Abstract
Study Design
Retrospective analysis.
Objective
To determine the epidemiology and prognostic indicators in patients with chondrosarcoma of the osseous spine.
Summary of Background Data
Chondrosarcoma of the spine is rare, with limited data on its epidemiology, clinicopathologic features, and treatment outcomes. Therapy centers on complete en bloc resection with radiotherapy reserved for subtotal resection or advanced disease.
Methods
The Surveillance, Epidemiology, and End Results Registry was queried for patients with chondrosarcoma of the osseous spine from 1973 to 2012. Study variables included age, sex, race, year of diagnosis, size, grade, extent of disease, and treatment modality.
Results
The search identified 973 cases of spinal chondrosarcoma. Mean age at diagnosis was 51.6 years, and 627% of patients were males. Surgical resection and radiotherapy were performed in 75.2% and 21.3% of cases, respectively. Kaplan-Meier analysis demonstrated overall survival (OS) and disease-specific survival (DSS) of 53% and 64%, respectively, at 5 years. Multivariate Cox regression analysis showed that age (OS, P < 0.001; DSS, P = 0.007), grade (OS, P < 0.001; DSS, P < 0.001), surgical resection (OS, P < 0.001; DSS, P < 0.001), and extent of disease (OS, P < 0.001; DSS, P < 0.001) were independent survival determinants; tumor size was an independent predictor of OS (P = 0.006). For confined disease, age (P = 0.013), decade of diagnosis (P = 0.023), and surgery (P = 0.017) were independent determinants of OS. For locally invasive disease, grade (OS, P < 0.001; DSS, P = 0.003), surgery (OS, P = 0.013; DSS, P = 0.046), and size (OS, P = 0.001, DSS, P = 0.002) were independent determinants of OS and DSS. Radiotherapy was an independent indicator of worse OS for both confined (P = 0.004) and locally invasive disease (P = 0.002). For metastatic disease, grade (OS, P = 0.021; DSS, P = 0.012) and surgery (OS, P = 0.007; DSS, P = 0.004) were survival determinants for both OS and DSS, whereas radiotherapy predicted improved OS (P = 0.039).
Conclusion
Surgical resection confers survival benefit in patients with chondrosarcoma of the spine independent of extent of disease. Radiotherapy improves survival in patients with metastatic disease and worsens outcomes in patients with confined and locally invasive disease.
Keywords: chondrosarcoma, dedifferentiated, epidemiology, mesenchymal, radiation therapy, spine, surgery, survival
Malignant primary osseous tumors of the spine are rare, accounting for 5% of all osseous neoplasms.1 Chondrosarcoma is a malignant tumor comprised of transformed cells producing a cartilaginous matrix without tumor osteoid.2 Its estimated annual incidence is 1 in 200,000,3 with 6.5% to 10% of cases arising within the mobile spine and 5% located within the sacrum.4–7 Chondrosarcoma may arise de novo in normal bone or may undergo malignant transformation from a previously benign cartilaginous tumor (e.g., enchondroma or osteochondroma).2,7 Within the osseous spine, chondrosarcoma has a predilection for the thoracic vertebrae, but can arise anywhere along the length from cervical spine to sacrum.5 It typically develops in the posterior elements with extension into the vertebral body (45% of cases) or confined to the posterior elements (40%), with some cases (15%) confined to the vertebral body.8
Despite occurring uncommonly in the spine,2 chondrosarcoma is estimated to comprise 26% of primary osseous spinal tumors9 and when present, carries with it significant risk of morbidity and mortality secondary to local invasion and destruction of adjacent structures and metastasis to distant sites.10 Treatment has typically involved a surgical approach centered on complete en bloc resection with adjuvant radiotherapy reserved for subtotal resection or advanced disease.6,11–14 Unlike other primary osseous tumors such as osteosarcoma or Ewing sarcoma for which multimodal therapy is more often utilized, chondrosarcoma has been shown to be relatively resistant to radiation and chemotherapy.14–17 One notable exception to this are the mesenchymal and dedifferentiated subtypes, wherein a low grade tumor reverts to a primitive cell type and loses its characteristic chondroid features. Here, chemotherapy and sometimes radiation therapy are used a last resort where surgical resection alone is ineffective for such aggressive tumors.18,19 For these reasons, the optimal treatment for chondrosarcoma of the spine remains controversial.
Small case series of treatment outcomes for patients with spinal chondrosarcoma at individual institutions have been reported,4,5,11,12,20–22 but partially because of the rarity of this malignancy, reports have been limited in terms of number of patients despite data spanning several decades. The Surveillance, Epidemiology, and End Results (SEER) registry began collecting cancer-related information in 1973 and today represents 30% of the total US population, serving as the only comprehensive source of population-level cancer data.23 Advantages of utilizing such a database includes multiinstitutional data with a large patient pool for greater statistical power. The SEER database has been queried in a series of reports in the past to include all malignant tumors of the osseous spine, including chondrosarcoma from 1973 to 2003.1,9,10,24 However, analysis of treatment modalities have not been performed using multivariate regression to account for confounding factors and identify independent prognostic indicators in the treatment of spinal chondrosarcoma to date. The purpose of this study is to report updated data on demographics and clinicopathologic features, and to use multivariate regression modeling to determine specific prognostic indicators and treatment outcomes for patients with chondrosarcoma of the osseous spine from 1973 to 2012.
