Abstract
Aim:
Glioblastoma multiforme is a devastating disease with no curative options due to the difficulty in achieving sufficient quantities of effective chemotherapies into the tumor past the blood–brain barrier. Micelles loaded with temozolomide (TMZ) were designed to increase the delivery of this drug into the brain.
Materials & methods:
pH-responsive micelles composed of distearoyl phosphoethanolamine-PEG-2000-amine and N-palmitoyl homocysteine were surface-functionalized with PDGF peptide and Dylight 680 fluorophore.
Results & conclusion:
PDGF-micelles containing TMZ have specific uptake and increased killing in glial cells compared with untargeted micelles. In vivo studies demonstrated selective accumulation of PDGF-micelles containing TMZ in orthotopic gliomas implanted in mice. Targeted micelle-based drug carrier systems hold potential for delivery of a wide variety of hydrophobic drugs thereby reducing its systemic toxicity.
Keywords: : glioblastoma, micelle, nanocarrier, PDGF, temozolomide
Glioblastoma multiforme (GBM) occurs in 2–3 people per 100,000 [1]. This relatively rare tumor of the brain has a significant overall mortality due to its refractory response to treatment. The standard of care therapy for GBMs is maximal safe surgical resection, radiation therapy and concurrent chemotherapy with temozolomide (TMZ). However, even with temozolomide therapy, GBMs have a dismal prognosis with a median survival of only 14 months [1]. Challenges to offering curative therapy to patients with GBMs are multifocal: surgery is unable to remove 100% of the tumor [2], radiation is able to temporarily halt the growth of a GBM, but is unable to eradicate GBMs [3], and chemotherapy delivery is limited by systemic toxicity [4], thereby reducing the amount of active drug that can be delivered past the blood–brain barrier (BBB) into the tumor.
The standard of care chemotherapy, TMZ, is a second-generation imidazotetrazine prodrug that is converted by pH changes in the cytoplasm of cells to the active alkylating agent 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide [5,6]. This drug has been used for decades in the treatment of GBM, but is unable to achieve a cure for patients with GBMs in spite of activity in mouse models [7]. At standard of care dosages, hematologic toxicity in those animals administered TMZ is dose limiting [8]. This implies that, in spite of its bioavailability and ability to cross the BBB, an increased intratumoral concentration of TMZ could be beneficial in treatment of GBMs.
Delivery of chemotherapy to tumors while reducing systemic toxicity has become more feasible as technology improves in the development of nanoparticles [9]. Polymeric micelles have been developed for delivery of chemotherapy into tumors [10,11] because they are stable nanoparticles that can release their contents under specific environmental circumstances (e.g., a change in pH can trigger the release of micellar chemotherapy). In addition, these polymeric micelles can be designed with targeting to tumor cells in mind [12]. This can decrease systemic toxicity while increasing the local concentration of chemotherapy into the desired tissues.
As is common in breast cancer and other cancer cells, GBMs have specific cell surface receptors that are frequently overexpressed, and there is an expanding field of targeted biomarkers available for GBMs. GBM targets that have been utilized in development of polymeric micelles include EGF receptor (EGFR), transferrin receptor (TfR), aminopeptidase N and some integrins [13]. This expanding field of discovery can allow for better inclusion of a range of GBM tumor markers in the targeting of micelle delivered chemotherapies. Ultimately, this will allow chemotherapies to be targeted to specific tumor markers in each GBM, allowing for truly personalized therapy with minimization of systemic toxicity.
EGFR has been used as a tumor marker for GBMs [14], and is used for targeting of micelles, as above. However, it is present in only certain subtypes of GBMs. Specifically, it is present in classical-like GBMs, though it is not present in proneural-like GBMs. This relationship is therapeutically relevant when designing therapies and targeted micelle therapies. Proneural-like GBMs have an increase in PDGF receptors (PGDFRs) on their cell surfaces [15]. In fact, PDGFR is noted on more than 50% of analyzed GBMs [15] and is also noted as a cell surface receptor in oligodendrogliomas and diffuse and anaplastic astrocytomas [16]. Given this frequent upregulation of PDGR in GBMs and other gliomas, PDGFR is an appropriate target for micelle delivery of chemotherapeutic agents into GBMs in the human brain.
Here, we improve upon previous micelle compositions in a number of ways to create an efficient and effective nanocarrier. First, we conjugate the micelle with a novel moiety against an overexpressed cell surface receptor, PDGR. TMZ-loaded micelles are targeted to GBM cells for increased uptake. Second, we incorporate a biocompatible coating to reduce cytotoxicity and improve circulation. Micelles loaded with TMZ are utilized for increased permeation across the BBB, due to the smaller size of the lipid structure. Finally, we add a triggered-release (i.e., pH) mechanism of the carrier for drug release in a localized environment. The pH-dependent release of TMZ from the micelles thus occurs preferentially within the GBM cells, thereby reducing the risks of systemic toxicity when administered in vivo. Taken in combination, these modifications increase the concentration of TMZ released into GBM tumor cells while simultaneously decreasing the risks of systemic toxicity.
Materials & methods
Synthesis of micelle-encapsulated TMZ
Micelle encapsulation of TMZ (MTMZ) was carried out as described by Dubertret et al. [17]. Dimethyl sulfoxide (DMSO) was added to TMZ and sonicated for 30 min in a water bath at room temperature. The TMZ was then mixed with amino-PEG-PE ([1,2-diacyl-sn-glycero-3-phosphoethanolamine-N-(amino-poly[ethylene glycol)]; 870320P; Avanti Polar Lipids, AL, USA) and 0.5 mg of PHC (N-palmitoyl homocysteine [ammonium salt]); 880128P; Avanti Polar Lipids, AL, USA) and suspended in chloroform. The solvent was evaporated in a vacuum oven for 1 h at room temperature. The pellet obtained after evaporation was heated to 80°C and dissolved in nanopure water (18 mΩ) to produce amine functionalized micelles. The micelle solution was sonicated for 1 h in a water bath and subsequently filtered using a 0.2 μm syringe filter to remove aggregates. For the synthesis of PDGFR-targeted MTMZ (PMTMZ), MTMZ solution was used for peptide conjugation (1:1 ratio of carboxyl group on peptide to amine group on the micelles at 30% coverage of amines). The PDGF peptide (PDGFpep) sequence was yITLPPPRPFFK (Peptide International, KY, USA) [18]. After 15 min of incubation at room temperature, phosphate buffered saline (PBS; pH ˜12) was added to bring the pH back to 7.5. PDGF peptide solution was added to the micelle solution and left incubating for 2 h at room temperature. After 2 h, excess peptide was purified using 10K MWCO ultracentrifugal device (EMD Millipore, MA, USA) at least three-times at 4000 rpm for 15 min at 4°C. For dye labeling, MTMZ and PMTMZ solution was added to NHS Dylight 680 (ratio of covering 30% amines on the micelles, Thermo Scientific, IL, USA), respectively. PBS buffer (pH 7.2) was added to the solution. The solution was incubated for 1 h at room temperature. After 1 h, excess dye was purified using 10K MWCO ultracentrifugal device three-times.
