Abstract
The aim of the present study was to investigate the effect of the mammalian target of rapamycin (mTOR) signaling pathway on thoracic aortic aneurysm (TAA) development. The study used a calcium chloride (CaCl2)-induced rat TAA model to explore the potential role of mTOR signaling pathway in the disease development. Adult male Sprague-Dawley rats underwent the periarterial exposure of thoracic aorta to either 0.5 M CaCl2 or normal saline, and a subgroup of CaCl2-treated rats received rapamycin 1 day prior to surgery. Without pre-administering rapamycin, significantly enhanced phosphorylation of mTOR and expression of proinflammatory cytokines [i.e., tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and interleukin (IL)-1β] were observed in the CaCl2-treated aortic segments 2 days post-treatment compared with the NaCl-treated segments. At 2 weeks post-treatment, hematoxylin and eosin and Verhoeff-Van Gieson staining revealed aneurysmal alteration and disappearance of normal wavy elastic structures in the aortic segments exposed to CaCl2. In contrast, the CaCl2-induced TAA formation was inhibited by pre-administering rapamycin to CaCl2-treated rats, which demonstrated attenuated mTOR phosphorylation and downregulation of the proinflammatory mediators (i.e., TNF-α, IL-6, IL-1β, matrix metallopeptidases 2 and 9) to the control level. Further in vitro cell culture experiments using aortic smooth muscle cell (SMC) suggested that the inhibition of the mTOR signaling pathway by rapamycin could promote the differentiation of SMCs, as reflected by the reduced expression of S100A4 and osteopontin. The present study indicated that the early enhanced mTOR signaling pathway in the TAA development and mTOR inhibitor rapamycin may inhibit CaCl2-induced TAA formation.
Keywords: rapamycin, thoracic aortic aneurysm formation, mTOR-mediated suppression, proinflammatory, mediators
Introduction
Thoracic aortic aneurysm (TAA) formation is the 15th leading cause of death in patients of >65 years, and accounts for ~20% of aortic aneurysm cases (1). A previous study (2) has revealed that both environmental (smoking, high blood pressure, high cholesterol) and genetic (a family history of aneurysms or other genetic syndromes) risk factors are involved in the disease development. A common pathogenic feature of TAA is progressive medial degeneration characterized by degeneration of the extracellular matrix, destruction of elastic lamina and loss of vascular smooth muscle cells (SMCs) (3). Although the surgical repair of lesions has become increasingly effective and less invasive, an urgent requirement exists to determine the underlying disease mechanism for early prevention and diversion of treatment from surgical to medical approaches.
Previous studies have demonstrated stimulation of T lymphocytes and significant changes in the expression of extracellular matrix proteolytic system during TAA formation, specifically an imbalance in the system of matrix metallopeptidases (MMPs) and endogenous tissue inhibitors of MMPs (TIMPs) (4,5). T lymphocytes and macrophages increased in the media and adventitia from Marfan syndrome, familial TAA and sporadic cases, when compared with control aortas (6). During aneurysm formation, infiltrating inflammatory cells, for example, macrophages, SMCs, fibroblasts and endothelial cells of blood vessels all can produce MMPs, leading to the degradation of extracellular matrix and remodeling of artery walls (7). Previously, high levels of MMP expression and activity have been observed in natural and experimentally induced aneurysms (8,9). However, the exact mechanism behind these changes remains elusive.
Tuberous sclerosis complex (TSC) is a genetic disorder with pleiotropic manifestations caused by heterozygous mutations in either TSC1 or TSC2. One of the less investigated complications of TSC is the formation of aneurysms, which are pathologically characterized by smooth muscle cell (SMC) proliferation in the aortic media. In vitro SMC studies have revealed that TSC1/TSC2-regulated mammalian target of rapamycin complex 1 (mTORC1) signaling pathway plays a pivotal role in SMC differentiation and proliferation (10,11). Inhibiting mTORC1 signaling with the macrolide antibiotic, rapamycin, promotes SMC differentiation through the activation of the Akt pathway and the induction of contractile protein expression (10). In contrast, the activation of the mTORC1 pathway with TSC2 deficiency leads to SMC proliferation and de-differentiation in vitro and in vivo, which can be reversed with rapamycin treatment (11).
The role of mTOR, the major component of mTORC, in the pathogenesis of human TAA has received little attention. To explore the potential role of the mTOR signaling pathway in TAA development, a series of animal studies were previously conducted using a CaCl2-induced TAA rat model (12). The results provided novel evidence indicating that the activation of the mTOR signaling pathway and enhanced expression of proinflammatory cytokines are important in early TAA development. Most important, CaCl2-induced TAA formation in rats maybe inhibited by rapamycin through suppressing the mTOR signaling pathway and cytokine expression.
Materials and methods
Animal experiments
The experimental study was conducted in accordance with the Guide for the Care and Usage of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA, 2011) and approved by the Committee for Animal Research of Shanghai Jiao Tong University (Shanghai, China).
Male Sprague-Dawley rats (n=70; 8-weeks-old; 250–300 g) were kept in a specific-pathogen-free facility by Ruijin hospital (Shanghai, China). A 12-h light/dark cycle was used and a temperature of 22°C was maintained in the facility. Rats had free access to food and water at all times. All rats were supplied by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and were randomly allocated to TAA group (CaCl2-treated; n=18), sham group (NaCl-treated; n=18), DMSO group (treated with CaCl2 and DMSO; n=12) or rapamycin group (treated with CaCl2 and rapamycin; n=12). The rat TAA model was established with CaCl2, as previously described (12). Briefly, all animals were subjected to orotracheal intubation and mechanical ventilation. The chest cavity was opened, and the descending thoracic aortic segment was wrapped with a strip (sterilized medical cotton pad) presoaked in 0.5 M CaCl2 solution or normal saline for 15 min. The experimental mortality for the treatment and control groups was ~10–20% due to anesthesia side effects or surgical trauma.
