Abstract
The molecular mechanisms underlying protection and pathogenesis in spinal degenerative diseases remain unclear. Tumor necrosis factor-α (TNF-α) has been demonstrated to induce apoptosis of intervertebral disc (IVD) cells during IVD degeneration, and 17β-estradiol (17β-E2) has a protective effect against IVD cell apoptosis. However, the underlying molecular mechanism by which 17β-E2 protects nucleus pulposus (NP) cells remains to be investigated. The aim of the present study was to evaluate whether 17β-E2 modulates apoptosis of human NP cells induced by TNF-α. In addition, the concentration-response effect of 17β-E2 on human NP cells was investigated. Human NP cells were cultured in complete medium, which was replaced every three days until the culture was ~80% confluent. Cells were treated with 100 ng/ml TNF-α for 48 h, with or without pretreatment with various concentrations of 17β-E2, and ICI 182,780, for 30 min. Morphologic alterations characteristic of apoptosis were observed by inverted phase-contrast microscopy and Hoechst 33258 staining; the apoptosis rate was analyzed by flow cytometry. A Cell Counting kit-8 assay was used to assess cell proliferation. Furthermore, caspase-3 activity was determined and proteins associated with apoptosis were analyzed by western blotting. The level of apoptosis and caspase-3 activity in human NP cells increased, whereas proliferation and the expression of poly ADP-ribose polymerase decreased following TNF-α treatment. These effects of TNF-α were abolished by pretreatment with 17β-E2 in a concentration-dependent manner. The results of the present study indicated that 17β-E2 serves a critical role in the survival of degenerative human NP cells.
Keywords: 17β-estradiol, tumor necrosis factor-α, nucleus pulposus, apoptosis
Introduction
The intervertebral disc (IVD) consists of three distinct sections: The outer annulus fibrosus (AF) which contains abundant type I collagens; the inner nucleus pulposus (NP) which is a gelatinous substance containing type II collagen and proteoglycans; and the cartilage end plates that connect the adjacent vertebral bodies (1–3). With age, morphological and molecular alterations in the IVD may induce progressive degeneration and pathologic alterations of this tissue (4). IVD degeneration may eventually result in conditions including disc herniation, facet joint osteoarthritis, spinal canal stenosis, segmental instability and spondylolisthesis. The mechanisms underlying IVD degeneration are considered to be associated with decreased cell viability and adhesion, reduced diffusion of nutrients and proteoglycan synthesis (5,6). Accelerated aging of the IVD due to abnormal apoptosis of IVD cells is a primary cytological process (7–9), and apoptosis of IVD cells serves a key role in the process of IVD degeneration.
Tumor necrosis factor (TNF)-α is an important member of the TNF superfamily, and is involved in immunity, cellular remodeling, apoptosis and cell survival (10). It acts primarily via TNF receptor-1 to induce apoptosis by activating caspases through mitochondria-dependent and -independent pathways (11). In addition to secretion by infiltrating immune cells, resident NP and AF cells produce high levels of TNF-α and interleukin (IL)-1β to promote inflammation (12–14). TNF-α has been confirmed to have a central role in degenerative osteoarthropathy (15). Additionally, it has been reported that TNF-α may induce apoptosis of NP cells in vitro (16). Furthermore, TNF-α released during the inflammatory process induced by the herniated IVD serves a fundamental role in the development of mechanical and thermal hyperalgesia (16–18). The inhibition of TNF-α-induced abnormal apoptosis of human NP cells may delay the degeneration of the IVD, preventing degenerative scoliosis and other spinal degenerative diseases.
17β-estradiol (17β-E2) has been extensively investigated due to its anti-apoptotic activity (19–22). Bozzo et al (23) reported that 17β-E2 protects nerve cells from β-amyloid peptide-induced apoptosis, and that 17β-E2 exerts anti-apoptotic effects by upregulating the expression of essential cell membrane components including α1β1 integrin, and affecting cell cycle progression. In addition, 17β-E2 has been reported to inhibit caspase-3/9 activity and increase B-cell lymphoma 2, cyclin D1 and survivin expression (24–26). Wang et al (6) and Yang et al (22) demonstrated that 17β-E2 protects rat IVD cells from apoptosis induced by IL-1β and levofloxacin. However, whether 17β-E2 inhibits TNF-α-induced apoptosis in human NP cells, and the concentration-response effect of 17β-E2 on TNF-α-induced human NP cell apoptosis, remains unclear.
Therefore, the aim of the present study was to investigate whether 17β-E2 modulates apoptosis induced by TNF-α in human NP cells, the concentration-response effect and whether 17β-E2 exerts protective effects via the caspase-3/poly (ADP-ribose) polymerase (PARP) pathway.
