Abstract
The cochlear blood-labyrinth barrier (BLB), located in the stria vascularis, is critical for the homeostasis of cochlear solutes and ion transport. Significant disruption to the BLB occurs early during noise-induced hearing loss. Matrix metalloproteinase (MMP)-2 and −9 are important molecules known to be capable of degrading tight junction (TJ) proteins. The TJ proteins are important components of the extracellular matrix (ECM), required to maintain BLB integrity and permeability. Previous studies have demonstrated that MMP-2 and −9, rich in healthy cochlea, serve an essential role in regulating the cochlear response to acoustic trauma. The present study investigated the localization and function of MMP-2 and −9 in the BLB by determining their associated gene expression and activity under normal conditions and after noise exposure. Analysis of gene expression by RNA-sequencing (RNA-seq) revealed expression of 15 MMP-associated genes, including genes for MMP-2 and −9, in healthy stria vascularis. Expression of these MMP genes was dynamically regulated by noise trauma to the cochlea, and accompanied by alterations in tissue inhibitors of metalloproteinases (TIMPs) and the TJ protein zona-occludens 1 (ZO-1). These alterations suggested that MMP-2 and −9 serve an important role in maintaining the integrity of BLB and in response to acoustic trauma. MMP-2, MMP-9 and ZO-1 protein expression levels in the stria vascularis by immunofluorescence, and observed that the stable expression of MMP-2 and −9 in healthy stria was markedly increased following noise exposure, consistent with the RNA-seq results. The compact structure of ZO-1 in the BLB loosened, and strial capillaries exhibited markedly increased leakage of Evans blue dye following acoustic trauma. These data indicated that mediation of MMP-2 and −9 in structural damage to TJ proteins, including ZO-1, may be an important mechanism in the breakdown of the BLB following acoustic trauma. Additionally, these results indicated that MMPs are involved in regulating the integrity and permeability of the BLB, which may provide a theoretical basis for the prevention of noise-induced hearing loss.
Keywords: matrix metalloproteinase, blood-labyrinth-barrier, noise-induced hearing loss, RNA-sequencing, zona-occludens 1
Introduction
The cochlear blood-labyrinth-barrier (BLB), located in the stria vascularis, is analogous to the blood-brain-barrier (BBB) and serves a critical role in maintaining homeostasis of cochlear solutes and ion transport (1,2). Tracer studies of uptake of sodium, calcium and albumin from blood into the perilymph have indicated how difficult it is for these substances to penetrate the BLB into the inner ear (3). The low permeability of the BLB limits the entry of inflammatory and infectious agents into the central nervous system (2).
It is well known that tight junction (TJ) proteins between adjacent microvascular endothelial cells in brain are the primary contributor to low paracellular permeability and high electrical resistance of the BBB (4). Downregulation of TJ proteins disrupts the BBB (5). Similarly, strial capillaries are also enriched in TJ and cell adhesion proteins, which suggests they serve a role in the impermeability of the BLB (6). Significant disruption of the BLB occurs early in noise-induced hearing loss, and a loosening of TJs and significantly increased BLB permeability are also observed (7–9).
TJs are transmembrane proteins are linked to the actin cytoskeleton through cytoplasmic accessory proteins, including zonula occludens (ZO) (10). ZO proteins, particularly ZO-1, are ubiquitous as scaffolds which provide the foundation for assembly of multi-protein complexes on the cytoplasmic surface of the plasma membrane. ZO proteins are actively involved in the remodeling of junctional complexes in a number of cellular systems (11). As ZO-1 often serves as a crucial central regulator of structural organization, it is used as an observation index of blood-tissue-barrier function (5,10).
A previous study demonstrated that matrix metalloproteinase (MMP)-2 and −9, secreted by leukemic cells, affect ZO-1 and disrupt the integrity of the BBB (5). MMP-2 and −9 are known to degrade collagen IV, the major component of extracellular matrix (ECM) (12). Previous studies have also revealed MMP-2 and −9 to be extensively expressed in the basal membrane, spiral ganglion and stria vascularis. Expression of MMP-2 and −9 dynamically alters following noise exposure (13,14). However, the involvement of MMP-2 and −9 in noise-induced impairment of the BLB remains to be fully demonstrated. Based on the results of previous studies, the present study hypothesized that degradation of the TJ protein ZO-1 by MMP-2 and −9 is an important mechanism in the noise-induced breakdown of the BLB. This hypothesis was tested in noise-exposed guinea pigs and subsequently assessed by RNA-sequencing (RNA-seq) and immunofluorescence.
