Abstract
Introduction
Insulin resistance is one of the most common features of polycystic ovary syndrome, and some studies suggest that vitamin D deficiency may have role in insulin resistance.
Objective
To study the effect of vitamin D supplementation on the clinical, hormonal and metabolic profile of the PCOS women.
Study Design
Randomized, placebo-controlled, interventional, double-blind study.
Materials and Methods
PCOS women were evaluated and enrolled after considering inclusion and exclusion criteria. They were randomized by block randomization with sealed envelope system done in two groups. In the study group (n = 25), patients were supplemented with vitamin D 60,000 IU weekly for 12 weeks, whereas control group (n = 25) was given placebo weekly for the same period. Both the groups were compared pre- and post-supplementation for variables like clinical profile, biochemical profile and metabolic profile. Statistical analysis was performed by the SPSS program for Windows, version 10.1 (SPSS, Chicago, IL).
Result
In the study (n = 50), PCOS patients were enrolled; 34 patients (68%) were vitamin D deficient (≤20 ng/ml) out of which 10 patients (29%) were severely deficient (<10 ng/ml). Twelve patients (24%) were vitamin D insufficient showing high prevalence of vitamin D deficiency in the PCOS women. The difference in mean serum fasting glucose pre- and post-supplementation of vitamin D in study group was found to be statistically significant with p value of 0.041. There was significant difference seen in insulin resistance (IR) (2.38 ± 4.88–1.00 ± 0.58, p = 0.003), serum fasting insulin (10.34 ± 20.00–5.00 ± 3.25, p = 0.021), and increase in insulin sensitivity determined by QUICKI (0.37 ± 0.04–0.394 ± 0.009, p = 0.001) after supplementation with vitamin D.
Conclusion
The study concluded that there was a beneficial effect of vitamin D supplementation on ovulatory dysfunctions and blood pressure. Post-supplementation, there were decrease in insulin resistance and increase in insulin sensitivity. In the study decreased serum fasting insulin level and fasting blood sugar after vitamin D supplementation suggest underlying role of vitamin D in glucose homeostasis.
Keywords: Vitamin D, PCOS, Insulin resistance, Glucose homeostasis
Introduction
Polycystic ovary syndrome is a common endocrine disorder among reproductive aged women. Women with PCOS are at increased risk of insulin resistance, inflammation, obesity, type 2 diabetes and cardiovascular diseases, and all of these diseases states have been linked with vitamin D insufficiency [1].
Vitamin D insufficiency may contribute to the pathogenesis of PCOS by promoting insulin resistance, which increases the risk of T2DM and cardiovascular diseases. Several PCOS studies demonstrate that serum 25-hydroxyvitamin D concentration is negatively correlated with body mass index, body fat and insulin resistance [2, 3]. Additionally, vitamin D insufficiency-induced alteration in intracellular calcium may contribute to ovulatory dysfunction and reproductive abnormalities in PCOS.
The primary aim of the study was to determine the effects of vitamin D supplementation on the clinical, hormonal and metabolic profile of PCOS women.
Materials and Methods
The randomized controlled interventional double-blind study was conducted in the Department of Obstetrics and Gynaecology, ESI PGIMSR, Delhi, from September 2014 to September 2016. Women aged 18–45 years, diagnosed with PCOS as per Rotterdam criteria, were eligible. PCOS was diagnosed as per Rotterdam criteria, having two of the following three features: (a) oligo or anovulation (oligomenorrhea/infertility), (b) clinical and/or biochemical signs of hyperandrogenism (hirsutism/acne/alopecia) and (c) polycystic ovaries on ultrasound examination (defined as the presence of ≥12 follicles measuring 2–9 mm in diameter and/or ovarian volume ≥10 cm). Women were excluded if they were pregnant or taking vitamin D or calcium supplements or if they had diabetes, uncontrolled hypertension, untreated hypothyroidism or hyperthyroidism, liver disease, osteopenia, osteomalacia, renal disease or cardiovascular disease. Women on medication known to affect metabolic parameters such as metformin and corticosteroid drugs were excluded from studies. Women having Cushing syndrome, congenital adrenal hyperplasia, hyperprolactinemia and an androgen-secreting tumor were also excluded from studies. Participants were advised to maintain their usual diet and lifestyle habits, including sun exposure, physical activity and dietary intake of vitamin D and calcium. Written informed consent was obtained from all participants. Institutional ethical clearance was obtained before the start of study.