Materials and methods
Reagents
Human NP cells, NP Cell Growth Supplement and NP cell medium (NPCM) were purchased from ScienCell Research Laboratories, Inc. (Carlsbad, CA, USA), and Dulbecco's modified Eagle's medium (DMEM)/F-12 and fetal bovine serum (FBS) were obtained from HyClone; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Trypsin, Cell Counting kit-8 (CCK-8) and 17β-E2 were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). PBS (phosphate buffered saline) was obtained from Gibco; Thermo Fisher Scientific, Inc. The Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) kit was purchased from BD Biosciences (Franklin Lakes, NJ, USA), and the caspase-3 activity kit, Hoechst staining kit and cell lysis buffer for western and immunoprecipitation were obtained from Beyotime Institute of Biotechnology (Shanghai, China). Z-DEVD-FMK was purchased from MedChem Express (Monmouth Junction, NJ, USA), ICI 182,780 was obtained from Sigma-Aldrich, Merck KGaA; and TNF-α was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
Initiation of human NP cell culture
The purchased cryopreserved human NP cells were originally isolated from the NP of human IVD. NPCM (500 ml) was supplemented with 10 ml FBS, 5 ml NP Cell Growth Supplement and 5 ml penicillin/streptomycin solution (P/S) to make complete human NP cell medium prior to cell recovery. Subsequently, cells were thawed in their original vials in a 37°C water bath and placed in a 50-ml culture flask containing 15 ml complete HNPC as soon as possible and with minimal handling. Cells were cultured in a humidified atmosphere of 5% CO2 at 37°C. The culture medium was replaced with fresh the next day to remove residual dimethyl sulfoxide and non-adherent cells. The medium was replaced every three days thereafter. Once the culture reached 70% confluence, the medium was replaced every other day until the culture was ~90% confluent.
Subculture and drug treatment
When the culture reached ≥90% confluence, the cells were digested, plated into appropriate culture plates and cultured as described in the previous section. When the culture reached 80–90% confluence, cells were washed twice with PBS and the medium was replaced with DMEM/F-12 without phenol red, FBS and P/S. Cells were divided into 7 groups according to treatment, the conditions of which are listed in Table I. All groups were incubated for 48 h in serum-free medium without phenol red.
Table I.
Experimental groups.
| Group | Treatment |
|---|---|
| Control | Ethanol (<0.1%) |
| TNF | 100 ng/ml TNF for 48 h |
| TNF + 0.1 E2 | 100 ng/ml TNF for 48 h, with pretreatment of 0.1 µmol/l E2 for 30 min |
| TNF + 1 E2 | 100 ng/ml TNF for 48 h, with pretreatment of 1 µmol/l E2 for 30 min |
| TNF + 10 E2 | 100 ng/ml TNF for 48 h, with pretreatment of 10 µmol/l E2 for 30 min |
| TNF + 10 E2 + ICI | 100 ng/ml TNF for 48 h, with pretreatment of 10 µmol/l E2 and 10 µmol/l ICI for 30 min |
| TNF + FMK | 100 ng/ml TNF for 48 h, with pretreatment of 20 µmol/l FMK for 2 h |
TNF, tumor necrosis factor-α; E2, 17β-estradiol; ICI, ICI 182,780; FMK, Z-DEVD-FMK.
Morphological observations
Human NP cells were plated in 6-well plates at a density of 2×105 cells/well in NPCM. A total of 48 h after treatment, the apoptotic morphological alterations in the human NP cells were observed under an inverted phase-contrast microscope (Olympus Corporation, Tokyo, Japan) and imaged using a digital camera (Nikon Corporation, Tokyo, Japan). Cells were subsequently washed twice with PBS and stained with Hoechst 33258 staining solution according to the manufacturer's protocol. Stained nuclei were detected under a fluorescence microscope (Olympus Corporation) with an excitation wavelength of 350 nm and an emission wavelength of 460 nm.
Proliferation assay
The effects of 17β-E2 on cell viability and proliferation were evaluated using CCK-8. Human NP cells were seeded into 96-well plates at a density of 2×104 cells/well (100 µl/well). Following treatment for 48 h, 10 µl CCK-8 solution was added to each well of the plate, and the plates were incubated for an additional 3 h in a 5% CO2 incubator at 37°C. The proliferation of cells was measured using a microplate reader at a wavelength of 450 nm.
Caspase-3 activity assay
Caspase-3 activity was measured using a Caspase-3 Activity kit, which measures the conversion by caspase-3 of N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide into a yellow formazan product, p-nitroaniline. Human NP cells were cultured in 6-well plates as aforementioned, and the assay was performed according to the manufacturer's protocol. Briefly, the treated NP cells were washed twice with cold PBS and lysed with lysis buffer (100 µl/2×106 cells) for 15 min on ice. Following incubation of 10 µl cell lysates, 80 µl reaction buffer and 10 µl caspase-3 substrate (2 mM) in 96-well microtiter plates at 37°C for 2 h, caspase-3 activity was quantified at a wavelength of 405 nm using a microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). Caspase-3 activity is expressed as the fold change in enzyme activity over control.