Materials and methods
Animals
40 Adult guinea pigs (weight, 250–400 g; 20 male and 20 female) were purchased from Chinese PLA General Hospital Laboratory Animal Center (Beijing, China) and normal tympanic membrane, and Preyer's reflex were used in our experiments. All animals were maintained in the same conditions, constant temperature of 21–24°C and humidity of 40–70%, 12-h light/12-h dark cycle, with free access to food and water. The experiment protocol on animal use and care was reviewed and approved by the Institutional Animal Care and Use Committee of the Chinese People's Liberation Army General Hospital (Beijing, China).
Noise exposure
After evaluating baseline hearing, the guinea pigs were randomly assigned to noise-exposure or control groups (n=20 per group). Animals in the noise-exposure group were placed in a wire mesh cage and exposed to white noise at 120 dB SPL for 4 h on 2 consecutive days. This noise exposure regime is routinely used in this laboratory and produces a permanent loss in cochlear sensitivity.
Auditory brainstem response (ABR)
ABRs to pure tone bursts were used to evaluate hearing function prior to and following noise exposure. Each animal was anesthetized with an intraperitoneal injection of 10% chloral hydrate (4 ml/kg, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and placed in a sound-isolated chamber. The body temperature was maintained at 37.5°C with a warming blanket. Stainless-steel needle electrodes were placed subdermally at the vertex (noninverting input) and behind the stimulated and non-stimulated ear (inverting input and ground, respectively). Each ear was stimulated separately with a closed tube sound delivery system sealed into the ear canal. ABRs were induced with clicks at 90 dB SPL and the stimulus level was decreased in 10 dB steps until no response was identifiable. The signal was band-pass filtered (100~3,000 Hz), amplified (x50,000), and averaged using Tucker Davis Technologies (TDT) System II hardware and SigGen/BioSig version 4.4.1 (TDT, RX6, Alachua, FL, USA) software. Responses were stored and displayed on a computer. The ABR threshold was defined as the lowest stimulus intensity which reliably induces a detectable response.
Collection of the stria vascularis tissue
Animals were anesthetized with 10% chloral hydrate and decapitated. The cochlea was quickly removed from the skull as previously described (15). For analysis of the transcriptional expression pattern of the MMPs and associated genes, the cochlea was perfused with an RNA stabilization reagent (RNAlater; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and dissected in the same reagent. Each turn of the bony cochlear lateral wall, including the spiral ligament and stria vascularis, was separated from the organ of Corti, and the stria vascularis was gently peeled away from the spiral ligament. The strial tissue was immediately frozen in dry ice for 10 min before being stored at −80°C. Dissection of the strial vasculature from both cochleae was completed within 30 min to ensure the quality of the subsequent RNA analysis. For immunohistological and histological examinations, the cochlea was fixed in 4% paraformaldehyde overnight. The cochlea was then dissected in PBS to harvest the stria vascularis.
RNA-seq
The stria vascularis from 8 cochleae (4 animals) was pooled to generate one sample. A total of three biological repeats were performed. Total RNA was extracted from the tissue using an Agilent RNA 6000 Pico kit (Agilent Technologies, Inc., Santa Clara, CA, USA) as per the manufacturer's protocol. Total RNA of the collected sample was assessed with an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) and a RiboMinus Eukaryote kit (Ambion; Thermo Fisher Scientific, Inc.).
Synthesis of cDNA from 8–10 ng of total RNA per sample was performed with an Agilent High Sensitivity DNA kit (Agilent Technologies, Inc.). A sequencing library was prepared for each cDNA sample with an Ion PI™ Template OT2 200 Kit v2 (Ambion; Thermo Fisher Scientific, Inc.) used according to the manufacturer's protocol. The average insert size of the libraries was 124 bp. Each cDNA library was sequenced in a 50-cycle single read flow cell lane on an Illumina HiSeq 2000 system.