For the rapamycin treatment, 2 mg/kg rapamycin (AG Scientific, Inc., San Diego, CA, USA) in DMSO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or DMSO alone (vehicle) was administered once intraperitoneally 1 day prior to the injury and continued for 2 weeks daily.
Tissue isolation
A ketamine/xylazine cocktail made with normal saline was used for deep anesthesia prior to performing terminal tissue collection. Specifically, 80 mg/kg ketamine (Zhejiang Jiuxu Pharmaceutical Co., Ltd., Jinhua, China) and 10 mg/kg xylazine (Selleck Chemicals, Houston, TX, USA) were administered as a single intraperitoneal injection. All rats were euthanized 2 days or 2 weeks following induction. The thorax was opened beneath the xiphoid process. The rats' right atrium was incised to provide an outlet for blood and perfusate. The cardiovascular system was perfused with normal saline from the left ventricle at a pressure of 100 mmHg for 10 min, and then the entire aorta was harvested. Only the part that was treated with the medical cotton pad (CaCl2 or NaCl) was used in the following experiment. Aortic tissues were stored at −80°C until protein isolation was conducted for western blotting analysis. For histological and immunohistochemical studies, the cardiovascular system was subjected to perfusion with 4% formalin for 10 min, stored in 4% formalin solution at 4°C, and then embedded in paraffin. Sections of 5 µm thickness were cut.
Histological and immunohistochemical studies
Formalin-fixed, paraffin-embedded sections were deparaffinized at 56°C, washed with xylene and rehydrated using serial concentrations of ethanol. For immunohistochemistry staining, heat-mediated antigen retrieval (98°C for 6 min) was performed to enhance antigen exposure prior to detection. H2O2 (0.3%) was used to suppress endogenous peroxidase activity to reduce background staining at room temperature for 15 min. Normal goat serum (10%; Fuzhou Maixin Biotech, Co., Ltd., Fuzhou, China) was used prior to immunostaining and incubated with membranes at room temperature for 1 h to reduce non-specific staining. Hematoxylin and eosin (H&E) staining was performed for morphometric analysis. Sections were stained using a modified Verhoeff-Van Gieson (VVG) stain kit (Sigma-Aldrich; Merck KGaA) to evaluate the elastic fiber content following the manufacturer's instructions. Elastin breaks were quantified in triplicate by two independent investigators. The disruption of elastic fibers was graded on a scale of 1–4 (1, normal or disruption <25%; 2, disruption 25–49%; 3, disruption of 50–75%; 4, disruption >75% or total absence).
The antibodies and dilutions used in the present study are listed in Table I. All primary antibodies were diluted and incubated with slides at 4°C overnight. Biotin-conjugated antibodies (cat. no. BA-1300; 1:200; Vector Laboratories, Inc., Burlingame, CA, USA) were used as secondary antibody and were incubated with membranes at room temperature for 1–2 h. For immunohistochemistry, the positively stained area was determined using the threshold-based digital planimetry software (Image-Pro Plus 6.0; Media Cybernetics, Inc., Rockville, MD, USA), and expressed as the percentage of the positive area in the intima-media or adventitia.
Table I.
Antibodies and dilutions.
| Primary antibodies | ||||
|---|---|---|---|---|
| Antibody name | Cat. no. | Company | Species | Dilution |
| MMP-2 | 87809 | Cell signal | Rabbit | 1:100 |
| MMP-9 | 13667 | Cell signal | Rabbit | 1:100 |
| SM α-actin | A2547 | Sigma | Mouse | 1:200 |
| Calponin | sc70487 | Santa cruz | Mouse | 1:100 |
| OPN | ab8448 | Abcam | Rabbit | 1:200 |
| CD68 | ab125212 | Abcam | Rabbit | 1:200 |
| S100A4 | ab41532 | Abcam | Rabbit | 1:400 |
| TNF-α | 8184 | Cell signal | Rabbit | 1:200 |
| IL-6 | 12912 | Cell signal | Rabbit | 1:400 |
| IL-1β | 12703 | Cell signal | Rabbit | 1:200 |
Cell Signal, Cell Signaling Technology, Inc.; Sigma, Sigma-Aldrich; Merck KGaA; Santa Cruz, Santa Cruz Biotechnology, Inc.; Abcam; MMP, matrix metallopeptidase; SM, smooth muscle; OPN, osteopontin; TNF, tumor necrosis factor; IL, interleukin.
Smooth muscle cell isolation, culture, and treatment
SMCs were explanted from the descending thoracic aortas, as previously described (11). SMCs were subcultured in a complete SMC medium (Lonza Group, Basel, Switzerland) supplemented with 20% fetal bovine serum, antibodies, L-glutamine, HEPES, sodium pyruvate, insulin, recombinant human epidermal growth factor, and recombinant human fibroblast growth factor) in a 37°C, 5% CO2-humidified incubator. SMCs from passages 2–6 were used for experiments, and all experiments were confirmed in at least three different explants of SMCs.
SMCs were divided into different groups: group 1 was divided into subgroups treated with different concentrations of epidermal growth factor (EGF; 0, 1.25, 2.5 and 5.0 µg/ml). Group 2 was divided into four subgroups treated with either saline, rapamycin (20 nmol/l), EGF (2.5 µg/ml) or rapamycin (20 nmol/l) + EGF (2.5 µg/ml). Group 3 was divided into four subgroups treated with either saline, rapamycin (20 nmol/l), siTSC2 (small interfering RNA targeting TSC2), and rapamycin (20 nmol/l) + siTSC2.
