Abstract
Severe asthma is typically characterized by chronic airway inflammation that is refractory to corticosteroids and associated with excess morbidity. Patients were recruited into the National Heart, Lung, and Blood Institute–sponsored Severe Asthma Research Program and comprehensively phenotyped by bronchoscopy. Bronchoalveolar lavage (BAL) cells were analyzed by flow cytometry. Compared with healthy individuals (n = 21), patients with asthma (n = 53) had fewer BAL natural killer (NK) cells. Patients with severe asthma (n = 29) had a marked increase in the ratios of CD4+ T cells to NK cells and neutrophils to NK cells. BAL NK cells in severe asthma were skewed toward the cytotoxic CD56dim subset, with significantly increased BAL fluid levels of the cytotoxic mediator granzyme A. The numbers of BAL CD56dim NK cells and CCR6−CCR4− T helper 1–enriched CD4+ T cells correlated inversely with lung function [forced expiratory volume in 1 s (FEV1) % predicted] in asthma. Relative to cells from healthy controls, peripheral blood NK cells from asthmatic patients had impaired killing of K562 myeloid target cells despite releasing more cytotoxic mediators. Ex vivo exposure to dexamethasone markedly decreased blood NK cell lysis of target cells and cytotoxic mediator release. NK cells expressed airway lipoxin A4/formyl peptide receptor 2 receptors, and in contrast to dexamethasone, lipoxin A4–exposed NK cells had preserved functional responses. Together, our findings indicate that the immunology of the severe asthma airway is characterized by decreased NK cell cytotoxicity with increased numbers of target leukocytes, which is exacerbated by corticosteroids that further disable NK cell function. These failed resolution mechanisms likely contribute to persistent airway inflammation in severe asthma.
INTRODUCTION
The immunology of the asthmatic airway is a complex mixture of innate and adaptive cellular responses. Whereas most asthmatic patients have increased type 2 inflammation that is responsive to anti-inflammatory therapy with corticosteroids (1), many patients have a mixed inflammatory response in their airways, and about 10% of the patients have severe asthma (SA) that is refractory to corticosteroids (2). SA is associated with chronic airway inflammation, frequent symptoms, and excess morbidity, representing an important unmet clinical health need. More detailed airway immunophenotyping is needed, especially for non–type 2 inflammation in SA, to identify cellular and molecular mechanisms responsible for the persistent inflammation (3).
T helper 2 (TH2) CD4+ T cells promote type 2 inflammation in asthma through secretion of interleukin-5 (IL-5) and IL-13, but the role of other CD4+ TH cell classes in asthma pathogenesis is less clear. TH1, TH2, and TH17 CD4+ T cells are present in asthma bronchoalveolar lavage (BAL) without clear difference between asthmatic patients and healthy donors (4). Natural killer (NK) cells are key members of the innate lymphocyte family with essential roles in host defense (5). Human NK cells are CD3−NKp46+ cells with lymphoid morphology that are characterized by levels of CD56 expression. CD56bright NK cells are a less mature subset of NK cells that predominantly secrete cytokines [interferon-γ (IFN-γ), tumor necrosis factor–α (TNF-α), and IL-17] and have limited cytotoxic capacity (6). CD56dim NK cells are derived from CD56bright NK cells through progressive differentiation and are primarily responsible for lysis of target cells through release of cytotoxic mediators (granzymes, perforin, and granulysin) (6). Preclinical and translational research has uncovered a pivotal role for NK cells in the clearance of T cells and granulocytes for the resolution of inflammation (7, 8). Thus, in addition to provoking inflammation in host defense, NK cells are also important cellular effectors for the resolution of inflammation.
Resolution of acute inflammation is an active process, with specialized proresolving mediators acting as agonists at cognate receptors to signal for cell-specific mechanisms that halt inflammation and clear affected organs (9). Lipoxins are lead members of this class of specialized proresolving mediators. Of interest, lipoxin A4 (LXA4) levels are reduced in SA, suggesting that the persistent airway inflammation in SA may stem in part from a defect in inflammation resolution (10). Peripheral blood NK cells express lipoxin A4/formyl peptide receptor 2 (ALX/FPR2) receptors for LXA4, and NK cell–mediated granulocyte apoptosis is increased by LXA4 in human non-SA (NSA) (7). In contrast, corticosteroids are commonly used as anti-inflammatory therapy in asthma, and in vitro, these agents can suppress the cytotoxic function of NK cells from healthy individuals (11).
Here, NK cells and CD4+ T cells were identified and quantitated in BAL collected from healthy and asthmatic individuals recruited into the Severe Asthma Research Program (SARP). BAL NK cells were decreased in asthma and skewed toward the CD56dim subtype. Peripheral blood NK cells from asthmatic patients were less effective at killing target leukocytes despite increased release of cytotoxic mediators relative to cells from healthy control (HC) individuals. Corticosteroids, but not LXA4, decreased NK cell cytotoxicity and mediator release in vitro. Together, these changes in NK cells in asthma and the actions of steroids on NK cell effector functions suggest an underlying defective cellular mechanism for the resolution of airway inflammation that is operative in asthma and not enhanced by current asthma therapies.
RESULTS
SA patients have uncontrolled symptoms and reduced lung function despite multimodality therapies
Patients with SA and NSA and HC individuals were recruited to the SARP by seven research centers in the United States, and some patients agreed to bronchoscopy with BAL (Table 1). Patients with SA had worse symptom control than those with NSA, as evidenced by a lower Asthma Control Test (ACT) score and higher Asthma Control Questionnaire (ACQ) score. Lung function was lower in SA despite nearly all patients being treated with high-dose inhaled corticosteroids, long-acting β-agonists, and additional adjunct therapies.
Table 1. Patient characteristics.
Results are expressed as means ± SD (range). BMI, body mass index; n/a, not applicable; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IgE, immunoglobulin E; ACQ, asthma control questionnaire; ACT, asthma control test.
| Healthy | NSA | SA | |
|---|---|---|---|
| Number of patients | 21 | 24 | 29 |
| Demographics | |||
| Age (years) | 40.5 ± 13.3 (23.0–62.1) | 38.2 ± 12.9 (18.1–60.6) | 42.9 ± 13.3 (16.4–67.3) |
| Gender (% male) | 48 | 38 | 34 |
| Race (% white) | 71 | 83 | 69 |
| Ethnicity (% Hispanic) | 0 | 0 | 0 |
| BMI | 28.1 ± 4.4 (20.9–40.5) | 29.4 ± 7.5 (19.0–52.4) | 29.4 ± 6.4 (19.0–41.7) |
| Medications | |||
| Inhaled corticosteroids (%) | 0 | 67 | 97* |
| Oral corticosteroids (%) | 0 | 0 | 21* |
| Long-acting β-agonists (%) | 0 | 46 | 90* |
| Leukotriene receptor antagonists (%) | 0 | 17 | 38 |
| Omalizumab (%) | 0 | 0 | 10 |
| Symptoms | |||
| Asthma duration (years) | n/a | 14.7 ± 13.2 (1.0–45.0) | 17.4 ± 14.9 (0–57.0) |
| ACQ score | n/a | 1.1 ± 0.8 (0–3.1) | 1.8 ± 1.0 (0.4–4.7)* |
| ACT score | n/a | 19.8 ± 3.7 (11–25) | 15.5 ± 4.6 (5–23)* |
| Lung function | |||
| FEV1 (% predicted) | 101.7 ± 10.9 (84.3–126.5) | 94.0 ± 17.0 (57–123.0) | 76.1 ± 18.8 (38.7–116.5)* |
| FVC (% predicted) | 100.8 ± 14.2 (82.1–128.8) | 104.7 ± 19.6 (57.8–144.0) | 92.8 ± 19.2 (56.5–136.3)* |
| IgE (IU/ml) | 109.6 ± 191.4 (3.5–737.5) | 211.4 ± 320.1 (2.2–1473.0) | 422.4 ± 646.7 (7.2–2981.0) |
P < 0.05 compared with patients with NSA by t test.
