Abstract
The most commonly utilized PET radionuclide is fluorine-18 (18F) because of its convenient half-life and excellent imaging properties. In this review, we present the first analysis of patents issued for radiotracers labeled with fluorine-18 (between 2009 and 2015), and provide perspective on current trends and future directions in PET radiotracer development.
There are an estimated 80 million advanced diagnostic radiology exams performed annually in the USA. Out of these, over 1.5 million are positron emission tomography (PET) scans [1], in which patients are injected with a radiotracer (a bioactive molecule tagged with a positron-emitting radionuclide) [2]. The functional information that is then obtained can be used in a healthcare setting as part of a move toward individualized therapy and personalized medicine [3]. PET is also increasingly used in drug discovery where it can be employed early on to confirm target engagement, estimate receptor occupancy and determine dosing regimens or late stage development, where it can be used to enrich clinical trial enrollment, predict response to therapy and monitor therapeutic response [4].
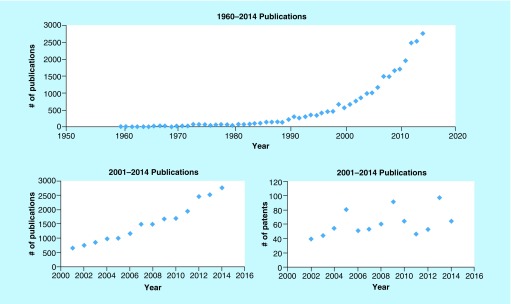
The most commonly utilized PET radionuclide is fluorine-18 (18F) because of its convenient half-life (110 min) and excellent imaging properties [5]. Moreover, the growing popularity for incorporating fluorine into pharmaceutical scaffolds offers many rich opportunities for adapting them into PET radiotracers [6,7]. Reflecting this, the last two to three decades have seen a rise in the number of publications containing fluorine-18 since the 1990s (Figure 1), which likely coincides with approval of [18F]fludeoxyglucose ([18F]FDG) by the US FDA and subsequent agreement to cover reimbursement by the Centers for Medicare and Medicaid Services (CMS) in the same decade. This trend is also apparent in the fluorine-18 patent literature, with an average of 50–100 fluorine-18 containing patents per year being issued since 2009. While synthesis and applications of PET radiotracers from the mainstream literature have been reviewed (see [8–11] for recent examples), the extensive development of fluorine-18 radiochemistry and novel radiotracers reported in the patent literature remains largely undiscussed; this review aims to meet this need.
Figure 1. . Fluorine-18 scientific and patent literature.
Fluorine-18 patent search (January 2009-March 2015)
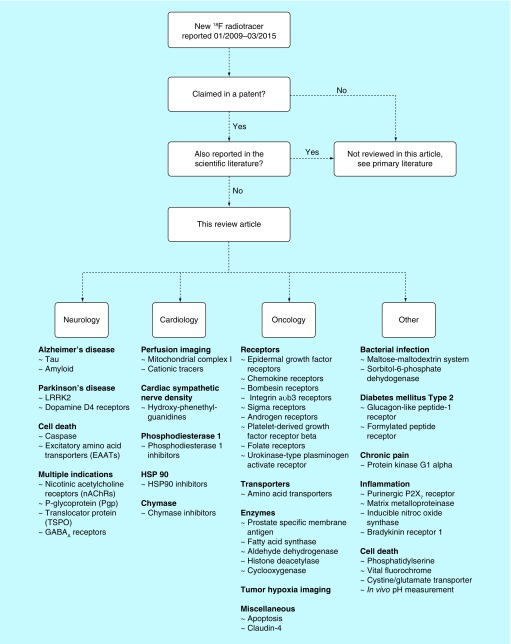
Patent searches were performed in March 2015 using SciFinder®. Patents containing ‘fluorine-18’ and ‘18F’ and issued between January 2009 and March 2015 were identified. As there were almost 500 patents, a comprehensive review of this body of work was well beyond the scope of this article. The search was therefore refined further to those patents which contain an 18F compound or method that is only referenced in that patent (i.e., contents have not been reported in a subsequent article). We reasoned that the field is familiar with those methods and radiotracers already reported in the scientific literature. Moreover, we have also separated the patents based upon content: Part 1 of this series of articles focuses upon novel 18F radiotracers for neurology, cardiology, oncology and other applications (Figure 2), while Part 2 reviews new radiochemistry methodology developed using fluorine-18, and will be published in due course.
Figure 2. . Roadmap of this article.
New radiotracers for neurology imaging
The use of positron emission tomography in neurology began with the observed uptake of [18F]FDG in the brain [12]. Since the late 1970s, this radiotracer has been used to visualize brain abnormalities and also inspired many other PET radiotracers. Both industrial and academic labs are fascinated with visualizing the brain; this flurry of research has advanced PET radiotracers for many neurological disorders and diseases in result. In the past 5 years, the patent literature has been crowded with compounds proposed to probe Alzheimer's disease, Parkinson's disease, cell death and neural injury as well as other indications. To simplify the diverse landscape of neurological imaging, the patents will be grouped by targeted disorder.
Alzheimer's disease
Alzheimer's disease (AD), which affects nearly 44 million people as of 2014, is characterized by β-amyloid senile plaques and tau neurofibrillary tangles [13]. Current diagnosis relies on clinical observations and cognitive testing; brain atrophy can be assessed by structural magnetic resonance imaging and decreased blood flow. Specific PET radiotracers for amyloid and tau are preferred and some exist: [11C]Pittsburgh Compound B ([11C]PiB), AMYViD ([18F]florbetapir), Neuraceq ([18F]florbetaben) and Vizamyl ([18F]flutemetamol) [14]. The latter three fluorine-18 labeled radiotracers have all been granted marketing authorization from the European Medicines Agency and the US FDA.
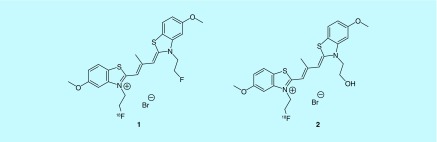
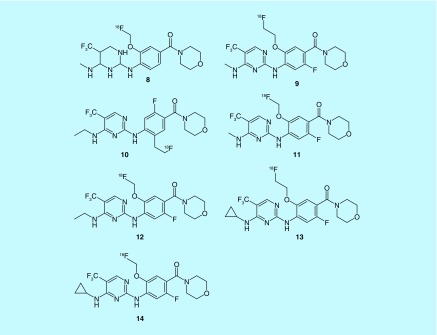
Recent efforts of fluorine-18 radiotracers for AD include both amyloid- and tau-specific compounds. For example, a scaffold based on a potent tau aggregation inhibitor thiacarbocyanine was evaluated for the detection of tau. These cyanine dye derivatives aimed to diagnose early stages of tauopathies, based on some research that indicates neurofibrillary tangles form before amyloid deposits. Two lead compounds, 1 and 2, from GE Healthcare's 2013 patent (Figure 3), were determined to have subnanomolar affinity for tau as well as over 20-fold selectivity for tau over amyloid plaque [15].
Figure 3. . Cyanine dye derivatives.
Cationic thiacarbocyanine derivatives have been shown to inhibit recombinant tau fibril formation. Other analogs included in the patent utilize longer alkyl substituents of the thiacarbocyanine in the O-methyl position.
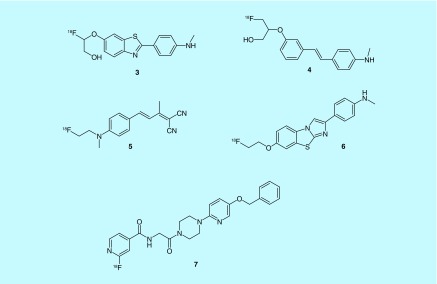
Common features in the amyloid-imaging patents include stilbene, dibenzo-thiazole, piperazine and FDDNP derivatives. Fluoro(hydroxy)alkoxybenzothiazoles and fluoro(hydroxy)alkoxystilbene derivatives were developed from Kudo and colleagues at the University of Tohoku; of note, compounds [18F]THK-837 and -853 (3 and 4) were reported to be blood–brain barrier permeable and have less osseous fluorine-18 accumulation than a previously published compound 18F-BF227 [16,17]. An FDDNP derivative, [18F]FBMP (5) was developed and evaluated in vivo; mouse models showed the compound could be more effective based on its binding ratio of 6–7 in the frontal cortex and 3.5–4.5 in the cerebellum [18]. Improving upon existing tracers for amyloid was commonplace; fluorine-18 versions of TAZA and Dalene were synthesized for imaging amyloid plaques and the norepinephrine transporter (NET) [19]. The NET has been implicated in multiple neurological disorders including AD and Parkinson's disease, as well as depression [20]. Quantification of amyloid peptide by using noninvasive imaging was the goal of Technische Universitat Munchen; their scaffold of benzothiazole-derived Aβ42 aggregation inhibitors afforded them a lead, 6, with a Ki of 4.2 nM and logP of 1.9 [21,22]. Bayer Schering patented a scaffold of piperazine derivatives with the goal of early detection; a lead compound was selected BAY1008472 (7) and has gone through nonhuman primate imaging [23,24]. For representative examples, see Figure 4.
Figure 4. . Selected amyloid imaging agents.
From each of the patents, key molecules are shown above; THK compounds were not clearly assigned to in vitro data; molecules shown best represent the scaffold.
Parkinson's disease
Parkinson's disease (PD) is one of the most common neurodegenerative disorders and is defined by the death of dopaminergic neurons in the substantia nigra. Some genes have been identified with the development of Lewy bodies: α-synuclein (SNCA), ubiquitin C-terminal hydrolase like 1 (UCH-L1), parkin (PRKN), leucine rich repeat kinase (LRRK 2), PTEN-induced putative kinase 1 (PINK 1) and protein deglycase DJ-1 (also known as Parkinson disease protein 7) [25]. Increased activity of LRRK2 has been associated with hereditary PD; Roche has been investigating small molecule modulators of LRRK2 activity in search of both therapeutics and imaging agents (8–14, Figure 5) [26–28]. Unfortunately, no imaging or in vitro binding data of labeled compounds has been published to date.
Figure 5. . Small molecule modulators of LRRK2.
In vitro evaluation of the small molecules (8–14) was reported for some of the selected molecules; the best compound reported (13) had a Ki of 0.6 nM.
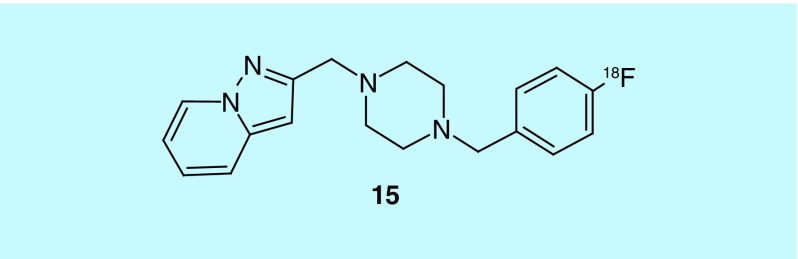
The effort to image in vivo function of the dopaminergic system has focused on synthesis and postsynaptic receptors: 6-[18F]fluoro-DOPA ([18F]FDOPA) and 6-[18F]fluoro-L-m-tyrosine (6-[18F]FMT) for synthesis and [11C]NNC 112 (D1), [11C]raclopride (D2/D3) and [18F]fallypride (D2) [29]. A patent from the Hunan Institute of Engineering described 15, specific to D4 (15, Figure 6). The radiotracer has a Ki for D4 of 1.2 nM and claims to pass through the blood–brain barrier (BBB) [30].
Figure 6. . D4-specific radiotracer.
In addition to the low Ki, the reported compound has selectivity for D4 over D2 of 1500-fold.
Cell death
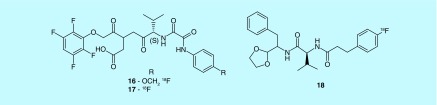
A recent effort has begun to image the biomarkers of traumatic brain injury (TBI). Neuronal calpain activation has been observed within minutes to hours; this suggests calpains are an early mediator of neuronal damage [31]. Another biomarker of apoptosis is caspase activation [32]. In this regard, Banyan Biomarkers investigated oxamyl dipeptides as caspase (16 and 17) and calpain (18) inhibitors to image protease overactivation, a hallmark of trauma (Figure 7) [33,34]. No further publications have reported radiosynthesis or in vivo imaging of the possible caspase-3 compounds. The calpain dipeptide derivatives build upon a scaffold from Cephalon, Inc. [35]. Similarly no imaging data are reported and no publications have succeeded this patent.
Figure 7. . Calpain and caspase inhibitors.
The left two compounds are caspase inhibitors, the uppermost with a Ki of less than 0.1 uM as reported in the patent. The right compound is a calpain inhibitor.
Glutamate excitotoxicity plays a role in the secondary injuries following TBI. This elevation in glutamate can be controlled by the excitatory amino acid transpor-ters (EAATs) [36]. In vivo imaging of the transporter could demonstrate over activation which would be valuable to treatment. Researchers at the University of Montana patented a scaffold of aspartylamide inhibitors of EAAT (19, Figure 8). No radiochemical yield of syntheses has been reported [37]; however, microPET imaging was performed in rodent and nonhuman primate. It was determined that EAAT2 was detectable in primate brain and in expected EAAT2 rich regions (thalamus, frontal cortex, and hippocampus regions).
Figure 8. . EAAT inhibitors.
Compound shown represents the scaffold. The stereocenter configuration appears to be important to binding, which was demonstrated in a previous publication in a cell-based assay [38].
Multiple indications
This section of the review is reserved for patents in which the compounds have multiple targets or the target specified is implicated in multiple disease states/disorders.
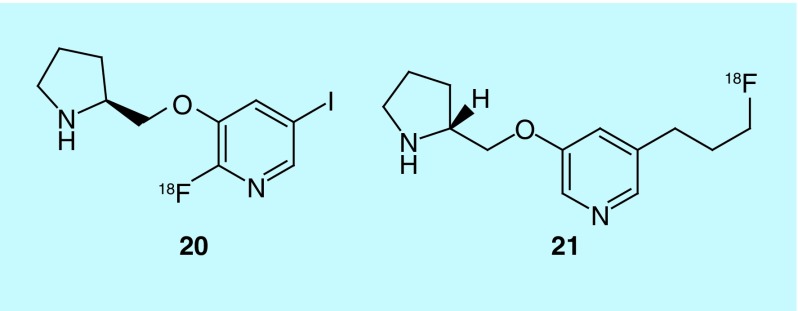
The nicotinic acetylcholine receptor (nAChR) subtype α4β2 is thought to play a role in various neurological disorders such as substance abuse, schizophrenia, and even be an early hallmark of AD [39]. Antagonists of the receptor have been synthesized for in vivo imaging out of the University of California, [18F]niofene (20) and [18F]nifrolidine (21) (Figure 9) [40,41]. Although both are BBB permeable, the rodent imaging data presented in the patent suggests the derivative of nifene, [18F]niofene, may be a better imaging agent because of the relatively short time (30–40 min) taken to reach equilibrium (cf. ˜2 h for [18F]nifrolidine).
Figure 9. . Nicotinic acetylcholine receptor agonists.
The kinetics of these mo-lecules for use as radiotracers has been a concern for the group. Other analogues included in the patent differ in alkaloid ring size and N-substituent in the same ring.
P-glycoprotein, Pgp, is the main export transporter for xenobiotics in the BBB; the transporter's involvement in epilepsy, AD, and PD makes it an interesting target for imaging. A known tracer for Pgp exists, [11C]verapamil [42]. Stichting Voor De Technische Wetenschappen (STW) patented a scaffold of verapamil-like compounds, which were labeled with fluorine-18; of the 10 compounds (R)-N-[18F]fluoroethylverapamil (22, Figure 10) was the most promising tracer when used in combination with a Pgp blocking agent [43].
Figure 10. . [18F]Fluoroethylverapamil.
Other analogs in the patent include different alkyl lengths of fluoro-substituents, O-alkyl-fluoro-substitution off of the benzyl ring, and the enantiomer of each compound.
Translocator protein 18 kDa (TSPO) may be involved in steroid biosynthesis, immunomodulation, porphyrin transport, calcium homeostasis, and programmed cell death, though its main neural function is ambiguous. TSPO levels increase during inflammation and TBI [44]. Nevertheless, TPSO is implicated in a variety of diseases: glioblastoma, multiple sclerosis, ischemic stroke, PD, human immunodeficiency virus (HIV), amyotrophic lateral sclerosis, corticobasal degeneration, Huntington's Disease, AD, depression, and cancer. Inventors at the University of Sydney patented the imaging agent [18F]PDAZ-FE (23, Figure 11), which was shown to have a Ki of 1.55 nM; no further studies using this tracer have been published [45].
Figure 11. . TSPO inhibitor [18F]PDAZ-FE.
The patent contained a multitude of in vitro data for other cold analogues of the above compound that also maintain low nanomolar inhibition. There is no mention of the SNP associated with TSPO in the patent or how these compounds preform with all isoforms.
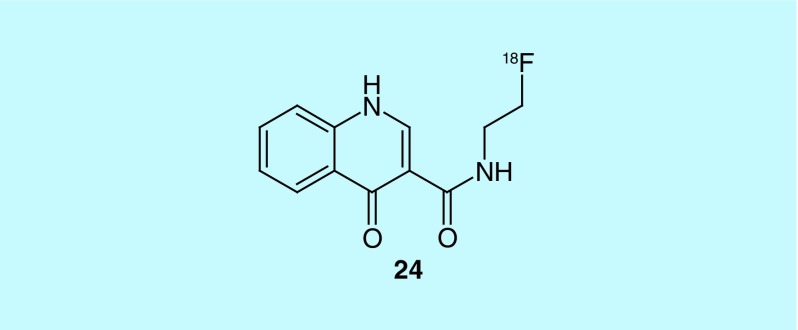
GABA (γ-Aminobutyric acid) is an inhibitory neurotransmitter; dysfunctions of GABAergic neurotransmission are implicated in epilepsy, and multiple neurological and psychiatric disorders [46]. To image the possible dysfunction, GABAA specific radiotracers exist: [11C]flumazenil ([11C]FMZ), [18F]fluoroflumazenil ([18F]FFMZ), [18F]fluoroethylflumazenil ([18F]FEFMZ), and [123l]iomazenil ([123I]IMZ). For a recent review of PET imaging of the GABAA/benzodiazepine receptor complex see: [47]. GE Healthcare looked to expand this series, developing a series of quinoline derivatives, all of which had a Ki below 10 nM (24, Figure 12). The synthesis and biodistribution data for some of the compounds was published and [48], although potent ligands, the scaffold is likely not suitable for further tracer development because of low brain uptake in rats [49].
Figure 12. . Quinoline derivatives for GABAA imaging.