The TSC2 siRNA and control nonspecific siRNA were synthesized by GeneChem Co., Ltd. (Shanghai, China). SMCs were transfected with TSC2 siRNA according to the manufacturer's instructions. In parallel, untreated cells and cells transfected with nonspecific siRNA were used as controls. The experiments were performed in triplicate for each experimental condition.
Western blot analysis
At each time point, the cells were harvested, washed in ice-cold phosphate-buffered saline (PBS), and lysed with radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck KGaA). The protein was extracted, and its concentration was determined using the Bio-Rad protein assay. Equal cell lysates (10 µg) were separated by 10% SDS-PAGE with Tris-HCl gel (Ready Gel; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and electrotransferred onto polyvinylidene difluoride membranes (Immobilon-P; EMD Millipore, Billerica, MA, USA). Following blocking in buffer (5% non-fat milk in T-PBS) for 3 h, the membranes were incubated overnight at 4°C with a primary antibody diluted in 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA). Western blot analysis was performed using primary antibodies against α-tubulin (cat. no. T8203; 1:2,000; Sigma-Aldrich; Merck KGaA), mTOR (cat. no. 2972; 1:1,000), phospho-mTOR (cat. no. 5536; 1:500), S6 (cat. no. 2317; 1:1,000), phospho-S6 (cat. no. 5364; 1:500), p70S6k (cat. no. 9202; 1:1,000) and phospho-p70S6k (cat. no. 9206; 1:500; all from Cell Signaling Technology, Inc., Danvers, MA, USA), smooth muscle myosin heavy chain (cat. no. ab53219; 1:1,000), osteopontin (OPN; cat. no. ab8448; 1:1,000) and S100A4 (cat. no. ab41532; 1:1,000; all from Abcam, Cambridge, MA, USA). The membranes were probed with horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary antibody (cat. nos. 115-035-003 and 111-035-003, respectively; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The secondary antibody was incubated with membranes at room temperature for 1 h, at a dilution of 1:5,000. Signals were developed using an enhanced chemiluminescence Western Blotting Detection kit (GE Healthcare Life Sciences, Chalfont, UK). α-tubulin was used as a control.
Statistical analysis
The morphometric analysis was performed blinded for the statistician. The results were statistically analyzed using one-way analysis of variance, with the Dunnett's C post hoc test. A two-sided probability level of P<0.05 was considered to indicate a statistically significant difference. All analyses were performed using the SPSS for Windows software (version, 13.0; SPSS, Inc., Chicago, IL, USA).
Results
Molecular alterations in rat TAA induction
Enhanced expression of proinflammatory cytokines
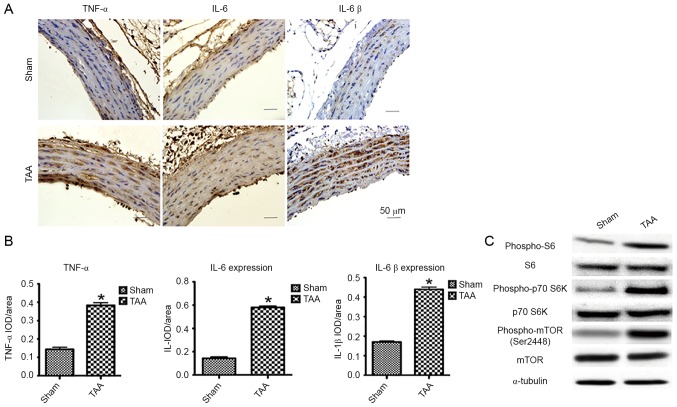
A CaCl2-induced rat TAA model was previously established to explore the potential role of mTOR signaling pathway in the disease development (12). In the present study, male Sprague-Dawley rats (250–300 g) underwent periarterial exposure of thoracic aorta to either 0.5M CaCl2 or normal saline (0.90% NaCl). Strikingly, immunohistochemical assessment of proinflammatory cytokines [i.e., tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β] by computerized planimetry in the aortic adventitia and aortic media revealed enhanced protein expression as early as 2 days following TAA induction (Fig. 1A and B). These results confirmed that the infiltration of inflammatory cells contributes to the pathogenesis of TAA.
Figure 1.
The effect of proinflammatory cytokines and mTOR signaling 2 days following TAA induction. (A) Representative pictures of the immunohistochemical study in aortic segments 2 days post-TAA induction. The slides were stained with TNF-α, IL-6 and IL-1β. The anti-rabbit horseradish peroxidase/diaminobenzidine detection system was used to visualize the expression (brown staining). Scale bar, 50 µm. Sham: NaCl-treated group (n=6). TAA: CaCl2-treated group (n=6). (B) Quantitation of the protein content of TNF-α, IL-6, and IL-1β was performed by computerized planimetry in the aortic adventitia and aortic media in immunohistochemically stained slides. Data are presented as the mean ± standard error of mean, *P<0.05 vs. the Sham group. (C) Representative western blots demonstrated the upregulation of phosphorylation of proteins involved in the mTOR signaling pathway in the CaCl2-treated segment of aorta as early as 2 days, including phospho-mTOR, phospho-p70 S6K and phospho-S6. TNF-α, tumor necrosis factor-α; IL, interleukin; TAA, thoracic aortic aneurysm; IOD, integral optical density.
