Abstract
Molecule-targeted therapy, such as sorafenib, is one of the effectively therapeutic options for advanced hepatocellular carcinoma (HCC). However, acquired resistance to sorafenib has been found in some HCC patients, resulting in poor prognosis. It is reported that PD-L1 and DNA methyltransferases (DNMTs) contribute to drug resistance. In this study, by inducing sorafenib-resistant HCC cell lines, we investigated their molecular and functional characteristics. Our data indicated that highly upregulated DNMT1 was positively correlated with PD-L1 overexpression in sorafenib-resistant HCC cells. We demonstrate that PD-L1 regulate DNMT1 through STAT3 signaling pathway. Knockdown of PD-L1 induced DNMT1-dependent DNA hypomethylation and restored the expression of methylation-silenced CDH1. Moreover, inactivation of NFκB blocked PD-L1/STAT3/DNMT1 pathway in sorafenib-resistant HCC cells. Functionally, genetic or pharmacological disruption of PD-L1 or/and DNMT1 sensitize HCC resistance to sorafenib. Importantly, dual inactivation of PD-L1 and DNMT1 by their inhibitor synergistically disrupts the colony formation of sorafenib-resistant HCC cells. These results demonstrate that targeting NFκB/PDL1/STAT3/DNMT1 axis is a new therapeutic strategy for preventing or overcoming the acquired resistance to sorafenib in HCC patients.
Keywords: hepatocellular carcinoma, sorafenib resistance, PD-L1, DNMT1, DNA methylation
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequently diagnosed cancer across the globe, and the most common primary liver tumor with increasing incidence worldwide. Targeted therapy is one of the effectively therapeutic options for advanced HCC during the past few years (1). Sorafenib is a multitargeted tyrosine kinase inhibitor for the treatment of HCC that blocks the Ras, VEGFR, PDGFR, FLT3 and KIT kinases, which increases the rate of apoptosis and inhibits cell proliferation, migration and tumor angiogenesis (2). However, acquired resistance to sorafenib has been found in HCC patients, which results in poor prognosis. The limited survival benefit from these clinical trials suggests the existence of primary and acquired sorafenib resistance mechanisms in HCC cells. Recently, some studies report novel molecular mechanism of sorafenib resistance in HCC cells. A low-molecular weight and cysteine-rich proteins, metallothionein (MT)-1G is reported as a critical regulator and promising therapeutic target of sorafenib resistance in human HCC cells (3). Using in vivo RNAi screening, Rudalska et al suggest that Mapk14 blockade is a promising approach to overcoming therapy resistance of human HCC (4). Tumor-associated neutrophils (TANs) mediated the intratumoral infiltration of macrophages and Treg cells by secreting CCL2 and CCL17, which stimulated neovascularization, enhanced HCC growth and metastasis, and contributed to sorafenib resistance, suggesting that TAN depletion could enhance the efficacy of sorafenib as an anti-HCC therapeutic (5). However, the resistance mechanism remains poorly understood.
Tumor cells often overexpress immune checkpoint proteins to allow them to evade the host immune system by inhibiting T-cell attack. One of these immune checkpoint proteins is programmed death-ligand-1 (PD-L1), which binds to programmed death-1 (PD-1) expressed on T-cells, B-cells, dendritic cells and natural killer T-cells to suppress anticancer immunity (6). Therefore, anti-PD-L1 and anti-PD-1 antibodies have been used for the treatment of cancer, showing promising outcomes (7,8). Moreover, PD-L1 also overexpressed drug-resistant cancer cells, such as enzalutamide-resistant prostate cancer (9), cisplatin-resistant small cell lung cancer cells (10). Despite the importance of PD-L1 in tumor immunity and drug resistance, the regulation of PD-L1 expression remains poorly understood. Zhu et al found PDL1 is a direct target of BRD4-mediated gene transcription. BET inhibitors suppress PD-L1 expression in both immune cells and ovarian cancer cells (11). Hypoxia upregulates PD-L1 on mouse and human tumor cell lines and on macrophages and DCs from naive C57BL/6 mice (12). Transcriptional factor MYC directly regulates CD47 and PD-L1 at the transcriptional level by binding to their promoters in human melanoma (13). Lo et al showed that inflammation increases PD-L1 expression in tumors through TNF-α-mediated activation of NFκB, leading to transactivation of CSN5 (14).
