Abstract
The convergence of wearable sensors and personalized medicine enhance the ability to sense and control the drug composition and dosage, as well as location and timing of administration. To date, numerous stimuli-triggered smart drug-delivery systems have been developed to detect changes in light, pH, temperature, biomolecules, electric field, magnetic field, ultrasound and mechanical forces. This review examines the major advances within the last 5 years for the three most common light-responsive drug delivery-on-demand strategies: photochemical, photoisomerization and photothermal. Examples are highlighted to illustrate progress of each strategy in drug delivery applications, and key limitations are identified to motivate future research to advance this important field.
Keywords: : controlled release, drug delivery, light, light-responsive drug delivery, personalized medicine, photochemically triggered release, photocleavable, photoisomerization, photothermal release, smart drug-delivery system
One objective of personalized medicine is to tailor drug therapies to each individual patient's pathophysiology by combining data on the patient's pharmacogenomics with information about their epigenetics, microbiome, diet, environment and lifestyle [1]. The goal is to not only match the right patient with the right drug but to also deliver the right dosage at the right time [2]. This requires innovative drug delivery technologies capable of customization. On-demand drug-delivery systems, also known as smart drug-delivery systems, are capable of exerting explicit control over where, when and how much of a drug is released by making release stimuli-dependent. To date, there have been many different stimuli used (both alone and in combination) in smart drug-delivery systems, including pH, temperature, light, biomarkers, electric field, magnetic field and ultrasound, and excellent reviews of these delivery systems are available [3–5]. A concise summary of the advantages and limitations of these categories of stimuli-responsive drug-delivery systems is highlighted in Table 1, and each bioactive molecule and clinical application has characteristics that will make them good candidates for some of these smart drug-delivery systems and less than ideal for others. Regardless, all of these systems modulate release as a function of a specific stimuli and they work in either a closed or open circuit [6,7]. Closed-loop systems are self-regulated and respond to changes in the physiological environment to control release [8,9]. Open-loop systems are independent of the physiological environment and release biologically active molecules in response to a remote or external stimulation [6,8,9]. Ideally, the release profile of these systems is dependent upon both the intensity and duration of the stimulation. This degree of control and adaptability makes open-loop systems attractive candidates for drug carriers in personalized medicine applications. In particular, light-responsive on-demand drug-delivery systems are well suited for these applications since they allow for explicit temporal and spatial control.
Table 1. . Advantages and limitations of closed- and open-loop on-demand drug-delivery systems.
| Stimulus | Advantage(s) | Limitation(s) | Ref. |
|---|---|---|---|
| Biomarker |
High target specificity |
Stability, potential leakage and possible immunogenicity of enzymes or antibodies used in the delivery system |
[104,105] |
| pH |
Small changes in pH can result in significant chemical and physical changes in drug carrier |
Limited to the clinical conditions that alter local pH |
[106] |
| Electrical | Iontophoresis devices make electrical fields accessible in the clinic | Risk of damage to healthy tissue from electric source needed for deep tissue penetration (attenuation of stimulus) | [3,107] |
| |
Safe levels of electrical field strengths have been extensively studied |
Electroresponsiveness is affected by many environmental factors (e.g., composition of aqueous medium, concentrations of electrolytes, presence of ionizable molecules) |
|
| Heat | Cancer cells are sensitive to hyperthermia (i.e., apoptosis, increased sensitivity to radiation and chemotherapeutics) | Risk of superficial tissue damage from external heating source needed for deep tissue penetration (attenuation of stimulus) | [3,105,108] |
| |
Thermally responsive drug-delivery systems are in clinical trials |
|
|
| Light | Ability to sequentially trigger multiple payloads | Questionable safety and/or biodegradability of materials | [,3,6,51] |
| |
High degree of spatiotemporal precision |
Safety risks and low tissue penetration for UV-Vis light |
|
| Magnetic | In addition to triggered delivery, capable of magnetically guided drug targeting and can act as a contrast agent for imaging | Potential toxicity from iron oxide | [3,109,110] |
| |
Magnetically responsive drug-delivery systems are in clinical trials |
Requires complex equipment set-up for adequate focusing, intensity and penetration depth |
|
| Ultrasound | Minimal safety risks with low intensity and short exposures | Risk of damage to tissues with high intensity and long exposures | [111–113] |
| High degree of spatiotemporal precision | Low drug carrier stability |
Light as an external stimulus for smart drug-delivery systems is advantageous for a number of reasons including its noninvasive nature, high spatial resolution and temporal control, and convenience and ease of use. For these reasons, light has been extensively applied in a variety of biomedical applications beyond drug delivery, including image-guided surgery [10], the photopolymerization and -degradation of tissue engineering scaffolds [11] and photodynamic therapy for cancer [12]. In this review, the major advances within the last 5 years for each of the major light-responsive smart drug-delivery mechanisms – photochemically triggered release, photoisomerization and photothermal release – are described. Examples are highlighted to illustrate progress for each mechanism, and key limitations are identified to motivate future research and advance the field. The scope has been limited to light actuated drug-delivery systems that have demonstrated triggered cargo release. There are many exciting approaches currently under way to advance the clinical effectiveness of light-actuated on-demand drug-delivery systems. Many have demonstrated successes as in vitro proof-of-concept systems, and some have produced promising preclinical in vivo results. However, there have not been any clinical trials for light-actuated on-demand drug-delivery systems. In fact, the only open-loop systems to reach clinical trials are thermosensitive liposomes and iron oxide nanoparticles [13,14]. This is partly due to the complex design of many open-loop systems including light-responsive systems [3]. Therefore, going forward, it will be advantageous to keep the KISS principle in mind: keep it simple and straightforward.
Light-responsive smart drug-delivery systems
Ideal light-responsive delivery systems have high spatial and temporal control over drug release; utilize nonionizing radiation; are composed of biocompatible materials; and can be easily tailored to the desired clinical application. Many light-based strategies have been used to design novel delivery systems and they can be classified into three broadly defined categories: photochemically triggered, where the absorbed light energy is sufficient to break covalent bonds directly or by a photochemical reaction; photoisomerization, where the excess energy causes structural changes; and photothermal, where the absorbed photon energy is dissipated via vibrational motion.
Photochemical
Drug-delivery systems under this classification use covalent bond cleavage regulated by light irradiation to facilitate the release of the encapsulated cargo. The work horse for photochemically triggered drug-delivery systems is the ortho-nitrobenzyl (o-nitrobenzyl) moiety, which upon irradiation with UV light irreversibly cleaves to release a free carboxylic acid and o-nitrosobenzaldehyde [15]. Other commonly used photoresponsive moieties include coumarin- and pyrene-derivatives, both of which contain ester bonds that are readily cleaved upon UV irradiation [16,17]. These systems require wavelengths of light that have sufficient energy per photon to break covalent bonds like UV and high-energy visible light. Recently, various strategies have been employed to replace UV light with NIR light, which achieves greater tissue penetration than UV light [18]. These strategies include two-photon absorption, triplet–triplet annihilation upconversion, second harmonic generation, upconverting nanoparticles and the use of NIR photosensitizers [19], and they have been successfully utilized in many different form factors.
Liposomes
Liposomes have been extensively used as drug carriers, and their popularity in the pharmaceutical industry is due to their ability to deliver both hydrophilic and hydrophobic drugs in addition to being biocompatible, biodegradable and having low toxicity [20–22]. Additionally, liposomes can be made at the micro- to nano-scale. The stability of the liposome's lipid bilayer ultimately controls the drug release profile. Liposome stability is dependent on the amphiphilic nature of the comprising lipids, which have a polar head group and hydrophobic tails that will spontaneously form bilayers in an aqueous environment to sandwich the hydrophobic tails between hydrophilic heads that are exposed to water [23]. Strategies that have employed light to trigger the release of drugs have used photochemical reactions to disrupt the lipid's hydrophilic/hydrophobic balance. In this section, the addition of photosensitizers rather than the use of two-photon absorption or upconverting nanoparticles is highlighted. However, it is important to note that these strategies have been previously used in phototriggerable liposomes [24–26], and recent reviews of phototriggerable liposomes are available [27–29].
