Duration of alcohol use was associated with decreased low frequency power of the right frontoparietal network, salience network, dorsal attention network, and several sensory networks.
Our findings suggest that adolescent cannabis and alcohol use are associated with widespread differences in resting-state time course power spectra, which may persist even after abstinence.
Keywords: Cannabis, Alcohol, Adolescence, Resting-state functional connectivity
1. Introduction
Adolescence is a period of significant growth and developmental change. Psychologically, one of the most marked changes may be the rise in risk-taking behavior, which places the adolescent at an increased risk for antisocial behaviors, such as substance use. In 2013, 34.9% of United States high-school students reported to have drunk alcohol in the last month, while 23.4% reported the use of cannabis (Kann et al., 2014). In adolescent detainees, these numbers are even higher, with 41–47% of youth reporting heavy alcohol use and 36–48% reporting use of cannabis (Ewing et al., 2015). Paradoxically, as the adolescent brain undergoes major changes in synaptic receptors density as well as in myelination (Crews et al., 2007), it may be particularly vulnerable to the effects of substance exposure. Elucidating the effects of substance use on the adolescent brain may provide important information for prevention, the development of intervention programs, and policy-making.
Both cannabis and alcohol are believed to have widespread effects on the brain. The main psychoactive component of cannabis, Δ9-tet-rahydrocannabinol (THC), binds to endogenous cannabinoid receptor-1 (CB1). CB1 receptors are widely distributed throughout the brain, but are especially concentrated in the cerebellum, prefrontal cortex, striatum, amygdala, and hippocampus (Hirvonen et al., 2012), and play a role in neurotransmitter release and concentrations across neural systems. Cannabinoid receptor density is also greater in children and adolescents than in adults (Glass et al., 1997). By affecting glutamate and gamma-aminobutyric acid (GABA) systems, THC may interfere with brain maturational processes (Bossong and Niesink, 2010). Exposing the cellular and molecular targets for alcohol’s actions has proven more challenging. However, like cannabis, alcohol is believed to affect many neurotransmitter systems in the brain (Paul, 2006). Three important neurotransmitter systems that undergo substantial changes during adolescence and are affected by alcohol consumption are dopamine, glutamine, and GABA (Hiller-Sturmhofel and Swartzwelder, 2004).
Cannabis and alcohol use during adolescence have been associated with both immediate and long-term outcomes that include disruptions in task-relevant neural activity and small or no effects on cognition (Gonzalez et al., 2012; Harvey et al., 2007; Jacobsen et al., 2007; Jager et al., 2010; Lane et al., 2007; Tapert et al., 2007; Tait et al., 2011). Structural magnetic resonance imaging (MRI) analyses suggest that adolescent cannabis use may be associated with decreased gray matter volume in widespread regions of the brain, such as the prefrontal cortex, amygdala, and hippocampus (Churchwell et al., 2010; Cousijn et al., 2012; Filbey et al., 2014; Yucel et al., 2006). Youth who drink alcohol show decreased brain volume or cortical thickness in the frontal, temporal, and parietal cortex, and hippocampus (Nagel et al., 2005; Luciana et al., 2013; Squeglia et al., 2014; Squeglia et al., 2015). Generally, earlier initiation of use and more frequent use have been associated with poorer outcomes (Buchy et al., 2015; Pope et al., 2003), and while some studies suggest that effects of alcohol and cannabis use decrease after prolonged periods of abstincence (Fortier et al., 2014; Hanson et al., 2010; Jacobus et al., 2012), other studies suggest long-lasting effects of adolescent alcohol and cannabis use (Ashtari et al., 2011; van Eijk et al., 2013). However, study results vary widely and await replication.
Functional connectivity, defined as the relation between the neuronal activation patterns of anatomically separated brain regions (Aertsen et al., 1989), describes the organization, inter-relationship and integrated performance of functionally coupled brain regions (Rogers et al., 2008). Most commonly, studies on functional connectivity describe the temporal correlation between two or more regions, or compare the spatial maps of resting-state networks. This latter measure examines the correspondence of the network’s time course and the time course of each voxel in a network (Balsters et al., 2013), thus providing a measure of a region’s strength of connectivity within a given network. The literature on cannabis use mostly describes increased functional connectivity in adult or adolescent cannabis users compared to controls in (regions of) the default mode network (DMN), salience network, and executive control network (ECN) (Cheng et al., 2014; Filbey et al., 2014; Harding et al., 2012; Houck et al., 2013; Pujol et al., 2014). However, more recently, several studies have reported negative associations between cannabis use and functional connectivity (Camchong et al., 2016; Orr et al., 2013; Peters et al., 2015).
Alcohol use has also been shown to be associated with functional connectivity. Both weaker and stronger functional connectivity have been reported in the DMN, salience network, subcortical reward network and ECN (Müller-Oehring et al., 2014; Weiland et al., 2014; Zhu et al., 2017), as well as stronger functional connectivity in the left frontoparietal network (Jansen et al., 2015) basal ganglia network and primary visual network (Weilandt et al., 2014). Moreover, disturbances in frontoparietal connectivity have been observed even in substance-naïve youth with a family history of alcohol (Wetherill et al., 2012), which suggest that weaker frontoparietal connectivity may be a neurobiological marker for alcohol use disorders. Thus, both alcohol and cannabis use appear to be associated to functional connectivity, however, studies are inconclusive whether associations are positive or negative.
Although described less frequently, functional connectivity can also be examined by measuring time course power spectra. The MR signal is dominated by oscillations in the 0.0–0.1 Hz frequency band (Cordes et al., 2001). The magnitude of these oscillations may differ per brain region and per person, and thus can be examined as a marker of individual differences. The time course power spectra reflect the degree of fluctuation in amplitude of the intrinsic activity within the network (Calhoun et al., 2011). The time courses of the different networks can also be correlated, resulting in a measure called functional network connectivity (FNC, Arbabshirani et al., 2013). The present study will examine associations between alcohol and cannabis use and network spatial map, network time course power spectra and functional network connectivity.