Enhanced mTOR signaling
Besides the enhanced expression of proinflammatory cytokines, immunoblot analyses revealed a significantly increased phosphorylation of mTOR (Ser2448) and S6K (Thr389) as early as 2 days following TAA induction (Fig. 1C). The activation of S6K was further confirmed by the increased phosphorylation of its substrate, S6, at both Ser240 and Ser244. Interestingly, mTOR signal enhancement was not detected 2 weeks post-induction, suggesting a role of the mTOR signaling pathway in the early development of TAA.
Dedifferentiation of SMCs
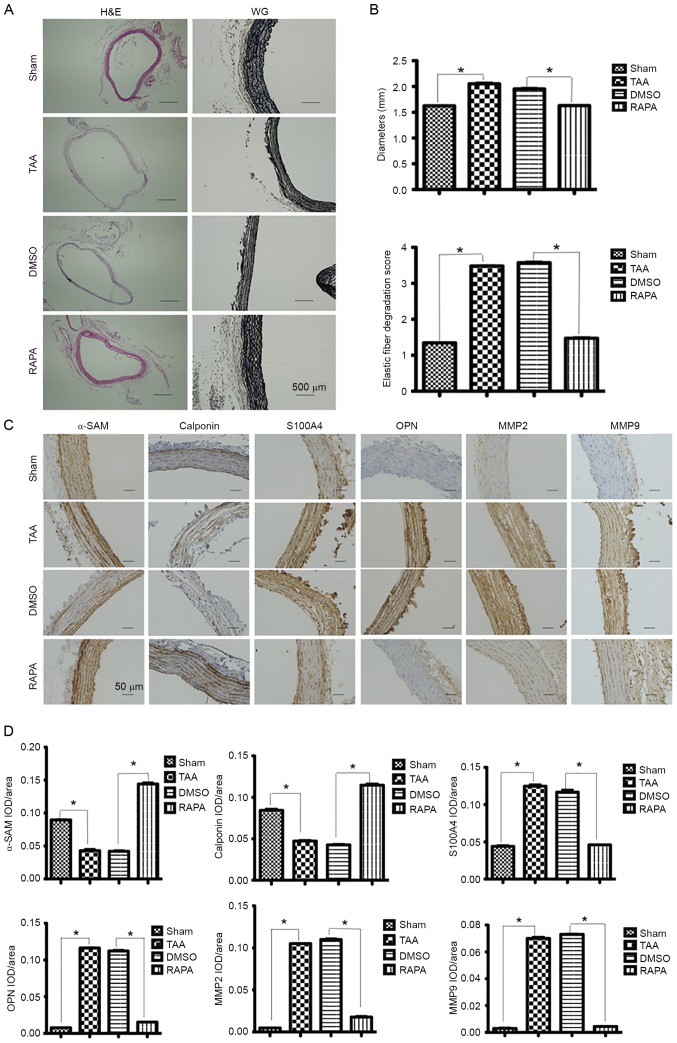
At 2 weeks post-induction, H&E staining was performed for measuring the aortic diameter in each group, presenting aneurysmal alteration in the CaCl2-treated segments (Fig. 2A). In addition, more severely disrupted elastic fibers were observed in CaCl2-treated segments than in CaCl2-untreated segments by VVG staining (Fig. 2A).
Figure 2.
Expression of contractile proteins and dedifferentiation markers in aortic segments 2 weeks post-rapamycin treatment. (A) Representative lower-power micrographs of the hematoxylin and eosin and Verhoeff-Van Gieson staining of NaCl-treated (sham group, n=6) and CaCl2-treated aortas (TAA group, n=6) pretreated with rapamycin (RAPA group, n=8) and DMSO (DMSO group, n=8). Scale bar, 500 µm. (B) Measurements of external media diameter and grading of elastin degradation in the four groups (2 weeks post-TAA induction). Data are presented as the mean ± standard error of mean, *P<0.05 as indicated. (C) Representative pictures of immunohistochemical study in aortic segments 2 weeks post-TAA induction. The slides were stained with αSMA, calponin, S100A4, OPN, MMP2 and MMP9. The anti-rabbit horseradish peroxidase/diaminobenzidine detection system was used to visualize the expression (brown staining). Scale bar, 50 µm. (D) The quantitation of the protein content of αSMA, calponin, S100A4, OPN, MMP2 and MMP9. The immunohistologically stained slides were assessed by computerized planimetry in the aortic adventitia and aortic media. Data are presented as the mean ± standard error of mean. *P<0.05 as indicated. TAA, thoracic aortic aneurysm; RAPA, rapamycin; MMP, matrix metallopeptidase; OPN, osteopontin; αSMA, α-smooth muscle actin; IOD, integral optical density.
SMCs contract in response to changes in pulse pressures through a cyclic interaction between thin and thick contractile filaments composed of SMC-specific isoforms of α-actin (α-SMA) and myosin heavy chain, respectively. Heterozygous mutations in α-SMA, encoded by the ACTA2 gene, predispose TAAs and acute aortic dissections (13). Therefore, the present study aimed to determine the expression of contractile proteins including α-SMA and calponin in aortic SMCs. Immunohistochemical staining revealed decreased expression of both proteins in the CaCl2-treated segments. In contrast, an increased expression of SMC dedifferentiation markers S100A4 and osteopontin (OPN) was observed in the CaCl2-treated segments (Fig. 2C).
Enhanced expression of MMP2 and MMP9
MMPs are a family of zinc-dependent endopeptidases capable of degrading different extracellular matrix components including collagens and elastin. Under normal physiological conditions, the enzymatic activity of MMPs is tightly controlled by their TIMPs that always keep a balance in the MMP/TIMP system, but in vascular pathologies (e.g., aneurysm, vasculitis, atherosclerosis), the increased expression of MMPs has been often observed (14–16). At 2 weeks post-induction, MMP-2 expression was detected diffusely across the entire layers of CaCl2-treated segments, which was significantly higher than the expression across the NaCl-treated segments (Fig. 2C). Similar changes were also observed for MMP-9 expression (Fig. 2C).