Immunofluorescence confocal microscopy
Primary antibodies used in the experiment included monoclonal mouse anti-MMP-2 (catalog no. MAB3308; EMD Millipore, Billerica, MA, USA), polyclonal rabbit anti-MMP-9 (catalog no. AB19016; EMD Millipore) and polyclonal rabbit anti-ZO-1 (catalog no. 617300; Invitrogen; Thermo Fisher Scientific, Inc.). Secondary antibodies included Alexa Fluor 488-conjugated goat anti-mouse IgG (catalog no. A11001) and Alexa Fluor 568-conjugated donkey anti-rabbit IgG antibodies (catalog no. A10042), both purchased from Invitrogen; Thermo Fisher Scientific, Inc. Stria vascularis samples were fixed in 4% paraformaldehyde at 4°C for 2 h, washed in PBS for 30 min, permeabilized in 0.5% Triton X-100 for 1 h, and immunoblocked in a solution of 5% goat serum (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in PBS for 1 h. The specimens were incubated overnight at 4°C with the primary antibody diluted (1:200) in PBS. After several washes in PBS, tissues were incubated with secondary antibodies (1:200) at room temperature for 1 h. The fluorescence was visualized under an Olympus IX81 inverted microscope fitted with an Olympus Fluoview FV1000 confocal laser system. The samples were examined as above, and Z-series stacks were acquired at 1-um intervals. The Z-series images were visualized using Image J 1.30 software (National Institutes of Health, Bethesda, MD, USA).
Evaluation of BLB permeability
BLB integrity was assessed by evaluating the extravasation and diffusion of a non-permeable dye (Evan's blue; EBD) around strial capillaries. Under deep anesthesia with 10% chloral hydrate as aforementioned, 2% EBD (20 mg/ml/kg; E2129; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was injected into the femoral vein 2 h prior to sacrifice. The cochlea was perfused with 4% paraformaldehyde in 0.1 M PBS in the vicinity of the round and oval windows, and the stria vascularis gently dissected from the bony cochlear lateral wall and fixed overnight in 4% paraformaldehyde at 4°C. The degree of EBD extravasation in the stria vascularis was assessed by reading the fluorescence under an Olympus IX81 inverted microscope fitted with an Olympus Fluoview FV1000 confocal laser system. Image processing and fluorescence analysis of the images were performed using Image J 1.30 software.
Statistical analysis
Data are expressed as the mean ± standard deviation. Comparisons between two groups were analyzed by Student's t-test and one-way analysis of variance with Turkey honest significant difference post hoc test was used for multiple comparisons on SPSS version 17 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Noise exposure causes loss in cochlear sensitivity
Exposure to white noise at 120 dB SPL for 4 h on 2 consecutive days caused significant loss in hearing sensitivity in the experimental animals. A total of 2 days after noise exposure, ABR thresholds at all test frequencies were significantly elevated (P<0.05; Fig. 1A), a result consistent with previous studies (9,16).
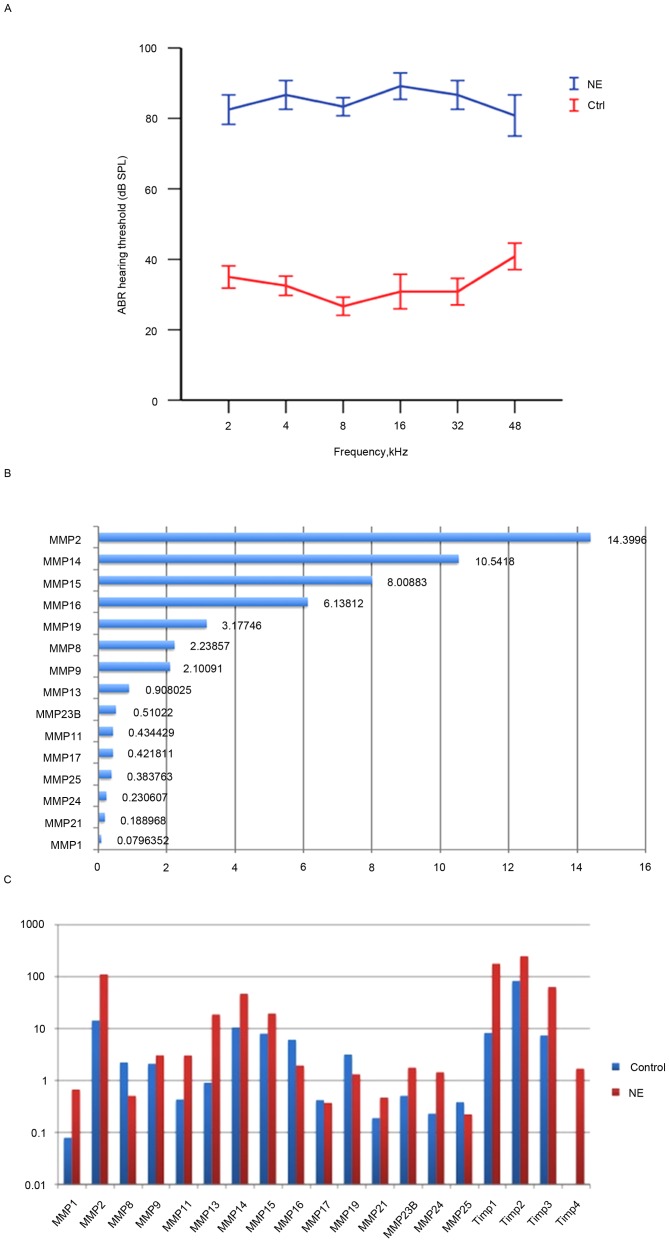
Figure 1.
