Abstract
In 1997, the human corneal epithelium was reconstructed in vitro and transplanted on patients. Later, it became a routine treatment, before regulations considered advanced therapy medicinal products and drugs on the same lines. Manufacturing, before and after good manufacturing practice setting, was established in different facilities and the clinical application in several hospitals. Advanced therapy medicinal products, including stem cells, are unique products with different challenges than other drugs: some uncertainties, in addition to benefit, cannot be avoided. This review will focus on all recent developments in the stem cell-based corneal therapy.
Keywords: : adult corneal stem cells, artificial organs, ATMP commercialization, cell therapy, culture/differentiation of stem cells (niche), ophthalmology, regenerative medicine, tissue engineering, translational studies, transplantation
Advanced therapy medicinal products (ATMPs), including cell/gene therapy and tissue engineering, have been regulated in recent years by pharmaceutical rules applying to this new area of medicine. This has introduced additional bottlenecks in the already difficult passage from bench to bedside. Pharmaceutical rules were originally tailored for manufacturing and control of chemical molecules and not of living tissues: as a matter of fact, the latter contain cells continuously changing and endowed with proliferative and differentiation potential, enabling them to secrete many different molecules, depending on their status. In addition, these products have a very short shelf life, limiting the time for controls before release, compared with chemical compounds. Therefore, most examples of cell therapy or tissue engineering have been shown only in in vitro models or in animals; few have entered the phase of clinical trials on humans. Altogether, the requested knowledge and adjustments increase the hurdles to the spread of advanced therapies, reducing the number of clinical applications, subsequently leading to low confidence among entrepreneurs investing in the sector and thus slowing down their diffusion. In the meantime, scientific papers – in addition to huge research investments in this area of medicine – increase expectations with regards to clinical trials and routine clinical treatments. In Europe and the USA, few ATMPs have been approved; though they are still in their infancy, it is useful to understand their procedures and share experiences, in order to facilitate the development of this area of personalized medicine. The first example of a therapeutic use of cells extracted from human tissue was the use of selected hematopoietic cells in hematologic diseases and in oncology [1]. However, this tissue is considered a typical transplant and not an ATMP, since transplanted cells are not extensively manipulated. Legally speaking, selecting and transplanting cells is considered a ‘minimal manipulation’ to the same extent as an organ transplant, since the same risks do not apply to cell pathway stimulation and extensive proliferation ex vivo. Stimulation is usually obtained through natural or artificial cell–drug contact, with related toxicity, potentially inducing uncontrolled cell behavior. One of the first examples of extensive in vitro manipulation of cells destined for transplantation in tissue reconstruction was successfully obtained with epidermal cells for the treatment of severely burnt patients [2,3].
Corneal cell therapy
After these first trials, in 1997 the human limbo-corneal epithelium was reconstructed in vitro and transplanted into two patients [4]. It was obtained by culturing cells extracted from a small biopsy of the ocular surface; the two patients were suffering from a limbal stem cell deficiency with a resultant defect in corneal repair and opacification of the anterior surface of the eye. The clinical outcome was good and results were proven to be stable up to the 2 years of documented follow-up period, suggesting that the selected biopsy area contained the cells needed for a long-term regeneration. This was one of the first examples of application of regenerative medicine in the field of ophthalmology, and it was successfully reproduced, with some modifications, by Ivan Schwab in the USA [5], Ray Tsai in Taiwan [6], and later by Geeta Vemuganti in India [7]. The treatment became a routine patient treatment in Italy in 2004 (reimbursed by the National Health System), was accepted in India in 2008, but never spread out of these specific countries. In the USA, the treatment was stopped due to regulatory requirements.
After the first proof of principle, lengthy studies were needed to investigate the mechanisms controlling stable regeneration and enabling high reproducibility in the manufacturing process.
Location/identification of stem cells
Since adult stem cells maintain a population of highly differentiated but short-lived cells such as epithelia, data suggest that human corneal stem cells can be found in the limbus.
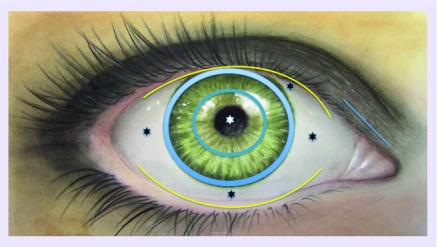
The first step of investigation was mapping the stem cells of the two epithelia covering the ocular surface: the cornea and the conjunctiva (Figure 1). Since the two epithelia in vivo behave differently, it had to be assessed whether a single stem cell could produce both epithelia, under the control of a local microenvironment, or whether two stem cells committed to different cell fates could be located in the areas to be defined [8].
Figure 1. . Corneal and conjunctival areas selected for biopsy retrieval.
Superior and inferior fornix (yellow line), superior, inferior, nasal and temporal bulbar conjunctiva (black asterisk), superior, inferior, temporal and nasal limbus (light blue line), paracentral cornea (blue line), central cornea (white asterisk). All defined areas were cultured in vitro and analyzed for stem cell content.
Studies in humans have shown the presence of two distinct adult stem cells, capable of corneal and conjunctival tissue regeneration, respectively; in particular, stem cells from the conjunctiva were shown to be bipotent (able to generate epithelium and goblet cells) and ubiquitous in the tissue, whereas corneal stem cells were proven to be segregated in the limbus [9], giving rise to progenitors covering the corneal surface. Different results on other mammals were reported by Majo et al., confirming, however, the human findings [10]. It should be noticed that strong evidence of localization of corneal epithelial stem cells in the limbus and of central pedal migration of clonal cells has been previously shown in live cell tracing experiment in mice [11].
In humans, both cells can be cultured in vitro, in similar culture conditions, maintaining their fundamental properties such as self-renewal, clonogenicity, long-term proliferative potential and specific differentiation properties. Corneal culture therapy has been reproduced by many different groups all over the world. Modifications in the culture process have been made by different research groups, and were related to supports for cell growth, presence/absence of feeder layer or serum [7,12–14] and use of tissue explant.