Characterization of micelle-encapsulated TMZ
The concentrations of MTMZ and PMTMZ were determined by ultraviolet-visible (UV-Vis) absorption using a Biotek microplate spectrophotometer (Winooski, VT, USA). Dynamic light scattering (DLS) analysis and zeta potential analysis of MTMZ and PMTMZ in aqueous solution was performed on a ZetaPALS particle analyzer (Brookhaven Instruments, NY, USA). PBS (pH 7.2) was used as the starting solvent. The respective aqueous master solution was diluted fivefold and sonicated for 1 h to prevent aggregation. The solution was filtered using a 0.2 μm syringe filter before taking the measurements. Zeta (ξ) potential was automatically calculated from electrophoretic mobility based on the Smoluchowski equation, v=(εE/η)ξ, where v is the measured electrophoretic velocity, η is the viscosity, ε is the electrical permittivity of the electrolytic solution and E is the electric field.
Negative-stain transmission electron micrographs (TEM) of MTMZ and PMTMZ were taken by spreading 10 μl of MTMZ or PMTMZ solution (˜1 μM) on a carbon-coated copper grid. Excess solution was removed with filter paper after 10 min, followed by the addition of 10 μl of saturated uranyl acetate solution (2% w/v). After another 10 min, the excess stain was removed with filter paper. The sample was visualized with a JEOL 200CX transmission electron microscope (JEOL, MA, USA) at 80 kV equipped with a digital camera.
For pH change experiments, PBS buffers of pH 4–10 were prepared. The pH of PBS buffer (pH 7.2) was changed to alternate pHs (pH 4-10) by adding sodium hydroxide or hydrochloric acid to assess the stability of the micelle at various pHs, thereby keeping the salt concentration in the PBS constant. 5 μl of MTMZ or PMTMZ (˜10–4 M) were placed in a 96 well plate. 200 μl of the respective PBS buffers were added to a well. The wells were incubated for 4 h. After 4 h, UV-Vis measurements were recorded at 325 nm (TMZ excitation).
In vitro treatment of micelle encapsulated TMZ
Two types of GBM cell lines are used in this work, U87 and LN229 (ATCC, VA, USA). These are the most commonly used glioma cell lines in research studies. U87 is a primary human GBM cell line with an epithelial morphology which was acquired from a stage IV 44-year-old cancer patient [19]. LN229 is another human GBM cell line derived from brain/right frontal parieto-occipital cortex of a 60-year old female GBM patient with similar epithelial morphology [20]. LN229 or U87 cells were plated on a 25 × 25 mm coverslip at a density of 30,000 cells per coverslip and maintained overnight in cDMEM at 37°C in an incubator supplied with 5% CO2. Twenty-four hours after plating, cells were treated with increasing TMZ concentrations of MTMZ and PMTMZ with a final volume of 300 μl for a total of 4 h. Immunostaining was done to observe the co-localization of the drug and receptors. After treatment, the cells were washed with media and then fixed with 8% paraformaldehyde for 10 min followed by three washes with PBS buffer. The fixed cells were blocked with 3% goat serum for 1 h. Following blocking, the cells were incubated with primary anti-PDGFR (1:500; sc-432; Santa Cruz Biotech, TX, USA) for 2 h. The cells were washed with PBS buffer followed by incubation with secondary goat antirabbit Alexa 488 antibody (1:1000; A11034; Life Technologies, NY, USA). For staining of nuclei, cells were incubated with DAPI (4′,6-diamidino-2-phenylindole) (1:7500). The uptake and co-localization of particles was visualized by fluorescence microscope using a Leica DM 4000B microscope (Leica Microsystems, IL, USA). The images were analyzed using ImageJ (NIH) software for relative normalized intensities for comparison analysis.
The prior experimental protocol was then replicated in a longitudinal study using MTMZ and PMTMZ at concentrations of 0.5 μM or 1 μM. Cells plated on coverslips were incubated with either MTMZ or PMTMZ over increasing time. Coverslips were fixed with paraformaldehyde at 5, 15, 30 and 60 min after incubation for short-term observation and at 4, 8, 16 and 24 h for long-term examination. The uptake and co-localization of particles was visualized by fluorescence microscope using a Leica DM 4000B microscope (Leica Microsystems). The images were analyzed using ImageJ (NIH) software for relative normalized intensities for comparison analysis.
Cytotoxicity of TMZ micelles
Three cytotoxicity experiments, using Guava ViaCount Assay and flow cytometry (EMD Millipore, MA, USA), were performed using increasing concentrations of free TMZ, MTMZ and PMTMZ over increasing time. Cells (30,000 cells per well) were plated in 24-well plates and incubated overnight at 37°C with 5% CO2. A TMZ solution (1 mg ml-1) in DMSO was prepared and stored at 4°C. The stock solution was diluted with alpha-MEM and used to prepare free TMZ at increasing concentrations of 0–100 μM. Independently, both 1 μM and 10 μM samples of PMTMZ and MTMZ were prepared with alpha-MEM. The triplicate wells were treated with concentrations at a constant volume of 200 μl per well. After 24 h, cell viability was performed using Guava ViaCount Assay and flow cytometry (EMD Millipore). The remaining wells were retreated with the appropriate concentrations over a 10 days period. Viability data were collected and analyzed.