Possibly due to relatively small sample sizes, studies examining substance use and functional connectivity mostly describe associations between a few regions or networks only rather than a comprehensive whole-brain analysis. Moreover, studies that do perform whole-brain analyses generally only assess differences in network spatial maps. The present study describes the results of a dose-response analysis of the association between duration of regular (comorbid) cannabis and alcohol use and whole-brain resting-state functional connectivity in a large sample of male juvenile delinquents, whom due to their imprisonment were abstinent. The majority of the sample has been dependent on cannabis or alcohol, many on both. We therefore expected that cannabis and alcohol use would be associated with widespread differences in functional connectivity despite current abstinence. In the spatial map domain, both cannabis and alcohol use are expected to affect the DMN, salience network, and ECN. Moreover, we hypothesize alcohol to be associated with frontoparietal network connectivity. However, as time course power spectra and between network connectivity are less frequently studied, we have no specific hypotheses regarding these domains.
2. Methods
2.1. Participants
The present sample was drawn from the NIMH-funded Southwest Advanced Neuroimaging Cohort, Youth sample (SWANC-Y), collected between June 2007, and May 2011 in a maximum-security facility in New Mexico and from ongoing (2011–15) research at a youth detention facility in Wisconsin. This research was approved by the University of New Mexico Human Research Review Committee. Both youth and parent/guardian provided written informed assent/consent. Participants were compensated comparable to the pay for general labor work in the facilities. Participants were excluded from participation if they had a history of seizures, psychosis, traumatic brain injury, other major medical problems, or failed to show fluency in English at or above a grade four reading level. Resting-state scans, information on duration of substance use, and Psychopathy Checklist-Youth Version. (PCL-YV) scores were available from n = 227 male adolescents. Nine youth were excluded for excessive motion, and seventeen were determined to meet the above exclusion criteria after scanning (MRI incidental findings of trauma or supplemental file review). The final sample consisted of n = 201 participants. Associations between drug use and functional connectivity were tested for complete cases only (n = 180 for duration analyses, and n = 167 for supplemental analyses on substance dependence). Demographic information on the participants is provided in Table 1.
Table 1.
Sample characteristics.
| n | M(SD)/n(%) | M(SD)/n(%) complete cases duration (n=180) | M(SD)/n(%) complete cases dependence (n=167) |
M(SD)/n(%) complete cases dependence Cannabis dependent (n=130) |
M(SD)/n(%) complete cases dependence Not cannabis dependent (n = 47) |
M(SD)/n(%) complete cases dependence Alcohol dependent (n=89) |
M(SD)/n(%) complete cases dependence Not alcohol dependent (n = 78) |
|
|---|---|---|---|---|---|---|---|---|
| Age | 201 | 17.19 (1.14) | 17.18 (1.13) | 17.19 (1.13) | 17.31 (1.09)a | 16.90 (1.19)b | 17.44 (1.06)c | 16.91 (1.16)d |
| IQ | 168 | 90.56 (13.22) | 90.79 (13.68) | 90.45 (13.71) | 91.36 (13.55)a | 88.13 (13.97)a | 92.35 (11.52)c | 88.28 (15.63)c |
| Handedness§ | 164 | |||||||
| Left | 16 (9.76) | 15 (10.27) | 14 (10.22) | 12 (11.32) | 2 (6.45) | 2 (2.32) | 4 (7.84) | |
| Right | 145 (88.41) | 128 (87.67) | 120 (87.59) | 93 (87.73) | 27 (87.10) | 74 (86.05) | 46 (90.20) | |
| Ambidextrous | 3 (1.83) | 3 (2.05) | 3 (2.19) | 1 (0.94) | 2 (6.45 | 10 (11.63) | 1 (1.96) | |
| PCL-YV | 201 | |||||||
| Total score | 25.02 (6.15) | 25.00 (6.12) | 25.05 (6.21) | 25.04 (5.69)a | 25.06 (7.44)a | 25.01 (5.28)c | 25.10 (7.16)c | |
| Factor 1 | 7.49 (3.29) | 7.41 (3.21) | 7.37 (3.20) | 7.28 (3.04)a | 7.61 (3.61)a | 7.08 (2.79)c | 7.71 (3.61)c | |
| Factor 2 | 15.10 (2.89) | 15.13 (2.91) | 15.17 (2.95) | 15.27 (2.73)a | 14.89 (3.47)a | 15.31 (2.67)c | 15.01 (3.26)c | |
| Smoker | 200 | 138 (69.00) | 123 (67.58) | 117 (70.06) | 91 (75.83)a | 26 (55.32)b | 73 (82.02)c | 44 (56.41)d |
| Mood disorder (current) | 141 | 6 (4.26) | 6 (4.65) | 6 (4.69) | 4 (4.04)a | 2 (6.67)a | 3 (3.70)c | 3 (6.38)c |
| Anxiety disorder (current) | 149 | 11 (7.38) | 11 (8.09) | 11 (8.21) | 9 (8.65)a | 2 (6.67)a | 8 (9.30)c | 3 (6.25)c |
| Cannabis abuse (lifetime) | 181 | 160 (88.95) | 147 (89.02) | 148 (89.70) | 120 (100.00)a | 29 (61.70)b | 88 (98.88)c | 59 (75.64)d |
| Cannabis dependent (lifetime) | 181 | 120 (66.30) | 118 (71.42) | 120 (71.86) | 120 (100.00)a | 0 (0.00)b | 78 (87.64)c | 42 (53.85)d |
| Duration of cannabis use (months) | 196 | 42.83 (31.37) | 42.24 (31.65) | 43.57 (31.72) | 52.35 (28.67)a | 21.53 (28.34)b | 55.67 (29.88)c | 29.39 (27.84)d |
| Alcohol abuse (lifetime) | 181 | 129 (67.96) | 117 (71.34) | 118 (71.52) | 94 (78.33)a | 23 (48.94)b | 89 (100.00)c | 78 (100.00)d |
| Alcohol dependent (lifetime) | 181 | 96 (53.03) | 89 (53.94) | 89 (53.29) | 78 (65.00)a | 11 (23.40)b | 89 (100.00)c | 78 (100.00)d |
| Duration of alcohol use (months) | 198 | 23.06 (28.19) | 20.83 (26.54) | 20.86 (26.32) | 25.52 (27.86)a | 8.96 (17.08)b | 29.62 (25.59)c | 10.86 (23.58)d |
| Comorbid cannabis and alcohol dependency (lifetime) | 181 | 84 (46.40) | 78 (46.70) | 78 (46.70) | 78 (65.00)a | 0 (0.00)b | 78 (87.64)c | 0 (0.00)d |
Note: In case of categorical variables, numbers represent n(%). Significance was tested using crosstabs. In case of continuous variables, numbers represent M(SD). Significance was examined using independent sample t-tests.Values that do not share the same subscript (a,b for cannabis dependence and c,d for alcohol dependence) are significantly different (p < .05).