New radiotracers for cardiology imaging
Perfusion imaging
Myocardial perfusion imaging (MPI) agents are currently used for the assessment of coronary artery disease (CAD) and left ventricular (LV) dysfunction; however, current SPECT and PET radiotracers utilized have several limitations. Clinical SPECT tracers (e.g., [99Tc]annexin V, [99Tc]sestamibi, [99Tc]tetrofosmin, 201Tl) provide less spatial resolution, require attenuation correction and have lower myocardial extraction compared with PET agents, which are currently limited due to the requirement of on-site cyclotron production (13NH3, H2 15O, [11C]acetate), except for generator-based 82RbCl. Unfortunately, 82RbCl has a lower than desired extraction fraction, short half-life (76 s) and high positron energy (3.15 MeV). Other (potentially) limiting factors include high cost, complications arising from strontium-82 breakthrough [50], and long-term availability of strontium-82 [51]. The short half-lives of all the currently available MPI PET agents limit options for studies and do not allow for multiple dose preparation or distribution. Fluorine-18 PET radiotracers with better myocardial extraction and pharmacokinetics have been developed by several laboratories to improve assessment of myocardial viability for selecting treatments for CAD and LV dysfunction through several different strategies.
Mitochondrial complex I
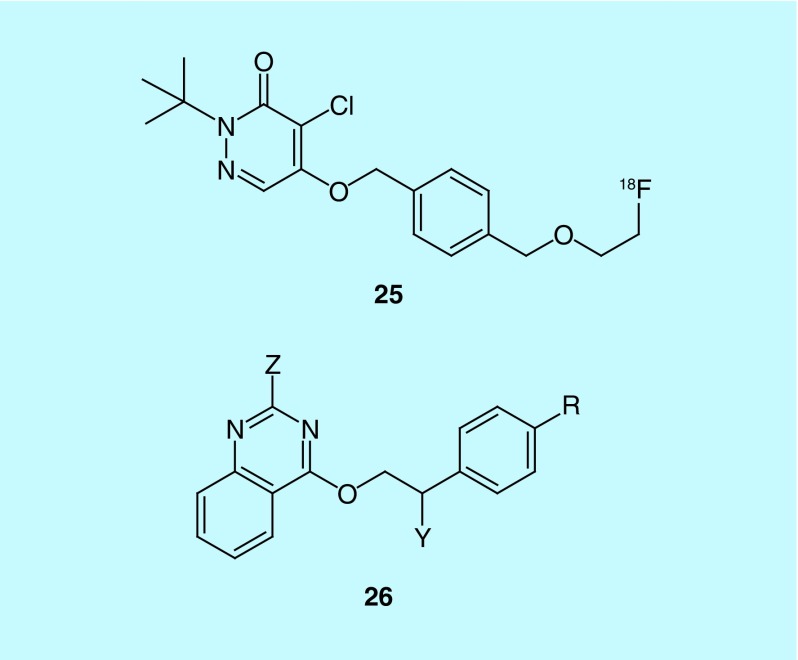
A patent from Lantheus Medical Imaging contains a series of fluorine-18 labeled quinazolines and pyridazinones (Figure 13), with the latter leading to the discovery of [18F]flurpiridaz (25), which has gone through Phase II and Phase III clinical trials [52,53]. The inventors selected mitochondrial complex 1 (MC1) as a target for developing an MPI agent based on the action of rotenone, a common insecticide that potently inhibits MC1, an enzyme in the electron transport complex that is located in the mitochondria. Cardiomyocytes have a large amount of mitochondria in comparison to other tissues, making MC1 a good target for the measure of myocardial perfusion provided the developed tracer has the required specificity and pharmacokinetics. The inventors sought out alternate scaffolds to that of rotenone for developing the properties required for the tracer, including a better safety profile. This led them to develop the pyridazione (e.g., 25) and quinazoline (26) series where alternative sites of labeling were also investigated with the pyridazinone flurpiridaz selected to be advance to clinic trials. In a later patent from Lantheus Medical Imaging, the production method for [18F]flurpiridaz is described and analogs where the 18F of flurpiridaz is replaced with 123I, 125I, 13N or 11C are claimed [54].
Figure 13. . The 25 is the structure of pyridazinone [18F]flurpiridaz, where further analogs have various chains attached to the phenyl ring and various positions for the 18F label; 26 is the general structure of the quinazoline series where R is an 18F labeled alkyl chain when Z and Y are H or an unlabeled alkyl chain when either Z or Y is 18F labeled.
Cationic tracers
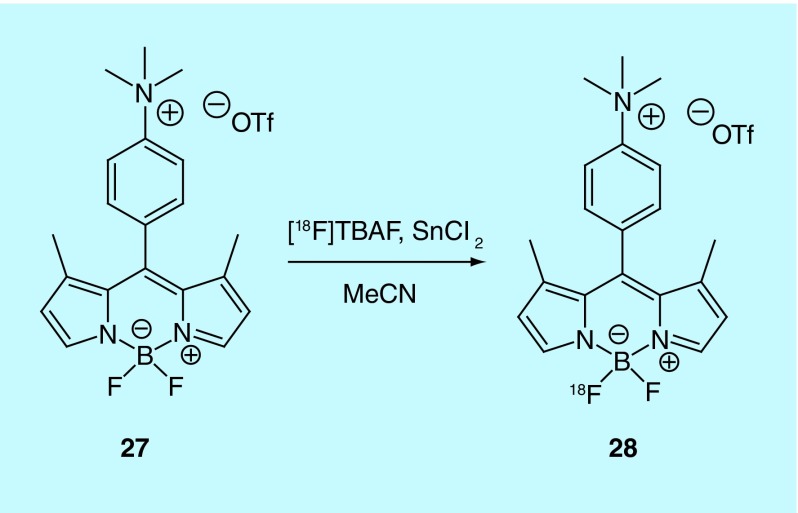
The Conti lab at the University of Southern California describe the production and evaluation of a boron-dipyrromethene (BODIPY) compound for use as an MPI agent [55]. The BODIPY tracer, given its fluorophore core, can be used as a dual-mode tracer, providing PET and fluorescent imaging data. The inventors believe the BODIPY compound is similar in behavior to better known triphenyl-phosphonium ions and rhodamine dyes that accumulate selectively in the mitochondrial matrix based on their lipophilic cationic structures [56]. The inventors had previously published a paper on the fluorine-18 radiolabeling of the BODIPY scaffold but did not describe a use [57]; in this patent they describe an improved method to generate the radiotracer 28 through isotopic exchange with 27 (Figure 14), and further experiments to claim its utility as an MPI agent. Preclinical evaluation was carried out with the use of two animal models: female nude mice (BALB/c nu/nu) and 2–3 week old male rats. Heart uptake was higher than that seen in blood, muscle and liver with a high amount of kidney uptake indicating renal clearance. Additional tissue studies in HEK-293 were undertaken that demonstrated [18F]BODIPY uptake was driven by the plasma and mitochondrial membrane potentials.
Figure 14. . Synthesis of fluorine-18 labeled BODIPY.
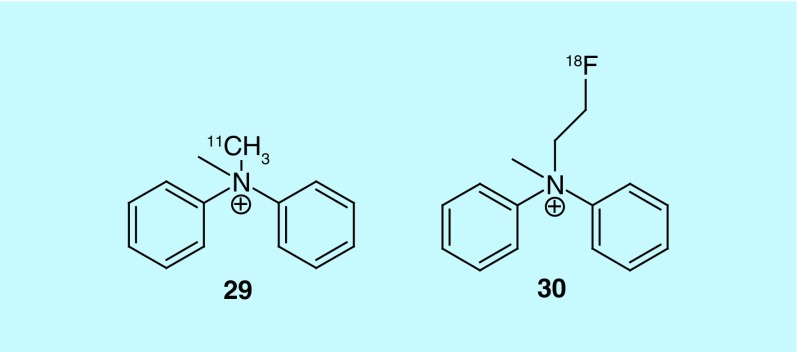
An additional class of cationic radiotracers for use in MPI has been patented in the USA by Mishani et al., of Hadasit Medical Research Services & Development Limited [58]. They image myocardial blood flow due to their cationic and lipophilic characteristics. A series of carbon-11-labeled ammonium salt derivatives were prepared and the best performing relative to commonly used 99mTc SPECT agents and [13N]ammonia was found to be compound 29 (Figure 15). A related [18F]fluoroethyl ammonium salt (30) was prepared but not evaluated. The radiotracers evaluated were found to be cleared renally. Compound 29 compared well to [13N]ammonia in rat and pig models, but further work is required with the fluorine-18 radiotracer to validate it as an MPI agent.
Figure 15. . Cationic radiotracers for myocardial perfusion imaging.
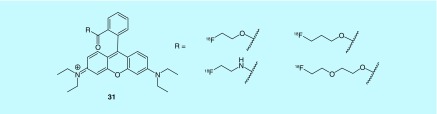
A short series or fluorine-18-labeled rhodamine dye derivatives (31) were prepared by Packard et al. (Children's Medical Center Corporation) as MPI agents (Figure 16) [59]. The cationic and lipophilic characteristics of xanthene dyes like rhodamine cause them to be accumulated in the mitochondria. The patent does not provide evaluation of the radiotracers as MPI agents and relies instead on previous reports of the properties of related xanthene dyes.
Figure 16. . Short series of fluorine-18 rhodamine dye derivatives.
Cardiac sympathetic nerve density
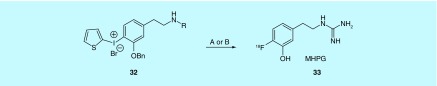
The preparation and examples of the use of 4-[18F]fluoro-meta-hydroxy-phenethylguanidine ([18F]MHPG, 33) are described with claims to encompass related phenethylguanidine structures prepared by fluorination of novel diaryliodonium salts (e.g., 32) in a patent from the Raffel group at the University of Michigan [60]. The development of MHPG was undertaken to provide a fluorine-18 alternative to [11C]meta-hydroxyephedrine ([11C]HED) and [123I]meta-iodobenzylguanidine ([123I]MIBG) for the measurement of regional cardiac sympathetic nerve density for use in estimating dysfunction in CAD, heart failure, cardiac arrhythmias, diabetic autonomic neuropathy, LV dysfunction and possibly an indication for adrenergic tumors like neuroblastoma and pheochromocytoma. The goal was not only to create a fluorine-18 analog that overcame the limitations of SPECT in the case of [123I]MIBG and the requirement of on-site production for [11C]HED as a carbon-11 radiotracer, but also to develop a tracer for measurement of sympathetic nerve density that was not flow-limited [61]. All three of these radiotracers are transported by the norepinephrine transporter (NET) into presynaptic sympathetic nerves and are then transported by the second isoform of the vesicular monoamine transporter into norepinephrine storage vesicles. The rapid uptake of [11C]HED and [123I]MIBG by NET makes kinetic analysis for quantitative measure of nerve density impractical. The inventors accomplished their goal of slower uptake by NET with MHPG and describe the preclinical evaluation in rats and nonhuman primates [60,61]. Out of the five fluorine-18-labeled molecules: [18F]MHPG, labeled intermediates to produce MHPG and possible metabolites, all but one of the metabolites have been reported in the literature [61,62]. The electron-rich arene of MHPG makes the fluorine-18 labeling difficult and requires the use of diaryliodonium salts to activate the site for fluorination. The use of the diaryliodonium salt method presents challenges due to the reactivity of the iodonium, which lead to the inventors investigating several different routes for the preparation of MHPG (Figure 17).
Figure 17. . MHPG preparation is described by two routes.
(A) The terminal amine is Boc protected and after 18F fluorination is transformed into the guanidine in a multiple purification and multiple pot synthesis; (B) a single pot fluorination of the tetraBoc-protected guanidine precursor.
Phosphodiesterase 1
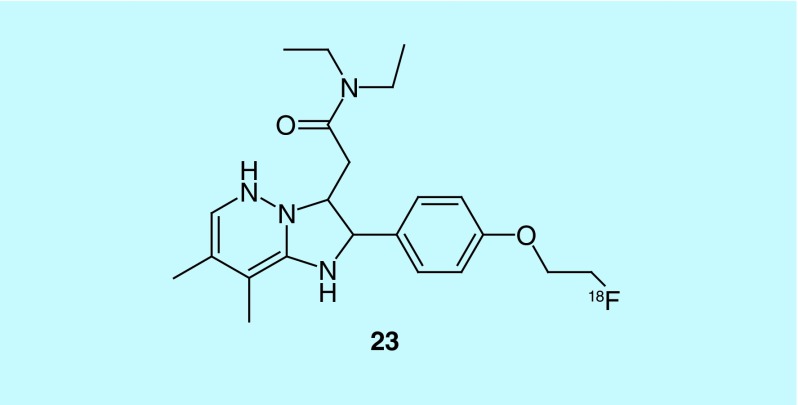
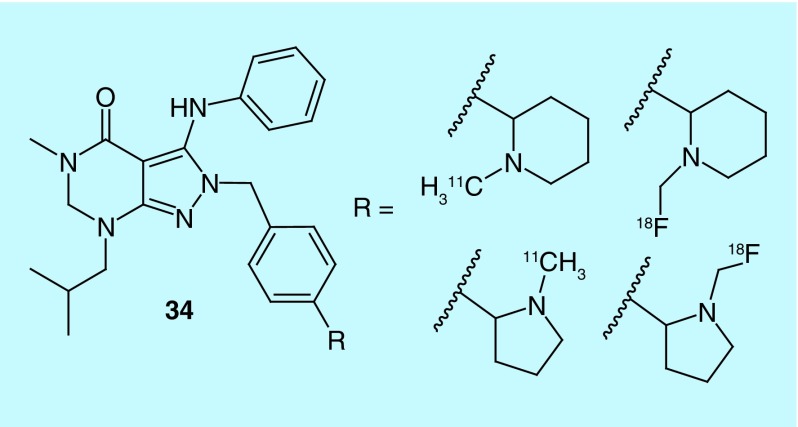
In work by Li et al. of Intra-Cellular Therapies, Inc., a short series of pyrazolo-pyrimidin-4-ones (34, Figure 18) were investigated as selective phosphodiesterase 1 (PDE1) inhibitors and have advanced to clinical trials [63]. The PDE1C isoform is associated with smooth muscle proliferation and thus could serve as a biomarker for tissue at risk of degradation as seen in conditions such as pulmonary arterial hypertension [64]. The agents described are claimed to have nanomolar potencies and are specific to PDE1, with at least tenfold selectivity over other PDE enzyme families, though data are not provided.
Figure 18. . Short series of carbon-11 and fluorine-18 pyrazolo-pyrimidin-4-ones.
Heat shock protein 90
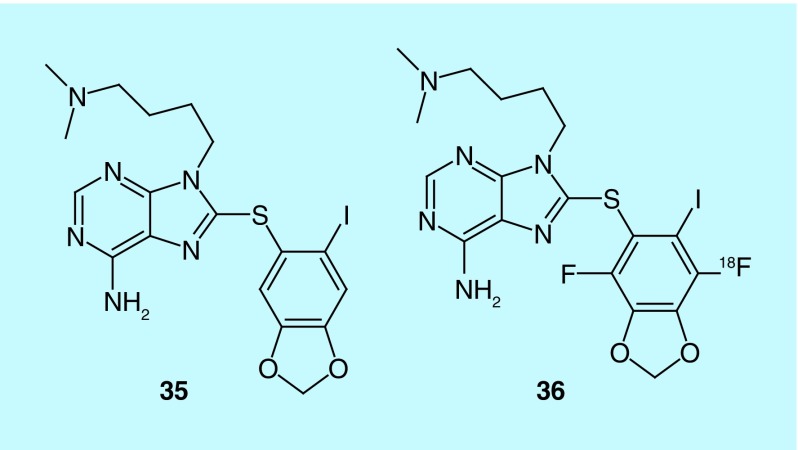
Dunphy et al. from Memorial Sloan–Kettering Cancer Center describe the evaluation of their previously developed heat shock protein 90 (Hsp90) scaffold for imaging cardiac disease in a 2014 patent [65]. The iodine-131 SPECT agent [66,67] and iodine-124 PET agent [68] were previously described in earlier patents as antitumor agents with the labeled forms functioning as companion diagnostics for imaging the tumor. Hsp90 is a chaperone that participates in oncogenesis by stabilizing growth receptors and signaling molecules with earlier work demonstrating Hsp90 inhibition can induce apoptosis. The authors cite previous work that shows Hsp90 involvement in ischemic heart disease and dilated cardiomyopathy, through increased expression over healthy controls [69], and they expand the claims from earlier patents to include cardiac indications and uses. In addition, the inventors prepared and claimed more radiolabeled analogs of their purine scaffold (e.g., 35 and 36, Figure 19).
Figure 19. . Purine scaffold of Hsp90 companion diagnostic agents.
In 35 the iodine can be labeled with iodine-124, -131 or -123; the dimethylamine is claimed as the nitrogen-13; the carbon-11-labeled methylene of the dioxole; the oxygen-15 labeled dioxole and the analog 36 is claimed as a fluorine-18 radiotracer.
