Abstract
Viral vectors are promising gene carriers for cancer therapy. However, virus-mediated gene therapies have demonstrated insufficient therapeutic efficacy in clinical trials due to rapid dissemination to nontarget tissues and to the immunogenicity of viral vectors, resulting in poor retention at the disease locus and induction of adverse inflammatory responses in patients. Further, the limited tropism of viral vectors prevents efficient gene delivery to target tissues. In this regard, modification of the viral surface with nanomaterials is a promising strategy to augment vector accumulation at the target tissue, circumvent the host immune response, and avoid nonspecific interactions with the reticuloendothelial system or serum complement. In the present review, we discuss various chemical modification strategies to enhance the therapeutic efficacy of viral vectors delivered either locally or systemically. We conclude by highlighting the salient features of various nanomaterial-coated viral vectors and their prospects and directions for future research.
Keywords: : adeno-associated virus, adenovirus, bioreducible polymers, cationic polymers, gene therapy, hydrogels, lentivirus, local delivery, systemic administration, viral vectors
Gene therapy is a powerful and promising strategy for treatment of various malignant diseases [1]. Viral vectors have been used more extensively than nonviral vectors to deliver therapeutic genes to a target site, due to significantly higher transgene expression [2]. In this regard, adenovirus (Ad), adeno-associated virus (AAV), retrovirus (RV) and lentivirus (LV) [3] have been actively explored.
To date, most virus-mediated clinical gene therapy has focused on direct and local administration of viral vectors to the disease site [4]. Many preclinical studies have reported potent therapeutic efficacy of locally administered viral vectors against a wide-range of localized illnesses. However, multiple administrations are required to achieve a sufficient therapeutic index in clinical trials. Viral vectors administered to particular physiological locations often diffuse to nontarget tissues and organs distant from the original administration site. This nonspecific shedding of viral vectors can induce an adverse host immune response or toxicity, thus limiting the administrable dose and the efficacy of viral vectors. Further, disseminated disease with multiple loci cannot be treated efficiently with local injection as virus-mediated transgene expression is attenuated with increasing distance from the injection site, resulting in poor therapeutic efficacy of viral vectors at distant disease sites. Systemic administration of viral vectors also has several limitations, as highly immunogenic viruses are rapidly cleared by nonspecific liver uptake and the host immune response, resulting in poor blood retention and limited viral accumulation at target tissues. Furthermore, viral vectors can be nonspecifically internalized into normal tissues, which can induce undesirable side effects. These nonspecific interactions with immune cells and nontarget cells hamper the efficiency of viral vectors delivered through systemic delivery.
Recent advances in nanotechnology have facilitated the development of innovative products for diagnosis and therapy [5,6]. Nanomaterial-based vector systems exhibit low immunogenicity, easily tunable molecular weight and structure, and facile conjugation of functional moiety to the nanomaterial backbone. The generation and synthesis of nanomaterials are straightforward, and they can be stored for long term. However, the efficiency of transgene expression mediated by nanomaterial-based vectors is markedly lower than that of viral vectors, limiting their therapeutic efficacy. To address the challenges of systemic delivery of viral vectors and the low therapeutic index of nanomaterial-based vectors, various nanomaterials, such as liposomes, polymers and nanoparticles, have been used to shield the surfaces of viral vectors, generating a hybrid vector system [7,8]. Hybrid vectors utilizing the strength of both viral and nanomaterial-based vectors can overcome each system's drawbacks while complementing their strengths. Nanomaterial coating can protect the viral vectors from host immune-surveillance and enzymatic degradation in vivo. Further, nanomaterials can harbor a targeting moiety that enhances target-specific localization and accumulation of the viral vectors. Following nanomaterial-mediated delivery and internalization into target cells, the viral portion of the hybrid system can facilitate endosome escape and nuclear localization, resulting in high transgene expression [9].
To date, a variety of natural and synthetic nanomaterials have been used for surface modification of viral vectors. In this review, we discuss the recent advancements in nanomaterial-coated viral vectors for gene therapy.
Frequently used viral vectors for gene delivery
Viral vectors can more efficiently deliver therapeutic genes to cells than nonviral vectors, making them attractive candidates for gene therapy [10]. Among viral vectors, Ad, AAV and RV have been extensively researched as gene delivery vectors. Each viral vector system has unique advantages and limitations, leading to diverse therapeutic applications (Table 1).
Table 1. . Basic characteristics of frequently used viral vectors for gene delivery.
| Adenovirus | Adeno-associated virus | Retrovirus | |
|---|---|---|---|
| Genome |
dsDNA |
ssDNA |
ssRNA(+) |
| Virion diameter |
70–100 nm |
˜20 nm |
80–120 nm |
| Coating |
Nonenveloped |
Nonenveloped |
Enveloped |
| Genome size |
36 kb |
4.7 kb |
8 kb |
| Tropism |
Dividing and nondividing cell |
Dividing and nondividing cell |
Dividing cell |
| Host genome interaction |
No integration |
Integration |
Integration |
| Vector yield (transducing units/ml) |
High (1013) |
High (1012) |
Moderate (109) |
| Transgene expression (duration) |
Transient (week to month) |
Long-term (>1 year) |
Long-term (>1 year) |
|
In vivo transgene expression |
High |
Low |
Low |
| Advantage for gene therapy | – Infection of both dividing and nondividing cells | – Infection of both dividing and nondividing cells | – Long-term transgene expression |
| – Strong gene expression | – Long-term transgene expression | – Transgene capacity up to 8 kb | |
| – Production of high-titer viral stocks | – Easy manipulation of viral genome | ||
| – No risk of insertional mutagenesis | |||
| – Transgene capacity up to 36 kb | |||
| |
– Easy manipulation of viral genome |
|
|
| Limitation for gene therapy | – Nonspecific liver accumulation | – Low transgene expression | – Poor target specificity |
| – Immunogenicity against Ad | – Nonspecific cellular uptake | – Limited gene transfer to dividing cells | |
| – Limited transgene capacity | – Mutation/recombination during reverse transcription | ||
| – Immunogenicity against AAV | – Risk of insertional mutagenesis | ||
| – Generation of replication-competent retrovirus | |||
| – Immunogenicity against retrovirus |
Adenovirus
Ad is a medium-sized (70–100 nm), nonenveloped (naked) icosahedral virus composed of nucleocapsid and double-stranded linear DNA genome [11]. Ad has several advantages for gene therapy application, including the ability to infect both dividing and nondividing cells, strong gene expression, the ability to produce high-titer viral stocks and no risk of insertional mutagenesis [12–14]. Replication-incompetent Ad, which lacks the Ad E1A gene essential for viral replication, has been widely used to transiently express therapeutic genes in a wide range of host cells [11]. Direct and local injection of Ad into target tissues has been frequently utilized to transduce a variety of target tissues, while systemic injection results in preferential trafficking to the liver [15]. Local injection of Ad in preclinical settings has been shown to effectively transduce skeletal muscle, heart, brain, lung, pancreas and tumors in order to treat a myriad of diseases such as Duchenne muscular dystrophy, ischemic heart disease, cystic fibrosis and cancer [16,17].
Gendicine (Shenzhen SiBiono GeneTech, China) was the first Ad gene therapy product approved for treatment of head and neck squamous cell carcinoma in the clinic [18]. Gendicine is a replication-incompetent Ad expressing wild-type p53, which activates the apoptotic pathway in tumor cells and decreases the expression of multidrug resistance, VEGF and MMP-2 genes. Further, Gendicine has been shown to induce synergistic therapeutic efficacy in combination with conventional treatments, such as chemo- and radiotherapy [19]. However, clinical applications of Gendicine have demonstrated insufficient therapeutic efficacy due to host immune response-mediated clearance of Ad and poor viral accumulation in tumor tissues, resulting in limited therapeutic efficacy and adverse side effects [20].
Oncolytic Ad, which conditionally replicates in cancer cells, is a promising therapeutic for cancer treatment as it selectively lyses cancer cells and expresses therapeutic genes at prominent levels [21–28]. Cancer-selectivity of oncolytic Ad can be achieved by modification of the endogenous Ad E1 region, which is integral for viral replication, resulting in prevention of viral replication in normal tissues [29–31]. E1A Rb binding site-mutated or E1B 55kDa-deleted oncolytic Ad resulted in cancer-selective viral replication, cytolysis and antitumor efficacy [25,32]. Alternatively, Ads have been endowed with cancer specificity using tumor-specific promoters to restrict the expression of E1A to cancer cells [33–36]. hTERT promoter-regulated oncolytic Ad showed active viral replication in hTERT-positive cells, while viral replication was significantly restricted in hTERT-negative normal cells [32,37]. Further, oncolytic Ads have been extensively modified to express therapeutic genes at high levels in targeted cancer cells. Insertion of anticancer therapeutic genes with antiangiogenic [21–23], proapoptotic [38,39] or immune-stimulatory [40–42] properties has demonstrated significantly improved therapeutic efficacy in comparison with control oncolytic Ad lacking these therapeutic genes. The Ad E1B 55 kDa-deleted oncolytic Ad Oncorin (H101), which selectively replicates in cancer cells with p53 mutation, was approved in 2005 by China's State Food and Drug Administration for the treatment of head and neck cancer [43].
Despite these advantages of Ad-mediated gene therapy, there are several drawbacks associated with their use, such as nonspecific liver accumulation and immunogenicity of Ad, which induce hepatotoxicity and acute inflammation [44]. Due to these limitations, the use of oncolytic Ad in clinical trials has been restricted to local administration. Furthermore, Ad is rapidly cleared from the blood and thus requires multiple doses to achieve an adequate therapeutic index. To overcome these issues associated with Ad-mediated gene therapy, a novel and systemically administrable Ad delivery method is required in order to minimize hepatic uptake and immunogenicity, while enhancing delivery and accumulation at the disease site.
Adeno-associated virus
AAV is a small nonenveloped virus (˜20 nm in diameter) that exhibits several advantageous attributes for gene therapy, including high titer production capability, the ability to transduce both dividing and nondividing cells and long-term expression of transgenes [45]. Despite these benefits, the cloning capacity of the vector is relatively limited. Thus, large therapeutic genes are not suitable for inclusion in the AAV vector [46]. There are 13 known AAV serotypes, and AAV serotype 2 (AAV2) has been widely studied and utilized as a vector for gene transfer. The AAV genome consists of two core genes (Rep and Cap) required for viral replication and for the production of structural proteins, respectively. AAV2 is a replication-defective parvovirus that requires a helper virus (dependovirus) such as Ad, herpes virus, or vaccinia virus for efficient replication [47,48]. The VA, E2A and E4 genes mediate replication of the AAV vector; thus, these genes have been incorporated into a helper plasmid [49]. AAV vectors have thus been produced by transfection of three separate plasmids each containing one of these attributes: ITRs flanking the therapeutic gene cassette, rep/cap genes or essential helper genes [50,51].
AAV has been shown to induce long-term transgene expression in a variety of tissues; thus, it has been actively explored for the treatment of genetic and acquired diseases, such as cystic fibrosis, hemophilia, Parkinson's disease and muscular dystrophy [52–55]. Although AAV-mediated gene therapy is efficient in tissues of varying origin, the most efficient transduction has been reported in skeletal muscle in vivo [52,56]. Intramuscular delivery of AAV was able to induce sustained expression of therapeutic genes over a 5-year period in a clinical trial [57]. Moreover, a Phase I clinical trial of AAV for the treatment of Duchenne muscular dystrophy demonstrated that AAV was able to stably transduce the follistatin gene, resulting in increased muscle size and strength [58].
Cancer gene therapy with AAV vectors is still in its infancy; however, AAV-mediated cancer gene therapy has shown promising results in preclinical studies. AAV-mediated cancer gene therapy has been explored to induce anti-angiogenesis, immune regulation and apoptosis [59–62]. Following either intratumoral or intramuscular injection of AAV expressing angiostatin, which prevents tumor vascularization, approximately 40% of treated mice in a glioma xenograft model survived for longer than 10 months. Conversely, 100% of the tumor-bearing mice treated with control AAV lacking angiostatin died of excessive tumor burden by 6 weeks [59,60]. Of note, the recombinant human angiostatin protein is unstable and is rapidly cleared from the blood, thus requiring multiple administrations at high doses [63,64]. Therefore, AAV-mediated gene therapy can be a more effective and expedient treatment option than conventional angiostatin protein therapy. AAV-mediated expression of an apoptotic gene has also demonstrated potent therapeutic efficacy for the treatment of cancer. TRAIL-expressing AAV has been shown to induce potent tumor growth inhibition in a colorectal tumor model by stimulating cytochrome c release from the mitochondria and caspase-3 activation [61]. Together, these findings indicate that AAV-mediated gene therapy can induce long-term and stable transgene expression at the disease site, resulting in prolonged therapeutic benefit with a single administration.