Flow cytometric analysis of apoptosis
Human NP cells were seeded into 6-well plates at a density of 2×105 cells/well and cultured as aforementioned. Following 48 h treatment, adherent cells and cells in suspension were collected and washed twice with cold PBS. Apoptotic cells were detected by staining with annexin V-FITC and PI according to the manufacturer's protocol. Briefly, cells were resuspended in 100 µl 1X binding buffer at a concentration of 1×106 cells/ml, and 100 µl (1×105 cells) were transferred to 5 ml culture tubes. PI (5 µl) and 5 µl annexin V-FITC was added to each tube and incubated for 15 min at room temperature in the dark, followed by addition of 400 ml 1X binding buffer. Samples were analyzed on a flow cytometer (BD Biosciences) with CellQuest software (version 5.2, BD Biosciences).
Western blot analysis
Protein expression levels of PARP and caspase-3 were determined by western blot analysis. Cells were incubated in 100 µl lysis buffer containing 1% protease inhibitors. The cell lysate was collected by centrifugation at 4°C for 10 min at 16,000 × g. The protein concentrations were determined using a Bicinchoninic Acid Protein assay kit (Beyotime Institute of Biotechnology). Proteins (50 µg) from each sample were separated on 12% SDS-polyacrylamide gels according to molecular weight and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% non-fat milk powder in TBS for 1 h at room temperature and incubated with primary antibodies against caspase-3 p17 (#9664; rabbit; 1:1,000), PARP (#9532; rabbit; 1:1,000) (both from Cell Signaling Technology, Inc., Danvers, MA, USA), and GAPDH (10494–1-AP; rabbit; 1:5,000; Wuhan Sanying Biotechnology, Wuhan, China) diluted in 5% non-fat milk powder in PBS containing Tween 20 (PBST) at 4°C overnight. Membranes were washed 3 times for 15 min in PBST and incubated with a peroxidase labeled anti-rabbit IgG (H+L) antibody (074–1506; goat; 1:1,000; KPL, Inc., Gaithersburg, MD, USA) with agitation for 1 h at room temperature. Following washing 3 times for 15 min with PBST, protein bads were detected using Enhanced Chemiluminescence reagent (Amercontrol Biosciences, San Francisco, CA, USA) and quantified with Quantity One imaging software version 4.6.2 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Groups were compared by one-way analysis of variance followed by pairwise comparison using the Student-Newman-Keuls post hoc test. All statistical tests were two-sided. P<0.01 was considered to indicate a statistically significant difference.
Results
Morphology of apoptotic human NP cells
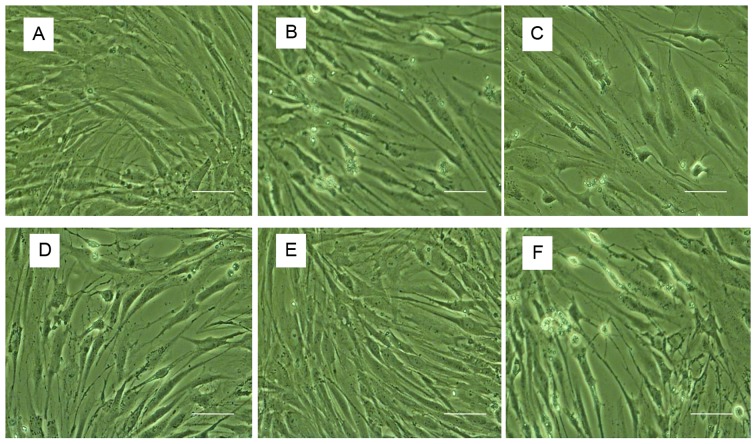
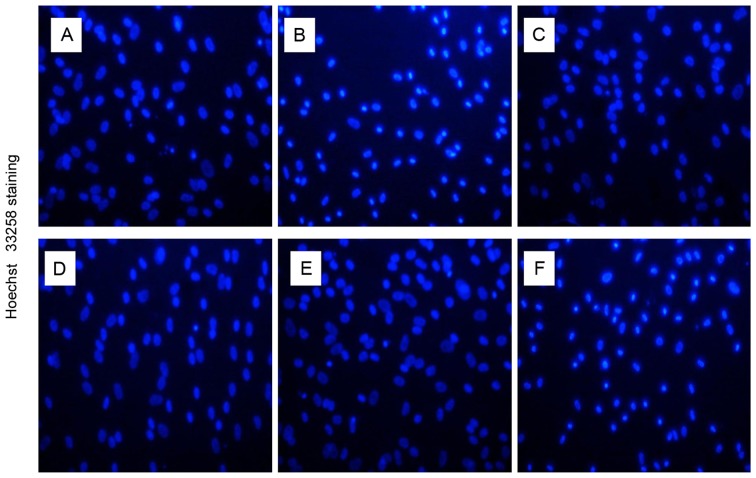
The morphological characteristics of human NP cells were observed using inverted phase-contrast microscopy (Fig. 1). Cell nuclei were stained using Hoechst 33258 and detected under a fluorescence microscope with an excitation wavelength of 350 nm and an emission wavelength of 460 nm (Fig. 2). Control untreated cells were well-adhered, exhibiting typical human NP cell morphology. By contrast, treatment with TNF-α induced apoptosis, as demonstrated by detachment from the plate, cell shrinkage, and nuclei that were compact, condensed and brightly stained with Hoechst 33258. Following pretreatment with various concentrations of 17β-E2, the number of apoptotic cells markedly decreased compared with those treated with TNF-α only. The effect of 17β-E2 was blocked by the estrogen receptor (ER) antagonist ICI 182,780.
