Abstract
To evaluate the effect of melatonin supplementation in maturation medium for human ‘rescue IVM’ and investigate differences in transcriptomic profile of blastocysts developed from oocytes matured in vitro with/without melatonin treatment and in vivo, a total of 314 GV oocytes and 320 MI oocytes were collected from 200 patients younger than 35 years old undergoing ICSI cycle. The oocytes were randomly distributed in the control group (no melatonin) and four other groups of varying melatonin concentrations (10−11, 10−9, 10−7, 10−5 mol/l). Gene profiling was performed on blastocysts developed from in vivo maturation oocytes (in vivo group), and in vitro maturation (IVM) oocytes with an optimal concentration of melatonin treatment (IVM-anti group) or without melatonin (IVM group). The ratio of high quality blastocysts was significantly higher in the groups treated with 10−5 mol/l melatonin compared with others groups. The large-scale analysis of the transcriptome revealed significant differences in mRNA expression levels. In each group, nine blastocysts were selected for gene expression profiling. The differentially expressed genes were involved in cysteine and methionine metabolism, regulation of apoptotic process, mineral absorption, steroid hormone biosynthesis, Wnt signaling, p53 signaling pathway and other functions. The findings indicated that the IVM procedure may potentially affect DNA methylation and the canonical Wnt signaling pathway. Exogenous melatonin positively influenced quality of blastocysts, which may be mediated via upregulation of p53 signaling and correcting DNA methylation changes caused by ‘rescue IVM’. However, this study reflected what was generally referred to as ‘rescue IVM’ and was not a true reflection of clinical IVM techniques. Therefore, melatonin required further investigation as a promising supplement for use in IVM.
Keywords: in vitro maturation, blastocyst, oocyte, melatonin, gene expression, microarray
Introduction
In human assisted reproduction technologies (ARTs), in vitro maturation (IVM) of oocytes had emerged as an important field, especially when applied to women with a diagnosis of polycystic ovary syndrome (1,2). However, embryos produced from IVM oocytes differ greatly from their in vivo counterparts in multiple aspects, including developmental competence and subsequent pregnancy rate (3). A lack of the required maturation factors (growth factors and others) and proper hormonal milieu are likely to cause incomplete cytoplasmic maturation in IVM. Additionally, the procedure of IVM involves several steps, including the isolation, handling and culture of oocytes, which may exert more environmental stress on oocytes and early embryos compared with oocytes matured in vivo. Oxidative stress is one of the most harmful stress factors. In vivo, oocytes and embryos produce endogenous reactive oxygen species (ROS) that have an important role as second messengers in cellular functions through activation of cell signaling cascades (4). However, excessive ROS can lead to serious consequences, including DNA fragmentation, enzymatic inactivation and cell death (5). Progress in embryo developmental competence was obtained by optimizing culture mediums and IVM protocols. Supplementation of basic culture medium with serum and gonadotropins, amino acids, epidermal growth factor, vascular endothelial growth factor, cysteamine and other factors improved embryo developmental competence following in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) of oocytes matured in vitro.
N-acetyl-5-methoxytryptamine (melatonin), one of the most effective antioxidants, is a pleiotropic molecule with an important role in animal reproductive activities (6) and reducing oxidative damage and may improve the survival environments of cells (7). Melatonin is a derivative of tryptophan predominantly produced in the pineal gland of vertebrates and a potent free radical scavenger and antioxidant. Takasaki et al (8) reported that oral melatonin can improve the quality of oocytes and may be useful as a new auxiliary drug for the treatment of infertility. When melatonin was added to semen extender or culture medium, sperm viability, oocyte competence and blastocyst development in vitro were significantly improved (9). Kim et al (10) reported that melatonin supplementation may be used to improve the clinical outcomes of typical IVM IVF-embryo transfer. Carr et al (11) demonstrated the long-term safety of melatonin use. They reported open label follow-up data of 41 cases of the safety of melatonin use in 50 children. They concluded that there were no reported late-onset adverse effects.
The majority of the literature supports a protective/beneficial role for melatonin; however, some controversy remains. Li et al (12) demonstrated that exogenous melatonin had no effect on development of cryopreserved metaphase II oocytes in mice.
To examine the potential beneficial effects of melatonin, immature oocytes from superovulation cycles were used in the present study, which was generally referred to as ‘rescue IVM’; these were used instead of immature oocytes from typical IVM cycles because this was a pilot study and this may also be applied on human oocytes. Different to immature oocytes in typical IVM cycles, the immature oocytes in ‘rescue IVM’ were inhibited by the dominant follicle during development. Normally, these immature oocytes are considered to be of no value and are discarded. However, several studies have demonstrated their value (13,14). In the current study, germinal vesicle (GV) or metaphase I stage (MI) oocytes were incubated with different concentrations of melatonin (10−11, 10−9, 10−7, 10−5 mol/l).
The advent of commercial microarrays and high-fidelity RNA amplification techniques have made it possible to profile gene expression in rarely available human oocytes and embryos to search for developmental regulators. In the current study, the transcriptomic profiles of the blastocysts developed from in vivo matured oocytes and in vitro matured oocytes with or without melatonin treatment were compared in an attempt to identify a molecular basis for the effect of melatonin on oocyte development.
Materials and methods
Experimental patients and ethics
The current study was approved and reviewed by the Ethics Committee of Anhui Medical University (Hefei, China; approval no. 2015012). All patients in the study had provided informed consents for this research. A total of 200 women undergoing ICSI cycles at the Reproductive Medicine Center of the First Affiliated Hospital of Anhui Medical University (Hefei, China) were enrolled from December 2014 to September 2015. The enrolled patients met the following criteria: i) Patients were <35 years old; ii) cycles were stimulated with standard long pituitary downregulation protocol as previously described (15); iii) patients had undergone ICSI cycles; and iv) no chromosomal abnormalities. No significant differences were detected in sperm parameters and major clinical characteristics among the five groups that received varying MT concentrations (Table I).
Table I.
Clinical characteristics among groups treated with varying melatonin concentrations.
| Melatonin treatment group (mol/l) | Age (years) | Body mass index | Total Gn dose (IU) | Gn using days |
|---|---|---|---|---|
| 0 | 27.0±2.62 | 20.42±1.52 | 1,730±400.10 | 11.2±1.69 |
| 10−5 | 27.3±3.62 | 21.85±3.33 | 1,862±541.98 | 12.1±2.33 |
| 10−7 | 27.5±3.59 | 22.41±2.88 | 2,104±533.11 | 12.4±2.67 |
| 10−9 | 28.4±3.03 | 21.16±2.71 | 1,982±453.28 | 10.9±1.37 |
| 10−11 | 27.6±3.37 | 22.99±2.31 | 2,280±420.49 | 12.8±2.65 |
n=40 per group. Gn, gonadotropin.