Despite these advancements in AAV-mediated gene therapy, the low-transduction efficiency and nonspecific cellular uptake limit its effective clinical application. Viral dissemination to nontarget tissues and subsequent nonspecific gene expression can lead to limited therapeutic efficacy and ectopic therapeutic gene expression at nontarget tissues, resulting in adverse side effects. [45]. Furthermore, the high prevalence of neutralizing Abs against AAV in the human population shortens the duration of therapeutic gene expression, leading to insufficient therapeutic efficacy in clinical settings [65].
Retroviruses
RVs, large enveloped RNA viruses, have been extensively studied for gene delivery. RVs possess ˜8 kb genomes and range in size from approximately 80–120 nm. When a RV infects a host cell, it introduces its RNA genome into the cell along with reverse transcriptase and can interact with the host machinery to undergo reverse transcription to form complementary DNA. Importantly, all RVs integrate therapeutic genes into the chromosomes of target cells, establishing long-lasting transgene expression and therapeutic efficacy. RV genomes commonly contain three open reading frames that encode gag, pol and env. To generate a recombinant retroviral vector suitable for gene therapy, these essential viral genes have often been replaced by therapeutic genes of interest, thus requiring packaging cells for the production of recombinant retroviral vectors [66]. Packaging cell lines, which express the gag, pol and env genes, provide essential viral proteins for capsid production and virion maturation. Among the wide-range of RVs, oncoretrovirus [67], LV [68] and spumavirus [69] have been frequently used in gene therapy. Oncoretrovirus-based vectors have been associated with several limitations in clinical settings, including poor target specificity, limited gene transfer to dividing cells, mutation/recombination during reverse transcription, risk of insertional mutagenesis, activation of the serum complement system against RVs and generation of replication-competent RVs. To overcome these limitations, retroviral vectors have been extensively modified to generate self-inactivating vectors [70]. Further, packaging cell lines have been modified to attenuate vector inactivation by serum complement [71] or to endow target-specificity to RVs [72], thus leading to enhanced safety and therapeutic profiles.
Recently, a therapeutic gene-expressing replicating retroviral vector has been developed to effectively kill cancer cells, making it a promising alternative to conventional cancer treatments [73]. This vector exhibits intrinsic tumor-selectivity due to its inability to infect quiescent cells, resulting in selective and stable therapeutic gene transfer to cancer cells. Suicide gene therapy, in which an apoptosis-inducing prodrug enzyme gene is introduced into cancer cells to catalyze the conversion of an inactive prodrug into an active cytotoxic form, is well-suited for replicating RV-mediated cancer gene therapy. Toca 511, developed by Tocagen, has been investigated as a promising candidate for RV-mediated suicide gene therapy in Phase I/II clinical trials against glioblastoma, lung cancer, breast cancer and colorectal cancer with liver metastases. Toca 511 is a replicating retroviral vector encoding cytosine deaminase, which catalyzes the conversion of inactive 5-FC into an active and cytotoxic metabolite (5-FU). Intravenous or intracranial injection of Toca 511 and cyclic administration of 5-FC to patients with high-grade gliomas resulted in prolonged survival since replicating RV can function as a long-term reservoir and catalyzes multiple cycles of prodrug administration. Further, integrated RV can spread to distant metastases as tumor cells migrate, leading to efficient prodrug enzyme gene expression at both primary and metastatic tumor sites [73,74].
LV-based vectors have gained prominence due to their unique ability to integrate into the genomes of nondividing cells [75,76]. LV-mediated gene therapy has shown therapeutic potential against chronic granulomatous disease, hemophilia A and diabetes [77–79]. Although LV-mediated gene therapy has achieved promising results in preclinical studies, insufficient safety and therapeutic efficacy have led to extensive modification of lentiviral vectors. To address the safety concerns associated with the generation of replication-competent LV, packaging systems have been modified by removing nonessential viral genes and separating functional viral components into separate expression plasmids [80]. To increase the transduction efficacy of LVs, additional elements, such as a lentiviral central polypurine tract, WPRE or PRE variants devoid of the X protein open reading frame, are routinely being introduced into lentiviral systems [81–83]. For example, incorporation of WPRE into a lentiviral system has been shown to increase lentiviral titer and transgene expression by improving the half-life, export and polyadenylation of mRNA [82]. WPRE-incorporated LV was able to efficiently transduce smooth muscle cells, hepatocytes, neural cells and blood cells [84–87], demonstrating that extensive modification of lentiviral vectors can induce safe, efficient and long-term transgene expression in perennial diseases.
Even though there are many advantages associated with the use of LV as a gene delivery vector, the majority of developed lentiviral vectors is associated with many safety concerns since mutational integration of the virus into the host genome and mobilization of structural viral genes can lead to cancer [88]. Further, the host can develop an adoptive immunity toward the lentiviral vector [89,90]. Lastly, lentiviral vectors have insufficient serum stability, leading to attenuated therapeutic efficacy in vivo. Therefore, these limitations of lentiviral vectors must be amended to achieve a safe and enhanced therapeutic outcome.
Local delivery of viral vectors using nanomaterials
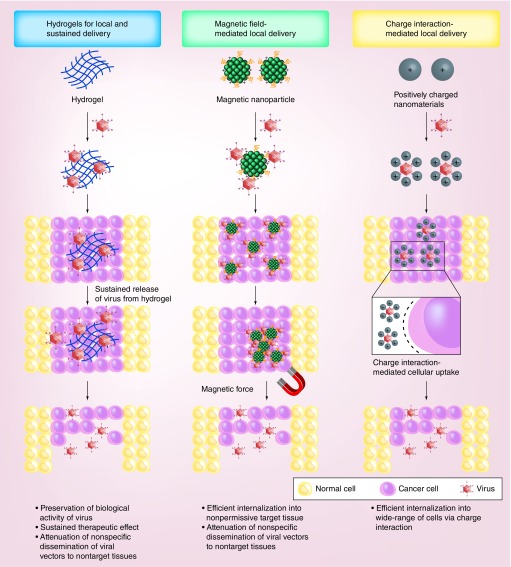
Direct injection of viral vectors into diseased tissues is a conventional route for viral gene delivery. However, viral vectors often disseminate from the injection site to surrounding nontarget tissues; thus, multiple injections are required to achieve an appropriate therapeutic index. Further, multiple administrations of the same viral vector can induce the production of neutralizing Abs that significantly attenuate the therapeutic efficacy of the viral vectors. To overcome rapid dissemination to nontarget tissues and antiviral immune responses, viral vectors have been encapsulated in various polymeric matrices, such as nanoparticles, microparticles, microspheres and hydrogels (Figure 1) [91–98].
Figure 1. . Local delivery of nanomaterial-complexed viral vectors.
Nanomaterial-complexed viral vectors are protected from the host immunity and degradative enzymes, resulting in the preservation of their biological activity in vivo. Viral vectors encapsulated in hydrogel can be released from the gel matrix over a prolonged periods of time to induce sustained therapeutic effect in target tissue with single administration [91,92]. Magnetic nanoparticle complexed virus can efficiently be internalized into nonpermissive target tissue upon exposure to magnetic field [93,96]. Both hydrogel- and magnetic nanoparticle-mediated local delivery of viral vectors can attenuate nonspecific dissemination of viral vectors from initial injection site to nontarget tissues, resulting in superior safety profile. Cationic nanoparticle-complexed virus are internalized into the cells through distinct charge interactions between the anionic cell surface and the cationic surface of the complex, thus enabling to transduce a wide range of cells with or without primary receptor for viral uptake [97,98].
Hydrogels for local & sustained delivery of viral vectors
Hydrogel is formed by self-assembly or cross-linking of natural polymers or synthetic polymers. Hydrogels have been broadly used as carrier systems and reservoirs for the sustained release of therapeutic agents. Hydrogels can be formed by linking a wide range of water-soluble polymers into a network, resulting in varying chemical and bulk physical properties. The highly porous and hydratable structure of hydrogels induces gelation and swelling in the biological microenvironment. These attributes enable the local administration of aqueous hydrogel by injection without invasive surgery, ultimately enhancing its application and safety [99]. Furthermore, the diameter of hydrogel pores can be easily manipulated to customize and control the release kinetics of a hydrogel's therapeutic cargo, making hydrogels attractive carriers for the sustained release of a wide range of therapeutics. The slow elution can also maintain a high local concentration of therapeutics in the local and surrounding target tissue for an extended time period [100]. Further, hydrogel can protect viral vectors from degradative enzymes and the host immune response [101,102]. Consequently, the sustained release of viral vectors from hydrogel can induce stable and sufficient transgene expression over prolonged periods of time [91].
Hydrogels generated from natural polymers have shown several superior characteristics over synthetic polymers, such as the capacity to emulate the natural tissue microenvironment and high biocompatibility. The easily tunable molecular size and chemical structure of synthetic polymer-based hydrogels are strengths associated with their use. However, synthetic hydrogels have inherently weak mechanical strength and insufficient biocompatibility that limit their application in biomedicine. Therefore, natural polymer-derived hydrogels have been more extensively studied for various biomedical applications, such as gene, drug and cell delivery [94,103–106].
Zeng et al. have generated a GFP-expressing recombinant AAV (rAAV2-GFP) encapsulated in a pH-sensitive polymeric hydrogel (PEG-polyHis) for the controlled release of viral vector in an hypoxic tumor microenvironment [107]. Hypoxia, which is characterized by low oxygen availability and subsequent acidification of the tumor microenvironment, is a clinical hallmark of human tumors. PEG-polyHis hydrogel, which is composed of PEG and poly (His) block copolymers, can swell and increase water uptake in an acidic cellular environment, leading to degradation of hydrogel and release of rAAV2-GFP at a hypoxic tumor site. As pH decreased from 7.4 to 6.0, the swelling and degradation rates of PEG-polyHis hydrogel increased 875 and 135%, respectively, demonstrating that both swelling ratio and degradation rate of the PEG-polyHis hydrogel increase inversely with environmental pH. The transduction efficiency of rAAV2-GFP released from PEG-polyHis hydrogel (pH 7.4) was similar to that from PEG hydrogel (pH 6.0 or 7.4). In marked contrast, rAAV2-GFP released from PEG-polyHis hydrogel at pH 6.0 showed markedly higher transduction efficacy than those released from PEG-polyHis hydrogel (pH 7.4) or PEG hydrogel (pH 6.0 or 7.4), indicating the preferential release of AAV in an acidic cellular environment. These results imply that PEG-polyHis hydrogel is a suitable candidate to preferentially release of AAV into hypoxic tumor tissue and for the induction of efficient therapeutic gene expression.
Entrapment of a lentiviral vector within a hydrogel has the potential to increase the stability of the LV through protection from denaturing enzymes and the host immune system [101]. Shin et al. investigated the potential of hydroxyapatite (HA) nanoparticle-incorporated collagen hydrogel loaded with LV to enhance long-term gene transfer [108]. The HA-incorporated collagen gel was able to retain and stabilize LV by HA-mediated immobilization within the gel, resulting in prolonged and enhanced gene expression in comparison with control collagen gel lacking HA. Importantly, the LV-mediated transgene expression was markedly higher for 4 weeks when virus was released from HA-incorporated collagen gel in comparison to those from control collagen gel. These results suggest that encapsulation of LV with HA-incorporated hydrogel can enhance the therapeutic profile of LV-mediated gene therapy by prolonged maintenance of lentiviral activity and transgene expression.
Kidd et al. demonstrated that addition of HA nanoparticles during the formation of fibrin hydrogel (fibrin-HA gel) enhanced lentiviral gene delivery in vitro and in vivo compared with fibrin gel lacking HA nanoparticles [101]. The presence of HA slowed collagenase-mediated hydrogel degradation in comparison to control fibrin gel, resulting in a lower rate of cell migration into the hydrogel. Interestingly, in comparison with control fibrin hydrogel, fibrin-HA gel maintained the transgene expression level for an additional two weeks in vivo before declining, suggesting that HA within fibrin hydrogel enhances the long-term gene transfer efficacy by increasing the stability of the LV. Taken together, these studies suggest that localized delivery of LV by HA-incorporated hydrogels can increase the duration of transgene expression by prolonged stabilization of the LV within the gel matrix.
