Abstract
Although surgery remains the standard therapy for the treatment of bladder cancer (BCa), the data from previous clinical studies suggest that there is an increase in the number of patients with a preference for bladder preservation strategies, including radiotherapy, to improve their life quality. Our preliminary results showed that disabled homolog 2 interactive protein (DAB2IP), a putative tumor suppressor gene, is often downregulated in BCa with a radioresistant phenotype. Subsequent investigations revealed that elevated expression of ataxia-telangiectasia mutated (ATM) induced by DAB2IP-knockdown may be the key event in BCa cell resistance to ionizing radiation (IR). However, how ATM is involved in the survival of DAB2IP-deficient cells exposed to IR remains to be fully elucidated. The present study knocked down the expression of ATM in DAB2IP-deficient BCa cells using RNA interference technology. Activation of mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways were detected by western blot analysis and immunofluorescence assay, respectively. It was demonstrated that knockdown of ATM enhanced the response of DAB2IP-deficient BCa cells to IR, which may have resulted from delayed DNA double-strand break repair kinetics, compromised nuclear factor-κB translocation, inhibited phosphorylation of p38 and the induced activation of c-Jun N-terminal kinase. Taken together, these findings suggested that ATM may be an effective target in the radiotherapy of patients with DAB2IP-deficient BCa.
Keywords: disabled homolog 2 interactive protein, bladder cancer, ataxia-telangiectasia mutated, nuclear factor-κB, mitogen-activated protein kinases
Introduction
In addition to the ageing population and serious environmental pollution, bladder cancer (BCa) is one of the most common malignancies in China (1). Although surgery remains the standard therapy for the treatment of BCa, an increasing number of patients have a preference for bladder preservation strategies to improve their quality of life (2). In 2012, James et al (3) completed a multicenter randomized phase III trial to compare the efficacy of radiotherapy alone or concomitant chemoradiotherapy for patients with muscle-invasive BCa. The 5-year overall survival rates for chemoradiation and radiotherapy were 48 and 35%, respectively. In addition, Zehnder et al (4)reported that the 5-year recurrence-free survival rates of patients with pT2pN0–2 and pT3pN0–2 BCa undergoing radical cystectomy and extended lymph node dissection were 57, vs. 67% and 32, vs. 34%, respectively. Although no studies have directly compared the outcome of bladder preservation therapy with that of standard surgery in BCa treatment, the data from previous clinical studies suggest that radiotherapy or chemoradiotherapy may be an alternative to surgery, particularly in less medically fit patients (5).
Human disabled homolog 2 interaction protein (DAB2IP), a putative tumor suppressor gene, belongs to the Ras GTPase-activating protein family (6). It is often downregulated in BCa with aggressive phenotypes (7) and confers BCa cell resistance to ionizing radiation (IR) (8) and antineoplastic drugs (9). Therefore, it may serve as a promising biomarker of prognosis for patients with BCa treated with radiotherapy or chemoradiotherapy. In previous investigations (8), it was found that ataxia-telangiectasia mutated (ATM), a key signal protein initiating DNA damage repair upon IR (10), was upregulated at the mRNA and protein levels in DAB2IP-deficient BCa cells. In addition, inhibiting the expression of ATM or its activation markedly enhanced the sensitivity of DAB2IP-deficient BCa cells to IR, suggesting that ATM-targeted drug screening may be an effective approach to improve the response of patients with DAB2IP-deficient BCa to radiotherapy. In order to elucidate the mechanism underlying the ATM-loss-induced enhancement of radiosensitivity of small interfering (si) RNA-transfected DAB2IP cells, the effect of γ-rays on the activation of nuclear factor-κB (NF-κB) and the mitogen-activated protein kinase (MAPK) signaling pathway were investigated in the present study.
Materials and methods
Cell culture
The 5637 human bladder urothelial cancer cell line, purchased from Shanghai Cell Bank of China (Shanghai, China), was cultured in Dulbecco's modified Eagle's medium (high glucose, HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 8% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere with 5% carbon dioxide.
RNA interference
The siRNA oligonucleotides against human DAB2IP, ATM, catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) and control siRNA have been described previously (8). In brief, transient inhibition of the target genes was performed on 2×105 cells per ml by transfection with 20 nM siRNA using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The resulting 5637 cells were termed siControl, siDAB2IP, siDAB2IP+siATM and siDAB2IP+siDNA-PKcs, respectively.