Epigenetic changes such as DNA methylation act to regulate gene expression in normal mammalian development. However, promoter hypermethylation also plays a major role in cancer through transcriptional silencing of critical growth regulators such as tumor suppressor genes (15). DNA methylation is controlled at several different levels in normal and tumor cells. The addition of methyl groups is carried out by a family of enzymes, DNA methyltransferases (DNMTs). DNMTs are enzymes that catalyze the addition of methyl groups to cytosine residues in DNA. DNMTs found in mammalian cells include DNMT1, DNMT3a, and DNMT3b (16,17).
In this study, we modeled sorafenib resistance in HCC cell lines, and explored the molecular and functional characteristics of resistant cells. We demonstrate NFκB/PDL1/STAT3/DNMT1 axis as a mechanism by which HCC cells develop sorafenib-resistance phenotypes and established its potential as a new therapeutic target for preventing or overcoming the acquired resistance to sorafenib.
Materials and methods
Plasmids, cell lines and chemicals
The shRNA and control vectors for PD-L1, DNMT1, STAT3 and NFκB were obtained from BMGC RNAi (University of Minnesota). Cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). HepG2 and Huh7 cell lines were grown in DMEM with 10% fetal bovine serum (FBS) (Life Technologies, Grand Island, NY, USA) at 37°C under 5% CO2. For the drug treatment, cells were treated with the following reagents used at concentrations, times and schedules indicated in Results. Sorafenib, decitabine (5-aza-2′-deoxycytidine or Dacogen) and Bay 11–7082 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Generation of sorafenib-resistant cells
HepG2 and Huh7 sorafenib-resistant cells (HepG2SR and Huh7SR) were cultured continuously with a step-wise increase of sorafenib concentrations for 8 weeks (0–20 µM). HepG2 and Huh7 parental cells (HepG2C and Huh7C) were cultured in parallel without sorafenib and served as control.
Transfections
Approximately 1×106 cells were seeded into 6-well plates overnight before transfection. The shRNA or plasmids were introduced into cells using Lipofectamine™ RNAiMAX or Lipofectamine™ 2000 reagent (Life Technologies), respectively, according to the manufacturer's instructions.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as described previously using EZ-ChIP assay kit (Millipore, Billerica, MA, USA). Briefly, ~2×106 transfected cells were cross-linked with 1% formaldehyde (Sigma-Aldrich), washed and resuspended in 1% SDS lysis buffer for sonication, in order to yield DNA fragments with an average size of 300–500 bp. The lysates were immunoprecipitated by 5 µg of antibody. Aliquots (1%) were reserved for the negative control (input DNA). ChIP DNA was quantified by qPCR with Power SYBR® Green PCR Master Mix. Fold change in binding was compared using the corresponding input DNA. The primers specific for DNMT1 or PDL1 gene promoter were: hDNMT1-ChIP1 forward, AATAGATGGAGGTTGGAT; reverse, AGGCATTCATTCATTCAT. hDNMT1-ChIP2 forward, CTATACACTGTGAGATTCTTG; reverse CTGGC TATACGACCTTAG. The anti-STAT3, anti-phospho-STAT3 (Tyr705), anti-NFκB and anti-phospho-NFκB (Cell Signaling Technology) were used.
Clonogenic assays
Methylcellulose colony formation assays were performed in MethoCult® medium (Stem Cell Technologies, Canada) according to the manufacturer's instructions. Briefly, at 6 h after transfection or exposure to drugs, 500 cells were harvested and diluted in 0.3 ml of IMDM + 2% FBS (Stem Cell Technologies), then mixed diluted cells in MethoCult® medium. Subsequently 1.1 ml of the MethoCult mixture was dispensed into a 35-mm dish. Colonies were scored in 7–10 days.