Inhibition of receptor cycling using brefeldin A in vitro
U87 cells were plated on 25 × 25 mm coverslips at a density of 30,000 cells per coverslip and maintained overnight in media at 37°C in an incubator supplied with 5% CO2. Twenty four hours after plating, one set of cells were treated with 250 μl of brefeldin A (BA) solution (10 μg ml-1 in media) and were incubated for 1 h (+BA). Another set of coverslips was left with 250 μl of media as -BA controls. For the +BA set of cells, the BA solution in media was replaced with 250 μl of 500 nM MTMZ or PMTMZ solutions. The -BA cells were treated with 250 μl of 500 nM MTMZ or PMTMZ solutions. Both set of cells were incubated with the NPs for 0.5, 1, 4 and 6 h, respectively. After treatment, the cells were washed with media and then fixed with 4% paraformaldehyde for 10 min followed by three washes with PBS buffer. For staining of nuclei, cells were incubated with DAPI (1:7500). Uptake and co-localization of NPs were visualized by fluorescence microscope using a Leica DM 4000B microscope (Leica Microsystems, IL, USA). The images were analyzed using ImageJ software for relative normalized intensities for comparison analysis
Orthotopic tumor implantation
For orthotopic brain tumor implants, athymic nude mice (NCR Nu;Nu; Charles River Laboratory, MD, USA) were anesthetized by intraperitoneal injection of 50 mg kg-1 bodyweight ketamine/xylazine and fitted into a stereotaxic rodent frame (David Kopf Instruments, CA, USA). A small incision was made just lateral left to midline to expose the bregma suture. A small (1.0 mm) burr hole was drilled at AP = +1, ML = -2.5 from bregma. Glioblastoma cells (U87, 300,000 cells in 3 μl) were slowly deposited at a rate of 1 μl per minute in the left striatum at a depth of –3 mm from dura with a 10 μl Hamilton syringe (26G blunt needle, Fisher Scientific, PA, USA). The needle was slowly withdrawn and the incision was closed with 2–3 sutures. The tumors developed for 9 days prior to tail vein injection. Tumor burden and location was evaluated using luciferase activity. At 9 days, luciferin (150 μg ml-1; substrate for luciferase) was injected within the peritoneal cavity. Luminescence measurements were taken using an IVIS 200 imager (PerkinElmer, MA, USA). Animals were fed exclusively on a special rodent diet (Tekland 2018S; Harlan Laboratories, Inc., IN, USA) to reduce autofluorescence. Animal experiments were performed according to policies and guidelines of the Institutional Animal Care and Use Committee (IACUC) at Medical University of South Carolina under approved protocols.
In vivo fluorescence imaging
Mice with orthotopic tumors were anesthetized with isoflurane and injected intravenously via the tail with either PMTMZ or MTMZ at a dosage of 0.001 mg kg-1 of TMZ per total mouse body weight. Mice were imaged at 0, 1, 4, 6 and 24 h. After live imaging, the mice were euthanized and excised organs were imaged after necropsy. Fluorescent multispectral images were obtained using the Maestro In Vivo Imaging System (PerkinElmer, MA, USA). Multispectral in vivo images were acquired under a constant exposure of 2000 ms with an orange filter acquisition setting of 630–850 nm in 2 nm increments. Multispectral images were unmixed into their component spectra (Dylight 680, autofluorescence, and background) and these component images were used to gain quantitative information in terms of average fluorescence intensity by creating regions of interest (ROIs) around the organs in the Dylight 680 component images.
Results
Micelles composed of PEG-PE amine (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000 and PHC (N-palmitoyl homocysteine (ammonium salt)) were prepared to encapsulate hydrophobic TMZ (Figure 1A). PHC, a pH sensitive lipid [21] was used to assist in the micelle rupture at acidic pH to ensure the delivery of the cargo inside the micelle core. Amine functionality on PEG-PE amine was utilized for further tailoring of the micelle with targeting peptides (PDGF, yITLPPPRPFFK) containing a carboxyl group and labeling with fluorescent dyes (Dylight 680) for tracking the micelle in in vitro cellular uptake studies.
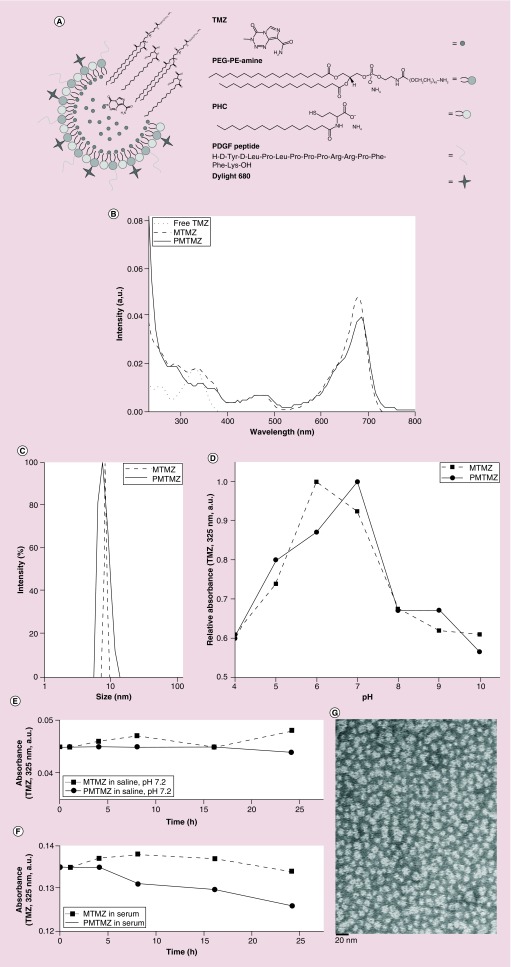
Figure 1. . Characterization of size, drug loading, disruption at varying pH, and stability of micelles in saline and serum.
(A) Schematic of PMTMZ (PDGFR-targeted micelles containing TMZ) construction. (B) Micelle concentrations using ultraviolet-visible spectroscopy of free temozolomide (TMZ), MTMZ and PMTMZ identify TMZ (325 nm) and Dylight 680 (692 nm). Concentration of each batch calculated based on the TMZ peak. (C) Size calculation using dynamic light scattering of MTMZ (untargeted) and PMTMZ (targeted) demonstrates micelle size between 10 and 15 nm. (D) An increase in intensity of TMZ (325 nm)-filled nanoparticles between pH 6 and 7 and is lost outside of the physiologic range due to rupture. (E & F) MTMZ and PMTMZ were evaluated for stability over time in both phosphate-buffered saline and serum. Both micelles were able to maintain their composition over a 24-h period. (G) The assembly of PMTMZ was examined using electron microscopy (transmission electron microscopy). Transmission electron microscopy reveals presence of spherical micelles with a diameter of approximately 12–13 nm.
MTMZ: Micelle-encapsulated TMZ; PE: Phosphatidylethanolamine; PHC: N-palmitoyl homocysteine (ammonium salt); PMTMZ: PDGFR-micelles containing TMZ; TMZ: Temozolomide.