NE affects auditory function and elevates expression of MMP-associated genes in the stria vascularis. (A) ABR thresholds to tone bursts in the control and NE groups. (B) 15 MMP genes were constitutively expressed in healthy stria vascularis. (C) Noise exposure caused dynamic; however, not significant alterations in the expression level of MMP-associated genes in the stria vascularis. The vertical axis is scaled logarithmically. NE, noise exposure; ABR, auditory brainstem response; MMP, matrix metalloproteinase.
Multiple MMPs and associated genes are constitutively expressed in healthy guinea pig strial tissue
The healthy cochlea has been demonstrated to be enriched in MMP enzymatic activity (13). However, a comprehensive understanding of the distribution and expression patterns of MMPs and their associated genes in the stria vascularis has remained elusive. Therefore, the transcriptional expression of these genes was profiled in the healthy stria vascularis.
RNA-seq was used to screen mRNA transcripts in the cochlear stria vascularis. A total of three biological repeats were performed, and transcript levels were quantified in Reads Per Kilobase of exon model per Million mapped reads (RPKM). An RPKM value >0.1 was considered the threshold. The expression of 15 MMP genes and 3 tissue inhibitor of matrix metalloproteinase (TIMP) genes in the stria vascularis were assessed. Among the 15 MMP genes detected were 2 gelatinases (MMP-2 and −9), 3 collagenases (MMP-1, −8 and −13), 2 stromelysins (MMP-11 and −19), 6 membrane-type MMPs (MMP-14, −15, −16, −17, −24 and −25), and 2 other MMPs (MMP-21 and −23B), data not shown.
To further assess the expression pattern, RNA-seq was performed to assess the transcriptional expression levels of the 15 MMP and 3 TIMP genes. A total of 7 high expression genes were identified, including Timp2, Mmp2, Mmp14, Timp1, Mmp15, Timp3, Mmp16, Mmp19, Mmp8 and Mmp9. Interestingly, Timp-2 was also highly expressed in healthy stria vascularis, consistent with Hu's data (13). In contrast, the expression levels of Mmp13, Mmp23B, Mmp11, Mmp17, Mmp25, Mmp24, Mmp21 and Mmp1 were low (Fig. 1B).
Noise trauma causes dynamic alterations in the expression level of MMP-associated genes in the stria vascularis
To determine alterations in the expression of MMPs and their associated genes following acoustic trauma, qRT-qPCR was performed immediately after noise exposure to assess MMP expression. The results revealed that 10 MMP genes were upregulated (Mmp1, Mmp2, Mmp9, Mmp11, Mmp13, Mmp14, Mmp15, Mmp21, Mmp23B, Mmp24), while 5 MMP genes were downregulated (Mmp8, Mmp16, Mmp17, Mmp19, Mmp25). Notably, expression levels of the two gelatinases (Mmp2 and −9) were both elevated, while the other MMP genes demonstrated a mixed pattern. In addition, the 3 MMP inhibitors (Timp1, Timp2 and Timp3) were upregulated. No genes appeared to remain unaltered, which highlighted the marked effect that noise trauma has on MMP-associated genes (Fig. 1C).