MATERIALS AND METHODS
A population-based search for patients diagnosed with chondrosarcoma of the spine was performed using the case-listing session protocol of the National Cancer Institute’s SEER 18 databases [www.seer.cancer.gov]. No Internal Review Board approval was required in this study because the database uses publicly available information with no personal identifiers. The SEER database is widely used and has been validated independently for analysis of primary osseous tumors of the spine.1,9,10,24
Patients diagnosed with chondrosarcoma of the spine from 1973 to 2012, the widest date ranges available in the latest version of the software were reviewed. Site specific codes were first used to identify all primary tumors that originated in the osseous spine: C41.2 (vertebral column) and C41.4 (pelvic bones, sacrum, coccyx, and associated joints). Histologic ICD-0–3 codes were then reviewed for all cases to identify the following histological subtypes with at least one case: “chondrosarcoma not otherwise specified,” “juxtacortical chondrosarcoma,” “myxoid chondrosarcoma,” “mesenchymal chondrosarcoma,” “clear cell chondrosarcoma,” and “dedifferentiated chondrosarcoma.” The following primary data were extracted for analysis: patient age, year of diagnosis, sex, race, histologic subtype (ICD), tumor extent, and tumor size from both extent of disease (EOD) and collaborative stage (CS) coding methods, tumor grade, treatment with surgery and/or radiation therapy, cause of death, and survival months. Tumor grade was reclassified as either low grade for well- or moderately-differentiated histology, or high grade for poorly differentiated or undifferentiated histology. EOD was manually reclassified using EOD and CS coding into three main categories as previously established in the literature24: confined (defined as tumor encasement within the periosteum), locally invasive (defined as further contiguous extension beyond the periosteum without distant involvement), and metastatic.
Primary outcome was defined as time in months from diagnosis to death from any cause for overall survival (OS), and time from diagnosis to death specific to the cancer-related diagnosis for disease-specific survival (DSS). Descriptive epidemiological and survival statistics were calculated for all variables. OS and DSS curves were calculated using the Kaplan-Meier method. Differences in survival were inferentially tested using the log-rank test. Covariates were assessed for predictive performance with multivariate Cox proportional hazards regression models,25 using hazard ratios (HR) with corresponding 95% confidence intervals (CI), with regard to OS and DSS. Comparisons between groups were deemed statistically significant at the P <0.05 threshold. Statistical analyses were performed using SPSS 21 software (IBM Corp., Armonk, NY). For all comparisons of therapy as separate variables, no therapy was the reference category. Differences in baseline characteristics between groups were analyzed using one-way ANOVA for continuous variables (age and size) and Pearson χ2 test or Fisher exact test for categorical variables (sex, race, decade of diagnosis, grade, extent of disease, and histologic subtype).
RESULTS
The search identified 973 patients with primary chondrosarcoma of the osseous spine from 1973 to 2012. Among these, myxoid (5.8%), dedifferentiated (3.5%), and mesenchymal (2.1%) were the most common histologic variants with the majority of cases being listed as chondrosarcoma, not otherwise specified (Table 1). Demographically, 62.7% of patients were males and 86.6% were White (Table 1). The mean and median age of diagnosis was 51.6 and 51 years, respectively. At diagnosis, 61.2% of cases were from the year 2000 and beyond. Histologically, 63.4% of cases were of low grade, 16.8% were high grade, and 19.7% were of unknown tumor grade. Extent of disease was known in 81.8% of cases, with the majority of cases presenting as locally invasive disease (58.6%). The mean and median tumor size at the time of diagnosis was 8.7 and 7.5 cm, respectively. After diagnosis, 12.3% of patients received both surgery and radiation, 57.9% underwent surgery alone, and 9.0% underwent radiation alone, whereas 16.2% received neither and 4.5% had an unknown treatment regimen.
TABLE 1.