Cell irradiation
The cells were irradiated at room temperature in ambient air using a 137Cs source (γ-ray; MDS Nordion, Toronto, ON, Canada) with a central dose rate of 0.77 Gy/min and a volume of radiation cavity of 7.5 L.
Colony formation assay
A total of 2×105 log-phase 5637 cells were seeded into 35 mm culture dishes (Thermo Fisher Scientific, Inc.) and subjected to increasing doses of γ-rays (0, 2 and 5 Gy). At 4 h post-irradiation, the cells were diluted serially to appropriate concentrations (100, 200 and 800 cells per 3 ml) and seeded into 60 mm dishes in triplicate. Following 14 days of incubation at 37°C, the colonies were rinsed twice with phosphate-buffered saline (PBS; Beyotime Institute of Biotechnology, Haimen, China), fixed for 15 min using methyl alcohol (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China), stained with 0.1% crystal violet solution (Sangon Biotech Co., Ltd., Shanghai, China). The visible colonies (>50 cells) were counted and the surviving fraction was calculated.
Western blot analysis
The cell lysates were extracted with radio immunoprecipitation assay lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) and mixed with 10 mM PMSF (cat. no. ST506; Beyotime Institute of Biotechnology) 2 h post-IR. The supernatant was collected following centrifugation at 12,000 × g for 10 mins at 4°C. The protein concentration was quantified using a Micro BCA Protein Assay kit (cat. no. SK3061; Sangon Biotech Co., Ltd.) and equal quantities of total protein (40 µg) were subjected to 10% SDS-PAGE followed by transfer onto PVDF membranes (0.45 µm; Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% bovine serum albumin (AR2440; Sangon Biotech Co., Ltd.) for 1 h at room temperature and incubated with primary antibodies at 4°C overnight. The primary antibodies used were as follows: DAB2IP rabbit polyclonal antibody (1:2,000; cat. no. A302-440A; Bethyl Laboratories, Inc., USA), ATM rabbit monoclonal antibody (1:1,000; cat. no. 1549-1; Epitomics, Burlingame, CA, USA), DNA-PKcs rabbit monoclonal antibody (1:1,000; cat. no. 1579-1; Epitomics), phosphorylated (p)-p38 mouse monoclonal antibody (1:2,000; cat. no. 9216; Cell Signaling Technology, Inc., Danvers, MA, USA), p38 rabbit polyclonal antibody (1:1,000; cat. no. 9212; Cell Signaling Technology, Inc.), p-c-Jun N-terminal kinase (JNK) rabbit polyclonal antibody (1:1,000; cat. no. 9251; Cell Signaling Technology, Inc.), JNK rabbit polyclonal antibody (1:1,000; cat. no. sc-571; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-extracellular signal-regulated kinase (ERK) rabbit monoclonal antibody (1:1,000, cat. no. 4370; Cell Signaling Technology, Inc.) and ERK rabbit monoclonal antibody (1:1,000; cat. no. ER31218; HuaAn Biotechnology, Inc., Hangzhou, China). Anti-GAPDH was purchased from Xianzhi Biotechnical Co., Ltd. (Hangzhou, Zhejiang, China). The membranes were washed three times and then incubated with anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) or anti-rabbit (1:1,000; cat. no. A0216; Beyotime Institute of Biotechnology) for 1 h at room temperature. Finally, the protein bands were visualized using chemiluminescence (BeyoECL Plus; cat. no. P0018; Beyotime Institute of Biotechnology) and detected using the ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence assay
The cells were diluted to the appropriate concentration, seeded onto cover slips and exposed to γ-rays of 2 Gy. At 30 min, 2 h, 8 h or 24 h post-IR, the cells were rinsed three times with pre-cooled PBS and fixed with fixing solution (cat. no. P0098; Beyotime Institute of Biotechnology, Inc.) for 15 min. Subsequently, 0.5%Triton X-100 was used to permeate the membrane at room temperature (RT) for 30 min, followed by blocking with goat serum (cat. no. P0102; Beyotime Institute of Biotechnology) for 30 min. The cells were then incubated with primary antibody γ-H2AX (1:100; cat. no. 2212-1; Epitomics) or NF-κB p65 (1:50; cat. no. sc-372; Santa Cruz Biotechnology, Inc.) at RT for 1 h. Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:300; cat. no. A0423; Beyotime Institute of Biotechnology) was used as the secondary antibody and incubated with samples at RT for 1 h, protected from light. The cells were mounted in Vectashield® mounting medium containing 4′,6-diamidino-2-phenylindole and examined using a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard error of the mean of at least three independent experiments. The results were analyzed for significance using Student's t test (unpaired). P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS 18.0 statistics software (SPSS, Inc., Chicago, IL, USA).