Steady MMP-2 and MMP-9 immunolabeling in the healthy stria vascularis, and marked alterations in immunoreactivity following noise trauma
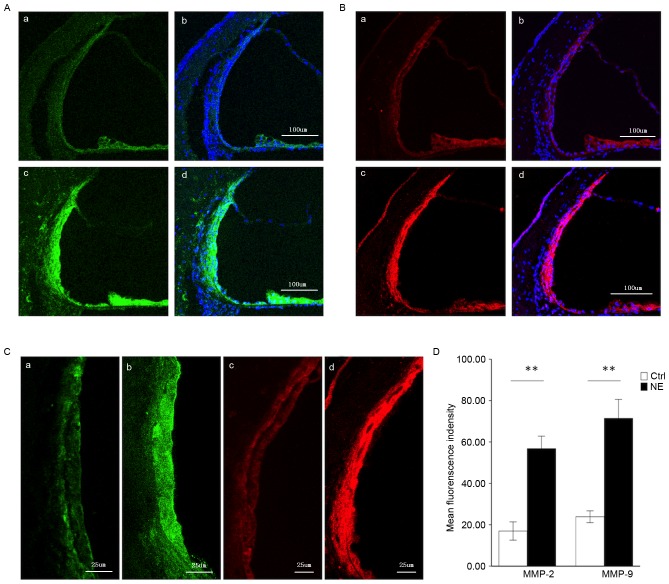
MMP-2 and −9 genes were demonstrated in the healthy stria vascularis by RNA-seq. An immunolabeling assay was employed to assess the distribution of MMP-2 (Fig. 2A) and −9 (Fig. 2B) in the stria vascularis. As both MMP-2 and −9 have been demonstrated to degrade the ECM, the present experiment focused on these two MMPs. In the healthy stria vascularis, weak MMP-2 and −9 immunoreactivity was present in marginal cells and basal cells, with no reactivity in intermediate cells, consistent with RNA-seq results. In noise-traumatized cochleae, there was a significant increase in MMP-2 and −9 immunoreactivity in the stria vascularis (Fig. 2A and B, respectively). Labeling was intense not only in marginal cells and basal cells, but significant immunoreactivity was also seen in intermediate cells (Fig. 2C).
Figure 2.
Immunofluorescence assessment of MMP-2 and MMP-9 expression in the stria vascularis prior to and following NE. (A) Tissue double-labeled for MMP-2 (green) and DAPI (blue) prior to (a, b) and following (c, d) noise exposure. Scale bar, 100 µm. (B) Tissue double-labeled for MMP-9 (red) and DAPI (blue) prior to (a, b) and following (c, d) noise exposure. Scale bar, 100 µm. (C) Weak MMP-2 (green) and −9 (red) immunoreactivity was observed in marginal cells and basal cells in the stria vascularis of controls (a, c), which significantly increased following noise-trauma (b, d). Scale bar, 25 µm. (D) Quantification of localized fluorescence density of MMP-2 (green) and −9 (red) in the stria vascularis. Data are expressed as the mean ± standard deviation. **P<0.01. MMP, matrix metalloproteinase; NE, noise exposure; Ctrl, control.
Fig. 2D compares MMP-2 and −9 immunoreactivity in the stria vascularis prior to and following noise exposure (images were visualized using Image J software).
Noise trauma causes alterations in the expression of the TJ protein, ZO-1
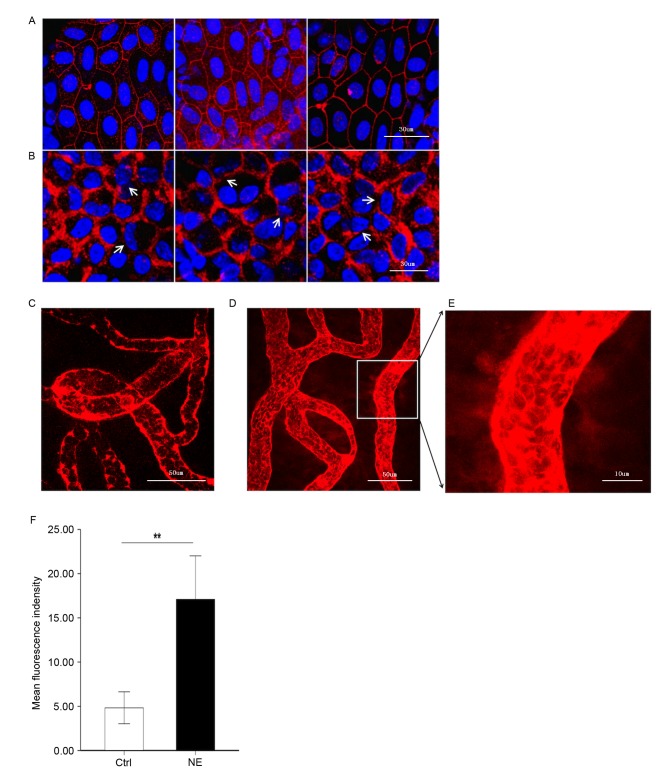
It was hypothesized that upregulation of MMP-2 and −9 following noise trauma would induce increased permeability of the BLB by disrupting the TJ protein ZO-1. To test this hypothesis, ZO-1 in the stria vascularis was immunolabeled. Confocal imaging demonstrated that ZO-1 formed a compact linear structure on the plasma membrane in areas of cell-cell contact between marginal cells in healthy cochlear stria vascularis (Fig. 3A). Following noise trauma, the ZO-1 structure became loose and intermittent regional breaks were seen (Fig. 3B).