Chymase
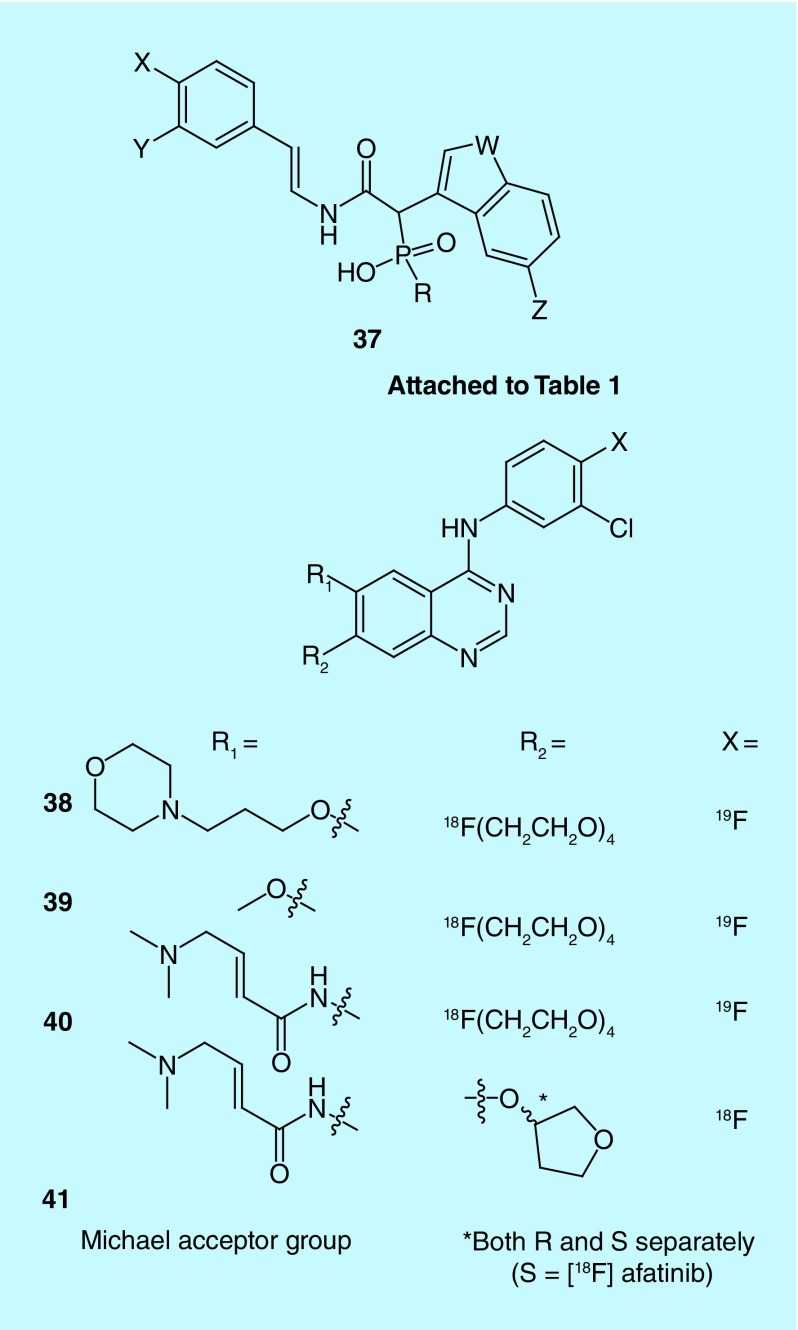
The role of chymase in the production of angiotensin II, a potent vasoconstrictor, from angiotensin I in patients with congestive heart failure (CHF) and related diseases is not fully understood, but evidence indicates that activated cardiac chymase is important in addition to angiotensin-converting enzyme (ACE) [70,71]. The inventors from Molecular Insight Pharmaceuticals generated a series of benzothiophene phosphonic acid derivatives and indole phosphinic acid derivatives for investigating chymase expression for therapeutic management in hypertension, diabetes, left ventricular dysfunction, CHF and atherosclerotic plaque [70]. For both series of chymase inhibitors PET radiotracers labeled with fluorine-18, and SPECT radiotracers labeled with iodine-123, were prepared (37, Table 1). This work complements other recent research that involves fluorine-18-labeled ACE inhibitors, for example, [18F]fluorocaptopril [72] and [18F]fluorobenzoyllisinopril [73], in that the evaluation of the role of chymase and ACE in CHF and related diseases will lead to better treatments and therapeutic management [70]. Work with these probes could facilitate the development of chymase inhibitors for the clinic or new strategies for treating CHF and related diseases with existing rennin-angiotensin system therapeutics.
Table 1. . Chymase radiotracers prepared for evaluation.
| W | R | X | Y | Z |
|---|---|---|---|---|
| S |
OH |
18F |
F |
Cl |
| S |
Me |
18F |
F |
Cl |
| N-Me |
OH |
18F |
F |
Cl |
| N-Me | Me | 18F | F | Cl |
Data taken from [70].
New radiotracers for oncology imaging
In oncology, PET imaging is a powerful diagnostic tool that allows for in vivo, noninvasive imaging for the improvement of diagnosis, management of therapy and improving overall patient outcome. The development of the radiotracer [18F]FDG to measure glucose metabolism was reported in 1977 by Gallagher et al. [74]. This would come to be a significant milestone for PET imaging once [18F]FDG's value to examine tumors and identify metastases was realized [75]. Currently, the vast majority (˜90%) of PET scans utilize [18F]FDG [76]. In addition to glucose metabolism, a number of other processes relating to oncology have been targeted for PET imaging. Notable examples include DNA synthesis, protein synthesis, lipid/membrane synthesis, hypoxia and receptor expression [77]. The vast majority of patents for 18F-labeled probes investigating these processes can be further categorized by target type, including receptors, transporters, enzymes and other targets.
Receptors
The majority of targets for fluorine-18 oncology radiotracers to emerge in the recent patent literature falls under the class of cellular receptors. Receptors localized to the cell membrane are particularly popular targets because their imaging does not require agents efficient in cell penetration. This enables facile use of larger molecules such as antibodies, peptides and scaffolds with bulky prosthetic groups.
EGFR and HER2 are members of the erbB family of receptor tyrosine kinases that promote cell proliferation and are overexpressed in various cancers [78]. They are commonly exploited for molecular imaging and therapy (for recent examples, see Figure 20) [79]. For example, in 2014, Baozhong (Harbin Medical University) reported three related fluorine-18-labeled quinazoline derivatives (38–40) targeting EGFR receptors, including an irreversible binder via inclusion of a Michael acceptor moiety and [18F]fluoride displacement of a polyethylene glycol (PEG)4-spaced tosyl group involving an ionic liquid ([1-butyl-3-methylimidazolium] triflate) [80–82]. Compound 38 shares its terminal morpholine group with gefitinib [79]. Similarly, Windhorst and colleagues, at Boehringer Ingelheim International Gmbh and Stichting Vu-Vumc, had a patent issued on fluorine-18-labeled afatinib: an irreversible (via inclusion of a Michael acceptor) inhibitor of EGFR and HER2 utilizing the same quinazoline core [83]. [18F]Afatinib (41) showed similar imaging properties when compared with [11C]erlotinib [84], but with an improved signal to background ratio.
Figure 20. . Fluorine-18-labeled quinazoline derivatives targeting EGFR receptors.
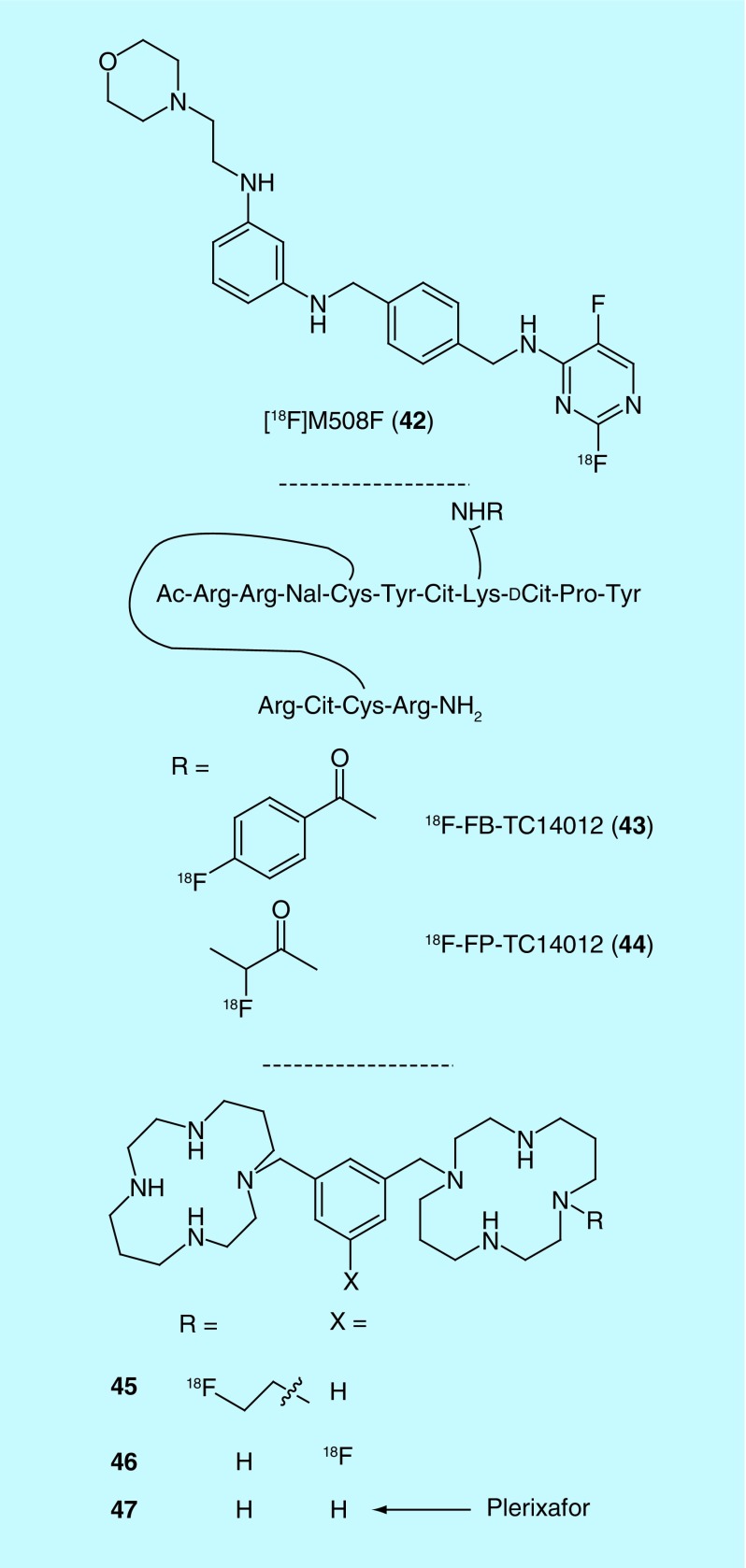
Chemokine receptors (CXCR) are a family of GPCR implicated as instigators of metastasis and inflammation and, reflecting this, they have been the target of numerous PET tracer discovery efforts (Figure 21) [85]. In 2011, a small-molecule CXCR4 antagonist, [18F]M508F (42), was developed by Liotta et al. at Emory University, with a binding affinity of 1.85 µM [86]. In 2013, Xianzhong et al. of the University of Xiamen, patented a pair of peptidomimetic tracers for CXCR4 based on high affinity CXCR4 antagonist TC14012 (19.3 nM IC50 against CXCR4 mediated HIV infection) [87] that are N-terminally acetylated and labeled via fluorobenzoate (43) or fluoropropionate (44) [88]. Finally, Shen and colleagues, of the General Hospital PLA, disclosed two (45 and 46) fluorinated plerixafor (1.9 nM Kd, 47) derivatives as radiotracers for CXCR4 [89,90].
Figure 21. . PET radiotracers for chemokine receptors.
Cit: Citrulline; Nal: 3-(2-naphthalenyllalanine).
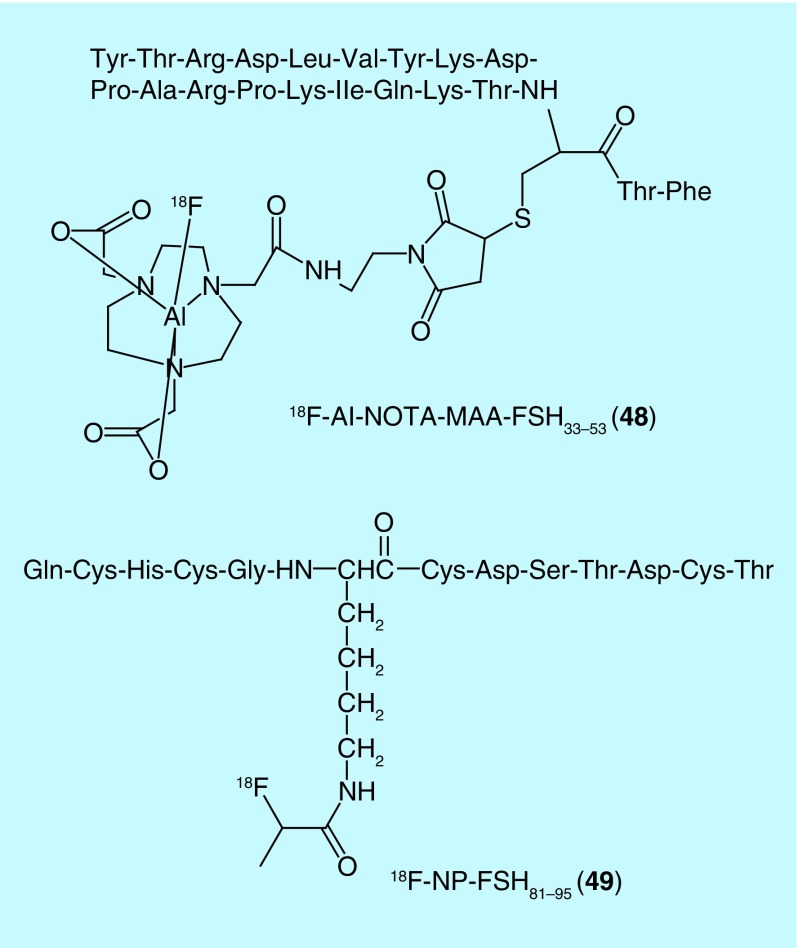
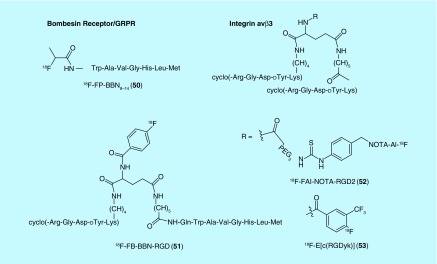
In addition to chemokine receptors, other GPCRs have been popular targets for F18 radiotracers for oncology. One such example is the follicle-stimulating hormone receptor (FSHR): a GPCR implicated in precipitating neoplasia in tissue related to the reproductive system [91]. Yang and colleagues of the Jiangsu Institute of Nuclear Medicine recently patented a number of radiolabeled FSH fragment derivatives [92]. Out of the fluorine-18-labeled compounds, one includes a NOTA-chelated Al-18F complex (48), while the other is labeled by lysine fluoropropionation (49) (Figure 22). Another example of GPCRs as recently exploited targets is the gastrin-releasing peptide receptor (GRPR; also known as Bombesin receptors or BB2): a protein overexpressed in numerous cancers [93]. In 2014, a [18F]fluoropropionate-labeled, C-terminal fragment of bombesin (BBN) emerged in the patent literature (from Guangdong Pharmaceutical University) as a radiotracer for bombesin receptors (50) [94]. Additionally, GPCRs formed one targeting axis of a set of peptidic, bivalent radioligands patented in 2010 by Chen [95]. In addition to the GPCR affinity, the ligands included a targeting moiety for integrin (51): a family of cell-surface receptors with important roles in tumor angiogenesis (Figure 23). The targeting moieties for GPCR and integrin αvβ3 are BBN7–14 and Arg-Gly-Asp (RGD) peptide derivatives, respectively. The bivalent approach greatly enhanced the percent of injected dose per gram in the tumor relative to either targeting axis alone [95]. Out of the 18F radiotracers patented, the majority have been previously reported outside of the patent literature [96,97] with the exception of the fluorobenzamide-labeled analog (51).
Figure 22. . Radiotracers for the follicle-stimulating hormone receptor.
Figure 23. . Radiotracers for Bombesin receptor and integrin αvβ3.
Integrin αvβ3 receptors have also been the target for radiotracer development by other groups. For example, Siemens Medical Solutions, USA. received a patent in 2009 for radiolabeled cyclic polypeptides composed of a cyclic RGD backbone and tagged with 18F for PET imaging [98]. In addition to the heterobivalent approach for targeting integrin αvβ3 described above, homobivalent probes have also been disclosed by Chen from the Jiangsu Institute of Nuclear Medicine. In 2011, they received patents for a pair of labeled peptide dimers, [18F]FAl-NOTA-PRGD2 (52) and 18F-E[c(RGDyk)2] (53), with a similar Arg-Gly-Asp targeting moiety as mentioned above (Figure 23) [99,100]. The former includes a PEGylated, NOTA chelated Al-18F complex, while the latter includes a substituted fluorobenzoate label. Interestingly, the integrin αvβ3, cyclized-peptide targeting groups, reported in the two patents have opposite polarity with regards to the peptide bond direction. Notably, the authors also included a kit-based preparation of [18F]FAl-NOTA-PRGD2. This scaffold has since been investigated to various extents outside of the patent literature [101,102].
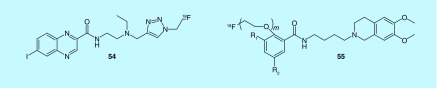
Other targets of interest to the oncology imaging community include sigma receptors (σ1 and σ2), and while their exact function is not known, human and murine tumors were found to show overexpression of σ2 receptors [103]. A series of aromatic and heteroaromatic analogs of halo-benzamides were reported by Chezal et al. (Institut National De La Sante et De La Recherche Medicale, Laboratoires Cyclopharma, Universite D'auvergnein) [104–106]. More specifically quinoxaline derivatives (54, Figure 24), labeled with fluorine-18 (or iodine-125) exhibited affinity for σ2 receptors. They displayed good tumor uptake and, depending upon choice of radionuclide, could be used for diagnosis (by PET or SPECT) or targeted radionuclide therapy of melanomas. Additionally, in 2012, Mach et al. developed additional compounds that selectively bind σ2 preferentially over σ1, as tumor cells are thought to have a high density of σ2 receptors, and as such these ligands are thought to more selectively bind tumor cells. This includes a selection of 16 pegylated fluorobenzamide analogs (55, Figure 24) that can be used as tracers for PET imaging through the incorporation of 18F, or for SPECT imaging when labeled with 123,124,125I or 76Br [107,108]. With these compounds, fluorine labeling was also reported by Gouverneur and patented in 2010 through Isis Innovation, Ltd. [109].
Figure 24. . PET radiotracers for σ1 and σ2 receptors (m = 1 – 10).
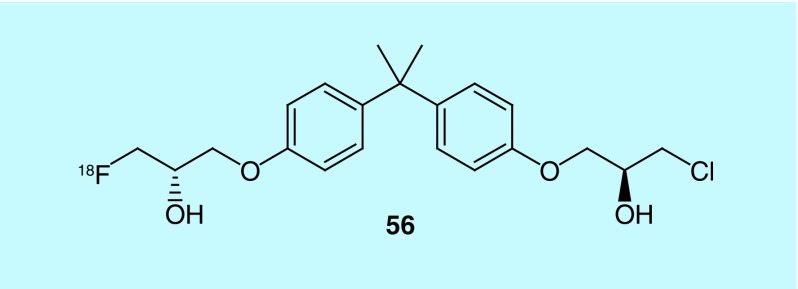
Androgen Receptor (AR) imaging in prostate cancer currently utilizes PET with 16β-[18F]-fluoro-5α dihydrotestosterone ([18F]FDHT), which binds to AR LBD (carboxy terminal ligand binding domain) [110]. This method, however, cannot detect splice variants without LBD. To address this need, 18F-labeled compounds (e.g., 56), described in a 2013 patent from the British Columbia Cancer Agency Branch and The University of British Columbia, were designed such that they may be used to alter AR activity either in vivo or in vitro for both research and therapeutic purposes, including splice site variants (see Figure 25 for a representative example) [111]. These molecules can be used to determine the presence or absence of a tumor and, when coupled with [18F]FDHT imaging, can also identify the presence of splice variants, or overexpression of splice variants lacking the ligand-binding domain [111].
Figure 25. . Radiotracer for androgen receptor imaging.