Potency marker
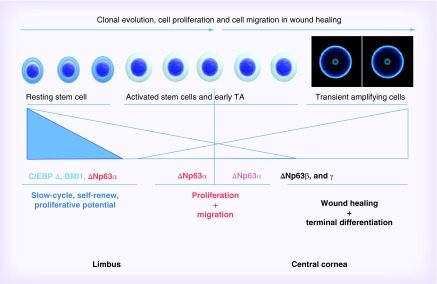
A specific challenge to this therapy is monitoring the maintenance of the therapeutic effect, and therefore the presence of stem cells in the cultures; the selection of culture conditions should expand these stem cells and guarantee their maintenance in a sufficient number, so as to produce a good clinical outcome in a long-term run. In the late 1990s, the only way to identify these cells was to look for the presence of holoclones, the only clonal type endowed with self-renewal, clonogenicity and long-term proliferative potential in epithelia [15,16]. The holoclone can be isolated by clonal analysis, resulting in less than 5% of aborted colonies in isolated daughter colonies. Stem cell identification can be completed approximately 1 month after cell isolation, allowing only a retrospective evaluation of their presence [17]. Attempts at holoclone identification by measuring the colony mass failed, showing no difference between the size of colonies generated by progenitors (meroclone) or by a stem cell (holoclone), whereas the capability of sustaining proliferation over time was very different [18]. When, at the same time in 1999, McKeon and Roop [19,20] tried to understand the role of the p63 transcription factor in a knockout mouse, they found a complete absence of all stratified epithelia, in addition to cranial abnormalities. The observation of areas of terminally differentiated epithelial cells, despite the absence of whole epithelia, could mean that the lack of p63 did not block epithelia morphogenesis but impeded their self-renewal, allowing only terminal differentiation. On this basis, p63 expression was investigated in subpopulations of proliferating cells: stem cells (identified by holoclones), progenitors (meroclones) and clones with limited residual proliferative potential [21]. Results highlighted a strong p63 expression in stem cell nuclei, presence in some nuclei of progenitor clones (meroclones) at a lower extent and absence in clones with limited residual proliferative potential, suggesting a correlation between the nuclear expression of p63 and long-term proliferative potential in ocular epithelial cells and epidermis. On the other hand, markers for proliferation such as PCNA, (a DNA polymerase D-associated protein synthesized in G1 and S phases of the cell cycle) [22], or Ki67, expressed during all active phases of the cell cycle (G1, S, G2 and mitosis) [23], were expressed by most cells forming the basal layer of the human limbus, at similar extent. The p63 gene produces full-length (TAp63) and N terminally truncated (ΔNp63) transcripts initiated by different promoters. The C-terminal is alternatively spliced to encode ten different isoforms, designated α, β, γ, δ, ϵ [24,25], to produce at least ten different p63 transcriptional variants. Some p63 isoforms were shown to be required for morphogenesis and proliferation of stratified epithelia in particular, ΔNp63-α sustains the proliferative potential of basal epithelial cells [26,27], whereas ΔNp63-β and ΔNp63-γ correlate with corneal regeneration and differentiation [21,28]. Further studies highlighted that p63 expression, in particular ΔN-α isoform in the resting limbus, is associated with the expression of at least two additional proteins: C/EBP-δ and BMI-1. The expression of C/EBP-δ is present in the subset of mitotically quiescent cells positive both for ΔNp63-α and BMI-1, a polycomb family transcriptional repressor. The induction of C/EBP-δ-induced slow cycling, maintenance of a long-term proliferative potential and persistence of p63 expression at high level (the findings are summarized in Figure 2) [29].
Figure 2. . Transcription factors and their related corneal cell functions.
Adapted from [29].
In human limbal stem cells, proliferative potential relies on the expression of ΔNp63, whereas self-renewal also requires C/EBPδ. Similarly, BMI-1 is also necessary for the self-renewal of neural stem cells but does not control the proliferative capacity of the committed progeny [30]. The Wnt signaling pathway has recently been implicated in the regulation and hemostasis of limbal stem cells [31]. Activation of the canonical Wnt pathway apparently improve the proliferation of limbal epithelial cells in culture [32]; and the Wnt receptor frizzled 7 was necessary for the maintenance of limbal stem cells [33].
Additional markers have recently been proposed, such as ABCG2 and ABCB5 [34–36]. They raised the general interest since surface markers are suitable for living cells sorting. Data proposed by the same authors or other groups showed neither absence of corneal epithelium or repair defects in knockout mice for both markers, raising doubts on their role in stem cells and tissue maintenance [36,37]. An advantage of this cultured tissue is that purification of a subpopulation of less differentiated cells is not suggested, since transit-amplifying cells orchestrate stem cell activity and tissue regeneration [38], and the short-term therapeutic effect is mediated by progenitors/differentiated cells, providing a barrier, whereas in the long-term a sufficient number of stem cells is needed to restore the epithelium. Most importantly, the culture system provides a high homogeneity of cell types, hence mainly epithelial cells, at several differentiation stages, composing the final product. This hypothesis was confirmed by the correlation with clinical success, later on in retrospective studies [rama p, lambiase a, pocobeli a et al. (2016) Manuscript in Preparation].
Carrier selection
In recent years, the environment of keratinocyte stem cells has been amply reviewed, but not in relation to the supports for tissue regeneration [39]. The proliferative compartment of epithelia, including stem cells, is located in the basal layer, which is in contact with the matrix and the supports used for regeneration and surgery. Stimuli from materials translate chemical interaction and mechanotransduction systems into biochemical signals, controlling multiple aspects of cell behavior, including adhesion, migration, growth, differentiation and cell fate [40,41].
Many supports for human tissue regeneration have been proposed, by different authors, hardly ever showing stem cell maintenance in comparative analysis with consolidated techniques. The most common support for cell growth was the amniotic membrane, used in clinical applications for a long time, and frequently associated with explant cultures [42]. The technique does not include enzymatic processing of biopsies, and the tissue explant is placed on support until cell migration and proliferation slowly cover the surface once. In the cultured corneal epithelium composing the product Holoclar®, the proposed matrix was fibrin, a support resembling the wound-healing environment. This carrier was introduced in 1999 [15,16], showing that epithelial cells grown on fibrin matrix have the same growth capacity and stem cell content as those cultured on plastics, but the enzymatic detachment and shrinking of the epithelium can be avoided and the basal cells protected during transport and handling. Despite the big variation in stiffness between plastic and fibrin, no differences were found in the number of stem cells between the two supports [43]. This culture system originates from a cell suspension obtained from biopsy, and allows multiple treatments, as opposed to amniotic membrane: cells dispersed on the surface do not need much migration to form the epithelial layer. The specific advantage of fibrin support is its rapid in vivo degradation, allowing a ready engraftment of the epithelium on the corneal wound bed, rapidly restoring transparency in the absence of deep stromal damage. In the case of human materials, biocompatibility should be granted, but their use raises the problem of disease transmission control within humans, in case of the absence of appropriate certification of the materials.