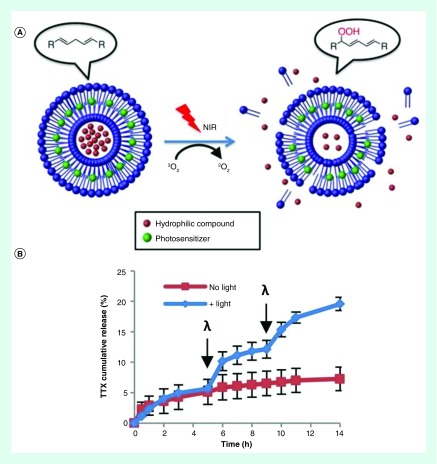
One popular mechanism to disrupt the bilayer stability is by light-induced oxidation. This is accomplished by: adding a photosensitizer to the liposome bilayer to generate reactive oxygen species, and fabricating liposomes containing unsaturated lipids. This was demonstrated by previous studies that showed visible light irradiation of photosensitizer-containing liposomes fabricated using unsaturated lipids could trigger the release of the encapsulated dye [30,31]. Recently, to increase the tissue penetration depth, the light-triggered release of the local anesthetic TTX from liposomes was achieved by adding an NIR-absorbing photosensitizer (PdPC[OBu]8) (Figure 1). A lipid containing biallylic hydrogens was used in the fabrication of the liposomes, which reacts with singlet oxygen to make lipid peroxides. This renders the lipids hydrophilic and destabilizes the hydrophobic interactions maintaining liposome integrity. In vivo studies demonstrated that injections of both phototriggerable liposomes and nonphototriggerable liposomes induced sciatic nerve blockade in rats for over 13 h due to the passive release of TTX. However, only the liposomes containing the photosensitizer could induce additional periods of nerve block with light irradiation at the 24- and 48-h time points [32]. Additional in vivo studies using reactive oxygen species to destabilize liposomes have been recently reported [33,34]. Most recently, phototriggerable liposomes were shown to increase the mean survival of female nude mice with tumors from human pancreatic cancer xenografts to 80.5 days compared with 22.5 days for the same treatment without light irradiation [33]. These liposomes were loaded with Dox and fabricated using DSPC along with the unsaturated phospholipid, DOPC. Small amounts of PoP were added to act as a photosensitizer and NIR-triggered release was achieved via the oxidation of DOPC by reactive oxygen species generated from irradiating PoP.
Figure 1. . Photochemically triggerable liposomes.
(A) Schematic of photochemically triggered release of TTX from liposomes. Irradiation of the photosensitizer with NIR generates singlet oxygen, which induces lipid peroxidation and subsequent liposome destabilization. (B) Percent release of TTX from liposomes at body temperature with and without near infrared irradiation (730 nm, 50mW/cm2, 10 min). Arrows at 5 h and 9 h time points indicate light exposure (λ).
Figure adapted from [32].
Micelles
Micelles use molecules with hydrophilic heads and hydrophobic tails to create vesicles with hydrophobic cores for encapsulating and retaining hydrophobic drugs. This is in contrast to liposomes, which use lipid bilayers to separate an aqueous core from the bulk aqueous phase and are capable of delivering both hydrophilic and hydrophobic drugs. However, as is the case with liposomes, the on-demand delivery of drug from micelles is achieved by disrupting the hydrophilic/hydrophobic balance of the carrier. In this section, the use of two-photon absorption rather than the addition of photosensitizers or upconverting nanoparticles is highlighted and the reader is directed to recent reviews of photoresponsive micelles for a more extensive review [35,36].
A primary objective for many studies designing photochemically-triggered micelle drug-delivery systems has been to synthesize biocompatible materials for micelle fabrication [37–39]. For about the last 10 years, the goal has been to further enhance both the biocompatibility and utility of these systems by using nontoxic wavelengths of light that readily penetrate tissues [40]. This is being done by functionalizing micelles with photocleavable moieties that also have large two-photon absorption cross-sections. For instance, polymeric micelles have been prepared using a biocompatible diblock copolymer made from a hydrophilic PEO block and a PGA block, which is made hydrophobic by linking it to a coumarin containing compound via the free carboxylic groups of the polypeptide. The coumarin pendant group has a high NIR two-photon absorption cross-sectional area, and upon removal by light irradiation converts the PGA block back to being hydrophilic, thereby destabilizing the hydrophilic/hydrophobic balance [41]. These light-responsive micelles were used for the triggered release of rifampicin (antibacterial) and paclitaxel (anticancer), and the authors found that the two-photon removal rate of coumarin containing compound was significantly slower (220 min) than the UV light removal rate (37 min).
Another group recently designed micelles using amphiphilic chitosan that had a o-nitrobenzyl group attached to the hydrophobic block that demonstrated low cytotoxicity at low doses (12.5 mg/kg) in vivo [42,43]. Similar to previously mentioned systems, the payload is released due to hydrophilic/hydrophobic imbalance that occurs when the o-nitrobenzyl pendant group is degraded into two hydrophilic parts by two-photon absorption of NIR light. Photolysis of the micelles was aided by the addition of cypate (Ex/Em: 780/808 nm), a nontoxic (up to 10 μmol/kg) hydrophobic dye and analogue of the US FDA approved NIR imaging agent, cardiogreen [44,45]. Specifically, the fluorescence emission of cypate promoted the two-photon-induced cleavage of the o-nitrobenzyl group. This addition increased the percent release of a model drug from NIR light irradiation within the first 2 h from 25% without cypate to 72% with cypate [42]. The addition of cypate further improves the micelles’ functionality since it has also demonstrated utility as both an imaging agent and a photothermal agent [46]. After functionalizing these micelles with target ligands, they demonstrated enhanced targeting and residence time in tumor sites. Furthermore, these micelles were capable of generating a strong photothermal response with NIR irradiation for energy-based ablation of cancer cells and photolysis for the release of anticancer drugs in nude mice with tumors from human breast cancer cell lines [43].
Hydrogels
Hydrogels are promising delivery vehicles because of their stability in aqueous environments and their mild processing conditions are compatible with fragile cargo such as proteins. Because light offers precise spatiotemporal control, there is considerable interest in photodegradable hydrogels for on-demand delivery of protein therapeutics for applications ranging from wound healing and tissue regeneration to disease treatment [47–51]. Much of the work in this field has been to fabricate photodegradable hydrogels out of different synthetic polymers by incorporating a photoresponsive moiety to the polymer backbone. One of the most popular photoresponsive moieties for photodegradable hydrogels is the o-nitrobenzyl groups and work has been done to create a library of polymerizable o-nitrobenzyl macromers with varying functionalities to allow for direct conjugation to various bioactive molecules and polymers [52]. For example, crosslinks containing o-nitrobenzyl groups were used to hold together hydrogels made of PEG and PAM. To avoid using UV light, upconverting nanoparticles were loaded into the hydrogel and the in vitro release of large biomacromolecules was triggered with continuous wave NIR light [53]. The nanoparticles were able to convert the NIR light to UV light within the hydrogel and cause photo-oxidation of the o-nitrobenzyl moieties. This caused the hydrogel to break down and release the trapped biomacromolecules. Similarly, hydrogels fabricated by a Michael addition reaction between dextran (modified with the o-nitrobenzyl moiety) and PEG underwent photodegradation resulting in 50% release of the model protein after 60 min of UV irradiation in vitro [54].
Since many different cellular processes or disease regulation would benefit from the delivery of more than one protein, hydrogels have recently been functionalized with two or more different photocleavable molecular groups to selectively release two or more different proteins using different wavelengths of light. One such study incorporated up to three different photocleavable groups into the backbone of PEG macromers to make photodegradable hydrogels [55]. All of the photocleavable groups contained the o-nitrobenzyl moiety but they each had varying modifications that changed their reactivity to different wavelengths of light. When exposed to low intensity (<45 mW/cm2) wavelengths of light ranging from 365 to 436 nm for 5 min, the authors demonstrated the triggered sequential release of three different dyes (fluorescein, rhodamine and aminomethylcoumarin acetate). In another study, two different photoresponsive moieties (o-nitrobenzyl and coumarin methylester) were incorporated into PEG hydrogels in order to sequentially release BMPs for the osteogenic differentiation of mesenchymal stem cells in vitro [51]. Specifically, the o-nitrobenzyl (405 nm) was used to tether BMP-2 and the coumarin moiety (365 nm) was used to tether BMP-7 to the hydrogel. Using ALP activity as a measure of osteogenic differentiation, the authors found that the greatest ALP activity were in cells exposed to both wavelengths of light in a sequential manner and that the ALP activity observed during sequential exposure was comparable to cells that had been exposed to native BMP-2 and BMP-7 also in a sequential manner. Together, these two studies provide an example for researchers to reference as they design new materials to deliver multiple proteins in sequence and with unique release profiles.