BAL NK cells are decreased relative to CD4+ T cells in SA
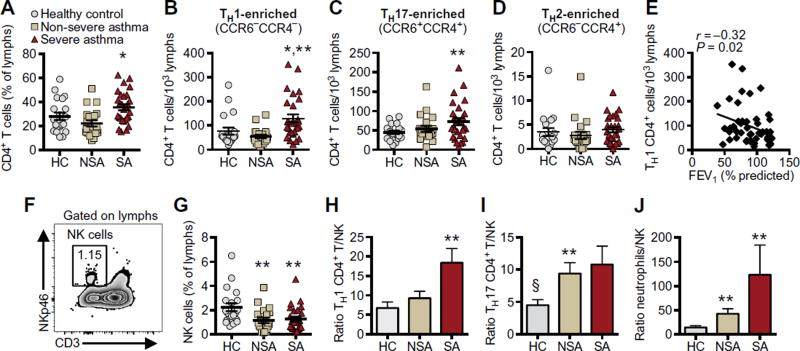
Total BAL cell counts and leukocyte differentials were enumerated (Table 2). BAL samples were more cellular in SA, with a higher percentage of neutrophils and trends for higher percentages of lymphocytes and eosinophils, as compared with those in NSA and HC. Lymphocytes comprised about 6 to 7% of total BAL cells in healthy and asthmatic individuals (Table 2). Lymphocyte subsets were further characterized by flow cytometric analyses (gating strategies are shown in fig. S1). In HC individuals, CD4+ T cells comprised 28 ± 3% (means ± SEM) of total BAL lymphocytes, which was similar to NSA (22.2 ± 2.5%; Fig. 1A). Relative to NSA, SA had a significantly higher percentage of BAL CD4+ T cells (35.5 ± 2.5%; P = 0.002; Fig. 1A) and total number of CD4+ T cells (fig. S2A). In contrast, the percentage of CD8+ T cells in BAL of healthy and asthmatic individuals was similar, accounting for about 25% of total BAL lymphocytes (fig. S2B). BAL CD4+ T cells were further classified on the basis of surface expression of the chemokine receptors CCR6 and CCR4, which in peripheral blood can differentiate populations of TH1-, TH2-, and TH17-enriched TH cell subclasses without stimulation and intracellular cytokine staining (12). In SA, BAL CD4+ T cells were skewed toward CCR6−CCR4− TH1–enriched and CCR6+CCR4+ TH17–enriched populations. Both TH1-enriched CD4+ T cells (Fig. 1B; P = 0.015) and TH17-enriched CD4+ T cells (Fig. 1C; P = 0.046) were more abundant in SA BAL relative to HC. CCR6−CCR4+ TH2–enriched CD4+ T cells were much fewer in number (about 2% of total CD4+ T cells), and there were no significant differences between clinical cohorts (Fig. 1D). Of interest, the number of TH1-enriched CCR6−CCR4− CD4+ T cells was inversely correlated with lung function (i.e., FEV1% predicted) (Fig. 1E; Spearman r = −0.32, P = 0.020). There was no significant correlation between lung function and the number of TH17-enriched CCR6+CCR4+ CD4+ T cells (fig. S2C).
Table 2. BAL cell counts and leukocyte differentials.
Total cell numbers and leukocyte differentials were determined in BAL by manual counting. Results are expressed as means ± SD (range).
| Healthy (n = 21) |
NSA (n = 24) |
SA (n = 29) |
|
|---|---|---|---|
| Total cell count, millions | 5.2 ± 3.1 (0.3–11.3) | 4.2 ± 2.9 (0–12.3) | 6.3 ± 6.2 (1.3–35) |
| Macrophages (%) | 91.1 ± 6.7 (73.0–98.6) | 91.9 ± 5.6 (78.8–99.1) | 86.5 ± 10.7 (53–98.2)* |
| Lymphocytes (%) | 6.7 ± 5.0 (0.8–19.3) | 5.8 ± 5.2 (0–5.8) | 7.5 ± 5.3 (0.3–18.7) |
| Neutrophils (%) | 1.9 ± 2.3 (0–9.6) | 1.2 ± 1.2 (0–4.0) | 3.8 ± 6.4 (0–23.6)* |
| Eosinophils (%) | 0.3 ± 0.5 (0–2.3) | 1.2 ± 2.4 (0–8.6) | 2.1 ± 4.2 (0–17.3) |
P < 0.05 when compared with patients with NSA by t test.
Fig. 1. BAL NK cells are decreased relative to CD4+ T cells in SA.
BAL samples were obtained from HC individuals and NSA and SA patients (see Materials and Methods). (A) CD4+ T cells were enumerated in BAL as percent of total lymphocytes by flow cytometry. (B to D) CD4+ T cells were characterized into functional phenotypes by surface expression of chemokine receptors CCR6 and CCR4. TH1-enriched (CCR6−CCR4−) (B), TH17-enriched (CCR6+CCR4+) (C), and TH2-enriched (CCR6−CCR4+) (D) CD4+ T cells were enumerated per 103 lymphocytes. (E) The relationship between BAL TH1-enriched CD4+ T cells and lung function (FEV1% predicted) was determined in asthma (diamonds), and Spearman correlation r value and significance are noted. (F) BAL NK cells were identified as CD3−NKp46+ cells with lymphoid morphology. Representative flow plot from an asthmatic patient is shown, and inset notes NK cells as percent of total lymphocytes. (G) BAL NK cells were enumerated as percent of total lymphocytes. To normalize comparisons among cohorts, we calculated individual ratios of TH1-enriched CD4+ T cells (H), TH17-enriched CD4+ T cells (I), and neutrophils (J) to NK cells in BAL for each individual. Scatter plots show individual data points with means ± SEM, and bar graphs express means ± SEM in HC individuals (gray) and NSA (tan) and SA (red) patients. *P < 0.05 compared with NSA, **P < 0.05 compared with HC, and §P < 0.10 compared with SA by Kruskal-Wallis test and post hoc Dunn’s multiple comparisons test.
BAL NK cells were identified as cells with lymphoid morphology that were CD3−NKp46+ (Fig. 1F). Relative to HC individuals, both SA and NSA patients had a significantly decreased percentage of BAL NK cells (Fig. 1G), with trends for decreased absolute numbers (fig. S2D). The ratio of TH1-enriched CD4+ T cells to NK cells in SA BAL was nearly three times that of HC individuals (18.4 versus 6.7; Fig. 1H), and the ratio of TH17-enriched CD4+ T cells to NK cells in NSA and SA patients was twice that of HC individuals (10.8 versus 4.5 for SA; Fig. 1I). The relative ratio of neutrophils to NK cells was also increased in NSA and SA patients relative to HC individuals (Fig. 1J). Together, these data identify a mixed inflammatory response in SA relative to HC, with reduced numbers of NK cells compared with CD4+ T cells and neutrophils in BAL.