Human oocyte collection and gamete manipulation
Controlled ovarian stimulation was achieved using a standard long pituitary downregulation protocol with gonadotropin-releasing hormone agonists and recombinant follicle stimulating hormone (FSH; Gonal-F; Merck KGaA, Darmstadt, Germany) for treatment of infertility. Follicular development was monitored by vaginal ultrasound. When at least three follicles reached 18 mm in diameter, 10,000 IU human chorionic gonadotropin (Livzon Pharmaceutical Co., Ltd., Shanghai, China) was administered. At ~36 h later, oocyte cumulus complexes (OCC) were collected under ultrasound guidance. The day prior to oocyte retrieval, serum was collected from each of the patients. The oocytes were from ICSI cycles and denuded so as to judge meiotic status as soon as possible. The immature oocytes at the GV or MI stage were donated for the research.
Rescue IVM with or without melatonin treatment
The immature oocytes were placed in pre-equilibrated in vitro maturation (IVM) medium which was TCM-199 (Sigma-Aldrich; Merck KGaA) medium supplemented with 0.075 IU/ml human recombinant FSH, 0.1 mg/ml 17β-estradiol, 0.22 mM pyruvic acid, 0.6 g/l penicillin and streptomycin, 0.5 IU/ml human chorionic gonadotropin, 20% (v/v) patient serum with different concentrations of melatonin (0, 10−11, 10−9, 10−7,10−5 mol/l; Sigma-Aldrich; Merck KGaA) and incubated at 6% CO2, 5% O2 for ~24 h at 37°C. Oocytes with a visible polar body in the perivitelline space were regarded as matured. The ICSI procedure was carried out with fresh sperm from different samples. No significant differences were detected in sperm parameters, which included concentration and motility among the different groups.
The embryos developed to the blastocyst stage were vitrified on day 5. The quality of the blastocyst was assessed based on Gardner's grading system (16).
Blastocyst thawing for total RNA extraction, amplification, and quality measurement
Blastocysts developed from in vivo maturation oocytes, and in vitro maturation oocytes with (IVM-anti group) or without (IVM group) a certain concentration of melatonin treatment were thawed. Patients that had given birth to healthy babies donated surplus frozen blastocysts for the research, which were developed from in vivo maturation oocytes in ICSI cycles. The three groups of blastocysts, which had similar quality, were pooled in TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA was extracted from each group using the All-Prep DNA/RNA Micro kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. The purified RNA was then amplified in two rounds and digoxigenin-labeled according to the NanoAmp™ RT-IVT Labeling kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The quality and quantity of extracted and amplified RNA were measured using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA). RNA integrity was assessed by standard denaturing agarose gel electrophoresis.
RNA labeling and array hybridization
Sample labeling and array hybridization were performed according to the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technologies, Inc., Santa Clara, CA, USA). Briefly, total RNA from each group was linearly amplified and labeled with Cy3-UTP. The labeled cRNAs were purified by RNeasy Mini kit (Qiagen, Inc.). The concentration and specific activity of the labeled cRNAs (pmol Cy3/µg cRNA) were measured using the NanoDrop ND-1000. Each labeled cRNA (1 µg) was fragmented by adding 11 µl 10X blocking agent and 2.2 µl 25X fragmentation buffer, then heated at 60°C for 30 min, and lastly 55 µl 2X GE hybridization buffer was added to dilute the labeled cRNA from Agilent Gene Expression Hybridization kit (Agilent Technologies, Inc.). Hybridization solution (100 µl) was dispensed into the gasket slide and assembled to the gene expression microarray slide (cat no. G4845A; Agilent Technologies, Inc.). The slides were incubated for 17 h at 65°C in an Agilent Hybridization Oven. The slides were washed and the arrays were scanned by a G2565BA Agilent scanner. Three independent repetitions were performed, and a total of nine chips were used in this study.
Microarray data analysis
Genes in three groups of blastocysts were compared in pairs. Agilent Feature Extraction software (version 11.0.1.1) was applied to analyze the acquired array images. Quantile normalization and subsequent data processing were performed with using the GeneSpring GX v12.1 software package (Agilent Technologies, Inc.). Following quantile normalization of the raw data, genes that at least three out of nine samples had flags in Detected (‘All Targets Value’) within the analysis software were selected for further analysis. Data were progressively filtered as follows: Measurements for each condition with 80% confidence were removed; less precise measurements based on control strength were removed; probes recorded as absent in all samples were removed and the measurements for probes representing the positive and fiducial controls were removed. Differentially expressed genes with statistical significance between the two groups were identified through fold change filtering (fold change ≥2.0; P≤0.05). Hierarchical clustering was performed using the R scripts (17). Biological functional analysis of the differentially expressed genes was done using Gene Ontology (GO; www.geneontology.gov) and the Kyoto Encyclopedia of Genes and Genomes (KEGG; www.genome.jp/kegg/pathway.html) pathway database.
Validation of important genes derived from microarray analyses by reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The levels of relevant mRNAs derived from microarray analyses including the products of certain important genes involved in a specific pathway, were determined by RT-qPCR using a One-Step SYBR PrimeScript RT-PCR kit (Takara Bio, Inc., Tokyo, Japan). The primers used in this study are presented in Table II. Briefly, 2 mg total RNA was converted to cDNA according to the manufacturer's protocol. PCR was performed in a total reaction volume of 25 µl, including 10 µl SYBR Premix Ex Taq (2X), 0.5 µl ROX reference dye II (50X) ×3, 2 µl cDNA, 8 µl double-distilled water, 1 µl 10 mmol/l PCR forward and reverse primer. The RT-qPCR was set at an initial denaturation step of 10 min at 95°C, and 95°C (5 sec), 63°C (30 sec), and 72°C (30 sec) in a total of 40 cycles with a final extension step at 72°C for 5 min. RT-qPCR was used to quantify the mRNA levels of methionine adenosyltransferase 2A (MAT2A), catechol-O-methyltransferase (COMT), period circadian clock 1 (PER1), serine/threonine kinase 4 (STK4), cyclin G1 (CCNG1), interleukin 4 induced 1 (IL4I1), and forkhead box P3 (FOXP3) with GAPDH mRNA as the endogenous reference gene using the 2−∆∆Cq method to perform the normalization (18). Melting curve analysis was performed to confirm the quantitative PCR products. All experiments were carried out in three repetitions. Statistical comparisons were performed using the Student's t-test and the SPSS 16.0 statistical software (P≤0.05 was considered statistically significant).
Table II.