Limitations & discussion of recent advances
These examples show that the majority of work done on photochemically triggered delivery systems has focused on: fabricating delivery vehicles that are biocompatible, have little-to-no leakage in the absence of stimulation and efficiently encapsulate drug; designing photolabile groups that can take advantage of strategies to avoid the use of UV light, such as two-photon absorption and using upconverting nanoparticles [19]; and achieving multiple release cycles [32]. However, there are still challenges to overcome. For instance, photochemical triggering usually creates an irreversible change in the carrier, which means these systems are often ‘one-and-done’ [23,43], which could require multiple administrations of the drug carrier, and for those that have demonstrated pulsatile release, they fail to produce uniform release profiles from each light exposure [32,56]. Additionally, the same pool of photoresponsive moieties are being used to design these new photolabile groups, and there are concerns about their biocompatibility. For example, the cleavage of o-nitrobenzyl groups from polymeric side chains results in the release of nitrosobenzaldehyde, which is highly reactive and was previously shown to inhibit enzyme activity [57]. This photo-dependent enzyme inhibition was eliminated by the addition of thiols to the irradiated solution, but it is still commonly reported in the literature that this by-product may produce unwanted side effects in vivo [15]. However, there has been little reported evidence to support this claim and it is likely that the presence of reduced thiols in the cell protect them from damage [58,59]. Also, many of the photochemically triggered drug-delivery systems discussed in this section either require UV light or they are more efficiently actuated by UV light. These wavelengths can damage biological molecules (e.g., DNA and growth factors) and have poor tissue penetration, which has not only limited these systems to surface applications but also hindered their translation to the clinic [60]. Some systems have employed upconverting nanoparticles to overcome this limitation. However, the impact nanoparticles that upconvert NIR light to UV light have on human health is not well established and further studies are required [61]. Other systems have used two-photon absorption of NIR light on photolabile groups that are designed for UV degradation. However, the lower energy associated with NIR light, coupled with the use of photolabile groups that have low two-photon absorption cross-sectional areas, requires irradiation times that are significantly longer than that of UV irradiation.
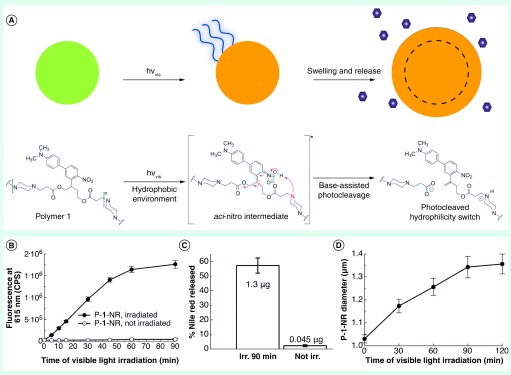
Clearly, novel strategies that move away from the use of UV responsive materials traditionally used in photochemically triggered drug delivery are needed. Indeed, work is already being done to create the next generation of photochemically triggered delivery systems. One such example was recently reported where a photocage designed for the delivery of active molecules [62] was further modified to fabricate photochemically triggered microparticles that delivered molecular cargo in vitro and in vivo with visible light irradiation [63]. Specifically, microparticles fabricated from ANBB, which contains a photolabile bond in the polymer backbone, were loaded with hydrophobic cargo (Nile red for in vitro studies and dexamethasone for in vivo studies) and irradiated with blue visible light to trigger release. The in vitro results and proposed mechanism of release are shown in Figure 2 and show that 2% of Nile red was released from nonirradiated controls while 57% was released from samples irradiated for 90 min. The in vivo experiment studied the anti-inflammatory effectiveness of dexamethasone released by blue light irradiation. The results showed that inflammation caused by carrageenan injections was lowered with greater efficiency by light triggered formulations than the free drug, which could readily diffuse from the injection site. Further research into the design of visible light and NIR responsive materials that are also biocompatible, such as this example as well as others [64,65], would help overcome some of the barriers photochemically triggered drug-delivery systems face reaching the clinic.
Figure 2. . Photochemically triggerable microparticles.
(A) Particles made of polymer 1 swell in aqueous media when irradiated with visible light, which triggers drug release. This is caused by the deprotonation of the aci-nitro intermediate by tertiary amines within the polymer backbone, which leads to photocleavage and an increase in hydrophilicity. (B) Change in fluorescence intensity due to the triggered release of Nile red and (C) the relative and actual amount of Nile red released from photoexpansile polymer 1 particles (P-1-NR) with and without blue light irradiation (180 mW, 210 mW/cm2). (D) Change in particle diameter overtime with blue light irradiation.
Figure adapted from [63]. Published by The Royal Society of Chemistry.
Photoisomerization
Light actuated drug delivery can also be achieved by the reversible conformational change of molecules induced by irradiation with UV and visible light. Azobenzenes are the most commonly used moieties for photoisomerization reactions, and they contain two phenyl groups joined by a N=N bond that transitions from trans to cis confirmation with UV light irradiation and back with blue light irradiation [5]. A significant advantage of stimuli-responsive drug-delivery systems using photoisomerization is that these molecules create a valve that can ‘turn-on/turn-off’ drug release with good temporal resolution. These systems can also be used in single release systems [66,67]. A recent review of light-activated molecular switches, their mechanisms of action and strategies to engineer visible light-activated photoswitchable molecules is available [68].
Liposomes & micelles
A great deal of work in this field has aimed to fabricate stable liposomes and micelles out of a variety of different phospholipids that either incorporate photoresponsive molecules into the lipid bilayer or modify the phospholipid's hydrophobic tails to include a photoresponsive moiety [69]. One mechanism of action for these photoisomerizable groups is to use the conformational change to create a leaky lipid bilayer upon light exposure. Specifically, the transition from the tightly packed trans confirmation to the cis conformation is used to disrupt liposome bilayers [28]. This strategy was recently used in nonphospholipid liposomes made of palmitic acid and cholesterol sulfate functionalized with azobenzene such that it was located at the center of the bilayers [70]. Using the fluorescent molecule sulforhodamine B as the model drug, they demonstrated triggered release with UV light irradiation and the cessation of release with blue light irradiation. Photo-triggered release is not only achieved through the steric effect of the trans to cis conformation change, it can be triggered by the increased polarity caused by the conformation change [69]. This polarity change disrupts the hydrophobic/hydrophilic balance and carrier stability. For instance, a recent study reported using the hydrophobic, photoisomerizable group spiropyran, which switches to the zwitterionic merocyanine with UV light irradiation, in light-responsive SP-PMPC micelles [71]. In vitro release studies of Dox showed that UV irradiation accelerated release compared with systems without UV irradiation. Additionally, cytotoxicity studies of the micelles showed that they had good biocompatibility, which the authors attributed to the stealth phosphorylcholine outer shell. Finally, Dox-loaded micelles irradiated with UV light had better anticancer activity against HeLa cells than nonirradiated micelles.
An active area of research has been to utilize the various strategies to overcome the dependence on UV light in these systems. For instance, one study recently published a number of modified azobenzene groups with responsiveness to visible and NIR light that could soon find utility in drug delivery applications [72]. Another strategy that has been investigated is the inclusion of upconverting nanoparticles. Micelles fabricated from the copolymer poly(isopropylacrylamide-co-spiropyran methacrylate) self-assembled around lanthanide-doped upconverting nanoparticles [73]. In vitro studies demonstrated that these composite nanoparticles could deliver Dox with 7 min of NIR light irradiation (4.3 W/cm2) and be used to kill U-87 glioblastoma cells. Most recently, azobenzene-doped liposomes with lanthanide-doped upconverting nanoparticles were studied as a drug delivery system for chemotherapy in vivo. Specifically, DSPC was used to fabricate the liposomes, which contained azobenzene derivatives, and the upconverting nanoparticles were outfitted with a monolayer of phospholipids to facilitate their encapsulation into the hydrophilic cavities of the liposomes [74]. In vitro studies demonstrated 57% of the encapsulated Dox could be released after 6 h with intermittent NIR laser irradiation (2.2 W/cm2) and that the release profile could be modulated by the light intensity and duration of light exposure. The in vivo studies showed that the tumor growth was inhibited by the Dox-loaded light-responsive liposomes with 980 nm irradiation (2.2 W/cm2, 20 min) in mice with multidrug-resistant human breast cancer tumors and the authors also reported negligible systemic toxicity from light-responsive liposomes.