Sample Size Calculation
Vitamin D supplementation and its effects on polycystic ovary syndrome were observed by few studies. Study conducted by Khan et al. [4] observed significant difference in i-PTH before and after treatment in vitamin D group with mean difference as −24.28 and 95% CI (−39.80, −8.76) having p values (.004) and in the placebo group with the mean difference as −16.45 and 95% CI (−31.40, −1.49) having p value (.03). Taking these values as reference, the minimum required sample size with 90% power of study and 5% level of significance was 11 per group. To lower margin of error, sample size taken was 25 per group and total sample size was 50.
Formula used was comparison of independent means:
where Z α is the value of Z at two-sided alpha error 5% and Z β is the value of Z at power of 90%.
Study Design
This was a 12-week randomized, double-blind, placebo-controlled trial. Participants were randomized in a double-blind fashion to receive either vitamin D, 12,000 IU or placebo weekly for 12 weeks. The randomization was done by double block seal technique, so that the participants, research coordinator who administered the intervention and investigators who assessed the outcomes were blinded.
Methodology
Eligible participants presented in a 12-h fasting state for baseline visit. Blood pressure, pulse, anthropometrics and signs of hirsutism were recorded. Urine pregnancy test excluded pregnancy. Fasting sample was drawn to measure serum LH, FSH, prolactin, serum cortisol, serum estradiol, serum testosterone, serum prolactin, serum TSH, serum PTH, serum fasting insulin, serum vitamin D, serum calcium, fasting and/or 2-h postprandial glucose, total cholesterol, serum triglyceride levels, high-density lipoprotein (HDL) cholesterol.
Insulin resistance was calculated using HOMA-IR method. It is calculated multiplying fasting plasma insulin (FPI), expressed in mU/L, by fasting plasma glucose (FPG), expressed in mg/dL, and then dividing by the constant 405, i.e., HOMA-IR = FPI × FPG/405. Values above 2.5 signified insulin resistance. Insulin sensitivity check index (QUICKI) = 1/{log[fasting insulin (milliunits per litre)] + log[fasting glucose(milligrams per deciliter]} was calculated using this formula.
The clinical profile and biochemical parameters were measured before and after supplementation to assess the change from baseline. Based on 25(OH) D levels comparison done pre- and post-supplementation of vitamin D, vitamin D status would be classified as: Value above 30 ng/ml considered as vitamin D sufficient, value between 20 and 30 ng/ml considered as vitamin D insufficient, value between 10 and 20 ng/ml considered as vitamin D deficient, and value below 10 ng/ml considered as severely vitamin D deficient.
Statistical analysis was performed by the SPSS program for Windows, version 10.1 (SPSS, Chicago, IL). Continuous variables were presented as mean ± SD, and categorical variables were presented as absolute numbers and percentage. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t test, whereas the Mann–Whitney U test was used for those variables that were not normally distributed. For within the group comparisons from pre-post, paired t test or Wilcoxon signed-rank test was used. Categorical variables were analyzed using either the Chi-square test or Fisher’s exact test. p < 0.05 was considered statistically significant.
Result
Fifty PCOS women were randomized to vitamin D or placebo once weekly for 12 weeks. Both the groups were similar in age and BMI. The mean age was 26.04 ± 2.73 and 26.64 ± 3.73 years in the study and control groups, respectively. The mean BMI was 24.93 ± 2.81 and 25.55 ± 1.98 kg/m2 in the vitamin D and placebo group, respectively.
The majority of the subjects were vitamin D deficient at the baseline. In the study 92% of the enrolled PCOS patients were found to have vitamin D level less than 30 ng/ml. Thirty-four patients (68%) were vitamin D deficient (≤20 ng/ml) out of which 10 patients (29%) were severely deficient (<10 ng/ml). Twelve patients were vitamin D insufficient (24%) (20–30 ng/ml), showing high prevalence of vitamin D deficiency in the PCOS women.