Results
Decreased expression of ATM enhances DAB2IP-deficient cell radiosensitivity
The endogenous expression of DAB2IP, ATM and DNA-PKcs in the 5637 cells were respectively knocked down by siRNA transfection and their levels were detected using western blot analysis (Fig. 1A). The cells were then exposed to γ-irradiation at increasing doses (0, 2 and 5 Gy). IR caused a dose-dependent reduction in clonogenic survival of the siControl cells, siDAB2IP cells, siDAB2IP+siATM cells and siDAB2IP+siDNAPKcs cells (Fig. 1B). As expected, the siDAB2IP cells exhibited higher radioresistance, compared with the siControl cells. The surviving fractions at 2 Gy (SF2) for the siDAB2IP and siControl cells were 0.69±0.06 and 0.38±0.07 (P=0.0022), respectively. When the expression of ATM was inhibited in the siDAB2IP cells the SF2 value was reduced to 0.37±0.08, which was significantly lower, compared with that in the siDAB2IP cells (P=0.0026). This radiosensitization effect was not observed for the siDAB2IP cells treated with DNA-PKcs-knockdown (SF2=0.59±0.10, vs. siDAB2IP cells; P=0.1058).
Figure 1.
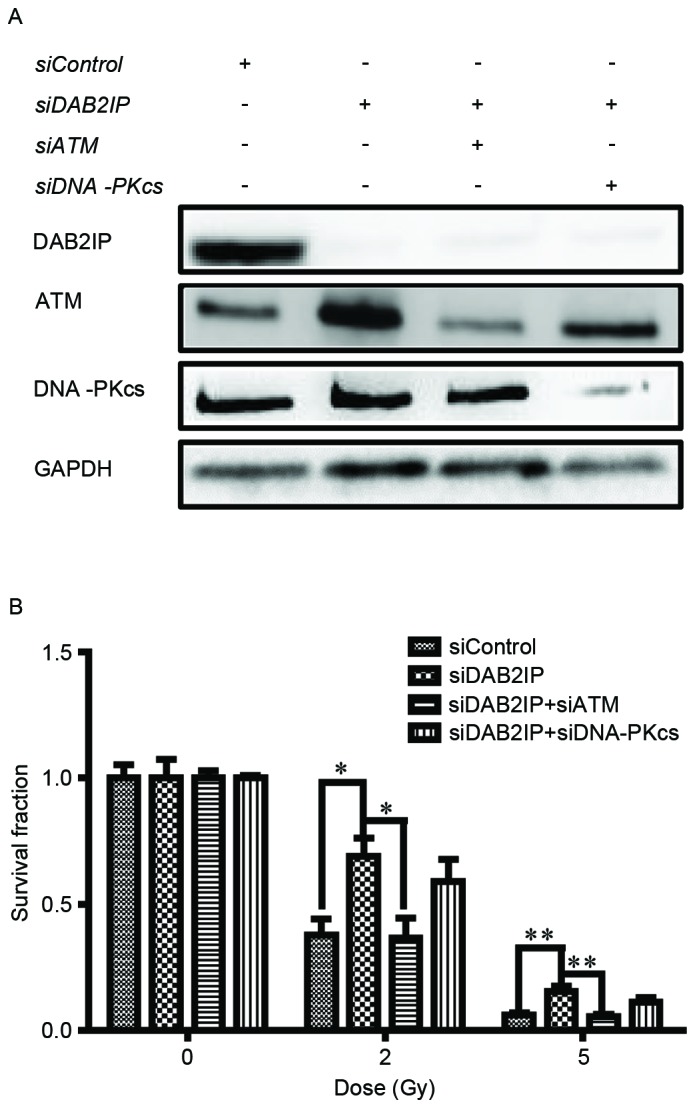
Loss of ATM enhances sensitivity of DAB2IP-deficient cells to γ-ray. (A) Cells were transfected with different siRNA as indicated. The expression levels of DAB2IP, ATM and DNA-PKcs in the sublines were detected using western blot analysis. GADPH was loaded as an internal control. (B) All sublines were subjected to increasing doses of radiation (0, 2 and 5 Gy). The colonies were counted and the surviving fraction was calculated following 14 days of incubation. The results are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05, compared with the 5637 siDAB2IP cells; **P<0.05, compared with the 5637 siDAB2IP cells. ATM, ataxia-telangiectasia mutated; DAB2IP, disabled homolog 2 interactive protein; DNA-PKcs, catalytic subunit of DNA-dependent protein kinase; si, small interfering RNA.