Nanoparticles
Mesoporous silica nanoparticles are popular candidates for on-demand drug-delivery systems that work via photoisomerization. This is due to their desirable properties for drug delivery, including biocompatibility, high pore volume, tunable pore size and versatile chemistry for surface functionalization. Excellent reviews of mesoporous silica nanoparticles are available [75,76]. The high pore volume and ability to tune the pore size means that high drug loading of both small molecule drugs as well as large biomacromolecules is possible. Furthermore, the versatility of silane chemistry means the surfaces of these nanoparticles can be easily functionalized such that these pores can be gated by molecules that are responsive to light [77]. For example, mesoporous silica nanoparticles functionalized with azobenzene, and containing a two-photon fluorophore, demonstrated triggered release of camptothecin and subsequent cancer cell death in vitro [78]. Drug release was achieved by NIR light irradiation because the two-photon fluorophore emitted 420 nm light, which matched the azobenzene absorption band leading to successful isomerization of the azobenzene gates.
Most of the light-responsive systems have been used for the delivery of small molecule drugs but peptides, proteins and DNA and RNA for gene therapy have significant therapeutic potential. Traditionally, hydrogels and nanogels have been used to deliver these cargos. Recently, self-assembled nanoparticles made of amphiphilic cyclodextrins, which are capable of host–guest complexation with azobenzene in the trans conformation, were used for the in vitro light-triggered capture and release of proteins and DNA [79]. This was achieved by adding appendages to the azobenzene group – polyamines to produce cationic nanoparticles and tricarboxylate produce anionic nanoparticles – to electrostatically bind anionic proteins, and DNA and cationic proteins, respectively. Irradiation with 365 nm light induced dissociation of the self-assembly and subsequent cargo release because UV-light causes photoisomerization into the cis conformation, which is not a suitable guest for cyclodextrin nanoparticles and leads to nanoparticle dissociation. Ultimately, this modular system has the potential to be adapted for a variety of applications.
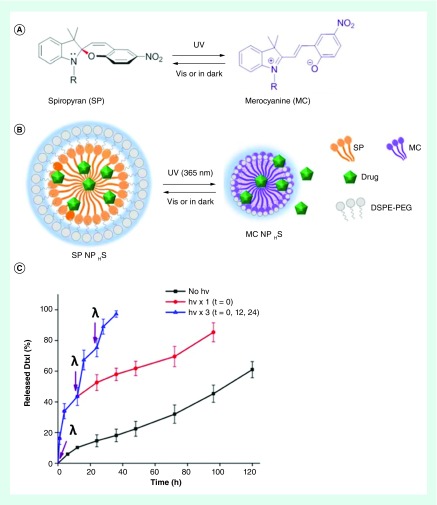
Efforts have also been made to demonstrate in vivo efficacy, safety and stability. A recent in vivo study reported using spiropyran in light-responsive lipid-PEG nanoparticles. The change in polarity caused the nanoparticles to shrink from 103 to 49 nm as spiropyran switches to merocyanine with UV light irradiation (Figure 3) [80]. The shrinking of the drug carrier had two impacts on the efficacy of the delivery system. First, the nanoparticle's penetration into mice fibrosarcoma tissue tumor was enhanced because the smaller particles could more readily diffuse through the dense ECM of tumor tissue. Second, the decrease in volume was accompanied with expulsion of the encapsulated docetaxel. The authors note that skin's high attenuation of UV light required greater light intensity to trigger release and ways to overcome this include using two-photon absorption of NIR light or incorporating upconverting nanoparticles.
Figure 3. . Photoswitchable nanoparticles.
(A) Photoisomerization reaction between spiropyran and merocyanine, and (B) schematic of the photoswitch ablenano-particles. (C) Percent release of the chemotherapeutic drug docetaxel from photoswitchable nano-particles in the absence of irradiation, and with 10 s UV irradiation (1 W/cm2, purple arrows, λ) at t = 0 as well as at t = 0, 12 and 24 h.
Figure adaptedfrom reference [80].
Limitations & discussion of recent advances
There are many different approaches being taken to improve the photoisomerizable on-demand drug-delivery systems as evidenced by the systems reviewed here. Some of the work has been to design new photoisomerizable groups that respond to visible or NIR light and can be readily incorporated into various types of delivery vehicles [72]. Additionally, work has been done to design photoisomerization systems that can take advantage of strategies to avoid the use of UV light. These strategies include two-photon absorption and using upconverting nanoparticles [73,74]. However, there are a limited number photoisomerizable molecules being used currently, and there are concerns with their biocompatibility. For instance, a fraction of azobenzene molecules will be irreversibly degraded with light irradiation [81] and azobenzene can also be degraded by azoreductase – an enzyme produced by bacteria in the GI tract [82]. Unfortunately, some of the degradation products, including nitrobenzene, are considered toxic by the US FDA [6,83,84]. As is the case with other categories of light actuated on-demand drug-delivery systems, these systems have been limited to in vitro models due to biocompatibility issues not only with the photoresponsive moieties but also the light wavelength, intensity and duration. Fortunately, in vivo studies have provided promising results and as more are conducted, the safety and efficacy of these systems can be better established. Another trend that is likely to receive greater attention in future studies is the design of delivery vehicles with additional functionalities beyond the triggered release of drug (e.g., size change with light irradiation). This is advantageous for certain clinical applications, such as cancer treatment, where the tissue microenvironment can be leveraged to improve drug targeting and localize drug delivery to prevent the indiscriminate destruction of normal cells that cause the characteristic side effects of chemotherapy.
Photothermal
Photothermally triggered drug-delivery systems use materials that generate heat upon photoexcitation to affect thermally sensitive components of drug-delivery systems, which culminates with drug being released. There are two key components required to successfully fabricate photothermally triggered drug-delivery systems: a chromophore that efficiently converts light energy into thermal energy; and a thermally responsive material that rapidly responds to temperature changes in some manner, and leads to the release of drug. Perhaps two of the most studied materials for these delivery systems are gold nanoparticles [85,86], and NiPAAm hydrogels [87]. Gold nanoparticles are popular because they are inert and nontoxic depending on their size, shape and surface chemistry [88]. Additionally, they have tailorable optical and photothermal properties at the nanoscale also dependent upon their size and shape, which includes particles that absorb strongly in the NIR region of the spectrum [89]. A recent review of additional photothermal agents used for triggered drug delivery is available [90,91]. NiPAAm is commonly used for triggered drug-delivery systems because it undergoes a reversible, temperature-induced change in hydrophobicity at its lower critical solution temperature. Above the lower critical solution temperature, NiPAAm goes from a swollen hydrogel to a globular state – expelling water and dissolved drug from the dehydrated network. The temperature at which this transition occurs can be tailored to physiological temperatures by controlling the hydrophilic/hydrophobic balance with the addition of co-monomers to the polymer network [92]. Beyond these materials, there are many different chromophores (e.g., carbon nanotubes and graphene oxide nanoparticles) and thermally responsive materials that have been used for photothermally triggered drug delivery, and various examples of form factors that have been developed.
Hydrogels & other form factors
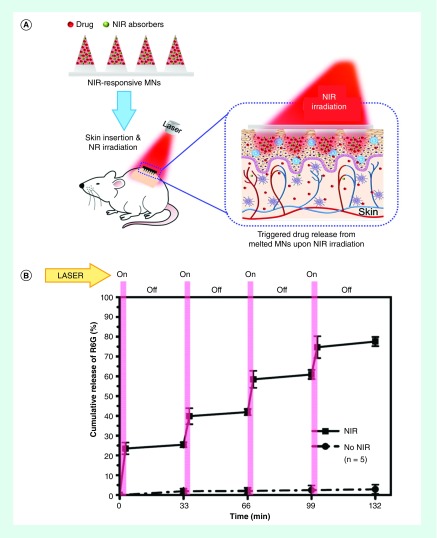
Recent studies have aimed to identify suitable chromophores that absorb either visible or NIR light. For instance, the photothermal response of the biocompatible chromophores cardiogreen, methylene blue and riboflavin were shown to rapidly generate heat (<2 min) when irradiated with either NIR or visible light. Further, the triggered release of model protein from NiPAAm hydrogels spiked with cardiogreen was achieved with 2 min of NIR light irradiation every 24 h for 4 days [93]. In another recent study, the photothermal response of lanthanum hexaboride nanostructures that were imbedded in PCL microneedles was used to melt the microneedles for the on-demand transdermal delivery of Dox in vivo (Figure 4) [94,95]. Investigators reported a pulsatile release profile with no detectable drug leakage during the off state. Significantly, the investigators reported that a single application of the microneedles that subsequently underwent three cycles of laser treatment (808 nm, 5 W/cm2) completely eradicated 4T1 tumors (stage IV human breast cancer) in mice within 1 week with no tumor recurrence and no significant body weight loss observed [95].