Difference could not be tested due to too little left-handed and ambidextrous participants.
2.2. Measures
2.2.1. Substance use
Trained researchers administered the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) (Kaufman et al., 1997). KSADS alcohol and drug use data was supplemented with questionnaires that asked youth how many months they used regularly (3 or more times/week). For 19 participants, only information on time of usage (at any frequency) was available. Of the 181 participants with available KSADS data, 131 (72%) once were cannabis dependent and 96 (53%) once were alcohol dependent. Eighty-four participants (46%) once were cannabis as well as alcohol dependent. Mean months of usage was mean (μ) = 42.83, standard deviation (σ) = 31.37 for cannabis and mean (μ) = 23.06, standard deviation (σ) = 28.19 for alcohol. Due to skew, the duration of alcohol use was log transformed. Cannabis and alcohol duration were strongly correlated, r = 0.48, p < 0.001. Cannabis dependence and cannabis duration were moderately correlated, r = 0.41, p < 0.001. Alcohol dependence and alcohol duration had a correlation of r = 0.45, p < 0.001. All participants were in forced abstinence for at least 30 days, many for at least 6 months. Unfortunately, no information on length of abstinence is available.
2.2.2. Callous/Unemotional traits
We assessed callous/unemotional traits (i.e., youth psychopathy) using the Hare PCL-YV (Forth et al., 2003). The PCL-YV includes a review of institutional records and a semi-structured interview regarding individuals’ school, family, work, and criminal histories, and their interpersonal and emotional skills. We examined a two-factor model of psychopathic traits in addition to a Total PCL-YV score, with Factor 1 composed of interpersonal and affective traits, and Factor 2 composed of lifestyle and antisocial traits.
2.2.3. IQ
IQ was estimated from the Vocabulary and Matrix Reasoning sub-tests of the Wechsler Adult Intelligence Scale-Third Edition for participants older than 16 years of age, and from the Wechsler Intelligence Scale for Children–Fourth Edition for participants younger than 16 years of age (Wechsler, 1997, 2003).
2.2.4. Imaging data
All participants were scanned at the maximum-security facilities using The Mind Research Network’s 1.5 T Avanto SQ Mobile MRI scanner. The EPI gradient-echo pulse sequence (TR/TE 2000/39 ms, flip angle 90°, FOV 24 × 24 cm, 64 × 64 matrix, 3.4 × 3.4 mm in-plane resolution, 5 mm slice thickness, 30 slices) effectively covered the entire brain (150 mm) in 2.0 s. Head motion was minimized using padding and restraint. During the 5-min rest scan the participant was asked to look at the fixation cross hair and keep eyes open. Participants were monitored by video.
2.3. Data analysis
2.3.1. Preprocessing
EPI data were preprocessed using a custom SPM pipeline (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). The first four volumes are discarded to remove T1 equilibration effects. To correct residual head motion, “bad” images (confounded by motion or radio-frequency spikes) were estimated and removed using ART-Repair (Mazaika et al., 2007). These images were determined by calculating the mean intensity for a given time series and identifying individual images whose intensity was greater than four standard deviations from the mean. The offending image(s) were replaced in the time series by a rolling mean image, and regressed in the statistical model. Images were motion-corrected using INRIalign (Freire and Mangin, 2001; Freire et al., 2002). Data were spatially normalized into the MNI space and re-sampled into 3 × 3 x 3 mm voxels, resulting in 53 × 63 × 46 voxels. Next, the data were spatially smoothed with a six mm full width at half-maximum Gaussian kernel. The MRI coordinates were converted to the Talairach and Tournoux standard space to assist with anatomical labeling. However, all (x,y,z) coordinates listed in the manuscript are in Montreal Neurological Institute (MNI), the default coordinate system in SPM.
2.3.2. Independent component analysis
Following preprocessing, a group independent component analysis (ICA) was performed (Calhoun et al., 2001; Calhoun and Adali, 2012). EPI time series data for all participants were compressed using principal component analysis (PCA). There were two PCA data reduction stages, which reduced the impact of noise and made the estimation computationally tractable (Calhoun et al., 2009; Erhardt et al., 2011; Schmithorst and Holland, 2004). The first data reduction stage was set to 45 components. The final dimensionality/number of components was 30. The data reduction was followed by a group spatial ICA, resulting in the 3nal estimation of our independent components (ICs) using the infomax algorithm (Bell and Sejnowski, 1995; Calhoun et al., 2001). From the group spatial ICA, we reconstructed spatial maps and their corresponding ICA time courses that represented the spatial and temporal characteristics of each component and subject using group ICA (GICA) (Erhardt et al., 2011). These maps and time courses were then inspected to determine which components reflected plausible non-artifact networks. ICs that depicted peak cluster locations in gray matter with minimal overlap with white matter, ventricles and edges of the brain and also exhibit higher low frequency temporal activity were retained for further analysis. A total of 15 components was retained after visual inspection.
2.3.3. Statistical analyses
Associations between drug use and functional connectivity were tested for complete cases only (n = 180 for duration analyses and n = 167 for supplemental analyses on substance dependence) using the Mancovan toolbox implemented in GIFT (Allen et al., 2011). We examined three connectivity measures: component spatial maps, component time course power spectra, and between component FNC (Jafri et al., 2008). For each measure, a multivariate selection strategy was first performed in order to identify potential significant associations between component measures and variables of interest. For these analyses, separate response vectors (e.g., spectral power) are considered as a whole. FNC timecourses were despiked and temporally bandpass filtered (0.01 Hz-0.15 Hz) followed by calculation of the among network connectivity matrices. The initial design matrix included duration of cannabis and alcohol use as predictors, and psychopathy scores, age, smoking, and IQ as covariates. As many participants used both cannabis and alcohol, analyses on cannabis use were corrected for alcohol use and vice versa. Head movement estimates were included as nuisance regressors (Allen et al., 2011), defined as the average of translation and rotation parameters. However, as model results were similar, we only report the model excluding motion parameters. In a second analysis, cannabis and alcohol dependency were examined. These analyses, as well as cannabis use analyses (both duration, and dependency models) uncorrected for alcohol use and vice versa can be found in the Supplemental Materials1 (Figs. S4 t/m S9). Once we retained the covariates of interest in the final model, we performed univariate tests to understand the nature and extent of the relationships between these variables and a given component property (e.g., spectral power). An alpha level of 0.05 was used for all analyses. Results were corrected for multiple comparisons using false discovery rate (FDR) (Genovese et al., 2002). FDR correction only considered the networks which showed a significant effect and corrects for each column of the response data matrix (i.e., voxels for spatial maps, spectral bins for power spectra).