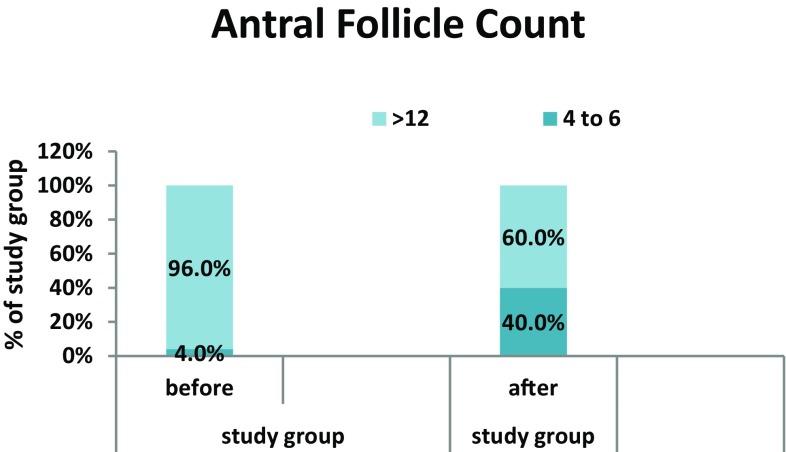
We found improvement in the menstrual irregularities after supplementation of vitamin D. In study group, before supplementation 20% of the patients had normal regular menstrual cycle which was found to be increased to 48% after supplementation with vitamin D. One patient in study group conceived with supplementation of vitamin D. Although 48% of patients after vitamin D supplementation resumed normal menstrual cycles, low conception rate could be because of short duration of study or because of other causes of infertility. There was 40% reduction in the number of antral follicles count in the ovaries after vitamin D supplementation (Fig. 1). We found no difference in waist–hip ratio (WHR) with vitamin D supplementation in study group. We had observed protective effect of vitamin D on systolic blood pressure in women with PCOS.
Fig. 1.
Comparison of antral follicle count before and after supplementation in study group
We have not found significant difference in the hormonal profile of the vitamin D-supplemented group (Table 1). In this study no changes were observed in the serum cholesterol and triglyceride after supplementation with vitamin D. However, we had found significant change in the HDLC values in the vitamin D-supplemented group (18.56 ± 9.68–44.90 ± 9.04, p = 0.035).
Table 1.
Comparison of hormonal and metabolic parameters of study group before and after supplementation of vitamin D
| Study group | Before (mean ± SD) supplementation | Median | After (mean ± SD) supplementation | Median | p value |
|---|---|---|---|---|---|
| FSH (mIU/ml) | 5.55 ± 1.22 | 5.50 | 5.51 ± 1.14 | 5.50 | 0.939 |
| LH (mIU/ml) | 9.59 ± 2.40 | 8.90 | 9.73 ± 1.80 | 9.60 | 0.979 |
| Estradiol (pg/ml) | 66.78 ± 22.76 | 61.40 | 69.78 ± 16.69 | 65.50 | 0.440 |
| S. cortisol (ng/ml) | 112.57 ± 72.26 | 81.50 | 105.90 ± 42.60 | 100.00 | 0.737 |
| S. prolactin (ng/ml) | 18.42 ± 12.42 | 17.00 | 16.56 ± 4.25 | 18.30 | 0.767 |
| S. TSH (mIU/ml) | 2.91 ± 1.08 | 2.80 | 2.76 ± 0.66 | 1.5–4.1 | 0.657 |
| S. testosterone | 1.65 ± 2.40 | 1.00 | 0.96 ± 0.24 | 9.00 | 0.362 |
| 17α-OH progesterone | 1.55 ± 0.93 | 1.20 | 1.42 ± 0.50 | 1.20 | 0.360 |
| DHEAS (μg/ml) | 1.28 ± 0.82 | 1.00 | 1.36 ± 0.39 | 1.20 | 0.085 |
We had found significant reduction in the fasting blood sugar levels of PCOS women in study group (88.24 ± 9.25–82.36 ± 8.03, p = 0.041) after supplementation of vitamin D, and no significant change was seen in the placebo group (Table 2).
Table 2.