Untargeted, micelle-encapsulated TMZ (MTMZ) and PDGFR-targeted TMZ (PMTMZ) were characterized by DLS, UV-Vis spectroscopy and micelle integrity in physiological buffer. The UV-Vis spectra of MTMZ and PMTMZ showed peaks from TMZ (325 nm) and the fluorophore (680 nm) demonstrating the presence of hydrophobic TMZ inside the core and the fluorescent label on the exterior of the micelles (Figure 1B). DLS data showed both MTMZ and PMTMZ have an average hydrodynamic diameter of around 10 ± 1.2 and 12 ± 2.3 nm, respectively, with a polydispersity index of 0.1 and 0.2% (Figure 1C). The size distribution is determined by the polydispersity index. The lower the value is, the narrower the size distribution or the more uniform the nanoparticle sample. However, attachment of PDGF increased the polydispersity index due to the steric hindrance caused by the cyclic structure of PDGF. The DLS size distribution is identical to the instrumental response function corresponding to a monodispersed sample, indicating that aggregation is negligible [22]. Zeta potential is an indicator of surface charge, which determines particle stability in dispersion. Zeta potentials of MTMZ and PMTMZ were -40.35 ± 4.46 and -45.18 ± 3.71 mV, respectively, as shown in Table 1. The micelles in the present study were found stable in the dispersion state, possessing high absolute values of zeta potential and having negative surface charges. Particle surface conjugation slightly increased the absolute value of the zeta potential.
Table 1. . Characteristics of micelle-encapsulated temozolomide and PDGF-micelles containing temozolomide nanoparticles.
| Nanoparticle properties | Sample group: MTMZ | Sample group: PMTMZ |
|---|---|---|
| Size ± SD (nm) |
10 ± 1.2 |
12 ± 2.3 |
| Polydispersity (%) |
0.1 |
0.2 |
| Zeta potential ± SD (mV) | -40.35 ± 4.47 | -45.19 ± 3.71 |
MTMZ: Micelle-encapsulated TMZ; PMTMZ: PDGFR-micelles containing TMZ; TMZ: Temozolomide.
Stability and rupture efficiency of the micelles were evaluated using a pH change assay (Figure 1E). The pH change studies were performed to evaluate the range at which the micelle would rupture. This is particularly important, as the micelles were engineered to be taken up by receptor-mediated endocytosis. The micelles would need to rupture at an endosomal of pH approximately 5.5 in order to deliver the encapsulated TMZ cargo. These studies illustrated that for both MTMZ and PMTMZ increased absorbance intensity at 325 nm was seen between pH 6 and 7 indicated that the micelles were intact, holding the hydrophobic TMZ inside its core. Upon decreasing the pH from 7 to 4 (acidic milieu), the intensity was reduced by approximately 34% for MTMZ and 40% for PMTMZ. Upon increasing the pH from 7 to 9 (basic milieu), the intensity declined by approximately 33% for both MTMZ and PMTMZ. This depletion of intensity is attributed to the loss of micelle membrane integrity, which is due to the pH-responsive lipid composition. TMZ was able to leach out of the micelle and then aggregated within the aqueous solution. TMZ was removed from the optical path of the excitation wavelength. This demonstrates the functional capability of the micelle to release the TMZ at an acidic pH that mimics the endosomal pH.
Stability of the MTMZ and PMTMZ was assessed over a 24 h period. To mimic the physiologic environment, the micelles were suspended in saline (PBS, pH 7.2) and absorbance of the drug was examined (Figure 1E). Both carriers were relatively stable over the 24 h since the change in absorbance of the drug was negligible. The slight increase in absorbance for MTMZ can be attributed to instrumental error. In addition, the stability of these micelles was also evaluated in serum since the presence of lipids, amino acids and proteins in the serum could aid to micelle instability (Figure 1F). The micelles were slightly less stable than those suspended in saline over the same period with overall loss of absorbance at 325 nm of approximately 5–7%. These micelle stability experiments established the robust nature of the micelles for potential use in in vivo studies. The self-assembly of the two lipids and structural integrity of the micelles were examined using electron microscopy (Figure 1G). TEM reveals the presence of spherical micelles for PMTMZ with a diameter of approximately 12–13 nm.
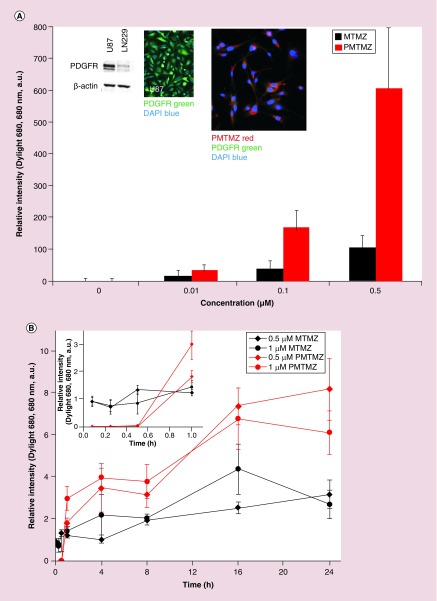
Micelles were functionalized with a PDGF peptide (PDGFpep) to target the PDGFR expressed on the glioma cell surfaces to facilitate targeting and cellular uptake. A schematic for the final structure of PMTMZ is shown in Figure 1A. Theoretical calculations predict that there are 242 PDGF peptides per micelle. Calculations were made as follows: assuming the micelle is a sphere of 10 nm, the surface area (SA) of the sphere was calculated first. Then the total number of lipid molecules in one micelle was calculated by dividing the SA of the lipid molecules by the total SA. Using the molar ratio of the lipids used, the number of PEG-PE amine molecules was calculated, which is equivalent to number of PDGF peptides (assuming 100% coupling). Accumulation of the micelles after targeting with the PDGFpep was assessed in vitro utilizing immunofluorescence (Figure 2). U87 cells that overexpress the PDGFR (Figure 2A, inset left) were treated with increasing concentrations of either PMTMZ (targeted) or MTMZ (untargeted) for 4 h at 37°C. PMTMZ was internalized in PDGFR-expressing U87 cells with as little as 0.01 μM micelles, indicating the threshold concentration for uptake (Figure 2A, graph). At 0.5 μM, significant uptake was observed suggesting receptor-mediated endocytosis. The uptake was quantified and the relative intensity graph demonstrated that PMTMZ uptake is threefold higher than that of MTMZ at 0.1 μM and sixfold higher at 0.5 μM. The targeted micelles (red) co-localized with the receptor (green) with a Pearson's correlation coefficient of 0.83 (Figure 2A, inset right). In contrast, few untargeted micelles were taken up during treatment with increasing concentrations and did not co-localize with the receptors (Pearson's correlation coefficient = 0.32). A longitudinal study was performed to demonstrate uptake of PMTMZ and MTMZ over a total period of 24 h (Figure 2B). Overall, PMTMZ uptake for 0.5 μM or 1 μM was consistently higher than MTMZ at every time point (1, 4, 8, 16, 24 h). A significant increase was first observed within 30 min to 1 h after treatment was initiated (Figure 2B, inset). After 24 h, PMTMZ uptake at 0.5 μM was significantly higher (166%) as compared with MTMZ uptake.