Reversal effects of rapamycin pretreatment
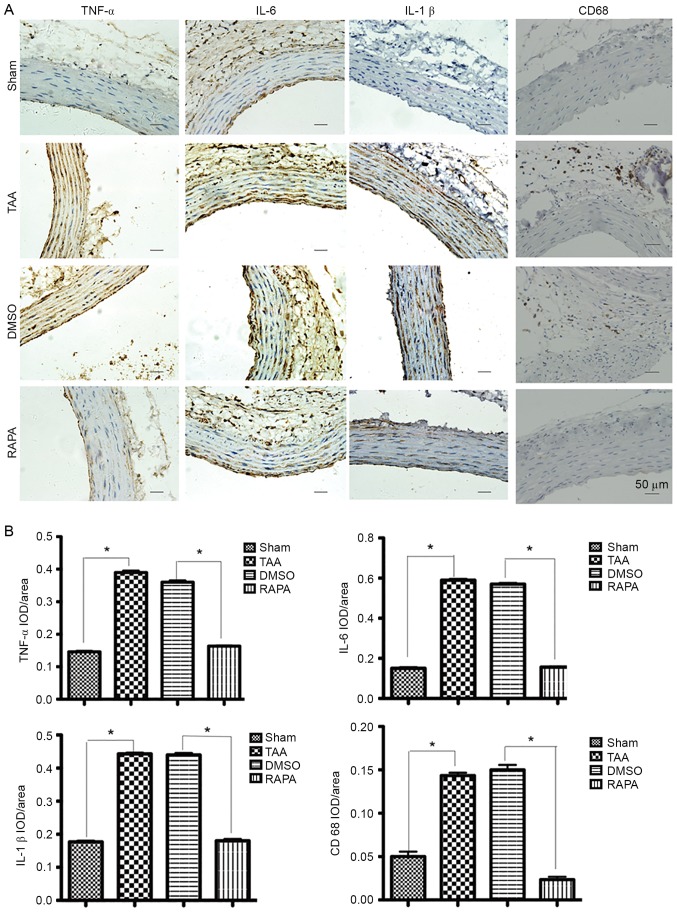
Pretreatment with rapamycin, a pharmacological inhibitor of the mTOR signaling pathway, reversed the augmentation of CaCl2-treated segments (Fig. 2A) in both diameters and elastic fiber degradation. Fig. 3 demonstrated that, with rapamycin pretreatment (RAPA group), IOD (integral optical density) values of TNF-α, IL-6 and IL-1β significantly decreased (P<0.01) compared with those without treatment (TAA and DMSO groups). As presented in Fig. 3A, in the RAPA and sham groups, immunostaining-positive cells in the media layer of aorta were arranged in line with less cytoplasm coloring. In contrast, in the TAA and DMSO groups, a large number of immunostaining-positive cells, in a mass, presented deep cytoplasm coloring. Staining with a macrophage-specific antibody indicated that all aneurysm tissue sections contained CD68+ macrophages that were frequently present diffusely in the adventitia of the aortas from the CaCl2-treated group at 2 weeks, but were rarely found in the control aortas (Fig. 3A, CD68 staining). IOD values of CD68 in the RAPA group significantly decreased (P<0.01) compared with those without treatment (TAA and DMSO groups). The results suggested that rapamycin pretreatment suppressed the hypersecretion of TNF-α, IL-6, IL-1β and CD68 and therefore protected the aorta against proinflammatory stress.
Figure 3.
Representative pictures of immunohistochemical study in aortic segments at 2 weeks post-rapamycin treatment. (A) The slides were stained with TNF-α, IL-6, IL-1β and CD68. The anti-rabbit horseradish peroxidase/diaminobenzidine detection system was used to visualize the expression (brown staining). Scale bar, 50 µm. (Sham group, n=6. TAA group, n=6. RAPA group, n=8. DMSO group, n=8). (B) The quantitation of the protein content of TNF-α, IL-6, IL-1β and CD68. The immunohistologically stained slides were assessed by computerized planimetry in the aortic adventitia and aortic media. Data are presented as the mean ± standard error of mean. *P<0.05 as indicated. RAPA, rapamycin; TNF, tumor necrosis factor; IL, interleukin; IOD, integral optical density.
Immunohistochemical assays of α-actin and calponin contractile proteins revealed that the expression of both proteins progressively increased following treatment with rapamycin (Fig. 2C). In contrast, following treatment with rapamycin, a significant reduction in expression was observed for dedifferentiation markers S100A4 and OPN (Fig. 2C).
As described previously, in the CaCl2-treated segments, MMP-2 significantly increased in the entire layers of aorta, while the NaCl-treated segments presented only mild staining (Fig. 2C). MMP-9 protein was also demonstrated at a peak level in the adventitia and elastic lamellae 2 weeks post-TAA induction. Following treatment with rapamycin, a significant reduction in MMP-2 and MMP-9 was observed (Fig. 2C).