Western blotting
After the various treatments, the whole cellular lysates were prepared by harvesting the cells in 1X cell lysis buffer [20 mM HEPES (pH 7.6), 150 mM NaCl and 0.1% NP40] supplemented with 1X phosphatase inhibitor Cocktail 2 and 3 (Sigma-Aldrich), 1 mM PMSF (Sigma-Aldrich) and 1X protease inhibitors (protease inhibitor cocktail set III, Calbiochem-Novabiochem, San Diego, CA, USA). Protein was resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Amersham, Piscataway, NJ, USA). The antibodies used were β-actin (Santa Cruz Biotechnology); DNMT1 (New England Biolabs, Ipswich, MA, USA), the anti-STAT3, anti-phospho-STAT3 (Tyr705), anti-NFκB and anti-phospho-NFκB, anti-PD-L1 (Cell Signaling Technology).
RNA isolation, cDNA preparation and quantitative PCR
RNA was isolated using miRNAeasy kit (Qiagen) according to the manufacturer's instructions. Reverse transcription for obtaining cDNA was performed according to the manufacturer's instructions (Invitrogen). The expression of DNMT1, PD-L1, CDH1 and GAPDH gene was evaluated by SYBR Green Quantitative PCR. Expression of the target genes was measured using the ∆CT approach. The primers were: DNMT1 forward, 5′-CCAGATGAGGACAATGAG-3′; reverse, 5′-AGCAAGACAACCATAATCA-3′. PD-L1 forward, 5′-TCCACTCAATGCCTCAAT-3′; reverse, 5′-GAAGACCTCACAGACTCAA-3′. GAPDH forward, 5′-ACAGGATTGACAGATTGA-3′; reverse, 5′-TATCGGAATTAACCAGACA-3′. CDH1 forward, 5′-AGAACGCATTGCCACATACAC-3′; reverse, GAGGATGGTGTAAGCGATGG-3′.
Bisulfite sequencing
Total DNA sample (2 µg) was converted and purified using EpiTect Bisulfite kit (Qiagen) according to the manufacturer's instructions. The −251 to +139 region within the CDH1 CpG was amplified from bisulfite-treated DNA sample by PCR using the following primers: forward, 5′-TTTTTTTTGATTTTAGGTTTTAGTGAG-3′; reverse, 5′-ACTCCAAAAACCCATAACTAACC-3′. The PCR products were subcloned using the TA Cloning® kit (Invitrogen), and sequenced by Genewiz Company.
Statistical analysis
The qPCR and colony assay were analyzed using the Student's t-test. Correlation data were performed with Pearson correlation coefficients. The statistical analysis were carried out using GraphPad Prism 5.0. Differences were considered statistically significant at P<0.05. All P-values were two-tailed.
Results
Highly upregulated DNMT1 is positively correlated with PD-L1 overexpression in sorafenib-resistant HCC cells
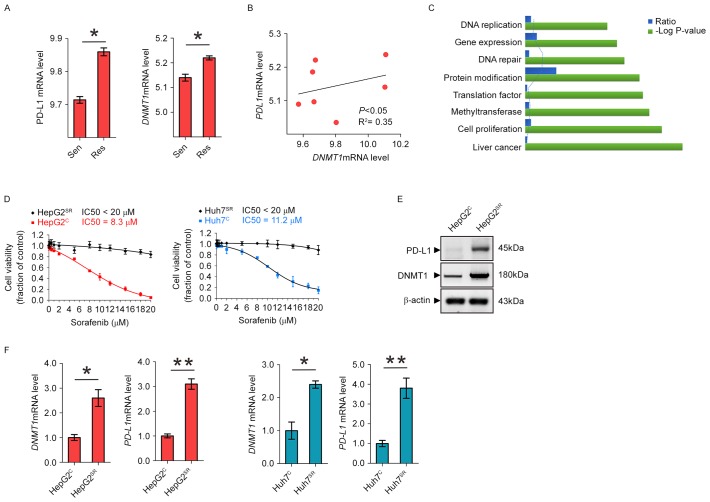
To determine whether PD-L1 and DNMT1 expression is upregulated in sorafenib-resistant HCC cells, we analyzed the GEO data discovered a significant PD-L1 and DNMT1 upregulation in sorafenib-resistant HCC mice (Fig. 1A). In addition, as shown in Fig. 1B, we found that in HCC mice, higher levels of DNMT1 are accompanied by PD-L1 overexpression, while lower expression of DNMT1 is observed in mice carrying lower PDL1 mRNA level, indicating of a positive correlation between these variables (P<0.05). To better understand which biological functions are affected by long-term sorafenib treatment, we conducted functional annotations using DAVID bioinformatics resources 6.7. Enrichment scores for Gene Ontology (GO) categories in overlapped genes (Fig. 1C). To further understand the sorafenib resistance mechanism, we established two HCC sorafenib-resistant cell lines, HepG2SR and Huh7SR, by the stepwise increase of drug dosages and continuous culture in drug-containing medium for 2 months. The final concentrations were 20 µM of sorafenib, which exerted sufficient inhibitory action and were in the range of clinically achievable levels (18). HepG2SR and Huh7SR exhibited significantly higher IC50 value than their parental control HepG2C and Huh7C (Fig. 1D). Notably, comparing to the parental cells, the protein expression and RNA level of DNMT1 and PD-L1 were all increased in HepG2SR and Huh7SR (Fig. 1E and F). These results support the highly upregulated DNMT1 is positively correlated with PD-L1 overexpression in sorafenib-resistant HCC cells.