Figure 1.
Morphological observations by phase-contrast microscopy. (A) Control untreated human NP cells. Human NP cells treated with (B) 100 ng/ml TNF-α, (C) 100 ng/ml TNF-α following pretreatment with 0.1 µmol/l 17β-E2, (D) 100 ng/ml TNF-α following pretreatment with 1 µmol/l 17β-E2, (E) 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 and (F) 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 and 10 µmol/l ICI 182,780. Apoptotic cells were characterized by shedding of smaller fragments from the cells, cell shrinkage and condensed nuclei. Original magnification, ×200; scale bar, 200 nm. NP, nucleus pulposus; TNF-α, tumor necrosis factor-α; 17β-E2, 17β-estradiol.
Figure 2.
Hoechst 33258 staining. (A) Control untreated human NP cells. Human NP cells treated with (B) 100 ng/ml TNF-α, (C) 100 ng/ml TNF-α following pretreatment with 0.1 µmol/l 17β-E2, (D) 100 ng/ml TNF-α following pretreatment with 1 µmol/l 17β-E2, (E) 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 and (F) 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 and 10 µmol/l ICI 182,780. The nuclei of living cells were a homogeneous blue, whereas apoptotic nuclei were compact, condensed and brightly stained with Hoechst 33258. Original magnification, ×200. NP, nucleus pulposus; TNF-α, tumor necrosis factor-α; 17β-E2, 17β-estradiol.
17β-E2 promotes proliferation of human NP cells damaged by TNF-α
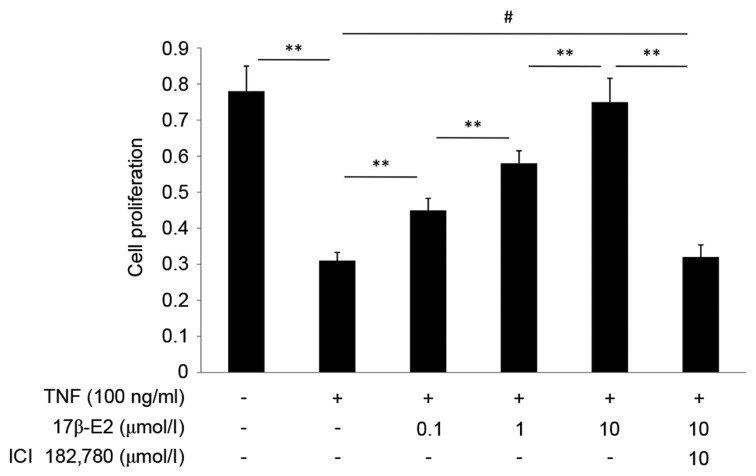
The effect of 17β-E2 on proliferation of human NP cells was detected by CCK-8 assay. As presented in Fig. 3, TNF-α inhibited the proliferation of human NP cells (P<0.001). This effect was abrogated when the cells were pretreated with 17β-E2 (P<0.001). The effect of 17β-E2 was blocked by ICI 182,780 (P<0.001). 17β-E2 inhibited the effect of TNF-α in a concentration-dependent manner.
Figure 3.
Proliferation of human NP cells, as assessed by CCK-8 assay. The CCK-8 assay reflects the activity of mitochondrial dehydrogenase. Human NP cells were untreated (control), treated with 100 ng/ml TNF-α, or treated with 100 ng/ml TNF-α following pretreatment with various concentrations of 17β-E2 with or without ICI 182,780. Data are expressed as the mean ± standard deviation from three independent experiments. **P<0.001; #P>0.05. NP, nucleus pulposus; CCK-8, Cell Counting kit-8; TNF-α, tumor necrosis factor-α; 17β-E2, 17β-estradiol.
17β-E2 reduces caspase-3 activity
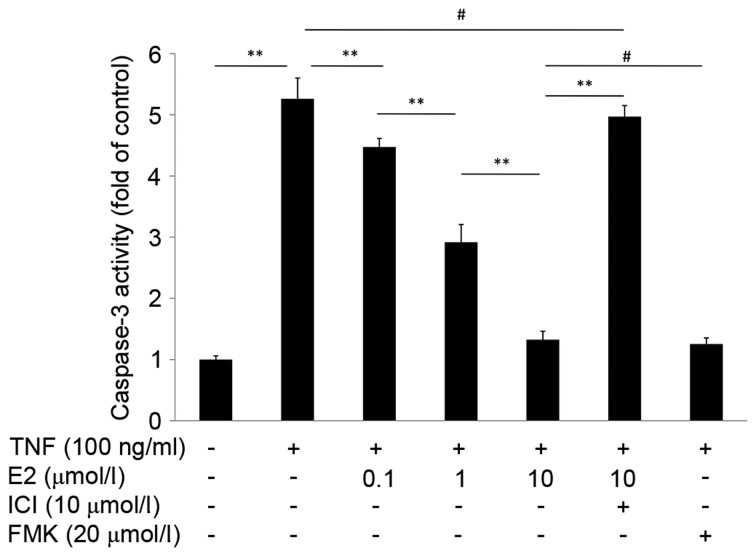
The effect of 17β-E2 on caspase-3 activity in human NP cells was measured using a caspase-3 activity assay. As presented in Fig. 4, the caspase-3 activity of cells treated with TNF-α alone was 5-fold greater compared with the control untreated group (P<0.001). However, cells pretreated with 17β-E2 demonstrated reduced caspase-3 activity compared with cells treated with TNF-α only; the effect of 17β-E2 was concentration-dependent. The effect of pretreatment with 10 µmol/l 17β-E2 for 30 min was similar to pretreatment with 20 µmol/l Z-DEVD-FMK (a specific, irreversible caspase-3 inhibitor) for 2 h (P>0.05). Addition of the ER antagonist ICI 182,780 abolished the protective effects of 17β-E2 (P<0.001).