Further evidence revealed by in vitro cell culture studies
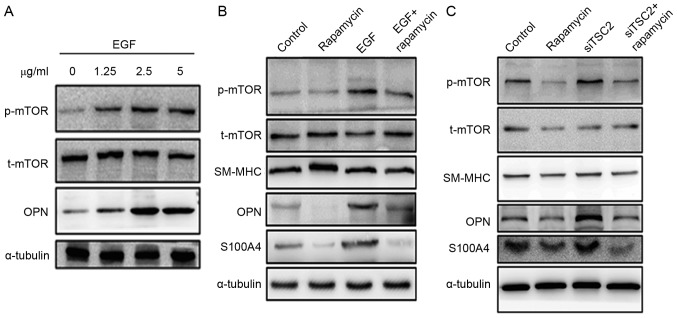
To further explore the mechanism underlying the rapamycin-associated inhibition of TAA formation, rat aortic SMCs were isolated to conduct the in vitro cell culture assay. mTOR signaling was induced by treating cells with different concentrations of EGF (0, 1.25, 2.5 and 5.0 µg/ml). The EGF treatment of SMCs was indicated to activate mTOR signaling and led to an increased expression of dedifferentiation marker OPN (Fig. 4A). However, pretreating aortic SMCs with rapamycin inhibited EGF-induced mTOR activation, leading to decreased expression of OPN and S100A4 (Fig. 4B).
Figure 4.
Expression of the change in mTOR, αSMA and OPN/S100A4 in aortic SMCs. (A) SMCs explanted from the aorta of rats were cultured at EGF concentrations of 0, 1.25, 2.5 and 5 µg/ml for 72 h. Western blot analysis demonstrated that EGF induced mTOR signaling in a dose-dependent manner. In addition, OPN expression increased. (B) Rapamycin treatment inhibited the expression of OPN and S100A4. Pretreating SMCs with rapamycin could inhibit EGF-induced mTOR activation. (C) Downregulation of TSC2 expression with SiTSC2 would activate the mTOR signaling pathway. Also, the expression of OPN and S100A4 increased when SMCs were pretreated with siTSC2. On pretreating SMCs with rapamycin, the activation of mTOR signaling pathway was inhibited. In the siTSC2 and rapamycin-double-treated cells, the expression levels of OPN and S100A4 were similar to the levels observed in rapamycin-treated cells. α-tubulin was used as a control. αSMA, α-smooth muscle actin; OPN, osteopontin; SMC, smooth muscle cell; EGF, epidermal growth factor; SM-MHC, smooth muscle, myosin heavy chain.
In another study, siRNA technology was used to downregulate TSC2 expression (11). TSC2 is a negative regulator of mTOR signaling (11). Therefore, when TSC2 was downregulated, the mTOR signaling pathway was activated. An increased expression of OPN and S100A4 was observed with TSC2 downregulation. However, when the cells were pretreated with rapamycin, the activation of the mTOR signaling pathway was inhibited, which is consistent with the fact that mTOR is a downstream mediator of TSC2. In the siTSC2 and rapamycin-double-treated cells, the expression levels of OPN and S100A4 were similar to the levels observed in rapamycin-treated cells (Fig. 4C).
Discussion
Aortic aneurysms are classified in terms of their anatomical location, and most commonly occur in the infrarenal abdominal aorta and thoracic aorta. AAAs primarily affect the elderly population and are characterized by atherosclerotic changes with the chronic inflammation of the aortic wall (17). By contrast, TAAs affect a younger population; the pathology present in the aortic wall of these patients is medial degeneration, which is described as a lesion characterized by the triad of loss of SMCs, fragmented and diminished number of elastic fibers and increased accumulation of proteoglycans (18). Medial degeneration that is associated with TAAs and TADs (thoracic aortic dissections) was originally described by Erdheim as a noninflammatory lesion (18). However, more recent previous evidence indicated that T lymphocytes and macrophages were common features in the aortas of patients with medial degeneration (19–22). Previous studies have documented an inflammatory infiltrate in the aortic wall of patients with TAA. In aortas of patients undergoing the prophylactic repair of TAAs, a significant increase in the number of CD3+ and CD68+ cells was observed throughout the aortic media and adventitia when compared with control aortas (6). Other investigators have also documented an inflammatory infiltrate in the aortic wall, which was associated with the IFN-γ production (23). The present study confirmed the aforementioned findings and further characterized the inflammatory infiltrate in aortas of the rat TAA model. Staining with the macrophage-specific antibody indicated that CD68+ macrophages were frequently present in the adventitia of the aortas from the TAA model, but were rarely found in the control aortas (Fig. 3A, CD68 staining). In addition, the results of the present study parallel the results obtained with a novel mouse model of TAAs, the IL-1 receptor antagonist-deficient mouse (IL1-Ra−/−) (24). In this mouse model, inflammatory aortitis of the ascending aorta developed in the deficient mice, which was not present in the wild-type mice. The medial infiltration of T lymphocytes and macrophages was observed, although the phenotype and biological activity of invading T cells and macrophages in TAAs should be further characterized before drawing conclusions regarding their relevance to the disease process (24).
Increased circulating levels of inflammatory cytokines, such as IL-1, IL-6 and TNF-α, were identified in patients with AAA (25–28). IL-6 is of major importance for the aneurysmal process in humans (26). IL-1β protein levels were measured in human TAA and control aortas; it increased ~20-fold in human TAAs (29). Genetic deletion of IL-1β and IL-1R significantly decreased thoracic aortic dilation in the mice TAA model (29). In the present TAA rat model, the enhanced expression of proinflammatory cytokines, such as IL-6, IL-1β, and TNF-α, was observed 2 days following TAA induction (Fig. 1A and B).