Figure 1.
PD-L1 and DNMT1 are frequently overexpressed and positively correlated in HCC resistance to sorafenib. (A) GEO data were analyzed for PD-L1 and DNMT1 expression in sorafenib-sensitive mice versus sorafenib-resistant mice. (B) The analysis of GEO dataset GSE73571 showing the correlation between PD-L1 and DNMT1 expression in sorafenib-resistant mice, GSE73571. Correlation between PD-L1 and DNMT1 was assessed by Pearson correlation. P<0.05 was considered statistically significant. (C) Enrichment scores for Gene Ontology (GO) categories in overlapped genes. The -log (P-value) axis indicates the statistical significance of the functions to the dataset. (D) Sorafenib-resistant cells were treated with sorafenib for 72 h. The cell proliferation was assessed by CCK-8 assays. (E) Western blotting for PD-L1 and DNMT1 expression in HepG2C and HepG2SR cells. (F) qPCR measuring the expression levels of indicated genes in HepG2C and Huh7C vs HepG2SR and Huh7SR cells. Data are mean ± SD, *P<0.05, **P<0.01. The data represent three independent experiments.
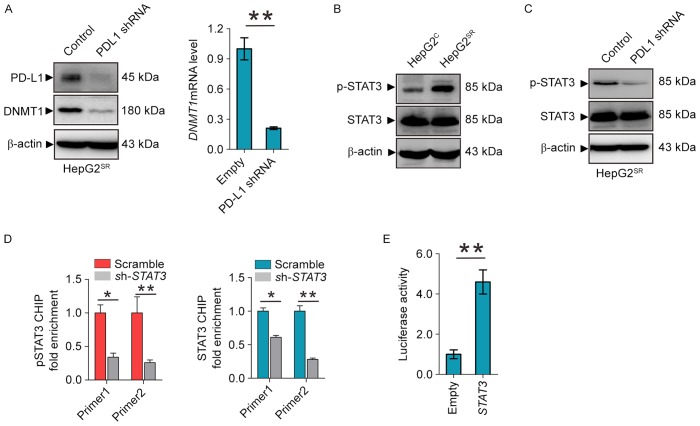
PD-L1 regulates DNMT1 through STAT3 signaling pathway
Given the positive correlations of DNMT1 and PD-L1 expression in sorafenib-resistant HCC, we proposed the existence of a regulatory loop between DNMT1 signaling and PD-L1 machinery, in which PD-L1 regulates the expression of DNMT1. As shown in Fig. 2A, PD-L1 depletion by shRNA in HepG2SR and Huh7SR, as expected, decrease PD-L1 and DNMT1 protein and mRNA levels. To elucidate how PD-L1 regulates the DNMT1 gene, we focused on the STAT3/DNMT1 network, since STAT3 has been shown to regulate DNMT1 transcription in cancer and play a key role in drug-resistant (19). First, we found that phosphor-STAT3 was overexpressed in HepG2SR compared with HepG2C (Fig. 2B). Second, PD-L1 knockdown dephosphorylates STAT3 at Tyr705 in HepG2SR and Huh7SR (Fig. 2C). In addition, ChIP showed that STAT3 knockdown diminished the binding of total and phospho-STAT3 in DNMT1 promoter (Fig. 2D). Reporter assays revealed that STAT3 inactivation by shRNA disrupted the luciferase activities driven by DNMT1 promoter region containing STAT3 binding elements (Fig. 2E). These results confirmed STAT3 transcript regulates the DNMT1 gene, and suggest that PD-L1 specifically regulate DNMT1 through STAT3 signaling in sorafenib resistance to HCC.