Demographics and Clinicopathologic Features (n = 973)
| Age | Years |
| Mean | 51.6 ± 18.8 |
| Median | 51 |
| Minimum | 8 |
| Maximum | 93 |
| Characteristic | Percentage (n) |
| Sex | |
| Female | 37.3 (363) |
| Male | 62.7% (610) |
| Race/Ethnicity | |
| White | 86.6 (843) |
| Black | 7.4 (72) |
| Other | 4.9 (49) |
| Unknown | 1.0 (10) |
| Decade | |
| 1970s | 8.2 (80) |
| 1980s | 11.2 (109) |
| 1990s | 19.4 (189) |
| 2000s | 61.2 (595) |
| Tumor grade | |
| Low grade | 63.4 (617) |
| High grade | 16.9 (164) |
| Unknown | 19.7 (192) |
| Histologic subtype | |
| Chondrosarcoma, not otherwise specified | 87.5 (851) |
| Juxtacortical chondrosarcoma | 0.7 (7) |
| Myxoid chondrosarcoma | 5.8 (56) |
| Mesenchymal chondrosarcoma | 2.1 (20) |
| Clear cell chondrosarcoma | 0.5 (5) |
| Dedifferentiated chondrosarcoma | 3.5 (34) |
| Extent of disease | |
| Confined | 13.6 (132) |
| Locally invasive | 58.6 (570) |
| Metastasis | 9.7 (94) |
| Unknown | 18.2 (177) |
| Surgical resection | |
| Yes | 75.2 (732) |
| No | 22.1 (215) |
| Unknown | 2.7 (26) |
| Radiation therapy performed | |
| Yes | 21.3 (207) |
| No | 76.4 (743) |
| Unknown | 2.4 (23) |
| Treatment modality | |
| Surgery + radiation | 12.3 (120) |
| Surgery only | 57.9 (563) |
| Radiation only | 9.0 (88) |
| No therapy | 16.2 (158) |
| Unknown | 4.5 (44) |
| Size (cm) | |
| Mean | 8.7 ± 6.2 |
| Median | 7.5 |
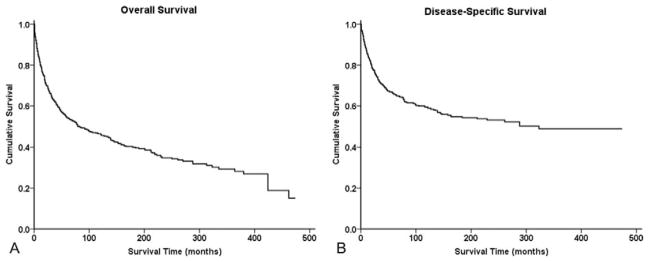
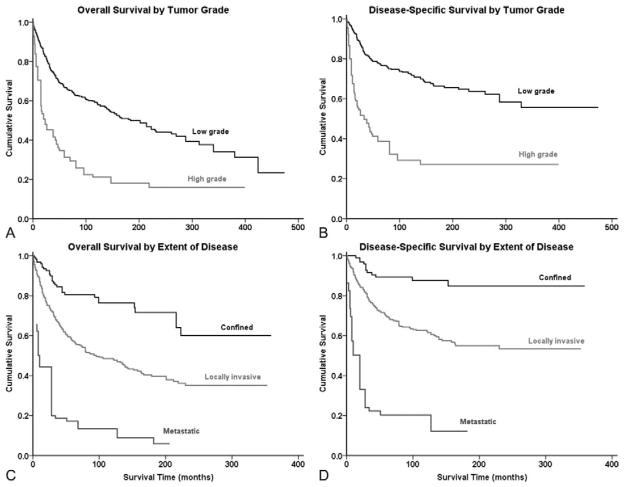
Survival analysis from Kaplan-Meier curves (Figure 1A, B) revealed that the 5-year OS and DSS for all patients with chondrosarcoma of the spine was 53% and 64%, respectively (Table 2); the median OS was 6.9 years. Demographically, the Kaplan-Meier univariate survival analysis revealed that greater age was associated with worse survival (OS log-rank P <0.001, DSS log-rank P <0.001) (Table 3). As a cohort, White patients had significantly better outcomes than Black patients (OS log-rank P = 0.031, DSS log-rank P = 0.026). Both OS and DSS showed a statistically significant difference in survival based on extent of disease at presentation (OS log-rank P <0.001, DSS log-rank P <0.001) (Figure 2A, B) with metastatic disease portending a dismal prognosis (OS 0.9 years, DSS 1.0 years) (Table 2). High tumor grade was also associated with worse survival prognosis (Figure 2C, D, Table 3) compared with low tumor grade in both OS and DSS (OS log-rank P <0.001, DSS log-rank P <0.001). Similarly, increasing tumor size was associated with worse survival (OS log-rank P <0.001, DSS log-rank P <0.001). For both OS and DSS, sex and decade of diagnosis were not associated with significant differences in survival. Among histologies, mesenchymal (OS 2.0 years, DSS 2.0 years) and dedifferentiated (OS 2.4 years, DSS 3.0 years) chondrosarcomas had particularly dismal prognoses compared with the rest of the cohort (Table 2 and 3). Decade of diagnosis was not associated with improved survival for the entire cohort, nor for any of the three most common histologic variants (myxoid, mesenchymal, and dedifferentiated).
Figure 1.
Survival analysis of patients with chondrosarcoma of the spine using Kaplan-Meier analysis. (A) Kaplan-Meier estimates of overall survival and (B) disease-specific survival are shown for all patients.
TABLE 2.
Survival Data
| Median Survival (Years) | OS | (DSS) |
|---|---|---|
| Overall | 6.9 | N/A |
| Subtype | ||
| Chondrosar coma, NOS | 8.2 | N/A |
| Juxtacortical chondrosarcoma | N/A | N/A |
| Myxoid chondrosarcoma | 6.3 | 9.1 |
| Mesenchymal chondrosarcoma | 2.0 | 2.0 |
| Clear cell chondrosarcoma | N/A | N/A |
| Dedifferentiated chondrosarcoma | 2.4 | 3.0 |
| Decade of diagnosis | ||
| 1970s | 4.5 | 11.1 |
| 1980s | 4.3 | 15.1 |
| 1990s | 11.6 | N/A |
| 2000s | 6.9 | N/A |
| Tumor grade | ||
| Low grade | 15.3 | N/A |
| High grade | 1.9 | 2.8 |
| Extent of disease | ||
| Confined | N/A | N/A |
| Locally invasive | 7.9 | N/A |
| Metastatic | 0.9 | 1.0 |
| Treatment modality | ||
| Surgery + radiation therapy | 5.7 | 9.0 |
| Surgery only | 17.6 | N/A |
| Radiation only | 1.4 | 2.0 |
| No therapy | 1.4 | 2.2 |
| Percent survival (%) | ||
| at 2 years | 63 | 72 |
| at 5 years | 53 | 64 |
| at 10 years | 45 | 58 |
N/A signifies where median survival time incalculable because of death event occurring in fewer than 50% of cases in the cohort.
TABLE 3.