Figure 2. . Concentration-based and kinetic internalization and accumulation of micelle-encapsulated temozolomide and PDGF-micelles containing temozolomide into U87 cells.
(A) Confocal microscopic imaging was performed to assess the uptake of both MTMZ and PMTMZ by glioma cells (U87). U87 cells were incubated with either MTMZ or PMTMZ (0.01, 0.1 and 0.5 μM). MTMZ and PMTMZ (red) were taken up in a concentration-dependent fashion. PMTMZ (red) accumulates in PDGFR-positive (green) U87 cells. PMTMZ appeared to internalize more rapidly than MTMZ and were present at higher levels at all the concentrations. (B) To evaluate kinetic-based uptake of MTMZ and PMTMZ, mean fluorescence imaging of internalized micelles at 0.5 or 1 μM at 0–1, 4, 8, 16 and 24 h was performed. Targeted-TMZ (0.5 or 1 μM) shows a significant increase in fluorescence intensity when compared with the same concentration of untargeted-TMZ. Experiments were conducted three-times in triplicate. Error bars represent standard deviation.
MTMZ: Micelle-encapsulated TMZ; PMTMZ: PDGFR-micelles containing TMZ; TMZ: Temozolomide.
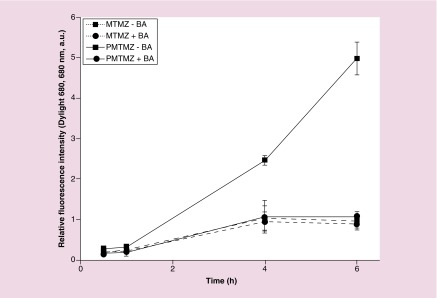
To demonstrate that uptake of PMTMZ was predominantly due to endocytosis associated with the PDGF peptide and not diffusion of the micelles, U87 were treated with brefeldin A (BA), a fungal metabolite that reversibly interferes with intracellular transport and receptor cycling and examined for uptake (Figure 3). BA acts by inducing major structural changes in the morphology of endosomes, the trans-Golgi network, and lysosomes by causing the formation of an extensive tubular network and preventing new endosome formation [23]. As seen previously in Figure 2, significant fluorescence was observed when U87 were incubated with PMTMZ (-BA, 15.8% increase) over a 6 h period. Fluorescence intensity increased by only 5.06% when U87 were treated with MTMZ (-BA). Preincubation with BA (+BA) decreased the relative fluorescence intensity of PMTMZ incubated cells by 78.2% over time. MTMZ uptake was inhibited to a much lesser extent with BA (8.2%).
Figure 3. . Inhibition of receptor-mediated uptake using brefeldin.
U87 were preincubated with BA (+BA) or without (-BA), then treated with PMTMZ (red) or MTMZ (black) for 1 h and assessed for fluorescence accumulation (680 nm) over a 6-h period. PMTMZs were rapidly internalized in the absence of BA. BA pretreatment significantly reduced internalization of PMTMZ. For MTMZ in the presence of BA, the decrease in fluorescence was minimal. Experiments were conducted three-times with triplicate experimental groups. Error bars represent standard deviation.
BA: Brefeldin A; MTMZ: Micelle-encapsulated TMZ; PMTMZ: PDGFR-micelles containing TMZ; TMZ: Temozolomide.
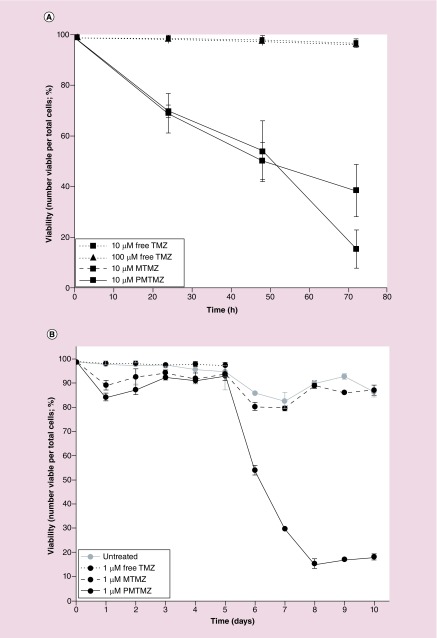
PMTMZ was first evaluated for cell killing efficacy using a short-term cell viability assay (Figure 4A). Glioma cells (U87) were treated with PMTMZ or MTMZ (10 μM each) or free TMZ (10 or 100 μM each) over the course of 24–72 h with treatments added to fresh media once a day. PMTMZ results were compared directly to either free TMZ or MTMZ measurements. After only 24 h, both PMTMZ and MTMZ began to exhibit more killing (˜30%) than that of equal or increased concentrations of free TMZ. By 72 h, there was a significant difference in the killing between treatment groups; with PMTMZ killing approximately 84% more cells than 10 or 100 μM of free TMZ; and MTMZ killing approximately 61% more cells than either treatment concentration of free TMZ. Overall, between 24 and 72 h, PMTMZ exhibited compelling cell death of approximately 78% with MTMZ following at compelling 44%. In comparison, free TMZ (10 or 100 μM) only illustrated a cell death of compelling 2%.
Figure 4. . Temozolomide delivered by targeted micelles increases the efficiency of cell killing at lower concentrations of temozolomide.
(A) Cell toxicity and death of U87 cells treated with PDGF-micelles containing TMZ (10 μM) versus micelle-encapsulated TMZ (10 mM) and free Pc 4 (10 or 100 μM) show increased cell killing after 72 h of treatment. (B) PDGF-micelles containing TMZ (1 μM) resulted in significant cell toxicity and death over a course of 10 days when compared with identical concentrations of micelle-encapsulated TMZ and free TMZ in addition to untreated cells. Experiments were conducted three-times in triplicate. Error bars represent standard deviation.