Figure 4. . Photothermally responsive microneedles.
(A) Schematic of NIR light actuated on-demand transdermal drug delivery from polycaprolactone microneedles spiked with photothermally responsive silica-coated lanthanum hexaboride nanostructures. (B) Percent release of Rhodamine 6G(R6G) from microneedles with four NIR laser on/off cycles (808 nm, 5 W/cm2, 3 min) or without laser treatment in vitro.
NIR: Near infrared.
Figure adapted with permission from [94]. Copyright 2015 American Chemical Society.
Micro- & nano-particles
Micro- and nano-gels of NiPAAm have recently been studied as form factors for the photothermally triggered delivery of drugs. For instance, magnetite (iron oxide) nanoparticles, which produce a photothermal response with visible light irradiation, were added to NiPAAm microgels. These microgels were encapsulated inside alginate hydrogels and further incorporated into PDMS skin patches for transdermal drug delivery [96]. Typically, transdermal drug-delivery systems are used for long-term delivery and are unable to achieve immediate release when drug action is needed. Instead, investigators successfully demonstrated triggered release of dexamethasone – a steroid medication used for the treatment of skin and rheumatological disorders – with 4 × 1 h cycles of blue light exposure (474 mW/cm2) in vitro. Without light exposure, drug still diffuses from the microgels and with a cumulative release of 24% after the first 10 h and 50% after 40 h. This leakage was observed in the ex vivo study as well but fluorescence measurements of the rat skin showed a twofold higher fluorescence in areas exposed to light than skin blocked from irradiation.
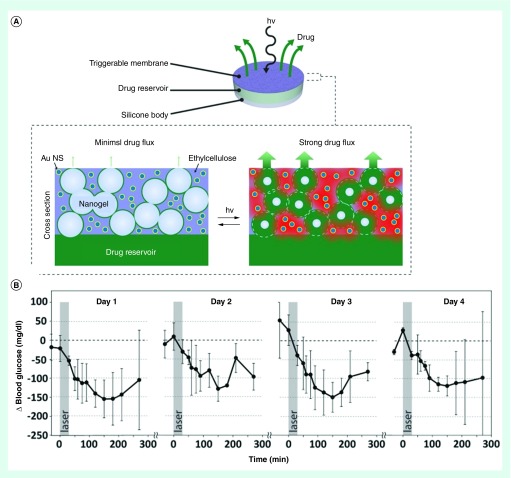
In another recent study, NiPAAm nanogels were imbedded in an impermeable ethylcellulose membrane containing gold nanoparticles (the NIR light chromophore) to create a membrane whose permeability was modulated by irradiation with light for the controlled release of aspart, a fast-acting insulin analog, from a drug reservoir [97]. These devices were implanted in rats rendered diabetic with streptozotocin, and repeated triggered release of aspart was demonstrated on days 1–3 and 14 with 30 min of 808 nm light irradiation (570 mW/cm2). On each of the four separate exposures to light over 14 days, the serum glucose levels were reduced (Figure 5) and in a separate in vivo experiment, the investigators reported a negligible effect on blood glucose levels from control devices – aspart-loaded devices without irradiation and saline-loaded devices with irradiation. While this study focused on systemic delivery of drug to regulate blood glucose levels, the authors note that these devices could: be loaded with a range of different drugs for a variety of clinical applications; and be utilized for local drug delivery applications.
Figure 5. . Photothermally modulated permeable membrane.
(A) Schematic of the reported device and a cross-section membrane containing NiPAAm nano-gels. (B) Decrease in serumglucose levels of diabetic rats after 4 cycles of dosing over 14 days at the same irradiance (808 nm, 570 mW/cm2, 30 min).
Figure adapted from reference [97].
Liposomes
Heating of the liposomal lipid bilayer over its transition temperature destabilizes the liposome, as it goes from an ordered gel phase to a liquid crystalline phase, and allows drug to diffuse away. The phase transition temperature of the constituent lipids should be near but slightly above body temperature to be suitable candidates. Recently, photothermally triggered liposomes of the same diameter but designed to respond to different light wavelengths have been fabricated [98]. This was accomplished by depositing gold nanoparticles with different resonances (760 and 1210 nm) onto the surface of the liposomes through the formation of zero-valent metal-lipid complexes. The liposomes were primarily composed of DPPC and stabilized with DPPE-PEG2000 and had a phase transition temperature around 40°C. MPPC was added to the formulation to increase the phase transition-induced permeability of the bilayer to the encapsulated cargo. The investigators demonstrated spectral selectivity of release. Specifically, 3 min of exposure to 760 nm promoted full release of encapsulated fluorescein from the liposomes with a resonance peak at 760 nm while less than 13% release was seen from the gold-coated liposomes with a plasmon resonance peak at 1210 nm. Exposure to 1210 nm light had the opposite effect: full release was achieved after 4 min of exposure from the liposomes with corresponding resonance peak at 1210 nm and less than 16% release from the liposomes resonant at 760 nm.
Gold-coated liposomes were recently used to deliver local anesthesia in vivo. Specifically, liposomes fabricated using DPPC and DPPG were used to locally deliver tetrodotoxin and dexmedetomidine in the footpad of rats [56]. These liposomes had a transition temperature around 41°C, and gold nanorods were chemically attached to liposomes in order to convert NIR light into heat. In vivo results showed investigators were able to do multiple light triggered releases over 4 days (808 nm, 10 min). This repeatable transient response to light irradiation is due to stabilization of the liposomes, which traps the remaining drug, after the light irradiation has stopped and the temperature falls below the transition temperature. However, the duration of local anesthesia was reduced with each subsequent irradiation. For postoperative pain management, the ability to repeatedly trigger local anesthesia over a 5-day period after the initial nerve block had worn off is advantageous. Additionally, the decreased dosage delivered with each light exposure coincides with the natural decrease in acute pain severity experienced by patients with each passing day. However, there may be clinical indications that require uniform drug dosages be delivered with each triggering event to be effective.
Limitations & discussion of recent advances
The systems reviewed here show that many different approaches are being pursued to improve the efficacy of photothermal on-demand drug-delivery systems and test them in vivo. There have been efforts to identify and characterize visible light and NIR chromophores suitable for photothermally triggered drug-delivery systems besides gold nanoparticles. As a result, the selection of candidate chromophores is greater than the selection of photolabile moieties and photoswitchable materials. Gold nanoparticles remain the popular choice, but the long-term in vivo effects of gold nanoparticles are currently unknown [99–101]. This strategy is perhaps most limited by the availability of thermally responsive materials that are both biocompatible and demonstrate a robust thermal response at physiologically relevant temperatures. Work is being done to identify candidate materials with both favorable thermodynamic properties and biocompatibility. NiPAAm is generally well tolerated and still remains the go-to material; however, NiPAAm monomers have been shown to be cytotoxic [102] and the noncytotoxic or low cytotoxic molecular weight range for NiPAAm is not well defined. Consequently, further studies are required so that the toxicity of NiPAAm delivery systems can be minimized while still taking advantage of the physical and mechanical properties various molecular weights can produce in the final delivery system. Going forward, it is important that photothermal on-demand delivery system use both thermally responsive materials and chromophores that respond to biocompatible wavelengths of light and ideally have a proven track-record of being safe in vivo. Perhaps one of the most promising (and simplest) photothermally triggered delivery system developed used water encapsulated in PLGA nanoparticles as the NIR light absorber. Upon irradiation with 1 W of NIR light for 5 min, the temperature rose above the glass transition temperature of PLGA, which increased the PLGA chain flexibility thereby allowing drug to diffuse out [103]. The high power and long exposure time required raises a concern with photothermal systems that the heat used to trigger release could also damage the surrounding tissues. This highlights the need for either lighting equipment that can specifically target the delivery vehicle or the design of systems that achieve rapid heating within the delivery vehicle thereby reducing the amount of time exposed to light and the risk of raising the temperature of nearby tissue.