Primer sequences.
| Gene | Bidirectional primer sequences | Size (bp) |
|---|---|---|
| GAPDH | F: 5′GGGAAACTGTGGCGTGAT3′ | 299 |
| R: 5′GAGTGGGTGTCGCTGTTGA3′ | ||
| MAT2A | F: 5′TGGAGACCAGGGCTTAATGTTTG3′ | 114 |
| R: 5′TTACGGCGTAGTTCTGCCAGTTT3′ | ||
| PER1 | F: 5′TACCAGCCATTCCGCCTAAC3′ | 196 |
| R: 5′ CAGCCCTTTCATCCACATCC3′ | ||
| STK4 | F: 5′TGAAACTGAAACGCCAGGAAT3′ | 120 |
| R: 5′TGCCCATCTCATCACCCACT3′ | ||
| CCNG1 | F: 5′ATGACAAGCCTGAGAAGGTAAA3′ | 142 |
| R: 5′TGTGGGAAGACTGATAGTTGATAG3′ | ||
| IL4I1 | F: 5′ CCCTCAAAGACCTCAAGGCACT3′ | 168 |
| R: 5′ CCTCGGCGAAGCTGAGATAGA3′ | ||
| COMT | F: 5′GCTGAAGAAGAAGTATGATGTGGA3′ | 220 |
| R: 5′CAGGAACGATTGGTAGTGTGTG3′ | ||
| FOXP3 | F: 5′AGGAAAGGAGGATGGACGAA3′ | 124 |
| R: 5′GGCAGGCAAGACAGTGGAA3′ |
All primers had a Tm of 60°C. F, forward; R, reverse; MAT2A, methionine adenosyltransferase 2A; PER1, period circadian clock 1; STK4, serine/threonine kinase 4; CCNG1, cyclin G1; IL4I1, interleukin 4 induced 1; COMT, catechol-O-methyltransferase; FOXP3, forkhead box P3.
Statistical analysis
Data were expressed as the mean ± standard error, Statistical comparisons were performed using the Student's t test or a one-way analysis of variance followed by post-hoc Tukey's honest significant difference test and the SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). P≤0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of the patients, effect of melatonin on maturation of human oocyte and embryo development
A total of 314 GV oocytes and 320 MI oocytes were collected from 200 patients younger than 35-years-old that had undergone ICSI cycle, and they were randomly distributed in the control group (no melatonin) and four other groups of varying MT concentrations. There was no significant difference in the ratio of GV/MI in each group. Maturation rate (no. of matured oocytes/total no. of oocytes), fertilization rate (no. of fertilized oocytes/no. of matured oocytes), cleavage rate (no. of cleaved oocytes/no. of fertilized oocytes), blastocyst rate (no. of blastocysts/no. of cleaved oocytes) and high quality blastocyst rate (no. of high quality blastocysts/no. of blastocysts) in the five groups are presented in Table III. The ratio of high quality blastocysts was significantly higher (P<0.05) in 10−5 mol/l melatonin-treated group compared with the others groups. The fertilization rate, cleavage rate, and the blastocyst rate in 10−5 mol/l melatonin-treated group were also higher than others groups, but had no statistically significance. As for maturation rate, there were also no significant differences among these groups.
Table III.
Embryo development in groups of varying MT concentrations.
| MT treatment group (mol/l) | Total no. of oocytes (GV/MI) | Total no. of matured oocytes maturation rate %) | Total no. of fertilized oocytes (fertilization rate %) | Total no. of cleaved oocytes (cleavage rate %) | Total ho. of blastocysts (Blastocyst rate %) | Total ho. of high quality blastocyst (high quality blastocyst rate %) |
|---|---|---|---|---|---|---|
| 0 | 135 (70/65) | 101 (76.1±2.2) | 80 (84.8±1.9) | 73 (86.4±2.6) | 14 (23.3±3.5) | 2 (11.1±3.3)a |
| 10−5 | 159 (66/93) | 112 (78.9±2.9) | 101 (94.0±1.5) | 93 (97.9±1.4) | 26 (29.1±3.6) | 14 (54.4±4.7)b |
| 10−7 | 103 (48/55) | 79 (81.1±2.5) | 71 (90.7±2.2) | 64 (82.4±3.6) | 11 (14.0±2.4) | 3 (27.3±4.6)a |
| 10−9 | 117 (64/53) | 93 (79.7±3.2) | 82 (89.7±2.1) | 78 (92.1±2.1) | 14 (16.3±2.8) | 3 (21.1±4.2)a |
| 10−11 | 120 (66/54) | 82 (69.6±3.7) | 68 (85.4±2.4) | 66 (94.2±2.2) | 11 (14.6±2.3) | 2 (15.4±3.2)a |
Percentage data are presented as the mean ± standard error. Values marked with different letters are statistically different to each other (P<0.05).
P>0.05 between the 0, 10−7, 10−9 and 10−11 mol/l groups
P<0.05 10−5 mol/l vs. all other groups. Maturation rate, no. of matured oocytes/total no. of oocytes; fertilization rate, no. of fertilized oocytes/no. of matured oocytes; cleavage rate, no. of cleaved oocytes/no. of fertilized oocytes; blastocyst rate, no. of blastocysts/no. of cleaved oocytes; high quality blastocyst rate, no. of high quality blastocysts/no. of blastocysts.
Comparative gene expression for human blastocysts from oocytes matured in vitro and in vivo
For each group, [IVM group, IVM-anti group (treated with 10−5 mol/l melatonin) and in vivo maturation] 9 blastocysts matured from oocytes were thawed for total RNA extraction. For each group, 9 blastocysts were assigned to three PCR tubes filled with 400 µl TRIzol reagent on average, so as to be replicated three times. In order to clarify a gene prolife that may better reflect the differences between oocytes matured in vivo and in vitro, a cut-off of >2-fold difference was applied. The three groups were compared pairwise. In blastocysts from the IVM group compared with the in vivo group, of 15,827 analyzed genes, 412 genes were identified as differentially expressed (P<0.05), of which 180 were upregulated, and 232 genes were downregulated. For the IVM-anti group compared with the IVM group, of 15,827 analyzed genes, 821 genes were identified as differentially expressed (P<0.05), of which 587 were upregulated, and 234 were downregulated. For the IVM-anti group compared with the in vivo group, of 15,827 analyzed genes, 1,312 genes are identified as differentially expressed (P<0.05), of which 877 genes were upregulated, and 435 were downregulated. GenBank accession numbers, false discovery rate (FDR), regulation direction, biological processes and P-values of several representative differentially expressed genes are listed in Table IV. Supervised hierarchical clustering analysis was performed based on the data of differentially expressed genes. In clustering maps (Fig. 1), genes from different groups were clearly distinguished, illustrating that the microarray data represent the genomic transcripts well. Significantly enriched GO and KEGG pathways among blastocysts of the three groups are demonstrated in Tables V–VII. The certain expression changes were confirmed by RT-qPCR. MAT2A, COMT, PER1, STK4 were analyzed by RT-qPCR in the IVM group and in vivo group (Fig. 2) MAT2A, COMT and STK4 were significantly differentially expressed in the RT-qPCR analysis and were upregulated in IVM group. CCNG1, IL4I1, MAT2A, FOXP3 were analyzed by RT-qPCR in IVM-anti group and IVM group (Fig. 3). MAT2A and CCNG1 were significantly differentially expressed. MAT2A were downregulated and CCNG1 were upregulated in IVM-anti group.
Table IV.