Comparison of biochemical parameters of study group before and after supplementation of vitamin D
| Study group | Before (mean ± SD) | Median | After (mean ± SD) | Median | p value |
|---|---|---|---|---|---|
| S. Vit D | 18.56 ± 9.68 | 16.00 | 44.90 ± 9.04 | 44.60 | <0.001 |
| S. calcium | 9.14 ± 0.63 | 9.00 | 9.77 ± 0.33 | 9.80 | 0.001 |
| S. PTH | 149.36 ± 130.56 | 140.00 | 65.23 ± 17.53 | 58.90 | <0.001 |
| Cholesterol (mg/dl) | 164.16 ± 36.28 | 167.00 | 167.88 ± 25.11 | 110–205 | 0.619 |
| HDLC (mg/dl) | 38.04 ± 8.34 | 36.00 | 39.48 ± 4.88 | 38.00 | 0.035 |
| TG (mg/dl) | 101.04 ± 39.65 | 88.00 | 95.32 ± 20.64 | 60–150 | 0.782 |
| S. fasting glucose (mg/dl) | 88.24 ± 9.25 | 88.0 | 82.36 ± 8.03 | 80.3 | 0.041 |
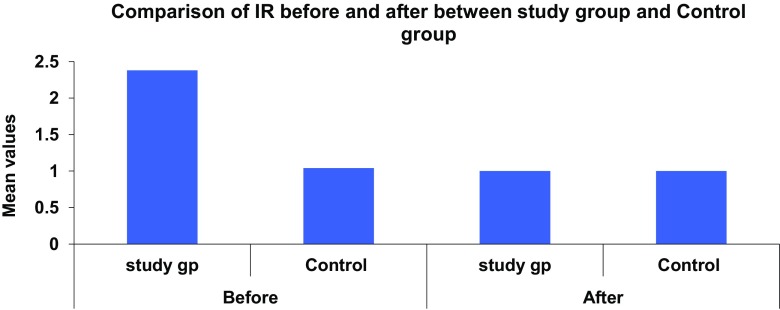
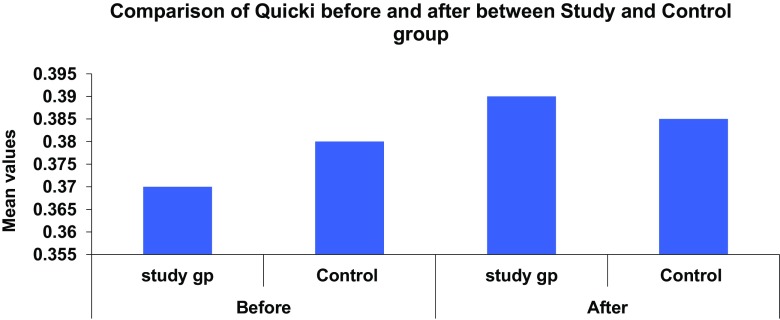
In the study group there was significant difference seen in the value of IR (2.38 ± 4.88–1.00 ± 0.58, p = 0.003), QUICKI (0.37 ± 0.04–0.394 ± 0.009, p = 0.001) and serum fasting insulin (10.34 ± 20.00–5.00 ± 3.25, p = 0.021). We found positive correlation between vitamin D levels and insulin sensitivity and negative correlation between vitamin D and insulin resistance and serum fasting insulin levels (Figs. 2, 3).
Fig. 2.
Comparison of serum insulin resistance in study and control group before and after supplementation
Fig. 3.
Comparison of serum insulin sensitivity in study and control group before and after supplementation
Discussion
Polycystic cystic ovary syndrome is a common endocrine disorder among women in their reproductive years, and it is associated with metabolic abnormalities.
The majority of the subjects were vitamin D deficient at the baseline. Change in the serum calcium level in the study group after supplementation with vitamin D is via its role in extracellular calcium regulation and normal calcium influx across cell membranes.
Li et al. [5] demonstrated that vitamin D deficiency is highly prevalent in women with PCOS in Scotland and their study supported the evidence that vitamin D deficiency is associated with multiple metabolic risk factors in PCOS women. Similar finding was observed in our study where 92% of enrolled patients were vitamin D insufficient.
Ardabili et al. [6] conducted a randomized, placebo-controlled, double-blind trial with 50 women with PCOS, assigned to receive 3 oral treatments consisting of 50,000 IU of vitamin D3 or a placebo (1 every 20 days) for 2 months. The fasting blood glucose, insulin, 25-hydroxyvitamin D and parathyroid hormone levels, as well as the homeostasis model assessment (HOMA) of insulin resistance and quantitative insulin sensitivity check index (QUICKI), were measured at baseline and after treatment. In the vitamin D group, the serum level of 25-hydroxyvitamin D increased (6.9 ± 2.8–23.4 ± 6.1 ng/mL, p < 0.0001), and the parathyroid hormone level decreased (70.02 ± 43.04–50.33 ± 21.99 μ IU/mL, p = 0.02). They found no significant effect of vitamin D on the BMI of PCOS of patients similar to our study.
In our study 54% of the enrolled patients had normal BMI and 46% were overweight. Obesity is a well-recognized risk factor for vitamin D deficiency. Wehr et al. [2] and Yildizahan et al. [3] found an inverse correlation between BMI and serum 25(OH)D concentration in PCOS women. In obese, higher proportion of vitamin D which is fat soluble is sequestered in adipose tissues; hence, bioavailability of vitamin D is lowered [7].
Wehr et al. [8] also had not found any change in waist–hip circumference after supplementation of vitamin D. We also found no difference in waist–hip ratio (WHR) and BMI with vitamin D supplementation in the study group.