Loss of ATM delays siDAB2IP cell DNA double-strand break (DSB) repair kinetics
Enhanced DSB repair is an important mechanism by which DAB2IP-deficient cells become resistant to IR (11). The rapid phosphorylation of H2AX and foci formation at damage sites occurs early in DSB repair. In the present study, the cells treated with the indicated siRNAs were subjected to a total dose of 2 Gy. The DSB repair kinetics were determined over a 24 h period by counting the γH2AX foci (green; Fig. 2A). The results revealed that ~80% repair was completed at 8 h post-IR in the siDAB2IP cells, whereas the siContol cells retained almost 50% of the foci at this time. Compared with the DNA-PKcs, inhibition delayed the DSB repair rate in DAB2IP-deficient cells, and the loss of ATM caused siDAB2IP cells to exhibit unique DSB repair kinetics. Firstly, the peak of γH2AX foci formation was observed 2 h following radiation in the siDAB2IP+siATM cells, whereas this peak occurred immediately following IR in the other groups. Secondly, >40% of the DSB remained unrepaired in cells lacking DAB2IP and ATM at 24 h post-IR. This value was double that in the siControl or siDAB2IP+siDNA-PKcs cells (~20%), and ~10 times higher than that in the siDAB2IP cells (~3%; Fig. 2B).
Figure 2.
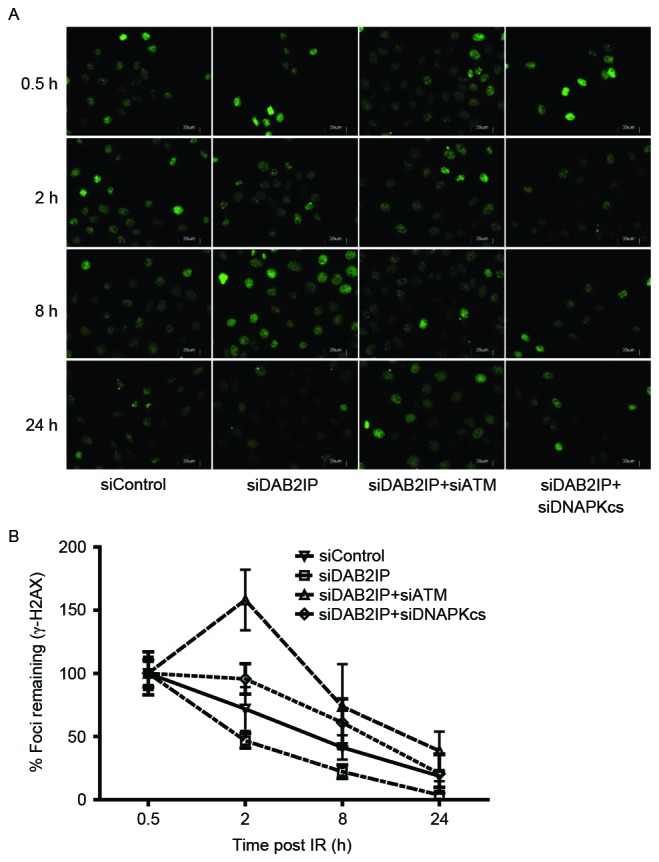
Loss of ATM delays DSB repair kinetics of siDAB2IP cells. (A) All sublines were exposed to γ-rays of 2 Gy and immunostained for γH2AX (green) foci at 0, 2, 8 and 24 h post-irradiation (average 100 nuclei; magnification ×40). (B) DNA repair kinetics in the four sublines are presented as a plot of the percentage of remaining foci against time. The results are presented as the mean ± standard error of the mean of triplicate experiments. ATM, ataxia-telangiectasia mutated; DSB, double-strand break; DAB2IP, disabled homolog 2 interactive protein; DNA-PKcs, catalytic subunit of DNA-dependent protein kinase; si, small interfering RNA.