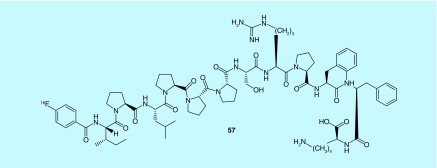
PDGFR-β PDGFR3/β is a transmembrane protein belonging to the tyrosine kinase family that binds to its dimeric ligand PDGF and has been shown to be upregulated in a variety of solid tumors. In conjunction with PDGFR3, Cluster of Differentiation 276 (CD276) is also found to be upregulated in cancer [112,113]. Taken together, these are enticing targets for cancer imaging and drug design. In a 2013 invention, eight novel peptides specific to these targets were identified and demonstrated suitable pharmacokinetic properties for use as a diagnostic imaging agent. Peptides and pentamers contain either fluorine-18 (57) or iodine-125 (suitable for PET or SPECT imaging, respectively; Figure 26) [114]. Peptides described therein contain a radioisotope or complex comprising a radioactive metal ion and appropriate chelating group; also included is the radiosynthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) and subsequent coupling to the appropriate peptide, as well as other indirect radiolabeling techniques involving a prosthetic group (such as [18F]FBAM and [18F]FBABM), as well as direct labeling via substitution [112,114–117].
Figure 26. . Peptides for PDGFR3 and CD276 imaging.
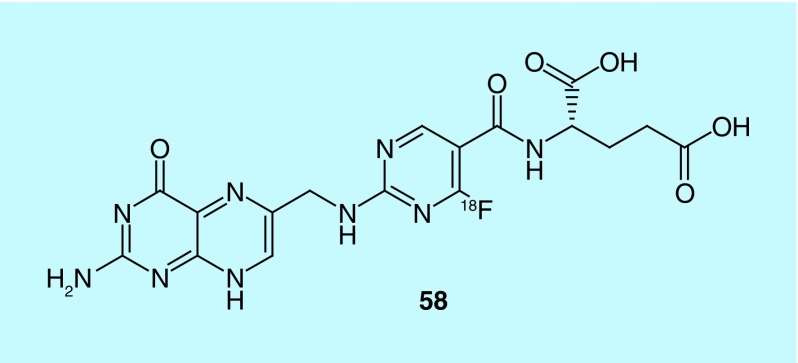
The folate receptor (FR) has several isoforms, including FR-α (FRA) and FR-β (FRB), which are frequently overexpressed in a variety of cancers, including a vast collection of specific cell types for FRA, and primarily leukemia cells and tumor-associated macrophages (TAMs) for FRB [118]. Folates and appropriate derivatives have been subject to much study for use as diagnostic agents and from this various probes have been developed for use as radiotracers [119–122]. Many folate radiotracers for use in PET or SPECT imaging are chelate-based and have been successfully evaluated in successful imaging of FR-positive tumors [123–126]. More recent advances in imaging of FR are PET diagnostic agents carrying the F-18 nuclide [127–135]. While the current F-18 folate radiotracers are promising, higher FR specificity and increased radiochemical yields for routine clinical imaging were explored in a recent application. New folate/antifolate analogs were reported whereby the phenyl group within folate structure has been substituted with 18F-five- or six-membered heterocycles; the new 18F analogs can be efficiently synthesized in high radiochemical yield, and show high selectivity with no negative effect on binding affinity at the FR (see compound 58, Figure 27, for a representative radiotracer) [136].
Figure 27. . PET radiotracer for the folate receptor.
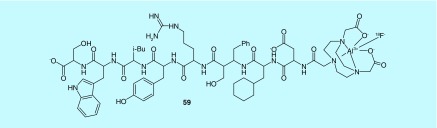
Urokinase-type Plasminogen Activate Receptor (uPAR) is overexpressed in many human cancers and is associated with poor prognosis in the case of advanced disease. For these reasons, it is considered an attractive target for nuclear imaging, as a means to noninvasively quantify uPAR density in vivo [137]. Small radiolabeled peptides with Cu-64 and Ga-68 were previously reported to specifically differentiate between tumors of varying uPAR expression and establish a correlation between uPAR PET probe uptake in tumors and uPAR expression [138,139]. Moreover, uPAR imaging has used DOTA-conjugated peptide, DOTA-AE105 radiolabeled with 64Cu and 68Ga and using NODAGA (NODAGA-AE105) radiolabeled with 68Ga [139,140]. Taken together, a 2014 application reported improvement upon uPAR PET radiotracer in vivo characteristics with specific and high tumor uptake through both [68Ga]- and [64Cu]-NOTA-AE105 in tumor models, as well as [AI18F]-NOTA-AE105's utility to efficiently detect uPAR-positive human prostate cancer lesions. Within this context, a series of positron-emitting radionuclide-labeled peptide conjugates utilizing a uPAR binding peptide coupled via a chelating agent or the covalently linked radionuclide were reported (e.g., compound 59, Figure 28) [141,142].
Figure 28. . uPAR radiotracers.
Transporters
The following subset of oncology-related fluorine-18 patents target transporters, particularly amino acid transporters: L-type, cationic or amino acid transporter generally. Radiolabeled amino acids are of interest as PET agents due to the increased rate of amino acid transport in many types of cancer (for a recent review on the use of amino acid transporter-targeted radiotracers in molecular imaging see: [143]). L-type amino acid transporters (LATs) prefer leucine and similar amino acids and have been shown to be upregulated in breast cancer and glioma. The described radiolabeled amino acids are mostly substrates for transport by LATs and A-type amino acid transporters. In addition, radiolabeled cationic amino acids targeting the cationic amino acid transporter are also represented in the patent literature (vide infra).
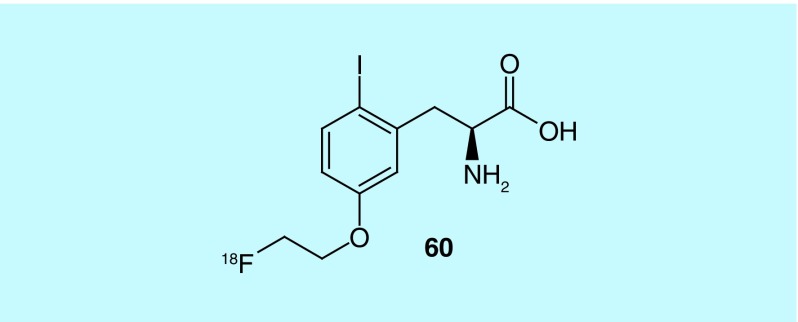
Inventors from Osaka University and the Nard Institute, Ltd. describe the design and synthesis of 5-[2-(fluoro-18F)ethoxy]-2-iodo- L-phenylalanine (60, Figure 29), an amino acid derivative shown to have strong affinity and selectivity toward LAT1. Additionally, 60 was found to accumulate in tumors at higher levels than [18F]-α-methyltyrosine, a previously described radiotracer for LAT-1 [144].
Figure 29. . 5-[2-(Fluoro-18F)ethoxy]-2-iodo-L-phenylalanine.
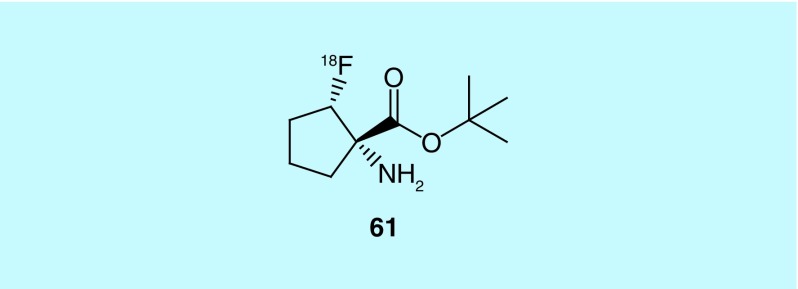
In work from Emory University, Goodman et al. report the design and synthesis of 1-amino-2-([18F]fluoro)-,1,1-dimethylethyl ester, (1S,2S) cyclopentanecarboxylic acid (61, Figure 30) and its enantiomer. The fluorine-18 labeled non-natural amino acids showed high specificity and selectivity for brain tumors. The authors posit these compounds have two advantages over [11C]methionine ([11C]MET), a radiotracer used clinically for brain tumor imaging (for a recent review, see: [145]). Unlike [11C]MET, 61 and the other reported compounds are not as susceptible to in vivo metabolism and, due to the fluorine-18 label instead of carbon-11, have a longer half-life, which facilitates offsite production and a greater range of experiments for clinical studies [146]. This work has been cited in subsequent literature concerning the use of heterocyclic amino acids in imaging proliferative disease [147].
Figure 30. . 1-Amino-2-(fluoro-18F)-1,1-dimethylethyl ester, (1S,2S) cyclopentanecarboxylic acid.
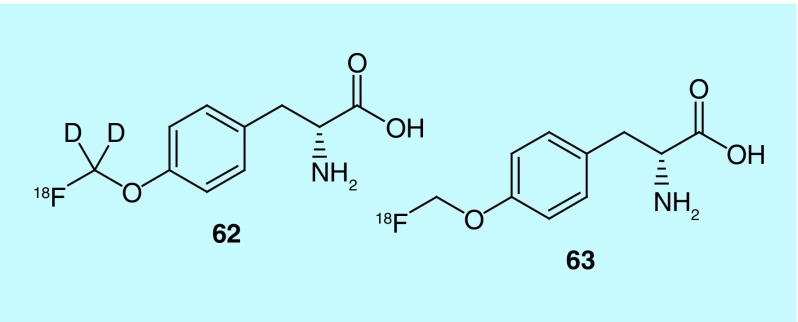
In work from Bayer Pharma the design, synthesis and evaluation of O-([18F]fluoro[2H2]methyl)-D-tyrosine ([18F]D-DFMT, 62) as a potential tumor imaging agent is described. [18F]D-FMT (63) has been previously identified as a possible tumor imaging agent, with the D-isomer showing higher tumor uptake (Figure 31). Citing previous research, the inventors investigate the substitution of deuterium for hydrogen on the [18F]fluroalkyl chains as a means of increasing metabolic stability. Biodistribution studies were used to directly compare [18F]D-DFMT and [18F]D-FMT in female NMRI mice with NCI-H292 lung tumors. [18F]D-DFMT was shown to have a higher tumor-to-organ ratio, high tumor uptake and low background activity with the exceptions being high uptake seen in the pancreas and bladder [148].
Figure 31. . [18F]D-DFMT and [18F]D-FMT.
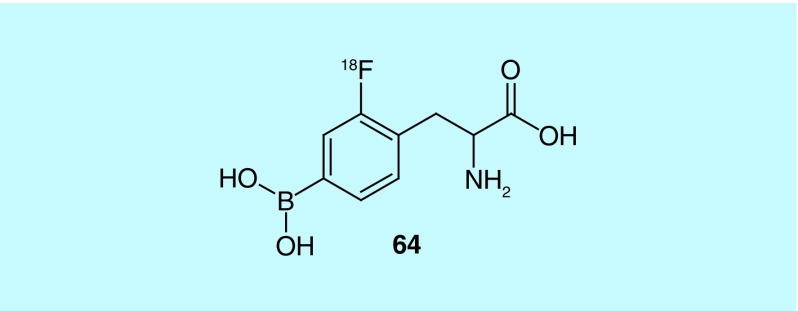
18F-2-Fluoro-4-borono-L-phenylalanine (18F-BPA [64], Figure 32), first developed by Ishiwatari, is used as a PET probe to determine the extent of accumulation of BPA in solid tumors. In turn, it can be determined if boron neutron capture therapy (BNCT) using 10B-BPA is a viable treatment option for the patient [149]. Takenaka et al. of the Stella Pharma Corporation and Osaka Prefecture University Public Corporation, describe the design and synthesis of novel, chiral 4-boronophenylalanine (BPA) derivatives, and their use as means of synthesizing 18F-BPA. These derivatives contain various protecting groups for the amine, carboxylic acid and boronic acid [150].
Figure 32. . 18F-2-Fluoro-4-borono-L-phenylalanine.
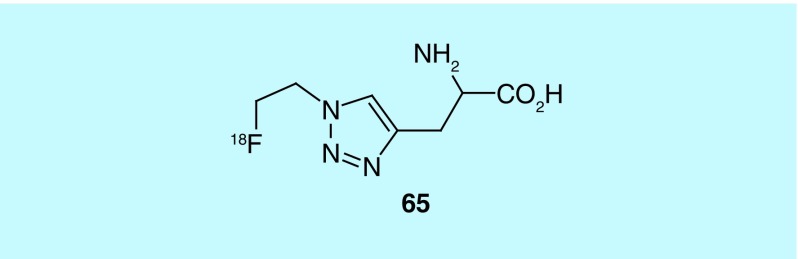
In a departure from targeting L-type amino acids Mach et al., of Washington University, investigated the design and synthesis of triazyl alkyl acids, varying in length of alkyl chain and substituents [151]. These acids primarily target cationic amino acid transporters, though studies show that they are, in part, transported via L-type amino acid transporters. The most promising compound, (S)-[18F]2-amino-3-(1-(2-fluoroethyl)-1H-1,2,3-triazol-4-yl)propanoic acid derivative (65, Figure 33), was found to have superior tumor imaging properties in comparison to its (R)-enantiomer, most specifically in gliosarcoma. Upon completion of in vivo studies, the authors assert it to be a potentially promising radiotracer. While this work has not been referenced in further studies relating to cationic amino acid transporter, it has been cited in a report on the synthesis of lanthanide-hydroporin dyads [152].
Figure 33. . (S)-[18F]2-Amino-3-(1-(2-fluoroethyl)-1H-1,2,3-triazol-4-yl)propanoic acid.
Enzymes
A number of reviewed patents for oncology imaging focus on targeting enzymes including: prostate-specific membrane antigen (PSMA), fatty acid synthase (FAS), aldehyde dehydrogenase, histone deacetylase (HDAC) and cyclooxygenase (COX). Enzymes are a desirable target for PET imaging because of the ability to design radiolabeled inhibitors or substrates that can measure enzyme expression levels and the important role played by enzymes in pathology due to those changes.
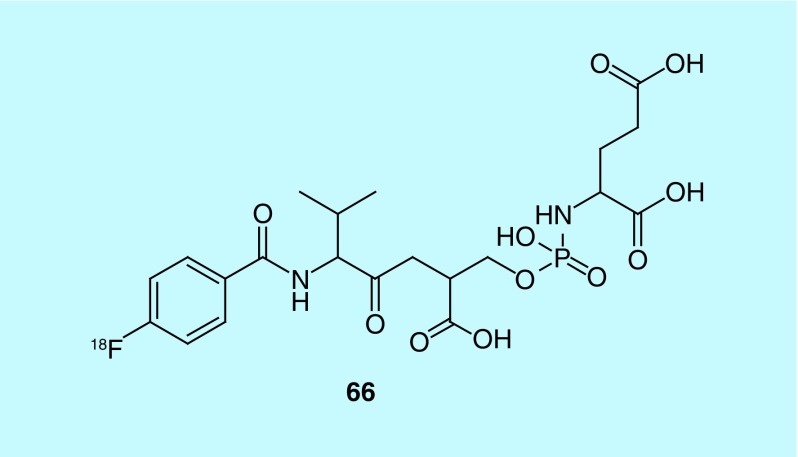
PSMA is uniquely overexpressed on the surface of prostate cancer cells, making it a promising clinical biomarker for the detection and management of prostate cancer. Between 2012 and 2014, Cancer Targeted Technology, LLC and collaborators put forward three patents concerning the synthesis and evaluation of small molecules for potential use as imaging agents for PSMA [153–155]. Small molecules are preferred over antibodies, given that antibodies are prone to immunogenicity and poor vascular permeability. The initial work concerned the synthesis and evaluation of N-[4-([18F]fluoro)benzoyl]-L-valyl-O-[[[(1S)-1,3-dicarboxypropyl]amino]hydroxyphosphinyl]-L-serine (66, Figure 34), and a related series of fluorine-18 analogs based on this scaffold. This work included inhibitors labeled by coordination with radiometals such as 99mTc, 64Cu, 68Ga or 111In. The inventors also discuss methods for the detection of PSMA containing cells, inhibiting and treating prostate cancer via the administration of a therapeutically effective dose, and a method for the blocking or destabilizing the neovasculature of a tumor [153]. This work has been referenced in a subsequent patent concerning the invention of a diagnostic device the inventors claim to be a specific and efficient detection method for a given biomarker based on the probe utilized [156]. Additional patents by Berkman cite this work, the first describes chelated antigen inhibitors for PSMA imaging and the later two patents from 2013 and 2014 describe the synthesis, design, in vitro and in vivo evaluation of PSMA antigen small molecule inhibitors based on [18F]dipeptidic-linked N-phosphoryl L-aspartic and glutamic acid derivatives. The series was developed to produce potential PET probes for detection and management of prostate cancer [154,155].
Figure 34. . Novel PSMA radiotracer.