Differentially expressed genes in the IVM, IVM-anti and in vivo groups.
| A, IVM group vs. in vivo group | |||||
|---|---|---|---|---|---|
| Gene symbol | GenBank accession | Regulation direction | FDR | P-value | Biological processes |
| MAT2A | NM_005911 | Up | 0.99997 | 0.03405 | S-adenosylmethionine synthetase |
| COMT | NM_000754 | Up | 0.70388 | 0.00733 | Catechol-O-methyltransferase |
| ZNF335 | NM_022095 | Up | 0.96627 | 0.01522 | Methylation |
| PER1 | NM_002616 | Up | 0.99997 | 0.01977 | Cation channel sperm-associated protein 1 |
| FOXO3 | NM_001455 | Up | 0.99997 | 0.02969 | Transcription factors |
| STK4 | NM_006282 | Down | 0.97854 | 0.01614 | Phosphorylating |
| NPHP4 | NM_015102 | Down | 0.99997 | 0.03147 | Chromosome and associated proteins |
| B, IVM-anti group vs. IVM group | |||||
| Gene symbol | GenBank accession | Regulation direction | FDR | P-value | Biological processes |
| CCNE1 | NM_001238 | Up | 0.47303 | 0.01166 | G1/S-specific cyclin E1 |
| CCNG1 | NM_004060 | Up | 0.30861 | 0.00296 | Cyclin G1 |
| MAT2A | NM_005911 | Down | 0.58916 | 0.03081 | S-adenosylmethionine synthetase |
| IL4I1 | NM_152899 | Down | 0.60742 | 0.04633 | L-amino-acid oxidase |
| ATP8 | AK128101 | Down | 0.44454 | 0.00934 | Mitochondrial biogenesis |
| C, IVM-anti group vs. in vivo group | |||||
| Gene symbol | GenBank accession | Regulation direction | FDR | P-value | Biological processes |
| DUSP4 | NM_001394 | Up | 0.10966 | 0.00199 | Dual specificity MAP kinase phosphatase |
| LRG1 | NM_052972 | Up | 0.12045 | 0.00249 | DNA repair and recombination proteins |
| ATP5J | NM_001003703 | Down | 0.36829 | 0.03604 | ATP synthase |
| COX6B | NM_144613 | Down | 0.13547 | 0.00334 | Cytochrome c oxidase subunit 6b |
FDR was estimated to evaluate the probability of false positive associations. FDR, false discovery rate; IVM, in vitro maturation; MAT2A, methionine adenosyltransferase 2A; COMT, catechol-O-methyltransferase; ZNF335, zinc finger protein 335; PER1, period circadian clock 1; FOXO3, forkhead box O3; STK4, serine/threonine kinase 4; NPHP4, nephrocystin 4; CCNE1, cyclin E1; CCNG1, cyclin G1; IL4I1, interleukin 4 induced 1; ATP8, ATP synthase F0 subunit 8; DUSP4, dual specificity phosphatase 4; LRG1, leucine rich α-2-glycoprotein 1; ATP5 J, ATP synthase H+ transporting, mitochondrial Fo complex subunit F6; COX6B, cytochrome c oxidase subunit 6B1.
Figure 1.
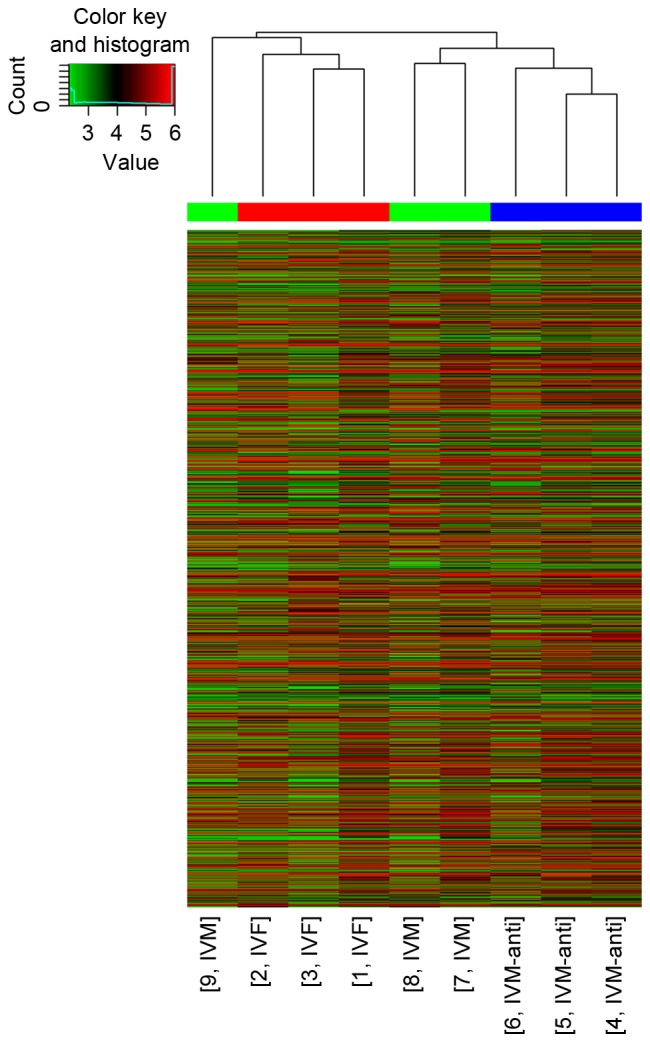
Clustering map of differentially expressed genes in the three groups of blastocysts. IVF, in vivo maturation group; IVM represents in vitro maturation group; IVM-anti, IVM with 10−5 mol/l melatonin group.
Table V.
Significantly enriched Gene Ontology and pathways of blastocysts between in IVM group vs. in vivo group.
| GO/Pathway ID | Regulation direction | Count | Description | Gene symbols |
|---|---|---|---|---|
| GO:0015711 | Up | 9 | Organic anion transport | PQLC2, PRAF2, SLC38A6, GRM7, SLC26A6, ABCB11, SLC15A4, MFSD10, SCP2 |
| GO:0000096 | Up | 3 | Sulfur amino acid metabolic process | GCLM, COMT, MAT2A |
| GO:0034754 | Up | 4 | Cellular hormone metabolic process | SCP2, HSD17B3, DHRS9, COMT |
| GO:0032259 | Up | 5 | Methylation | ZNF335, CMTR2, COMT, MAT2A, PRDM15 |
| GO:0043523 | Up | 4 | Regulation of neuron apoptotic process | GCLM, LRP1, BCL2L11, FOXO3 |
| hsa00270 | Up | 2 | Cysteine and methionine metabolism | MAT2A, SRM |
| hsa00480 | Up | 2 | Glutathione metabolism | GCLM, SRM |
| hsa04978 | Up | 2 | Mineral absorption | MT1A, SLC26A6 |
| hsa00140 | Up | 2 | Steroid hormone biosynthesis | COMT, HSD17B3 |
| GO:0030178 | Down | 6 | Negative regulation of Wnt signaling pathway | G3BP1, MAD2L2, NPHP4, SHH, STK4, GNB2L1 |
| GO:0090090 | Down | 5 | Negative regulation of canonical Wnt signaling pathway | G3BP1, MAD2L2, NPHP4, SHH, STK4 |
| GO:0035329 | Down | 3 | Hippo signaling | NPHP4, STK4, TEAD3 |
| GO:0021510 | Down | 5 | Spinal cord development | SHH, MDGA2, MDGA1, EVX1, NEUROG3 |
Pathway IDs were produced from KEGG analysis. Count indicates the number of differentially expressed genes associated with the listed GO/pathway ID. IVM, in vitro maturation; GO, gene ontology.