Loss of ATM compromises the activation of NF-κB in siDAB2IP cells
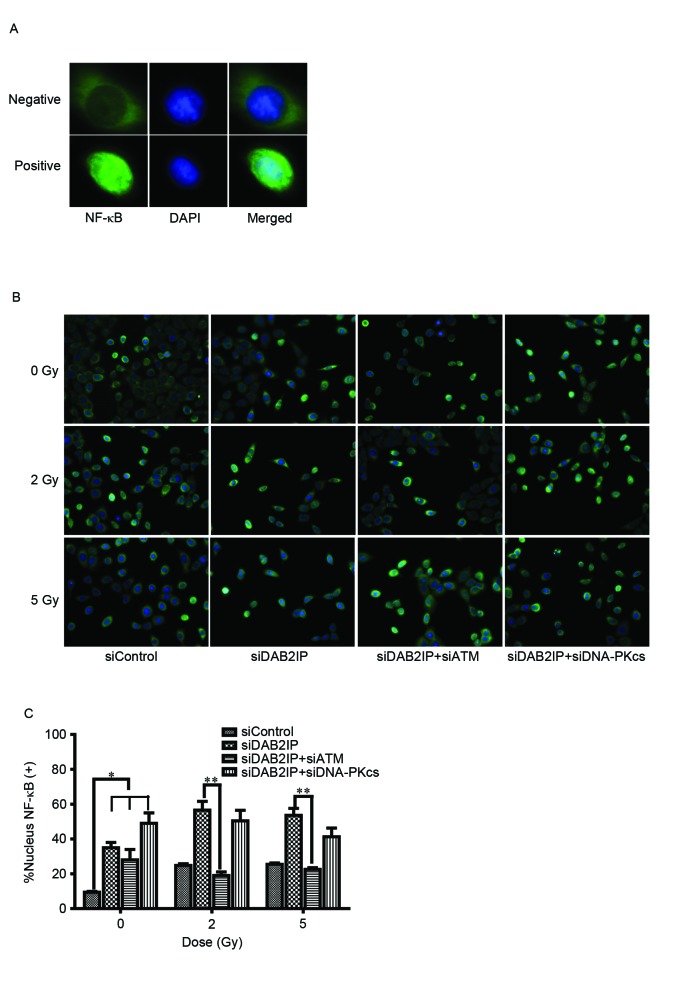
To elucidate the mechanism underlying the ATM deficiency-enhanced siDAB2IP cell radiosensitivity, the effect of γ-rays on the activation of NF-κB was investigated using an immunofluorescence assay (Fig. 3A). The translocation of p65 (green) into the nucleus following radiation was monitored (Fig. 3B) and the number of p65-posive nuclei were counted (12). Compared with the siControl cells, the activation of NF-κB was significantly increased in the cells lacking DAB2IP. IR induced the increased movement of cytoplasmic p65 into the nuclei in DABIP-deficient and DAB2IP-proficient cells. By contrast, knocking down the endogenous expression of ATM in the siDAB2IP cells resulted in dose-independent impairment of the response of NF-κB to IR. However, these changes were not observed in cells deficient in DNA-PKcs and DAB2IP, suggesting that ATM was involved in the activation of NF-κB.
Figure 3.
Loss of ATM delays DSB repair kinetics of siDAB2IP cells. (A) Distribution of NF-κB (green) was determined using an immunofluorescence assay (magnification ×100). DAPI (blue) was utilized to mark the location of nuclei. Above, NF-κB distributed in the cytoplasm only was determined as negative. Below, NF-κB translocated into the nucleus was determined as positive. (B) All sublines were irradiated with 2 Gy and immunostained for NF-κB at 2 h post-IR (magnification ×40). (C) Percentage of nuclear NF-κB (+) cells against time were plotted (average 100 nuclei). The results are presented as the mean ± standard error of the mean of triplicate experiments. *P<0.05, compared with the siControl cells; **P<0.05, compared with the siDAB2IP cells. ATM, ataxia-telangiectasia mutated; DSB, double-strand break; DAB2IP, disabled homolog 2 interactive protein; DNA-PKcs, catalytic subunit of DNA-dependent protein kinase; si, small interfering RNA; NF-κB, nuclear factor-κB; DAPI, 4′,6-diamidino-2-phenylindole.