Medical plausibility
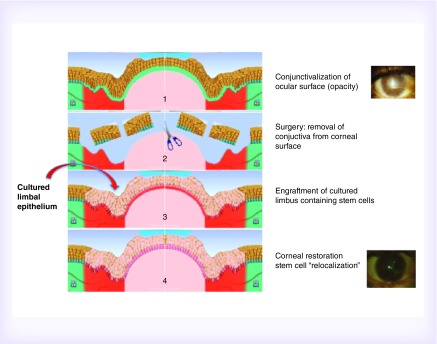
In the human cornea, stem cells are segregated on the border with the conjunctiva, in the only vascularized area, called the limbus. On the other hand, the transparent and avascular area of the cornea is maintained by a continuous centripetal migration of progenitors from the limbus. Pathologies characterized by loss of limbal stem cells or their dysfunction produce a condition called limbal stem cell deficiency (LSCD), resulting in migration of adjacent conjunctiva and blood vessel overgrowth on the cornea (‘conjunctival pannus’), with involvement of the visual axis in severe cases and subsequent loss of visual function. Under these pathologic conditions, patients suffer from pain, photophobia, burning, recurrent erosions, with the risk of both infections and/or melting of the corneal stroma. Pathologic conditions producing anatomical or functional loss of stem cells can be of genetic, degenerative, infective or traumatic origin [44]. Each pathology generates a different corneal environment modifications and cell-signaling alterations, involving more than one cell-type and damaging multiple anatomical structures due to altered cell signaling. In the course of this deterioration, the ocular surface is chronically inflamed and the resulting microenvironment abnormalities might prevent the engraftment of cultured stem cells. In damages due to viral infections, such as herpes virus, elimination of viruses is not possible, and the following risk of reactivation after treatment is feasible. On the other hand, trauma or eradicated bacterial infections are not progressive and leave stable damage on anatomical structures, surrounding cells could still be able to respond to physiological stimuli. Conjunctival migration over the corneal area can be hindered by limbal restoration through transplantation of cultured autologous limbal grafts obtained from the healthy eye [4,45,46]. The fibrovascular conjunctival pannus, grown on the corneal surface, is removed to enable the treatment of LSCD by transplanting the cultured limbal corneal epithelium obtained from stem cells of the same patient (Figure 3).
Figure 3. . Clinical application of cultured limbal stem cells.
The fibrovascular conjunctival pannus, grown on the corneal surface (panel 1), is removed (panel 2) to enable the transplant of the cultured limbal corneal epithelium (panel 3). Stem cell re-localization follows the cornea restoration (panel 4).
The ability to culture stem cells, with its enormous proliferative potential, permits under appropriate conditions, to originate cultures even from a very small biopsy sample. Indeed, a small amount of stem cells can give rise to a huge amount of daughter colonies, sufficient for whole cornea coverage. The eye is kept closed for several days after the transplant, so as to enable the engraftment of the cultured epithelium, reducing risks related to accidental friction forces or discomfort due to incomplete engraftment. The drugs used before and after surgery, such as anesthestics, corticosteroids to reduce inflammatory reactions, antibiotics or analgesics, can produce cell damage; their toxicity is amplified when the latter are administered before the full integration of cultured cells on the wound bed. None of them appear to be free of toxicity to some extent, hence a screening of drugs on cell cultures and appropriate dosage/mode of administration is thought to maximize the success of engraftment [44,47]. Autologous cultures of limbal stem cells have been confirmed successful in chemical/thermal burn-dependent corneal destruction in total LSCD. In this clinical setting, the mechanism of action of the cultures is the long-term replacement of missing limbal stem cells, as shown by several authors [47–49]. The engrafted stem cells should be restricted to the limbal area and preserve their self-renewal capacity, as formally proven by their potential to regenerate a normal corneal epithelium again after the keratoplasty. The latter is usually performed 12–24 months after limbal cell grafting, in order to eliminate the stromal scarring and to restore full visual acuity.
Evaluation of alternative techniques
Lamellar and/or penetrating keratoplasty will not be successful, in the presence of severe/total corneal epithelial stem cell deficiency, because donor graft corneal re-epithelialization will not take place. This will result in consequent epithelial absence and a final recurrence of conjunctivalization, associated with a risk of rejection and graft failure. Thus, unilateral LSCD has been treated for years by grafting approximately 30–40% of the healthy limbus from the other eye [45,50,51]. Some concerns have been expressed about this approach, regarding donor eye risks [52], in that patients should be compliant to accept a large damage of the only remaining eye, and surgeons feel this responsibility. Finally, further harvesting of additional limbal areas following possible failure is not possible.
A surgical technique, simple limbal epithelial transplantation (SLET), resembling a previous burnt-patient treatment, was recently proposed for limbal transplantation [53]. A 2 × 2 mm biopsy of donor limbal tissue from the healthy eye is split into several small pieces, containing epithelium, keratocytes and other cell types. After surgical preparation of the cornea, these tiny limbal transplants are dispersed evenly over an amniotic membrane placed on a recipient ocular surface. A competition between engraftment, migration of limbal cells from the biopsy fractions and resident conjunctiva overgrowth on the corneal surface occurs, leading to uncertainty about the final results, with a dependence on the patient microenvironment too. It is well known that direct transplantation of limbal tissue can repopulate the LSCs, however, the major concern is how much stem cells can promptly migrate to fully cover the whole corneal surface. Any delay in covering the wounded surface results in inflammation, infection and recurrence of partial conjunctivalization. In order to really evaluate the success rate, standardized criteria to categorize patient selection and severity of LSCD are needed, and a comprehensive report of adverse events too. SLET might well be effective in milder LSCD, but for severe disease, cultivation of LSCs to regenerate a large LSCD is still necessary.