TMZ: Temozolomide.
PMTMZ was then evaluated for cell killing efficacy using a longitudinal cell viability assay with a tenfold decrease in TMZ concentration (Figure 4B). Glioma cells (U87) were treated with PMTMZ, MTMZ or free TMZ (1 μM each) over a 10 days period with treatments added to fresh media once a day. PMTMZ results were compared directly to either free TMZ or MTMZ measurements. Between 1 and 5 days, there was no significant difference between the treatment groups; free TMZ killed approximately 4% of the cells and PMTMZ and MTMZ had little to no effect on cell death. However, after day 5, PMTMZ dramatically killed the cells at 1 μM (˜82% by day 8). In contrast, MTMZ after day 5 showed no appreciable cell death, maintaining a 1–2% death rate comparable to that of untreated cells.
For Figure 4A the concentration is tenfold higher for MTMZ and PMTMZ administered to the cells than that of Figure 4B. We would expect that a decrease in administered concentration would take longer (5 days) to show efficacy as compared with that of a higher concentration over a shorter period of time (3 days). Our data show a consistent decrease in cell viability over 3 days at 10 μM PMTMZ (Figure 4A) and after 5 days 1 μM PMTMZ (Figure 4B). It appears that untargeted, MTMZ is unable to deliver a significantly toxic dose of TMZ to the cells when only 1 μM is administered.
Mice containing orthotopic gliomas from implanted luciferase expressing U87 cells were first evaluated for tumor burden using in vivo bioluminescence imaging (Supplementary Information 1). Luciferase expressing glioma cells were used in conjuction with luciferin substrate (150 μg ml-1) in order to confirm the presence of tumor in the brain and verify the location of the tumor (Supplementary Information 1B). A standard curve for luciferase activity was generated using increasing cell numbers of U87 (without luciferase expression as control) and U87-luciferase cells incubated with luciferin (Supplementary Information 1A). U87-luciferase expressing cells showed a linear increase in luminescence with increasing cell numbers. Tumor burden in vivo was approximated to cell number using the standard in vitro curve (Supplementary Information 1B). After 7 days of growth, tumors contained approximately 12.3 million cells.
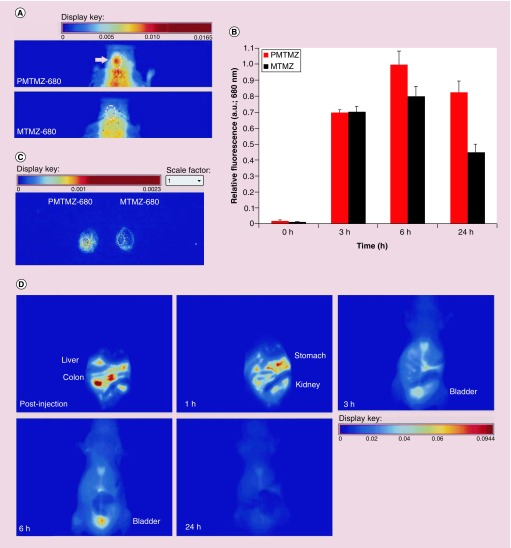
Mice treated with PMTMZ accumulate the nanocarrier in the brain over a 24 h period (Figure 5A, top panel) as compared with those animals treated with untargeted MTMZ (bottom panel). Multiple controls were conducted, including mice sham-implanted with PBS instead of cells and mice orthotopically implanted but administered PBS instead of either MTMZ or PMTMZ. No fluorescence was observed in these control mice as compared with the experimental PMTMZ and MTMZ administered mice. A ROI modeled around the craniums of the mice showed significant fluorescence associated with PMTMZ treated animals. Quantitation of fluorescence intensity in an ROI created around the brain tumor (white dashed circle) confirmed the trend observed in the whole animals (Figure 5B). During short incubation periods (3–6 h), both targeted PMTMZ and untargeted MTMZ micelles were found in the brain tumor. However, after 24 h the untargeted micelles washed away and the tumors retained 40% more of the targeted micelles containing TMZ. To verify that the fluorescence from PMTMZ was attributable to uptake specifically within the brain, the brains of mice injected with either PMTMZ (left) or MTMZ (right) were then excised from euthanized mice and imaged (Figure 5C). Biodistribution of PMTMZ was also observed using real time in vivo fluorescence (Figure 5D). Animals were imaged in the ventral position during the 24-h period. Since this is topographic fluorescence imaging, the 3 mm depth of the tumor in the dorsal striatum of the brain would not be seen from the ventral side because of tissue and bone scattering. PMTMZ was found quickly in the colon just postinjection and declined rapidly over 6 h. PMTMZ then was excreted through the urinary bladder until almost all fluorescence washed away after 24 h. No difference was observed in the excretion pattern of MTMZ as compared with PMTMZ (Supplementary Information 2).
Figure 5. . Accumulation of PDGF-micelles containing temozolomide in orthotopic gliomas in mice.
(A) Mice with orthotopically implanted with U87-luciferase cells in the left hemisphere of the brain were intravenously injected with either PMTMZ or MTMZ (0.001 mg kg-1). After 24 h, the animals were imaged using an in vivo fluorescence imaging system. Representative fluorescence hotmap images are shown (n = 4 per each group). Images were scaled evenly. (B) Relative fluorescence was quantified over time from region of interest indicating the brain tumor. Error bars represent standard deviation. (C) The micelle fluorescence was observed in excised mouse brains from respectively treated animals using an in vivo fluorescence imaging system. (D) Biodistribution of the PMTMZ was imaged over the 24-h period in mice placed to show ventral organs (n = 4 per group). Fluorescence intensity decreases over time postinjection.
MTMZ: Micelle-encapsulated TMZ; PMTMZ: PDGFR-micelles containing TMZ; TMZ: Temozolomide.
Discussion
Temozolomide is an effective, US FDA-approved chemotherapeutic known for its comprehensive antitumor activity in tumor models, and it is the current standard of care for glioblastoma multiforme. In previous studies TMZ has proven potent in in vivo systems by traversing the CNS, demonstrating accumulation in malignant tissues [5]. Despite its exceptional tumor regression activity, TMZ is extremely hydrophobic thereby reducing its bioavailability. In addition, it also hampers its ability to cross the BBB, which remains considerable obstacle in glioma therapy [24]. This necessitates formulation of a drug delivery system which can encompass these requirements: a tailored surface on the carrier to attach biomolecules for targeted drug delivery; a biocompatible coating which can efficiently encapsulate the hydrophobic drug thereby reducing cytotoxicity; stimuli-induced (i.e., pH) disruption of the carrier agent for drug release to the desired environment.