Loss of ATM affects the phosphorylation of p38MAPK and JNK in siDAB2IP cells subjected to γ-rays
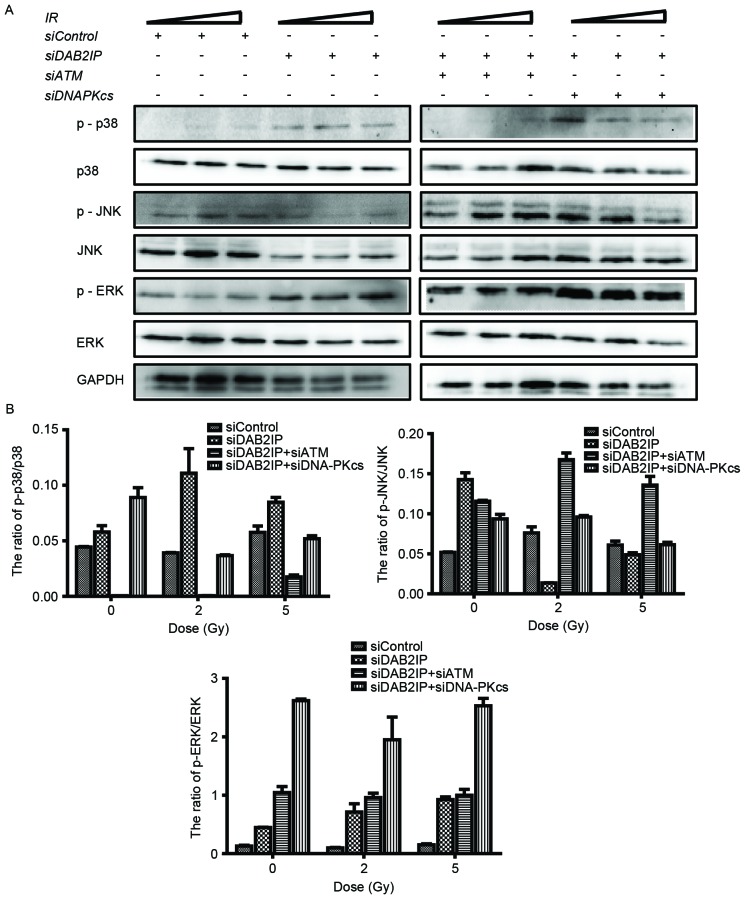
It is known that the mitogen-activated protein kinase (MAPK) signaling pathway is involved in a variety of fundamental cellular processes, including the stress response, apoptosis and survival. In the present study, the cells treated with the indicated siRNAs were exposed to IR of 2 or 5 Gy. The phosphorylation and the total protein levels of p38, JNK and ERK were detected using western blot analysis (Fig. 4A) and the ratios were calculated using QuantityOne software, version 4.6.2 (Bio-Rad Laboratories, Inc.) (Fig. 4B). Neither RNA interference nor IR altered the total protein expression levels of p38, JNK or ERK. However, the levels of p-MAPK were altered markedly in the cells in response to all types of stress. Compared with the siControl cells, knocking down DAB2IP alone caused elevated expression levels of p-p38, p-JNK and p-ERK. When exposed to IR, a simultaneous increase in the expression levels of p-p38 and p-ERK, and a decrease in the expression of p-JNK were observed in the siDAB2IP cells. In the siControl cells, only the phosphorylation of JNK was enhanced upon IR. ATM and DAB2IP deficiency in the cells led to differences in MAPK activation compared with the siDAB2IP cells, in which IR enhanced the phosphorylation of JNK and decreased the activation of p38.
Figure 4.
Loss of ATM affects the phosphorylation of MAPK in irradiated siDAB2IP cells. (A) All sublines were subjected to increasing doses of IR (0, 2 and 5 Gy). The phosphorylation and the total protein levels of p38, JNK and ERK were detected using western blot analtsis GADPH was loaded as an internal control. (B) Ratios of p-MAPK/MAPK were determined using QuantityOne software and plotted against the radiation dose. The results are presented as the mean ± standard error of the mean of triplicate experiments. IR, irradiation; ATM, ataxia-telangiectasia mutated; DSB, double-strand break; DAB2IP, disabled homolog 2 interactive protein; DNA-PKcs, catalytic subunit of DNA-dependent protein kinase; si, small interfering RNA; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p-, phosphorylated.