Silk-elastin-like protein (SELP) polymer-based hydrogel has been utilized for Ad delivery into solid tumors via local injection [92,109]. SELP polymer can be prepared in an aqueous solution through an irreversible sol-to-gel transition at physiological temperature, facilitating its injection into tumor tissue. Furthermore, SELP polymers can be easily manipulated with respect to polymer length and sequence in order to change the release kinetics of therapeutics. Jung et al. examined C-Met-specific shRNA-expressing oncolytic Ad (Ad-C-Met) encapsulated in a SELP hydrogel matrix to induce sustained local delivery of Ad to tumors [110]. They found that naked Ad-C-Met elicited the highest cancer cell killing activity (˜87%) on day 3, and that this killing activity progressively decreased over the incubation period; negligible activity was observed on days 21 and 28. Conversely, the biological activity of Ad-C-Met released from a SELP hydrogel disc was conserved at 14 days (˜80% cancer cell killing efficacy) and partially conserved on days 21 and 28 (˜30%), suggesting that oncolytic Ad captured in SELP hydrogel matrix could elicit extended cancer cell killing efficacy. Human xenograft tumor-bearing mice treated by intratumoral administration of SELP hydrogel matrix encapsulating Ad-C-Met (Ad + SELP) showed markedly greater tumor growth inhibition than treatment with PBS control or naked Ad-C-Met. Further, a histological study revealed that more abundant Ad particles were present three weeks following virus administration in tumor those treated with Ad encapsulated in SELP hydrogel in comparison to tissues treated with naked Ad. These results are in a good agreement with previous studies in which intratumorally administered naked Ad was shown to rapidly disseminate into the surrounding tissue and cleared out by host immunity [109,111,112], resulting in poor viral accumulation in tumor tissues. Ad-mediated suppression of C-Met expression in tumor tissue was also significantly enhanced and prolonged by encapsulation with SELP matrix. Taken together, these results demonstrate that SELP hydrogel matrix can preserve the in vivo biological activity of Ad for long periods of time, making it an ideal candidate for sustained delivery of Ad.
Choi et al. demonstrated that injectable alginate gel is a suitable matrix for local and sustained delivery of oncolytic Ad into tumor tissues [111]. Alginate gel can function as a reservoir for the controlled release of Ad to tumor tissues while preserving the biological activity of Ad. Oncolytic Ad (DWP418) encapsulated in alginate gel (DWP418/gel) elicited a 1.9- or 2.4-fold greater antitumor effect than did naked Ad by the 41st day of treatment in a C33A or U343 xenograft model, respectively, suggesting that local administration of Ad encapsulated in alginate gel can enhance oncolytic Ad-mediated antitumor efficacy. On the 22nd day after intratumoral administration, a 4.51-fold higher Ad genome copy number was quantified in DWP418/gel-treated tumor tissues than in tissues treated with naked DWP418, indicating that the DWP418/gel overcomes the well-documented poor viral localization and maintenance of Ad in tumor tissues for extended time periods. Importantly, the DWP418/gel exhibited markedly attenuated accumulation of Ad in normal tissues in comparison to naked oncolytic Ad, demonstrating that alginate gel can efficiently prevent dissemination of oncolytic Ad to nontarget tissues, thus increasing oncolytic Ad's safety index. These results imply that local delivery of oncolytic Ad using an alginate gel matrix can enhance viral accumulation and prolong viral activity in tumor tissues, leading to a potent therapeutic outcome.
In summary, these studies illustrate the feasibility of biocompatible hydrogels for controlled and sustained release of viral vectors. Hydrogels can protect viral vectors from nonspecific interactions with the host environment, such as host immunity and degrading enzymes, while maintaining the biological activity of viral vectors for prolonged periods of time. These attributes make biocompatible hydrogels promising candidates for local and sustained delivery of viral vectors.
Magnetic field-mediated local delivery of viral vectors
Magnetic nanoparticles have been approved for clinical use as contrast agents for MRI [113]. Magnetic carriers complexed with drugs or viral vectors can be guided to the target area by exposure to a magnetic field gradient (MFG) [96]. These attributes of magnetic nanoparticles make them promising candidates to actively monitor the pharmacokinetics of therapeutics and can enhance delivery of therapeutics to target tissue. Recently, Sapet et al. developed a magnetic nanoparticle-complexed Ad vector and showed accelerated vector accumulation at target sites under external MFG [114]. The Ad-magnetic hybrid system (Ad-MnMEIO) has been developed for gene delivery and concurrent MR imaging [115]. This hybrid system was able to induce transgene expression in a coxsackie and adenovirus receptor (CAR)-dependent manner as it demonstrated higher GFP expression in CAR-positive U251N cancer cells compared with CAR-negative CHO-1 cells, suggesting that MnMEIO complex formation did not significantly hinder the internalization activity of Ad capsid. Further, MRI demonstrated that Ad-MnMEIO was visible as dark MR contrast in CAR-positive cells. These results imply that the magnetic nanoparticle-complexed Ad system has a potential to efficiently monitor the delivery of viral vectors in vivo for gene therapy. Bhattarai et al. coated the surface of a LacZ-expressing Ad with hexanoyl chloride-modified chitosan-stabilized magnetic nanoparticles (Nac-6-IOPs), generating Ad/LacZ/Nac-6-IOPs for viral gene delivery using magnetofection [116]. Ad/LacZ/Nac-6-IOPs-treated K562 leukemia cells exhibited two–tenfold increased level of β-galactosidase expression compared with cells treated with control (PBS) or naked Ad under MFG. Further, 15–30-fold higher β-galactosidase expression was observed in the lung tissues of mice after intratracheal administration of Ad/lacZ/Nac-6-IOPs in comparison to the level of expression achieved by naked Ad under MFG. Together, these results demonstrate that Nac-6-IOP is a suitable nanomaterial to mediate target-specific accumulation of Ad and to enhance Ad-mediated transgene expression. This enhanced gene transfer efficiency can further enable dose-reduction, leading to improved safety and therapeutic profiles.
Magnetofection of E1A-mutant oncolytic Ad (Ad520) has been explored for the potential enhancement of therapeutic efficacy after coating with polybrene-, silica- or branched PEI 25-kDa-modified magnetic nanoparticles (PB-Mag1, SO-Mag2 or PEI-Mag2-MNPs, respectively) [117]. PB-SO-Mag2-coated Ad elicited more potent antitumor efficacy than did the PBS control, PB-Mag1- or PEI-Mag-2MNPs-coated Ad, demonstrating the high therapeutic value of SO-Mag2-coated oncolytic Ad. Twenty-five days after treatment, SO-Mag2-coated oncolytic Ad-treated tumors showed 1.6- and 3.0-fold higher levels of accumulation of Ad particles than did tumors in the naked Ad or PEI-Mag2-coated Ad group, respectively, suggesting that SO-Mag2 coating can efficiently shield and internalize oncolytic Ad into tumor tissues for enhanced therapeutic efficacy. The potent antitumor efficacy of SO-Mag2-coated oncolytic Ad was attributed to higher MFG-induced velocity and magnetic moment compared with other complexes, resulting in accelerated viral accumulation and oncolysis. Further, SO-Mag2-coated oncolytic Ad was more resistant to the presence of Ad-specific neutralizing Abs than was naked Ad or the other magnetic particle-complexed Ads. These results indicate that SO-Mag2 coating can be an effective method for overcoming the limited therapeutic efficacy of naked Ad-mediated gene therapy in clinical settings where a significant portion of the patient cohort possesses some degree of adaptive immunity against Ad. Taken together, these results imply that the high magnetophoretic mobility and shielding efficiency of SO-Mag2 can lead to efficient viral accumulation and subsequently potent oncolysis in tumor tissues.
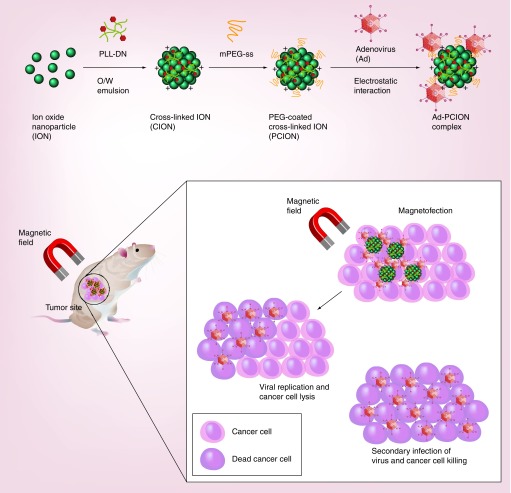
Choi et al. demonstrated that PEGylated PLL cross-linked iron oxide nanoparticle (PCION)-coated Ad complex (PCION/Ad) can enhance the transduction efficiency of Ad in the absence or presence of MFG, irrespective of cellular CAR expression (Figure 2) [118]. Further, the oncolytic Ad (HmT)-PCION complex demonstrated increased induction of apoptosis when used in combination with an external magnetic field compared with naked HmT or HmT-PCION without MFG. Intratumoral injection of HmT-PCION with MFG exposure elicited 2.7- and 2.1-fold higher antitumor effects compared with naked HmT under MFG or HmT-PCION without MFG, respectively. This potent tumor growth inhibition of HmT-PCION was caused by MFG-mediated enhancement of viral uptake and subsequent oncolysis in tumor tissues. Viral vectors complexed with cationic polymers have been shown to be internalized into the liver and nontarget tissues due to charge-mediated endocytosis. Of interest, Ad complexed with cationic PLL-based PCION under MFG showed markedly higher tumor-specific accumulation of Ad without substantial nonspecific liver uptake in comparison with naked Ad plus MFG or HmT-PCION without MFG. HmT-PCION plus MFG exhibited a 450-fold higher tumor-to-liver ratio than did HmT-PCION without MFG. These results imply that localized MFG exposure of a tumor can enhance vector accumulation in the target tissue and prevent vector dissemination into nontarget tissue, leading to enhanced therapeutic efficacy and an improved safety profile. Furthermore, immunohistochemical analysis of tumor tissue demonstrated increased oncolytic Ad replication in tumors following infection with HmT-PCION when directed by MFG, suggesting that PCION facilitates uptake of HmT in the presence of MFG and enhances viral replication in situ.
Figure 2. . Local delivery of magnetic nanomaterial-complexed viral vectors via magnetofection.
Viral vector complexed with a magnetic nanoparticle, such as PCION, can be guided by magnetic field to disease loci and internalized into disease tissue at accelerated rate. Importantly, magnetic field exposure to disease loci can prevent rapid diffusion of viral vector to nontarget tissues.
In sum, these studies demonstrate the potential use of magnetic nanoparticles for local delivery of viral vectors. Magnetic nanoparticles can further protect viral vectors from nonspecific interactions with the host environment, including macrophages, the reticuloendothelial system and virus-specific neutralizing Abs, while promoting efficient transgene expression at the target site through enhanced vector accumulation. These attributes make magnetic nanoparticle-coated viruses well-suited for targeted local cancer gene therapy as they can be utilized to enhance the therapeutic efficacy of the viral vector and to simultaneously image vector localization in vivo.
Charge interaction-mediated local delivery of viral vectors
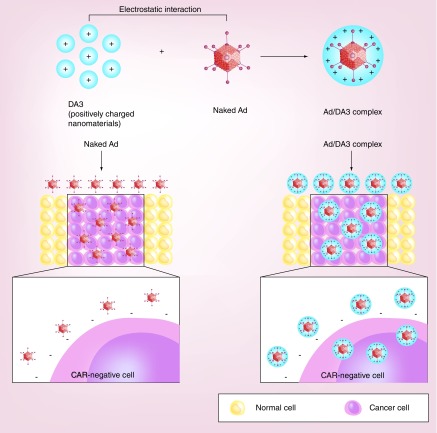
Surface charge is an important parameter in the transduction efficiency of nanomaterial-coated virus in vitro and in vivo. Anionic glycosaminoglycans abundantly expressed on the cell surface can interact with positively charged complexes through electrostatic interaction, subsequently increasing the cellular uptake of nanocomplexes. Based on this fundamental knowledge, several cationic polymers have been utilized to enhance the transduction efficacy of viral vectors [119]. Recently, Lee et al. have developed a bile acid-conjugated PEI (DA3)-modified Ad complex (Ad/DA3) that achieved significantly higher transduction efficiency in both CAR-positive and -negative cancer cells compared with naked Ad (Figure 3) [120]. TEM imaging revealed that the Ad/DA3 complex accumulated more rapidly (by 5 min post transduction) near the cell membrane than did naked Ad. Further, multiple Ad particles were observed in a single endocytic vesicle present in Ad/DA3-treated cells, whereas only a single Ad particle was detected in a vesicle present in naked Ad-treated cells. These results imply that the Ad/DA3 complex can be internalized more rapidly and efficiently into cancer cells than naked Ad, contributing to enhanced transgene expression. DA3-complexed oncolytic Ad showed significantly enhanced therapeutic efficacy compared with naked oncolytic Ad in an HT1080 fibrosarcoma tumor model. In support of this finding, more efficient viral replication and accumulation were observed in tumor tissues treated with DA3-coated oncolytic Ad than in naked Ad-treated tumors. Together, these results suggest that the potent antitumor efficacy of DA3-coated oncolytic Ad is most likely due to DA3-mediated rapid internalization into the tumor tissues and subsequent viral replication.