Figure 2.
PD-L1 regulates DNMT1 through STAT3 signaling. (A) Western blotting (left) and qPCR (right) for DNMT1 and PD-L1 in HepG2SR transfected with PD-L1 empty vector or shRNA. (B and C) Western blotting for the protein expression of p-STAT3 and total STAT3 in HepG2SR and HepG2C (B) or HepG2SR transfected with PDL1 empty vector or shRNA (C). (D) HepG2SR cells were transfected with STAT3 siRNA or its control vector for 48 h and subjected to ChIP. The change of STAT3 binding on DNMT1 promoter was assessed by qPCR. (E) 293T cells were transfected with pGL3-DNMT1 alone or plus STAT3 vectors for 48 h, followed by the measurement of luciferase activity. Data are mean ± SD, *P<0.05, **P<0.01. The data represent three independent experiments.
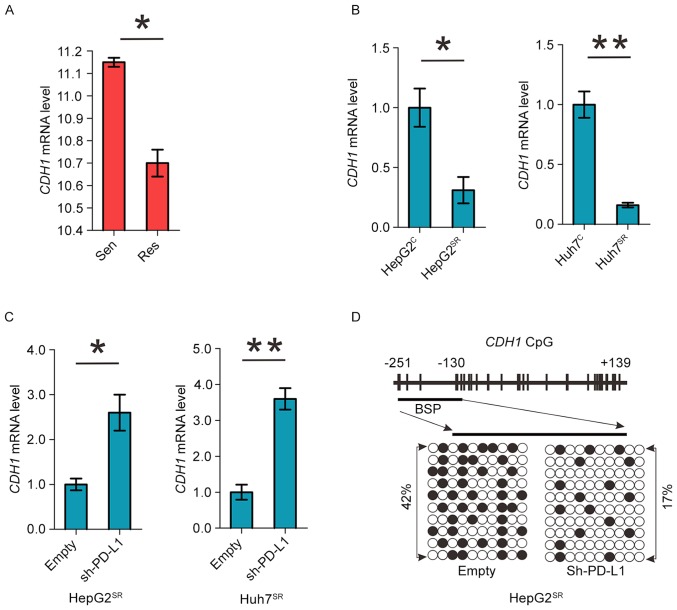
PD-L1 induces DNMT1-dependent DNA hypomethylation and restores the expression of methylation-silenced CDH1
Cadherin 1 (CDH1) is a cell-cell adhesion molecule and functions as a metastasis suppressor in HCC. It is epigenetically silenced and its downregulation associates with poor prognosis in HCC (20,21). As shown in Fig. 3A and B we found significant CDH1 downregulation in sorafenib-resistant HCC mice and cell model. Important, knockdown of PDL1 can increase CDH1 mRNA level in HepG2SR and Huh7SR cells (Fig. 3C). As PD-L1 regulates DNMT1, we proposed that PD-L1 downregulation might mediate CDH1 restoration via promoter DNA hypermethylation. We analyzed CDH1 promoter methylation status using bisulfite sequencing in PD-L1 shRNA-transfected HepG2SR and Huh7SR cells, and found a >25% change (42% in empty versus 17% in PDL1 shRNA) from hyper- to unmethylated in CDH1 promoter (Fig. 3D). Thus, PD-L1 induces DNMT1-dependent DNA hypomethylation and restores the expression of methylation-silenced CDH1.
Figure 3.