Discussion
Our previous study demonstrated that BCa cells deficient in DAB2IP exhibited increased clonogenic survival in response to IR, compared with cells expressing endogenous levels of DAB2IP, and was associated with elevated expression and activation of ATM, increased S phase cell distribution and faster DSB repair kinetics (8). Therefore, the present study aimed to determine whether knocking down ATM enhanced the radiosensitivity of DAB2IP-deficient cells and examine the possible underlying mechanism. Firstly, it was observed that the downregulation of ATM decreased the survival fraction of the irradiated siDAB2IP cells, however, this enhanced sensitivity to IR was not detected in siDAB2IP cells with DNA-PKcs deficiency (Fig. 1). It has been reported that ATM and DNA-PKcs, which belong to the phosphatidyl inositol 3-kinase related kinase superfamily, are involved in DNA damage repair following IR (13). The data obtained in the present study showed that ATM, but not DNA-PKcs, may be the major protein mediating radioresistance in DAB2IP-deficient BCa cells. Secondly, the present study monitored the dynamic course of DSB repair following IR in cells with different phenotypes. A unique time-lag of γH2AX foci formation and high levels of unpaired DSB were detected in the cells treated with siDAB2IP+siATM (Fig. 2). Similar results were shown previously in KU55933-treated DAB2IP-deficient 5637 cells in response to IR (8). KU55933 specifically inhibited the phosphorylation of ATM (14), whereas siRNA transfection decreased the total expression of ATM, the results of the present study supported these findings that the activation of ATM is important in mediating cell response to DSB repair.
To clarify the mechanism by which ATM affected the radiosensitivity of DAB2IP-deficient cells, activation of the NF-κB and MAPK signaling pathways were identified using immunofluorescence and western blot analyses, respectively. NF-κB is usually maintained in an inactive state in the cytoplasm and enters the nucleus in response to various stimuli, including IR exposure (15). The activation of NF-κB is recognized as a key feature in protecting cells from apoptosis and is associated with reinforcing radioresistance in the majority of cell types (16). In the preγΔβδσπκχsent study, knocking down DAB2IP resulted in the significant nuclear translocation of NF-κB. Neither siATM nor siDNA-PKcs resulted in such activation of NF-κB (Fig. 3). Although radiation exposure led to the activation of NF-κB in the DAB2IP-positive and DAB2IP-negative cells, ATM deficiency markedly suppressed the induction of NF-κB by IR in the siDAB2IP cells. By contrast, the downregulation of DNA-PKcs did not affect the activation of NF-κB in the IR-treated DAB2IP-deficient cells. These results suggested that ATM, but not DNA-PKcs, was involved in the activation of NF-κB by IR. Of note, the activation of NF-κB was similar in the siDAB2IP and siDAB2IP+siATM cells, and no differences in the percentage of cells positive for nuclear NF-κB were found between the IR-treated and unirradiated cells with DAB2IP and ATM deficiency. Of note, the increase of IR dose did not alter the above trend. These results suggested that siDAB2IP exhibits a robust DNA damage/repair system, in which ATM occupies a pivotal position. Following the induction of DSB in the nucleus upon IR, ATM is exported to the neoplasm and triggers the activation of NF-κB (17). In unirradiated cells, the increased activation of NK-κB observed in the DAB2IP-deficient cells may be associated with the protein-protein interaction between p53 and DAB2IP (18). It was hypothesized that mutated p53 in 5637 cells (19) bound to and inhibited the tumor suppressor, DAB2IP, in the cytoplasm. By contrast, in DAB2IP-negative cells, an increase in mutated p53 release and accumulation in the neoplasm induced the activation of NF-κB and reduced the activation of JNK (Fig. 4).
The present study also revealed that the elevated activation of p38 and ERK, and decreased phosphorylation of JNK occurred simultaneously in the IR-treated siDAB2IP cells, but not in the siControl cells exposed to IR. The downregulation of ATM inhibited the expression of p-p38 and stimulated the activation of JNK, but did not affect the activation of ERK in the DAB2IP-negative cells in response to IR. These findings indicated that DAB2IP deficiency induced the radioresistance of the BCa cells, and this may be due to ATM-dependent enhancement of DSB repair kinetics, sensitization of NF-κB and MARK signaling cascade transfer following IR.
Acknowledgements
This study was supported by National Nature Science Foundation of China (grant no. 31270896), the Shanghai Nature Science Foundation (grant no. 11ZR1402100), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (grant no. 44-8) and the Zhuoxue Project of Fudan University to Dr Zhaolu Kong.