Figure 3. . Charge-mediated cellular uptake of nanomaterial-complexed viral vectors.
Cellular uptake of naked Ad is mediated by specific interaction of Ad capsid with complementary receptor (CAR) and integrins (αvβ3 and αvβ3) expressed on the cell surface, which limits its internalization into the cells with low expression level of CAR. In marked contrast, cationic nanomaterial-coated Ad is internalized into wide range of cells independently of Ad capsid's interaction with complementary receptor via charge interaction, leading to efficient internalization into various cells with varying CAR expression levels.
Arginine-grafted bioreducible polymer has also been utilized to coat the Ad surface through ionic interactions, and this complex showed enhanced transduction and reduced Ad-associated immunogenicity [121]. Jung et al. developed a hybrid gene delivery system using bioreducible PEI (mPEG-PEI-g-Arg-S-S-Arg-g-PEI-mPEG; PPSA) [122]. Ad coated with PPSA (Ad/PPSA) showed increased transduction efficiency in both CAR-negative and -positive cells compared with naked Ad, demonstrating that the Ad/PPSA complex can be internalized independent of cellular CAR expression. Overcoming the CAR-dependency of Ad is integral for successful Ad-mediated cancer gene therapy as the heterologous tumors encountered in the clinical environment often exhibit attenuated CAR expression, limiting the therapeutic efficacy of oncolytic Ad [123]. Importantly, the DWP418/PPSA nanocomplex elicited a 2.24-fold greater antitumor efficacy than did naked Ad in a CAR-negative MCF7 xenograft tumor, demonstrating that the PPSA coating can increase the infection ability of Ad regardless of CAR expression and leads to potent antitumor efficacy against a heterogenic tumor population with varying levels of CAR expression.
RV has also been complexed with cationic polymers to increase the viral delivery efficacy to target cells [124–126]. Katakura et al. developed RV coated with poly(ethylene glycol)-poly-(L-lysine) block copolymer (PEG-PLL) that showed three- to sevenfold higher transduction efficiencies compared with naked retroviral vector in the Lewis carcinoma cell line and primary cultured brain cells without substantial toxicity, respectively [126]. Positively charged PLL facilitated complexation of the polymer with the anionic retroviral vector, leading to stable modification of the viral surface that was maintained after several rounds of centrifugation, while PEG endowed biocompatibility and reduction in PLL-mediated toxicity. Overall, these results suggest that PEG-PLL can be utilized to stably modify RV to achieve enhanced transduction efficacy without substantial toxicity.
Liposomes are a biomimicking carrier with unique attributes in that they can form a vesicular structure from single and multiple phospholipid bilayers and encapsulate hydrophilic and hydrophobic drugs within the bilayer membrane. Over the past two decades, cationic lipids have been utilized as carriers to transfect cells with various nucleic acids and viruses [127,128]. Further, liposomes can be easily engineered to become environment-sensitive and to incorporate targeting moieties, such as peptides, Abs or small molecules, for enhanced endosomal escape and target-specific delivery of their therapeutic cargo [129–131]. Wang et al. attempted to enhance the therapeutic efficacy of endostatin-expressing Ad (Ad-hE) in a CAR-deficient CT26 colon carcinoma murine model by encapsulating Ad in cationic liposome (Ad-hE/Lipo), which showed 68% greater transduction efficiency compared with naked Ad [132]. Treatment of a murine CT26 tumor by intratumoral injection of Ad-hE/Lipo resulted in more effective tumor growth inhibition compared with naked Ad. Moreover, a survival rate study showed that animals treated with Ad-hE/Lipo lived significantly longer than those treated with naked Ad.
Wan et al. developed an enzyme-responsive liposome for complexation with Ad [133]. The enzymatically cleavable PEG-lipids (PPC-Al) containing PEG and MMP substrate can be easily degraded by the type IV collagenases (MMP-2 and MMP-9) abundantly expressed in the tumor microenvironment. Ad complexed with MMP-cleavable PPC-Al (PPC-Al-Ad) exhibited higher transduction than naked Ad or noncleavable PEG-lipid-modified Ad vectors in MMP-positive cancer cells, demonstrating that MMP-mediated cleavage of PEG from cationic lipid can enhance tumor-specific transduction of Ad through increased charge interaction-mediated internalization. Further, complexation of Ad with PPC-Al markedly attenuated both the innate and adaptive immune responses against Ad in comparison with naked Ad, implying that PPC-Al can efficiently shield the surface of Ad from interacting with the host immune system. In addition, PPC-Al-Ad showed lower liver toxicity than naked Ad. Together, these results suggest that enzyme-responsive cationic liposomes can efficiently deliver Ad into MMP-expressing cancer cells via local administration and enhance the safety profile of Ad by reducing its immunogenicity.
Altogether, these studies demonstrate that cationic nanomaterials can enhance the transduction efficacy of nanomaterial-coated virus and protect the virus from the host immune response. Further, the charge-mediated cellular uptake mechanism of cationic nanomaterial-complexed viral vectors can enhance the delivery of viral vectors to cells lacking complementary cellular receptors necessary for viral internalization, thus expanding the application of virus-mediated gene therapy to efficiently treat a wide range of cells.
Systemic delivery of viral vectors using nanomaterials
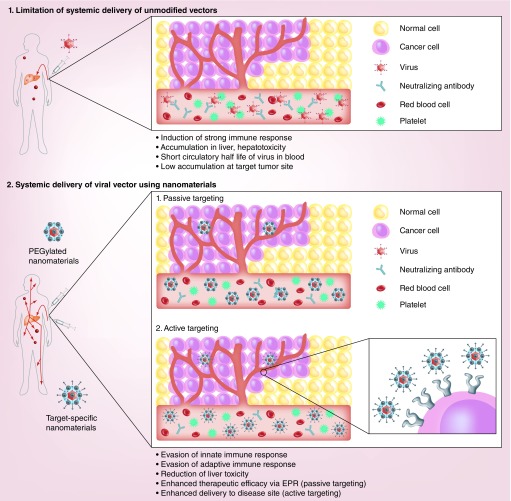
Over the last two decades, many clinical trials have demonstrated the effective use of intratumoral administration of viral vectors for cancer gene therapy against primary tumors. However, locally administered viral vectors are inefficient as a treatment for metastases, which are key contributors to cancer mortality and morbidity in the clinical environment. To treat both primary and disseminated metastases, a systemically injectable vector system is required. Systemic administration of viral vectors is associated with several issues, such as vector neutralization by the host immune response, short blood circulation time, ectopic gene expression in nontarget tissue and insufficient vector accumulation at the disease site, which limit their therapeutic efficacy. To address these challenges, surface modification of viral vectors with nanomaterials, such as polymers, liposomes and nanoparticles, has been widely attempted for systemic delivery of viral vectors (Figure 4) [98,134–136].
Figure 4. . Systemic delivery of nanomaterial-complexed viral vectors.
Overcoming poor pharmacokinetic and Biodistribution profile of systemically administered viral vector: The modification of the virus with nanomaterials allows prolonged retention of viral vectors in the blood and efficient targeting of disease loci. Passive tumor targeting: PEGylation of viral vectors can enhance pharmacokinetics of viral vectors and preferential accumulation of virus at tumor tissue by enhanced permeability and retention effect [98]. Active tumor targeting: viral vectors complexed with targeting moiety-conjugated nanomaterials can be internalized into targeted cells through specific interactions between the targeting moieties and complementary receptors expressed on the surface of target cells [136]. Alternatively, active tumor targeting of viral vectors can be achieved by complexation with tumor microenvironment-responsive polymers, which can be activated by tumor-specific conditions. Under tumor-specific conditions, such as hypoxia and high levels of MMPs, nanocomplex can be internalized into cancer cells [152,155].
PEGylation for systemic delivery of viral vectors
Poly(ethylene glycol) (PEG) is a hydrophilic, neutral polymer with negligible cytotoxicity. PEG has been frequently used as a delivery vehicle in biomedical applications and is approved by the US FDA, making it a safe polymer for clinical application. PEGylation of virus is a promising strategy to reduce the activation of the antiviral immune response and to enhance the blood circulation profile, as confirmed by reduced induction of pro-inflammatory cytokines (IL-6, IL-12 and TNF-α) and the increased half-life of systemically administered virus. PEGylation of virus minimizes nonspecific uptake by macrophages, hepatocytes and the reticuloendothelial system due to the shielding and steric hindrance effects of PEG molecules over the viral surface. O'Riordan et al. pioneered the covalent conjugation of PEG with Ad using monofunctional PEGs [137]. The active functional group of PEG can react with the free amine of lysine within the viral capsid protein, resulting in a covalent linkage between Ad and PEG. That study showed that PEGylating 15–20% of the Ad surface was sufficient to protect Ad from Ad-specific neutralizing Ab, whereas less than 10% PEGylation offered no protection, suggesting that a critical threshold of Ad surface must be PEGylated to achieve protection from interactions with the host immune response [138].
Yao et al. demonstrated the effect of PEG molecular size on the transduction and biodistribution of Ad [139]. In their study, Ad was covalently conjugated with 5 or 20 kDa PEG at various modification ratios. Ad conjugated with 20 kDa PEG at a modification ratio of 45% (PEG [20K/45%]-Ad) showed fivefold higher transgene expression in tumor tissue and 185-fold lower expression in liver tissue than unmodified Ad after systemic administration. In contrast, PEG [5K/90%]-Ad exhibited 40-fold higher transgene expression in tumor tissue while exhibiting twofold higher hepatic transgene expression, suggesting that the greater molecular size of PEG was more effective in preventing nonspecific interaction of Ad with nontarget tissues than was degree of Ad surface modification. Further, PEG [20K/45%]-Ad exhibited significantly improved blood retention and viral accumulation in tumor tissues compared with naked Ad or PEG [5K/90%]-Ad, suggesting that PEG [20K/45%]-Ad preferentially accumulated in tumor tissues due to an enhanced permeability and retention (EPR) effect mediated by leaky tumor vasculature. Herpes simplex virus thymidine kinase (HSVtk) converts ganciclovir (GCV) into an active cytotoxic metabolite. PEG [20K/45%]-conjugated Ad expressing hTERT-driven HSVtk (PEG-Ad-TERT/HSVtk) induced cancer-specific HSVtk expression in primary tumor and lung metastases after systemic administration. Importantly, PEG-Ad-TERT/HSVtk and GCV-treated mice exhibited a markedly attenuated number of metastatic colonies in their lungs compared with animals treated with PEG-Ad-TERT/HSVtk alone, GCV alone or naked Ad expressing hTERT-driven HSVtk, indicating that PEGylation of Ad can enhance the therapeutic profile of an Ad-mediated prodrug system against distant metastases. Together, these results demonstrate that EPR effect-mediated preferential accumulation of PEGylated vectors at tumor tissues and tumor-specific gene expression can augment the potency and safety of systemically administered Ad.
Although PEGylation of viral vectors can be an efficient strategy to enhance blood retention time and attenuate the immunogenicity of systemically administered viral vectors, insufficient transduction capacity of PEGylated vectors has been a major limitation. In order to preserve Ad's transduction capacity following PEGylation, Ad incorporating a biotin acceptor peptide (BAP) in the hypervariable region (HVR) 5 of hexon (Ad-BAP-L2) was generated [140]. HVR5 of Ad hexon is not involved in CAR-mediated endocytosis of Ad; thus, modification of HVR5 does not affect the innate transduction capacity of Ad. Biotin was then conjugated to the BAP of Ad-BAP-L2 and 5 kDa PEG, generating Ad-BAP/Bio-L2 and Bio-PEG-L2, respectively. Subsequently, Ad/BAP/Bio-L2 and Bio-PEG-L2 were mixed with avidin to generate PEGylated Ad (Ad-BAP/Bio/Avi/Bio-PEG-L2) via avidin–biotin interaction. Treatment with Ad-specific neutralizing Ab at increasing concentrations did not markedly alter the transgene expression efficiency of Ad-BAP/Bio/Avi/Bio-PEG-L2 in comparison with Ab-free conditions, whereas the transgene expression of naked Ad-BAP-L2 was significantly attenuated by neutralizing Ab in a dose-dependent manner. Furthermore, Ad-BAP/Bio/Avi/Bio-PEG-L2 exhibited transgene expression efficacy similar to that of Ad-BAP-L2. These results demonstrate that PEGylation of HVR5 does not significantly alter the endogenous transduction ability of Ad, while providing efficient protection against the host immune system. Further, a pharmacokinetic study revealed that PEGylation of HVR5 can efficiently prolong the half-life of Ad in vivo. Importantly, the tumor-to-liver ratio of Ad-BAP/Bio/Avi/Bio-PEG-L2 was markedly higher than that of unmodified Ad or Ad-BAP-L2, implying that Ad-BAP/Bio/Avi/Bio-PEG-L2 preferentially accumulates in tumor tissue through an EPR effect while still achieving a liver detargeting similar to other PEGylated Ad vectors. However, transgene expression of Ad-BAP/Bio/Avi/Bio-PEG-L2 in tumor tissue was significantly lower than that of unmodified Ad, suggesting that further modification is required to achieve a sufficient therapeutic index in vivo.