BAL NK cells are skewed toward a CD56dim cytotoxic phenotype in SA
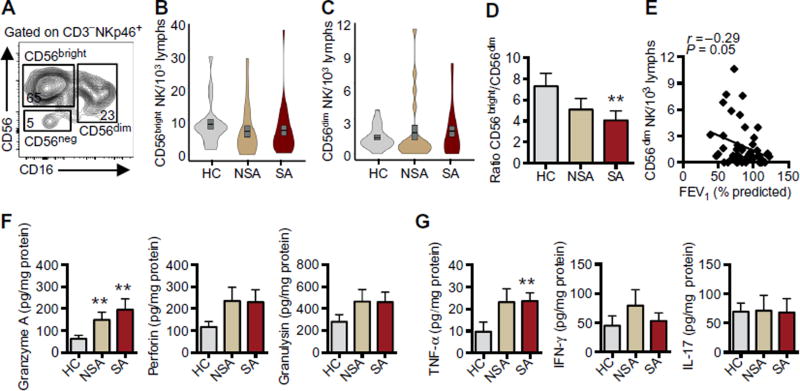
To determine the distribution of NK cell functional phenotypes, we further characterized BAL NK cells based on expression of CD56 and CD16 into CD56bright, CD56dim, and CD56neg subpopulations (Fig. 2A). Relative to those in healthy individuals, BAL NK cells in asthmatic patients trended toward fewer CD56bright (Fig. 2B) and more CD56dim NK cells (Fig. 2C). Violin plots highlight probability density of data at each value by the width of the plot. The violin plots demonstrate that the increase in BAL CD56dim NK cells was present in a larger proportion of SA than NSA patients (Fig. 2C). The ratio of CD56bright to CD56dim NK cells was significantly lower in SA patients than in healthy individuals [CD56bright to CD56dim ratio of 4 (SA) versus 7.3 (HC), P = 0.032; Fig. 2D]. In asthma, lung function (FEV1% predicted) was inversely correlated with the numbers of BAL CD56dim NK cells (Fig. 2E; Spearman r = −0.29, P = 0.050), but not the numbers of BAL CD56bright NK cells (fig. S2E). To determine whether this skewing of NK cell phenotype toward the CD56dim subset in the asthmatic BAL was reflected in differences in levels of mediators, we measured representative classes of NK cell–derived mediators in cell-free BAL fluid (BALF). Granzyme A levels were significantly higher in BALF from SA and NSA than in HC, and there was a trend for increased perforin and granulysin (Fig. 2F). There were no significant differences in levels of granzyme B, soluble Fas (sFas), or sFas ligand (sFasL) among the clinical cohorts (fig. S3A). Levels of TNF-α were increased in SA BALF relative to HC (P = 0.012), but no significant differences were present in levels of IFN-γ or IL-17 (Fig. 2G).
Fig. 2. BAL NK cells are skewed toward a CD56dim cytotoxic phenotype in SA.
BAL NK cells (CD3−NKp46+) were phenotyped by CD56 and CD16 expression into CD56bright, CD56dim, and CD56neg subtypes. (A) Representative flow plot from an asthmatic patient notes percent of total NK cells for each subtype. (B and C) Violin plots (combined box plot and kernel density plot) show the density distribution of CD56bright (B) and CD56dim (C) NK cells in each clinical cohort (gray, HC; tan, NSA; red, SA). Overlaid gray box plots represent means (black line) ± SEM. (D) Ratio of CD56bright to CD56dim NK cells was calculated for each individual. (E) The relationship between CD56dim NK cell number and lung function (FEV1% predicted) was determined in asthma (diamonds), and Spearman correlation r value and significance are noted. Cytotoxic mediators (F) and cytokines (G) were measured in cell-free BALF by cytokine bead array and normalized to protein (see Materials and Methods). Values represent means ± SEM. **P < 0.05 compared with HC by Kruskal-Wallis test and post hoc Dunn’s multiple comparisons test.
Systemic triamcinolone alters BAL NK cell phenotype and suppresses cytotoxic mediator release in asthma
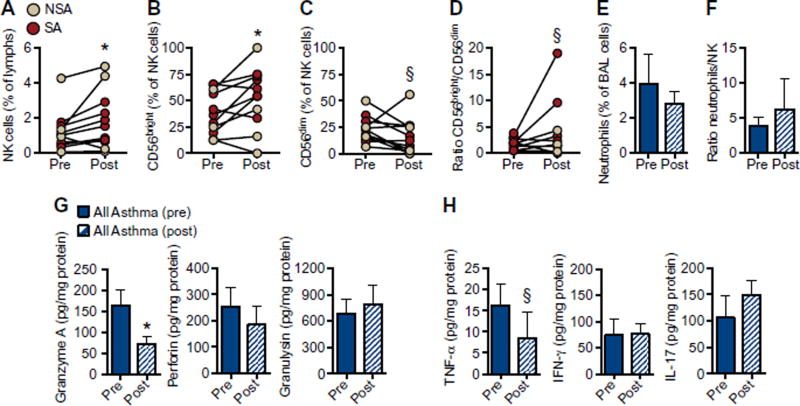
Intramuscular triamcinolone was administered to asthmatic patients, and a few patients agreed to a second bronchoscopy and BAL 3 to 6 weeks after the steroid injection. Although the sample size was limited, BAL NK cell percentage increased after systemic triamcinolone (Fig. 3A; P = 0.019). Relative numbers of BAL CD56bright NK cells significantly increased (Fig. 3B; P = 0.042), with a trend to decreased CD56dim NK cells (Fig. 3C; P = 0.051) and resultant trend to an increase in the ratio of CD56bright to CD56dim NK cells after systemic steroids (Fig. 3D; P = 0.100). The number of neutrophils (Fig. 3E) and the ratio of neutrophils to NK cells (Fig. 3F) did not change after triamcinolone. There was also no significant change in the number of CD4+ T cells (fig. S4A) or the ratio of CD4+ T cells to NK cells after triamcinolone (fig. S4, B to D). BALF levels of granzyme A were decreased after triamcinolone (Fig. 3G; P = 0.030), and levels of TNF-α also trended lower (Fig. 3H; P = 0.055), but there was no discernible change in BALF levels of perforin, granulysin, IL-17, or IFN-γ (Fig. 3, G and H) or granzyme B, sFas, or sFasL (fig. S4E).
Fig. 3. Systemic triamcinolone alters BAL NK cell phenotype and suppresses cytotoxic mediator release in asthma.
BAL was performed in asthmatic patients at baseline and 3 to 6 weeks after intramuscular triamcinolone. BAL NK cell number (percent of lymphocytes) (A), CD56bright NK cells (B) and CD56dim NK cells as percent of total NK cells (C), and the ratio of CD56bright/CD56dim NK cells (D) were measured [n = 4 NSA (tan); n = 7 SA (red)]. Neutrophils (percent of total BAL cells) (E) and ratio of neutrophils (percent of BAL cells) to NK cells (percent of lymphocytes) (F) were compared before and after triamcinolone (n = 11 asthmatic patients). Cytotoxic mediators (G) and cytokines (H) were measured in cell-free BALF before and after triamcinolone by cytokine bead array and normalized to protein in n = 18 asthmatic patients. *P < 0.05 and §P ≤ 0.10 compared with pre-triamcinolone by Wilcoxon matched-pairs signed-rank test.