Patient selection
LSCD includes very different diseases that all result in a damaged limbus, and it presents differently based on the severity of the condition [54,55]. In partial or sectorial LSCD, stippled late fluorescein staining in a vortex pattern can be seen in the affected region of the cornea. In moderate-to-severe cases, recurrent epithelial defects and superficial vascularization of the cornea occur. Vision is severely compromised. If there is also presence of tear deficiency, keratinization may occur. Total LSCD is characterized by a total absence of limbal stem cell populations associated with conjunctivalization of the entire corneal surface. Neovascularization was frequently seen but is not the only symptom of LSCD.
Although LSCD can be diagnosed clinically based on the presentation described above, it is rather challenging in many mild-to-moderate disease stages because many signs present in LSCD are not specific and are also seen in many other common eye diseases. Laboratory tests are important to confirm the diagnosis of LSCD and measure the outcome of treatment. Impression cytology showing the presence of goblet cells or the absence of corneal markers has been the gold-standard diagnostic test for LSCD [56]. However, impression cytology is limited to analysis of superficial layers.
In recent years, cytokeratin (K) 7, 13 and 19, mucin (MUC) 1, and MUC5AC have been suggested as a conjunctival epithelial marker [57–61]. K19, K13 and MUC5AC might be more specific and reverse transcriptase-PCR might be more sensitive than immunohistochemistry in detecting these molecules. However, the specificity and sensitivity of these new markers still needs to be confirmed by comparison in larger studies on LSCD patients.
Another recent development in the diagnosis of LSCD is the use of in vivo laser scanning confocal microscopy to delineate the microstructural changes in patients’ eyes with different degrees of LSCD. In a normal cornea, epithelial cells are seen as well demarcated with a hyper-reflective border. The deep basal epithelial cells are smaller in size, with not big difference between the cytoplasm and nuclei area, while the cell border is still very well defined. In addition, palisades of Vogt may be detected as hyper-reflective, double-contour linear structures in confocal microscopy of the scleral limbus [62,63]. By contrast, the basal cell density of corneal epithelium becomes progressively reduced and the cell size becomes larger in the more advanced states of LSCD. The normally clear cell–cell border becomes indistinguishable. There is also a significant decrease in sub-basal nerve density [63,64]. The loss of innervation suggests that LSCD pathophysiology can also have a neurotrophic component. Therefore, a combination of morphological changes in the corneal epithelium, and a significant reduction in both basal epithelial cell density and sub-basal nerve density might aid in the diagnosis and classification of LSCD. These criteria, however, should become standardized measures to evaluate the clinical success of LSC transplantation.
Accurate diagnosis and staging of the disease severity is a prerequisite in the management of LSCD. In mild LSCD, surgical intervention might not be necessary, whereas in the severe state or total corneal involvement, transplantation of LSC is necessary to reconstruct the entire corneal surface. Accurate evaluation of LSC function in vivo will be critical to monitor the disease progression and measure the success of LSC transplantation.
The proper selection and preparation of the receiving bed is of paramount importance for a positive clinical outcome of limbal cultures, since the microenvironment where the cell cultures or tissues should be engrafted can be not permissive of cell integration due to pathologic alterations.
Abnormality of the corneal stroma, endothelium, nerves, eyelids, conjunctiva and the lacrimal and immune systems could also be included in the pathogenesis of LSCD. Scrupulous step-by-step reconstruction of these structures was performed, to prepare the best receiving bed for the cultured cells. Once the eyelids and conjunctiva were treated, tear film and inflammation were analyzed. Tear film and inflammation were carefully evaluated, after treatment of eyelids and conjunctiva; however, it is still not clear how much is a minimum admissible tear film and a maximum inflammation permitting the successful long-term survival of the grafted stem cells. In previous clinical trials [43,47], patients showing Schirmer test below 5 mm/5 min, arbitrarily selected, were excluded. However the quality of tears appears to be even more important than the quantity. At present, there is still no agreement on its assessment. Patients showing severe active inflammation were not included in the clinical protocol for limbal transplantation. We are still far from having a standardized clinical assessment and inflammation grading, with the exception of redness scoring.
On this basis, exclusion criteria also exempted patients with unknown etiology/pathogenesis, or active viral infections.
Patients with severe or total, unilateral or partial burn related bilateral limbal stem cell deficiency were therefore included in the study. The prevalence of those patients is well below 1:10,000, therefore it is defined as a rare disease.
Further applications can be considered, with the same medical plausibility; however, any additional inclusion criteria should be carefully evaluated in a significant number of patients in a specific clinical trial, to avoid the variability faced in anecdotal cases.
Clinical protocol & retrospective trials
The first manufacturing of corneal tissue and the related clinical trials were done before regulatory changes catagorized this stem cell therapy as an advanced therapy in the same group as chemical drugs.
In the course of development, the manufacturing process was subsequently based in different facilities and the clinical application was conducted in many different ophthalmology departments over the Italian territory. In order to maintain a very limited/null variability, all parts of the procedure were described in standard operating procedures, so as to enable the biological and clinical work to be reproduced at the different sites. Each cell parameter was registered, as well as the clinical data, with photos and follow-up analysis, defined in the clinical protocol, making the analysis of data possible. When drug regulation was put into force for these therapies, good clinical practice retrospective studies were possible, allowing a ‘masked’ re-evaluation of treated patients and related biological data by external evaluators. Adverse event analysis through re-evaluation of all the follow-up data of each patient, up to 10 years post-transplant, allowed risk–benefit investigation on 130 patients [holostem terapie avanzate, data on file; rama p, lambiase a, pocobeli a et al. (2016) Manuscript in Preparation]. Later, the collection of additional data early after good manufacturing practice (GMP) setting allowed a comparative analysis of procedure before and after regulatory changes.
This work represents one of the largest official datasets on the topic available in the world. In particular, the homogeneity of patient selection, grading and the evaluation of long-term safety reassure us about the spread of this technology. Comparison of data published on different culture systems shows a difference in the long-term stability of the cultured corneal epithelium.
In process controls
During the industrialization process of an ATMP, as well as in routinary clinical application, one major problem is the reproducibility of the technology for scaling up.