References
- 1.Han SJ, Zhang SW, Chen WQ, Li CL. Analysis of the status and trends of bladder cancer incidence in China. Oncology Progress. 2013;11:89–95. [Google Scholar]
- 2.Eapen L. A review of the 2014 English Language publications pertinent to the treatment of invasive bladder cancer by radiotherapy. Curr Opin Support Palliat Care. 2015;9:245–248. doi: 10.1097/SPC.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 3.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder P, Studer UE, Skinner EC, Dorin RP, Cai J, Roth B, Miranda G, Birkhäuser F, Stein J, Burkhard FC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: A comparative study. J Urol. 2011;186:1261–1268. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a united kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–463. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Pong R, Wang Z, Hsieh JT. Differential regulation of the human gene DAB2IP in normal and malignant prostatic epithelia: Cloning and characterization. Genomics. 2002;79:573–581. doi: 10.1006/geno.2002.6739. [DOI] [PubMed] [Google Scholar]
- 7.Shen YJ, Kong ZL, Wan FN, Wang HK, Bian XJ, Gan HL, Wang CF, Ye DW. Downregulation of DAB2IP results in cell proliferation and invasion and contributes to unfavorable outcomes in bladder cancer. Cancer Sci. 2014;105:704–712. doi: 10.1111/cas.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Shen Y, Chen Y, Hsieh JT, Kong Z. The ATM inhibitor KU55933 sensitizes radioresistant bladder cancer cells with DAB2IP gene defect. Int J Radiat Biol. 2015;91:368–378. doi: 10.3109/09553002.2015.1001531. [DOI] [PubMed] [Google Scholar]
- 9.Wu K, Wang B, Chen Y, Zhou J, Huang J, Hui K, Zeng J, Zhu J, Zhang K, Li L, et al. DAB2IP regulates the chemoresistance to pirarubicin and tumor recurrence of non-muscle invasive bladder cancer through STAT3/Twist1/P-glycoprotein signaling. Cell Signal. 2015;27:2515–2523. doi: 10.1016/j.cellsig.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Guleria A, Chandna S. ATM kinase: Much more than a DNA damage responsive protein. DNA Repair (Amst) 2016;39:1–20. doi: 10.1016/j.dnarep.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Kong Z, Xie D, Boike T, Raghavan P, Burma S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT, Saha D. Downregulation of Human DAB2IP gene expression in prostate cancer cells results in resistance to ionizing radiation. Cancer Res. 2010;70:2829–2839. doi: 10.1158/0008-5472.CAN-09-2919. [DOI] [PubMed] [Google Scholar]
- 12.Otterson MF, Nie L, Schmidt JL, Link BJ, Jovanovic N, Lyros O, Rafiee P. EUK-207 protects human intestinal microvascular endothelial cells (HIMEC) against irradiation-induced apoptosis through the Bcl2 pathway. Life Sci. 2012;91:771–782. doi: 10.1016/j.lfs.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrivastav M, Miller CA, de Haro LP, Durant ST, Chen BP, Chen DJ, Nickoloff JA. DNA-PKcs and ATM co-regulate DNA double-strand break repair. DNA Repair (Amst) 2009;8:920–929. doi: 10.1016/j.dnarep.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, Rajendran A, Papa A, Spencer K, Lyssiotis CA, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:SARIAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Bai M, Ma X, Li X, Mei Q, Li X, Wu Z, Han W. The Accomplices of NF-κB Lead to Radioresistance. Curr Protein Pept Sci. 2015;16:279–294. doi: 10.2174/138920371604150429152328. [DOI] [PubMed] [Google Scholar]
- 17.Hadian K, Krappmann D. Signals from the nucleus: Activation of NF-kappaB by cytosolic ATM in the DNA damage response. Sci Signal. 2011;4:pe2. doi: 10.1126/scisignal.2001712. [DOI] [PubMed] [Google Scholar]
- 18.Di Minin G, Bellazzo A, Dal Ferro M, Chiaruttini G, Nuzzo S, Bicciato S, Piazza S, Rami D, Bulla R, Sommaggio R, et al. Mutant p53 reprograms TNF signaling in cancer cells through interaction with the tumor suppressor DAB2IP. Mol Cell. 2014;56:617–629. doi: 10.1016/j.molcel.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhu HB, Yang K, Xie YQ, Lin YW, Mao QQ, Xie LP. Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J Surg Oncol. 2013;11:22. doi: 10.1186/1477-7819-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]