Micelles are the preferred choice of nanocarrier in comparison to other potential carriers based on their composition. Micelles are composed of amphiphilic lipid molecules with a hydrophobic core and hydrophilic exterior. The hydrophobic core of the micelles serves as a container for weakly water-soluble drugs while the outer shell can protect encapsulated drugs and prevent the drugs from leaching out. Recently, polymeric micelles have been utilized as drug carriers due to their properties of hydrophilicity and degradability and due to the ability to tailor their exterior surface with multiple functionalities to attach various biomolecules [12]. In addition, PEG (hydrophilic polymer) can be incorporated in the micellar composition in order to block nonspecific interaction and prolong the blood circulation times of the micelles in a biological milieu. Other hydrophobic drugs like lomustine, carmustine and 5-fluorouracil have been encapsulated inside these micelles composed of poly(propylene oxide) (PPO), poly(D, L-lactic acid) (PDLLA), poly(ε-caprolactone) (PCL), poly(L-asparate) and poloxamers against brain tumors [25]. Our system is distinct because it incorporates a pH-sensitive lipid (PHC), a targeting ligand (PDGF), and PEG for prolonged circulation, making it a multifunctional micelle within the tumor environment. Since MTMZ and PMTMZ range between 10 and 15 nm, their size (<100 nm) is advantageous for these carriers to cross the BBB, which prohibits larger nanocarriers [26]. The main mechanisms by which micelles target brain tumors are passive diffusion through a disrupted BBB via permeability and enhanced permeability and retention (EPR) effect to reach glioma cells or active receptor-mediated endocytosis to the tumor region [27].
Treatment of GBMs has been limited by a number of medical obstacles, not least of which is the challenge of achieving adequate chemotherapeutic concentrations in the tumor without systemic toxicities. As discussed above, micelle-encapsulated therapies address this obstacle. Outside of the brain, micelle packaged chemotherapies are being tested in clinical trials and are demonstrating improved tolerability compared with similar free chemotherapies [28]. However, many micelle formulations rely upon ultrasound-guided hyperthermia for release of the packaged chemotherapies [29]. Though this is a practical approach in mouse and rat models [30], ultrasound guided localized delivery into the human brain poses quite a logistic challenge in the human brain.
This is one motivation for specifically investigating pH-sensitive micelles for treatment of GBMs. Although PDGFR is expressed at low to moderate levels in other organs, focal amplification of the PDGFR gene and overexpression of PDGFR is frequently observed in aggressive brain tumors. The PDGFR targeting induces the GBM tumor cells to internalize the micelles via receptor-mediated endocytosis. These internalized micelles are then within an appropriate pH environment for the intracellular release of TMZ. This approach increases the accumulation of micelles in the relevant regions of the brain (specifically, in the tumor tissue) in order to increase the release of TMZ, which leads to an elevated concentration of TMZ in the tumor itself. Simultaneously, this approach also potentially reduces the risk of systemic toxicity as the micelles are targeted to the GBM, such that lysis of the micelle should preferentially occur in the tumor rather than systemically. The pattern of fluorescence observed in the biodistribution study suggests that the micelles are processed through both hepatobiliary and urinary excretory paths with over 80% clearance from initial excretory organ uptake within 72 h.
Due to the high concentration of TMZ (˜100 μM) utilized in the clinical setting, this concept of an effective and efficient targeting moiety (PMTMZ) is vital to the cause [31,32]. Consistent treatment at these increased concentrations does not guarantee that all of the chemotherapeutic will reach the tumor, but instead leads to concern of unintentionally prompting drug resistance [33]. Through the use of the targeted, pH-responsive chemotherapeutic, the dosage can be reduced from approximately 100 μM of free TMZ to a minimum of 1 μM PMTMZ resulting in more than double the efficacy of glioma cell death and diminished overall systemic effects.
Conclusion
Targeted micelles loaded with TMZ were designed to increase the delivery of the drug into the brain. TMZ-packaged pH-responsive micelles composed of PEG-PE amine and PHC surface functionalized with PDGF peptide and Dylight 680 fluorophore (PMTMZ) have specific uptake and increased cell killing in glial cells compared with untargeted micelles (MTMZ). In vivo studies demonstrated selective and increased accumulation of PMTMZ in orthotopic gliomas implanted in mice.
Future perspective
This study validates the use of a pH-responsive, receptor-mediated targeting moiety for the effective delivery of chemotherapeutics in the treatment of GBM. Through its ability to overcome the widespread clinical obstacle of crossing the BBB, in addition to significantly decreasing overall systemic toxicity, this hydrophobic drug-loaded carrier creates potential for the selective delivery of other anticancer agents. The next clear steps for investigating targeted micelles for treatment of GBMs will be to test the treatment regime of these micelle-encapsulated agents in orthotopic GBM models.
Executive summary.
Synthesis & characterization
Temozolomide (TMZ) encapsulated micelles composed of PEG-PE amine and PHC were self-assembled and further conjugated with PDGF peptide via covalent amide bond. These conjugated micelles were labeled with a fluorescent dye for tracking applications.
Both targeted and untargeted micelles were 10–15 nm in diameter (dynamic light scattering). They were stable for a period of 24 h, both in serum and phosphate-buffered saline.
In vitro studies
Due to the targeting moiety, PDGF-micelles containing TMZ (PMTMZ) demonstrated higher uptake than MTMZ in U87 cells via receptor-mediated endocytosis. This was further confirmed with the brefeldin-blocking assay in U87 cells.
PMTMZ demonstrated efficient cell death overall when compared with either micelle-encapsulated TMZ (at similar concentrations) or free TMZ.
In vivo & biodistribution studies
PMTMZ accumulated at a higher level in the brain over a period of 24 h as compared with micelle-encapsulated TMZ.
Biodistribution of PMTMZ was immediately observed in the colon, stomach and bladder postinjection, and declined rapidly over a period of 6 h.
Conclusion
This work represents a targeted, pH-responsive micelle system for drug delivery of chemotherapeutics across the BBB in the treatment of GBM.
Supplementary Material
Acknowledgements
The authors would like to thank Xingju Nie for his technical support and animal handling during ex vivo fluorescence imaging.