3. Results
We performed a 30 component GICA using resting-state functional MRI (rs fMRI) data from 201 participants. Based on visual inspection of the spatial maps and power spectra, 15 components were identified as ventricular, vascular, susceptibility or motion-related artifacts (Fig. S1). The 15 remaining components corresponded to known resting-state networks and are shown in Fig. S2. Coordinates of their peak activation are provided in Table S1. Fig. S3 shows the FNC. The ICA parcellation resulted in similar networks as reports in typical samples (Beckmann et al., 2005; Calhoun et al., 2008).
3.1. Duration of cannabis and alcohol use
3.1.1. Inter-network connectivity
3.1.1.1. Spatial maps
There were no significant associations between cannabis use and network spatial maps, nor were there significant associations between alcohol use and network spatial maps
3.1.1.2. Power spectra
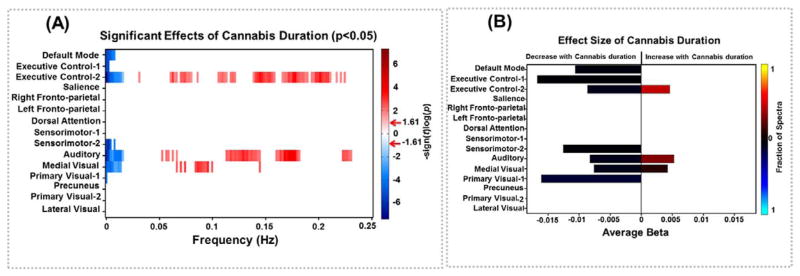
In order to analyze the power spectra results, we first identified components that showed an association to a particular covariate (for example, alcohol or cannabis duration). Next, we explored whether the associations were related to any particular frequency band (lower range, both lower and higher range, or higher range). Once we identified the dominating frequencies showing drug-related associations, we then analyzed those particular frequencies of the power spectra. Fig. 1 shows the association between duration of cannabis use and component time-course power spectra. Duration of cannabis use was associated with power spectra of the DMN (component 5, multivariate p = 0.008), the ECNs (component 19, 30, multivariate p = 0.002 and p = 0.001), sensorimotor network (component 10, multivariate p = 0.001), auditory network (component 15, multivariate p < 0.001), a medial visual network (component 2, multivariate p = 0.02), and a primary visual network (component 7, multivariate p = 0.04). For all networks, longer duration of cannabis use was associated with decreased amplitude in the lower frequencies (0.00-0.05) which typically dominate the fMRI signal (Cordes et al., 2001). For one of the ECNs (component 30), the auditory (component 15), and medial visual network (component 2), longer duration of cannabis use was associated with increased amplitude at higher frequencies (0.05–0.20). Results of the cannabis dependence analysis were highly similar, see Fig. S6.
Fig. 1.
Univariate test results showing the association between cannabis duration and power spectra. Univariate tests were performed only on covariates of interest retained in the reduced MANCOVA model. Left panel (A) depicts the significance and direction of cannabis duration as a function of frequency for each component, displayed as - sign(t)log10(p). Red arrows on the color bar designate the FDR-corrected threshold (α = 0.05). Right panel (B) shows bar plots of the average β-values for cannabis duration term. β -Values were averaged over frequency bands with associations of the same directionality where test statistics exceeded the FDR threshold. The color of the bar is proportional to the fraction of the contributing frequency bins; the absence of a bar indicates that either univariate tests were not performed or test
Fig. 2 shows the association between duration of alcohol use and component time course power spectra. Alcohol use was associated with power spectra of the right fronto-parietal network (component 13, multivariate p < 0.001), salience network (component 3, multivariate p = 0.001), dorsal attention network (component 4, multivariate p = 0.004), a sensorimotor network (component 1, multivariate p = 0.008), and a lateral visual network (component 21, multivariate p = 0.008). For all networks, longer duration of alcohol use was associated with decreased amplitude in lower frequencies (0.00–0.05). Only for the lateral visual network (component 21), a longer duration of alcohol use was associated with greater amplitude in higher frequencies. As can be seen from the plot showing the effect sizes of alcohol duration (Fig. 2b), the negative associations with spectral power are slightly more significant in the salience and sensorimotor networks, however the associations are fairly consistent over all networks suggesting that these trends are fairly global across networks. Results of the alcohol dependence analysis were highly similar, see Fig. S7.
Fig. 2.
Univariate test results showing the association between alcohol duration and power spectra. Univariate tests were performed only on covariates of interest retained in the reduced MANCOVA model. Left panel (A) depicts the significance and direction of alcohol duration as a function of frequency for each component, displayed as - sign(t)log10(p). Red arrows on the color bar designate the FDR-corrected threshold (α = 0.05). Right panel (B) shows bar plots of the average β-values for alcohol duration term. β -Values were averaged over frequency bands with associations of the same directionality where test statistics exceeded the FDR threshold. The color of the bar is proportional to the fraction of contributing the frequency bins; the absence of a bar indicates that either univariate tests were not performed or test statistics were not significant. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1.2. Intra-Network connectivity
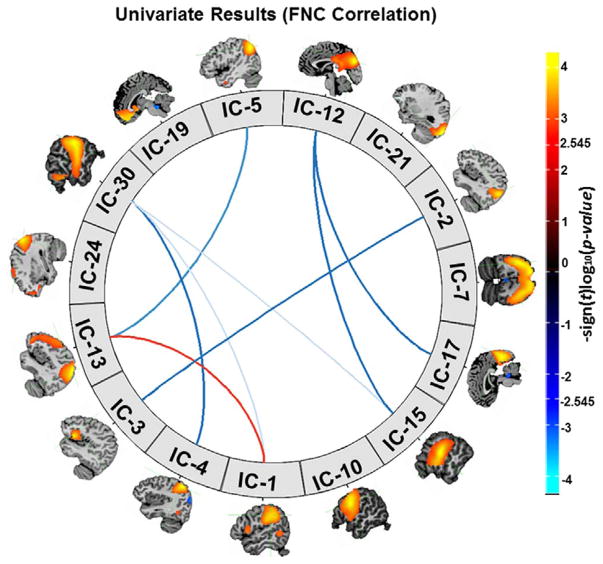
The association between cannabis use and FNC is shown in Fig. 3. Longer duration of cannabis use was associated with decreased network connectivity between the ECN (component 30), and the auditory network (component 15), a sensorimotor network (component 1), and the dorsal attention network (component 4). Moreover, we found a negative association between duration of cannabis use and network connectivity between the DMN (component 5), and the right fronto-parietal network (component 13), and between the salience network (component 3) and the medial visual network (component 2). Duration of cannabis use was negatively associated with connectivity between the precuneus network (component 12) and the auditory network (component 15), and a primary visual network (component 17). Finally, a longer duration of cannabis use was associated with increased connectivity between the right fronto-parietal network (component 13) and one of the sensorimotor network (component 1).