BAL leukocytes express the proresolving receptor ALX/FPR2
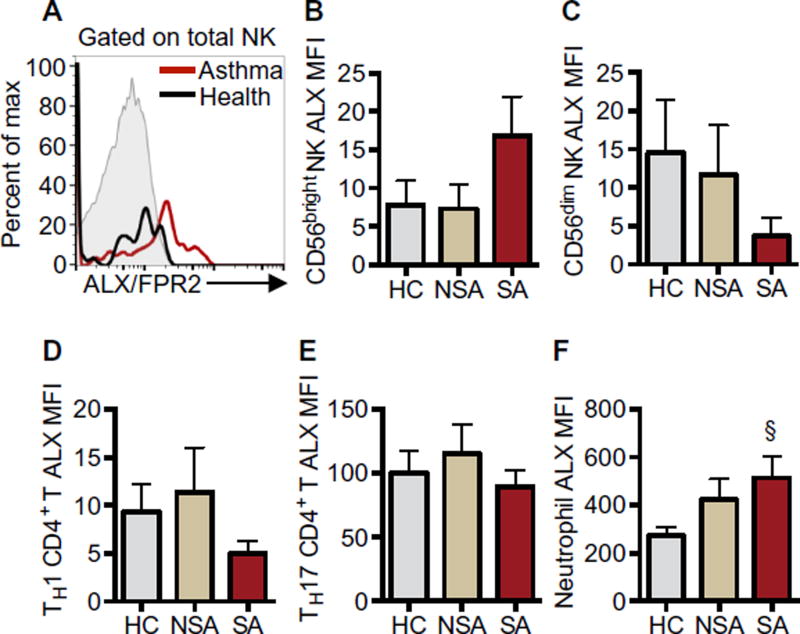
Because the specialized proresolving mediator LXA4 can influence NK cell function (7), expression of the LXA4 cognate receptor ALX/FPR2 was determined. BAL NK cells from healthy and asthmatic individuals expressed low levels of surface ALX/FPR2 (Fig. 4A). ALX/FPR2 expression on BAL NK cells from SA trended higher on CD56bright cells (Fig. 4B) and lower on CD56dim cells (Fig. 4C). TH1-enriched CCR6−CCR4− CD4+ T cells expressed ALX/FPR2 at similar median fluorescence intensity (MFI) as NK cells (Fig. 4D), and TH17-enriched CCR6+CCR4+ CD4+ T cells expressed ALX/FPR2 at 10-fold higher levels (Fig. 4E). ALX/FPR2 MFI was similar among the clinical cohorts. Neutrophils expressed the highest levels of ALX/FPR2 among the leukocyte subsets tested, and BAL neutrophil ALX/FPR2 expression trended higher in SA relative to HC (Fig. 4F; P = 0.085).
Fig. 4. BAL leukocytes express the proresolving receptor ALX/FPR2.
(A) Flow cytometry histograms show ALX/FPR2 expression on BAL total NK cells from a representative SA patient (red line) and a HC individual (black line) compared with isotype control (gray, filled). (B to F) MFI of ALX/FPR2 minus MFI of the isotype control was calculated to quantitate ALX/FPR2 surface expression on leukocyte subsets including CD56bright NK cells (B), CD56dim NK cells (C), TH1-enriched CD4+ T cells (D), TH17-enriched CD4+ T cells (E), and neutrophils (F). Values represent means ± SEM in each cohort. §P = 0.085 compared with HC by Kruskal-Wallis test and post hoc Dunn’s multiple comparisons test.
Corticosteroids impair peripheral blood NK cell cytotoxic function and inhibit NK cell release of cytotoxic mediators in vitro
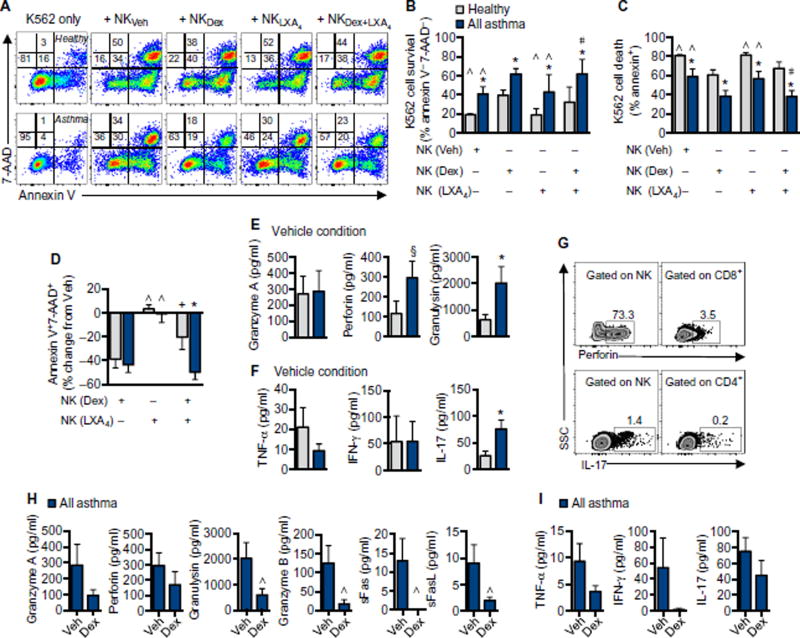
Because increased doses of inhaled and systemic corticosteroids are used to treat SA (Table 1) and intramuscular triamcinolone altered NK cell phenotype (Fig. 3), the direct impact of corticosteroids on NK cell function was tested in vitro. Peripheral blood mononuclear cells (PBMCs) from asthmatic or healthy individuals were exposed to vehicle or dexamethasone (1 µM) for 48 hours, followed by coincubation with the K562 myeloid cell line (an NK cell target) at an effector/ target ratio of 20:1 (PBMC/K562) for 4 hours. NK cell–specific lysis of K562 cells was assessed by flow cytometry staining for annexin V and 7-aminoactinomycin D (7-AAD) (Fig. 5A). K562 target cells did not undergo significant apoptosis over the course of 4 hours when incubated in the absence of effector NK cells (Fig. 5A). NK cell–mediated killing of K562 cells was consistently lower with NK cells from asthmatic patients relative to healthy individuals (Fig. 5, A to D). Relative to cells from healthy donors, more K562 cells survived when incubated with NK cells from asthmatic donors (annexin V−7-AAD−; Fig. 5, A and B), and fewer K562 cells underwent apoptosis (annexin V+; Fig. 5, A and C). This decrease in NK cell cytotoxicity was despite a trend to higher numbers of peripheral blood CD56dim NK cells in asthma (fig. S5A). Dexamethasone exposure reduced the numbers of both CD56dim and CD56bright NK cells and increased the number of CD56neg NK cells in asthmatic and healthy individuals (fig. S5, B to D). Relative to vehicle, dexamethasone significantly inhibited the ability of NK cells to lyse K562 cells by about 40% in both asthmatic and healthy individuals (Fig. 5, A and D). Despite decreased lysis of target K562 cells, peripheral blood NK cells from asthmatic patients released more cytotoxic mediators, including perforin and granulysin, as well as more IL-17 relative to NK cells from healthy individuals (Fig. 5, E and F). Intracellular staining confirmed that the cellular source of perforin and IL-17 was NK cells (Fig. 5G). Dexa-methasone exposure inhibited NK cell release of cytotoxic mediators, including granzymes, perforin, granulysin, sFas, and sFasL (Fig. 5H). There were trends to decreased release of TNF-α, IFN-γ, and IL-17 with dexamethasone exposure as well, but the changes did not reach statistical significance secondary to donor- to- donor variation with vehicle alone (Fig. 5I).
Fig. 5. Corticosteroids decrease peripheral blood NK cell functional responses in vitro.