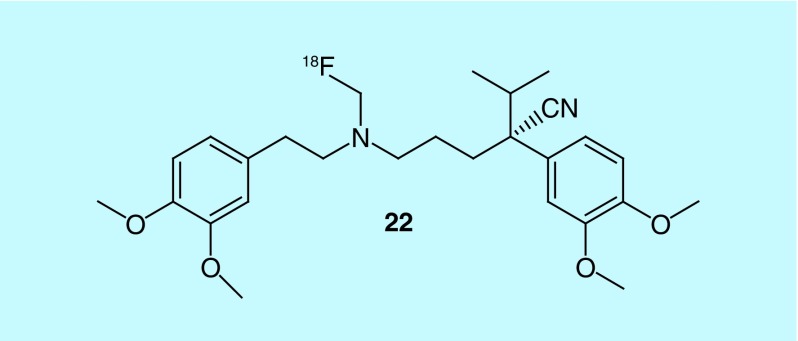
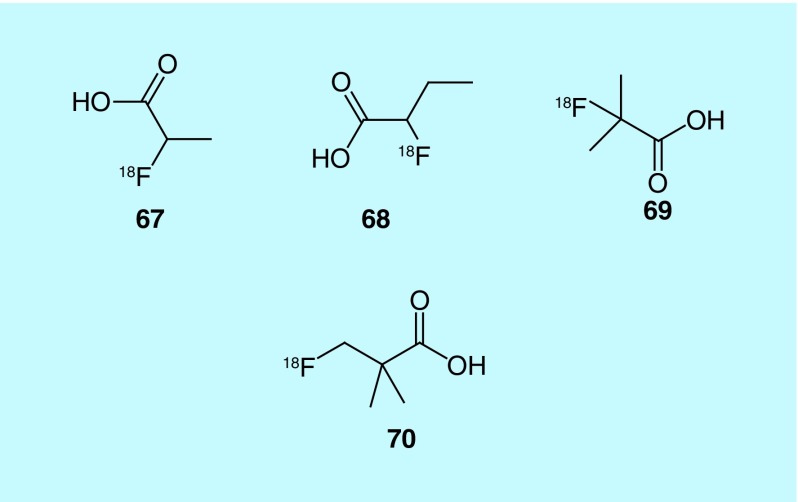
Fatty acid synthase (FAS), which is responsible for synthesizing long fatty acid chains, is believed to be overexpressed in prostate tumors, as there is significant uptake of [11C]acetate, a substrate of FAS. The short half-life of carbon-11 (20 min) requires on-site production, which has given rise to demand for a fluorine-18 analog of acetate. Though [18F]-fluoroacetate mimics [11C]acetate, it is highly toxic [157]. In related work, fluorine-18-labeled derivatives of choline are also currently being investigated as possible prostate imaging agents, though work remains to fully evaluate their potential. As previous metabolic studies have shown that propionate could be a possible precursor for the synthesis of fatty acids, in a patent from GE Healthcare the inventors describe the use of 2-[18F]fluoropropionic acid (2-[18F]FPA, 67), 2-[18F]fluorobutanoic acid (68) and 2-[18F]-fluoro-2-methylpropionic acid (69) as possible prostate tumor imaging agents (Figure 35). They focused on 2-[18F]FPA for further evaluation of its imaging potential for oncology applications [158]. It is important to note that 2-fluoropropanoic acid has much lower toxicity, with rats treated with 212 mg/kg showing no observable toxicity. In mouse models of prostate cancer, CWR22RV1 xenografts, 2-[18F]FPA showed high tumor uptake, with high brain and heart uptake and notable kidney uptake observed. In comparison to [18F]FDG, 2-[18F]FPA was shown to be a better imaging agent for prostate tumors. It should further be noted, 2-[18F]FPA has also been used for imaging αvβ3 integrin expression. This patent is cited in later studies where 2-[18F]FPA was compared with 3-fluoro-2,2-dimethylpropanonic acid ([18F]FDMP, 70) [159]. [18F]FDMP was described as a suitable imaging agent for cancer and other diseases, including Alzheimer's disease and multiple sclerosis (MS). In a comparison between [18F]FDMP and [18F]FDG uptake in breast adenocarcinoma xenografts, the patent reports that differentiation was impossible, but noted that [18F]FDMP showed significantly higher retention in prostate tumors. In vivo studies with [18F]FDMP showed that after 60 min, it had a tumor to muscle ratio of 1.93, a tumor-to-blood ratio of 2.32 and high metabolic stability. In additional studies, the favorable uptake and high metabolic stability suggested that [18F]FDMP outperformed 2-[18F]FPA.
Figure 35. . Radiotracers for fatty acid synthase.
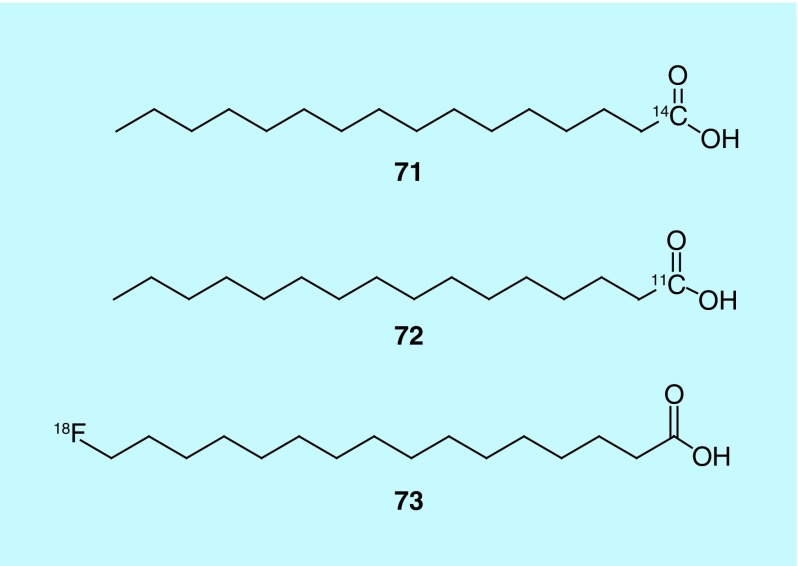
Reske et al. describe the design, synthesis and evaluation of several possible fatty acids for use as radiotracers for prostate cancer. The inventors describe several different radiolabeled fatty acids that differ only in the length of the alkyl chain, and the position and identity of the radioisotope used to label, though [14C]palmitic acid (Figure 36) is focused on in the patent for evaluating the potential of the molecule. The synthesis of 16-([18F]fluoro)hexadecanoic acid is described in detail. Alternate isotopic labeling described includes carbon-14 (71), carbon-11 (72) and fluorine-18 (73). In vivo studies with [14C]palmitic acid show that there is significant uptake of the radiotracer in the tumor, with low background activity and some uptake in the liver and prostate. Palmitic acid is a preferred fatty acid, in part, because of its low toxicity; the LD50, determined in rats, was greater than 2 g/kg and palmitic acid has been used in a number of cosmetics, approved drugs and food [160].
Figure 36. . Radiolabeled fatty acids.
The work of Cuthbertson and co-workers at GE Healthcare describes tracer design and methods for targeting aldehyde dehydrogenase (ALDH), prompted by development of the stem cell model of cancer [158]. One finding of this model is a benefit of purging samples with 4-hydroperoxycyclophosphamide (4-HC) prior to bone marrow transplantation. Sensitivity of stem cells to 4-HC has been correlated to intracellular activity of ALDH. Additionally, breast cancer studies have demonstrated a correlation between ALDH expression in tumors and poor clinical outcome. The work in question focuses on ALDH substrates, preferably those with no other known biological activity, and methods for detection of tumor stem cells using these substrates, labeled with not only 18F but in some cases 11C, 13N, 75,76,77Br, 123,124,122I or chelated metals such as 64Cu, 48V, 52Fe, 55Co, 94m,99mTc,67,68Gd, 67,68Ga or 111,113mIn. This work has been referenced in additional patents by Cuthbertson focusing on 6-[2-(fluoro-18 F)ethoxy]-2-naphthalenecarboxaldehyde, [161,162] work by Kulangara et al. (of GE Healthcare and Medi-Physics) on aldehydes for in vivo imaging of ALDH in cancer cells [163], and work by Li et al. (University of Southern California) on boron-based dual imaging (PET and fluorescence) probes, their composition, and use in rapid aqueous 18F labeling [164].
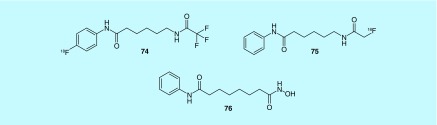
Histone deacetylases’ (HDAC) primary function is to regulate gene transcription by making DNA inaccessible through deacetylating histones. HDAC expression has been linked to a number of different cancers. Ronen et al. describes substrates of HDAC that could be used for magnetic resonance spectroscopy (MRS) imaging, or PET imaging of HDAC activity (Figure 37), including Boc-lysine tri[18F]fluoroacetic acid ([18F]BLT, 74) and 6-([18F]fluoroacetamide)-1-hexanoicanilide ([18F]FAHA, 75), although only [18F]FAHA was described in detail and evaluated with in vivo imaging. [18F]FAHA was administered to nude rats with human breast cancer cells injected subcutaneously into the neck area. At 60 min, [18F]FAHA showed a%ID/g of 0.79 ± 0.13, and a tumor to muscle (T/M) ratio of 1.95 ± 0.19. When also treated with suberoylanilide hydroxamic acid (SAHA, 76), a known HDAC substrate, both %ID/g and T/M ratio saw significant drops, indicating that [18F]FAHA is a substrate of HDAC. Brain uptake was evaluated and without SAHA present, [18F]FAHA was shown to have a %ID/g of 0.44 ± 0.03 with a brain to muscle ratio of 2.56 ± 0.33 at 5 min. Both values decreased as time progressed, and both showed inhibition by SAHA. The inventors conclude that [18F]FAHA could also be a useful tool for monitoring therapeutic response following treatment with SAHA [165].
Figure 37. . PET radiotracers for histone deacetylases.
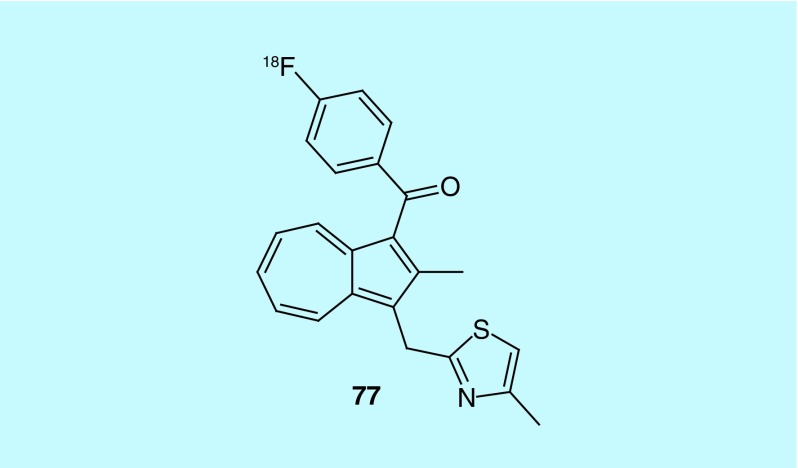
Cyclooxygenase (COX) is the key enzyme in converting arachidonic acid to prostanoids, which include prostaglandin, prostacyclin and thromboxane. The isoform COX2 is undetectable in most cells but highly inducible in cells involved in inflammation as seen in cancer, as well as being shown to be involved in the early stages of tumorigenesis. COX2 not only continues to be expressed during tumor progression, but this expression indicates an aggressive tumor phenotype and consequently poor prognosis. COX2 overexpression is well documented in various human carcinomas including colon, stomach, lung, breast, head and neck, bladder and pancreas. The work of Pham et al. from Vanderbilt University describes the design and synthesis of [4-(fluoro-18 F)phenyl][2-methyl-3-[(4-methyl-2-thiazolyl)methyl]-1-azulenyl]- methanone (77, Figure 38), a COX2 probe and methods for its use in imaging, diagnosing and monitoring disease [166].
Figure 38. . A novel COX2 radiotracer.
Tumor hypoxia imaging
Tumor hypoxia imaging continues to be of interest to the field, and is dominated primarily by radiolabeled 2-nitroimidazole derivatives. However, no new 18F-labeled 2-nitroimidazoles have been the subject of a patent application during our 2009–2015 search criteria (the most recent was [18F]HX4, reported by Siemens Medical Solutions, USA in 2008) [167]. Other approaches to hypoxia imaging are also being considered. For example, the same group also received a patent in 2008 for hypoxia imaging with [18F]VM4–037, a carbonic anhydrase IX inhibitor [168]. Rather than applications for new PET radiotracers for imaging hypoxia, it appears that the existing agents continue to advance through clinical trials. Progress in this regard is beyond the scope of this patent review, but has been summarized in recent review articles [169–171].
Miscellaneous oncology imaging
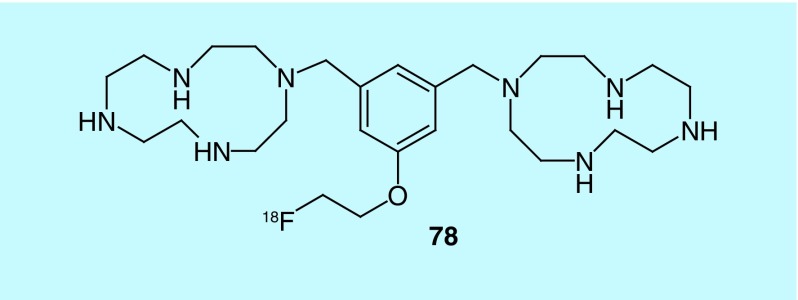
The quantification of apoptosis by PET imaging could be used as an indicator of chemotherapy response. Historically, the detection of apoptosis has typically been accomplished using annexin V labeled with 99mTc. Labeled annexin V, however, can be expensive and is moderately unstable. There continues to remain a need for imaging agents capable of monitoring and quantifying apoptosis. Zitzmann-Kolbe et al., of Bayer Schering Pharma, describe the design and synthesis of novel molecules for imaging apoptosis by targeting phosphatidylserine with cyclic polyamines [172]. All of the molecules described in the patent contain at least one cyclic polyamine moiety for complexation with a bi- or trivalent metal ion, and at least one moiety for binding a radiolabel, or a linker and radiolabel. The radiolabel can be replaced with florescent dye, or a protein for uses other than PET imaging. Though several 18F-radiolabeled molecules are described, only a labeled 1,4,7,10-tetraazacyclododecane derivative (78, Figure 39) had biodistribution data included. The study was performed in male mice with induced hepatic apoptosis. Injection of the radiotracer 1.5 and 3 h after hepatic apoptosis induction showed accumulation in the apoptotic liver after 30 min. In a comparison study using a control tumor and an irradiated tumor, transplanted into the same rat, the irradiated tumor showed an increase in the accumulation of the radiotracer when compared with the control, demonstrating the radiotracers ability to identify cell death.
Figure 39. . 1,4,7,10-tetraazacyclododecane derivatives for imaging apoptosis.
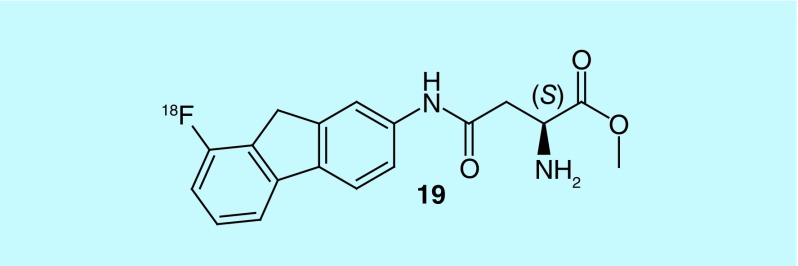
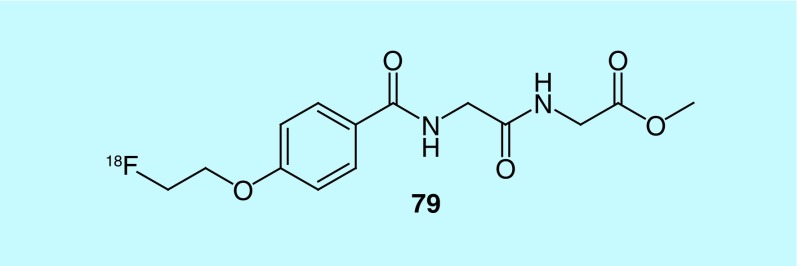
In their patent, Qi et al., of Bejing Normal University, claim the design and synthesis of a novel 18F-labeled polypeptide, with a substituted alkoxyben-zoyl structure and an amino acid structure opposite ends (glycine, N-[4-[2-(fluoro-18F)ethoxy]benzoyl]glycyl, methyl ester [79], Figure 40) [173]. According to the patent, biodistribution studies indicate that the novel radiotracer has better cellular uptake, retention and metabolism, when compared with O-(2-[18F]fluoroethyl)-L-tyrosine, a known amino acid transport substrate used for imaging brain tumors.
Figure 40. . Glycine, N-[4-[2-(fluoro-18F)ethoxy]benzoyl]glycyl-, methyl ester.
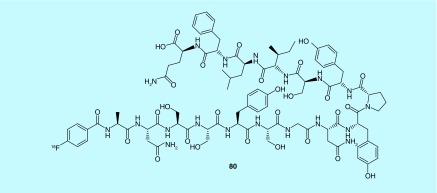
Damage to tight junctions can lead to enhanced proliferation of tumors. Claudin-4, a tight junction protein in epithelial cells, can be expressed in cancer cells; its distribution and expression could reflect important changes to the function of tight junctions, and as a result give information about the proliferation of tumors. Guo and co-workers at Shandong Cancer Hospital describe a fluorine-18-labeled 16 amino acid polypeptide (80) as an imaging agent for claudin-4 (Figure 41). The polypeptide has the amino acid sequence Ala-Asn-Ser-Ser-Tyr-Ser-Gly-Asn-Tyr-Pro-Tyr-Ser-Ile-Leu-Phe-Gln, and has no effect on epithelial permeability. No in vivo or in vitro evaluation of the described polypeptide is provided [174].
Figure 41. . 16 amino acid polypeptide as an imaging agent for imaging clau-din-4.
New radiotracers for other miscellaneous applications
Bacterial infection
Maltose-Maltodextrin system
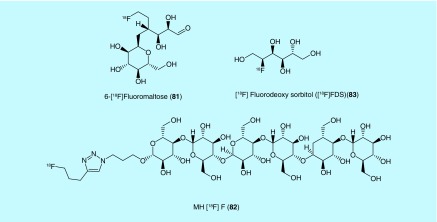
Inventors from Stanford University have described a series of fluorine-18-labeled maltose and acarbose probes for detecting bacterial infection by PET [175]. Current imaging methods (MRI and CT) cannot easily distinguish bacterial infection from inflammation, so the inventors sought to find a noninvasive method to identify bacterial infection from sterile inflammation or viral infection. As maltose is a substrate in multiple pathways in many types of pathogenic bacteria but not used in mammalian cells, it was reasoned that fluorine-18-labeled maltose analogs would provide an imaging method to identify bacterial infection. In addition, many pathogenic bacteria also contain maltose transporters, which allows for imaging of those infections with labeled maltose or acarbose (a clinically used inhibitor of amylases, glucosidases and cyclodextrin glycosyltransferases for the treatment of Type II diabetes) that is also transported but not metabolized by the maltose-maltodextrine system in Escherichia coli. and presumably other bacteria [176]. The fluorine-18 labeling was achieved by standard nucleophillic substitution methods and both 6-[18F]fluoromaltose (81, Figure 42) and the 1-[18F]fluoromaltose were confirmed by in vitro uptake assays to be specific for bacteria and dependent on the maltose pathways as cold maltose blocked uptake; however, 1-[18F]fluoromaltose was found to be unstable in vivo, making 6-[18F]fluoromaltose the most promising analog described in the patent.
Figure 42. . Bacterial imaging agents described for use in detecting infection.
The connection of an oligosaccharide to a fluorine-18 label via azide-alkyne click chemistry conjugation was explored by researchers from Emory University, Georgia Tech and Georgia State as a means to image bacterial infection [177]. The inventors decided that maltodextrin transport was the most promising target and decided to generate a fluorine-18-labeled maltodextrin analog, given previous work with fluorescent dye conjugated maltohexaose, which was found to have a 1000-fold selectivity for bacteria over mammalian host uptake. The developed radiotracer, MH18F (82, Figure 42), was prepared from the 4-pentyn-1-yl brosyl ester that, once fluorinated, was treated with the azide containing oligosaccharide in the prescence of copper to afford the triazole click product. MH18F was found in a thigh infection model using Sprague–Dawley rats to be able to detect an infection as small as 105 colony forming units with specific uptake of the radiotracer into bacterial cells and fast clearance from mammalian tissue. The maltodextrin transporters are common to pathogenic bacteria and have high homology, which leads the inventors to expect that MH18F will be able to detect a variety of bacterial infections at early stages of infection.