Conclusion & Future perspective
A summary of the reviewed light-actuated on-demand drug-delivery systems is presented in Table 2. It shows a strong early research pipeline but there is still much work to do. These systems have been designed to deliver therapeutics for clinical applications ranging from pain management and chemotherapy to fighting infections and inflammation. Unfortunately, there are no US FDA approved light-actuated on-demand drug-delivery systems. These systems have been limited by issues with biocompatibility, and stability and increasing complexity places even more obstacles for approval since the methods to synthesize these materials and prepare the delivery vehicles are not standardized [4]. Over the past 5 years, researchers have made progress addressing these limitations by: employing strategies that replace UV with NIR light (i.e., upconverting nanoparticles and two-photon absorption); designing photoresponsive moieties to respond to either visible or NIR light and testing cytocompatibility in vitro; and evaluating in vivo performance and biocompatibility of light-actuated delivery systems. Going forward, the biocompatibility of these drug-delivery systems will remain a critical consideration for researchers. Additionally, most in vivo studies have been performed on small animals and as a result, the ability of light-actuated systems to deliver practical levels of the desired drug in larger animals (including humans) remains unknown and needs to be evaluated. Also, the response times of these systems to stimulation by light will be an important practical consideration for researchers designing on-demand delivery systems for clinical use. Finally, further research into quality control issues (e.g., shelf life, sterilization, reproducibility) will be required for clinical translation.
Table 2. . Summary of reviewed light actuated on-demand drug-delivery systems.
| Classification | Carrier | Payload (application) | In vitro and in vivo study | λ (nm) | Ref. |
|---|---|---|---|---|---|
| Photochemical | Liposomes | TTX (anesthetic) | In vitro and in vivo in rats | 730 | [32] |
| Dox (chemotherapy) | In vitro and in vivo in mice | 665 | [33] | ||
| Micelles | Rifampicin (antibacterial) Paclitaxel (chemotherapy) |
In vitro | 794 | [41] | |
| Paclitaxel (chemotherapy) | In vitro and in vivo in mice | 765 | [42,43] | ||
| Microparticles | Dexamethasone (anti-inflammatory) | In vitro and in vivo in mice | 400–500 | [63] | |
| Hydrogels | Model protein | In vitro | 980 | [53] | |
| Model protein | In vitro | 365 780 |
[54] | ||
| Dye | In vitro | 365–436 | [55] | ||
| |
|
BMP-2 BMP-7 (stem cell differentiation) |
In vitro |
365–405 |
[51] |
| Photoisomerization | Liposomes | Dye | In vitro | 350–450 | [70] |
| Dox (chemotherapy) | In vitro and in vivo in mice | 980 | [74] | ||
| Micelles | Dox (chemotherapy) | In vitro | 365 | [71] | |
| Dox (chemotherapy) | In vitro | 980 | [73] | ||
| Nanoparticles | Camptothecin (chemotherapy) | In vitro | 760 | [78] | |
| Docetaxel (chemotherapy) | In vitro and in vivo in mice | 365 | [80] | ||
| |
|
Model proteins DNA |
In vitro |
365 |
[79] |
| Photothermal | Hydrogels | Model protein | In vitro | 780 | [93] |
| Microneedles | Dox (chemotherapy) | In vitro and in vivo in mice and rats | 808 | [94,95] | |
| Microparticles | Dexamethasone (anti-inflammatory) | In vitro and ex vivo in rat skin | 400–500 | [96] | |
| Nanoparticles | Aspart (diabetes management) | In vitro and in vivo in rats | 808 | [97] | |
| Dye | In vitro | 980 | [103] | ||
| Liposomes | Dye | In vitro | 760–1210 | [98] | |
| TTX DMED (anesthetic) | In vitro and in vivo in rats | 808 | [56] |
BMP: Bone morphogenetic protein; DMED: Dexmedetomidine; Dox: Doxorubicin; TTX: Tetrodotoxin.
Although not a focus of this review, the instrumentation used to administer the light will also be a key consideration. Currently, many of the light-actuated on-demand drug-delivery systems require expensive lasers in complex setups. For widespread utilization of light-triggered release systems, equipment that patients and physicians can safely and easily use need to be designed. Ideally, these units would have a small footprint and be easily movable as well as reasonably affordable. In 2001, Kost and Langer envisioned a wristwatch-like system for on-demand drug-delivery systems that patients could wear and that could be either preprogrammed to trigger release at predetermined time points or the patient could turn it on as needed [104]. The emergence of smartphones and smartwatches – many of which are already outfitted with green and infrared LEDs – suggests such a system for administering light to trigger drug delivery is one-step closer to becoming a reality.
The convergence of wearable sensors and personalized medicine enhances the ability to sense and control the drug composition and dosage in real-time. This requires novel drug-delivery systems to control the location and timing of drug administration. Light-actuated on-demand drug-delivery systems are an excellent choice for such applications and this review has shown that there are a number of promising systems in development. Future work should aim to make these systems clinically acceptable with greater sensitivity to light stimulation.
Executive summary.
Photochemical drug-delivery systems
Most photochemically reactive molecules used in light-triggered drug-delivery systems are responsive to UV light and strategies to overcome this limitation include red shifting light responsive moieties through chemical modifications, two-photon absorption and including upconverting nanoparticles.
In vivo studies in small animals (i.e., mice and rats) have shown success in anesthetic, chemotherapy, antibacterial and anti-inflammatory applications with no detectable systemic toxicity.
Photoisomerization drug-delivery systems
In vivo studies in small animals showed success in chemotherapy applications with no detectable systemic toxicity.
The design of delivery vehicles with additional functionalities beyond the triggered release of drug (e.g., size change with light irradiation) is likely to receive greater attention in order to improve drug targeting and localize drug delivery.
Photothermal drug-delivery systems
Gold nanoparticles are the most popular chromophore since their resonance wavelength is tunable but other chromophores with favorable photothermal response include visible and near infrared dyes, iron oxide and entrapped water.
In vivo studies in small animals have shown success in anesthetic, chemotherapy and diabetes management applications with no detectable systemic toxicity.
Future perspective
Despite a strong early research pipeline, there are no US FDA approved light-actuated on-demand drug-delivery systems.
Areas of focus for future research include evaluating the ability of light-actuated systems to deliver practical levels of the desired drug in larger animals, improving the response times of these systems to stimulation by light and resolving quality control issues (e.g., shelf life, sterilization, reproducibility).
Equipment to administer light should be designed to be small portable units to promote widespread utilization of light-triggered release systems.
Footnotes
Financial & competing interests disclosure
The authors acknowledge funding support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH under award number R21AR064437. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Acosta-Vélez GF, Wu BM. 3D pharming: direct printing of personalized pharmaceutical tablets. Polym. Sci. 2016;2(1):11. [Google Scholar]
- 2.Sadee W, Dai ZY. Pharmacogenetics/genomics and personalized medicine. Hum. Mol. Genet. 2005;14:R207–R214. doi: 10.1093/hmg/ddi261. [DOI] [PubMed] [Google Scholar]
- 3.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]; • A comprehensive review (through 2013) covering the various stimuli-responsive nanocarriers developled for smart drug-delivery systems.