PBMCs from healthy donors or asthmatic patients were exposed to vehicle (Veh), dexamethasone (Dex) (1 µM), LXA4 (50 nM), or dexamethasone and LXA4 for 48 hours, followed by coincubation with K562 cells at a 20:1 ratio (effector/target) for 4 hours (at 37°C in 5% CO2). (A) K562 cell lysis was assessed by annexin V and 7-AAD staining as in representative flow cytometry plots from a healthy individual (top) and an asthmatic patient (bottom). Numbers indicate percent of target cells in each quadrant. (B) K562 target cell survival (percent annexin V−7-AAD−) was assessed with NK cells from healthy individuals (gray bars; n = 6) and asthmatic patients (blue bars; n = 3 NSA and n = 3 SA) for each condition. (C) NK cell–mediated K562 target cell lysis (percent annexin V+) was determined for each condition. (D) Percent change in target cell lysis relative to vehicle was assessed for each condition. Cytotoxic mediators (E) and cytokines (F) were quantified by cytokine bead array in the cell-free supernatant of coincubations of NK and K562 cells in the vehicle condition. (G) To determine the cellular source of these mediators, we exposed PBMCs to K562 cells at a 20:1 ratio for 2 hours followed by 4 hours in the presence of a Golgi inhibitor. Intracellular perforin and IL-17 were measured in NK cells and CD8+ and CD4+ T cells, respectively, as shown in a representative donor. Numbers note percent of parent population positive for perforin (top) or IL-17 (bottom). Cytotoxic mediators (H) and cytokines (I) were measured in cell-free supernatants, with paired comparisons made between vehicle and dexamethasone (n = 5). Values represent means ± SEM. *P < 0.05 and §P < 0.10 compared with healthy individuals, ∧P < 0.05 and +P = 0.08 compared with dexamethasone, and #P < 0.05 compared with LXA4 by ANOVA with Holm-Sidak correction for multiple comparisons (B to D) or by unpaired (E and F) or paired t test (H and I).
LXA4-exposed peripheral blood NK cells effectively lyse target cells, and NK cell cytotoxic mediator release is preserved
To determine whether the specialized proresolving mediator LXA4 had similar actions to the anti-inflammatory corticosteroid dexamethasone on NK cell function, we exposed PBMCs from asthmatic or healthy individuals in parallel to vehicle, LXA4 (50 nM), or LXA4 and dexa-methasone (1 µM) for comparison with dexamethasone alone for 48 hours before coincubation with K562 cells at an effector/target ratio of 20:1 (PBMC/K562) for 4 hours (Fig. 5, A to D). LXA4-exposed NK cells killed K562 cells similarly to vehicle and significantly more effectively than dexamethasone-exposed NK cells in both asthmatic and healthy individuals (Fig. 5, A and C). ALX/FPR2 expression is higher on BAL CD56dim NK cells from healthy donors than SA donors (Fig. 4C), and the combination of LXA4 and dexamethasone partially prevented the steroid-mediated suppression of target cell lysis by peripheral blood NK cells from healthy individuals but not asthmatic patients (Fig. 5D). NK cells from healthy individuals exposed to dexamethasone alone had 40% reduced killing of target cells, as opposed to a 20% decrease when exposed to both LXA4 and dexa-methasone (Fig. 5D; P = 0.08). In contrast, NK cells from asthmatic patients exposed to both dexamethasone and LXA4 had 50% reduction in killing compared with vehicle (Fig. 5D; P = 0.014 compared with healthy individuals). LXA4 exposure increased the number of CD56bright NK cells in healthy individuals but not in asthmatic patients (fig. S5B, P = 0.012) and did not significantly alter the number of CD56dim NK cells relative to vehicle in contrast to dexamethasone (fig. S5C). Unlike dexamethasone, LXA4 exposure did not substantially increase the number of CD56neg NK cells (fig. S5D). LXA4-exposed NK cells had preserved release of cytotoxic mediators (fig. S5E) and cytokines (fig. S5F) when coincubated with K562 cells.
DISCUSSION
In this multicenter study, patients with SA had increased lung inflammation characterized by abundant BAL proinflammatory granulocytes and CD4+ T cells and a relative paucity of NK cells. NK cells can promote the resolution of inflammation by mediating apoptosis of autologous leukocytes (7). Here, there was a clear correlation between reduced BAL NK cell number and increased proinflammatory leukocyte numbers in patients with SA relative to healthy individuals. Although BAL NK cell total number was lower in asthmatic patients compared with healthy individuals, there was a relative increase in cytotoxic CD56dim NK cells and release of the cytotoxic mediator granzyme A. BAL CD56dim NK cell number was inversely correlated with lung function in asthma. Peripheral blood NK cells in asthma were less effective at killing target leukocytes in vitro despite releasing more cytotoxic mediators. Corticosteroid exposure reduced the number of CD56dim NK cells and their release of cytotoxic mediators in vivo in BAL and in vitro from peripheral blood NK cells and decreased NK cell– mediated target cell lysis in vitro. In contrast to corticosteroid inhibition of NK cell function, the proresolving mediator LXA4 preserved NK cell cytotoxic mediator release and lysis of target leukocytes in vitro. Together, these findings are consistent with an altered inflammatory milieu in the asthma airway characterized by deficient NK cells relative to inflammatory leukocytes, particularly in SA, and suggest that disabled NK cells are linked to asthma pathogenesis. Because all SA patients are treated with corticosteroids, which here disabled NK cell function, the loss of NK cell protective action is likely to be an important mechanism underlying the persistent airway inflammation and dysfunction in SA.
In preclinical murine models of allergic lung inflammation, NK cells are important for the clearance of activated leukocytes, including T cells and granulocytes (8). For inflammation resolution, NK cells can promote granulocyte apoptosis (7) for clearance from inflamed lung by macrophage efferocytosis (13). In patients meeting international criteria for human SA (14), TH1 CD4+ T cells (15) and granulocytes (4) are increased in BAL. Here, BAL neutrophils and CD4+ T cells with CC chemokine receptor expression consistent with TH1-enriched (CCR6−CCR4−) and TH17-enriched (CCR6+CCR4+) cells were increased in SA relative to HC, with a concomitant decrease in NK cells. In SA, the patients’ ratios of CD4+ T cells to NK cells and neutrophils to NK cells were elevated, suggestive of ineffective leukocyte clearance by NK cells. The number of TH1-enriched CD4+ T cells was inversely correlated with lung function, supporting recent studies linking IFN-γ–secreting TH1 CD4+ T cells to the pathophysiology of SA (15, 16).
In healthy individuals, NK cells in secondary lymphoid organs are primarily CD56bright cells that, when activated, produce cytokines, such as IFN-γ, but are poor mediators of cytotoxicity (6, 17). Proinflam-matory cytokines and chemokines produced by CD56bright NK cells shape the adaptive immune response and recruit leukocytes to sites of inflammation (6). Here, BAL CD56bright NK cells were more abundant than CD56dim NK cells in both asthmatic and healthy individuals; however, in SA, the CD56bright to CD56dim NK cell ratio was decreased secondary to a relative increase in CD56dim cells. Of interest, lung function in asthma was inversely correlated with the number of BAL CD56dim NK cells. Unlike BAL (7), CD56dim NK cells are the dominant NK cell population in peripheral blood and in some lobectomy specimens from patients with lung cancer (18). CD56dim NK cells can cause lysis of target cells by releasing cytotoxic mediators, including perforin that forms pores in target cell membranes and granzymes and granulysins that enter target cells through these pores to induce apoptosis (19, 20). Relative to HC, increased CD56dim NK cells in SA BAL were associated with increased BALF levels of the cytotoxic mediator granzyme A. Excess extracellular release of granzymes has been linked to chronic inflammatory disease and matrix turnover (21). Together, these data suggest a potential pathogenic mechanism for dysregulated NK cells in airway inflammation and remodeling in SA, with excess production of cytotoxic mediators that may promote chronic inflammatory changes and compromise lung function.