Sorbitol-6-phosphate dehydrogenase
In work from inventors at Johns Hopkins University, several different tritium-labeled probes for bacterial pathways were explored for subsequent development into a PET radiotracer with the inventors generating one fluorine-18-labeled probe, 2-[18F]fluorodeoxy sorbitol ([18F]FDS [83], Figure 42) [178]. [18F]FDS was generated from commercially available [18F]FDG by reduction with sodium borohydride at 35°C over 15 min. Although originally reported for tumor imaging [179], in this patent the inventors demonstrate that [18F]FDS is also selectively taken up by Gram-negative bacteria. Further studies with excess unlabeled soribitol inhibited uptake of the radiotracer, also demonstrating specificity. The inventors postulate that sorbitol uptake could be a function of a pathway that includes sorbitol-6-phosphate dehydrogenase with follow-up experiments on bacteria with and without the gene accumulating the probe or not as predicted. In vivo experiments were then conducted with [18F]FDS in CBA/J mice that were inoculated with E. coli. in one thigh. [18F]FDS showed a 12-fold higher signal for the infected thigh over the control thigh, while a control experiment with [18F]FDG did not show a more intense signal for the infected thigh. A related experiment with K. pneumoniae for pulmonary infection in CBA/J mice also showed PET signal at the site of infection. The authors work indicates that [18F]FDS has promise for imaging Gram-negative infections by PET.
Diabetes mellitus Type 2
Glucagon-like peptide-1 receptor
In work of inventors from Kyoto University and ARKRAY, Inc., a polypeptide that targets glucagon-like peptide-1 receptor was labeled by use of the prosthetic group [18F]SFB [180]. The two fluorine-18 labeled polypeptides were based on results found from modifying exendin-4, which is a marketed compound (Byetta) for treating diabetes based on a hormone found in Gila monster saliva. The radiotracers are intended to allow for the estimation of β-cell mass to aid in the determination of disease progression and to evaluate diabetes mellitus Type 2 therapeutics or treatments. The inventors give examples where their molecule successfully images a pancreas in mouse models with little bone uptake, demonstrating the molecule does target the pancreas and does not suffer from defluorination. More work is required to determine if the labeled polypeptide can accurately estimate β-cell mass and correlate it to disease progression.
Formylated peptide receptor
Saji and co-inventors have been exploring development of a radiotracer for early detection of diabetic foot to avoid the complications that result from the associated ulcers and destructive lesions and often lead to amputation [181]. In their work, they describe six N-succinimidyl 4-[18F]fluorobenzoate-labeled polypeptides (6–8 amino acids in length) that were based on known formylated peptides that bind to the formylated peptide receptor. This allows for the selective imaging of inflammation associated with diabetic foot as the formylated peptide receptor is expressed in leukocytes associated with the condition. The described peptides have Ki of 0.04–1.22 nM as determined from competition assay with an iodine-125-labeled peptide. The fluorine-18-labeled peptides were then investigated in a mouse dynamic PET experiment and revealed sites of inflammation with accumulation observed over the course of the experiment.
Chronic pain
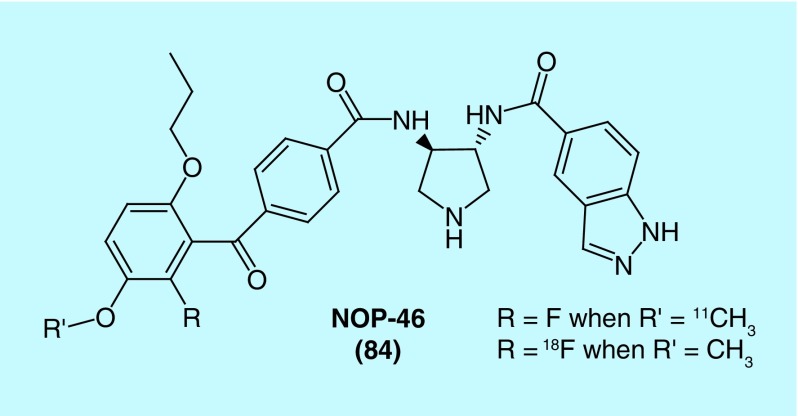
Protein kinase G1 alpha (PKG-1α)
In a patent from Columbia University in the city of New York, fluorine-18 and carbon-11-labeled radiotracers for imaging chronic pain with the intent to quantify neuropathic pain are described [182]. PKG-1α in nociceptive neurons is activated by injury or inflammation and can lead to long-term hyperexcitability, which increases activity at pain centers leading to hypralgesia and allodynia. The inventors refer to their radiotracer as NOP-46 (84, Figure 43), which is labeled with fluorine-18 or carbon-11 to generate the described radiotracers. In biological evaluation, the inventors found NOP-46 was selective for PKG-1α over the other 287 kinases they tested and that NOP-46 alleviated mechanical allodynia in a rat chronic pain model.
Figure 43. . Structure of NOP-46, an inhibitor of protein kinase G1 alpha.
Inflammation
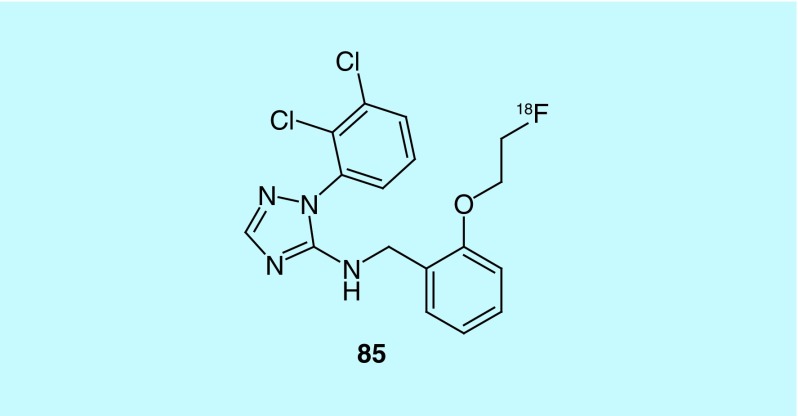
Purinergic P2X7 receptor
Building on their prior work to discover a potent antagonist of the purinergic P2X7 receptor [183], GE Healthcare describes the synthesis of an analog labeled through a [18F]fluoroethoxy group (85, Figure 44) [184]. The purinergic P2X7 receptor is an adenosine triphosphate (ATP) activated ion channel found in mast cells, macrophages, lymphocytes and other cell types associated with inflammation. Its role in the release of inflammatory cytokines and its expression in glial cells where it has been found to play a role in the release of glutamate have led to interest in the receptor as a therapeutic or as an imaging agent for inflammation. The inventors evaluated the fluorine analog 85 in a cell-based assay where HEK 293 cells were transfected with P2X7 and the amount of a fluorescent dye that entered through the large pore form of the receptor being measured to indicate inhibition by the antagonist, with more potent molecules requiring a lower concentration to reduce uptake of the dye. Further work with the compound is required to evaluate its potential as an imaging agent.
Figure 44. . Fluorine-18-labeled purinergic P2X7 receptor antagonist.
Matrix metalloproteinase
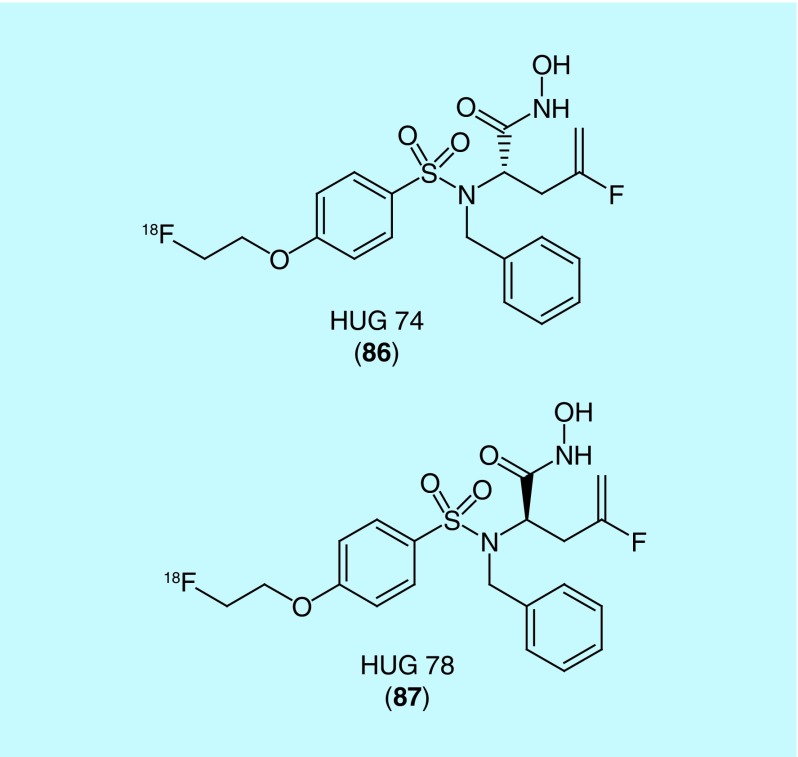
In a patent from University of Münster and Siemens Medical Solutions, two fluorine-18-labeled matrix metalloproteinase inhibitors are described [185]. Their work was recently published in the literature but did not include a description of their fluorine-18-labeled compounds HUG 74 (86) and HUG 78 (87) (Figure 45) [186]. The interest in matrix metalloproteinase stems from its upregulation in pathologies like atherosclerosis, tumorigenesis and other inflammatory diseases (authors claim usefulness in detecting the following diseases where matrix metalloproteinases are upregulated: atherosclerosis, congestive heart failure, cancer, arthritis, amyotrophic lateral sclerosis, brain metastases, cerebrovascular diseases, Alzheimer's disease, chronic obstructive pulmonary disease). Small animal PET experiments were performed with HUG 74 and found high initial uptake in liver and kidneys and rapid clearance through hepatic and renal elimination. Accumulation in organs and bone was not observed, indicating HUG 74 merits further investigation to evaluate its use as a radiotracer for disease related to matrix metalloproteinases.
Figure 45. . Structure of HUG 74 and HUG 78, HUG 74 was utilized in preclinical evaluation.
Inducible nitric oxide synthase
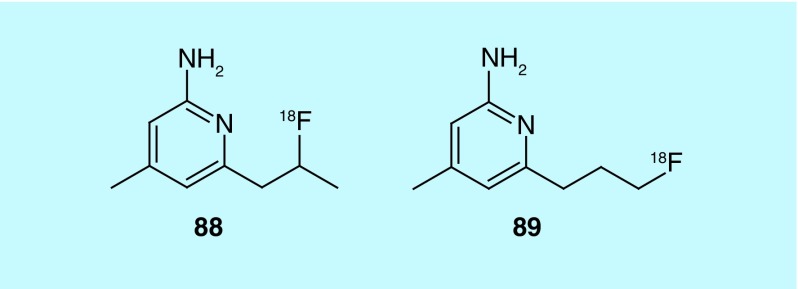
In a patent from Washington University Mach, Zhou and Welch describe two 4-methyl pyridine fluorine-18 labeled inhibitors (88 and 89, Figure 46) of inducible nitric oxide synthase (iNOS) [187]. The inventors’ interest in iNOS stemmed from the association of its overproduction with disease such as ischemia/reperfusion injury, septic shock, diabetes (vascular dysfunction) and transplant rejection. Production of nitric oxide by iNOS has been confirmed in many acute and chronic inflammatory diseases and is found in activated macrophages making it a potential biomarker for inflammation. The more promising of the two compounds (88) was reported in the literature [188], but its analog and related information can be found in the patent.
Figure 46. . 4-Methyl-pyridine inhibitors of inducible nitric oxide synthases.
Bradykinin receptor B1
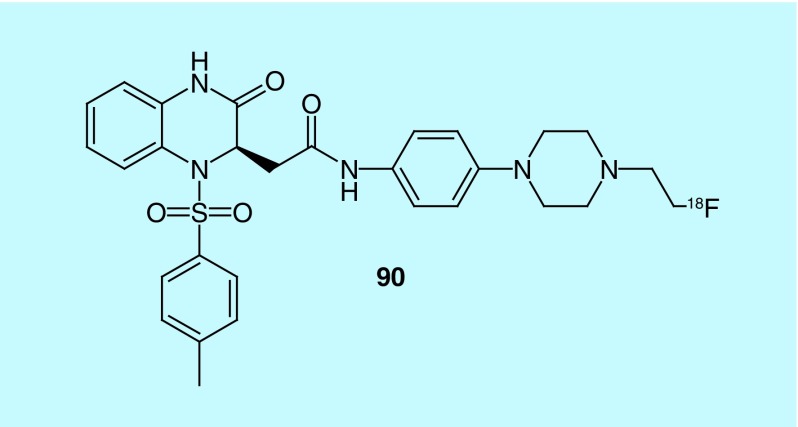
Bradykinin receptor B1 (B1) is a G-protein-coupled receptor known to be involved in pain, inflammation and cancer, which lead inventors at the British Columbia Cancer Agency to develop radiotracers for B1 [189]. The inventors describe both peptides and nonpeptidic tracers for B1 with in vivo evaluation only conducted with gallium-68 peptides with a DOTA connected via a linker for binding the gallium. Al-18F is also claimed for use with the peptides although it is not described. Out of the nonpeptide compounds, only one compound (90, Figure 47) had an nM Ki (108.3 nM) as determined in a competitive binding assay utilizing a tritium-labeled ligand.
Figure 47. . Radiotracer for bradykinin receptor B1.
Cell death
Phosphatidylserine
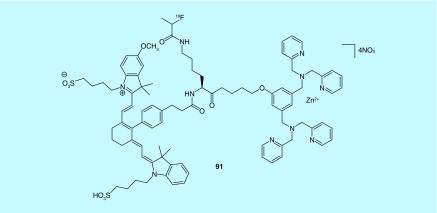
In two patents from Molecular Targeting Technologies, Inc., zinc (II) dipicoly-lamine coordination complexes were developed for imaging phosphatidylserine [190] for its use in detecting cell death and its use in examining ocular disease as a dual modality tracer, PET and fluorescence [191]. Phosphatidylserine is an anionic phospholipid that is typically found in the membrane inner leaflet but is externalized during most types of cell death. The zinc (II) dipicolylamine coordination complexes were designed by the inventors to mimic the annexin-V apoptosis sensing function [192]. The scaffold had previously been developed with 99mTc and 64Cu coordination, but the inventors sought to generate fluorine-18-labeled analogs using common fluorine-18 prosthetic groups: [18F]SFB, 2-18F-fluoroethyl tosylate, and 2-18F-fluoropropionate; as fluorine-18 is the most common PET isotope and would not affect the ability of the zinc (II) dipcolylamine coordination complex to interact with phosphatidylserine as much as the PET metal coordinate analogs would. Experiments in athymic nude mice with a human glioma xenograft demonstrated that the selected 18F-radiotracer (91, Figure 48), imaged the tumor successfully as confirmed by ex vivo and fluorescence imaging with the molecule [190]. Additionally, a benzalkonium chloride induced corneal toxicity model study in Wistar rats was preformed and demonstrated that the tracer examined by fluorescence imaging could detect cell death in the eye with greater benzalkonium chloride concentrations showing more damage and no signal detected with just vehicle [191].
Figure 48. . Inventors from Molecular Targeting Technologies, Inc. advanced this fluorine-18 radiotracer to preclinical evaluation for imaging cell death.
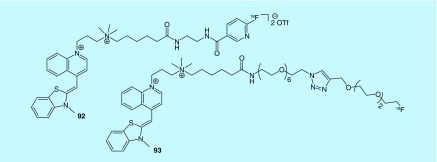
Vital fluorochrome
In a patent filed by the General Hospital Corporation, two fluorine-18-labeled vital fluorochromes (92 and 93) that bind to nucleic acids in cells that are dying or dead are described (Figure 49) [193]. The inventors envision using the imaging agent to detect cell death due to diabetes, organ transplant rejection, myocardial infarction or atherosclerosis with selective imaging based on the concept that the vital fluorochrome can pass through the permeable membrane of a dead or dying cell but not that of an intact cell. Biological evaluation or preclinical PET experiments are not described in the patent, so it is yet to be determined if they function as imaging agents for cell death.
Figure 49. . Structures of vital fluorochromes for imaging cell death.
Cystine/glutamate transporter
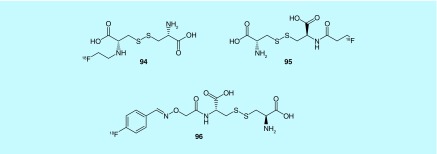
Three fluorine-18-labeled substrates for the cystine/glutamate transporter, which is an antiporter, are described in a patent from the GE Healthcare [194]. The transporter has low expression levels in most tissues unless the cells are exposed to oxidative stress. The transporter uptakes cystine into the cell which is then reduced to cysteine; therefore, the inventors reasoned that a fluorine-18-labeled cystine substrate could be a measure for oxidative stress in disease states due to apoptosis or tissue damage seen in stroke, traumatic injury, transplant rejection or as a measure of chemotherapy effectiveness. The cystine substrate analogs have been used previously to image the transporter with fluorescence and SPECT but have not seen use, likely due to limitations of those modalities. The three cystine analogs are described in the patent (94–96, Figure 50); the aminoxy-[18F]fluorobenzyl analog was evaluated in Jurkat and A549 cell lines to measure uptake of substrate, Balb-c mice to measure the biodistribution and an apoptosis model where mice were injected with anti-Fas antibody 2 h prior to small animal PET scan. The radiotracer is renally cleared and apoptosis model experiments showed increased uptake in the liver as expected.
Figure 50. . Fluorine-18-labeled cystine analogs for imaging the Cystine/glutamate transporter.
In vivo pH measurement
A series of fluorine-18 analogs of pH indicators were prepared with the chemistry described in a patent from the University of Pennsylvania [195]. Indicators that were used as starting points include phenol red, cresol red, thymol blue, bromophenol red, naphthol blue, phenolphthalein and other related structures. The synthesis of several of these fluorine-18-labeled indicators is also reported in the journal literature [196]. The inventors expect the radiolabeled indicators will allow for the measurement of extracellular pH to detect tumors or to measure pH changes associated with hypoxia or diabetes as pH changes will affect the electronics of the ring and thus there partitioning between tissues [196]. The probes are prepared by electrophilic aromatic fluorination ortho to a phenol in indicator molecules with [18F]F2 gas that unfortunately results in low specific activities. The inventors also envision utilizing Cherenkov detection to image the probes.