- 4.Alvarez-Lorenzo C, Concheiro A. Smart drug-delivery systems: from fundamentals to the clinic. Chem. Commun. 2014;50(58):7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 5.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug-delivery systems. Adv. Mater. 2010;22(44):4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug-delivery systems. Photochem. Photobiol. 2009;85(4):848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 7.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003;21(10):1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 8.Kost J, Langer R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2001;46(1–3):125–148. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 9.Sershen S, West J. Implantable, polymeric systems for modulated drug delivery. Adv. Drug Deliv. Rev. 2002;54(9):1225–1235. doi: 10.1016/s0169-409x(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 10.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJH, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013;10(9):507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 12.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landon CD, Park J-Y, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Sterner ES, Coughlin EB, Theato P. o-Nitrobenzyl alcohol derivatives: opportunities in polymer and materials science. Macromolecules. 2012;45(4):1723–1736. [Google Scholar]
- 16.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002;1(7):441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Zhang W, Gao C. Shape transformation of light-responsive pyrene-containing micelles and their influence on cytoviability. Biomacromolecules. 2015;16(8):2276–2281. doi: 10.1021/acs.biomac.5b00497. [DOI] [PubMed] [Google Scholar]
- 18.Weissleder R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001;19(4):316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 19.Barhoumi A, Liu Q, Kohane DS. Ultraviolet light-mediated drug delivery: principles, applications, and challenges. J. Control. Rel. 2015;219:31–42. doi: 10.1016/j.jconrel.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int. J. Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015:6. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eeman M, Deleu M. From biological membranes to biomimetic model membranes. Biotechnol. Agron. Soc. 2010;14(4):719–736. [Google Scholar]
- 24.Jayakumar MKG, Idris NM, Zhang Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc. Natl Acad. Sci. USA. 2012;109(22):8483–8488. doi: 10.1073/pnas.1114551109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He SQ, Krippes K, Ritz S, et al. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem. Commun. 2015;51(2):431–434. doi: 10.1039/c4cc07489k. [DOI] [PubMed] [Google Scholar]
- 26.Zhao LZ, Peng JJ, Huang Q, et al. Near- infrared photoregulated drug release in living tumor tissue via yolk-shell upconversion nanocages. Adv. Funct. Mater. 2014;24(3):363–371. [Google Scholar]
- 27.Puri A. Phototriggerable liposomes: current research and future perspectives. Pharmaceutics. 2013;6(1):1–25. doi: 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012;64(11):1005–1020. doi: 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda D, Lovell JF. Mechanisms of light-induced liposome permeabilization. Bioeng. Transl. Med. 2016;1(3):267–276. doi: 10.1002/btm2.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pashkovskaya A, Kotova E, Zorlu Y, et al. Light-triggered liposomal release: membrane permeabilization by photodynamic action. Langmuir. 2010;26(8):5726–5733. doi: 10.1021/la903867a. [DOI] [PubMed] [Google Scholar]
- 31.Randles EG, Bergethon PR. A photodependent switch of liposome stability and permeability. Langmuir. 2013;29(5):1490–1497. doi: 10.1021/la303526k. [DOI] [PubMed] [Google Scholar]
- 32.Rwei AY, Lee J-J, Zhan C, et al. Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc. Natl Acad. Sci. USA. 2015;112(51):15719–15724. doi: 10.1073/pnas.1518791112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo D, Li N, Carter KA, et al. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin–phospholipids. Small. 2016;12(22):3039–3047. doi: 10.1002/smll.201503966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter KA, Shao S, Hoopes MI, et al. Porphyrin-phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Dong RJ, Zhu XY, Yan DY. Photo-responsive polymeric micelles. Soft Matter. 2014;10(33):6121–6138. doi: 10.1039/c4sm00871e. [DOI] [PubMed] [Google Scholar]
- 36.Gohy J-F, Zhao Y. Photo-responsive block copolymer micelles: design and behavior. Chem. Soc. Rev. 2013;42(17):7117–7129. doi: 10.1039/c3cs35469e. [DOI] [PubMed] [Google Scholar]
- 37.Kotharangannagari VK, Sanchez-Ferrer A, Ruokolainen J, Mezzenga R. Photoresponsive reversible aggregation and dissolution of rod-coil polypeptide diblock copolymers. Macromolecules. 2011;44(12):4569–4573. [Google Scholar]
- 38.Liu XY, He JW, Niu YL, et al. Photo-responsive amphiphilic poly(alpha-hydroxy acids) with pendent o-nitrobenzyl ester constructed via copper-catalyzed azide-alkyne cycloaddition reaction. Polym. Adv. Technol. 2015;26(5):449–456. [Google Scholar]
- 39.Xie ZG, Hu XL, Chen XS, Sun J, Shi Q, Jing XB. Synthesis and characterization of novel biodegradable poly(carbonate ester)s with photolabile protecting groups. Biomacromolecules. 2008;9(1):376–380. doi: 10.1021/bm700906k. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin AP, Mynar JL, Ma Y, Fleming GR, Fréchet JMJ. Synthetic micelle sensitive to IR light via a two-photon process. J. Am. Chem. Soc. 2005;127(28):9952–9953. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, Allard J-F, Morris D, Dory YL, Lepage M, Zhao Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012;22(15):7252–7257. [Google Scholar]
- 42.Cao J, Huang S, Chen Y, et al. Near-infrared light-triggered micelles for fast controlled drug release in deep tissue. Biomaterials. 2013;34(26):6272–6283. doi: 10.1016/j.biomaterials.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Cao J, Chen D, Huang S, Deng D, Tang L, Gu Y. Multifunctional near-infrared light-triggered biodegradable micelles for chemo- and photo-thermal combination therapy. Oncotarget. 2016;7(50):82170–82184. doi: 10.18632/oncotarget.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang KA, Wang JT. Conditionally activating optical contrast agent with enhanced sensitivity via gold nanoparticle plasmon energy transfer: feasibility study. J. Nanobiotechnology. 2014;12(1):56. doi: 10.1186/s12951-014-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alford R, Simpson HM, Duberman J, et al. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol. Imaging. 2009;8(6):341–354. [PubMed] [Google Scholar]
- 46.Yang H, Mao HJ, Wan ZH, et al. Micelles assembled with carbocyanine dyes for theranostic near-infrared fluorescent cancer imaging and photothermal therapy. Biomaterials. 2013;34(36):9124–9133. doi: 10.1016/j.biomaterials.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011;3(12):925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Draper ER, Eden EGB, McDonald TO, Adams DJ. Spatially resolved multicomponent gels. Nat. Chem. 2015;7(10):849–853. doi: 10.1038/nchem.2347. [DOI] [PubMed] [Google Scholar]
- 50.Bao CY, Zhu LY, Lin QN, Tian H. Building biomedical materials using photochemical bond cleavage. Adv. Mater. 2015;27(10):1647–1662. doi: 10.1002/adma.201403783. [DOI] [PubMed] [Google Scholar]
- 51.Azagarsamy MA, Anseth KS. Wavelength-controlled photocleavage for the orthogonal and sequential release of multiple proteins. Angew. Chem. Int. Ed. Engl. 2013;52(51):13803–13807. doi: 10.1002/anie.201308174. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrated selective growth factor release in vitro to stimulate sequential signaling of human mesenchymal stem cells at different times by systematically switching the light from one wavelength to another.
- 52.Griffin DR, Schlosser JL, Lam SF, Nguyen TH, Maynard HD, Kasko AM. Synthesis of photodegradable macromers for conjugation and release of bioactive molecules. Biomacromolecules. 2013;14(4):1199–1207. doi: 10.1021/bm400169d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan B, Boyer J-C, Habault D, Branda NR, Zhao Y. Near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J. Am. Chem. Soc. 2012;134(40):16558–16561. doi: 10.1021/ja308876j. [DOI] [PubMed] [Google Scholar]
- 54.Peng K, Tomatsu I, van den Broek B, et al. Dextran based photodegradable hydrogels formed via a Michael addition. Soft Matter. 2011;7(10):4881–4887. [Google Scholar]
- 55.Griffin DR, Kasko AM. Photoselective delivery of model therapeutics from hydrogels. ACS Macro Lett. 2012;1(11):1330–1334. doi: 10.1021/mz300366s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan CY, Wang WP, McAlvin JB, et al. Phototriggered local anesthesia. Nano Lett. 2016;16(1):177–181. doi: 10.1021/acs.nanolett.5b03440. [DOI] [PubMed] [Google Scholar]; •• Demonstrated on-demand and repeated delivery of anesthesia in rat footpads in proportion to near infrared (NIR) light intensity and with minimal toxicity.