Univariate Analysis of Variables Using Kaplan-Meier Method
| Characteristic | OS (Log-rank P) | DSS (Log-rank P) |
|---|---|---|
| Age at diagnosis | <0.001 | <0.001 |
| Race/Ethnicity | ||
| White vs. Black | 0.031 | 0.026 |
| Black vs. Other | 0.113 | 0.078 |
| White vs. Other | 0.703 | 0.604 |
| Sex | 0.073 | 0.065 |
| Decade of diagnosis | 0.100 | 0.146 |
| Tumor grade | <0.001 | <0.001 |
| Subtypes | ||
| NOS vs. myxoid | 0.356 | 0.302 |
| NOS vs. mesenchymal | 0.074 | 0.003 |
| NOS vs. dedifferentiated | 0.001 | <0.001 |
| Myxoid vs. mesenchymal | 0.203 | 0.044 |
| Myxoid vs. dedifferentiated | 0.004 | 0.001 |
| Mesenchymal vs. dedifferentiated | 0.725 | 0.836 |
| Surgical resection | <0.001 | <0.001 |
| Radiation therapy performed | <0.001 | <0.001 |
| Treatment modality | <0.001 | <0.001 |
| Surgery + radiation vs. surgery only | <0.001 | <0.001 |
| Surgery + radiation vs. radiation only | <0.001 | <0.001 |
| Surgery + radiation vs. no therapy | <0.001 | 0.001 |
| Surgery vs. radiation only | <0.001 | <0.001 |
| Surgery vs. no therapy | <0.001 | <0.001 |
| Radiation vs. no therapy | <0.001 | 0.659 |
| Extent of disease | <0.001 | <0.001 |
| Size (cm) | <0.001 | <0.001 |
DSS indicates disease-specific survival; OS, overall survival.
Figure 2.
Kaplan-Meier analyses of patients with chondrosarcoma of the spine for overall survival and disease-specific survival by (A, B) tumor grade and extent of disease (C, D).
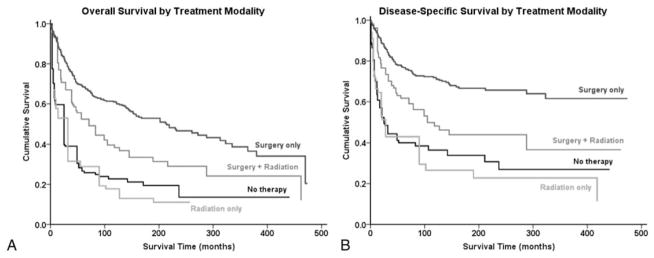
Therapeutically, patients who underwent surgical resection of the chondrosarcoma had significant improved survival (OS log-rank P <0.001, DSS log-rank P <0.001) on univariate Kaplan-Meier analysis, whereas patients who received radiation therapy had a worse prognosis (OS log-rank P <0.001, DSS log-rank P <0.001). Kaplan-Meier analysis was also used to determine the relative survival curves for patients receiving surgical resection, radiation therapy, both or neither (Figure 3A, B, Table 3). As a cohort, patients who underwent surgery alone had greater OS and DSS than patients who underwent both surgery and radiation therapy (log-rank P <0.001, log-rank P <0.001, respectively). All other pair-wise comparisons achieved statistical significance, except for DSS when comparing radiation therapy and no therapy (Figure 3A, B, Table 3). In addition, patients with histological variants did not receive radiation therapy at a significantly higher frequency than their counterparts (P = 0.357 for myxoid, P = 0.464 for mesenchymal, and P = 0.201 for dedifferentiated). To account for potential confounding baseline characteristics for patients receiving a particular treatment regimen, an analysis of baseline characteristics was performed and revealed age, race, grade, and extent of disease as potential confounders in assessing therapeutic benefit using univariate analysis (Table 4).
Figure 3.
Kaplan-Meier analyses of patients with chondrosarcoma of the spine by treatment modality. (A) Kaplan-Meier estimates of overall survival and (B) disease-specific survival are depicted for patients who underwent bimodal surgery and radiation therapy, surgery alone, radiation alone, or no therapy.
TABLE 4.
Analyses of Baseline Characteristics by Treatment Group
| Characteristic | Surgery + Radiation | Surgery Only | Radiation Only | No Therapy | P |
|---|---|---|---|---|---|
| Age (yrs, mean) | 51.1 ± 18.5 | 48.3 ± 17.0 | 61.5 ± 19.9 | 58.5 ± 20.4 | <0.001 |
| Tumor size (cm, mean)* | 7.3 ± 4.4 | 8.9 ± 6.3 | 9.2 ± 5.5 | 9.1 ± 8.2 | 0.205 |
| Sex† | 0.893 | ||||
| % Male | 65.0 | 62.2 | 64.8 | 61.4 | |
| Race† | 0.011 | ||||
| % White | 82.5 | 91.0 | 86.2 | 81.0 | |
| % Black | 9.2 | 5.2 | 9.2 | 12.0 | |
| % Other | 8.3 | 3.8 | 4.6 | 7.0 | |
| Decade of diagnosis† | 0.276 | ||||
| % 1970s | 7.5 | 6.7 | 13.6 | 10.8 | |
| % 1980s | 12.5 | 9.2 | 13.6 | 10.8 | |
| % 1990s | 23.3 | 20.1 | 19.3 | 18.4 | |
| % 2000s | 56.7 | 63.9 | 53.4 | 60.1 | |
| Grade† | 0.001 | ||||
| % High grade | 29.4 | 16.8 | 26.8 | 31.4 | |
| Extent of Disease† | <0.001 | ||||
| % Confined | 10.6 | 21.4 | 6.1 | 9.0 | |
| % Locally invasive | 79.8 | 73.7 | 56.1 | 64.9 | |
| % Metastatic | 9.6 | 4.9 | 37.9 | 26.1 |
Differences in age and tumor size distributions among treatment groups were assessed using one-way ANOVA.
Differences in sex, race, decade, grade, and extent of disease distributions among treatment groups were assessed using χ2 analysis.