Conclusion & future perspective
The number of patents containing fluorine-18 has risen steadily since the 1990s, with an average of 50–100 fluorine-18 containing patents per year being issued since 2009. These patent applications typically cover new radiotracers (this review) or new radiochemical methods (to be covered in part 2). In the radiotracer space, the majority of applications fall into neurology, oncology, cardiology or other miscellaneous categories. Neurological applications include extensive tracer discovery efforts targeting misaggregated proteins in Alzheimer's disease (amyloid, tau), dysfunctional systems in Parkinson's disease (leucine rich repeat kinase (LRRK2), dopamine receptors) and traumatic brain injury (e.g., tau, cell death), as well as neuroinflammation (TSPO), which has been implicated in many neurological disorders. In the oncology space, efforts continue to develop radiotracers that target a variety of biomarkers for cancer including systems such as receptors, transporters and enzymes that are dysregulated in the tumor environment. New radiotracers for cardiac PET imaging have focused upon perfusion imaging, quantification of cardiac sympathetic nerve density and new radiotracers targeting phosphodiesterase 1, chymase and Hsp90. Finally, the capabilities of PET are being pushed, with exciting new applications in imaging diabetes, chronic pain, bacterial infections, cell death and inflammation.
The wide range of PET radiotracers now available, and continuing to be developed, suggest a lasting role for PET imaging in drug discovery efforts and the move toward personalized medicine, although we recognize that the cost of a PET scan means the latter is heavily dependent upon healthcare reimbursement policies. Impact of many of these classes of radiotracer in both areas is already apparent. For example, the huge trials imaging amyloid and tau across multiple neurodegenerative disorders are increasing our understanding of the entire disease spectrum, while amyloid PET is being increasingly used to appropriately populate clinical trials. The need for development of new radiotracers is also clear, as many disease mechanisms remain poorly understood and functional PET imaging could have significant impact in this area. Development of PET radiotracers is a complicated business that requires close and careful collaboration between basic scientists and physicians so that those radiotracers being translated from the bench into clinical use address the bedside needs of radiologists and nuclear medicine physicians. While it is difficult to predict which radiotracers described herein will follow the translation pathway advance to widespread clinical use, we expect the growth of PET to continue for the foreseeable future, as the valuable functional information and exquisite sensitivity available from PET are not available from other imaging modalities.
Finally, in addition to the development of the PET radiotracers highlighted in this review, there have been a steady number of patents focusing upon hybrid or multimodality imaging agents. Although multimodality agents are outside the scope of this article, they are alluded to throughout as many of the tracers reported can also be tagged with SPECT radionuclides, fluorescent probes for optical imaging and gadolinium for MRI applications. Hybrid scanners such as PET/CT or PET/MRI are increasingly commonplace, although the latter remain extremely costly at the time of writing, and therefore hybrid probe development is also expected to continue.
Executive summary.
Positron emission tomography (PET) imaging is a form of functional molecular imaging increasingly used in personalized medicine (presymptomatic diagnosis, predict response to therapy, monitor response to therapy) and drug discovery (confirm target engagement, estimate receptor occupancy, determine dosing regimens, enrich clinical trial enrollment).
The most commonly utilized PET radionuclide is fluorine-18 because the convenient 110 min half-life enables commercial distribution from centralized nuclear pharmacies and the excellent imaging properties provide exquisite images. Moreover, the growing popularity for incorporating fluorine into pharmaceutical scaffolds offers rich opportunities for adapting them into PET radiotracers to function as companion diagnostics.
The number of publications and patents containing fluorine-18 has risen steadily since the 1990s, coinciding with approval of [18F]fludeoxyglucose by the US FDA and subsequent agreement to cover reimbursement by the Centers for Medicare and Medicaid Services.
An average of 50–100 fluorine-18 containing patents per year have been issued since 2009.
Patent applications typically cover new radiotracers (for applications in, for example, neurology, cardiology, oncology and other miscellaneous areas, that are covered in this review) or new radiochemical methods (to be covered in Part 2 of this review).
Footnotes
Financial & competing interests disclosure
Financial support of this work by NIH (T32-GM007767 and T32-EB005172), US DOE/NIBIB (DE-SC0012484), Alzheimer's Association (NIRP-14–305669) and the University of Michigan (College of Pharmacy, Rackham Graduate School, Undergraduate Research Opportunity Program [UROP] and Michigan Research Community [MRC]) is gratefully acknowledged. In addition to his employment at the University of Michigan, PJH Scott is an owner of SynFast Consulting, LLC, a consultant to JUAMA Medico and receives honoraria from Zevacor Molecular and John Wiley and Sons. His laboratory at the University of Michigan is supported through grants from NIH, US DOE, Alzheimer's Association, Avid Radiopharmaceuticals/Eli Lilly, Bristol-Myers Squibb, Merck, Adeptio, GE Healthcare, Threshold Pharmaceuticals and Ionetix. PJH Scott is also a former employee of Siemens Medical Solutions, USA (2007–2009) and is a coinventor on a patent mentioned in this review (Siemens Medical Solutions, USA WO 2008124703 [2008]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.2014 PET Imaging Market Summary Report. IMV Medical Information Division 2014 Des Plaines, IL 60018
- 2.Ametamey SM, Honer M, Schubiger PA. Molecular imaging with PET. Chem. Rev. 2008;108(5):1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]; • Provides a general review of PET imaging.
- 3.For an overview of the role of PET imaging in personalized medicine see: Pither R. PET and the role of in vivo molecular imaging in personalized medicine. Expert Rev. Mol. Diagn. 2003;3(6):703–713. doi: 10.1586/14737159.3.6.703. [DOI] [PubMed] [Google Scholar]
- 4.Matthews PM, Rabiner EA, Passchier J, Gunn RN. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 2012;73(2):175–186. doi: 10.1111/j.1365-2125.2011.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of the applications of PET imaging in drug discovery.
- 5.Brooks AF, Topczewski JJ, Ichiishi N, et al. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci. 2014;5(12):4545–4553. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Recent perspective from our group on the current state of the art in fluorine-18 radiochemistry, also see references therein.
- 6.Wang J, Sánchez-Roselló M, Aceña JL, et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 2014;114(4):2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 7.Gillis EP, Eastman KJ, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015;58(21):8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 8.Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging. 2012;2(1):55–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer L, Luxen A. PET radiotracers for molecular imaging in the brain: past, present and future. Neuroimage. 2012;61(2):363–370. doi: 10.1016/j.neuroimage.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Jones T, Rabiner EA. The development, past achievements, and future directions of brain PET. J. Cereb. Blood Flow Metab. 2012;32(7):1426–1454. doi: 10.1038/jcbfm.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantaleo MA, Nannini M, Maleddu A, et al. Conventional and novel PET tracers for imaging in oncology in the era of molecular therapy. Cancer Treat. Rev. 2008;34:103–121. doi: 10.1016/j.ctrv.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Singhal T, Alavi A, Kim CK. Brain: positron emission tomography tracers beyond [18F]fluorodeoxyglucose. PET Clinic. 2014;9(3):267–276. doi: 10.1016/j.cpet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 13.2015 Alzheimer's Statistics. http://www.alzheimers.net/resources/alzheimers-statistics/
- 14.Wurtman R. Biomarkers in the diagnosis and management of Alzhiemer's Disease. Metabolism. 2015;64(3):S47–S50. doi: 10.1016/j.metabol.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 15.2012. GE Healthcare Limited; Medi-Physics, Inc. WO2012068072.
- 16.Kudo Y, Furumoto S, Okamura N. 2009. WO2009004914.
- 17.Kudo Y, Okamura N, Furumoto S, et al. 2-(2-[2-dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer's disease patients. J. Nucl. Med. 2007;48(4):553–561. doi: 10.2967/jnumed.106.037556. [DOI] [PubMed] [Google Scholar]
- 18.The Regents of the University of California. 2009. US20090004107.
- 19.The Regents of the University of California. 2013. US20130315826.
- 20.Ding Y-S, Lin K-S, Logan J. PET imaging of norepinephrine transporters. Curr. Pharm. Des. 2006;12(30):3831–3845. doi: 10.2174/138161206778559687. [DOI] [PubMed] [Google Scholar]
- 21.Technische Universität München. 2010. EP2218464.
- 22.Yousefi BH, Drzezga A, von Reutern B, et al. A novel 18F-labeled imidazo[2,1-b]benzothiazole (IBT) for high-contrast PET imaging of β-amyloid plaques. ACS Med. Chem. Lett. 2011;2(9):273–277. doi: 10.1021/ml200123w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BAYER Schering Pharma aktiengesellschaft. 2010. WO2010028776.
- 24.Brockschnieder D, Schmitt-Willich H, Heinrich T, et al. Preclinical characterization of a novel class of 18F-labeled pet tracers for amyloid-β. J. Nucl. Med. 2012;53(11):1794–1801. doi: 10.2967/jnumed.112.104810. [DOI] [PubMed] [Google Scholar]
- 25.Davie CA. A review of Parkinson's Disease. Br. Med. Bull. 2008;86(1):109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann-La F, Roche AG. 2013. WO2013079496.
- 27.Chan BK, Estrada AA, Chen H, et al. Discovery of a highly selective, brain-penetrant aminopyrazole LRRK2 inhibitor. ACS Med. Chem. Lett. 2013;4(1):85–90. doi: 10.1021/ml3003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada AA, Liu X, Baker-Glenn C, et al. Discovery of Highly potent, selective, and brain-penetrable leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J. Med. Chem. 2012;55(22):9416–9433. doi: 10.1021/jm301020q. [DOI] [PubMed] [Google Scholar]
- 29.Elsinga PH, Hatano K, Ishiwata K. PET Tracers for imaging of the dopaminergic system. Curr. Med. Chem. 2006;13(18):2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- 30.Hunan Institute of Engineering. 2014. CN103110966.; •• Potential PET radiotracer selective for Dopamine D4 receptors.
- 31.Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7(1):31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MS, Kobeissya FH, Wanga KKW, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol. Psychiatry. 2009;66(2):118–127. doi: 10.1016/j.biopsych.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BANYAN BIOMARKERS. 2012. WO2012021800.
- 34.BANYAN BIOMARKERS. 2012. WO2012021788.
- 35.Chatterjee S, Ator MA, Bozyczko-Coyne D, et al. Synthesis and biological activity of a series of potent fluoromethyl ketone inhibitors of recombinant human calpain I. J. Med. Chem. 1997;40(23):3820–3828. doi: 10.1021/jm970197e. [DOI] [PubMed] [Google Scholar]
- 36.Yi J-H, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006;48(5):394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.The University of Montana. 2013. WO201334241.
- 38.Greenfield A, Grosanu C, Dunlop J, et al. Synthesis and biological activities of aryl-ether-, biaryl-, and fluorene-aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT-2. Bioorg. Med. Chem. Lett. 2005;15(22):4985–4988. doi: 10.1016/j.bmcl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Haydar SN, Dunlop J. Neuronal nicotinic acetylcholine receptors – targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer's disease. Curr. Top. Med. Chem. 2010;10(2):144–152. doi: 10.2174/156802610790410983. [DOI] [PubMed] [Google Scholar]
- 40.The Regents of the University of California. 2014. US2014050663.
- 41.Chattopadhyay S, Xue B, Collins D, et al. Synthesis and evaluation of nicotine α4β2 receptor radioligand, 5-(3′-18F-Fluoropropyl)-3-(2-(S)-Pyrrolidinylmethoxy)pyridine, in podents and PET in nonhuman primate. J. Nucl. Med. 2005;46(1):130–140. [PubMed] [Google Scholar]
- 42.Syvänen S, Eriksson J. Advances in PET imaging of p-glycoprotein function at the blood–brain barrier. ACS Chem. Neurosci. 2013;4(2):225–237. doi: 10.1021/cn3001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stichting Voor De Technische Wetenschappen, Stichting Vu-Vumc, Rijksuniversiteit Groningen, Academisch Ziekenhuis Groningen. 2014. WO2014098593.
- 44.Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp. Neurol. 2009;219(1):53–57. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassiou M, James ML, Luus CA. University of Sydney. 2009. WO 2009079683.; •• TSPO imaging is a hot topic. Current imaging agents suffer from complications arising from two-site binding associated with a single nucleotide polymorphism (SNP). This patent reports an alternate scaffold for TSPO imaging (although additional studies to determine if it will be affected by the same SNP issue are warranted).
- 46.Susanne N. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders – results from in vivo imaging studies. Rev. Neurosci. 2010;21(2):119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 47.Andersson JD, Halldin C. J. Label. Compd Radiopharm. 2013;56(3–4):195–206. doi: 10.1002/jlcr.3008. [DOI] [PubMed] [Google Scholar]
- 48.Gaeta A, Woodcraft J, Plant S, et al. Use of 2-[18F]fluoroethylazide for the Staudinger ligation – preparation and characterisation of GABAA receptor binding 4-quinolones. Bioorg. Med. Chem. Lett. 2010;50(15):4653–4656. doi: 10.1016/j.bmcl.2010.05.106. [DOI] [PubMed] [Google Scholar]
- 49.GE HEALTHCARE LTD. 2009. WO2009040377.
- 50.Pillai SK, Chang A, Murphy MW, et al. 2011 Investigation of internal contamination with radioactive strontium following Rb 82 cardiac PET scan. Biosecur. Bioterror. 2014;12(1):42–48. doi: 10.1089/bsp.2013.0072. [DOI] [PubMed] [Google Scholar]
- 51.Slart RJA, Bax J, van Veldhuisen D, van der Wall E, Dierckx RJO, Jager P. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int. J. Cardiovasc. Imaging. 2006;22(1):63–80. doi: 10.1007/s10554-005-7514-8. [DOI] [PubMed] [Google Scholar]
- 52.Berman DS, Maddahi J, Tamarappoo BK, et al. Phase II Safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J. Am. Coll. Cardiol. 2013;61(4):469–477. doi: 10.1016/j.jacc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lantheus Medical Imaging, Inc. 2009. WO2009110984.
- 54.Lantheus Medical Imaging, Inc. 2011. WO2011097649.
- 55.University of Southern California. 2014. WO2014065874.
- 56.Flewelling RF, Hubbell WL. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys. J. 49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Lin T-P, Liu S, et al. Rapid aqueous [18F]-labeling of a bodipy dye for positron emission tomography/fluorescence dual modality imaging. Chem. Commun. 2011;47(33):9324–9326. doi: 10.1039/c1cc13089g. [DOI] [PubMed] [Google Scholar]
- 58.Hadasit Medical Research Services & Development Limited. 2011. US20110293519.
- 59.Children's Medical Center Corporation. 2009. US20090257953.
- 60.The Regents of the Universiy of Michigan. 2014. WO2014052454.; •• Report of [18F]MHPG, a potential fluorine-18 PET replacement for [123I]MIBG SPECT.
- 61.Jang KS, Jung Y-W, Gu G, et al. 4-[18F]Fluoro-m-hydroxyphenethylguanidine: a radiopharmaceutical for quantifying regional cardiac sympathetic nerve density with positron emission tomography. J. Med. Chem. 2013;56(18):7312–7323. doi: 10.1021/jm400770g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang KS, Jung Y-W, Sherman PS, Quesada CA, Gu G, Raffel DM. Synthesis and bioevaluation of [18F]4-fluoro-m-hydroxyphenethylguanidine ([18F]4F-MHPG): a novel radiotracer for quantitative PET studies of cardiac sympathetic innervation. Bioorg. Med. Chem. Lett. 2013;23(6):1612–1616. doi: 10.1016/j.bmcl.2013.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Intra-Cellular Therapies, Inc. 2011. WO2011043816.
- 64.Chan S, Yan C. PDE1 isozymes, key regulators of pathological vascular remodeling. Curr. Opin. Pharmacol. 2011;11(6):720–724. doi: 10.1016/j.coph.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Memorial Sloan–Kettering Cancer Center. 2014. WO2014144715.
- 66.Sloan–Kettering Institute for Cancer Research. 2013. WO2013009655.
- 67.Sloan–Kettering Institute for Cancer Research. 2006. US7834181.
- 68.Sloan–Kettering Institute for Cancer Research. 2014. US8703942.
- 69.Kapustian L, Vigontina O, Rozhko O, et al. Hsp90 and its co-chaperone, Sgt1, as autoantigens in dilated cardiomyopathy. Heart Vessels. 2013;28(1):114–119. doi: 10.1007/s00380-011-0226-1. [DOI] [PubMed] [Google Scholar]
- 70.MOLECULAR INSIGHT PHARMACEUTICALS, INC. 2010. WO2009102384.
- 71.Takai S, Jin D, Miyazaki M. New approaches to blockade of the renin–angiotensin–aldosterone system: chymase as an important target to prevent organ damage. J. Pharmacol. Sci. 2010;113(4):301–309. doi: 10.1254/jphs.10r05fm. [DOI] [PubMed] [Google Scholar]
- 72.Hwang D-R, Eckelman WC, Mathias CJ, Petrillo EW, Lloyd J, Welch MJ. Positron-labeled angiotensin-converting enzyme (ACE) inhibitor: fluorine-18F-fluorocaptopril. Probing the ACE activity in vivo by positron emission tomography. J. Nucl. Med. 1991;32(9):1730–1737. [PubMed] [Google Scholar]
- 73.Dilsizian V, Eckelman WC, Loredo ML, Jagoda EM, Shirani J. Evidence for tissue angiotensin-converting enzyme in explanted hearts of ischemic cardiomyopathy using targeted radiotracer technique. J. Nucl. Med. 2007;48(2):182–187. [PubMed] [Google Scholar]
- 74.Gallagher BM, Ansari A, Atkins H, et al. Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoroD-glucose as radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. J. Nucl. Med. 1977;18(10):990–996. [PubMed] [Google Scholar]
- 75.Som P, Atkins HL, Bandoypadhyay D, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J. Nucl. Med. 1980;21(7):670–675. [PubMed] [Google Scholar]
- 76.Coenen HH, Elsinga PH, Iwata R, et al. Fluorine-18 radiopharmaceuticals beyond [18F]FDG for use in oncology and neurosciences. Nucl. Med. Biol. 2010;37(7):727–740. doi: 10.1016/j.nucmedbio.2010.04.185. [DOI] [PubMed] [Google Scholar]
- 77.Vallabhajosula S. 18F-labeled positron emission tomographic radiopharmaceuticals in oncology: an overview of radiochemistry and mechanisms of tumor localization. Sem. Nucl. Med. 2007;37(6):400–419. doi: 10.1053/j.semnuclmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Hynes NE, MacDonal G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 2009;21(2):177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 79.Mishani E, Abourbeh G, Eiblmaier M, Anderson CJ. Imaging of EGFR and EGFR tyrosine kinase overexpression in tumors by nuclear medicine modalities. Curr. Pharm. Des. 2008;14(28):2983–2998. doi: 10.2174/138161208786404326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Univ. Harbin Medical. 2014. CN103656693.
- 81.Univ. Harbin Medical. 2014. CN103656694.
- 82.Univ. Harbin Medical. 2014. CN103690972.
- 83.Boehringer Ingelheim International Gmbh, Stichting Vu-Vumc. 2014. WO2014118197.