Vitamin D insufficiency is a modifiable risk factor for atherogenesis and hypertensive disorder [9]. As little as 2 mmHg decrease in SBP has been suggested to reduce CVD-related morbidity and morbidity by almost 6% and all cause mortality by 3% [10]. A lowering in SBP was previously described with 800 IU of vitamin D and Ca in a cohort of elderly women similar to our study.
Rashidi et al. [11] investigated the effect of calcium (1000 mg) and vitamin D (400 IU) and metformin (1500 mg) in regulating menstrual cycle in 60 infertile women with the PCOS. The patients were treated for 3 months. The number of dominant follicles >14 mm during 2–3 months of follow-up was higher in calcium vitamin D and metformin group, though no significant difference was seen in rates of pregnancy and menstrual irregularity. Similarly, in our study there was significant reduction in number of antral follicles after vitamin D supplementation.
Wehr et al. [8] supplemented 46 PCOS women with 20,000 IU cholecalciferol weekly for 24 weeks found that 23 women out of 46 women improved menstrual frequency and 4 out of 16 seeking pregnancy conceived.
We found improvement in the menstrual irregularities after supplementation of vitamin D. In study group before supplementation 20% of the patients had normal regular menstrual cycle which increased to 48% after supplementation with vitamin D. One patient in study group conceived with supplementation of vitamin D. Low conception rate may be because of short duration of study or because of other cause of infertility.
Kotsa et al. [12] demonstrated that vitamin D supplementation had beneficial effect on insulin discharge and serum lipids in patients with PCOS, and our results also showed significant increase in HDLC.
In this study no changes were observed in the serum cholesterol and triglyceride after supplementation with vitamin D. However, we have found significant change in the HDLC values in the vitamin D-supplemented group (18.56 ± 9.68–44.90 ± 9.04, p = 0.035).
Vitamin D insufficiency induces elevations in PTH, which can adversely affect glucose metabolism. As expected PTH level decreased within the vitamin D supplementation. In our study we have found decrease in the levels of PTH with vitamin D supplementation in study group, while no difference was seen in control group.
Muscogiuri et al. [13] carried a study in 23 obese and lean PCOS women, and IR was evaluated using hyperinsulinemic–euglycemic clamp method, which is gold standard for determining IR. They found positive correlation between serum 25 OHD levels and glucose uptake.
Selimoglu et al. [14] did a small uncontrolled interventional trial in obese women with PCOS (n = 11) which demonstrated a significant decrease in insulin resistance (HOMA-IR; 4.41–3.67) 3 weeks after a single dose of vitamin D (300,000 IU). The single dose significantly increased 25 OHD levels from 16.9 to 37.1 ng/ml, and only two women had level lower than 25 ng/ml.
Wehr et al. [15] did pilot study in which 46 women with PCOS who had received 20,000 IU cholecalciferol weekly for 24 weeks demonstrated an increase in 25 OHD levels (28–51.3 ng/ml at 12 weeks and 52.4 ng.ml at 24 weeks). He found significant decrease in fasting insulin, and stimulated insulin HOMA levels were unchanged.
Wehr et al. [2] carried a study in which 206 PCOS women were stratified by hypovitaminosis D (<75 nmol/l, n = 150) and vitamin D sufficiency (>75 nmol/l, n = 56). They reported that the hypovitaminosis D group had a significantly higher HOMA-IR than the vitamin D sufficient group. There was inverse correlation with HOMA-IR and BMI, and there was positive correlation with HDL level.
The potential mechanism by which vitamin D can affect glucose metabolism could be the result of direct and indirect actions of serum 25 OHD: (1) direct stimulation of insulin release through the expression of VDR as well as the enzymes 1α-OHase in the pancreatic β-cells; (2) through the binding of 1,25(OH)2D-VDR complex to the vitamin D response element of the INSR at the tissue level and thereby enhancing insulin responsiveness for glucose transport; and (3) suppression of the release of pro-inflammatory cytokines that are believed to mediate IR [16]. Vitamin D plays indirect role through its influence on the extracellular and intracellular calcium regulation which is essential for the mediation of glucose transport in the target tissues.
In our study we found positive correlation between vitamin D levels and insulin sensitivity and negative correlation between vitamin D and insulin resistance and serum fasting insulin levels.