Figure 3.
Noise trauma upregulates expression of ZO-1 (red) and causes alterations in BLB permeability, nuclei are labeled by DAPI (blue). Magnification, ×60. (A) Representative confocal microscope images demonstrating the compact linear structure of ZO-1 (red) on the plasma membrane in the stria vascularis. Scale bar, 30 µm. (B) Noise exposure caused the structure of ZO-1 (red) to loosen, and regional breaks were observed (white arrows). Scale bar, 30 µm. BLB integrity was assessed by noting the degree of EBD extravasated around strial capillaries (C) prior to and (D) following noise exposure. Scale bar, 50 µm. (E) EBD outside capillaries was observed further away from the vessel with noise exposure, demonstrative of the significantly increased permeability following loud noise exposure. Scale bar, 10 µm. (F) Mean fluorescence density outside capillaries in the stria vascularis, as assessed with Image J software. Data are expressed as the mean ± standard deviation. **P<0.01. EBD, Evans blue dye; ZO-1, zona-occludens 1; Ctrl, control; NE, noise exposure; Ctrl, control.
BLB permeability alters following noise trauma
EBD (961 Da) is widely used as a tracer to study blood-tissue-barrier permeability. Once injected into the circulation, EBD binds strongly to serum albumin (69,000 Da) and becomes a high molecular weight protein tracer. Unlike sodium fluorescein, EBD becomes fully albumin-bound within 5 min of post-intravenous bolus injection (17,18). Consistent with previous reports on the BBB, EBD quickly colored the eyes, nose and paws of the guinea pigs a deep blue, and the coloration persisted for the 2-h duration of the experiment. The BLB is well known to restrict extravasation of inert tracers (3). Accordingly, in the control group, the red-fluorescence of albumin-bound EBD was mostly limited to the capillary lumen in the stria vascularis, which was expected as the BLB is non-permeable (Fig. 3C). Animals in the noise exposure group displayed markedly increased EBD extravasation in the stria vascularis (Fig. 3D-F). EBD concentration outside the capillaries exhibited marked alterations in distance from the vessel, demonstrative of significantly increased permeability following loud noise.
Discussion
The blood-labyrinth barrier (BLB) in the stria vascularis comprises a dense capillary network of endothelial cells, pericytes, basement membranes and perivascular resident macrophages (16,19,20). The capillary network in the stria vascularis is a sandwich of epithelial marginal cells and mesodermal basal cells interconnected by TJs. The network functions as a barrier, selectively excluding most blood-borne substances from entering the ear and protecting it from systemic influences (6,21). Consistent with the BLB's impermeability, a previous study has demonstrated that the stria vascularis is abundant with TJ proteins (6). Noise trauma significantly disrupts blood flow and diminishes the integrity of the BLB within a quick time frame (19,22). Under a light microscope, acute swelling of the stria vascularis is seen within 24 h of noise exposure, and electron microscopy has demonstrated the acute strial swelling is largely due to an increase in extracellular space between marginal and intermediate cells (8).