On our multivariate analysis model (Table 5), age at diagnosis (HR 1.03, CI 1.02–1.04, P <0.001), tumor grade (HR 2.86, CI 2.08–3.93, P <0.001), surgical resection (HR 0.44, CI 0.29–0.67, P <0.001), tumor size (HR 1.03, CI 1.01–1.06, P = 0.021), and EOD (HR 2.54, CI 1.77–3.66, P <0.001) were found to be independent predictors of OS. Age (HR 1.02, CI 1.01–1.03, P = 0.007), tumor grade (HR 3.21, CI 2.14–4.82, P <0.001), surgical resection (HR 0.37, CI 0.22–0.63, P <0.001), and EOD were found to be independent predictors of DSS (HR 4.61, CI 2.89–7.36, P <0.001). The multivariate analysis model was next used to ascertain the independent effects of these variables on survival in patients with confined, locally invasive disease and patients with metastatic disease as separate cohorts. For cases presenting with confined disease, age (HR 1.08, CI 1.02–1.14, P = 0.013), decade of diagnosis (HR 19.50, CI 1.50–253.85, P = 0.023), and surgical resection (HR 0.06, CI 0.01–0.61, P = 0.017) were independent OS determinants. For cases presenting with locally invasive disease, age (HR 1.03, CI 1.02–1.05, P <0.001), tumor grade (HR 2.70, CI 1.88–3.88, P <0.001), surgical resection (HR 0.52, CI 0.31–0.87, P = 0.013), radiation therapy (HR 1.70, CI 1.13–2.56, P = 0.001), and tumor size (HR 1.05, CI 1.02–1.08, P = 0.001) were independent determinants of OS. In addition, age (HR 1.02, CI 1.01–1.04, P = 0.003), tumor grade (HR 3.43, CI 2.17–5.41, P <0.001), surgical resection (HR 0.48, CI 0.23–0.99, P = 0.046), and size (HR 1.06, CI 1.01–1.09, P = 0.002) were determinants of DSS. For cases presenting with meta-static disease, tumor grade (HR 3.08, CI 1.19–7.98, P = 0.021), surgical resection (HR 0.23, CI 0.08–0.67, P = 0.007), and radiation therapy (HR 0.37, CI 0.14–0.95, P = 0.039) were independent determinants of OS, whereas tumor grade (HR 4.09, CI 1.37–12.20, P = 0.012) and surgical resection (HR 0.16, CI 0.05–0.56, P = 0.004) were independent determinants of DSS. It was noted that although radiation therapy was an independent predictor of improved OS in metastatic disease (HR 0.37, CI 0.14–0.95, P = 0.039), it predicted worse OS in patients with confined (HR 69.43, CI 3.79–1272.92, P = 0.004) and locally invasive disease (HR 1.70, CI 1.13–2.56, P = 0.011).
TABLE 5.
Cox Proportional Hazards Model for Multivariate Analysis
| Characteristic | OS | DSS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Overall (n = 974) | ||||
| Age | 1.03 (1.02–1.04) | <0.001 | 1.02 (1.01–1.03) | 0.007 |
| Race/Ethnicity | 1.14 (0.85–1.52) | 0.385 | 1.01 (0.68–1.50) | 0.967 |
| Sex | 0.96 (0.70–1.31) | 0.779 | 1.04 (0.70–1.55) | 0.849 |
| Decade of diagnosis | 1.16 (0.84–1.60) | 0.363 | 1.15 (0.78–1.72) | 0.479 |
| Grade | 2.86 (2.08–3.93) | <0.001 | 3.21 (2.14–4.82) | <0.001 |
| Surgical resection | 0.44 (0.29–0.67) | <0.001 | 0.37 (0.22–0.63) | <0.001 |
| Radiation therapy | 1.30 (0.90–1.86) | 0.163 | 1.18 (0.74–1.88) | 0.494 |
| Size (cm) | 1.03 (1.01–1.06) | 0.021 | 1.03 (1.00–1.06) | 0.053 |
| Extent of disease | 2.54 (1.77–3.66) | <0.001 | 4.61 (2.89–7.36) | <0.001 |
| Confined disease (n = 132) | ||||
| Age | 1.08 (1.02–1.14) | 0.013 | – | – |
| Race/Ethnicity | 1.28 (0.45–3.65) | 0.643 | – | – |
| Sex | 0.98 (0.25–3.84) | 0.978 | – | – |
| Decade of diagnosis | 19.50 (1.50–253.85) | 0.023 | – | – |
| Grade | 1.84 (0.28–12.03) | 0.527 | – | – |
| Surgical resection | 0.06 (0.01–0.61) | 0.017 | – | – |
| Radiation therapy performed | 69.43 (3.79–1272.92) | 0.004 | – | – |
| Size (cm) | 0.88 (0.69–1.11) | 0.287 | – | – |
| Locally-invasive disease (n = 570) | ||||
| Age | 1.03 (1.02–1.05) | <0.001 | 1.02 (1.01–1.04) | 0.003 |
| Race/Ethnicity | 0.90 (0.62–1.32) | 0.595 | 0.85 (0.51–1.43) | 0.546 |
| Sex | 0.95 (0.66–1.37) | 0.792 | 0.94 (0.59–1.50) | 0.808 |
| Decade of diagnosis | 1.19 (0.84–1.69) | 0.320 | 1.29 (0.83–1.99) | 0.257 |
| Grade | 2.70 (1.88–3.88) | <0.001 | 3.43 (2.17–5.41) | <0.001 |
| Surgical resection | 0.52 (0.31–0.87) | 0.013 | 0.48 (0.23–0.99) | 0.046 |
| Radiation therapy performed | 1.70 (1.13–2.56) | 0.011 | 1.60 (0.92–2.77) | 0.094 |
| Size (cm) | 1.05 (1.02–1.08) | 0.001 | 1.06 (1.02–1.09) | 0.002 |
| Metastatic disease (n = 94) | ||||
| Age | 1.03 (0.99–1.06) | 0.127 | 1.02 (0.99–1.06) | 0.224 |
| Race/Ethnicity | 1.47 (0.59–3.64) | 0.404 | 0.96 (0.35–2.66) | 0.938 |
| Sex | 1.60 (0.64–3.96) | 0.314 | 1.80 (0.72–4.53) | 0.210 |
| Decade of diagnosis | 1.29 (0.38–4.34) | 0.680 | 1.35 (0.32–5.70) | 0.682 |
| Grade | 3.08 (1.19–7.98) | 0.021 | 4.09 (1.37–12.20) | 0.012 |
| Surgical resection | 0.23 (0.08–0.67) | 0.007 | 0.16 (0.05–0.56) | 0.004 |
| Radiation therapy performed | 0.37 (0.14–0.95) | 0.039 | 0.49 (0.18–1.31) | 0.156 |
| Size (cm) | 1.01 (0.97–1.04) | 0.670 | 0.97 (0.90–1.05) | 0.424 |
CI indicates confidence interval; DSS, disease-specific survival; HR, hazard ratio; OS, overall survival.