One of the major hurdles of successful systemic administration of lentiviral vector is vector inactivation in serum. Corey et al. have conjugated PEG to VSV-G-pseudotyped lentiviral vector and have shown that PEG conjugation to the lentiviral surface extends blood circulation time by fivefold and reduces the rate of vector inactivation by 1000-fold in comparison to unmodified VSV-G-psuedotyped lentiviral vector [141]. Further, the PEGylated VSV-G lentiviral vector showed significantly enhanced transduction efficiency in bone marrow and spleen. Together, these results imply that PEGylation of lentiviral vectors can prevent serum complement-mediated inactivation and improve the transduction efficiency of VSV-G pseudotyped lentiviral vectors in vivo, suggesting that a potent therapeutic index can be achieved by PEGylated vectors.
Taken together, these studies demonstrate that viral vectors conjugated with different molecular weights of PEG exhibit significantly enhanced stability, pharmacokinetics and protection against the immune response, leading to improved therapeutic efficacy upon systemic administration.
Target-specific nanomaterials for systemic delivery of viral vectors
Target-specific delivery of viral vectors is essential for efficient systemic treatment of various illnesses as inadequate specificity leads to poor biodistribution and subsequently limits the bioavailability of viral vectors at the target site, resulting in an insufficient therapeutic index. Further, nontargeted vectors are sequestered into off-target tissues, resulting in adverse side effects. To overcome these hurdles, active targeting strategies that rely on specific binding affinity between targeting moiety of a nanocomplex and the cognate receptors on the surface of the cell membrane have been extensively explored as a means to modify virus vectors. Currently, various targeting moieties, such as small molecules [142–146], natural ligands [147] and macromolecules [136,148], have been utilized to maximize therapeutic efficacy while minimizing side effects.
Stevenson et al. developed laminin peptide (SIKVAV) conjugated HPMA for complexation with Ad in order to achieve tumor-specific targeting through interaction between laminin peptide and α6β1-integrin overexpressed on prostate cancer cells [149]. Competition studies demonstrated that cellular entry of the Ad nanocomplex was dependent on the cellular expression of α6β1 integrin rather than on CAR, implying that internalization of the Ad nanocomplex was mediated by laminin peptide conjugated on the surface of HPMA polymer. This Ad nanocomplex elicited markedly enhanced liver detargeting ability, as Ad-mediated transgene expression was attenuated by 300-fold in comparison with naked Ad, leading to reduced Ad-associated hepatotoxicity. Further, systemically administered Ad nanocomplex exhibited levels of transgene expression similar to those achieved with naked Ad in tumor tissue, indicating that α6β1 integrin-targeting Ad nanocomplex can achieve a therapeutic profile similar to that of naked Ad while attenuating Ad-associated hepatotoxicity.
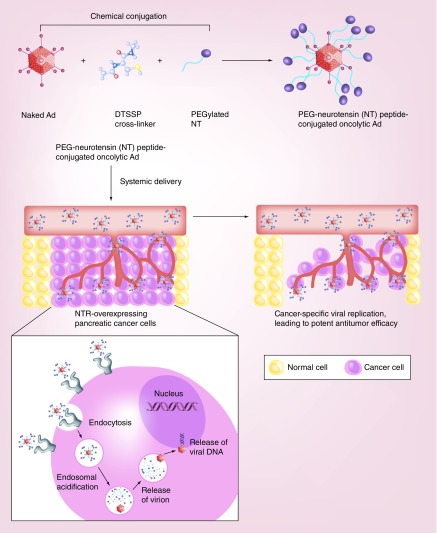
Recently, Na et al. investigated the therapeutic potential of oncolytic Ad conjugated with neurotensin peptide (NT)-incorporated PEG (oAd/DCN/LRP-PEG-NT) to treat neurotensin receptor (NTR)-overexpressing pancreatic cancer (Figure 5) [145]. Treatment with oAd/DCN/LRP-PEG-NT elicited NTR-specific transduction and cancer cell killing efficacy. Systemic administration of oAd/DCN/LRP-PEG-NT led to significantly attenuated induction of the innate and adaptive immune responses against Ad in comparison with naked Ad (similar to the baseline level in the PBS-treated group). Further, oAd/DCN/LRP-PEG-NT resulted in significantly greater accumulation of oncolytic Ad in tumor tissues compared with that achieved by naked oncolytic Ad or a PEGylated control nanocomplex lacking NT (oAd/DCN/LRP-PEG), indicating that active targeting mediated by NT induces more effective tumor-specific delivery of Ad than does EPR-mediated passive targeting. Importantly, oAd/DCN/LRP-PEG-NT elicited 2.7- and 2.0-fold higher antitumor efficacy in orthotopic pancreatic tumors than naked oncolytic Ad or oAd/DCN/LRP-PEG, respectively. These results suggest that the high targeting capacity of the nanocomplex led to an augmented therapeutic profile through enhanced accumulation of oncolytic Ad in the tumor tissues. Together, these results demonstrate that oAd/DCN/LRP-PEG-NT can achieve good therapeutic and safety indices by enhancing the tumor-specific accumulation of oncolytic Ad and preventing internalization into nontarget tissues.
Figure 5. . Systemic delivery of nanomaterial-complexed viral vectors to tumor tissue via tumor-specific targeting moiety.
PEGylated viral vectors are protected from host environment, resulting in enhanced half-life and pharmacokinetics in vivo. However, PEGylation alone results in inadequate vector accumulation at a disease loci. Systemic delivery of viral vectors to disease loci can be markedly enhanced by incorporating a targeting moiety on the surface of PEGylated virus. NT can function as a targeting moiety for NTR, which is overexpressed on pancreatic cancer cells. Ad covalently conjugated with NT-incorporated PEG demonstrated markedly enhanced localization and accumulation of Ad at NTR-positive pancreatic tumors in comparison with cognate control vector lacking a targeting moiety.
The Her2/neu-specific therapeutic Ab Herceptin is commonly used to treat a subset of breast cancer patients who overexpress Her2. Kim et al. developed Herceptin-conjugated PEG (PEG-HER) for complexation with an oncolytic Ad expressing relaxin (DWP418; DWP418-PEG-HER) [136]. One hour after systemic administration of DWP418-PEG-HER, significantly greater numbers (16-fold) of viral particles were retained in blood relative to naked Ad, implying that Ad can be efficiently protected from the host environment by conjugation with PEG-HER. Further, Her2/neu-positive SKOV3 and MDA-MB435 xenograft tumors treated with DWP418-PEG-HER showed significantly greater tumor growth inhibition in comparison to that of naked oncolytic Ad or PEGylated DWP418 (DWP418-PEG). In contrast, DWP418-PEG-HER-treated Her2/neu-negative MCF7-mot xenograft tumors elicited antitumor activity similar to that of DWP418-PEG, demonstrating that the potent antitumor efficacy of DWP418-PEG-HER is mediated by Her2/neu-specific internalization of oncolytic Ad. Moreover, DWP418-PEG-HER showed a tenfold higher tumor-to-liver ratio than did naked oncolytic Ad in Her2/neu-positive tumors, implying that Her2/neu-specific cellular uptake leads to liver detargeting and preferential tumor localization for improvements in safety and therapeutic indices.
Together, these studies demonstrate that incorporation of a targeting moiety into a nanomaterial can greatly augment the therapeutic and safety profiles of viral vectors via induction of the target-specific accumulation of viral vectors. Of note, targeting moiety-mediated active targeting resulted in more efficient intratumoral accumulation of viral vectors than did EPR-mediated passive targeting, suggesting that it is an indispensable platform for targeted systemic therapy. However, multistep chemical modification of viral vectors is required to conjugate the targeting moieties, and this may alter the biological activity of viral vector. Thus, single-step viral vector modification protocols to provide viral vector/nanocomplex systems should be investigated.
Tumor microenvironment-responsive polymers for systemic delivery of viral vectors
In most clinically apparent tumors, subpopulations of cancer cells within a single patient possess different genomes as the tumors evolve over time, resulting in heterogeneity among tumors [150]. As these heterogenic tumors express various cellular receptors at different levels, it is difficult to treat clinical tumors using therapeutics that target a single type of cellular receptor. To address this limitation, tumor microenvironment-responsive nanomaterials have been developed to target tumor-specific conditions that are commonly shared among the various subsets of tumor.
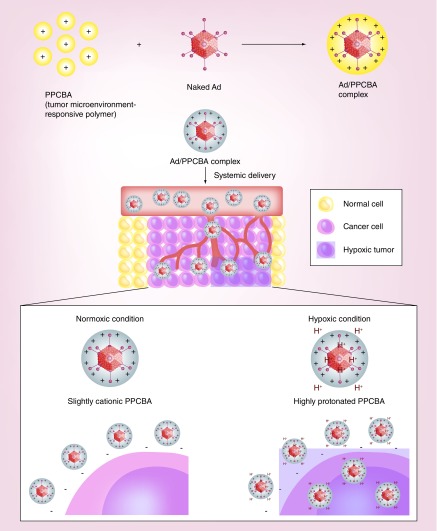
Hypoxia, which is characterized by low oxygen availability and subsequent acidification of the tumor microenvironment, is a hallmark of human tumors. Recently, Moon et al. have generated a pH-sensitive and bioreducible polymer (PPCBA) and coated the surface of oncolytic Ad to generate an Ad-PPCBA complex (Figure 6) [151]. The PPCBA polymer has a pH-responsive moiety that can be protonated under acidic conditions in tumor microenvironment, facilitating the release of its cargo into targeted cells. In CAR-negative cancer cells, the Ad-PPCBA complex exhibited 475.2- and 4.4-fold higher transduction efficacy than naked Ad at pH 6.0 or 7.4, respectively, confirming that protonation of PPCBA under acidic conditions can enhance the transduction efficiency. Importantly, systemic administration of VEGF-specific shRNA-expressing oncolytic Ad complexed with PPCBA (RdB/shVEGF-PPCBA) elicited significantly higher antitumor efficacy than naked RdB/shVEGF. Further, Ad-PPCBA significantly reduced the induction of IL-6 expression and ALT/AST levels relative to naked Ad (similar to basal level induced by PBS treatment), implying that PPCBA coating can effectively reduce the immunogenicity and hepatotoxicity of Ad.
Figure 6. . Systemic delivery of nanomaterial-complexed viral vectors to tumor tissue via tumor microenvironment targeting.
Hypoxia, an oxygen deprivation, occurs in tumor nests due to aberrant microcirculation and vascularization, contributing to acidic pH of tumor microenvironment. As hypoxia is a common attribute shared amongst the tumors, tumor microenvironment-targeting of viral vectors via complexation with pH-sensitive nanomaterials, such as PPCBA, can induce preferential accumulation of viral vectors in the tumor. PPCBA is protonated under acidic tumor microenvironment, which increases the surface charge of PPCBA-coated viral vector and induces cellular internalization of virus to tumor cells. In contrast, PPCBA has a low surface charge under physiological pH, which restricts internalization of viral vector into nontarget cells.
Choi et al. have examined the therapeutic profile of oncolytic Ad expressing VEGF promoter-targeting transcriptional repressor (KOX) complexed with a pH-sensitive block copolymer (methoxy poly(ethylene glycol)-b-poly(L-histidine-co-L-phenylalanine; PEGbPHF) [152]. At pH 6.4, KOX/PEG bPHF significantly suppressed VEGF gene expression, cancer cell migration and vessel sprouting compared with naked KOX or KOX/PEG bPHF at pH 7.4, demonstrating that an acidic cellular environment enhances the antiangiogenic efficacy of KOX/PEG bPHF. Additionally, PEGbPHF-coated Ad showed markedly improved endosomal escape capacity in comparison with naked Ad, leading to enhanced viral release and subsequent secondary infection of tumor cells. The efficient endosomal escape of KOX/PEGbPHF was likely due to the high buffering capacity of PEGbPHF, which can efficiently disrupt endosomes and subsequently facilitate release of Ad. Importantly, the antitumor and antiproliferative activities of systemically administered KOX/PEG bPHF were significantly higher than those of naked KOX. Moreover, KOX/PEGbPHF-treated tumor tissues exhibited markedly increased viral distribution in tumor tissues in comparison with naked oncolytic Ad, implying that KOX/PEG bPHF elicits potent antitumor efficacy through preferential intratumoral accumulation of Ad. Together, these results suggest that PEGbPHF-coated Ad elicits a potent therapeutic profile through tumor microenvironment-targeting and enhanced endosomal escape proficiency.