Table VII.
Significantly enriched Gene Ontology and pathways of blastocysts in IVM-anti group vs. IVM group.
| GO/pathway ID | Regulation | Count | Description | Gene symbol |
|---|---|---|---|---|
| GO:0010942 | Up | 20 | Positive regulation of cell death | ADIPOQ, BCL2L11, VAV3, TRIM35, NCSTN, NOTCH2, ADAMTSL4 |
| GO:0043549 | Up | 30 | Regulation of kinase activity | CDK5R2, CCNE1, CCNG1, GNAI2, MOS, NTRK3, THBS1, DUSP4 |
| hsa04115 | Up | 5 | p53 signaling pathway | CCNE1, CCNG1, CD82, STEAP3, THBS1 |
| hsa00270 | Down | 2 | Cysteine and methionine metabolism | IL4I1, MAT2A |
| hsa05016 | Down | 5 | Huntington's disease | ATP8, NDUFV3, POLR2G, POLR2J, SDHC |
Pathway IDs were produced from KEGG analysis. Count indicates the number of differentially expressed genes associated with the listed GO/pathway ID. IVM, in vitro maturation; GO, gene ontology.
Figure 2.
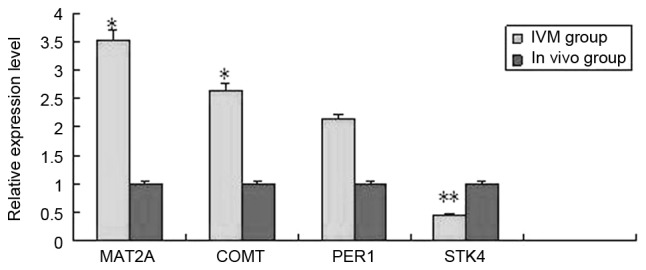
Results of reverse transcription-quantitative polymerase chain reaction analysis in the IVM and in vivo group. *P<0.05, **P<0.01 vs. in vivo group. IVM, in vitro maturation. MAT2A, methionine adenosyltransferase 2A; COMT, catechol-O-methyltransferase; PER1, period circadian clock 1; STK4, serine/threonine kinase 4.
Figure 3.
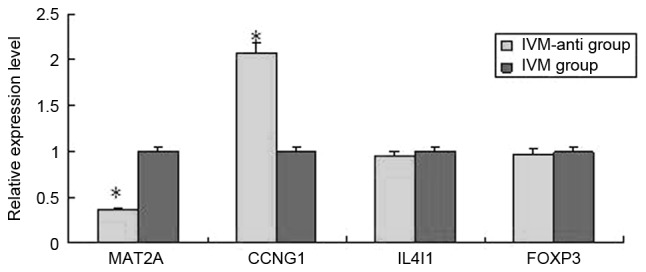
Results of reverse transcription-quantitative polymerase chain reaction analysis in the IVM and IVM-anti group. *P<0.05. IVM, in vitro maturation; MAT2A, methionine adenosyltransferase 2A; CCNG1, cyclin G1; IL4I1, interleukin 4 induced 1; FOXP3, forkhead box P3.
Detailed analysis of associated GO and KEGG pathways
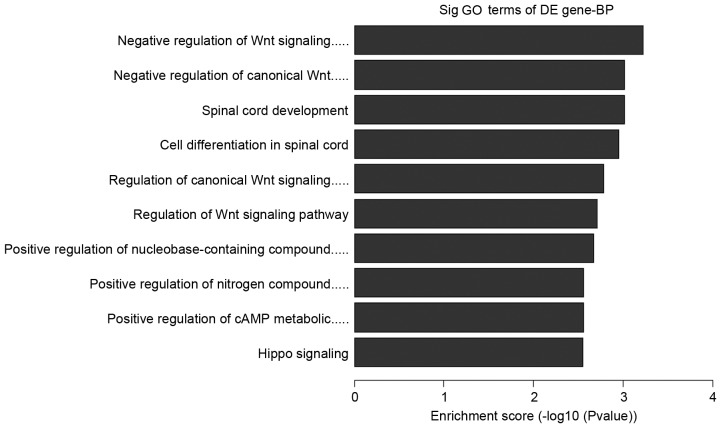
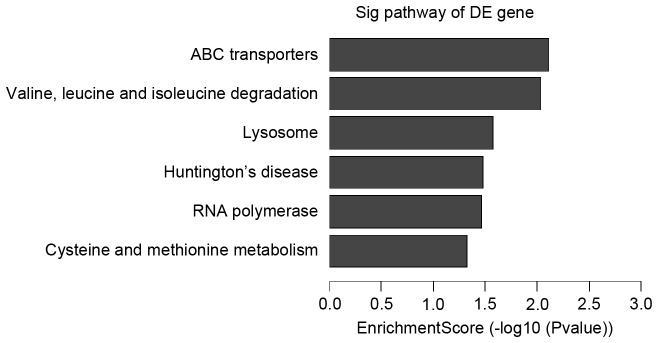
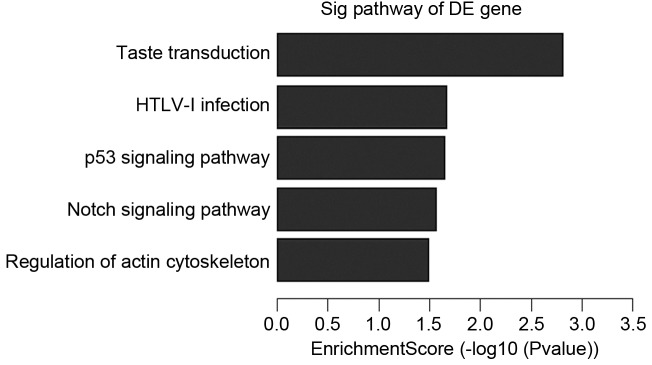
Further analysis to determine which of the functional categories assigned by GO and the KEGG pathways were overrepresented at the 95% confidence level with a minimum overlap set to two genes. GO analysis and KEGG pathway analysis were performed using the standard enrichment computation method. The results are presented in Figs. 4–7.
Figure 4.