Inhaled and oral corticosteroids are a mainstay of anti-inflammatory therapy in asthma, yet SA patients have persistent, refractory symptoms despite high-dose steroid therapy (2). In the SARP-3 cohort, only 20% of SA patients had improvement in FEV1 by ≥10% after a dose of intramuscular triamcinolone (22). Peripheral blood NK cell activity in asthmatic patients is reduced in vitro by fluticasone (23). Here, systemic administration of triamcinolone to asthmatic patients altered BAL NK cells by decreasing the CD56dim subset relative to CD56bright and decreasing BALF granzyme A. In addition, in vitro exposure to dexamethasone impaired the ability of blood NK cells to lyse target K562 myeloid cells by about 40%. Impaired NK cell cytotoxicity after dexamethasone exposure in vitro was associated with impaired release of CD56dim cytotoxic mediators, including granzymes, perforin, granulysin, sFas, and sFasL. The number of BAL neutrophils and CD4+ T cells and the ratio of these leukocytes to NK cells remained increased and were not significantly altered by systemic triamcinolone administration. Steroid-mediated reduction of CD56dim NK cell number and cytotoxic mediators may decrease NK cell inflammatory potential (21); however, CD56dim NK cells are the subset most important for clearance of virus-infected cells as well as antigen-specific T cells and granulocytes in the lungs (8). These data raise the possibility that by decreasing CD56dim NK cell number and function, corticosteroids may have the unintended effect of sustaining inflammation by impairing viral host defense and resolution of airway inflammation.
Peripheral blood NK cells in asthma released more cytotoxic mediators than cells from healthy individuals on contact with target cells in vitro yet killed target leukocytes less effectively than cells from healthy donors. Soluble mediators of cytotoxicity kill target cells in a rapid and coordinated manner, with perforin creating openings in cell membranes that provide portals of entry for granzymes and granulysins to promote apoptosis in nonredundant pathways (19, 20). The blunted cytotoxicity of the asthma NK cells despite increased release of their cytotoxic mediators suggests that the immune synapses formed by NK cells and their leukocyte targets were disrupted in asthma. This defect may in part be related to the influence of corticosteroids on NK cell phenotype. CD56neg NK cells were increased after exposure to dexamethasone and have been described as an activated mature NK cell phenotype with poor effector function (24). Peripheral blood CD56neg NK cells trended higher in asthmatic patients relative to healthy individuals, and dexamethasone exposure further increased this dysfunctional NK cell phenotype. Thus, these data provide further evidence that corticosteroids alter NK cells by promoting an NK cell phenotype that may secrete effector molecules but is poorly able to clear activated leukocytes.
Corticosteroids are commonly used in the treatment of asthma as anti-inflammatory agents. Patients with SA were more likely to be on inhaled and oral corticosteroids than those with NSA (Table 2). Here, BAL findings in SA differed from NSA in several respects. TH1-enriched CD4+ T cells were more prevalent in SA than in NSA, and the ratio of TH1-enriched CD4+ T cells to NK cells was increased relative to healthy individuals only in the SA population. Also, the ratio of CD56bright to CD56dim NK cells was only decreased in the SA cohort. It is likely that these differences between SA and NSA stem in part from more frequent and higher-dose exposure to corticosteroids. Whereas both triamcinolone in vivo and dexamethasone in vitro suppressed CD56dim NK cell function, responses in CD56bright and CD56neg NK cells diverged. The glucocorticoid potency of dexamethasone is about five times that of an equivalent dose of triamcinolone, and the concentration of dexamethasone used in vitro may have been more immuno-suppressive than the in vivo dose of triamcinolone (25). In addition, BAL NK cell phenotyping occurred 3 to 6 weeks after triamcinolone dosing, and cell turnover may have influenced the in vivo NK cell repertoire in that period of time.
Anti-inflammation is not synonymous with proresolution, because the latter term refers to the processes of catabasis that are responsible for clearing tissue-infiltrating cells and debris, restoring tissue homeostasis, and enhancing host defense. The resolution of airway inflammation is an active process regulated by specialized proresolving mediators derived from essential fatty acids that signal as receptor agonists for cell type–specific mechanisms to halt inflammation and clear affected organs (9). LXA4 is enzymatically derived from arachidonic acid and signals through ALX/FPR2 receptors to trigger proresolving actions [reviewed by Levy and Serhan (9)]. LXA4 biosynthesis is decreased in asthma in both blood (10) and BALF (26), and lower levels of LXA4 are associated with decreased lung function and more severe disease (10). Administration of a stable bioactive LXA4 analog decreases lung leukocyte infiltration and airway hyperresponsiveness in a murine model of asthma (27), and inhaled LXA4 reduces bron-choprovocation in human asthmatic patients (28). Peripheral blood NK cells express ALX/FPR2, and LXA4 promotes NK cell–mediated granulocyte apoptosis in asthma (7) and can influence NK cell cytotoxicity in vitro (29). Here, LXA4-exposed peripheral blood NK cells lysed target K562 cells similarly to cells exposed to vehicle control and significantly more effectively than dexamethasone-treated NK cells with preserved release of cytotoxic effector molecules. For peripheral blood NK cells from healthy individuals but not asthmatic patients, the exposure of NK cells to LXA4, in addition to dexamethasone, resulted in partial blunting of the dexamethasone-mediated inhibition of NK cell cytotoxicity. This partial inhibition of dexamethasone by LXA4 in cells from healthy individuals, but not asthmatic patients, may reflect a lower availability of ALX/FPR2 receptors on CD56dim NK cells in asthma, in particular SA. Thus, CD56dim BAL NK cells with lower expression of surface ALX/FPR2 and low airway levels of endogenous LXA4 (10, 26) may predispose patients with SA to being less able to overcome the immunosuppressive effects of chronic inhaled and oral corticosteroids on NK cells. Together, these data indicate that NK cells in asthma display phenotype-specific properties that can influence their responses to environmental cues from endogenous proresolving mediators and suggest that ALX/FPR2 receptor expression gated NK cell functional responses.
Here, we have uncovered several aspects of NK cell function that appear to contribute to SA pathogenesis. Our detailed immunological analyses of asthma BAL cells have leveraged the National Heart, Lung, and Blood Institute’s multicenter effort in the SARP-3 to rigorously determine endotypes for the clinical syndrome of SA; however, there are limitations to molecular phenotyping methods that are associated with the multicenter design of this study. In particular, using previously frozen BAL cell samples rather than fresh samples precluded analyses of intracellular cytokines. Indirect methods to define enriched populations of CD4+ TH subclasses by chemokine receptor expression were used, as has been described for peripheral blood T cells (12), but the assignment of CD4+ T cell subsets is less confident by these indirect methods. Lymphocytes account for 6 to 7% of total BAL cells and NK cells represent less than 10% of total BAL lymphocytes, so cell abundance limited the number of analyses performed. Some cell types, such as regulatory T cells and eosinophils, do not tolerate the sample handling procedures required in this trial, and some cells, such as innate lymphoid cells and mucosal-associated invariant T cells, were not present in sufficient number for routine analysis. In future studies, use of new analytical technologies, such as CyTOF, may prove useful in obtaining additional identification from SA BAL cells, and novel humanized mouse models may enable more detailed functional analyses of innate immune cells, such as NK cells, in disease models (30).
In summary, immunophenotyping of BAL cells uncovered a complex mixture of innate and adaptive inflammation in SA and suggested a contributory role for NK cells in asthma pathogenesis. NK cells from asthmatic patients were less effective at killing target cells in vitro despite increased release of cytotoxic molecules. The numbers of BAL TH1-enriched CCR6−CCR4−CD4+ T cells and CD56dim NK cells were inversely correlated with lung function, suggestive of a link between these immune mechanisms and asthma pathogenesis. These findings highlight an association between reduced BAL NK cell number and function relative to proinflammatory leukocytes in SA, consistent with impaired NK cell–mediated leukocyte clearance. In addition, the immunosuppressive effects of corticosteroids on NK cell function suggest that steroids administered for acute anti-inflammatory effects in SA may have the unintended consequences of perpetuating chronic lung inflammation by disabling the capacity of NK cells to promote leukocyte apoptosis for clearance in catabasis. Distinct from corticosteroids, the endogenous specialized proresolving mediator LXA4 preserved NK cell effector mechanisms for killing leukocyte targets. Together, these findings demonstrate that lung NK cells are nimble immune effectors that respond to environmental and pharmacological influences to serve as pivotal cellular regulators of inflammation in SA and carry the potential to serve as targets for molecular or cell-based therapies in this morbid condition.