To account for the potential of newer radiotherapy influencing survival, we additionally performed multivariate analysis on patients treated after the year 2000. We found no difference in outcomes from the cohort for all years with respect to radiotherapy (OS HR 1.44, CI 0.93–2.25, P = 0.107; DSS HR 1.41, CI 0.80–2.48, P = 0.231). This held true for the differential effect of radiotherapy in both confined/locally invasive where it predicted worse outcome (OS HR 2.18, CI 1.36–3.51, P = 0.001; DSS HR 2.44, CI 1.28–4.67, P = 0.007) and metastatic disease (OS HR 0.31, CI 0.10–0.97, P = 0.044; DSS (HR 0.37, CI 0.12–1.18, P = 0.092) where it improved survival.
DISCUSSION
Chondrosarcoma of the osseous spine is considered to be a rare malignancy, and has the potential for both locally invasive destruction and systemic metastasis. Although previous investigations have reported on demographics and prognostic determinants of primary osseous neoplasms of the spine using the SEER registry,1,10 this study is the first to analyze population-level data on the role and outcomes of surgical resection and radiation therapy for spinal chondrosarcoma, an entity that is frequently resistant to chemotherapy. Furthermore, this study uses multivariate regression analysis to more confidently report on treatment outcomes in the presence of multiple known confounders.25,26
Our study found the average age at diagnosis of chondrosarcoma of the spine to be 51 years. We also found that there was a 3:2 predilection of males to females, which has been previously supported in smaller studies.21,27 In the pathology literature, the reported estimates for each of the histological variants of chondrosarcoma is <5% for all chondrosarcomas.28 However, to our knowledge there have been no epidemiologic studies reporting on their incidence in the spine. Our study is the first to demonstrate that the frequency of these variants is comparable to those found for the appendicular skeleton (myxoid 5.8%, mesenchymal 2.1%, dedifferentiated 3.5%, and clear cell 0.5%). In terms of survival outcomes, patients had an overall median survival of 6.9 years, and a 5-year OS and DSS of 53% and 64%, respectively. This is by-in-large consistent with prior case series4,5,11,12,20–22 and prior SEER database reports of primary osseous tumors.1,9,10,24 In general, the prognosis of chondrosarcoma of the axial skeleton appears to be less favorable than disease affecting the long bones, which has an estimated 5-year survival ranging from 50% to 80% in the literature.3,29 The findings of this study are congruent with these prior estimates, and implicate that the complexities of the spine anatomy may make curative treatment more difficult than in the extremities. Previous studies have found that high-grade chondrosarcomas of the axial skeleton have significantly worse long-term (at 10 years and beyond) conditional survival compared with high-grade disease of the extremity which further underscores this point.30,31
Among tumor histologies, we found that mesenchymal and dedifferentiated chondrosarcomas portended a comparatively dismal prognosis in the spine. These are recognized in the literature as particularly aggressive tumors and frequently necessitate chemotherapy in addition to primary surgery for treatment in the long bones.18,19 In congruence with prior literature, we also found tumor grade and extent of disease to be independent prognostic indicators of both OS and DSS. Given that the majority of the cases were poorly differentiated or anaplastic on histology and presented with at least local invasion at the time of diagnosis, this association stands to reason when considering that a 5% to 20% chondrosarcomas are high-grade (16.4% in this study), aggressive malignancies with significant soft tissue extension, particularly in the spine.3,11,29 Notably, tumor size was found to be an independent survival determinant, particularly for confined and locally invasive disease. Though this trend has not been exclusively reported for chondrosarcoma of the spine, it has been reported in other sarcomas, including chondrosarcomas of the appendicular skeleton and skull base.32,33
This study also found that patient age at presentation portended worse outcomes in both OS and DSS. This trend is understandable given the aggressive therapies needed to treat such a disease, and has been reported in other sarcomas, and chondrosarcoma in particular.34 In context, this finding is consistent with children tolerating higher doses of chemotherapy, which is used in instances of advanced disease in mesenchymal and dedifferentiated chondrosarcoma, that may be otherwise intolerable in older patients for therapeutic effect.18,19,35 Although there was an observed trend, the decade of diagnosis was not found to be associated with a statistically significant improvement in survival in either univariate or multivariate analysis, or for chondrosarcoma variants. Contextually, this may suggest that advances in radiation therapy and chemotherapy regimens have not been as successful for chondrosarcoma as for other bone tumors.2,3,36 Despite the male predilection for this disease, we also noted differences in survival based on sex.