In this study we had found higher IR with mean of 2.41 ± 4.87 in study group with hypovitaminosis D with mean of vitamin D 18.56 ± 9.68 pre-supplementation. Significant improvement was seen in IR (1.00 ± 0.58, p = 0.003) with vitamin D supplementation in study group. Our study also showed positive association of vitamin D with insulin sensitivity (QUICKI).
The strength of this study was that it was a double-blind, randomized controlled study and most of the patients were vitamin D deficient. The relatively short duration and small sample size could be the limiting factor. Vitamin D supplementation could be an element in the complex treatment of PCOS patients presenting with obesity and hypovitaminosis D, to prevent other serious health consequences.
Conclusion
We found that PCOS patients presented with menstrual irregularities, increased AFC, hyperandrogenism and had metabolic risk factors like insulin resistance and low HDLC levels, independent of obesity. Vitamin D supplementation has a protective effect on systolic blood pressure. It had no beneficial effect on hormonal profile of the PCOS patients. In women with PCOS, insulin sensitivity increased, whereas the insulin resistance and fasting glucose decreased with vitamin D supplementation. Our study concluded that there is beneficial effect of vitamin D supplementation on ovulatory dysfunctions and insulin resistance. Large randomized controlled trials are needed to better understand the effect of vitamin D supplementation in women with PCOS.
Funding
This study was not funded by any outsource.
Dr. Taru Gupta
is Professor and Academic Head, Department of Obstetrics and Gynaecology, ESI PGIMSR Basaidarapur, New Delhi. Her field of interest is high-risk pregnancy, endoscopy and Gynae Oncology.
Compliance with Ethical Statement
Conflict of interest
All authors declare that they have no conflict of interest.
Research Involving Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Taru Gupta is Professor and Academic Head, Department of Obstetrics and Gynaecology, ESI PGIMSR Basaidarapur, New Delhi, India; Mukta Rawat is a Postgraduate Student in Department of Obstetrics and Gynaecology, ESI PGIMSR Basaidarapur, New Delhi, India; Nupur Gupta is a Assistant Professor, Obstetrics and Gynaecology, ESI PGIMSR Basaidarapur, New Delhi, India; Sarika Arora is Professor, Biochemistry, ESI PGIMSR Basaidarapur, New Delhi, India.
References
- 1.Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D glucose, insulin, and insulin sensitivity. Nutrition. 2008;24:279–285. doi: 10.1016/j.nut.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–582. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 3.Yildizhan R, Kurdoglo M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non obese women with polycystic ovary syndrome. Arch Gynaecol Obstet. 2009;280:559–563. doi: 10.1007/s00404-009-0958-7. [DOI] [PubMed] [Google Scholar]
- 4.Khan NR, Shah J, Stetter CM, Lott Mary EJ, Kunselman D. High dose vitamin D supplementation and measures of insulin sensitivity in PCOS:a randomized controlled pilot trial. Fertil Steril. 2014;101(6):1740–1746. doi: 10.1016/j.fertnstert.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li HWR, Brereton RE, Anderson RA, et al. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metab, Clin Exp. 2010;60:1475–1481. doi: 10.1016/j.metabol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;2012(32):195–201. doi: 10.1016/j.nutres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Lagunova Z, Porojnicu AC, Lindberg F, et al. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 8.Wehr E, Pieber TR, Obermayer-Pietsch B. Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in PCOS women—a pilot study. J Endocrinol Invest. 2011;34:757–763. doi: 10.3275/7748. [DOI] [PubMed] [Google Scholar]
- 9.Forman JP, Curhan GC, Taylor EN. Plasma 25- hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systemic review. Nat Rev Cardiol. 2009;6:621–630. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 11.Raishidi B, Haghollahi F, Shariat M, et al. The effects of calcium-vitamin D and Metformin on polycystic ovary syndrome; a pilot study. Taiwan J Obstetr Gynecol. 2009;48:142–147. doi: 10.1016/S1028-4559(09)60275-8. [DOI] [PubMed] [Google Scholar]
- 12.Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92:1053–1058. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- 13.Moscogiuri G, Policola C, Prioletta A et al. Low levels of 25(OH)D and insulin resistance: 2 unrelated features or a cause effect in PCOS? Clin Nutr 2012; PMID:22260937 [epub ahead of print]. [DOI] [PubMed]
- 14.Seglimoglu H, Duran C, Kiyici S, Ersoy C, Guclu M, Ozkaya G. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest. 2010;33:234–238. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 15.Wehr E, Trummer O, Giuliani A, et al. Vitamin D—associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–749. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010; 351385. doi:1155/2010/351385. [DOI] [PMC free article] [PubMed]