Contractile proteins and biomarkers associated with the differentiation status of SMCs were also examined in the present study, along with extracellular matrix proteolytic proteins. At 2 days post-TAA induction, the expression of contractile proteins, including α-SMA and calponin, significantly decreased compared with the control group (Fig. 2C and D). In contrast, the expression of biomarkers OPN and S100A4, as well as MMP2 and MMP9, increased. OPN is a multifunctional glycophosphoprotein with a regulatory function in bone remodeling and in the synthesis of collagen fibers (16). Increased OPN levels may indicate the transition phase from the contractile to the synthetic form of SMCs in the tunica media, which might lead to a higher aortic diameter or aortic dissection (30). Based on these observations, OPN might serve as an early marker for changes leading to SMC activation in the aortic wall (16,30). In a previous study, the S100A4/MMP-expressing cells were also identified as CD68 positive, suggesting an inflammatory property for the cells (9). S100A4 has been suggested as a proinflammatory factor due to its contribution to proliferation, inflammatory angiogenesis, and extracellular matrix remodeling (31–33). An early observation of increased MMP2 and MMP9 expression in the present study has presented another line of evidence supporting a critical role of extracellular matrix remodeling in TAA development.
So far, the role of mTOR signaling in the pathogenesis of human TAA has remained elusive and received little attention. In SMCs, however, mTORC1 signaling is already known to influence SMC differentiation, and inhibiting mTORC1 signaling with the macrolide antibiotic rapamycin promotes SMC differentiation through the activation of the Akt pathway and the induction of contractile protein expression (10,34). A previous study on the Tsc2+/− mice demonstrated that the activation of the mTORC1 pathway with Tsc2 deficiency leads to SMC proliferation and dedifferentiation in vitro and in vivo, which can be reversed with rapamycin treatment (11). In the present study, the enhanced expression of proinflammatory cytokines and mTOR signaling were observed as early as 2 days following TAA induction. In contrast, mTOR enhancement was not detected 2 weeks following induction, suggesting an important role of the mTOR signaling pathway in the early development of TAA.
The most exciting observation in the present study is that rapamycin pretreatment was capable of effectively reversing the formation of aortic aneurysms augmented likely by downregulating the expression of proinflammatory molecules. Inhibiting mTORC1 using rapamycin may also promote differentiation through the activation of the Akt pathway and the induction of SMC contractile protein expression (10) (as also presented in the present study). The effectiveness of rapamycin in vivo is illustrated by the success of rapamycin-eluting stents in preventing stent occlusion due to SMC proliferation (35). These clinical studies and the present data suggest a potential therapeutic use of rapamycin or rapamycin-eluting stents in treating aortic aneurysms.
In the in vitro cell culture assay used in the present study, mTOR signaling was induced by treating explanted vascular SMCs with different concentrations of EGF. SMCs are not terminally differentiated and maintain a phenotypic plasticity that allows for transition from quiescent, differentiated cells expressing a repertoire of proteins required for contractile function (α-actin, calponin and β-myosin heavy chain) to proliferating, migrating cells with the loss of contractile protein expression and increased synthesis of extracellular matrix proteins. SMCs are differentiated cells in a mature, functional artery, which dedifferentiate with vascular injury and environmental cues. In the current study, SMCs of wild-type rats were explanted from the descending thoracic aorta. The EGF treatment of SMCs was indicated to activate mTOR signaling and led to the increased expression of dedifferentiation marker OPN (Fig. 4A). Increased OPN levels may indicate the transition from the contractile to the synthetic form of SMCs in the tunica media, which may lead to a higher aortic diameter or aortic dissection. Consistent with the animal study, it was observed that pretreating aortic SMCs with rapamycin could inhibit EGF-induced mTOR activation, leading to decreased expression of OPN and S100A4 (Fig. 4B).
As the major effectors of the mTOR signaling pathway, TSC1 and TSC2 are ubiquitously expressed and form heterodimers that inhibit the activation of mTOR signaling. The TSC1/TSC2 complex negatively regulates mTOR through the GTPase-activating protein activity. siRNA technology was used to test whether TSC2 functions as a regulator of mTOR expression. When TSC2 was downregulated, the mTOR signaling pathway was activated. Consequently, the expression of OPN and S100A4 increased (Fig. 4C). However, when the cells were pretreated with rapamycin, activation of the mTOR signaling pathway was inhibited, which is consistent with the fact that mTOR is a downstream mediator of TSC2. In the siTSC2 and rapamycin-double treated cells, the expression levels of OPN and S100A4 were similar to the levels observed in rapamycin-treated cells.
In summary, the current experimental evidence indicated that the early enhanced mTOR signaling pathway and increased proinflammatory cytokines in the early stage of TAA development and mTOR inhibitor rapamycin can inhibit CaCl2-induced TAA formation. This effect appears to be because of the decrease in the proinflammatory factors.
Acknowledgements
The present study was supported by the grant from the National Natural Science Foundation of China (grant nos. 30971211, and 81170284 to Y.C. and 81570226 to J.C.).