Tseng et al. have developed a hybrid vector incorporating AAV and a pH-sensitive polyelectrolyte (AAV2-matrix) to enhance the tumor-specific delivery of AAV vector [153]. At an acidic pH of 4.0, the average size of the AAV2-matrix was enlarged by 4.5-fold in comparison with that at pH 7.4, as protonation of the amine group and absorption of water expands the polymer matrix, resulting in release of AAV in an acidic cellular environment. Importantly, the transduction efficacy of this AAV-matrix was markedly higher in tumor tissue relative to the efficiencies of naked AAV and PEI 25-kDa-complexed AAV, implying that tumor acidosis-mediated release of AAV from polyelectrolyte can induce more efficient and tumor-specific delivery of AAV compared with unmodified AAV.
MMPs are overexpressed in many types of tumor tissues and regulate cellular and extracellular components of the tumor microenvironment [154]. Fan et al. have developed a tumor microenvironment targeting β-cyclodextrin–PEI-MMP-cleavable-PEG polymer (CDPCP) in order to enhance the tumor-specific delivery potential of Ad [155]. CDPCP possesses an MMP-sensitive linker between the positively-charged β-cyclodextrin–PEI and the PEG molecule, which can be cleaved by MMP2. MMP2-mediated cleavage of this MMP-sensitive linker and detachment of PEG from cationic β-cyclodextrin–PEI increase the net surface charge of CDPCP-complexed Ad (CDPCP/Ad), facilitating the internalization of the complex into cells. CDPCP/Ad showed 2.9- to 14.0-fold higher transduction efficacies in MMP2-expressing cancer cells than did naked Ad, irrespective of the cellular CAR expression level. These results suggest that a high level of MMP2 expression in the tumor microenvironment can enhance internalization of Ad into tumor cells through an increase in the surface charge of the polyplex. Further, CDPCP/Ad elicited 1.5-fold higher transduction efficiency than an uncleavable control polymer-complexed Ad (CDPUP/Ad) in MMP2-positive cancer cells, whereas both CDPCP/Ad and CDPUP/Ad showed similar transduction efficiencies in MMP2-negative cancer cells, implying that MMP2-mediated cleavage of the PEG chain is essential for efficient internalization of Ad complex. Importantly, a biodistribution study in tumor-bearing mice revealed that accumulation of CDPCP/Ad in MMP2-positive tumors was 692- or 10.5-fold higher than that with naked Ad or CDPUP/Ad, respectively. Moreover, the hepatic accumulation of systemically administered CDPUP/Ad was 96.6% lower than that of naked Ad. Together, these results indicate that MMP-2-dependent dePEGylation of CDPCP/Ad can improve therapeutic efficacy and safety through effective tumor localization and liver detargeting.
Taken together, these studies demonstrate the feasibility of tumor microenvironment-responsive polymers for tumor-specific delivery of viral vectors via systemic administration. The increased surface charge of polymers in the tumor microenvironment facilitates tumor-specific accumulation and internalization of the nanocomplex, leading to enhanced tumor selectivity and therapeutic efficacy. Further, this tumor microenvironment-responsive polyplex can target a wide range of tumors from various cellular origins, thereby overcoming the constraints of targeting moiety-conjugated nanocomplex systems that target a specific cellular receptor.
Conclusion & future perspective
In this review, we highlighted recent developments in the modification of viral vectors using a variety of nanomaterials for local and systemic gene therapy applications. Hybrid vectors combining viral vectors and nanomaterials have shown increased virus stability in the host environment and shield virus from host immunity, leading to enhanced half-lives, safety profiles and therapeutic efficacies. Some of the hurdles associated with local delivery of viral vectors are poor viral accumulation and retention at disease sites. To this end, hydrogels, magnetic nanoparticles and cationic polymers/liposomes have been successfully used to improve viral accumulation at target tissues, leading to enhanced therapeutic efficacy. Hydrogels can protect viral vectors from degradative enzymes and host immunity, leading to improvement in half-life and in the biological activity of the virus at the target tissue for prolonged periods of time. Magnetic particle-coated viral vectors under magnetic field exposure exhibit accelerated vector accumulation, enhanced cellular uptake and prolonged retention of virus at the targeted tissue, ensuring potent therapeutic efficacy via local administration. Importantly, both hydrogels and magnetic nanoparticles prevented viral dissemination into nontarget tissue, assuring a good safety profile. Cationic nanomaterial-coated viral vectors demonstrated efficient evasion of the host immune system. Furthermore, cationic nanomaterial-coated virus exhibited enhanced cellular uptake and transgene expression in a wide range of cells with or without the complementary cellular receptors essential for virus internalization, thus expanding the potential therapeutic targets of these viral vectors.
Certain illnesses with multiple disease loci necessitate systemic administration of viral vectors. However, systemic administration of viral vectors poses significant problems due to poor target-specific delivery and the short serum half-lives of viral vectors. PEGylation of therapeutics has been extensively researched to attenuate recognition and uptake by the reticuloendothelial system, thereby decreasing plasma opsonin adhesion and increasing circulation time in the blood. PEGylated viral vectors showed efficient protection against degradative enzymes and the host immune response; however, internalization of PEGylated vectors and subsequent transgene expression were insufficient in most target diseases. To overcome these hurdles, targeted nanomaterials have been thoroughly investigated as ways to enhance vector accumulation at disease sites. Both targeting moiety-mediated active targeting and microenvironment-targeting strategies, which target a specific cellular receptor or physiological condition, respectively, have resulted in efficient target-specific accumulation of viral vectors and attenuated ectopic gene expression at nontarget tissues. These attributes of targeted vectors lead to enhanced therapeutic efficacy and better safety profiles than EPR-mediated passive targeting. Collectively, this review demonstrates that both local and systemic delivery of viral vectors can be enhanced by complexation with nanomaterials, which improves viral accumulation at disease sites, prevents vector dissemination into nontarget tissue and shields viral vectors from the host immune system.
Critical hurdles of virus-mediated gene therapy in clinic can mainly be divided into following categories; immunogenicity of viral vectors, native viral tropism and lack of specificity toward a target disease. In this regard, a hybrid vector system utilizing multifunctional nanomaterial and viral vector is a particularly promising platform to overcome the innate limitations of viral vectors as nanomaterial can attenuate the virus-induced immune response and enhance the target-specific delivery of these vectors. Although hybrid vectors have demonstrated high therapeutic efficacy in preclinical studies, there are insufficient data regarding the safety of these materials in human, which poses as a significant hurdle toward the clinical evaluation of these vectors. Therefore, extensive safety assessment of targeted and multifunctional nanomaterials must be preceded prior to potential clinical evaluation of hybrid vector system. Nevertheless, these hybrid vectors are of considerable interest due to immense potential and promise of this strategy. Presently, clinical evaluation of hybrid vector could be well-suited for the treatment of localized illness with precise disease locus, as systemic administration will require the assessment of broader array of tissues, organs and metabolic pathways that these vectors traverse inside the host. For the treatment of cancer, nanomaterial-coated immunomodulatory oncolytic viruses are particularly promising as enhanced delivery of these vectors to local tissue has demonstrated potent antitumor efficacy against both primary and distant metastases via induction of systemic antitumor immune response. Furthermore, immune stimulatory nanoparticles, which has demonstrated potent antitumor immune responses in preclinical models, in conjunction with immunomodulatory oncolytic viruses can be a promising strategy to achieve systemic antitumor immunity [156,157].
Executive summary.
Viral vectors
Viral vectors are frequently used as gene carriers for gene therapy due to their high transgene expression efficiency mediated by proficient endosomal escape and nuclear localization capacity.
Adenovirus, adeno-associated virus and retrovirus have been extensively explored for clinical gene therapy.
Local delivery of viral vectors
Encapsulation of viral vectors within hydrogels can induce stable transgene expression in local tissue for prolonged periods of time. Furthermore, hydrogel matrix can efficiently protect viral vectors from the host immune system and enzymatic degradation.
Magnetic nanoparticle-coated viral vectors under magnetic field exposure can be preferentially accumulated and retained in target tissue while preventing dissemination into nontarget tissue, resulting in good safety and therapeutic profiles.
Positively charged nanomaterial-coated viral vectors can be efficiently internalized into a wide range of cells lacking complementary cellular receptors integral for internalization of naked virus, thus increasing the potential therapeutic targets of virus-mediated gene therapy.
Systemic delivery of viral vectors
PEGylation of viral vectors can enhance pharmacokinetics and blood retention and provide protection against the host immune response. Further, PEGylated viral vectors preferentially accumulate in tumor tissue due to EPR-mediated passive targeting.
Targeting moiety-mediated active targeting by a nanocomplex can enhance accumulation of viral particles at a disease site, while attenuating nonspecific sequestration into nontarget tissues. Active targeting-mediated by a targeted nanocomplex exhibits superior intratumoral accumulation than EPR-mediated passive targeting.
A tumor microenvironment-responsive nanocomplex can target a wide subset of tumors from different cellular origins and heterogenic tumor populations by targeting cellular conditions commonly shared among multiple tumors.
Footnotes
Financial & competing interests disclosure
This work was supported by grants from the National Research Foundation of Korea (2015R1A2A1A13027811 and 2013M3A9D3045879; C-O Yun, 2013R1A1A2012483; D. Kasala). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60(2):249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wong HH, Lemoine NR, Wang Y. Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses. 2010;2(1):78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv. Drug Deliv. Rev. 2006;58(8):931–947. doi: 10.1016/j.addr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Khuri FR, Nemunaitis J, Ganly I, et al. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000;6(8):879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]; • Demonstrates the potential applications of oncolytic viruses for cancer therapy.