- 57.Kaplan JH, Forbush B, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analog – utilization by Na-K pump of human red blood-cell ghosts. Biochemistry. 1978;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 58.Corrie JET. Photoremovable protecting groups used for the caging of biomolecules. In: Goeldner M, Givens RS, editors. Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged Biomolecules. Wiley-VCH Verlag GmbH & Co.; Weinheim, Germany: 2005. pp. 1–28. [Google Scholar]
- 59.Lin WY, Peng DW, Wang B, Long L, Guo C, Yuan J. A model for light-triggered porphyrin anticancer prodrugs based on an o-nitrobenzyl photolabile group. Eur. J. Org. Chem. 2008;(5):793–796. [Google Scholar]
- 60.Tadokoro T, Kobayashi N, Zmudzka BZ, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003;17(6):1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- 61.Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA. Upconverting nanoparticles: assessing the toxicity. Chem. Soc. Rev. 2015;44(6):1561–1584. doi: 10.1039/c4cs00177j. [DOI] [PubMed] [Google Scholar]
- 62.Donato L, Mourot A, Davenport CM, et al. Water-soluble, donor–acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter? -aminobutyric acid at? =800 nm. Angew. Chem. Int. Ed. Engl. 2012;51(8):1840–1843. doi: 10.1002/anie.201106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carling C-J, Viger ML, Viet Anh Nguyen H, Garcia AV, Almutairi A. In vivo visible light-triggered drug release from an implanted depot. Chem. Sci. 2015;6(1):335–341. doi: 10.1039/c4sc02651a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fomina N, McFearin CL, Sermsakdi M, Morachis JM, Almutairi A. Low power, biologically benign NIR light triggers polymer disassembly. Macromolecules. 2011;44(21):8590–8597. doi: 10.1021/ma201850q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lux CD, Lux J, Collet G, et al. Short soluble coumarin crosslinkers for light-controlled release of cells and proteins from hydrogels. Biomacromolecules. 2015;16(10):3286–3296. doi: 10.1021/acs.biomac.5b00950. [DOI] [PubMed] [Google Scholar]
- 66.Lu J, Choi E, Tamanoi F, Zink JI. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4(4):421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angelos S, Yang YW, Khashab NM, Stoddart JF, Zink JI. Dual-controlled nanoparticles exhibiting and logic. J. Am. Chem. Soc. 2009;131(32):11344–11346. doi: 10.1021/ja9042752. [DOI] [PubMed] [Google Scholar]
- 68.Bleger D, Hecht S. Visible-light-activated molecular switches. Angew. Chem. Int. Ed. Engl. 2015;54(39):11338–11349. doi: 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- 69.Leung SJ, Romanowski M. Light-activated content release from liposomes. Theranostics. 2012;2(10):1020–1036. doi: 10.7150/thno.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui Z-K, Phoeung T, Rousseau P-A, et al. Nonphospholipid fluid liposomes with switchable photocontrolled release. Langmuir. 2014;30(36):10818–10825. doi: 10.1021/la502131h. [DOI] [PubMed] [Google Scholar]
- 71.Shen HJ, Zhou M, Zhang Q, Keller A, Shen Y. Zwitterionic light-responsive polymeric micelles for controlled drug delivery. Colloid Polym. Sci. 2015;293(6):1685–1694. [Google Scholar]
- 72.Dong MX, Babalhavaeji A, Samanta S, Beharry AA, Woolley GA. Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res. 2015;48(10):2662–2670. doi: 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- 73.Chen S, Gao Y, Cao Z, et al. Nanocomposites of spiropyran-functionalized polymers and upconversion nanoparticles for controlled release stimulated by near-infrared light and pH. Macromolecules. 2016;49(19):7490–7496. [Google Scholar]
- 74.Yao C, Wang P, Li X, et al. Near-infrared-triggered azobenzene-liposome/upconversion nanoparticle hybrid vesicles for remotely controlled drug delivery to overcome cancer multidrug resistance. Adv. Mater. 2016;28(42):9341–9348. doi: 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]; •• Near-infrared (NIR)-triggered liposomes with UCNP released Dox on-demand and inhibited the growth of drug-resistant tumors in vivo.
- 75.Mamaeva V, Sahlgren C, Linden M. Mesoporous silica nanoparticles in medicine-recent advances. Adv. Drug Deliv. Rev. 2013;65(5):689–702. doi: 10.1016/j.addr.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 76.Mai WX, Meng H. Mesoporous silica nanoparticles: a multifunctional nano therapeutic system. Integr. Biol. 2013;5(1):19–28. doi: 10.1039/c2ib20137b. [DOI] [PubMed] [Google Scholar]
- 77.Liu JA, Bu WB, Pan LM, Shi JL. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew. Chem. Int. Ed. Engl. 2013;52(16):4375–4379. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 78.Croissant J, Maynadier M, Gallud A, et al. Two-photon-triggered drug delivery in cancer cells using nanoimpellers. Angew. Chem. Int. Ed. Engl. 2013;52(51):13813–13817. doi: 10.1002/anie.201308647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moratz J, Samanta A, Voskuhl J, Nalluri SKM, Ravoo BJ. Light-triggered capture and release of dna and proteins by host-guest binding and electrostatic interaction. Chemistry. 2015;21(8):3271–3277. doi: 10.1002/chem.201405936. [DOI] [PubMed] [Google Scholar]; • Modular light-triggered nanoparticle system that uses host–guest binding of azobenzene and cyclodextrin to deliver DNA and both cationic and anionic proteins.
- 80.Tong R, Chiang HH, Kohane DS. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl Acad. Sci. USA. 2013;110(47):19048–19053. doi: 10.1073/pnas.1315336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galvan-Gonzalez A, Canva M, Stegeman GI, et al. Photodegradation of azobenzene nonlinear optical chromophores: the influence of structure and environment. J. Opt. Soc. Am. B. 2000;17(12):1992–2000. [Google Scholar]
- 82.Rao JY, Khan A. Enzyme sensitive synthetic polymer micelles based on the azobenzene motif. J. Am. Chem. Soc. 2013;135(38):14056–14059. doi: 10.1021/ja407514z. [DOI] [PubMed] [Google Scholar]
- 83.Joseph JM, Destaillats H, Hung HM, Hoffmann MR. The sonochemical degradation of azobenzene and related azo dyes: rate enhancements via Fenton's reactions. J. Phys. Chem. A. 2000;104(2):301–307. [Google Scholar]
- 84.National Toxicology P. Bioassay of azobenzene for possible carcinogenicity. Natl. Cancer Inst. Carcinog. Tech. Rep. Ser. 1979;154:1–131. [PubMed] [Google Scholar]
- 85.Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012;64(2):190–199. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A, Zhang X, Liang X-J. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013;31(5):593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Gandhi A, Paul A, Sen SO, Sen KK. Studies on thermoresponsive polymers: phase behaviour, drug delivery and biomedical applications. Asian J. Pharmacol. 2015;10(2):99–107. [Google Scholar]
- 88.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart. Res. 2010;12(7):2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000;19(3):409–453. [Google Scholar]
- 90.Song XJ, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8(2):340–354. [Google Scholar]
- 91.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release. 2011;149(1):65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 92.Feil H, Bae YH, Feijen J, Kim SW. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules. 1993;26(10):2496–2500. [Google Scholar]
- 93.Linsley CS, Quach VY, Agrawal G, Hartnett E, Wu BM. Visible light and near-infrared-responsive chromophores for drug delivery-on-demand applications. Drug Deliv. Transl. Res. 2015;5(6):611–624. doi: 10.1007/s13346-015-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen M-C, Ling M-H, Wang K-W, Lin Z-W, Lai B-H, Chen D-H. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules. 2015;16(5):1598–1607. doi: 10.1021/acs.biomac.5b00185. [DOI] [PubMed] [Google Scholar]
- 95.Chen MC, Lin ZW, Ling MH. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10(1):93–101. doi: 10.1021/acsnano.5b05043. [DOI] [PubMed] [Google Scholar]
- 96.Kim H, Lee H, Seong K-Y, Lee E, Yang SY, Yoon J. Visible light-triggered on-demand drug release from hybrid hydrogels and its application in transdermal patches. Adv. Healthc. Mater. 2015;4(14):2071–2077. doi: 10.1002/adhm.201500323. [DOI] [PubMed] [Google Scholar]
- 97.Timko BP, Arruebo M, Shankarappa SA, et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl Acad. Sci. USA. 2014;111(4):1349–1354. doi: 10.1073/pnas.1322651111. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrated on-demand release of fast-acting insulin analog from implantable, NIR-triggered drug reservoirs to regulate blood glucose levels in vivo.
- 98.Leung SJ, Kachur XM, Bobnick MC, Romanowski M. Wavelength-selective light-induced release from plasmon resonant liposomes. Adv. Funct. Mater. 2011;21(6):1113–1121. doi: 10.1002/adfm.201002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004;15(4):897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 100.Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 101.Albanese A, Tang PS, Chan WCW. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 102.Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. 2005;26(16):3055–3064. doi: 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Viger ML, Sheng W, Dore K, et al. Near-infrared-induced heating of confined water in polymeric particles for efficient payload release. ACS Nano. 2014;8(5):4815–4826. doi: 10.1021/nn500702g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kost J, Langer R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012;64:327–341. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 105.Oh KT, Yin HQ, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J. Mater. Chem. 2007;17(38):3987–4001. [Google Scholar]
- 106.Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov. Today. 2002;7(10):569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 107.Brudno Y, Mooney DJ. On-demand drug delivery from local depots. J. Control. Release. 2015;219:8–17. doi: 10.1016/j.jconrel.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 108.Tagami T, Foltz WD, Ernsting MJ, et al. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials. 2011;32(27):6570–6578. doi: 10.1016/j.biomaterials.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 109.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang HW, Hua MY, Liu HL, et al. Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials. 2011;32(27):6523–6532. doi: 10.1016/j.biomaterials.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 111.Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shankar H, Pagel PS. Potential adverse ultrasound-related biological effects a critical review. Anesthesiology. 2011;115(5):1109–1124. doi: 10.1097/ALN.0b013e31822fd1f1. [DOI] [PubMed] [Google Scholar]
- 113.Kennedy S, Hu J, Kearney C, et al. Sequential release of nanoparticle payloads from ultrasonically burstable capsules. Biomaterials. 2016;75:91–101. doi: 10.1016/j.biomaterials.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]