- 84.Memon AA, Jakobsen S, Dagnaes-Hansen F, Sorensen BS, Keiding S, Nexo E. Positron emission tomography (PET) imaging with [11C]-labeled erlotinib: a micro-pet study on mice with lung tumor xenografts. Cancer Res. 2009;69(3):873–878. doi: 10.1158/0008-5472.CAN-08-3118. [DOI] [PubMed] [Google Scholar]
- 85.Domanska UM, Kruizinga RC, Nagengast WB, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur. J. Cancer. 2013;49(1):219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Emory University. 2011. WO2011094389.
- 87.Tamamura H, Omagari A, Hiramatsu K, et al. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg. Med. Chem. Lett. 2001;11(14):1897–1902. doi: 10.1016/s0960-894x(01)00323-7. [DOI] [PubMed] [Google Scholar]
- 88.Univ. Xiamen. 2013. CN103275188.
- 89.Rosenkilde MM, Gerlach L-O, Hatse S, et al. Molecular mechanism of action of monocyclam versus bicyclam non-peptide antagonists in the CXCR4 chemokine receptor. J. Biol. Chem. 2007;282(37):27354–27365. doi: 10.1074/jbc.M704739200. [DOI] [PubMed] [Google Scholar]
- 90.The General Hospital of PLA. 2010. CN101780286.
- 91.Bose Chinmoy K. Follicle stimulating hormone receptor in ovarian surface epithelium and epithelial ovarian cancer. Oncol. Res. 2008;17(5):231–238. doi: 10.3727/096504008786111383. [DOI] [PubMed] [Google Scholar]
- 92.Jiangsu Institute of Nuclear Medicine. 2015. CN103041412.
- 93.Roesler R, Henriques JAP, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS & neurological disorders. Drug Targets. 2006;5(2):197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- 94.Guangdong Pharmaceutical University. 2015. CN103497235.
- 95.Stanford University. 2010. US2010015058.
- 96.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J. Med. Chem. 2009;52(2):425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z, Yan Y, Liu S, Wang F, Chen X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug. Chem. 2009;20(5):1016–1025. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siemens Medical Solutions USA, Inc. 2009. WO2009134382.
- 99.Jiangsu Institute of Nuclear Medicine. 2013. CN102295685.
- 100.Jiangsu Institute of Nuclear Medicine. 2013. CN102268074.
- 101.Guo N, Lang L, Li W, et al. Quantitative analysis and comparison study of [18F]AlF-NOTA-PRGD2, [18F]FPPRGD2 and [68Ga]Ga-NOTA-PRGD2 using a reference tissue model. PLoS ONE. 2012;7(5):e37506. doi: 10.1371/journal.pone.0037506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim HL, Sachin K, Jeong HJ, et al. F-18 labeled RGD probes based on bioorthogonal strain-promoted click reaction for pet imaging. ACS Med. Chem. Lett. 2015;6(4):402–407. doi: 10.1021/ml500464f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bem WT, Thomas GE, Mamone JY, et al. Overexpression of sigma receptors in nonneural human tumors. Cancer Res. 1991;51(24):6558–6562. [PubMed] [Google Scholar]
- 104.Institut National De La Sante et De La Recherche Medicale, Laboratoires Cyclopharma, Universite D'auvergne. 2013. WO2013160808.
- 105.Billaud EM, Rbah-Vidal L, Vidal A, et al. Synthesis, radiofluorination, and in vivo evaluation of novel fluorinated and iodinated radiotracers for PET imaging and targeted radionuclide therapy of melanoma. J. Med. Chem. 2013;56(21):8455–8467. doi: 10.1021/jm400877v. [DOI] [PubMed] [Google Scholar]
- 106.Maisonial A, Billaud EM, Besse S, et al. Synthesis, radioiodination and in vivo screening of novel potent iodinated and fluorinated radiotracers as melanoma imaging and therapeutic probes. Eur. J. Med. Chem. 2013;63:840–853. doi: 10.1016/j.ejmech.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 107.Washington University. 2012. US2012171119.
- 108.Mach RH, Wheeler KT. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo . Cent. Nerv. Syst. Agents Med. Chem. 2009;9(3):230–245. doi: 10.2174/1871524910909030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Isis Innovation Ltd. 2010. WO2010007363.
- 110.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16((-18F-fluoro-5(-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J. Nucl. Med. 2010;51(2):183–192. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.British Columbia Cancer Agency Branch, The University of British Columbia. 2013. WO2013028791.; •• Report of a PET radiotracer for imaging androgen receptors (AR) that potentially overcomes challenges caused by splice variants that have historically complicated AR imaging in prostate cancer.
- 112.Berndt M, Pietzsch J, Wuest F. Labeling of low-density lipoproteins using the 18F-labeled thiol-reactive reagent N-[6-(4-[18F]fluorobenzylidene)aminooxyhexyl]maleimide. Nucl. Med. Biol. 2007;34(1):5–15. doi: 10.1016/j.nucmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Universitätsklinikum Heidelberg, Bayer Pharma Aktiengesellschaft, Deutsches Krebsforschungszentrum. 2013. WO2013132094.
- 115.Wuest F, Köhler L, Berndt M, Pietzsch J. Systematic comparison of two novel, thiol-reactive prosthetic groups for 18F labeling of peptides and proteins with the acylation agent succinimidyl-4-[18F]fluorobenzoate ([18F]SFB) Amino Acids. 2009;36(2):283–295. doi: 10.1007/s00726-008-0065-2. [DOI] [PubMed] [Google Scholar]
- 116.Li X, Link JM, Stekhova S, et al. Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconjug. Chem. 2008;19(8):1684–1688. doi: 10.1021/bc800164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang G, Zeng WB, Yu MX, Kabalka G. Facile synthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) for protein labeling. J. Labelled Comp. Radiopharm. 2008;51(1):68–71. [Google Scholar]
- 118.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 119.Leamon CP, Low PS. Folate-mediated targeting: from diagnostics to drug and gene delivery. Drug Discov. Today. 2001;6(1):44–51. doi: 10.1016/s1359-6446(00)01594-4. [DOI] [PubMed] [Google Scholar]
- 120.Müller C, Schibli R. Folic acid conjugates for nuclear imaging of folate receptor-positive cancer. J. Nucl. Med. 2011;52(1):1–4. doi: 10.2967/jnumed.110.076018. [DOI] [PubMed] [Google Scholar]
- 121.Müller C. Folate based radiopharmaceuticals for imaging and therapy of cancer and inflammation. Curr. Pharm. Des. 2012;18(8):1058–1083. doi: 10.2174/138161212799315777. [DOI] [PubMed] [Google Scholar]
- 122.Al Jammaz I, Al-Otaibi B, Okarvi S, Amartey J. Novel synthesis of [18F]-fluorobenzene and pyridinecarbohydrazide-folates as potential PET radiopharmaceuticals. J. Labelled Comp. Radiopharm. 2006;49(2):125–137. [Google Scholar]
- 123.Siegel BA, Dehdashti F, Mutch DG, et al. Evaluation of 111In-DTPA-Folate as a Recepto-targeted diagnostic agent for ovarian cancer: initial clinical results. J. Nucl. Med. 2003;44(5):700–707. [PubMed] [Google Scholar]
- 124.Muller C, Vlahov IR, Santhapuram HK, Leamon CP, Schibli R. Tumor targeting using 67Ga-DOTA-Bz-folate: investigations of methods to improve the tissue distribution of radiofolates. Nucl. Med. Biol. 2011;38(5):715–723. doi: 10.1016/j.nucmedbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 125.Mathias CJ, Lewis MR, Reichert DE, et al. Preparation of 66Ga- and 68Ga-labeled Ga(III)-deferoxamine-folate as potential folate-receptor-targeted PET radiopharmaceuticals. Nucl. Med. Biol. 2003;30(7):725–731. doi: 10.1016/s0969-8051(03)00080-5. [DOI] [PubMed] [Google Scholar]
- 126.Merck Eprova Ag, Moser R, Schibli R, Müller CM, Groehn V, Michel U, Sparr C, Mindt TL. 2008. WO2008125618.
- 127.Bettio A, Honer M, Muller C, et al. Synthesis and preclinical evaluation of a folic acid derivative labeled with 18F for PET imaging of folate receptor-positive tumors. J. Nucl. Med. 2006;47(7):1153–1160. [PubMed] [Google Scholar]
- 128.Low PS, Varghese B, Vlahov IR. Purdue Research Foundation, Endocyte Inc. 2006. WO2006071754.
- 129.Low PS. Purdue Research Foundation. Kularatne. 2008. WO2008098112.
- 130.Merck Eprova Ag, Ametamey SM, Moser R, Ross TL, Phoebe L, Groehn V. 2008. WO2008125613.
- 131.Merck Eprova Ag, Ametamey SM, Moser R, Ross TL, Mindt TL, Groehn V. 2008. WO2008125615.
- 132.Ametamey SM, Groehn V, Moser R, Ross TL. 2008. WO2008125617.
- 133.Merck Eprova Ag. 2010. WO2010040854.
- 134.Ross TL, Honer M, Muller C, et al. A new 18F-labeled folic acid derivative with improved properties for the PET imaging if folate receptor-positive tumors. J. Nucl. Med. 2010;51(11):1756–1762. doi: 10.2967/jnumed.110.079756. [DOI] [PubMed] [Google Scholar]
- 135.Fischer CR, Müller C, Reber J, et al. [18F]fluoro-deoxy-glucose folate: a novel PET radiotracer with improved in vivo properties for folate receptor targeting. Bioconjug. Chem. 2012;23(4):805–813. doi: 10.1021/bc200660z. [DOI] [PubMed] [Google Scholar]
- 136.Merck & Cie. 2013. WO2013167653.
- 137.Rasch MG, Lund IK, Almasi CE, Hoyer-Hansen G. Intact and cleaved uPAR forms: diagnostic and prognostic value in cancer. Front. Biosci. 2008;13:6752–6762. doi: 10.2741/3186. [DOI] [PubMed] [Google Scholar]
- 138.Kriegbaum MC, Persson M, Haldager L, et al. Rational targeting of the urokinase receptor (uPAR): development of antagonists and non-invasive imaging probes. Curr. Drug Targets. 2011;12(12):1711–1728. doi: 10.2174/138945011797635812. [DOI] [PubMed] [Google Scholar]
- 139.Persson M, Madsen J, Ostergaard S, et al. Quantitative PET of human urokinase-type plasminogen activator receptor with 64Cu-DOTA-AE105: implications for visualizing cancer invasion. J. NucI. Med. 2012;53(1):138–145. doi: 10.2967/jnumed.110.083386. [DOI] [PubMed] [Google Scholar]
- 140.Persson M, Madsen J, Østergaard S, et al. 68Ga-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl. Med. Biol. 2012;39(4):560–569. doi: 10.1016/j.nucmedbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 141.Rigshospitalet. 2014. WO2014086364.
- 142.Persson M, Liu H, Madsen J, Cheng Z, Kjaer A. First 18F-labeled ligand for PET imaging of uPAR: in vivo studies in human prostate cancer xenografts. Nucl. Med. Biol. 2013;40(5):618–624. doi: 10.1016/j.nucmedbio.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kong FL, Yang DJ. Amino acid transporter-targeted radiotracers for molecular imaging in oncology. Curr. Med. Chem. 2012;19(20):3271–3281. doi: 10.2174/092986712801215946. [DOI] [PubMed] [Google Scholar]
- 144.Osaka University, Nard Institute, Ltd. 2014. WO2014126071.
- 145.Glaudemans AWJM, Enting RH, Heesters MAAM, et al. Value of 11C-methionine PET in imaging brain tumors and metastases. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(4):615–635. doi: 10.1007/s00259-012-2295-5. [DOI] [PubMed] [Google Scholar]
- 146.Emory University. 2009. WO2009129110.
- 147.Bayer Pharma Aktiengesellschaft. 2011. WO2011151348.
- 148.Bayer Pharma Aktiengesellschaft. 2012. WO 2012025464.
- 149.Menichetti L, Cionini L, Sauerwein WA, et al. Positron emission tomography and [18F]BPA: a perspective application to assess tumor extraction of boron in BNCT. Appl. Radiat. Isot. 2009;67(Suppl.7–8):S351–S354. doi: 10.1016/j.apradiso.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 150.Stella Pharma Corporation, Osaka Prefecture University Public Corporation. 2014. WO2014061508.
- 151.Washington University. 2010. US20100278732.
- 152.Xiong R, Andres J, Scheffler K, Borbas KE. Synthesis and characterization of lanthanide–hydroporphyrin dyads. Dalton Trans. 2015;44(6):2541–2553. doi: 10.1039/c4dt02448f. [DOI] [PubMed] [Google Scholar]
- 153.The Regents of the University of California, Washington State University Research Foundation. 2012. WO2012064914.; •• Report of a new 18F-labeled PET radiortracer targeting PSMA.
- 154.Cancer Targeted Technology LLC. 2013. WO2013173583.
- 155.Cancer Targeted Technology LLC. Washington State University Research Foundation. 2014. WO2014143736.
- 156.Gilupi Gmbh. 2015. WO2015028489.
- 157.Goncharov NV, Jenkins RO, Radilov AS. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 2006;26(2):148–161. doi: 10.1002/jat.1118. [DOI] [PubMed] [Google Scholar]
- 158.General Electric Company, GE Healthcare Limited. 2010. WO2010048144.
- 159.Imperial Innovations Ltd. 2014. WO2014181112.
- 160.Reske S. 2011. DE102010010666.
- 161.General Electric Company, GE Healthcare Limited. 2013. WO2013048832.
- 162.GE Healthcare Limited, Medi-Physics, Inc. 2013. WO2013048811.
- 163.GE Healthcare Limited, Medi-Physics, Inc. 2011. WO2011087823.
- 164.University of Southern California. 2013. WO2013012754.
- 165.Ronen S, Gelovani J, Tong W, Alauddin M, Mukhopadhyay U, Sankaranarayanapillai M, Pal A. Board of Regents of the University of Texas System. 2009. WO2009009179.
- 166.Vanderbilt University. 2014. US20140134107.
- 167.Siemens Medical Solutions, USA. 2008. WO 2008124651.
- 168.Siemens Medical Solutions, USA. 2008. WO 2008124703.
- 169.Lopci E, Grassi I, Chiti A, et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am. J. Nucl. Med. Mol. Imaging. 2014;4(4):365–384. [PMC free article] [PubMed] [Google Scholar]
- 170.Kurihara H, Honda N, Kono Y, Arai Y. Radiolabelled agents for PET imaging of tumor hypoxia. Curr. Med. Chem. 2012;19(20):3282–3289. doi: 10.2174/092986712801215964. [DOI] [PubMed] [Google Scholar]
- 171.Dubois LJ, Niemans R, van Kuijk SJA, et al. New ways to image and target tumour hypoxia and its molecular responses. Radiother. Oncol. 2015 doi: 10.1016/j.radonc.2015.08.022. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 172.Bayer Schering Pharma Aktiengesellschaft. 2010. WO2010006755.
- 173.Beijing Normal University. 2010. CN101768208.
- 174.Shandong Cancer Hospital. 2009. CN101574532.
- 175.The Board of Trustees of the Leland Stanford Junior University. 2014. US20140314671.
- 176.Brunkhorst C, Andersen C, Schneider E. Acarbose, a pseudooligosaccharide, is transported but not metabolized by the maltose-maltodextrin system of Escherichia coli . J. Bacteriol. 1999;181(8):2612–2619. doi: 10.1128/jb.181.8.2612-2619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Emory University, Georgia Tech Research Foundation, Georgia State Research Foundation. 2014. US20140219917.
- 178.The Johns Hopkins University. 2014. WO2014043606.
- 179.Li Z-B, Wu Z, Cao Q, et al. The synthesis of 18F-FDS and its potential application in molecular imaging. Mol. Imaging Biol. 2008;10(2):92–98. doi: 10.1007/s11307-007-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kyoto University, Arkray Inc. 2011. WO2011040460.; •• New approach to diabetes imaging targeting the glucagon-like peptide-1 receptor.
- 181.Saji H, Kimura H, Ono M, Seki I. 2012. US20120253010.
- 182.Columbia University. 2014. WO2014210167.; •• Novel approach to using functional PET imaging to improve our understanding of the mechanisms underlying chronic pain.
- 183.Florjancic AS, Peddi S, Perez-Medrano A, et al. Synthesis and in vitro activity of 1-(2,3-dichlorophenyl)-N-(pyridin-3-ylmethyl)-1H-1,2,4-triazol-5-amine and 4-(2,3-dichlorophenyl)-N-(pyridin-3-ylmethyl)-4H-1,2,4-triazol-3-amine P2X7 antagonists. Bioorg. Med. Chem. Lett. 2008;18(6):2089–2092. doi: 10.1016/j.bmcl.2008.01.095. [DOI] [PubMed] [Google Scholar]
- 184.GE Healthcare Limited. 2010. WO 2010115881.
- 185.Westfaelische Wilhelms-Universitaet Muenster, Germany; Siemens Medical Solutions USA, Inc. EP. 2012. 2520573.; •• New approach to imaging inflammation using 18F-labeled matrix metalloproteinase inhibitors.
- 186.Behrends M, Wagner S, Kopka K, et al. New matrix metalloproteinase inhibitors based on γ-fluorinated α-aminocarboxylic and α-aminohydroxamic acids. Bioorg. Med. Chem. 2015;23(13):3809–3818. doi: 10.1016/j.bmc.2015.03.078. [DOI] [PubMed] [Google Scholar]
- 187.Washington University. 2010. US20100278751.
- 188.Zhou D, Lee H, Rothfuss JM, et al. Design and synthesis of 2-amino-4-methylpyridine analogues as inhibitors for inducible nitric oxide synthase and <i>in vivo</i> evaluation of [18F]6-(2-Fluoropropyl)-4-methyl-pyridin-2-amine as a potential PET tracer for inducible nitric oxide synthase. J. Med. Chem. 2009;52(8):2443–2453. doi: 10.1021/jm801556h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.British Columbia Cancer Agency Branch. 2014. WO2014040192.
- 190.Molecular Targeting Technologies, Inc. 2013. US 20130323172.
- 191.Molecular Targeting Technologies, Inc. 2014. WO2014092958.
- 192.Li J, Gray BD, Pak KY, Ng CK. Optimization of labeling dipicolylamine derivative, N,N’-(5-(4-aminobutoxy)-1,3-phenylene)bis(methylene)bis(1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)methanamine), with three 18F-prosthetic groups as potential imaging agents for metastatic infectious disease. J. Labelled Comp. Radiopharm. 2012;55(4):149–154. [Google Scholar]
- 193.The General Hospital Corporation. 2010. WO 2010141833.
- 194.General Electric Company, GE Healthcare Limited. 2010. WO2010125068.
- 195.University of Pennsylvania. 2013. WO2013062751.
- 196.Kachur AV, Popov AA, Delikatny EJ, Karp JS, Popov AV. Synthesis of 18F-labeled phenolphthalein and naphtholphthalein. J. Fluor. Chem. 2013;151(1):1–6. doi: 10.1016/j.jfluchem.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]