PD-L1 induces DNMT1-dependent DNA hypomethylation and restores the expression of methylation-silenced CDH1. (A) GEO data were analyzed for CDH1 expression in sorafenib-sensitive mice versus sorafenib-resistant mice. (B) qPCR measuring the expression levels of CDH1 genes in HepG2C and Huh7C vs HepG2SR and Huh7SR cells. (C) qPCR for CDH1 expression in HepG2SR and Huh7SR cells transfected with empty or PD-L1 shRNA. (D) Bisulfite analysis for the change of DNA methylation in CDH1 promoter (transcription start site −251 to +139) in HepG2SR cells transfected with empty or PD-L1 shRNA. Data are mean ± SD, *P<0.05, **P<0.01. The data represent three independent experiments.
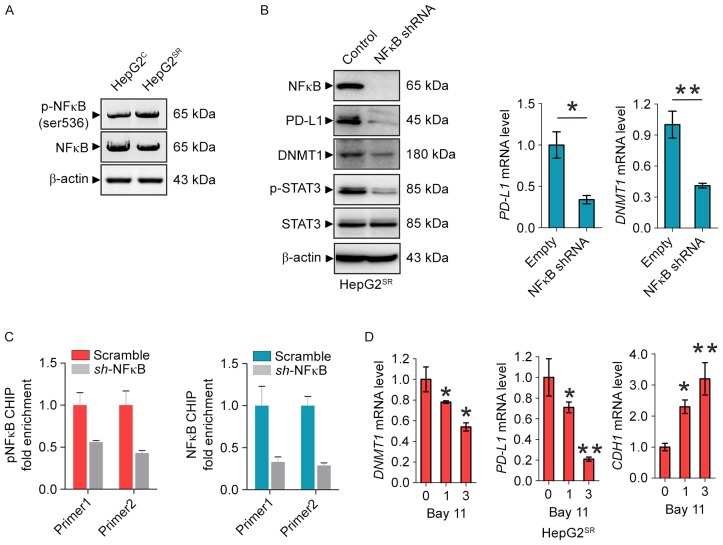
Inactivation of NFκB blocks PD-L1/Stat3/DNMT1 pathway in sorafenib-resistant HCC cells
To provide further insight into the mechanism underlying PD-L1/STAT3/DNMT1 signaling in sorafenib-resistant HCC, we considered the transcriptional factor NFκB may play a key role. Indeed, as shown in Fig. 4A, NFκB was activated in HepG2SR cells. NFκB knockdown by shRNA in HepG2SR cells significantly decreased PDL1 protein and mRNA levels followed by the dephosphorylation of STAT3 and downregulated DNMT1 expression (Fig. 4B). To elucidate how NFκB regulates PD-L1, we investigated whether NFκB can directly transcriptionally regulate PDL1. Firstly, it was predicted that PDL1 promoter area has an NFκB binding site. Secondly, ChIP demonstrated ~1.5-fold of enrichment reduction of NFκB on PD-L1 promoter in HepG2SR cells after NFκB shRNA transfection supporting that PD-L1 is one of the NFκB binding targets (Fig. 4C). Of note, as shown in Fig. 4D, when HepG2SR cells were treated at 1 and 3 M of Bay 11, a specific NFκB inhibitor (22), the mRNA level of PD-L1 and DNMT1 were significantly reduced, and the CDH1 was markedly increased. These data suggest that inactivation of NFκB blocks the PD-L1/Stat3/DNMT1 pathway in sorafenib-resistance HCC cells.
Figure 4.
Inactivation of NFκB blocks PD-L1/Stat3/DNMT1 pathway in sorafenib-resistant HCC cells. (A) Western blotting for the protein expression of p-NFκB and total NFκB in HepG2SR and HepG2C cells. (B) Western blotting (left) and qPCR (right) for the indicated genes in HepG2SR transfected with PD-L1 empty vector or shRNA. (C) HepG2SR cells were transfected with NFκB shRNA or its control vector for 48 h and subjected to ChIP. The change of NFκB binding on PD-L1 promoter was assessed by qPCR. (D) qPCR for DNMT1, PDL1 and CDH1 levels in HepG2SR cells treated with Bay 11 for 48 h. Data are mean ± SD, *P<0.05, **P<0.01. The data represent three independent experiments.