Footnotes
Financial & competing interests disclosure
This project was supported by the South Carolina Clinical & Translational Research (SCTR) Institute with an academic home at the Medical University of South Carolina (MUSC), through the NIH grant number UL1 TR000062 (to A-M Broome). This work was also supported in part by the Small Animal Imaging Unit of the Cell & Molecular Imaging Shared Resource, Hollings Cancer Center, MUSC (NIH grant number P30 CA138313). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Disclaimer
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or National Center for Advancing Translational Sciences (NCATS).
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Waters JD, Rose B, Gonda DD, et al. Immediate post-operative brachytherapy prior to irradiation and temozolomide for newly diagnosed glioblastoma. J. Neurooncol. 2013;113(3):467–477. doi: 10.1007/s11060-013-1139-x. [DOI] [PubMed] [Google Scholar]
- 2.Jelsma R, Bucy PC. The treatment of glioblastoma multiforme of the brain. J. Neurosurg. 1967;27(5):388–400. doi: 10.3171/jns.1967.27.5.0388. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey RG, Brand WN. Radiotherapy of glioblastoma multiforme. J. Neurosurg. 1973;39(2):197–202. doi: 10.3171/jns.1973.39.2.0197. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer JE, Hewlett JS, Bay J, Livingston RB. High dose BCNU with autologous bone marrow rescue in the treatment of recurrent malignant gliomas. J. Neurooncol. 1983;1(3):269–273. doi: 10.1007/BF00165611. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000;6(7):2585–2597. [PubMed] [Google Scholar]
- 6.Patil R, Portilla-Arias J, Ding H, et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(beta-L-malic acid) Pharm. Res. 2010;27(11):2317–2329. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plowman J, Waud WR, Koutsoukos AD, Rubinstein LV, Moore TD, Grever MR. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1994;54(14):3793–3799. [PubMed] [Google Scholar]
- 8.Bower M, Newlands ES, Bleehen NM, et al. Multicentre CRC Phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother. Pharmacol. 1997;40(6):484–488. doi: 10.1007/s002800050691. [DOI] [PubMed] [Google Scholar]
- 9.Hu CM, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Therapeut. Deliv. 2010;1(2):323–334. doi: 10.4155/tde.10.13. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Wang W, Yang J, Zhou C, Sun J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharmaceut. Sci. 2013;8(3):159–167. [Google Scholar]; •• Describes pH sensitive strategy of polymeric micelles to facilitate specific drug delivery.
- 11.Ding H, Wang X, Zhang S, Liu X. Applications of polymeric micelles with tumor targeted in chemotherapy. J. Nanopart. Res. 2012;14(11):1–13. [Google Scholar]
- 12.Nasongkla N, Bey E, Ren J, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6(11):2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 13.Morshed RA, Cheng Y, Auffinger B, Wegscheid ML, Lesniak MS. The potential of polymeric micelles in the context of glioblastoma therapy. Front. Pharmacol. 2013;4:157. doi: 10.3389/fphar.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y, Meyers JD, Agnes RS, et al. Addressing brain tumors with targeted gold nanoparticles: a new gold standard for hydrophobic drug delivery? Small. 2011;7(16):2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates a receptor-targeted nanoparticle capable of traversing the blood–brain barrier with high specificity.
- 15.Le Mercier M, Hastir D, Moles Lopez X, et al. A simplified approach for the molecular classification of glioblastomas. PLoS ONE. 2012;7(9):e45475. doi: 10.1371/journal.pone.0045475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu KW, Hu B, Cheng SY. Platelet-derived growth factor receptor alpha in glioma: a bad seed. Chin. J. Cancer. 2011;30(9):590–602. doi: 10.5732/cjc.011.10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science (NY) 2002;298(5599):1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 18.Marr A, Nissen F, Maisch D, et al. Mol. Imaging Biol. 2013;15(4):391–400. doi: 10.1007/s11307-013-0616-0. [DOI] [PubMed] [Google Scholar]
- 19.Clark MJ, Homer N, O'connor BD, et al. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010;6(1):e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii N, Maier D, Merlo A, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9(3):469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatvin MB, Kreutz W, Horwitz BA, Shinitzky M. pH-sensitive liposomes: possible clinical implications. Science (NY) 1980;210(4475):1253–1255. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Nieves A, Fernandez-Barbero A, De Las Nieves FJ. Particle-counterion clustering in highly charge-asymmetric complex fluids. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2001;63(4 Pt 1):041404. doi: 10.1103/PhysRevE.63.041404. [DOI] [PubMed] [Google Scholar]
- 23.Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67(3):617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- 24.Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin. Drug Deliv. 2009;6(10):1017–1032. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhujbal SV, De Vos P, Niclou SP. Drug and cell encapsulation: alternative delivery options for the treatment of malignant brain tumors. Adv. Drug Deliv. Rev. 2014;67–68:142–153. doi: 10.1016/j.addr.2014.01.010. [DOI] [PubMed] [Google Scholar]; •• Reviews the challenges associated with the treatment of brain tumors and the different encapsulation options available for drugs and living cells.
- 26.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochemistry. 2013;2013:18. doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kievit FM, Zhang M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 2011;23(36):H217–H247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deeken JF, Slack R, Weiss GJ, et al. A Phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2013;71(3):627–633. doi: 10.1007/s00280-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oude Blenke E, Mastrobattista E, Schiffelers RM. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013;10(10):1399–1410. doi: 10.1517/17425247.2013.805742. [DOI] [PubMed] [Google Scholar]
- 30.Alkins RD, Brodersen PM, Sodhi RN, Hynynen K. Enhancing drug delivery for boron neutron capture therapy of brain tumors with focused ultrasound. Neuro Oncol. 2013;15(9):1225–1235. doi: 10.1093/neuonc/not052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanzawa T, Germano IM, Kondo Y, Ito H, Kyo S, Kondo S. Inhibition of telomerase activity in malignant glioma cells correlates with their sensitivity to temozolomide. Br. J. Cancer. 2003;89(5):922–929. doi: 10.1038/sj.bjc.6601193. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Establishes activity of temozolomide and quantifies reduction in tumor size based on telomerase activity.
- 32.Qi Q, Liu X, Li S, Joshi HC, Ye K. Synergistic suppression of noscapine and conventional chemotherapeutics on human glioblastoma cell growth. Acta Pharmacol. Sin. 2013;34(7):930–938. doi: 10.1038/aps.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Yang XJ, Zhang WG, Ji YW, Pan Q. Mechanism of thalidomide to enhance cytotoxicity of temozolomide in U251-MG glioma cells in vitro . Chin. Med. J. (Engl.) 2009;122(11):1260–1266. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.