MATERIALS AND METHODS
Study design
The SARP-3 is an ongoing 3-year longitudinal study designed to characterize molecular, cellular, and physiological phenotypes in patients with SA and NSA. Asthmatic and healthy individuals were recruited and completed baseline characterization. Details regarding SARP recruitment methods, patient enrollment, study measurements, and study procedures can be found in recent publication (31). Adult patients 18 years of age and older with asthma and HC individuals were recruited to the SARP between November 2012 and February 2015 by seven research centers in the United States. Patients were defined as having SA or NSA, as outlined by the European Respiratory Society/ American Thoracic Society guidelines (14). Healthy individuals were nonsmokers with no history of lung disease, atopic disease, or allergic rhinitis. Written informed consent was obtained after institutional review board approval at each site.
Sample collection
BAL was performed by instilling three 50-ml aliquots of warm saline, and BALF was recovered by suctioning. BAL cells and cell-free BAL supernatant were frozen and stored at −80°C or liquid nitrogen and later shipped to Brigham and Women’s Hospital for analysis. Patients received intramuscular triamcinolone (1 mg/kg up to maximum of 40 mg), and some patients agreed to undergo a second bronchoscopy 3 to 6 weeks later. BAL samples were collected in the same manner. Baseline BAL cellular samples were obtained from a total of 172 patients. Samples with low cell viability were excluded from further analyses. Viability was less than 70% by trypan blue staining in 46 of 172 (26.7%), and 25 of 172 (14.5%) had <70% viability by annexin V staining by flow cytometry and were excluded. An additional 28 of 172 (16.3%) samples had too few cells for lymphocyte immunophenotyping. After these exclusions, 73 of 172 (42.4%) BAL cell samples were analyzed. In total, BAL cells from up to 21 HC individuals, 24 NSA patients, and 29 SA patients were used for each assay on the basis of sample availability (details are listed in table S1). For in vitro experiments, heparinized peripheral whole blood was collected by venipuncture from asthmatics (n = 6) from the Brigham and Women’s Hospital SARP cohort and HC individuals (n = 6). Healthy donors had no history of asthma or atopy and took no over-the-counter medications in the previous 2 weeks. PBMCs were isolated by density gradient centrifugation over Histopaque-1077 (Sigma-Aldrich) and used within 2 hours of phlebotomy.
Flow cytometry
The following antibodies specific for human proteins were used, with clones noted in parentheses: anti-CD4 allophycocyanin (APC)–Cy7 (RPA-T4), anti-CCR4 phycoerythrin (PE)–Cy7 (L291H4), anti-CCR6 APC (G034E3), anti-CD69 FITC (fluorescein isothiocyanate) or PE (FN50), anti-CD3 FITC (UHCHT1), anti-NKp46 APC (9E2), anti-CD56 PE-Cy7 (HCD56), anti-CD16 APC-Cy7 (3G8), anti-CD45 PE-Cy7 (HI30), anti-CD66b (G10F5), anti-CD8 FITC (RPA-T8), anti-perforin PE (B-D48), anti-CD4 PE (SK3), anti–IFN-γ PerCP (peridinin chlorophyll protein) (4S.B3), and anti–IL-17 APC (BL168) (all from BioLegend); anti-CD3 PerCP-Cy5.5 (SK7) (from eBioscience); and anti-ALX/FPR2 PerCP-Cy5.5 (304405) (from R&D Systems). For ex vivo staining of BAL, cells were incubated with purified human monoclonal immuno-globulin G (BioLegend) to block Fc receptors for 15 min at 4°C before specific surface staining for 30 min at 4°C. For intracellular cytokine staining in ex vivo PBMC experiments, cells were fixed/permeabilized with Cytofix/Cytoperm according to the manufacturer’s instructions (BD Biosciences), and then surface and intracellular staining was performed simultaneously for 30 min at 4°C. Data were acquired on a Canto II flow cytometer (Becton Dickinson) and analyzed using FlowJo software version 10.1 (Tree Star). NK cells were identified as a cell population with lymphoid morphology based on forward scatter and side scatter that lacked CD3 expression and expressed NKp46. Subtypes of NK cells were classified on the basis of surface expression of CD56 and CD16 (6). CD4+ T cells were identified as lymphocytes that expressed CD4 and were classified into populations of enriched functional subclasses based on the presence or absence of surface CCR6 and CCR4, as has been described for peripheral blood CD4+ T cells (12).
In vitro NK cell and K562 target cell coincubations
PBMCs were cultured in RPMI 1640 (Lonza) supplemented with 2 mM l-glutamine, 2% heat-inactivated fetal calf serum (Gibco), penicillin (100 IU/ml), streptomycin (100 µg/ml), and low-dose IL-2 (2 U/ml; Sigma) in the presence of vehicle (<0.1% ethanol), dexamethasone (1 µM; Sigma), LXA4 (50 nM; Cayman Chemical), or both dexamethasone and LXA4 for 48 hours. Dexamethasone and LXA4 were redosed after 24 hours. After 48 hours, PBMCs were washed and placed into coculture with K562 myeloid tumor cells that were labeled with the DNA dye eFluor 670 (eBioscience) at an effector/target ratio of 20:1 (PBMC/K562) for 4 hours at 37°C in 5% CO2. NK cell–mediated lysis of target cells was then assessed by gating on eFluor 670+ K562 cells and using annexin V and 7-AAD (BioLegend) staining to identify necrotic/ late apoptotic cells. To determine the cellular source of cytotoxic mediators and cytokines in coculture experiments, we exposed PBMCs to K562 cells at an effector/target ratio of 20:1 for 2 hours followed by 4 hours in the presence of GolgiStop (BD). Cells were then stained as described above, and intracellular perforin and IL-17 were measured in NK cells, CD8+ T cells, or CD4+ T cells by flow cytometry.
Cytokine and cytotoxic mediator analysis
Select cytokines and cytotoxic mediators were measured in cell-free BALF or cell culture supernatant using a flow cytometry bead-based immunoassay (LEGENDplex, BioLegend). BALF mediators were normalized to BALF protein levels as assessed by Pierce BCA Protein Assay (Thermo Scientific).
Statistical analysis
Data are expressed as means ± SEM. Data in the tables are expressed as means ± SD. The statistical significance of differences for parametric data was assessed by two-tailed Student’s t test or one-way analysis of variance (ANOVA) with Holm-Sidak correction for multiple comparisons. Differences between groups with nonparametric data were assessed using Wilcoxon matched-pairs signed-rank test or Kruskal-Wallis test with post hoc test and correction for multiple comparisons with Dunn’s test. Correlations for nonparametric data were evaluated by Spearman’s correlation coefficient (r). A P value of <0.05 was considered significant, although comparisons with significance P < 0.1 are also noted. Prism 6.0 (GraphPad) was used to analyze data. Violin plots were generated using R software (www.r-project.org).
Supplementary Material
Fig. S1. Flow cytometry gating strategies.
Fig. S2. BAL lymphocytes in healthy and asthmatic individuals.
Fig. S3. Levels of additional cytotoxic mediators in BALF from healthy and asthmatic individuals.
Fig. S4. Systemic triamcinolone does not alter BAL CD4+ T cell number or the ratio of effector CD4+ T cells to NK cells in asthma.