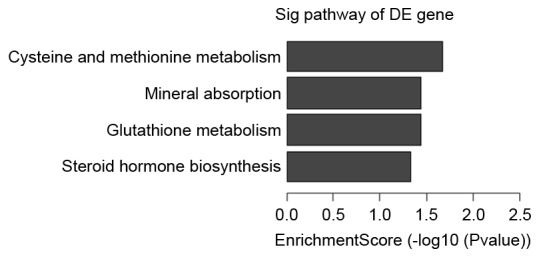
Upregulated pathways in the in vitro maturation group vs. in vivo group. DE, differentially expressed.
Figure 7.
Downregulated pathways in in vitro maturation group vs. in vivo group. GO, gene ontology; DE, differentially expressed; BP, biological process.
Discussion
In the majority of superovulation cycles, a small portion of oocytes remains immature after human chorionic gonadotropin stimulation. These oocytes are routinely abandoned because clinical outcomes of these oocytes are flawed (19). In order to make good use of these oocytes, melatonin was added to the IVM system. The aim of our study was to evaluate the effect of melatonin in maturation medium for human ‘rescue IVM’.
As an effective antioxidant, melatonin is known to be associated with regulation of various dynamic physiological functions, and also is present in human preovulatory follicular fluid and may be associated with reproduction. The beneficial effects of melatonin are concentration dependent. The current study demonstrated that 10−5 mol/l was the appropriate concentration of melatonin.
In a previous study, improved maturation and embryonic development were observed in IVM supplemented with 10−5 mol/l melatonin (10), which is consistent with the results of the present study. In another study on bovine oocyte maturation in vitro, the most effective melatonin concentrations ranged from 10−9-10−7 M; however, as the concentration of melatonin increased from 10−9-10−3 M, the cleavage rate and the blastocyst rate declined dramatically (20). This was not surprising as high concentrations of melatonin inhibit human leukemia cell division (21).
Consistent with the findings of the current study, Takasaki et al reported that oral melatonin supplementation had a beneficial effect on fertilization and embryo quality (8). In a prospective, longitudinal, cohort study, treatment with myo-inositol and melatonin improved ovarian stimulation protocols and pregnancy outcomes in infertile women with poor oocyte quality (22). Batıoğlu et al (23) demonstrated that melatonin was likely to improve oocyte and embryo quality in women undergoing IVF or ICSI (23). Rodriguez-Osorio et al (24) demonstrated that melatonin had a positive effect on porcine embryo cleavage rates and blastocyst cell numbers. Taken together, these data suggest that melatonin supplementation may have a beneficial effect on embryo quality. The melatonin in follicular fluid may be synthesized by the oocytes (25) and used to protect them from oxidative stress or other environmental insults by reducing the oxidation of macromolecules (26). The quality of the oocyte and the embryo are critical for ART. Poor oocyte or embryo quality is directly associated with low developmental potential of the embryo.
Regulation of gene expression has an important role in embryonic development. Transcripts accumulated throughout the human oocyte growth phase control development until a transcriptionally active embryonic genome is established at the 4- to 8-cell stage of embryo development (27). To further clarify the mechanisms by which melatonin mediates its beneficial effects on the quality of embryo, whole gene expression of human blastocysts from in vivo matured blastocysts, and ‘rescue IVM’ blastocysts with or without melatonin treatment were profiled. In addition, the most differentially expressed genes (MAT2A, COMT, PER1, CCNG1), but also the less differentially expressed genes (PER1, FOXP3 and IL4I1) were selected for RT-qPCR validation. These genes were selected out of the numerous differentially expressed genes as they are involved in the significantly enriched GO and pathways of blastocysts, which include methylation, cation channel sperm-associated protein 1, negative regulation of Wnt signaling pathway, p53 signaling pathway, L-amino-acid oxidase and regulation of neuron apoptotic process, which have an important role in regulating embryonic growth.
In vitro and in vivo
The large-scale analysis of the transcriptome revealed significant differences in mRNA expression levels. Firstly, a number of the genes that were differentially expressed when comparing the IVM group and in vivo group (PQ loop repeat containing 2, PRA1 domain family member 2, solute carrier family 38 member 6, glutamate metabotropic receptor 7, COMT, MAT2A, spermidine synthase, metallothionein 1A, STK4 and others) were involved in organic anion transport, cysteine and methionine metabolism, methylation, regulation of apoptotic process, mineral absorption, steroid hormone biosynthesis, Wnt signaling pathway. These changes likely reflected differences in restructuring of the cells of the blastocyst during maturation between the in vitro and in vivo conditions. Many genes that were involved in stress response and metabolism also varied between in vitro and in vivo (glutamate-cysteine ligase modifier subunit, LDL receptor related protein 1, BCL2 like 11 and forkhead box O3) suggesting sub-optimal oxidative and nutritive conditions in vitro compared with in vivo.
Cysteine and methionine metabolism pathway genes were upregulated in the IVM group compared with the vivo group. Genes, such as MAT2A, involved in this process were upregulated. Cysteine and methionine metabolism is an epigenetic pathway associated with DNA methylation that has an important role in regulating embryonic growth and establishment/maintenance of genomic imprints (28). DNA methylation differences between in vitro- and in vivo-conceived children were previously reported to be associated with ART procedures rather than infertility (29). The potential for ARTs to adversely affect the epigenetic status of the developing embryo had been widely discussed; particularly its potential role in increasing the relative risk of imprinting defect syndromes, such as Beckwith-Wiedemann and Angelman syndr`omes (30). Incomplete methylation of maternal loci during oocyte development may have serious consequences during development and is of particular concern for IVM.
By contrast, Kuhtz et al (31) demonstrated that human in vitro oocyte maturation was not associated with increased imprinting error rates at the KCNQ1 opposite strand/antisense transcript 1, small nuclear ribonucleoprotein polypeptide N, paternally expressed 3 and maternally expressed 3 genes (31). Pliushch et al (32) reported that in vitro maturation of oocytes was not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmic sperm injection (32).
The sample size of microarray analysis in the present study was relatively small. The MAT2A and COMT genes, which are associated with DNA methylation, were selected for RT-qPCR validation comparing expression in the IVM group and in vivo group; the expression of MAT2A and COMT were significantly different in these groups. However, whether IVM had an effect on epigenetics should be clarified in further research.
Melatonin treatment
To the best of our knowledge, establishing a mature epigenetic status in the genome is part of the maturation process of the oocyte and is essential for embryo development following fertilization. Certain reports have suggested that melatonin may regulate the genome methylation status (33–36). Li et al (6) reported that melatonin increased imprinted gene expressions of sirtuin 1, AKT serine/threonine kinase 2, and DNA polymerase γ 2. In the current study, MAT2A and COMT expression, which is involved in the pathway of cysteine and methionine metabolism, were significantly downregulated in the melatonin-treated group compared with no melatonin, suggesting that melatonin may regulate epigenetic status; this may be a factor that mediates the effect of melatonin on the quality of embryos. When comparing the expression between the IVM with melatonin treatment group and in vivo group, no differentially expressed genes involved in DNA methylation were detected. Thus, melatonin may correct DNA methylation changes caused by the ‘rescue IVM’ procedure.