With regards to treatment outcomes, the multivariate regression model used in this study assesses the effect of surgery and radiation independent of possibly confounding covariates such as age, tumor grade, and extent of disease. In our univariate Kaplan-Meier analysis of treatment modalities, we found that patients who underwent surgical resection of chondrosarcoma had significantly improved survival, whereas patients who received radiation therapy had an overall worse prognosis. When accounting for extent of disease in our multivariate model, the differential survival impact of radiation therapy was more clearly elucidated. The multivariate analysis suggests that surgical resection benefited patients with both metastatic and nonmetastatic disease, in terms of both OS and DSS. However, for patients with confined and locally invasive tumors, radiotherapy portended a poorer OS whereas it conferred an improvement in OS for metastatic disease. This result suggests that radiotherapy may be more effective if restricted to patients with metastatic or otherwise advanced disease burden. It is well established in the literature that chondrosarcomas are as an entity resistant to radiation,14–17 requiring doses in excess of 50 Gy, a threshold above which there has been an identifiable risk of paralysis and other neurological side effects because of nearby spine and nerve root anatomy.14,37 This is consistent with the finding that decade of diagnosis, and the contemporary improvement in radiotherapy technology, was not found to be associated with improved survival. However, we acknowledge that such a database study cannot clearly elucidate an explanation for such a differential effect of radiation therapy in survival. This observation may be caused by tumor dedifferentiation with radiotherapy.38 It may also be because of the fact that early radiation therapy may preclude a patient from being eligible for surgery. Future multiinstitutional studies may be warranted to delineate this as well the role of advances in targeted radiotherapy and chemotherapy regimens, particularly in the treatment of chondrosarcoma variants.
Surgical treatment for spinal chondrosarcoma has traditionally included both en bloc resection and curettage. Although en bloc resection is preferred, the inherent proximity of the neoplasm with neurovascular structures as well as the need for structural stabilization may make en bloc resection difficult, making curettage with a cytotoxic adjuvant more appropriate in some cases.7 However, results after curettage are inferior to those after en bloc therapy.22,39 Of note, his study found that patients with confined disease are the lone subgroup where survival has improved in the last several decades, which may reflect the improvement of surgical management of this disease. Although the SEER database reports whether surgical intervention was performed, it is limited in its ability to retrospectively analyze certain other variables, such as margin status, extent of surgical resection, and postoperative tumor recurrence. Similarly, no data on chemotherapy are available in the database. However, the effect of this shortcoming in terms of the current analysis is thought to be lessened by chondrosarcoma’s generally accepted lack of response to chemotherapy, except in advanced mesenchymal and dedifferentiated chondrosarcoma.7
Key Points.
We reviewed the SEER registry for patients with chondrosarcoma of the spine to determine patient outcomes and survival determinants.
The median OS was 6.9 years; greater age at diagnosis, increasing tumor size, Black race, tumors of high grade, greater extent of disease, and dedifferentiated and mesenchymal variants of the disease were associated with worse prognosis.
Surgical resection was an independent predictor of improved survival for both confined/locally invasive and metastatic disease.
Radiation therapy portended worse prognosis in patients with confined/locally invasive disease and portended better prognosis in metastatic disease.
Acknowledgments
The authors would like to thank the UCLA Statistical Consulting Group through the Institute for Digital Research and Education (IDRE) for assistance with statistical analysis.
Footnotes
No relevant financial activities outside the submitted work.
No funds were received in support of this work.
References
- 1.Mukherjee D, Chaichana KL, Gokaslan ZL, et al. Survival of patients with malignant primary osseous spinal neoplasms: results from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2003. J Neurosurg Spine. 2011;14:143–50. doi: 10.3171/2010.10.SPINE10189. [DOI] [PubMed] [Google Scholar]
- 2.Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am. 2009;40:21–36. doi: 10.1016/j.ocl.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg. 2009;91:1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 4.Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine (Phila Pa 1976) 2000;25:804–12. doi: 10.1097/00007632-200004010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Shives TC, McLeod RA, Unni KK, et al. Chondrosarcoma of the spine. J Bone Joint Surg Am. 1989;71:1158–65. [PubMed] [Google Scholar]
- 6.Gitelis S, Bertoni F, Picci P, et al. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Jt Surg Am. 1981;63:1248–57. [PubMed] [Google Scholar]
- 7.Stuckey RM, Marco RAW. Chondrosarcoma of the mobile spine and sacrum. Sarcoma. 2011;2011:274281. doi: 10.1155/2011/274281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphey MD, Walker EA, Wilson AJ, et al. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologicpathologic correlation. Radiographics. 2003;23:1245–78. doi: 10.1148/rg.235035134. [DOI] [PubMed] [Google Scholar]