In general, maintenance of identity, purity and potency should be guaranteed. In the case of Holoclar, the corneal marker expression defined the identity, the stem cell marker percentage represented the potency, and quantification of process-related impurities (i.e., feeder cells or conjunctival cells) or contaminants (i.e., infectious pathogens) defined the purity of this ATMP. A set of parameters for these quantifications would permit a comparison of the quality of different culture methods around the world.
The biopsy was obtained from culturing the limbus, at the boundary between the cornea and conjunctiva; therefore the percentage of corneal keratins was controlled, to confirm proper biopsy retrieval and cell identity. Indeed, epithelia from different body areas express keratin pairs that are specific for each location [65,66]. The corneal epithelium expresses the K3/K12 pair, in contrast to the conjunctival epithelium.
The in vivo long-term maintenance of the cultured tissue is dependent on the number of cultured stem cells; therefore their number should be carefully controlled to meet efficacy criteria.
Actually, it is important – and required by ATMP regulation – to define the applicability and the quality of cultured epithelial grafts, which are relevant to any clinical use of cultured cell types. Many biological parameters conceivably contributory to the clinical success of cultured corneal epithelium have been investigated. Quality attributes of cell culture and raw material properties were examined and, most of them, marked biological functions relevant to the product, but only some of them were later shown capable to measure biological activity, connected to efficacy/safety in the patient.
More generally, multiple in-process controls and in-process monitoring were necessary to evaluate cell cultures and to reveal/avoid eventual deviation from normal cell behavior; all their results should be on the same line.
Risk-based approach
A major concern over the use of stem cell therapies is the perceived risk of tumorigenicity.
Limbal-corneal cells contain adult stem cells, having defined differentiation capacity and more limited self-renewal capacity than embryonic and induced pluripotent stem cells; therefore these adult stem cells have a very low risk of uncontrolled proliferation. In the case of one treatment of spinal cord injury with olfactory mucosal cells, however, a mucosal-like mass was found at the transplant location [67] during follow-up analysis. This kind of finding highlights the importance of an extensive follow-up program to monitor and reduce patient risks. To date, tumorigenesis has not been reported in limbal stem cell therapy.
More than 3000 trials associated with stem cells are currently collected in the WHO International Clinical Trials Registry Platform. Most of them are adult stem cell-based therapies, likely attributable to the longer established use of these cells.
Extensive cultivation and expansion can make cells susceptible to chromosomal aberrations and karyotype abnormalities, therefore limbal cultured epithelia were transplanted at an early passage and controlled for chromosomal phenotype. Concerning the immunogenic potential, it is generally accepted that no rejection can occur due to autologous cells, even following in vitro culture; data on cultured limbal cells confirmed the absence of rejection, with an increased success rate after repeated transplants. This latter observation also reassures us about any potential effect due to residual traces of some culture components (such as a feeder layer), given that removal by extensive dilution of any culture components was achieved.
The issue of xenogenic components relates to attempts at preventing putative xenogenic contaminants. Human autologous serum has been suggested as a potential surrogate for fetal or calf serum; however, variability of hormones and growth-factor content due to individual genetic background could be detrimental to the reliability of the in-process controls, hampering the definition of well-defined quality criteria for the culture. Pools of sera minimize such variability, although risks of contamination by viral, nonviral infective agents and prions also apply to human-derived reagents [68].
With current technologies, serum has an impact on epithelial stem cell preservation, as well as a proper feeder layer of lethally irradiated fibroblasts [69]. Lethally irradiated murine 3T3 fibroblasts have been applied for decades to culture limbal and epithelial keratinocytes [3,15,16] and no adverse reactions have been described [69,70] in large case series. Considering the potential transmission of pathogens, the same implications described for fetal calf serum versus donor serum pertain to mouse cells versus allogeneic human fibroblasts. EU rules on master/working cell banks allow us to properly control risks by use of GMP-certified, clinical grade reagents/cell lines, which have been tested for specific bacteria, yeasts, fungi and viruses, or irradiated. This approach appears safer than proposed alternatives and has the advantage of reducing variability between batches of cultured ocular tissues.
An additional issue is biodistribution, since it relates to the risk associated with proliferation and the long-term survival of transplanted material in ectopic sites. In general, direct transplant to the targeted organ area reduces this risk; in the case of the cornea, the majority of limbal corneal cells engraft in an avascular area, over Bowman's membrane, reducing the possibility of distribution through the bloodstream; moreover, nontransformed epithelial cells, when losing adhesion, trigger the differentiation program, inducing loss of proliferative capacity. The absence of migration in underlying tissues was revealed by an analysis of follow-up data showing, in all cases, a well-defined transplanted corneal area and an intact basal membrane years after transplant; a loss of proliferation, due to absence of adhesion, was also shown in vitro by standard soft agar assay.
ATMPs are unique products, more demanding than other drug classes. To accommodate their challenges and facilitate appropriate scientific development, flexibility in the requirements is necessary to prove the safety and efficacy of this rapidly evolving drug class.
To provide some versatility, the EU has introduced a risk-based approach. This approach introduces the possibility of abstaining from strict guideline-based studies based on risk analyses. In the USA, new guidelines from the US FDA categorize stem cells as a drug and make them subject to the same regulatory requirements to obtain investigational new drug status before they can be offered to patients. The risk-based approach would be a balanced approach that would shorten regulatory process.
The existence of risk, together with potential benefit, remain difficult to eliminate; therefore we have sought a counterbalance between the minimization of potential risks and ensuring therapies are not unnecessarily kept away from patients. This therapy advanced into the clinic, but its safety was continuously evaluated.
The need for public–private partnerships
Developers of novel ATMPs come from small medium enterprises (SMEs), hospitals, academia and even charities. In spite of the scientific challenges, many products reach early clinical studies, but very few of them proceed to pivotal studies and marketing authorization, due to limited resources and high workload. It is difficult for scientists to accept the new standards and requirements for ATMPs that look very like the tissue preparations used in hospitals; therefore scientific advice is a very important tool in supporting development, since it is a forum in which to agree on methodological approaches for quality and nonclinical and clinical development.