Figure 4.
Caspase-3 activity in human NP cells was determined using a caspase-3 activity assay. Human NP cells were untreated (control), treated with 100 ng/ml TNF-α, or treated with 100 ng/ml TNF-α following pretreatment with various concentrations of E2 with or without ICI. An additional control group was pretreated with the capase-3 inhibitor FMK prior to treatment with TNF-α. Data are expressed as the mean ± standard deviation from three independent experiments. **P<0.001; #P>0.05. NP, nucleus pulposus; TNF-α, tumor necrosis factor-α; E2, 17β-estradiol; ICI, ICI 182,780; FMK, Z-DEVD-FMK.
17β-E2 reduces TNF-α-induced human NP cell apoptosis
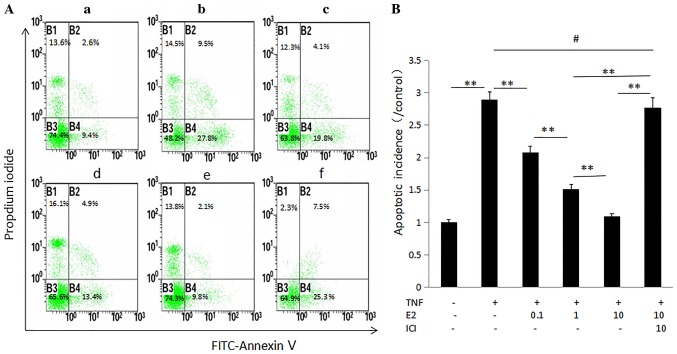
The effect of 17β-E2 on apoptosis of human NP cells was examined by flow cytometry following annexin V-FITC and PI double staining. The apoptotic rate is presented as apoptotic cells in the experimental group/apoptotic cells in the control group. As presented in Fig. 5, an increase in the apoptotic rate was observed following treatment with TNF-α. Pretreatment with 17β-E2 significantly decreased the rate of apoptosis in a concentration-dependent manner compared with cells treated with TNF-α only (P<0.001). Addition of the ER antagonist ICI 182,780 blocked the effect of 17β-E2 on TNF-α treatment, further demonstrating the potential contribution of 17β-E2 to TNF-α-induced human NP cell apoptosis.
Figure 5.
Apoptosis of human NP cells. (A) Annexin V/propidium iodide double staining of human NP cells; (a) Control untreated human NP cells. Human NP cells treated with (b) 100 ng/ml TNF-α, (c) 100 ng/ml TNF-α following pretreatment with 0.1 µmol/l E2, (d) 100 ng/ml TNF-α following pretreatment with 1 µmol/l E2, (e) 100 ng/ml TNF-α following pretreatment with 10 µmol/l E2 and (f) 100 ng/ml TNF-α following pretreatment with 10 µmol/l E2 and 10 µmol/l ICI. The cells in lower right quadrant (FITC+/PI-) were considered to be apoptotic. (B) Data are expressed as the mean ± standard deviation from three independent experiments. **P<0.001; #P>0.05. NP, nucleus pulposus; TNF-α, tumor necrosis factor-α; E2, 17β-estradiol; ICI, ICI 182,780.