Fig. 3.
Univariate test results showing the association between cannabis use and functional network connectivity (FNC). Figure depicts the significance and direction of the cannabis duration term for each pairwise correlation, displaying as the - sign(t)log10(p). The FDR-corrected threshold (α = 0.05) on the colorbar is represented by the values 2.545 and -2.545.
4. Discussion
The present study examined the association between duration of cannabis and alcohol use and resting-state functional connectivity in a large sample of male juvenile delinquents. The majority of participants have met criteria for cannabis and/or alcohol dependence. However, due to their detention, all participants were in forced abstinence. Although hypothesized, no associations with network spatial maps were found. However, cannabis and alcohol use had widespread yet distinct associations with network time course spectral power that were independent of psychopathic traits (including antisocial behavior). Generally, cannabis and alcohol use was found to be associated with less low frequency power and more high frequency power. Less low frequency power and more high frequency power has previously been described in several psychological disorders, such as bipolar disorder, schizophrenia and Alzheimer’s disease (Calhoun, 2011; Ongur et al., 2010; Xi et al., 2012). The origin of rs-fMRI spectral power at different frequencies currently is not well understood. Moreover, due to the lack of a particular task, different participants may be performing different mental activities, making it difficult to characterize brain processes underlying neural activation. Nevertheless, the similarities between the present study and studies on psychiatric patients suggest that cannabis and alcohol use are associated with aberrant network functioning.
Corresponding to the literature on cannabis use and resting-state temporal correlation and network spatial maps, cannabis use was associated with the DMN (Bossong et al., 2013; Pujol et al., 2014; Wetherill et al., 2015), a network implicated in self-referential thought, social perspective taking, future thought, and memory (Andrews-Hanna, 2012), and the ECNs (Houck et al., 2013). Interestingly, Pujol et al. (2014) showed that the association between activation of the DMN and memory was stronger in young adult cannabis users compared to controls. The aberrant DMN connectivity in cannabis users may thus be involved in disruptions in memory performance previously reported in association to cannabis use. However, as we do not have data on neuropsychological functioning, this interpretation remains speculative. The only other study examining the association between cannabis dependence and resting-state power spectra analyzed the fractional amplitude of the low-frequency fluctuations (fALFF, Zou et al., 2008) (Orr et al., 2013). Instead of examining the total power spectrum, this method quantifies the amplitude of low frequency oscillations only. Moreover, this method looks for voxel-wise associations, whereas the present study examined the power spectra of previously calculated neural networks. Orr et al. (2013) reported increased fALFF in regions of the ECN in adolescent cannabis users. The authors also report decreased intrahemispheric connectivity and suggest that the increase in fALFF may be a compensatory mechanism for the altered intrahemispheric connectivity. The only other studies examining associations of prolonged substance use and resting-state power spectra examined effects of heroin and cocaine. Similar to our findings, these studies did report decreased ALFF in substance users compared to controls in regions of the DMN (Ide et al., 2014; Jiang et al., 2011; Wang et al., 2007).
In addition to associations in the DMN and ECNs, we found associations between cannabis use and network time course power spectrum in several sensory networks (auditory, visual and sensorimotor). Interestingly, prolonged cannabis use has been shown to directly affect the retina (Zobor et al., 2015), and a positron emission tomography (PET) study on the acute effects of smoking marijuana reported decreased regional cerebral blood flow in sensorimotor, auditory, and visual regions (O’Leary et al., 2000). Moreover, a recent study on the effects of cannabis on tinnitus in rodents shows that cannabis may increase tinnitus, and suggests that CB1 receptors in the cochlear nucleus may be important for auditory function (Zheng et al., 2015a, 2015b). Repeated administration of THC may result in distorted activation in sensory systems even in the brain at rest.
Duration of alcohol use was associated with the right frontoparietal network (Jansen et al., 2015; Jansen et al., 2015; Wetherhill et al., 2012), the salience network (Muller-Oehring et al., 2014; Sullivan et al., 2013; Zhu et al., 2017), the ECN (Muller-Oehring et al., 2014; Zhu et al., 2017), the dorsal attention network (Muller-Oehring et al., 2014), a sensorimotor network, and a visual network (Weiland et al., 2014). These networks are involved in attention (frontoparietal network, dorsal attention network), cognitive control (ECN, frontoparietal network), and salience attribution (salience network). In recovering alcoholics, aberrant connectivity within the salience network has been associated with poorer visuospatial and verbal working memory, while connectivity of the attention network has been related to decreased depressive symptoms (Muller-Oehring et al., 2014). The only study examining the association between alcohol use and resting state power spectra, suggests that acute alcohol intake is associated with a mixed pattern of increased and decreased ALFF (Zheng et al., 2015a, 2015b), but with decreased ALFF in regions of the ECN. However, effects of acute and chronic alcohol and substance use cannot be one on one compared (Crean et al., 2011; Schulz et al., 1980).