Fig. S5. Exposure to dexamethasone or LXA4 changes peripheral blood NK cell phenotype, and LXA4 exposure does not impair NK cell release of cytotoxic mediators.
Table S1. Patient number (n) for each experiment.
Acknowledgments
We thank the study participants, the SARP-3 clinical research coordinators, and the data coordinating center. We also thank J. Barkas and G. Zhu for expert technical assistance. Funding: This study was conducted with the support of grants that were awarded by the National Heart, Lung, and Blood Institute: U10 HL109172 (to E.I. and B.D.L., Harvard Medical School), U10 HL109164 (to E.R.B., Wake Forest University), U10 HL109257 (M. Castro, Washington University), U10 HL109250 (to S.C.E., Cleveland Clinic; co-principal investigator, Virginia-Cleveland Consortium), U10 HL109146 (to J.V.F., University of California, San Francisco), U10 HL109250 (to B.M.G., Case Western Reserve University), U10 HL109168 (to N.N.J., University of Wisconsin), U10 HL109152 (to S.E.W., University of Pittsburgh), and U10 HL109086 (to D.T.M., Penn State University). The work was also supported in part by RO1-HL122531 (to B.D.L.), K12-HD047349 (to M.G.D.), and K23-HL116657 (to N.R.B.). In addition, this program is supported through the following NIH National Center for Advancing Translational Sciences awards: UL1 TR001420 to Wake Forest University, UL1 TR000427 to University of Wisconsin, UL1 TR001102 to Harvard University, and UL1 TR000454 to Emory University.
Footnotes
Author contributions: M.G.D., C.B., M. Cernadas, I.R., and N.K. designed and performed experiments, analyzed data, and wrote the manuscript. N.L.G., N.R.B., J.V.F., E.R.B., M. Castro, S.C.E., B.M.G., N.N.J., D.T.M., S.E.W., S.A.C., A.M.C., M.L.F., A.T.H., M.W.J., M.C.P., and B.R.P. collected specimens, analyzed data, and wrote the manuscript. D.T.M. provided expert statistical consultation. E.I. and B.D.L. conceived the study, designed experiments, analyzed data, and wrote the manuscript. All authors contributed to the editing of the final manuscript. All authors agreed to all of the content of the submitted manuscript.
Competing interests: B.D.L. is a co-inventor on patents assigned to Brigham and Women’s Hospital (“Method for treating airway hyper-responsiveness with lipoxin analogs,” patent 8,119,691). The interests of B.D.L. were reviewed and are managed by the Brigham and Women’s Hospital and the Partners HealthCare in accordance with their conflict-of-interest policies. The other authors declare that they have no competing interests.
"This manuscript has been accepted for publication in Science Immunology. This version has not undergone final editing. Please refer to the complete version of record at http://immunology.sciencemag.org. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS."
REFERENCES AND NOTES
- 1.Fahy JV. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, Ober C, Woodruff PG, Barnes KC, Bender BG, Camargo CA, Jr, Chupp GL, Denlinger LC, Fahy JV, Fitzpatrick AM, Fuhlbrigge A, Gaston BM, Hartert TV, Kolls JK, Lynch SV, Moore WC, Morgan WJ, Nadeau KC, Ownby DR, Solway J, Szefler SJ, Wenzel SE, Wright RJ, Smith RA, Erzurum SC. Future research directions in asthma. An NHLBI Working Group report. Am. J. Respir. Crit. Care Med. 2015;192:1366–1372. doi: 10.1164/rccm.201505-0963WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinks TSC, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, Walls AF, Gadola SD, Djukanović R. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J. Allergy Clin. Immunol. 2015;136:323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J. Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Severe Asthma Research Program; National Heart, Lung, and Blood Institute, Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen BK, Beyer JM. Characterization of the in vitro effects of glucocorticosteroids on NK cell activity. Allergy. 1986;41:220–224. doi: 10.1111/j.1398-9995.1986.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F. Heterogeneity of human CD4+ T cells against microbes. Annu. Rev. Immunol. 2016;34:317–334. doi: 10.1146/annurev-immunol-032414-112056. [DOI] [PubMed] [Google Scholar]
- 13.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving Gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2016;9:1278–1287. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 15.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-g and low SLPI mark severe asthma in mice and humans. J. Clin. Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Distinct endotypes of steroid-resistant asthma characterized by IL-17Ahigh and IFN-ghigh immunophenotypes: Potential benefits of calcitriol. J. Allergy Clin. Immunol. 2015;136:628–637. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 18.Marquardt N, Kekäläinen E, Chen P, Kvedaraite E, Wilson JN, Ivarsson MA, Mjösberg J, Berglin L, Säfholm J, Manson ML, Adner M, Al-Ameri M, Bergman P, Orre A-C, Svensson M, Dahlén B, Dahlén S-E, Ljunggren H-G, Michaëlsson J. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69CD56dim cells. J. Allergy Clin. Immunol. 2016 doi: 10.1016/j.jaci.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Saini RV, Wilson C, Finn MW, Wang T, Krensky AM, Clayberger C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J. Immunol. 2011;186:3497–3504. doi: 10.4049/jimmunol.1003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping IS, Bleackley RC, Kirchhausen T, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011;12:770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiebert PR, Granville DJ. Granzyme B in injury, inflammation, and repair. Trends Mol. Med. 2012;18:732–741. doi: 10.1016/j.molmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, Ly NP, Bacharier LB, Bhakta NR, Moore WC, Bleecker ER, Hastie AT, Meyers DA, Castro M, Fahy J, Fitzpatrick A, Gaston BM, Jarjour NN, Levy BD, Peters SP, Teague WG, Fajt M, Wenzel SE, Erzurum SC, Israel E. Severe Asthma Research Program, Effects of age and disease severity on systemic corticosteroid responses in asthma. Am. J. Respir. Crit. Care Med. 2016 doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Lorenzo G, Esposito Pellitteri M, Drago A, Di Blasi P, Candore G, Balistreri C, Listi F, Caruso C. Effects of in vitro treatment with fluticasone propionate on natural killer and lymphokine-induced killer activity in asthmatic and healthy individuals. Allergy. 2001;56:323–327. doi: 10.1034/j.1398-9995.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- 24.Milush JM, López-Vergès S, York VA, Deeks SG, Martin JN, Hecht FM, Lanier LL, Nixon DF. CD56negCD16+ NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology. 2013;10:158. doi: 10.1186/1742-4690-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 26.Planagumà A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am. J. Respir. Crit. Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat. Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 28.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am. Rev. Respir. Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 29.Ramstedt U, Serhan CN, Nicolaou KC, Webber SE, Wigzell H, Samuelsson B. Lipoxin A-induced inhibition of human natural killer cell cytotoxicity: Studies on stereospecificity of inhibition and mode of action. J. Immunol. 1987;138:266–270. [PubMed] [Google Scholar]
- 30.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, Jarjour NN, Coverstone AM, Castro M, Holguin F, Wenzel SE, Woodruff PG, Bleecker ER, Fahy JV. National Heart, Lung, and Blood Institute Severe Asthma Research Program, Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometry gating strategies.
Fig. S2. BAL lymphocytes in healthy and asthmatic individuals.
Fig. S3. Levels of additional cytotoxic mediators in BALF from healthy and asthmatic individuals.
Fig. S4. Systemic triamcinolone does not alter BAL CD4+ T cell number or the ratio of effector CD4+ T cells to NK cells in asthma.
Fig. S5. Exposure to dexamethasone or LXA4 changes peripheral blood NK cell phenotype, and LXA4 exposure does not impair NK cell release of cytotoxic mediators.
Table S1. Patient number (n) for each experiment.