Clinical trial requirements are frequently under the evaluation of national authorities, which may increase the workload for multicenter confirmatory trials requiring simultaneous submission of applications to more national authorities. Also different interpretations of the hospital exemption (article 28 of Regulation 1394/2007) raises concerns about ‘class B’ products and has led to conflicts with the ATMP industry.
Furthermore, challenges in getting funding for large pivotal clinical trials, and cost of these products, are higher compared with traditional medicinal products, due to the small batch sizes. The reimbursement negotiations for first ATMPs have also turned out difficult, leading to slow market access and limited use of the new products within the EU. On this basis, Holoclar certification and industrialization was based on a public–private partnership.
The public–private partnership, through specific agreements, can share the skills and assets of the public (i.e., research institute or academia) and the private (i.e., company) in delivering the product/service to patients. In addition to the sharing of resources, partnerships allow sharing the risks and rewards of the service, reducing the cost of the activity.
More people are involved in the partnership – not just the public and private partners: it involves employees, physicians and patients receiving products/services, the press and several stakeholders with varied opinions, and potentially misinterpretations about the value of the partnership to the public as a whole. It is important to interact openly with all interest groups to minimize potential resistance to maintaining the partnership and to meet the interest of all. Distinguished critical figures from public academia and private companies play an essential role in minimizing misunderstandings about that value to the public.
It was important to have a dedicated, specific team for the project, within academia and a private company, which were involved from its conceptualization, negotiation and the final control of the fulfillment of the partnership. The team supported proposals based on best value, not on lowest prices.
A statutory foundation was needed for the implementation of the partnership. A definition of mission was first delineated in the statute, as well as looking at transparency. The proposal was a positive catalyst for initiating new approaches to public issues. The contract between the public and private sectors included a detailed specification of the responsibilities, benefits and risks of both the public and private partners. Such an agreement can improve the likelihood of positive outcome from the partnership. Obviously, while the private partner provided significant funding, there should be various identifiable future revenues to retire the investment and produce an adequate rate of return, including satellite activities over the term of the alliance. On the other hand, the specific interests of public stakeholders should be met as well, even though they are often more complex because the public consists of many subgroups of stakeholders.
Finally, the best value in the partnership is critical in maintaining the long-term relationship that is instrumental to success. Partner experience in the specific area of activity is considered an important value and, equally, the financial capacity of the private partner should be considered in the selection process.
Conclusion
The field of stem cell therapies, representing one of the most advanced examples of personalized medicine, is moving ever closer to unrestricted application on patients.
However, despite the undoubted potential of these therapies, the balance between risk and advantage remains difficult to predict.
The use of the eye in a first application of these stem cells is ideal: the graft size is small, limbal epithelial cells are easily differentiated due to high purity, and the grafts can be visualized noninvasively. Regression of neovascularization is developed when these cells engraft, all contributing to a lower risk-profile and easier assessment than other cell types in less accessible organs.
As in any new field, in case of an absence of preceding application in humans and gaps in the basic science, investigators and regulators should continue to seek a balance between minimizing potential risk and ensuring therapies are not unnecessarily kept away from patients. In the case of Holoclar, attempts were made to identify the critical issues, estimating the advances in scientific data and how they were translated to clinical therapeutic application and later to industrialization strategy. The tools and procedures now available to researchers during preclinical, clinical and GMP development of a stem cell product, their benefits and constraints, were investigated, as well as how these tools can be used in the advance of these therapies.
Finally, placing safety at the forefront of science and undertaking robust measures, coupled with continuous and open discussions between regulators and academic/industrial investigators, is likely to be the most fruitful path to identifying the safest possible road to new products and an increase in common knowledge. Safe and efficacious ATMPs will be more easily exploited by entrepreneurs, and can prove the best cost–benefit balance at the stage of regulatory market approval and reimbursement negotiation.
Future perspective
ATMPs are a heterogeneous group of therapies; therefore no single approach can be used to make them compliant with new regulations and available to patients.
To transfer experience of people working in this field is essential, as well as tailoring regulatory rules to this type of product. New technologies are required to complete the process of assuring safety and efficacy in an acceptable time frame and at reasonable cost. All these approaches together will boost the distribution of these new products, which are the best example of personalized medicine.
Executive summary.
Corneal cell therapy
The first application of cultured corneal cells on patients was successfully undertaken in 1997.
The technology was reproduced with some variants in many countries.
Location/identification of stem cells
Clonal analysis of ocular epithelia has identified three types of clonogenic basal cells: holoclones, meroclones and paraclones.
The holoclone-forming cells are stem cells, and are able to regenerate all ocular epithelia in the long term.
Human corneal holoclones reside in the limbus.
The human cornea and conjunctiva derive from different stem cells; the holoclones from conjunctiva are bipotent and generate conjunctival epithelium and goblet cells.
Cells from both the cornea and conjunctiva can be cultured in vitro, producing the entire epithelium and maintaining their fundamental properties.
Potency marker
The potency marker is an assay providing a correlation of cell quality to clinical outcome.
The clinical outcome of corneal cell therapy is related to the presence of enough stem cells responsible for long-term cornea regeneration.
Long-term corneal regeneration is provided by holoclones.
Holoclones express a high level of nuclear p63 transcription factor, in particular the α isoform; C/EBP-δ and BMI-1 are also expressed in slow cycling cells.
Isoforms p63 β and γ appear to be related to proliferation and differentiation.
The Wnt signaling pathway is implicated in the regulation and hemostasis of limbal stem cells.
Knockout mice for other described markers, such as ABCG2 or ABCB5, did not show any corneal impairment.
Carrier selection
Many carriers were proposed for corneal regeneration.
Maintenance of a sufficient number of stem cells on the proposed carriers should be evaluated.
Fibrin matrix maintains holoclones.
Medical plausibility
Loss of stem cells produces limbal stem cell deficiency (LSCD), allowing corneal invasion by conjunctiva and vessels.
Limbus integrity should be restored to avoid conjunctival invasion.
Fibrovascular conjunctival pannus should be removed to enable engraftment of cultured corneal epithelium.
Cultured corneal epithelium can engraft and maintain a stable corneal surface over time.
The toxicity of drugs on cultured epithelium after grafting should be evaluated.