In previous studies of the central nervous system, disruption of TJ proteins, including occludin and ZO-1, and dissociation of ZO-1 from the junctional complex, have been associated with increased BBB permeability (5). Increased matrix metalloproteinase (MMP) activity, especially MMP-2 and −9, is a consequence of degradation of the TJ protein ZO-1, and occludin may also serve an important role in the BBB breakdown (5,23). As MMPs can degrade the ECM, MMPs are involved in regulation of tissue remodeling, embryonic development, modulation of inflammation, tumor invasion and metastasis, and wound healing (24–27). Healthy cochlea has been demonstrated to be rich in MMP enzymatic activity, and MMPs and their associated gene products were identified in the modulation of cochlear sensory epithelium response to acoustic trauma in rats (13,14). The expression profile of MMPs and their associated genes in healthy cochlear stria vascularis, as well as the role the MMPs play in BLB pathogenesis following acoustic trauma, remains largely unknown. The present study provided novel evidence that MMP-2 and −9 are involved in noise-induced disruption of the BLB by downregulating the TJ protein ZO-1.
Numerous members of the MMP family have been identified in the cochlear sensory epithelium (13). The present study used RNA-seq and RT-qPCR assays to profile the expression pattern of MMP-associated genes. A set of MMP genes were identified to be constitutively active and expressed in the stria vascularis. The gene types identified were slightly different from the previously known gene types in the cochlear sensory epithelium (13).
Based on their substrate specificity, MMPs can be subdivided into six groups: Interstitial collagenases (MMP-1, −8, −13 and −18); type IV collagenases or gelatinases (MMP-2 and −9); stromelysins (MMP-3, −10, −11 and −19); matrilysins (MMP-7 and −26); membrane-type MMPs (MMP-14, −15, −16, −17, −24 and −25); and 6) other MMPs (MMP-12, −20, −21, −22, −23, −27 and −28) (28,29). Among the 15 MMP genes detected in healthy stria vascularis, there are 2 gelatinases (MMP-2 and −9), 3 collagenases (MMP-1, −8 and −13), 2 stromelysins (MMP-11 and −19), 6 membrane-type MMPs (MMP-14, −15, −16, −17, −24 and −25), and 2 other type MMPs (MMP-21 and −23B). The abundance of MMP genes indicates the important role MMPs serve in maintaining the integrity of the BLB.
The TIMP family is composed of four members. A total of three TIMP genes (Timp1, 2 and 3) are detected in healthy stria vascularis. Although TIMPs do not exhibit high specificity for any particular MMP, TIMP-2 does preferentially bind with MMP-2, and TIMP-1 with MMP-9. In addition, TIMP-2, −3 and −4, but not TIMP-1, are effective inhibitors of membrane-type MMPs (30). In the present study, Timp2 was most highly expressed in normal stria vascularis, consistent with Hu's data on the sensory epithelium (13). Therefore, TIMP-2 may be the primary endogenous inhibitor of MMPs in the cochlea.
Consistent with the RNA-seq data, weak immunoreactivity for MMP-2 and −9 in marginal cells and basal cells was observed in healthy stria vascularis, and no immunoreactivity in intermediate cells. The distribution of MMP-2 and −9 in healthy stria vascularis conforms with the ultrastructural morphology of the three cell types in the BLB. Marginal cells comprise the epithelial lining of the endolymphatic duct and form a TJ barrier between endolymph and the intra-strial compartment, while basal cell plasma membranes line the lateral surface of the stria and form a TJ barrier between the intrastrial space and spiral ligament (8). The present study revealed that noise trauma causes a marked increase in immunoreactivity for MMP-2 and −9 not only in marginal and basal cells, but also in intermediate cells.
Previous studies have observed that noise exposure leads to disruption of the BLB and increased permeability of the stria vascularis (7,8,19,22). Transmission electron microscope images of the stria vascularis in noise-exposed animals reveals a reduced number of TJ contact points (6). The results of the present study are consistent with previous studies; albumin-bound EBD served as a tracer to indicate a marked increase in the permeability of the BLB, with destruction of TJ protein ZO-1 structures. As ECM components, including ZO-1, are major targets of MMP-2 and −9, it was hypothesized that MMP-2 and −9-mediated structural damage to ZO-1 is a potential underlying mechanism for noise-induced disruption of the BLB, leading to aberrations in cochlear ion transport and sensorineural hearing loss. Notably, analysis of immunolabeled ZO-1 did not reveal obvious downregulation in the stria vascularis following noise exposure, in contrast to a previous observation that other TJ proteins, including claudin-5 and occludin, were downregulated in the BLB (5,9). Therefore, there may be a variety of regulatory mechanisms involved in noise-induced disruption of the BLB.