Both cannabis and alcohol use were associated with less low frequency power and more high frequency power. Such a pattern of resting-state brain activity has previously been described in several psychological disorders, such as bipolar disorder, schizophrenia and Alzheimer’s disease (Calhoun, 2011; Ongur et al., 2010; Xi et al., 2012), and may point towards more rapid connectivity, and a lower degree of interconnection between the regions in the associated networks and other brain regions (Garrity et al., 2007). Although we excluded participants suffering from psychosis, and we controlled for psychopathic traits, still a similar pattern of resting-state activation was found between the present study and studies of psychopathology. One way of interpreting these similarities in power spectra is that the use of cannabis and alcohol may make the adolescent more vulnerable to psychiatric disorders. Alternatively, the similarities may be explained by comorbid cognitive deficits, or by psychiatric symptoms not controlled for in our analyses.
Other than associations in the power spectra domain, our results suggest that longer duration of cannabis use is associated with decreased inter network connectivity. Strongest inter network associations were found for the ECN, for which we also found evidence for aberrant within network connectivity. The exact neural mechanisms underlying functional (network) connectivity remain unclear. However, decreased inter network connectivity may point towards less effective communication between networks with longer cannabis use.
Despite abstinence, cannabis and alcohol use were associated with activation of the brain at rest. While some studies suggest that the effects of adolescent cannabis use decrease or disappear after prolonged periods of abstinence (Hanson et al., 2010; Jacobus et al., 2012), others suggest that cannabis use may have long-term effects on brain structure and function despite abstinence (Ashtari et al., 2011). To our knowledge, no study has examined associations of alcohol use and neural functioning in abstinent adolescents. However, animal literature suggests that adolescence limited binge drinking causes changes in brain structure that are observable still in adulthood (Coleman et al., 2014). Literature on adults suggests that alcohol use may have long-lasting effects on brain structure and function (Fortier et al., 2014; Fortier et al., 2014; Müller-Oehring et al., 2014), which may decrease over abstinence (Pfefferbaum et al., 2014; Segobin et al., 2014; van Eijk et al., 2013). Moreover, over four-to six-weeks of abstinence, Winward et al. (2014) reported some recovery in neurocognitive functioning in adolescent heavy drinkers. However, for most functions under investigation (e.g., prospective memory, cognitive switching and inhibition) heavy drinkers did not perform to levels of nondrinkers. Our results suggest that both alcohol and cannabis use may have prolonged effects on resting-state network frequency power despite forced abstinence.
As described above, previous studies on substance use and resting-state whole-brain functional connectivity generally study and report associations in network spatial maps (e.g., Houck et al., 2014; Zhu et al., 2017). However, our data suggest that, at least after abstinence, no such associations are present. The networks in which associations with power spectra were found do correspond well to the networks previously described to be related to cannabis and alcohol use. Our results, thus, could imply that associations of substance use with time course power spectra may be more substantial and/or enduring than associations previously found with network spatial maps. However, as no longitudinal data is available, this suggestion cannot be tested in the current data. Nevertheless, time course power spectra may provide a fruitful domain of resting-state data that may be studied concurrently with network spatial maps.
While this study has considerable strengths, such as the large sample size in a hard to reach at risk population, and the correction for baseline trait antisociality, several limitations should be noted. When comparing users to non-users, it is difficult to ascertain whether reported differences reflect pre-existing brain differences that have led individuals to substance use, or whether differences are the effect of substance use. Examining associations with duration of use rather than use versus non-use may provide some evidence for a causal link. However, we cannot fully discard the possibility of reversed causality. As is often the case, information on duration of drug use was based on self-report. Self-report relies on the truthfulness and memory accuracy of the respondent, which raises validity and reliability issues. All youth were in forced abstinence, but it is possible that some may still have procured drugs while incarcerated. However, the facilities performed regular drug tests in order to assure juveniles remained abstinent. Although this does not guarantee abstinence, the prospect of a drug test may have deterred youth from using alcohol or cannabis. Unfortunately, no data on length of abstinence was available. Several studies report that drug effects wear off after abstinence, thus, our results may change over time if youth are/are not abstinent. Moreover, only limited information on substance use was available. For example, age of substance use initiation has been shown to moderate the effect of substance use on brain structure/functioning (e.g., Weissman et al., 2015), and also daily or weekly frequency of use would provide information relevant to our analyses. However, this type of information unfortunately was not available. Moreover, many adolescents in the present sample used both cannabis and alcohol. Although analyses on duration of cannabis use were corrected for duration of alcohol use, we cannot rule out that some of our findings may be confounded by comorbid drug use. The present sample contains only boys. Due to the many differences between boys and girls in both substance use and brain structure and functioning (Kuhn, 2015; Mutlu et al., 2013; Wang et al., 2008), we believe it is a good strategy to examine boys and girls separately. However, it is currently unclear if and how our results correspond to girls. Our general impression was that participants were interested and motivated during the IQ assessment. However, some were more difficult. Unfortunately, we did not score their motivation, so we could not control our IQ scores for effects of disinterest. Finally, analyses were performed on a high-risk sample. By correcting for the effects of psychopathic traits, we aimed to control for confounding by antisocial behavior problems. However, as we did not include a healthy control group, we cannot ascertain our results can be extrapolated to low-risk youth in the general population.
5. Conclusion
Although no associations between cannabis and alcohol use and network spatial maps have been found, we have identified specific patterns of decreased coherent network activity (spectral power analysis) and FNC in relation to duration of cannabis and alcohol use. These findings may point towards less effective communication between brain regions/networks. As the analysis of spectral power is relatively uncommon in substance use research, our findings provide important information for hypothesis generation of future work using power spectra analyses of adolescent substance use related changes in abstinence. All in all, adolescent cannabis and alcohol use are associated with widespread differences in resting-state time course power spectra, which may persist after abstinence.
Supplementary Material
Acknowledgments
Role of funding source
Data collection was funded by NIMH; 1 R01 MH071896–01 (PI: Kiehl) and NICHD: 1R01HD082257-01 (PI: Kiehl). Thijssen was funded by a European Research Council grant (ERC AdG 669249) awarded to Marian J. Bakermans-Kranenburg.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2017.05.045.
Footnotes
* Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributions
Sandra Thijssen: conception, analysis, interpretation and writing
Barnaly Rashid: analysis
Shruti Gopal: analysis
Prashanth Nyalakanti: analysis
Vince D. Calhoun: analysis, interpretation
Kent A. Kiehl: conception, analysis, interpretation
All authors approved of the final manuscript.