Evaluation of alternative techniques
In Kenyon's technique 30–40% of limbus is removed from the healthy eye for transplantation on LSCD. In case of failure, the technique cannot be repeated. This technique works only in unilateral LSCD and was proven in long-term follow-up.
The simple limbal epithelial transplantation technique includes the transplant of many small biopsies on the ocular surface, waiting for the cell outgrowth to cover the corneal surface. This technique does not yet have very long-term follow-up data.
Patient selection
LSCD is an heterogeneous group of diseases, some of them producing irreversible alteration of corneal environment signaling.
Accurate diagnosis should be obtained to define the type of LSCD and to grade severity.
In addition to clinical evaluation, diagnosis of LSCD can be obtained by impression cytology and nonquantitative laser scanning confocal microscopy.
Proper selection of LSCD is of paramount importance for the positive clinical outcome.
Clinical protocol & retrospective trials
The therapy was developed before good manufacturing practice rules on advanced therapy medicinal products (ATMPs).
Good clinical practice retrospective clinical trials allowed collection of data on almost 200 patients.
Clinical trials confirmed the safety and efficacy of the procedure, up to 15 years follow-up.
In-process controls
Identity, purity and potency should be defined for ATMPs.
Relevant quality attributes of cells and raw materials were investigated to identify the appropriate markers.
Those markers/assays with their specification limits became in-process controls of manufacturing.
In-process monitoring however, is also important for evaluating process robustness.
Risk-based approach
Risks of tumorigenicity, immunogenic potential and xenotoxicity were evaluated in vitro and related to long-term follow-up analysis on patients.
Accurate controls on raw materials and use of low passage cultures assured the safety of the procedure.
Risk-based analysis, introduced by the EU, proposed analysis of balance between minimizing potential risks and ensuring benefits to patients.
The need for public–private partnerships
The amount of knowledge, funding and legal requirements needed for ATMPs is impressive.
The opportunity to have multidisciplinary knowledge and to share risk and benefits is met in public–private partnerships.
A definitional list of rules and documents, defining reciprocal dues, is instrumental to a successful partnership.
Acknowledgements
G Pellegrini and M De Luca, inventors of this technology, thank H Green (Harvard Medical School, Boston) for providing the original 3T3-J2 feeder cells and for his invaluable contribution to our knowledge in regenerative medicine. His recent loss left the research field devoid of an outstanding scientist. Finally, we want to thank IMI and Fondazione Cassa di Risparmio di Modena for their impressive contribution, making this project, possible.
Footnotes
Financial & competing interests disclosure
This work was partially supported by the Ministry of Health, Project number GR-2009-1580594, Ricerca finalizzata 2011; by the Ministry of Education, University and Research (MIUR), Project Number 2008JJTFCW, PRIN 2008; Regione Emilia–Romagna (area 1b, medicina rigenerativa) and POR-FESR 2007-13-Tecnopolo. P Rama is consultant of Chiesi Farmaceutici. G Pellegrini is a member of the Board of Directors of Holostem Terapie Avanzate S.r.l. and J-TEC consultant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Sugimura R. Bioengineering hematopoietic stem cell niche toward regenerative medicine. Adv. Drug Deliv. Rev. 2015 doi: 10.1016/j.addr.2015.10.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]; • The first example of regenerative medicine with cultured human cells.
- 3.Gallico GG, 3rd, O'Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 1984;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 5.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19(4):421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Tsai RJ, Li L, Chen J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells (1) Am. J. Ophthalmol. 2000;130(4):543. doi: 10.1016/s0002-9394(00)00746-7. [DOI] [PubMed] [Google Scholar]
- 7.Sangwan VS, Vemuganti GK, Singh S, Balasubramanian D. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci. Rep. 2003;23(4):169–174. doi: 10.1023/b:bire.0000007690.43273.73. [DOI] [PubMed] [Google Scholar]
- 8.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 1999;145(4):769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The limbal stem cells identification, later used in therapy.
- 10.Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456(7219):250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]; • The difference between human and other mammalian animal models.
- 11.Di Girolamo N, Bobba S, Raviraj V, et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells. 2015;33(1):157–169. doi: 10.1002/stem.1769. [DOI] [PubMed] [Google Scholar]; • The limbal stem cell behavior in vivo.
- 12.Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol. Vis. Sci. 2002;43(7):2114–2121. [PubMed] [Google Scholar]
- 13.Feng Y, Borrelli M, Reichl S, et al. Review of alternative carrier materials for ocular surface reconstruction. Curr. Eye Res. 2014;39(6):541–552. doi: 10.3109/02713683.2013.853803. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Lawrence BD, Liu A, et al. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generation. Invest. Ophthalmol. Vis. Sci. 2012;53(7):4130–4138. doi: 10.1167/iovs.12-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68(6):868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 16.Ronfard V, Rives JM, Neveux Y, et al. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70(11):1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA. 1987;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The identification of the three clonal types, critical for the definition of stem cell function.
- 18.Pellegrini G, Rama P, Matuska S, et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen. Med. 2013;8(5):553–567. doi: 10.2217/rme.13.43. [DOI] [PubMed] [Google Scholar]; • Proposes an analysis of several cell parameters, for identification of a potency marker.
- 19.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]; • Understanding the p63 role.
- 20.Mills AA, Zheng B, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398(6729):708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc. Natl Acad. Sci. USA. 2001;98(6):3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo R, Frank R, Blundell PA, et al. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 23.Malizia G, Trejdosiewicz LK, Wood GM, et al. The microenvironment of coeliac disease: T cell phenotypes and expression of the T2 ‘T blast’ antigen by small bowel lymphocytes. Clin. Exp. Immunol. 1985;60(2):437–446. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell. 1998;2(3):305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 25.Mangiulli M, Valletti A, Caratozzolo MF, et al. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res. 2009;37(18):6092–6104. doi: 10.1093/nar/gkp674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsa R, Yang A, McKeon F, et al. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Invest. Dermatol. 1999;113(6):1099–105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 27.Blanpain C, Fuchs E. p63: revving up epithelial stem-cell potential. Nat. Cell Biol. 2007;9(7):731–733. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]; • Understanding the p63 role.