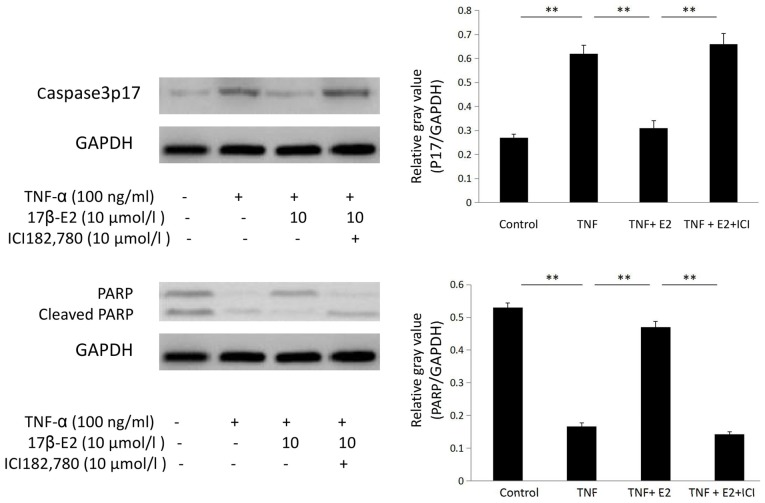
Western blot analysis
Human NP cells were divided into the following four groups: Control untreated, 100 ng/ml TNF-α, 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 for 30 min and 100 ng/ml TNF-α following pretreatment with 10 µmol/l 17β-E2 and 10 µmol/l ICI 182,780 for 30 min. Following treatment, protein expression levels of PARP, caspase-3p17 and GAPDH were determined by western blot analysis. Caspase-3p17 protein expression levels were increased in human NP cells following treatment with TNF-α compared with the control cells (P<0.001); this increased was inhibited by pretreatment with 17β-E2 (P<0.001). By contrast, PARP protein expression levels were decreased in cells subjected to TNF-α treatment (P<0.001); this decrease was abrogated by pretreatment with 17β-E2 (P<0.001). These effects of 17β-E2 were blocked by ICI 182,780 treatment (P<0.001; Fig. 6).
Figure 6.
Protein expression levels of caspase-3p17 and PARP in human NP cells. Human NP cells were untreated (control), or treated with 100 ng/ml TNF-α, 100 ng/ml TNF-α following pretreatment with 10 µmol/l E2 for 30 min, or 100 ng/ml TNF-α following pretreatment with 10 µmol/l E2 and 10 µmol/l ICI for 30 min. Data are expressed as the mean ± standard deviation from three independent experiments. **P<0.001. PARP, poly (ADP-ribose) polymerase; NP, nucleus pulposus; TNF-α, tumor necrosis factor-α; E2, 17β-estradiol; ICI, ICI 182,780.
Discussion
One of the primary causes of spinal degeneration is IVD degeneration. Apoptosis of NP cells and the loss of ECM are the central features of the aged and degenerated IVD (7,8,27,28). Gruber et al (29) reported that the ER gene is expressed in human IVD cells in vivo and in vitro, and further demonstrated that 17β-E2 significantly increased proliferation of annulus cells. Previous studies have revealed that TNF-α may induce NP cell apoptosis (16,30), and various in vitro studies have demonstrated that 17β-E2 protects against apoptosis in IVD cells (6,22,24). Therefore, the hypothesis of the present study was that 17β-E2 would attenuate TNF-α-induced apoptosis in human NP cells.
The results of the present study suggested that 17β-E2 abolished TNF-α-induced apoptosis in human NP cells by increasing proliferation and reducing apoptosis. A CCK-8 assay revealed that 17β-E2 enhanced the proliferative capacity of human NP cells. Morphological observations and flow cytometric analysis demonstrated that TNF-α-induced apoptosis in human NP cells was prevented by 17β-E2. Western blot analysis was used to detect alterations in the protein expression levels of PARP and caspase-3 in human NP cells. Caspase-3 is a crucial executioner protease, which activates a caspase-activated DNase and serves an important role in apoptosis (27,31–33). Previous studies have reported that caspase-3 may be a therapeutic target for regulation of IVD degeneration (34). Pro-caspase-3 may be sheared into two fragments, p17 and p10, upon activation. The protein expression levels of caspase-3 p17 are positively correlated with caspase-3 activity. PARP is a substrate of caspase-3, which may trigger inflammation and cell death (35). Shakibaei et al (36) demonstrated that the cleavage of PARP was activated in vitro in apoptotic human articular chondrocytes.
The present study investigated whether 17β-E2 inhibited human NP cell apoptosis induced by TNF-α. Estrogen has positive effects on bone structure, chondrocyte and nerve cells. Various recent studies have revealed the anti-apoptotic effect of 17β-E2 in rat IVD cells (6,22,37). However, whether 17β-E2 may prevent TNF-α-induced apoptosis in human NP cells remains to be investigated. In the present study, human NP cells were used to provide an environment approaching the physiological state for the in vitro test of the effect of 17β-E2 on human NP. Phenol red may increase cell proliferation (38); therefore, medium without phenol red was used in the present study.
IVD degeneration is demonstrated by alterations in cell proliferation and cell matrix metabolism, which includes inhibition of nuclear proteoglycan synthesis and enhanced matrix degradation. IVD cells have been reported to produce inflammatory cytokines, including IL-1β, IL-6, TNF-α, matrix metalloproteinases (MMPs), nitric oxide and prostaglandin E2 (39). As an important inflammatory molecule, TNF-α may induce the cellular and matrix alterations of IVD degeneration (30), and activate crosstalk between signaling pathways including nuclear factor-κB and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) (40,41).