In conclusion, the present study used RNA-seq and immunofluorescence analysis to demonstrated stable expression of MMP-2 and −9 in healthy stria vascularis, and marked upregulation of MMP-2 and −9 with noise trauma. The acoustic trauma caused the compact structure of ZO-1 in the BLB to loosen and substantially leak EBD out of the capillaries of the stria vascularis. These data implicated MMP-2 and −9 in the structural damage to TJ proteins, including ZO-1, with structural damage to TJ proteins contributing to the breakdown of the BLB with acoustic trauma. More generally, the present study demonstrated the involvement of MMPs in the regulation of BLB integrity and permeability, which may provide a theoretical basis for the prevention of noise-induced hearing loss.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 81170908 and 81470683). The authors would like to thank Dr Yongbing Shi for editing the language of this paper.
References
- 1.Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J. 2001;7:72–83. [PubMed] [Google Scholar]
- 2.Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014;15:707–719. doi: 10.1007/s10162-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juhn SK, Rybak LP, Prado S. Nature of blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol Laryngol. 1981;90:135–141. doi: 10.1177/000348948109000208. [DOI] [PubMed] [Google Scholar]
- 4.Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, Adamson P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/S0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 5.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and −9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS One. 2011;6:e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M, Yang Y, Omelchenko I, Nuttall AL, Kachelmeier A, Xiu R, Shi X. Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier. Am J Pathol. 2010;177:3089–3099. doi: 10.2353/ajpath.2010.100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YX, Zhu GX, Liu XQ, Sun F, Zhou K, Wang S, Wang CM, Jia JW, Song JT, Lu LJ. Noise alters guinea pig's blood-labyrinth barrier ultrastructure and permeability along with a decrease of cochlear Claudin-5 and Occludin. BMC Neurosci. 2014;15:136. doi: 10.1186/s12868-014-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 11.Hervé JC, Derangeon M, Sarrouilhe D, Bourmeyster N. Influence of the scaffolding protein Zonula Occludens (ZOs) on membrane channels. Biochim Biophys Acta. 2014;1838:595–604. doi: 10.1016/j.bbamem.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Hu BH, Cai Q, Hu Z, Patel M, Bard J, Jamison J, Coling D. Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium. J Neurosci. 2012;32:14927–14941. doi: 10.1523/JNEUROSCI.1588-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setz C, Brand Y, Radojevic V, Hanusek C, Mullen PJ, Levano S, Listyo A, Bodmer D. Matrix metalloproteinases 2 and 9 in the cochlea: Expression and activity after aminoglycoside exposition. Neuroscience. 2011;181:28–39. doi: 10.1016/j.neuroscience.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Neng L, Zhang W, Hassan A, Zemla M, Kachelmeier A, Fridberger A, Auer M, Shi X. Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat Protoc. 2013;8:709–720. doi: 10.1038/nprot.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi S, Ando M, Sato T, Kakigi A. Three-dimensional and ultrastructural relationships between intermediate cells and capillaries in the gerbil stria vascularis. Hear Res. 2001;155:103–112. doi: 10.1016/S0378-5955(01)00252-0. [DOI] [PubMed] [Google Scholar]
- 17.Wolman M, Klatzo I, Chui E, Wilmes F, Nishimoto K, Fujiwara K, Spatz M. Evaluation of the dye-protein tracers in pathophysiology of the blood-brain barrier. Acta Neuropathol. 1981;54:55–61. doi: 10.1007/BF00691332. [DOI] [PubMed] [Google Scholar]
- 18.Yen LF, Wei VC, Kuo EY, Lai TW. Distinct patterns of cerebral extravasation by evans blue and sodium fluorescein in rats. PLoS One. 2013;8:e68595. doi: 10.1371/journal.pone.0068595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol. 2009;174:1692–1704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- 21.Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 2010;459:521–533. doi: 10.1007/s00424-009-0754-z. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K. Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear Res. 2002;164:12–18. doi: 10.1016/S0378-5955(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 24.Szabova L, Son MY, Shi J, Sramko M, Yamada SS, Swaim WD, Zerfas P, Kahan S, Holmbeck K. Membrane-type MMPs are indispensable for placental labyrinth formation and development. Blood. 2010;116:5752–5761. doi: 10.1182/blood-2009-10-249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 28.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Semin Cancer Biol. 2002;12:131–138. doi: 10.1006/scbi.2001.0421. [DOI] [PubMed] [Google Scholar]
- 30.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J. 2012;40:766–782. doi: 10.1183/09031936.00209911. [DOI] [PubMed] [Google Scholar]