- 5.Hajitou A. Targeted systemic gene therapy and molecular imaging of cancer contribution of the vascular-targeted AAVP vector. Adv. Genet. 2010;69:65–82. doi: 10.1016/S0065-2660(10)69008-6. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Curr. Opin. Biotechnol. 2011;22(6):901–908. doi: 10.1016/j.copbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reetz J, Herchenroder O, Putzer BM. Peptide-based technologies to alter adenoviral vector tropism: ways and means for systemic treatment of cancer. Viruses. 2014;6(4):1540–1563. doi: 10.3390/v6041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemoto H, Miyata K, Nishiyama N, Kataoka K. Bioresponsive polymer-based nucleic acid carriers. Adv. Genet. 2014;88:289–323. doi: 10.1016/B978-0-12-800148-6.00010-9. [DOI] [PubMed] [Google Scholar]
- 10.Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biol. Direct. 2006;1:29. doi: 10.1186/1745-6150-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol. Biotechnol. 1995;3(3):207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 12.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat. Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green NK, Hale A, Cawood R, et al. Tropism ablation and stealthing of oncolytic adenovirus enhances systemic delivery to tumors and improves virotherapy of cancer. Nanomedicine. 2012;7(11):1683–1695. doi: 10.2217/nnm.12.50. [DOI] [PubMed] [Google Scholar]
- 14.Bachtarzi H, Stevenson M, Fisher K. Cancer gene therapy with targeted adenoviruses. Expert Opin. Drug Deliv. 2008;5(11):1231–1240. doi: 10.1517/17425240802507636. [DOI] [PubMed] [Google Scholar]
- 15.Bramson JL, Graham FL, Gauldie J. The use of adenoviral vectors for gene therapy and gene transfer in vivo . Curr. Opin. Biotechnol. 1995;6(5):590–595. doi: 10.1016/0958-1669(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 16.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J. Neurooncol. 2003;65(3):237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 17.Rosenecker J, Harms KH, Bertele RM, et al. Adenovirus infection in cystic fibrosis patients: implications for the use of adenoviral vectors for gene transfer. Infection. 1996;24(1):5–8. doi: 10.1007/BF01780642. [DOI] [PubMed] [Google Scholar]
- 18.Pearson S, Jia H, Kandachi K. China approves first gene therapy. Nat. Biotechnol. 2004;22(1):3–4. doi: 10.1038/nbt0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005;16(9):1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin. Biol. Ther. 2006;6(8):823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- 21.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol. Ther. 2008;16(6):1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 22.Yoo JY, Kim JH, Kim J, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15(9):635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 23.Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol. Ther. 2007;15(2):295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 24.Vile R. Cancer gene therapy—new approaches to tumour cell killing. J. Gene Med. 2000;2(2):141–143. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<141::AID-JGM93>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002;9(9):725–736. doi: 10.1038/sj.cgt.7700494. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Kim JH, Choi KJ, Kim PH, Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum. Gene Ther. 2007;18(9):773–786. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 28.Kirn D. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. Oncogene. 2000;19(56):6660–6669. doi: 10.1038/sj.onc.1204094. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 30.Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of Phase I and II trials. Expert Opin. Biol. Ther. 2001;1(3):525–538. doi: 10.1517/14712598.1.3.525. [DOI] [PubMed] [Google Scholar]
- 31.Makower D, Rozenblit A, Kaufman H, et al. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin. Cancer Res. 2003;9(2):693–702. [PubMed] [Google Scholar]
- 32.Kim J, Lee B, Kim JS, et al. Antitumoral effects of recombinant adenovirus YKL-1001, conditionally replicating in alpha-fetoprotein-producing human liver cancer cells. Cancer Lett. 2002;180(1):23–32. doi: 10.1016/s0304-3835(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 33.Kim E, Kim JH, Shin HY, et al. Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum. Gene Ther. 2003;14(15):1415–1428. doi: 10.1089/104303403769211637. [DOI] [PubMed] [Google Scholar]
- 34.Cascallo M, Alonso MM, Rojas JJ, Perez-Gimenez A, Fueyo J, Alemany R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol. Ther. 2007;15(9):1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 35.Ryan PC, Jakubczak JL, Stewart DA, et al. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004;11(8):555–569. doi: 10.1038/sj.cgt.7700735. [DOI] [PubMed] [Google Scholar]
- 36.Barry MJ. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med. 2001;344(18):1373–1377. doi: 10.1056/NEJM200105033441806. [DOI] [PubMed] [Google Scholar]
- 37.Hallenbeck PL, Chang YN, Hay C, et al. A novel tumor-specific replication-restricted adenoviral vector for gene therapy of hepatocellular carcinoma. Hum. Gene Ther. 1999;10(10):1721–1733. doi: 10.1089/10430349950017725. [DOI] [PubMed] [Google Scholar]
- 38.Yang WS, Park SO, Yoon AR, et al. Suicide cancer gene therapy using pore-forming toxin, streptolysin O. Mol. Cancer Ther. 2006;5(6):1610–1619. doi: 10.1158/1535-7163.MCT-05-0515. [DOI] [PubMed] [Google Scholar]
- 39.Son YG, Kim EH, Kim JY, et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;67(17):8274–8284. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 40.Choi KJ, Kim JH, Lee YS, et al. Concurrent delivery of GM-CSF and B7–1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 2006;13(13):1010–1020. doi: 10.1038/sj.gt.3302759. [DOI] [PubMed] [Google Scholar]
- 41.Lee YS, Kim JH, Choi KJ, et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7–1 in an immunocompetent murine model. Clin. Cancer Res. 2006;12(19):5859–5868. doi: 10.1158/1078-0432.CCR-06-0935. [DOI] [PubMed] [Google Scholar]
- 42.Huang JH, Zhang SN, Choi KJ, et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4–1BBL. Mol. Ther. 2010;18(2):264–274. doi: 10.1038/mt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen EE, Rudin CM. ONYX-015. Onyx Pharmaceuticals. Curr. Opin. Investig. Drugs. 2001;2(12):1770–1775. [PubMed] [Google Scholar]
- 44.Kang E, Yun CO. Current advances in adenovirus nanocomplexes: more specificity and less immunogenicity. BMB Reports. 2010;43(12):781–788. doi: 10.5483/BMBRep.2010.43.12.781. [DOI] [PubMed] [Google Scholar]
- 45.Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7(1):24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- 46.Fine EJ, Appleton CM, White DE, et al. Trans-spliced Cas9 allows cleavage of HBB and CCR5 genes in human cells using compact expression cassettes. Sci. Rep. 2015;5:10777. doi: 10.1038/srep10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 48.Buller RM, Janik JE, Sebring ED, Rose JA. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J. Virol. 1981;40(1):241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carter BJ, Marcus-Sekura CJ, Laughlin CA, Ketner G. Properties of an adenovirus type 2 mutant, Ad2dl807, having a deletion near the right-hand genome terminus: failure to help AAV replication. Virology. 1983;126(2):505–516. doi: 10.1016/s0042-6822(83)80008-7. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita T, Elliger S, Elliger C, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5(7):938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 51.Tal J. Adeno-associated virus-based vectors in gene therapy. J. Biomed. Sci. 2000;7(4):279–291. doi: 10.1007/BF02253246. [DOI] [PubMed] [Google Scholar]
- 52.Miao CH, Nakai H, Thompson AR, et al. Nonrandom transduction of recombinant adeno-associated virus vectors in mouse hepatocytes in vivo: cell cycling does not influence hepatocyte transduction. J. Virol. 2000;74(8):3793–3803. doi: 10.1128/jvi.74.8.3793-3803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riaz M, Raz Y, Moloney EB, et al. Differential myofiber-type transduction preference of adeno-associated virus serotypes 6 and 9. Skelet. Muscle. 2015;5:37. doi: 10.1186/s13395-015-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brimble MA, Reiss UM, Nathwani AC, Davidoff AM. New and improved AAVenues: current status of hemophilia B gene therapy. Expert Opin. Biol. Ther. 2015;16(1):79–92. doi: 10.1517/14712598.2015.1106475. [DOI] [PubMed] [Google Scholar]
- 55.Yan Z, Sun X, Feng Z, et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum. Gene Ther. 2015;26(6):334–346. doi: 10.1089/hum.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996;70(11):8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stedman H, Wilson JM, Finke R, Kleckner AL, Mendell J. Phase I clinical trial utilizing gene therapy for limb girdle muscular dystrophy: alpha-, beta-, gamma-, or delta-sarcoglycan gene delivered with intramuscular instillations of adeno-associated vectors. Hum. Gene Ther. 2000;11(5):777–790. doi: 10.1089/10430340050015671. [DOI] [PubMed] [Google Scholar]
- 58.Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci. Lett. 2012;527(2):90–99. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma HI, Guo P, Li J, et al. Suppression of intracranial human glioma growth after intramuscular administration of an adeno-associated viral vector expressing angiostatin. Cancer Res. 2002;62(3):756–763. [PubMed] [Google Scholar]
- 60.Ma HI, Lin SZ, Chiang YH, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9(1):2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 61.Mohr A, Henderson G, Dudus L, et al. AAV-encoded expression of TRAIL in experimental human colorectal cancer leads to tumor regression. Gene Ther. 2004;11(6):534–543. doi: 10.1038/sj.gt.3302154. [DOI] [PubMed] [Google Scholar]
- 62.Zhang JF, Hu C, Geng Y, Blatt LM, Taylor MW. Gene therapy with an adeno-associated virus carrying an interferon gene results in tumor growth suppression and regression. Cancer Gene Ther. 1996;3(1):31–38. [PubMed] [Google Scholar]
- 63.Kirsch M, Strasser J, Allende R, Bello L, Zhang J, Black PM. Angiostatin suppresses malignant glioma growth in vivo . Cancer Res. 1998;58(20):4654–4659. [PubMed] [Google Scholar]
- 64.O'reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat. Med. 1996;2(6):689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 65.Van Der Marel S, Comijn EM, Verspaget HW, et al. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm. Bowel Dis. 2011;17(12):2436–2442. doi: 10.1002/ibd.21673. [DOI] [PubMed] [Google Scholar]
- 66.Sinn PL, Sauter SL, Mccray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors – design, biosafety, and production. Gene Ther. 2005;12(14):1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- 67.Ellis J, Yao S. Retrovirus silencing and vector design: relevance to normal and cancer stem cells? Curr. Gene Ther. 2005;5(4):367–373. doi: 10.2174/1566523054546233. [DOI] [PubMed] [Google Scholar]
- 68.Poeschla E, Corbeau P, Wong-Staal F. Development of HIV vectors for anti-HIV gene therapy. Proc. Natl Acad. Sci. USA. 1996;93(21):11395–11399. doi: 10.1073/pnas.93.21.11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olszko ME, Trobridge GD. Foamy virus vectors for HIV gene therapy. Viruses. 2013;5(10):2585–2600. doi: 10.3390/v5102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu W, Russ JL, Eiden MV. Evaluation of residual promoter activity in gamma-retroviral self-inactivating (SIN) vectors. Mol. Ther. 2012;20(1):84–90. doi: 10.1038/mt.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rigg RJ, Chen J, Dando JS, Forestell SP, Plavec I, Bohnlein E. A novel human amphotropic packaging cell line: high titer, complement resistance, and improved safety. Virology. 1996;218(1):290–295. doi: 10.1006/viro.1996.0194. [DOI] [PubMed] [Google Scholar]
- 72.Chandrashekran A, Gordon MY, Casimir C. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood. 2004;104(9):2697–2703. doi: 10.1182/blood-2003-10-3717. [DOI] [PubMed] [Google Scholar]
- 73.Tai CK, Kasahara N. Replication-competent retrovirus vectors for cancer gene therapy. Front. Biosci. 2008;13:3083–3095. doi: 10.2741/2910. [DOI] [PubMed] [Google Scholar]
- 74.Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. 2012;20(9):1689–1698. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes D, Andersson DI. Evolutionary consequences of drug resistance: shared principles across diverse targets and organisms. Nat. Rev. Genet. 2015;16(8):459–471. doi: 10.1038/nrg3922. [DOI] [PubMed] [Google Scholar]
- 76.Khabou H, Dalkara D. [Developments in gene delivery vectors for ocular gene therapy] Med. Sci. (Paris) 2015;31(5):529–537. doi: 10.1051/medsci/20153105015. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, Liu G, Zhang W, et al. Inhibition of MEF2A prevents hyperglycemia-induced extracellular matrix accumulation by blocking Akt and TGF-beta1/Smad activation in cardiac fibroblasts. Int. J. Biochem. Cell Biol. 2015;69:52–61. doi: 10.1016/j.biocel.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 78.Narayan O, Cork LC. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev. Infect. Dis. 1985;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- 79.Kuether EL, Schroeder JA, Fahs SA, et al. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J. Thromb. Haemost. 2012;10(8):1570–1580. doi: 10.1111/j.1538-7836.2012.04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Higashimoto T, Urbinati F, Perumbeti A, et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14(17):1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- 82.Schambach A, Bohne J, Baum C, et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13(7):641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 83.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol. 1999;73(4):2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selden C, Mellor N, Rees M, et al. Growth factors improve gene expression after lentiviral transduction in human adult and fetal hepatocytes. J. Gene Med. 2007;9(2):67–76. doi: 10.1002/jgm.1000. [DOI] [PubMed] [Google Scholar]
- 85.Brun S, Faucon-Biguet N, Mallet J. Optimization of transgene expression at the posttranscriptional level in neural cells: implications for gene therapy. Mol. Ther. 2003;7(6):782–789. doi: 10.1016/s1525-0016(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 86.Robert D, Mahon FX, Richard E, Etienne G, De Verneuil H, Moreau-Gaudry F. A SIN lentiviral vector containing PIGA cDNA allows long-term phenotypic correction of CD34+-derived cells from patients with paroxysmal nocturnal hemoglobinuria. Mol. Ther. 2003;7(3):304–316. doi: 10.1016/s1525-0016(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 87.Dishart KL, Denby L, George SJ, et al. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: implications for gene therapy. J. Mol. Cell Cardiol. 2003;35(7):739–748. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 88.Nair V. Latency and tumorigenesis in Marek's disease. Avian Dis. 2013;57(Suppl. 2):360–365. doi: 10.1637/10470-121712-Reg.1. [DOI] [PubMed] [Google Scholar]