The mechanism underlying the protection of 17β-E2 against TNF-α-induced apoptosis in human NP cells is complex. Various potential mechanisms may be involved in the anti-apoptotic effect of 17β-E2 in human NP cells. Firstly, integrin production was induced in NP cells by 17β-E2. It has been reported that 17β-E2 may protect rat IVD cells by upregulating integrin α and β (22,42). Secondly, 17β-E2 may inhibit human NP cell apoptosis via the mitochondrial pathway. Yang et al (43) demonstrated that 17β-E2 protects against apoptosis by downregulating MMP-3 and −13 via a mitochondrial pathway in rat NP cells. Finally, the PI3K/Akt signaling pathway may be involved in the effect of 17β-E2. A further study by Yang et al (37) revealed that 17β-E2 protects against apoptosis via the activation of PI3K/Akt signaling pathway. This signaling pathway is associated with cell migration and invasion (44,45).
The present study investigated the effect of 17β-E2 on human NP cells; however, the findings of the present study are limited. The results of the present study were used as preliminary data for our subsequent study, which demonstrated that 17β-E2 inhibits TNF-α-induced apoptosis in human NP cells via the PI3K/Akt pathway (46). Further signaling mechanisms underlying the anti-apoptotic effects of 17β-E2 in human NP cells will require further investigation. In addition, 17β-E2 is a systemic hormone, and therefore further studies are required to determine the systemic effect of 17β-E2 in humans, to assess the suitability of 17β-E2 as a therapeutic strategy for the clinical treatment of IVD degeneration.
In conclusion, the results of the present study revealed that 17β-E2 protected against TNF-α-induced apoptosis in human NP cells in a concentration-dependent manner, and that the activation of caspase-3 and inhibition of PARP may participate in this process. The ability of 17β-E2 to protect human NP cells from apoptosis was blocked by ICI 172,780, an ER antagonist. The results of the present study may present a novel route for the prevention and therapy of IVD degeneration.
Acknowledgements
The present study was supported by the Natural Science Foundation of Hebei Province (grant no. H2014206075).
References
- 1.Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA. Aging and degeneration human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SJ, Ito K, Nolte LP. Fluid flow and convection transport of solutes within the intervertebral disc. J Biomech. 2004;37:213–221. doi: 10.1016/S0021-9290(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- 5.Podichetty VK. The aging spine: The role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand) 2007;53:4–18. [PubMed] [Google Scholar]
- 6.Wang H, Ding W, Yang D, Gu T, Yang S, Bai Z. Different concentrations of 17β-estradiol modulates apoptosis induced by interleukin-1β in rat annulus firosus cells. Mol Med Rep. 2014;10:2745–2751. doi: 10.3892/mmr.2014.2514. [DOI] [PubMed] [Google Scholar]
- 7.Zhou GQ, Yang F, Leung VVL, Cheung KMC. Molecular and cellular biology of the intervertebral disc and the use of animal models. Current Orthopaedics. 2008;22:267–273. doi: 10.1016/j.cuor.2008.05.008. [DOI] [Google Scholar]
- 8.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as atumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-a-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human Intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Moon CS, Sul D, Lee J, Bae M, Hong Y, Lee M, Choi S, Derby R, Kim BJ, et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Mourão AF, Caetano-Lopes J, Costa P, Canhão H, Santos MJ, Pinto P, Brito I, Nicola P, Cavaleiro J, Teles J, et al. Tumor necrosis factor-alpha-308 genotypes influence inflammatory activity and TNF-alpha serum concentrations in children with juvenile idiopathic arthritis. J Rheumatol. 2009;36:837–842. doi: 10.3899/jrheum.080615. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CC, Cui GP, Hu JG, Xiao YZ, Zhou XS, Shao C, Lin Q, Zhou JS. Effects of adenoviral vector expressing hIGF-1 on apoptosis in nucleus pulposus cells in vitro. Int J Mol Med. 2014;33:401–405. doi: 10.3892/ijmm.2013.1586. [DOI] [PubMed] [Google Scholar]
- 17.de Souza Grava AL, Ferrari LF, Defino HL. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. Eur Spine J. 2012;21:537–545. doi: 10.1007/s00586-011-2027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackermann S, Hiller S, Osswald H, Lösle M, Grenz A, Hambrock A. 17beta-Estradiol modulates apoptosis in pancreatic beta-cells by specific involvement of the sulfonylurea receptor (SUR) isoform SUR1. J Biol Chem. 2009;284:4905–4913. doi: 10.1074/jbc.M807638200. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi A, Nozawa K, Fujishiro M, Kawasaki M, Takamori K, Ogawa H, Sekigawa I, Takasaki Y. Estrogen inhibits apoptosis and promotes CC motif chemokine ligand 13 expression on synovial fibroblasts in rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2012;34:852–857. doi: 10.3109/08923973.2012.664149. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y, Kojima T, Kato D, Matsubara H, Takigawa M, Ishiguro N. A selective estrogen receptor modulator inhibits tumor necrosis factor-α-induced apoptosis through the ERK1/2 signaling pathway in human chondrocytes. Biochem Biophys Res Commun. 2012;421:418–424. doi: 10.1016/j.bbrc.2012.03.111. [DOI] [PubMed] [Google Scholar]