- 9.McGirt MJ, Gokaslan ZL, Chaichana KL. Preoperative grading scale to predict survival in patients undergoing resection of malignant primary osseous spinal neoplasms. Spine J. 2011;11:190–6. doi: 10.1016/j.spinee.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Chaichana KL, Parker SL, et al. Association of surgical resection and survival in patients with malignant primary osseous spinal neoplasms from the Surveillance, Epidemiology, and End Results (SEER) database. Eur Spine J. 2013;22:1375–82. doi: 10.1007/s00586-012-2621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenfeld AJ, Hornicek FJ, Pedlow FX, et al. Chondrosarcoma of the mobile spine: a review of 21 cases treated at a single center. Spine (Phila Pa 1976) 2012;37:119–26. doi: 10.1097/BRS.0b013e31823d2143. [DOI] [PubMed] [Google Scholar]
- 12.Bergh P, Gunterberg B, Meis-Kindblom JM, et al. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201–12. doi: 10.1002/1097-0142(20010401)91:7<1201::aid-cncr1120>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Henderson ED, Dahlin DC. Chondrosarcoma of bone: a study of two hundred and eighty-eight cases. J Bone Joint Surg Am. 1963;45:1450–8. [PubMed] [Google Scholar]
- 14.Holliday EB, Mitra HS, Somerson JS, et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine. Spine (Phila Pa 1976) 2015;40:544–9. doi: 10.1097/BRS.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 15.Hsu W, Kosztowski TA, Zaidi HA, et al. Multidisciplinary management of primary tumors of the vertebral column. Curr Treat Options Oncol. 2009;10:107–25. doi: 10.1007/s11864-009-0102-8. [DOI] [PubMed] [Google Scholar]
- 16.Zagars GK, Ballo MT. Significance of dose in postoperative radiotherapy for soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2003;56:473–81. doi: 10.1016/s0360-3016(02)04573-x. [DOI] [PubMed] [Google Scholar]
- 17.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–9. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi S, Sun T, Lin PP, et al. Does ifosfamide therapy improve survival of patients with dedifferentiated chondrosarcoma? Clin Orthop Relat Res. 2014;472:983–9. doi: 10.1007/s11999-013-3360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Italiano A, Mir O, Cioffi A, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. 2013;24:2916–22. doi: 10.1093/annonc/mdt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groves ML, Zadnik PL, Kaloostian P, et al. Epidemiologic, functional, and oncologic outcome analysis of spinal sarcomas treated surgically at a single institution over 10 years. Spine J. 2015;15:110–4. doi: 10.1016/j.spinee.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Strike Sa, McCarthy EF. Chondrosarcoma of the spine: a series of 16 cases and a review of the literature. Iowa Orthop J. 2011;31:154–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh PC, Xu R, Sciubba DM, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas. Spine (Phila Pa 1976) 2009;34:2233–9. doi: 10.1097/BRS.0b013e3181b61b90. [DOI] [PubMed] [Google Scholar]
- 23.Nathoo N, Mendel E. The National Cancer Institute’s SEER registry and primary malignant osseous spine tumors. World Neurosurg. 2011;76:531–2. doi: 10.1016/j.wneu.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee D, Chaichana KL, Adogwa O, et al. Association of Extent of local tumor invasion and survival in patients with malignant primary osseous spinal neoplasms from the Surveillance, Epidemiology,;1; and End Results (SEER) database. World Neurosurg. 2011;76:580–5. doi: 10.1016/j.wneu.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Katz MH, Hauck WW. Proportional hazards (Cox) regression. J Gen Intern Med. 1993;8:702–11. doi: 10.1007/BF02598295. [DOI] [PubMed] [Google Scholar]
- 26.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–68. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 27.Yin H, Zhou W, Meng J, et al. Prognostic factors of patients with spinal chondrosarcoma: a retrospective analysis of 98 consecutive patients in a single center. Ann Surg Oncol. 2014;21:3572–8. doi: 10.1245/s10434-014-3745-z. [DOI] [PubMed] [Google Scholar]
- 28.Kilpatrick SE. Chondrosarcoma Variants. Surg Pathol Clin. 2012;5:163–81. doi: 10.1016/j.path.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Duchman KR, Lynch CF, Buckwalter JA, et al. Estimated cause-specific survival continues to improve over time in patients with chondrosarcoma. Clin Orthop Relat Res. 2014;472:2516–25. doi: 10.1007/s11999-014-3600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duchman KR, Lynch CF, Buckwalter JA, et al. Estimated cause-specific survival continues to improve over time in patients with chondrosarcoma. Clin Orthop Relat Res. 2014;472:2516–25. doi: 10.1007/s11999-014-3600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bindiganavile S, Han I, Yun JY, et al. Long-term outcome of chondrosarcoma: a single institutional experience. Cancer Res Treat. 2015;47:897–903. doi: 10.4143/crt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones PS, Aghi MK, Muzikansky A, et al. Outcomes and patterns of care in adult skull base chondrosarcomas from the SEER database. J Clin Neurosci. 2014;21:1497–502. doi: 10.1016/j.jocn.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84:93–9. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 34.Kim H-S, Bindiganavile SS, Han I. Oncologic outcome after local recurrence of chondrosarcoma: analysis of prognostic factors. J Surg Oncol. 2015;111:957–61. doi: 10.1002/jso.23925. [DOI] [PubMed] [Google Scholar]
- 35.Bishop MW, Somerville JM, Bahrami A, et al. Mesenchymal chondrosarcoma in children and young adults: a single institution retrospective review. Sarcoma. 2015;2015:608279. doi: 10.1155/2015/608279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bilsky MH, Gerszten P, Laufer I, et al. Radiation for primary spine tumors. Neurosurg Clin N Am. 2008;19:119–23. doi: 10.1016/j.nec.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol. 1995;31:1093–112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 38.Davies BW, Prescott CR, Said SA, et al. Radiation-induced dedifferentiated chondrosarcoma with orbital invasion. Ophthal Plast Reconstr Surg. 2014;30:205–8. doi: 10.1097/IOP.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 39.Fourney DR, Rhines LD, Hentschel SJ, et al. En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine. 2005;3:111–22. doi: 10.3171/spi.2005.3.2.0111. [DOI] [PubMed] [Google Scholar]