- 89.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou HS, Liu DP, Liang CC. Challenges and strategies: the immune responses in gene therapy. Med. Res. Rev. 2004;24(6):748–761. doi: 10.1002/med.20009. [DOI] [PubMed] [Google Scholar]
- 91.Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol. Ther. 2011;19(8):1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Adv. Drug Deliv. Rev. 2010;62(15):1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Schwerdt JI, Goya GF, Calatayud MP, Herenu CB, Reggiani PC, Goya RG. Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr. Gene Ther. 2012;12(2):116–126. doi: 10.2174/156652312800099616. [DOI] [PubMed] [Google Scholar]
- 94.Seidlits SK, Gower RM, Shepard JA, Shea LD. Hydrogels for lentiviral gene delivery. Expert Opin. Drug Deliv. 2013;10(4):499–509. doi: 10.1517/17425247.2013.764864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rey-Rico A, Cucchiarini M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater. 2016;29:1–10. doi: 10.1016/j.actbio.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 96.Sensenig R, Sapir Y, Macdonald C, Cohen S, Polyak B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo . Nanomedicine. 2012;7(9):1425–1442. doi: 10.2217/nnm.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang C, Pham PT. Polymers for viral gene delivery. Expert Opin. Drug Deliv. 2008;5(4):385–401. doi: 10.1517/17425247.5.4.385. [DOI] [PubMed] [Google Scholar]
- 98.Kim J, Kim PH, Kim SW, Yun CO. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials. 2012;33(6):1838–1850. doi: 10.1016/j.biomaterials.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release. 2008;127(3):189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Todd R, Hoare DSK. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007. [Google Scholar]
- 101.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo . J. Control. Release. 2012;157(1):80–85. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sailaja G, Hogenesch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9(24):1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Rodrigues J, Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012;41(6):2193–2221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 104.Huang W, Rollett A, Kaplan DL. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2015;12(5):779–791. doi: 10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garg T, Goyal AK. Biomaterial-based scaffolds – current status and future directions. Expert Opin. Drug Deliv. 2014;11(5):767–789. doi: 10.1517/17425247.2014.891014. [DOI] [PubMed] [Google Scholar]
- 106.Lau HK, Kiick KL. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules. 2015;16(1):28–42. doi: 10.1021/bm501361c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeng YF, Tseng SJ, Kempson IM, Peng SF, Wu WT, Liu JR. Controlled delivery of recombinant adeno-associated virus serotype 2 using pH-sensitive poly(ethylene glycol)-poly-L-histidine hydrogels. Biomaterials. 2012;33(36):9239–9245. doi: 10.1016/j.biomaterials.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 108.Shin S, Shea LD. Lentivirus immobilization to nanoparticles for enhanced and localized delivery from hydrogels. Mol. Ther. 2010;18(4):700–706. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greish K, Frandsen J, Scharff S, et al. Silk-elastinlike protein polymers improve the efficacy of adenovirus thymidine kinase enzyme prodrug therapy of head and neck tumors. J. Gene Med. 2010;12(7):572–579. doi: 10.1002/jgm.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jung SH, Choi JW, Yun CO, et al. Sustained local delivery of oncolytic short hairpin RNA adenoviruses for treatment of head and neck cancer. J. Gene Med. 2014;16(5–6):143–152. doi: 10.1002/jgm.2770. [DOI] [PubMed] [Google Scholar]
- 111.Choi JW, Kang E, Kwon OJ, et al. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther. 2013;20(9):880–892. doi: 10.1038/gt.2013.10. [DOI] [PubMed] [Google Scholar]
- 112.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm. Res. 2007;24(4):773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 113.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur. Radiol. 2003;13(6):1266–1276. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 114.Sapet C, Pellegrino C, Laurent N, Sicard F, Zelphati O. Magnetic nanoparticles enhance adenovirus transduction in vitro and in vivo . Pharm. Res. 2012;29(5):1203–1218. doi: 10.1007/s11095-011-0629-9. [DOI] [PubMed] [Google Scholar]
- 115.Yong-Min Huh E-SL, Jae-Hyun Lee, Young-Wook Jun, et al. Hybrid nanoparticles for magnetic resonance imaging of target-specific viral gene delivery. Adv. Mater. 2007;19:3109–3112. [Google Scholar]
- 116.Bhattarai SR, Kim SY, Jang KY, et al. N-hexanoyl chitosan-stabilized magnetic nanoparticles: enhancement of adenoviral-mediated gene expression both in vitro and in vivo . Nanomedicine. 2008;4(2):146–154. doi: 10.1016/j.nano.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 117.Tresilwised N, Pithayanukul P, Holm PS, Schillinger U, Plank C, Mykhaylyk O. Effects of nanoparticle coatings on the activity of oncolytic adenovirus-magnetic nanoparticle complexes. Biomaterials. 2012;33(1):256–269. doi: 10.1016/j.biomaterials.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 118.Choi JW, Park JW, Na Y, et al. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials. 2015;65:163–174. doi: 10.1016/j.biomaterials.2015.07.001. [DOI] [PubMed] [Google Scholar]; • Demonstrates the efficient accumulation and retainment of magnetic nanoparticle-coated adenovirus at disease tissue following local delivery.
- 119.Kasala D, Choi JW, Kim SW, Yun CO. Utilizing adenovirus vectors for gene delivery in cancer. Expert Opin. Drug Deliv. 2014;11(3):379–392. doi: 10.1517/17425247.2014.874414. [DOI] [PubMed] [Google Scholar]
- 120.Lee CH, Kasala D, Na Y, et al. Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression. Biomaterials. 2014;35(21):5505–5516. doi: 10.1016/j.biomaterials.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 121.Kim PH, Kim TI, Yockman JW, Kim SW, Yun CO. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31(7):1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 122.Jung SJ, Kasala D, Choi JW, et al. Safety profiles and antitumor efficacy of oncolytic adenovirus coated with bioreducible polymer in the treatment of a CAR negative tumor model. Biomacromolecules. 2015;16(1):87–96. doi: 10.1021/bm501116x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim PH, Kim J, Kim TI, et al. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials. 2011;32(35):9328–9342. doi: 10.1016/j.biomaterials.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 124.Landazuri N, Le Doux JM. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. J. Gene Med. 2004;6(12):1304–1319. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- 125.Landazuri N, Krishna D, Gupta M, Le Doux JM. Retrovirus-polymer complexes: study of the factors affecting the dose response of transduction. Biotechnol. Prog. 2007;23(2):480–487. doi: 10.1021/bp060336y. [DOI] [PubMed] [Google Scholar]
- 126.Katakura H, Harada A, Kataoka K, et al. Improvement of retroviral vectors by coating with poly(ethylene glycol)-poly(L-lysine) block copolymer (PEG-PLL) J. Gene Med. 2004;6(4):471–477. doi: 10.1002/jgm.519. [DOI] [PubMed] [Google Scholar]
- 127.Singh R, Al-Jamal KT, Lacerda L, Kostarelos K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. ACS Nano. 2008;2(5):1040–1050. doi: 10.1021/nn8000565. [DOI] [PubMed] [Google Scholar]
- 128.Kwon OJ, Kang E, Kim S, Yun CO. Viral genome DNA/lipoplexes elicit in situ oncolytic viral replication and potent antitumor efficacy via systemic delivery. J. Control. Release. 2011;155(2):317–325. doi: 10.1016/j.jconrel.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 129.Van Den Bossche J, Al-Jamal WT, Yilmazer A, Bizzarri E, Tian B, Kostarelos K. Intracellular trafficking and gene expression of pH-sensitive, artificially enveloped adenovirusesin vitro and in vivo . Biomaterials. 2011;32(11):3085–3093. doi: 10.1016/j.biomaterials.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 130.Futaki S, Masui Y, Nakase I, et al. Unique features of a pH-sensitive fusogenic peptide that improves the transfection efficiency of cationic liposomes. J. Gene Med. 2005;7(11):1450–1458. doi: 10.1002/jgm.796. [DOI] [PubMed] [Google Scholar]
- 131.Ashizawa AT, Cortes J. Liposomal delivery of nucleic acid-based anticancer therapeutics: BP-100–1.01. Expert Opin. Drug Deliv. 2015;12(7):1107–1120. doi: 10.1517/17425247.2015.996545. [DOI] [PubMed] [Google Scholar]
- 132.Wang L, Yao B, Li Q, et al. Gene therapy with recombinant adenovirus encoding endostatin encapsulated in cationic liposome in coxsackievirus and adenovirus receptor-deficient colon carcinoma murine models. Hum. Gene Ther. 2011;22(9):1061–1069. doi: 10.1089/hum.2011.014. [DOI] [PubMed] [Google Scholar]
- 133.Wan Y, Han J, Fan G, Zhang Z, Gong T, Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34(12):3020–3030. doi: 10.1016/j.biomaterials.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 134.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol. Ther. 2008;16(1):16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 135.Fisher KD, Seymour LW. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv. Drug Deliv. Rev. 2010;62(2):240–245. doi: 10.1016/j.addr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 136.Kim PH, Sohn JH, Choi JW, et al. Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials. 2011;32(9):2314–2326. doi: 10.1016/j.biomaterials.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 137.O'riordan CR, Lachapelle A, Delgado C, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo . Hum. Gene Ther. 1999;10(8):1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]; • Demonstrates a strong masking effect of PEGylated adenovirus vectors and an efficient protection of virus from adenovirus-specific neutralizing antibodies.
- 138.Wortmann A, Vohringer S, Engler T, et al. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008;16(1):154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
- 139.Yao X, Yoshioka Y, Morishige T, et al. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene Ther. 2009;16(12):1395–1404. doi: 10.1038/gt.2009.95. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki-Kouyama E, Katayama K, Sakurai F, et al. Hexon-specific PEGylated adenovirus vectors utilizing avidin-biotin interaction. Biomaterials. 2011;32(6):1724–1730. doi: 10.1016/j.biomaterials.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 141.Croyle MA, Callahan SM, Auricchio A, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004;78(2):912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao XL, Yoshioka Y, Ruan GX, et al. Optimization and internalization mechanisms of PEGylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules. 2012;13(8):2402–2409. doi: 10.1021/bm300665u. [DOI] [PubMed] [Google Scholar]
- 143.Kim J, Nam HY, Choi JW, Yun CO, Kim SW. Efficient lung orthotopic tumor-growth suppression of oncolytic adenovirus complexed with RGD-targeted bioreducible polymer. Gene Ther. 2014;21(5):476–483. doi: 10.1038/gt.2014.18. [DOI] [PubMed] [Google Scholar]
- 144.Kim J, Nam HY, Kim TI, et al. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials. 2011;32(22):5158–5166. doi: 10.1016/j.biomaterials.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Na Y, Choi JW, Kasala D, et al. Potent antitumor effect of neurotensin receptor-targeted oncolytic adenovirus co-expressing decorin and Wnt antagonist in an orthotopic pancreatic tumor model. J. Control. Release. 2015;220(Pt B):766–782. doi: 10.1016/j.jconrel.2015.10.015. [DOI] [PubMed] [Google Scholar]; • Demonstrates an active tumor targeting and extended blood circulation of targeted polymer-coated adenovirus for systemic treatment.
- 146.Choi JW, Kim HA, Nam K, Na Y, Yun CO, Kim S. Hepatoma targeting peptide conjugated bio-reducible polymer complexed with oncolytic adenovirus for cancer gene therapy. J. Control. Release. 2015;220(Pt B):691–703. doi: 10.1016/j.jconrel.2015.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kwon OJ, Kang E, Choi JW, Kim SW, Yun CO. Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J. Control. Release. 2013;169(3):257–265. doi: 10.1016/j.jconrel.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 148.Morrison J, Briggs SS, Green NK, et al. Cetuximab retargeting of adenovirus via the epidermal growth factor receptor for treatment of intraperitoneal ovarian cancer. Hum. Gene Ther. 2009;20(3):239–251. doi: 10.1089/hum.2008.167. [DOI] [PubMed] [Google Scholar]
- 149.Stevenson M, Hale AB, Hale SJ, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007;14(4):335–345. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 150.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moon CY, Choi JW, Kasala D, Jung SJ, Kim SW, Yun CO. Dual tumor targeting with pH-sensitive and bioreducible polymer-complexed oncolytic adenovirus. Biomaterials. 2015;41:53–68. doi: 10.1016/j.biomaterials.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 152.Choi JW, Jung SJ, Kasala D, et al. pH-sensitive oncolytic adenovirus hybrid targeting acidic tumor microenvironment and angiogenesis. J. Control. Release. 2015;205:134–143. doi: 10.1016/j.jconrel.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates a potent therapeutic profile through tumor microenvironment-targeting and enhanced endosomal escape proficiency.
- 153.Tseng SJ, Kempson IM, Peng SF, et al. Environment acidity triggers release of recombinant adeno-associated virus serotype 2 from a tunable matrix. J. Control. Release. 2013;170(2):252–258. doi: 10.1016/j.jconrel.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 154.Hatakeyama H, Akita H, Kogure K, et al. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14(1):68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 155.Fan G, Fan M, Wang Q, et al. Bio-inspired polymer envelopes around adenoviral vectors to reduce immunogenicity and improve in vivo kinetics. Acta Biomater. 2016;30:94–105. doi: 10.1016/j.actbio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 156.Campbell DF, Saenz R, Bharati IS, et al. Enhanced anti-tumor immune responses and delay of tumor development in human epidermal growth factor receptor 2 mice immunized with an immunostimulatory peptide in poly(D,L-lactic-co-glycolic) acid nanoparticles. Breast Cancer Res. 2015;17:48. doi: 10.1186/s13058-015-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo Y, Wang D, Song Q, et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9(7):6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]