- 28.Di Iorio E, Barbaro V, Ruzza A, et al. Isoforms of ΔNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl Acad. Sci. USA. 2005;102(27):9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbaro V, Testa A, Di Iorio E, et al. C/EBPδ regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007;177(6):1037–1049. doi: 10.1083/jcb.200703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakatsu MN, Vartanyan L, Vu DM, et al. Preferential biological processes in the human limbus by differential gene profiling. PLoS ONE. 2013;8(4):e61833. doi: 10.1371/journal.pone.0061833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakatsu MN, Ding Z, Ng MY, et al. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol. Vis. Sci. 2011;52(7):4734–4741. doi: 10.1167/iovs.10-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei H, Nakatsu MN, Baclagon ER, et al. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells. 2014;32(4):938–945. doi: 10.1002/stem.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J. Cell Sci. 2005;118(Pt 8):1715–1724. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubota M, Shimmura S, Miyashita H, et al. The anti-oxidative role of ABCG2 in corneal epithelial cells. Invest Ophthalmol. Vis. Sci. 2010;51(11):5617–5622. doi: 10.1167/iovs.10-5463. [DOI] [PubMed] [Google Scholar]
- 36.Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511(7509):353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priya CG, Prasad T, Prajna NV, et al. Identification of human corneal epithelial stem cells on the basis of high ABCG2 expression combined with a large N/C ratio. Microsc. Res. Tech. 2013;76(3):242–248. doi: 10.1002/jemt.22159. [DOI] [PubMed] [Google Scholar]
- 38.Hsu YC, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157(4):935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr. Opin. Cell Biol. 2003;15(6):771–777. doi: 10.1016/j.ceb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Ng AH, Baht GS, Alman BA, et al. Bone marrow stress decreases osteogenic progenitors. Calcif. Tissue Int. 2015;97(5):476–486. doi: 10.1007/s00223-015-0032-3. [DOI] [PubMed] [Google Scholar]
- 41.Wanjare M, Agarwal N, Gerecht S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2015;309(4):C271–C281. doi: 10.1152/ajpcell.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhamodaran K, Subramani M, Jeyabalan N, et al. Characterization of ex vivo cultured limbal, conjunctival, and oral mucosal cells: a comparative study with implications in transplantation medicine. Mol. Vis. 2015;21:828–845. [PMC free article] [PubMed] [Google Scholar]
- 43.Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72(9):1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini G, Rama P, Di Rocco A, et al. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32(1):26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- 45.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709–722. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 46.Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br. J. Ophthalmol. 2000;84(3):273–278. doi: 10.1136/bjo.84.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 48.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br. J. Ophthalmol. 2011;95(11):1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 49.Satake Y, Higa K, Tsubota K, et al. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118(8):1524–1530. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 50.Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans. Am. Ophthalmol. Soc. 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 51.Frucht-Pery J, Siganos CS, Solomon A, et al. Limbal cell autograft transplantation for severe ocular surface disorders. Graefes Arch Clin. Exp. Ophthalmol. 1998;236(8):582–587. doi: 10.1007/s004170050125. [DOI] [PubMed] [Google Scholar]
- 52.Baylis O, Figueiredo F, Henein C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J. Cell Biochem. 2011;112(4):993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 53.Sangwan VS, Basu S, Macneil S, et al. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012;96(7):931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 54.Dua HS, Saini JS, Azuara-Blanco A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J. Ophthalmol. 2000;48(2):83–92. [PubMed] [Google Scholar]
- 55.Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr. J. Ophthalmol. 2013;20(1):5–10. doi: 10.4103/0974-9233.106381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10):1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 57.Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, et al. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol. Vis. 2011;17:1652–1661. [PMC free article] [PubMed] [Google Scholar]
- 58.Barbaro V, Ferrari S, Fasolo A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br. J. Ophthalmol. 2010;94(7):926–932. doi: 10.1136/bjo.2009.164152. [DOI] [PubMed] [Google Scholar]
- 59.Garcia I, Etxebarria J, Boto-De-Los-Bueis A, et al. Comparative study of limbal stem cell deficiency diagnosis methods: detection of MUC5AC mRNA and goblet cells in corneal epithelium. Ophthalmology. 2012;119(5):923–929. doi: 10.1016/j.ophtha.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 60.Poli M, Burillon C, Auxenfans C, et al. Immunocytochemical diagnosis of limbal stem cell deficiency: comparative analysis of current corneal and conjunctival biomarkers. Cornea. 2015;34(7):817–823. doi: 10.1097/ICO.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 61.Jirsova K, Dudakova L, Kalasova S, et al. The OV-TL 12/30 clone of anti-cytokeratin 7 antibody as a new marker of corneal conjunctivalization in patients with limbal stem cell deficiency. Invest. Ophthalmol. Vis. Sci. 2011;52(8):5892–5898. doi: 10.1167/iovs.10-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel DV, McGhee CN. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: a major review. Clin. Exp. Ophthalmol. 2007;35(1):71–88. doi: 10.1111/j.1442-9071.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 63.Deng SX, Sejpal KD, Tang Q, et al. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch. Ophthalmol. 2012;130(4):440–445. doi: 10.1001/archophthalmol.2011.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vera LS, Gueudry J, Delcampe A, et al. In vivo confocal microscopic evaluation of corneal changes in chronic Stevens–Johnson syndrome and toxic epidermal necrolysis. Cornea. 2009;28(4):401–407. doi: 10.1097/ICO.0b013e31818cd299. [DOI] [PubMed] [Google Scholar]
- 65.Franke WW, Schiller DL, Moll R, et al. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J. Mol. Biol. 1981;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- 66.Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 67.Tabakow P, Raisman G, Fortuna W, et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23(12):1631–1655. doi: 10.3727/096368914X685131. [DOI] [PubMed] [Google Scholar]
- 68.Stacey GN, Cobo F, Nieto A, et al. The development of ‘feeder’ cells for the preparation of clinical grade hES cell lines: challenges and solutions. J. Biotechnol. 2006;125(4):583–588. doi: 10.1016/j.jbiotec.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 69.De Luca M, Pellegrini G, Green H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regen. Med. 2006;1(1):45–57. doi: 10.2217/17460751.1.1.45. [DOI] [PubMed] [Google Scholar]
- 70.Green H. The birth of therapy with cultured cells. Bioessays. 2008;30(9):897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]