- 22.Yang SD, Ma L, Gu TX, Ding WY, Zhang F, Shen Y, Zhang YZ, Yang DL, Zhang D, Sun YP, Song YL. 17β-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis. 2014;19:789–800. doi: 10.1007/s10495-014-0965-4. [DOI] [PubMed] [Google Scholar]
- 23.Bozzo C, Graziola F, Chiocchetti A, Canonico PL. Estrogen and beta-amyloid toxicity: Role of integrin and PI3-K. Mol Cell Neurosci. 2010;45:85–91. doi: 10.1016/j.mcn.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen J, Brinton R Diaz. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Zhang X, Dhakal IB, Beggs M, Kadlubar S, Luo D. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer. 2012;12:29. doi: 10.1186/1471-2407-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge C, Yu M, Zhang C. G Protein-Coupled Receptor 30 mediates estrogen-induced proliferation of primordial germ cells via EGFR/Akt/β-catenin signaling pathway. Endocrinology. 2012;153:3504–3516. doi: 10.1210/en.2012-1200. [DOI] [PubMed] [Google Scholar]
- 27.Ding F, Shao ZW, Yang SH, Wu Q, Gao F, Xiong LM. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis. 2012;17:579–90. doi: 10.1007/s10495-012-0708-3. [DOI] [PubMed] [Google Scholar]
- 28.Nardinocchi L, Puca R, Sacchi A, D'Orazi G. Inhibition of HIF-1alpha activity by homeodomain-interacting protein kinase-2 correlates with sensitization of chemoresistant cells to undergo apoptosis. Mol Cancer. 2009;8:1. doi: 10.1186/1476-4598-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruber HE, Yamaguchi D, Ingram J, Leslie K, Huang W, Miller TA, Hanley EN., Jr Expression and localization of estrogen receptor-beta in annulus cells of the human intervertebral disc and the mitogenic effect of 17-beta-estradiol in vitro. BMC Musculoskelet Disord. 2002;3:4. doi: 10.1186/1471-2474-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber A, Wandinger KP, Mueller W, Aktas O, Wengert O, Grundström E, Ehrlich S, Windemuth C, Kuhlmann T, Wienker T, et al. Identification and functional characterization of a highly polymorphic region in the human TRAIL promoter in multiple sclerosis. J Neuroimmunol. 2004;149:195–201. doi: 10.1016/j.jneuroim.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Singh M, Sharma H, Singh N. Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion. 2007;7:367–373. doi: 10.1016/j.mito.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 32.He B, Tao H, Liu S, Wei A. Protective effect of carboxymethylated chitosan on hydrogen peroxide-induced apoptosis in nucleus pulposus cells. Mol Med Rep. 2015;11:1629–1638. doi: 10.3892/mmr.2014.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudo H, Minami A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 2011;63:1648–1657. doi: 10.1002/art.30251. [DOI] [PubMed] [Google Scholar]
- 35.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 36.Shakibaei M, John T, Seifarth C, Mobasheri A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci. 2007;1095:554–563. doi: 10.1196/annals.1397.060. [DOI] [PubMed] [Google Scholar]
- 37.Yang SD, Ma L, Yang DL, Ding WY. Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ. 2016;4:e1640. doi: 10.7717/peerj.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wesierska-Gadek J, Schreiner T, Gueorguieva M, Ranftler C. Phenol red reduces ROSC mediated cell cycle arrest and apoptosis in human MCF-7 cells. J Cell Biochem. 2006;98:1367–1379. doi: 10.1002/jcb.20960. [DOI] [PubMed] [Google Scholar]
- 39.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: A study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 2005;30:44–54. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandra F, Matsuki NA, Takeuchi H, Ikebe T, Kanematsu T, Ohishi M, Hirata M. TNF inhibited the apoptosis by activation of Akt serine/threonine kinase in the human head and neck squamous cell carcinoma. Cell Signal. 2002;14:771–778. doi: 10.1016/S0898-6568(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhao CM, Chen Q, Zhang WJ, Huang AB, Zhang W, Yang HL, Zhang ZM. 17β-estradiol protects rat annulus fibrosus cells against apoptosis via α1 integrin-mediated adhesion to type I collagen: An in-vitro study. Med Sci Monit. 2016;22:1375–1383. doi: 10.12659/MSM.897906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang SD, Yang DL, Sun YP, Wang BL, Ma L, Feng SQ, Ding WY. 17β-estradiol protects against apoptosis induced by interleukin-1β in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20:348–357. doi: 10.1007/s10495-015-1086-4. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Tumor necrosis factor alpha induces the expression of transforming growth factor alpha and the epidermal growth factor receptor in human pancreatic cancer cells. Proc Natl Acad Sci USA. 1993;90:863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, Yang SD, Liu S, Wang H, Liu H, Ding WY. 17β-estradiol inhibites tumor necrosis factor-a induced apoptosis of human nucleus pulposus cells via the PI3K/Akt pathway. Med Sci Monit. 2016;22:4312–4322. doi: 10.12659/MSM.900310. [DOI] [PMC free article] [PubMed] [Google Scholar]