Conflict of interest
No conflict declared.
References
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation - modulation of effective connectivity. J Neurophysiol. 1989;61:900–917. doi: 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. http://dx.doi.org/10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. http://dx.doi.org/10.1177/1073858411403316.1073858411403316 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbabshirani MR, Havlicek M, Kiehl KA, Pearlson GD, Calhoun VD. Functional network connectivity during rest and task conditions: A comparative study. Hum Brain Mapp. 2013;34:2959–2971. doi: 10.1002/hbm.22118. http://dx.doi.org/10.1002/hbm.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. http://dx.doi.org/10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, O’Connell RG, Galli A, Nolan H, Greco E, Kilcullen SM, Bokde AL, Lai R, Upton N, Robertson IH. Changes in resting connectivity with age: a simultaneous electroencephalogram and functional magnetic resonance imaging investigation. Neurobiol Aging. 2013;34:2194–2207. doi: 10.1016/j.neurobiolaging.2013.03.004. http://dx.doi.org/10.1016/j.neurobiolaging.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. http://dx.doi.org/10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous can-nabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. http://dx.doi.org/10.1016/j.pneurobio.2010.06.010.S0301-0082(10)00131-0 [pii] [DOI] [PubMed] [Google Scholar]
- Bossong MG, Jansma JM, van Hell HH, Jager G, Kahn RS, Ramsey NF. Default mode network in the effects of Delta9-Tetrahydrocannabinol (THC) on human executive function. PLoS One. 2013;8:e70074. doi: 10.1371/journal.pone.0070074. http://dx.doi.org/10.1371/journal.pone.0070074.PONE-D-13-03816 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchy L, Cannon TD, Anticevic A, Lyngberg K, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Bearden CE, Mathalon DH, Addington J. Evaluating the impact of cannabis use on thalamic connectivity in youth at clinical high risk of psychosis. BMC Psychiatry. 2015;15:276. doi: 10.1186/s12888-015-0656-x. http://dx.doi.org/10.1186/s12888-015-0656-x. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. http://dx.doi.org/10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson G, Pekar J. A method for making group inferences using independent component analysis of functional MRI data: exploring the visual system. Neuroimage. 2001;13:S88–S88. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. http://dx.doi.org/10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. doi: 10.3389/fpsyt.2011.00075. http://dx.doi.org/10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V. Intrinsic functional and structural networks in schizophrenia bipolar disorder, and healthy controls. Biol Psychiatry. 2011;69:119s–119s. [Google Scholar]
- Camchong J, Lim KO, Kumra S. Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb Cortex. 2016;27:1922–1930. doi: 10.1093/cercor/bhw015. bhw015 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, O’Donnell BF, Hetrick WP, Newman SD. Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users - a multi-voxel pattern analysis. J Psychopharmacol. 2014;28:1030–1040. doi: 10.1177/0269881114550354. http://dx.doi.org/10.1177/0269881114550354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010 doi: 10.3389/fpsyg.2010.00225. http://dx.doi.org/10.3389/fpsyg.2010.00225. 1 Artn 225. [DOI] [PMC free article] [PubMed]
- Coleman LG, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. http://dx.doi.org/10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in resting-state data. Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. http://dx.doi.org/10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. http://dx.doi.org/10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. http://dx.doi.org/10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. http://dx.doi.org/10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. http://dx.doi.org/10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Kosson D, Hare RD. The Hare Psychopathy Checklist: Youth Version. Multi-Health Systems; New York, N.Y: 2003. [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Lindemer E, Maksimovskiy AL, Shepel J, Williams V, Venne JR, Milberg WP, McGlinchey RE. Widespread effects of alcohol on white matter microstructure. Alcohol Clin Exp Res. 2014;38:2925–2933. doi: 10.1111/acer.12568. http://dx.doi.org/10.1111/acer.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. http://dx.doi.org/10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imag. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. http://dx.doi.org/10.1109/Tmi.2002.1009383. Pii S0278-0062(02)05532-5. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. http://dx.doi.org/10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RLM. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neurosci. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. http://dx.doi.org/10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. http://dx.doi.org/10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. http://dx.doi.org/10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takag M, Lorenzetti V, Lubman DI, Seal ML, Pantelis C, Yucel M. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacol. 2012;37:1923–1933. doi: 10.1038/npp.2012.39. http://dx.doi.org/10.1038/Npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–319. doi: 10.1080/09595230701247772. http://dx.doi.org/10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hiller-Sturmhofel S, Swartzwelder HS. Alcohol’s effects on the adolescent brain - what can be learned from animal models. Alcohol Res Health. 2004;28:213–221. [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. http://dx.doi.org/10.1038/mp.2011.82.mp201182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck JM, Bryan AD, Ewing SWF. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. 2013;39:414–423. doi: 10.3109/00952990.2013.837914. http://dx.doi.org/10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014 doi: 10.1016/j.drugalcdep.2013.09.004. http://dx.doi.org/10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. http://dx.doi.org/10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacol (Berl) 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. http://dx.doi.org/10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. http://dx.doi.org/10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. http://dx.doi.org/10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, van Wingen G, van den Brink W, Goudriaan AE. Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur Neuropsychopharmacol. 2015;25:2230–2239. doi: 10.1016/j.euroneuro.2015.09.019. http://dx.doi.org/10.1016/j.euroneuro.2015.09.019.S0924-977X(15)00321-1 [pii] [DOI] [PubMed] [Google Scholar]
- Jiang GH, Qiu YW, Zhang XL, Han LJ, Lv XF, Li LM, Lin CL, Zhuo FZ, Hu SY, Tian JZ. Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. Neuroimage. 2011;57:149–154. doi: 10.1016/j.neuroimage.2011.04.004. http://dx.doi.org/10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Hawkins J, Harris WA, Lowry R, Olsen EO, McManus T, Chyen D, Whittle L, Taylor E, Demissie Z, Brener N, Thornton J, Moore J, Zaza S. Youth risk behavior surveillance - United States, 2013. MMWR Surveill Summ. 2014;63:1–168. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. http://dx.doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kuhn DC. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol Ther. 2015;153:55–78. doi: 10.1016/j.pharmthera.2015.06.003. http://dx.doi.org/10.1016/j.pharmthera.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict Behav. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. http://dx.doi.org/10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39(6):345–355. doi: 10.3109/00952990.2013.837057. http://dx.doi.org/10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The resting brain of alcoholics. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu134. http://dx.doi.org/10.1093/cercor/bhu134.bhu134 [pii] [DOI] [PMC free article] [PubMed]