In the present study, the p53 signaling pathway was up regulated in the-melatonin treated group compared with no melatonin treatment. Differentially expressed genes cyclin E1, CCNG1, damage specific DNA binding protein 2 and protein phosphatase, Mg2+/Mn2+ dependent 1D were also up regulated. p53 is known as the guardian of the genome and has a major role in DNA repair and apoptosis (36). The cells undergo apoptosis in response to unrepaired DNA via p53-mediated activation (37). The p53 pathway is induced by a number of stress signals, including oxidative stress, DNA damage and activated oncogenes. Over the past three decades researchers have identified p53 as a multi-functional transcription factor. p53 affects highly diverse cellular processes, and is one of the most important and extensively studied tumor suppressors (38). p53-regulated gene functions communicate with adjacent cells, repair damaged DNA, and set up positive and negative feedback loops that enhance or attenuate the functions of the p53 protein, integrating these stress responses with other signal transduction pathways (39). It was previously reported that apoptosis is suppressed by p53 in post-mitotic spermatogenic cells to avoid excess death of spermatocytes to guarantee the robustness of spermatogenesis (40). Therefore, upregulated p53 signaling pathway may be another mechanism of action by melatonin.
Blastocyst formation is a crucial stage in early embryo development. Genes regulate the development and differentiation of the inner cell mass and trophectoderm of the embryo, which controls the transition from the undifferentiation to differentiation stage. The results of the current study also demonstrated that negative regulation of canonical Wnt signaling pathway was downregulated in the IVM group without melatonin treatment compared with the in vivo group [differentially expressed genes were G3BP stress granule assembly factor 1, MAD2L2, nephrocystin 4, sonic hedgehog, STK4, receptor for activated C kinase 1], but no difference in the Wnt signaling pathway were detected between the IVM with melatonin treatment and the in vivo group. Wnt signaling was first identified for its role in carcinogenesis, but had since been recognized for its function in embryonic development range from cell fate determination, cell cycle, body axis patterning to cell fate specification, cell proliferation, and cell migration (41). Wnts were highly evolutionarily conserved in animals (42). Melatonin may also act via effects on Wnt signaling.
NET working
The mechanisms of network of interaction between various signaling pathways had been investigated. Several authors have reported that apoptotic activation of p53 is mediated by activation of extracellular signal-regulated kinase, p38 mitogen-activated protein kinase and c-Jun N-terminal kinase pathways (43). Other studies have determined the effects of microRNAs (miRNAs). miRNAs are a group of noncoding regulatory RNAs known to influence the stability and translational efficiency of target mRNAs. Kim et al (44) reported that p53 and miRNA-34 re suppressors of the canonical Wnt signaling pathway; their data provided insight into the mechanisms by which a p53-miRNA-34 network restrained canonical Wnt signaling cascades in developing organisms and human cancer. In another study, miRNAs were reported to be associated with human embryo implantation defects (45). Melatonin was demonstrated to exert its biological functions by modulation of miRNA expression in human breast cancer cells (46). Thus, melatonin may affect miRNAs, which may regulate the network of interaction between the p53 and Wnt signaling pathways in in vitro maturation of oocytes.
In summary, gene expression profiling using whole human genome arrays and subsequent data analysis provided a molecular basis for the relative higher quality of IVM blastocysts treated with melatonin compared with those without melatonin treatment. Certain genes identified in the current study, including MAT2A, COMT, CCNG1, and STK4, may be useful as the biomarkers of IVM safety. The IVM procedure may potentially affect gametes and embryos by causing disorders of DNA methylation patterns and the canonical Wnt signaling pathway. Exogenous melatonin treatment positively influenced the quality of blastocysts, and regulated the p53 signaling pathway and genes associated with DNA methylation during ‘rescue IVM’. However, the current study was limited due to the suboptimal source of the oocytes and limited sample size. This study reflects what is generally referred to as ‘rescue IVM’ and was not a true reflection of clinical IVM techniques. Therefore, as a promising IVM supplement, the role of melatonin requires further investigation.
Figure 5.
Downregulated pathways in IVM-anti group vs. IVM group. IVM, in vitro maturation; DE, differentially expressed.
Figure 6.
Upregulated pathways in IVM-anti group vs. IVM group. IVM, in vitro maturation; DE, differentially expressed.
Table VI.
Significantly enriched Gene Ontology and pathways of blastocysts in the IVM-anti group vs. in vivo group.
| GO/pathway ID | Regulation direction | Count | Description | Gene symbol |
|---|---|---|---|---|
| GO:0010563 | Up | 29 | Negative regulation of phosphorus metabolic process | DUSP4, CAV1, MEN1, NTRK3, DNAJC10, PID1, MICAL1, ZBED3, PPAP2B |
| GO:0030511 | Up | 29 | Positive regulation of transforming growth factor β receptor signaling pathway | LRG1, FLCN, MEN1, THBS1, MYOCD |
| hsa04115 | Up | 7 | p53 signaling pathway | CCNE1, CCNG1, DDB2, PPM1D, SHISA5, STEAP3, THBS1 |
| hsa00190 | Down | 8 | Oxidative phosphorylation | ATP5J, ATP8, COX6B2, ND1, ND2, ND3, NDUFA6, SDHC |
| hsa05012 | Down | 2 | Parkinson's disease | ATP5J, ATP8, COX6B2, ND1, ND2, ND3, NDUFA6, SDHC, UBA7 |
Pathway IDs were produced from KEGG analysis. Count indicates the number of differentially expressed genes associated with the listed GO/pathway ID. IVM, in vitro maturation; GO, gene ontology.
Acknowledgements
The study has been supported by the Cultivating Youth Training Program (2015KJ03) in The First Affiliated Hospital of Anhui Medical University.