References
- 1.National Center for Injury Prevention and Control: WISQARS Leading Causes of Death Reports. 1999–2006 [Google Scholar]
- 2.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17vii:615–635. doi: 10.1016/S0733-8651(05)70105-3. [DOI] [PubMed] [Google Scholar]
- 3.El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–786. doi: 10.1038/nrcardio.2009.191. [DOI] [PubMed] [Google Scholar]
- 4.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, III, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–1036. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 6.He R, Guo DC, Sun W, Papke CL, Duraisamy S, Estrera AL, Safi HJ, Ahn C, Buja LM, Arnett FC, et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg. 2008;136:922–929, 929.e1. doi: 10.1016/j.jtcvs.2007.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Shen YH, LeMaire SA. Thoracic aortic dissection: Are matrix metalloproteinases involved? Vascular. 2009;17:147–157. doi: 10.2310/6670.2008.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JA, Barbour JR, Lowry AS, Bouges S, Beck C, McClister DM, Jr, Mukherjee R, Ikonomidis JS. Spatiotemporal expression and localization of matrix metalloproteinas-9 in a murine model of thoracic aortic aneurysm. J Vasc Surg. 2006;44:1314–1321. doi: 10.1016/j.jvs.2006.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J, Geng L, Wu Q, Wang W, Chen Q, Lu L, Shen W, Chen Y. Spatiotemporal expression of matrix metalloproteinases (MMPs) is regulated by the Ca2+-signal transducer S100A4 in the pathogenesis of thoracic aortic aneurysm. PLoS One. 2013;8:e70057. doi: 10.1371/journal.pone.0070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Gong L, Guo DC, Mietzsch U, Kuang SQ, Kwartler CS, Safi H, Estrera A, Gambello MJ, Milewicz DM. Thoracic aortic disease in tuberous sclerosis complex: Molecular pathogenesis and potential therapies in Tsc2+/− mice. Hum Mol Genet. 2010;19:1908–1920. doi: 10.1093/hmg/ddq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng L, Wang W, Chen Y, Cao J, Lu L, Chen Q, He R, Shen W. Elevation of ADAM10, ADAM17, MMP-2 and MMP-9 expression with media degeneration features CaCl2-induced thoracic aortic aneurysm in a rat model. Exp Mol Pathol. 2010;89:72–81. doi: 10.1016/j.yexmp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 14.Dodd T, Jadhav R, Wiggins L, Stewart J, Smith E, Russell JC, Rocic P. MMPs 2 and 9 are essential for coronary collateral growth and are prominently regulated by p38 MAPK. J Mol Cell Cardiol. 2011;51:1015–1025. doi: 10.1016/j.yjmcc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabek J, Glogowska-Ligus J, Szadorska B. Transcription activity of MMP-2 and MMP-9 metalloproteinase genes and their tissue inhibitor (TIMP-2) in acute coronary syndrome patients. J Postgrad Med. 2013;59:115–120. doi: 10.4103/0022-3859.113836. [DOI] [PubMed] [Google Scholar]
- 16.Huusko T, Salonurmi T, Taskinen P, Liinamaa J, Juvonen T, Pääkkö P, Savolainen M, Kakko S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2013;145:1117–1123. doi: 10.1016/j.jtcvs.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 18.Erdheim J. Medionecrosis aortae idiopathica cystica. Virchows Arch (Pathol Anat) 1930;276:187–229. doi: 10.1007/BF02275142. [DOI] [Google Scholar]
- 19.Girardi LN, Coselli JS. Inflammatory aneurysm of the ascending aorta and aortic arch. Ann Thorac Surg. 1997;64:251–253. doi: 10.1016/S0003-4975(97)00458-X. [DOI] [PubMed] [Google Scholar]
- 20.Biddinger A, Rocklin M, Coselli J, Milewicz DM. Familial thoracic aortic dilatations and dissections: A case control study. J Vasc Surg. 1997;25:506–511. doi: 10.1016/S0741-5214(97)70261-1. [DOI] [PubMed] [Google Scholar]
- 21.Roth M, Lemke P, Bohle RM, Klovekorn WP, Bauer EP. Inflammatory aneurysm of the ascending thoracic aorta. J Thorac Cardiovasc Surg. 2002;123:822–824. doi: 10.1067/mtc.2002.121291. [DOI] [PubMed] [Google Scholar]
- 22.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Tang PC, Yakimov AO, Teesdale MA, Coady MA, Dardik A, Elefteriades JA, Tellides G. Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J. 2005;19:1528–1530. doi: 10.1096/fj.05-3671fje. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist-deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111:3135–3140. doi: 10.1161/CIRCULATIONAHA.104.519132. [DOI] [PubMed] [Google Scholar]
- 25.Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–156. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 26.Jones KG, Brull DJ, Brown LC, Sian M, Greenhalgh RM, Humphries SE, Powell JT. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation. 2001;103:2260–2265. doi: 10.1161/01.CIR.103.18.2260. [DOI] [PubMed] [Google Scholar]
- 27.Schönbeck U, Sukhova GK, Gerdes N, Libby P. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. doi: 10.1016/S0002-9440(10)64206-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: Implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.CIR.100.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, Lu G, Owens GK, Upchurch GR, Jr, Ailawadi G. Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130(11 Suppl 1):51–59. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesauskaite V, Epistolato MC, Castagnini M, Urbonavicius S, Tanganelli P. Expression of matrix metalloproteinases, their tissue inhibitors, and osteopontin in the wall of thoracic and abdominal aortas with dilatative pathology. Hum Pathol. 2006;37:1076–1084. doi: 10.1016/j.humpath.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Boye K, Maelandsmo GM. S100A4 and metastasis: A small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senolt L, Grigorian M, Lukanidin E, Simmen B, Michel BA, Pavelka K, Gay RE, Gay S, Neidhart M. S100A4 is expressed at site of invasion in rheumatoid arthritis synovium and modulates production of matrix metalloproteinases. Ann Rheum Dis. 2006;65:1645–1648. doi: 10.1136/ard.2005.047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oslejsková L, Grigorian M, Gay S, Neidhart M, Senolt L. The metastasis associated protein S100A4: A potential novel link to inflammation and consequent aggressive behaviour of rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis. 2008;67:1499–1504. doi: 10.1136/ard.2007.079905. [DOI] [PubMed] [Google Scholar]
- 34.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 35.Holmes DR, Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, Brown C, Fischell T, Wong SC, Midei M, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109:634–640. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]