- Mutlu AK, Schneider M, Debbane M, Badoud D, Eliez S, Schaer M. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013;82:200–207. doi: 10.1016/j.neuroimage.2013.05.076. http://dx.doi.org/10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res Neuroimag. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. http://dx.doi.org/10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Block RI, Flaum M, Schultz SK, Ponto LLB, Watkins GL, Hurtig RR, Andreasen NC, Hichwa RD. Acute marijuana effects on rCBF and cognition: a PET study. Neurorep. 2000;11:3835–3841. doi: 10.1097/00001756-200011270-00047. http://dx.doi.org/10.1097/00001756-200011270-00047. [DOI] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res Neuroimag. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. http://dx.doi.org/10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, Kelly C, Weierstall K, Watts R, Smyth B, Garavan H. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39:372–381. doi: 10.3109/00952990.2013.848213. http://dx.doi.org/10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- Paul SM. Alcohol-sensitive GABA receptors and alcohol antagonists. Proc Natl Acad Sci U S A. 2006;103:8307–8308. doi: 10.1073/pnas.0602862103. http://dx.doi.org/10.1073/pnas.0602862103.0602862103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinol. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. S0306-4530(15)00018-9 [pii] [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu WW, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1:202–212. doi: 10.1016/S2215-0366(14)70301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. http://dx.doi.org/10.1016/S0376-8716(02)00334-4. Pii S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, Crippa JA, Fagundo AB, Deus J, de la Torre R, Nogué S, Farré M, Torrens M, Martin-Santos R. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. http://dx.doi.org/10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imag. 2008;26:146–146. doi: 10.1016/j.mri.2007.06.002. http://dx.doi.org/10.1016/j.mri.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Comparison of three methods for generating group statistical inferences from independent component analysis of functional magnetic resonance imaging data. J Magn Reson Imag. 2004;19:365–368. doi: 10.1002/jmri.20009. http://dx.doi.org/10.1002/Jmri.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Wüster M, Duka T, Herz A. Acute and chronic ethanol treatment changes endorphin levels in brain and pituitary. Psychopharmacol (Berl) 1980;68:221–227. doi: 10.1007/BF00428107. http://dx.doi.org/10.1007/BF00428107. [DOI] [PubMed] [Google Scholar]
- Segobin SH, Chetelat G, Le Berre AP, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel AL. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38:739–748. doi: 10.1111/acer.12300. http://dx.doi.org/10.1111/acer.12300. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. http://dx.doi.org/10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. http://dx.doi.org/10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, Rohlfing T, Pfefferbaum A. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry. 2013;74:547–555. doi: 10.1016/j.biopsych.2013.02.026. http://dx.doi.org/10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106:2195–2203. doi: 10.1111/j.1360-0443.2011.03574.x. http://dx.doi.org/10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacol (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. http://dx.doi.org/10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zhong Q, Zang Y. Gender effect on functional networks in resting brain. In: Gao X, Müller H, Loomes MJ, Comley R, Luo S, editors. Medical Imaging and Informatics: 2nd International Conference, MIMI 2007; Berlin, Heidelberg, Beijing, China. August 14–16, 2007; Berlin Heidelberg: Springer; 2008. pp. 160–168. Revised Selected Papers. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Psychological Corporation; New York: 1997. [Google Scholar]
- Wechsler D. Wechlser Intelligence Scale for Children. 4. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, Mayer AR, Hutchison KE. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res. 2014;38:2445–2453. doi: 10.1111/acer.12505. http://dx.doi.org/10.1111/acer.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Schriber RA, Fassbender C, Atherton O, Krafft C, Robins RW, Hastings PD, Guyer AE. Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev Cogn Neurosci. 2015;16:121–129. doi: 10.1016/j.dcn.2015.07.002. http://dx.doi.org/10.1016/j.dcn.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-nave youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. http://dx.doi.org/10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–123. doi: 10.1016/j.drugalcdep.2015.05.046. http://dx.doi.org/10.1016/j.drugalcdep.2015.05.046. S0376-8716(15)00308-7[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Bekman NM, Tapert SF, Brown SA. Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. J Int Neuropsychol Soc. 2014;20:218–229. doi: 10.1017/S1355617713001410. http://dx.doi.org/10.1017/S1355617713001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Zhao XH, Wang PJ, Guo QH, Yan CG, He Y. Functional MRI study of mild Alzheimer’s disease using amplitude of low frequency fluctuation analysis. Chin Med J. 2012;125:858–862. http://dx.doi.org/10.3760/cma.j.issn.0366-6999.2012.05.023. [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Velakoulis D, Wong MTH, Wood SJ, Condello A, Brewer W, Pantelis C. Structural brain correlates of alcohol and cannabis use in recreational users. Act Neuropsychiatr. 2006;18:226–229. doi: 10.1111/j.1601-5215.2006.00154.x. http://dx.doi.org/10.1111/j.1601-5215.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Kong L, Chen L, Zhang H, Zheng W. Acute effects of alcohol on the human brain: a resting-state fmri study. BioMed Res Int. 2015a;94752:9. doi: 10.1155/2015/947529. http://dx.doi.org/10.1155/2015/947529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Reid P, Smith PF. Cannabinoid cb1 receptor agonists do not decrease, but may increase acoustic trauma-induced tinnitus in rats. Front Neurol. 2015b;6:60. doi: 10.3389/fneur.2015.00060. http://dx.doi.org/10.3389/fneur.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol. 2017;22:206–217. doi: 10.1111/adb.12272. http://dx.doi.org/10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobor D, Strasser T, Zobor G, Schober F, Messias A, Strauss O, Batra A, Zrenner E. Ophthalmological assessment of cannabis-induced persisting perception disorder: is there a direct retinal effect? Doc Ophthalmol. 2015;130:121–130. doi: 10.1007/s10633-015-9481-2. http://dx.doi.org/10.1007/s10633-015-9481-2. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang TF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Method. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. http://dx.doi.org/10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res. 2013;37:67–74. doi: 10.1111/j.1530-0277.2012.01853.x. http://dx.doi.org/10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.