References
- 1.Fadini R, Dal Canto MB, Renzini MM, Brambillasca F, Comi R, Fumagalli D, Lain M, De Ponti E. Predictive factors in in-vitro maturation in unstimulated women with normal ovaries. Reprod Biomed Online. 2009;18:251–261. doi: 10.1016/S1472-6483(10)60263-5. [DOI] [PubMed] [Google Scholar]
- 2.Smitz JE, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29:24–37. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: Current status. Semin Reprod Med. 2012;30:199–213. doi: 10.1055/s-0032-1311522. [DOI] [PubMed] [Google Scholar]
- 4.Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction. 2002;123:479–486. doi: 10.1530/rep.0.1230479. [DOI] [PubMed] [Google Scholar]
- 5.Reshi ML, Su YC, Hong JR. RNA viruses: ROS-mediated cell death. Int J Cell Bio. 2014;2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Zhang Z, He C, Zhu K, Xu Z, Ma T, Tao J, Liu G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J Pineal Res. 2015;59:365–375. doi: 10.1111/jpi.12268. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D. Melatonin protects against isoproterenol-induced myocardial injury in the rat: Antioxidative mechanisms. J Pineal Res. 2010;48:251–262. doi: 10.1111/j.1600-079X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 8.Takasaki A, Nakamura Y, Tamura H, Shimamure K, Morioka H. Melatonin as a new drug for improving oocyte quality. Reprod Med Biol. 2003;2:139–144. doi: 10.1111/j.1447-0578.2003.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz MH, Leal CL, da Cruz JF, Tan DX, Reiter RJ. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim Reprod Sci. 2014;145:150–160. doi: 10.1016/j.anireprosci.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, Yoon TK. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod Biomed Online. 2013;26:22–29. doi: 10.1016/j.rbmo.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Carr R, Wasdell MB, Hamilton D, Weiss MD, Freeman RD, Tai J, Rietveld WJ, Jan JE. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J Pineal Res. 2007;43:351–359. doi: 10.1111/j.1600-079X.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Cheng K, Zhang Y, Meng Q, Zhu S, Zhou G. No effect of exogenous melatonin on development of cryopreserved metaphase II oocytes in mouse. J Anim Sci Biotechnol. 2015;6:42. doi: 10.1186/s40104-015-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isachenko EF, Nayudu PL. Vitrification of mouse germinal vesicle oocytes: Effect of treatment temperature and egg yolk on chromosomal normality and cumulus integrity. Hum Reprod. 1999;14:400–408. doi: 10.1093/humrep/14.2.400. [DOI] [PubMed] [Google Scholar]
- 14.Mandelbaum J, Belaїsch-Allart J, Junca AM, Antoine JM, Plachot M, Alvarez S, Alnot MO, Salat-Baroux J. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum Reprod. 1998;13:161–177. doi: 10.1093/humrep/13.suppl_3.161. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Yang D, Zhang Q. Clinical outcome of one-third-dose depot triptorelin is the same as half-dose depot triptorelin in the long protocol of controlled ovarian stimulation. J Hum Reprod Sci. 2012;5:14–19. doi: 10.4103/0974-1208.97785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 17.R Development Core Team R, corp-author. A language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2008. [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:5. doi: 10.1186/1757-2215-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X, Wang F, He C, Zhang L, Tan D, Reiter RJ, Xu J, Ji P, Liu G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J Pineal Res. 2014;57:239–247. doi: 10.1111/jpi.12163. [DOI] [PubMed] [Google Scholar]
- 21.Büyükavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, Savaşan S. Melatonin cytotoxicity in human leukemia cells: Relation with its pro-oxidant effect. Fundam Clin Pharmacol. 2006;20:73–79. doi: 10.1111/j.1472-8206.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 22.Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol Endocrino. 2011;27:857–861. doi: 10.3109/09513590.2011.564687. [DOI] [PubMed] [Google Scholar]
- 23.Batıoğlu AS, Sahin U, Gürlek B, Oztürk N, Unsal E. The efficacy of melatonin administration on oocyte quality. Gynecol Endocrinol. 2012;28:91–93. doi: 10.3109/09513590.2011.589925. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Osorio N, Kim IJ, Wang H, Kaya A, Memili E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J Pineal Res. 2007;43:283–288. doi: 10.1111/j.1600-079X.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi K, Itoh MT, Takahashi N, Tarumi W, Ishizuka B. The rat oocyte synthesises melatonin. Reprod Fertil Dev. 2013;25:674–682. doi: 10.1071/RD12091. [DOI] [PubMed] [Google Scholar]
- 26.Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, Zhu SE, Liu GS. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res. 2012;52:305–311. doi: 10.1111/j.1600-079X.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- 27.Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG. Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in vitro maturation. Hum Reprod. 1998;13:3132–3138. doi: 10.1093/humrep/13.11.3132. [DOI] [PubMed] [Google Scholar]
- 28.Chiba H, Hiura H, Okae H, Miyauchi N, Sato F, Sato A, Arima T. DNA methylation errors in imprinting disorders and assisted reproductive technology. Pediatr Int. 2013;55:542–549. doi: 10.1111/ped.12185. [DOI] [PubMed] [Google Scholar]
- 29.Song S, Ghosh J, Mainigi M, Turan N, Weinerman R, Truongcao M, Coutifaris C, Sapienza C. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin Epigenetics. 2015;7:41. doi: 10.1186/s13148-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: Epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18:2508–2511. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- 31.Kuhtz J, Romero S, de Vos M, Smitz J, Haaf T, Anckaert E. Human in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2. Hum Reprod. 2014;29:1995–2005. doi: 10.1093/humrep/deu155. [DOI] [PubMed] [Google Scholar]
- 32.Pliushch G, Schneider E, Schneider T, El Hajj N, Rösner S, Strowitzki T, Haaf T. In vitro maturation of oocytes is not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2015;103:720–727.e1. doi: 10.1016/j.fertnstert.2014.12.096. [DOI] [PubMed] [Google Scholar]
- 33.Schwimmer H, Metzer A, Pilosof Y, Szyf M, Machnes ZM, Fares F, Harel O, Haim A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol Int. 2014;31:144–150. doi: 10.3109/07420528.2013.842925. [DOI] [PubMed] [Google Scholar]
- 34.Lee SE, Kim SJ, Yoon HJ, Yu SY, Yang H, Jeong SI, Hwang SY, Park CS, Park YS. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J Pineal Res. 2013;54:80–88. doi: 10.1111/j.1600-079X.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 35.Korkmaz A, Reiter RJ. Epigenetic regulation: A new research area for melatonin? J Pineal Res. 2008;44:41–44. doi: 10.1111/j.1600-079X.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 36.Woods DB, Vousden KH. Regulation of p53 function. Exp Cell Res. 2001;264:56–66. doi: 10.1006/excr.2000.5141. [DOI] [PubMed] [Google Scholar]
- 37.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 38.Stegh AH. Targeting the p53 signaling pathway in cancer therapy-the promises, challenges and perils. Expert Opin Ther Targets. 2012;16:67–83. doi: 10.1517/14728222.2011.643299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine AJ, Hu W, Feng Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Zheng W, Lin A, Uyhazi K, Zhao H, Lin H. Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis. Curr Biol. 2012;22:420–425. doi: 10.1016/j.cub.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 42.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-U. [DOI] [PubMed] [Google Scholar]
- 43.Lin T, Mak NK, Yang MS. MAPK regulate p53-dependent cell death induced by benzo(a)pyrene: Involvement of p53 phosphorylation and acetylation. Toxicology. 2008;247:145–153. doi: 10.1016/j.tox.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Kim NH, Kim HS, Kim NG, Lee I, Choi HS, Li XY, Kang SE, Cha SY, Ryu JK, Na JM, et al. p53 and miRNA-34 are suppressors of canonical Wnt signaling. Sci Signal. 2012;4:ra71. doi: 10.1126/scisignal.2001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26:2830–2840. doi: 10.1093/humrep/der255. [DOI] [PubMed] [Google Scholar]
- 46.Lee SE, Kim SJ, Youn JP, Hwang SY, Park CS, Park YS. MicroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. J Pineal Res. 2011;51:345–352. doi: 10.1111/j.1600-079X.2011.00896.x. [DOI] [PubMed] [Google Scholar]