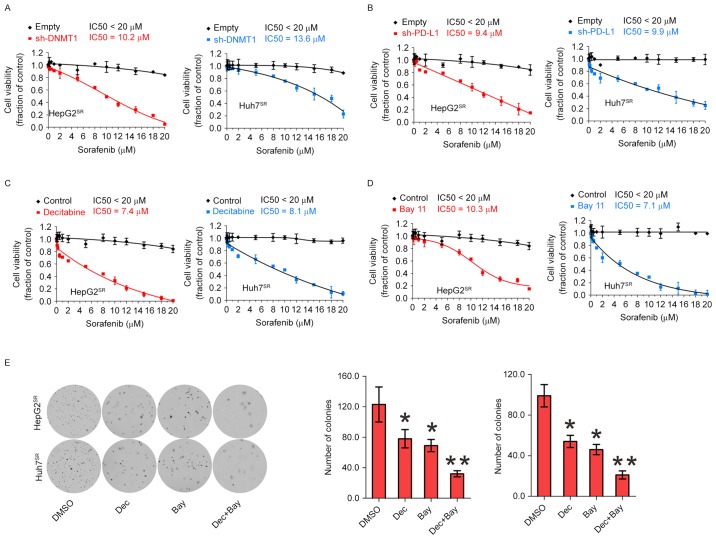
Genetic or pharmacological disruption of PD-L1 or DNMT1 sensitizes HCC resistance to sorafenib
Because of activation of PD-L1 and DNMT1 signaling in sorafenib resistance to HCC, we speculated that genetic or pharmacological disruption of PD-L1 and DNMT1 function could be an alternative strategy to impair sorafenib-resistant cell proliferation. When PD-L1 or DNMT1 was depleted by their specific shRNA in sorafenib-resistant cells, then exposing the cells to 0–20 µM sorafenib for 72 h, cell growth IC50 value for PD-L1 or DNMT1 knockdown was significantly decreased, respectively, compared with their empty control, suggesting that abrogation of PDL1 and DNMT1 restores sorafenib sensitivity (Fig. 5A and B). Next, we considered pharmacological inhibition using their inhibitor to sensitize resistant cells to sorafenib treatment. As shown in Fig. 5C, treatment with decitabine, a DNMT1 inhibitor, induced more pronounced inhibition on cell proliferation rate in HepG2SR and Huh7SR. Because anti-PD-L1 can not inhibit PD-L1 expression but activity. Therefore, we consider Bay 11 can be used as PD-L1 inhibitor (Fig. 4D). Indeed, 3 M of Bay 11 induced sensitized sorafenib-resistant cells (Fig. 5D). Decitabine and Bay 11 sensitized resistant cells to sorafenib treatment alone, and in a potential cooperation of NFκB/PDL1/DNMT1 in controlling sorafenib-resistant cell growth. We treated with decitabine and Bay 11 alone or both, and colony assays showed combination of decitabine and Bay 11 significantly disrupted the colony forming capability of HepG2SR and Huh7SR cells (Fig. 5E). Collectively, these data support the potential targeted combination therapies to enhance current and emerging sorafenib therapies for HCC.
Figure 5.
Genetic or pharmacological disruption of PD-L1 or DNMT1 sensitizes HCC resistance to sorafenib. (A) HepG2SR and Huh7SR cells were transfected with DNMT1 shRNA or empty vectors for 12 h, treated with sorafenib for additional 72 h and subjected to CCK-8 assays. (B) HepG2SR and Huh7SR cells were transfected with PD-L1 shRNA or empty vectors for 12 h, treated with sorafenib for additional 72 h and subjected to CCK-8 assays. (C) HepG2SR and Huh7SR cells were treated with decitabine for 12 h, treated with sorafenib for additional 72 h and subjected to CCK-8 assays. (D) HepG2SR and Huh7SR cells were treated with Bay 11 for 12 h, treated with sorafenib for additional 72 h and subjected to CCK-8 assays. (E) Resistant cells were treated with DMSO, decitabine, Bay 11, or decitabine plus Bay 11 for 6 h then subjected to colony-forming assay. Representative images of colony-forming assay (left) and the quantification of colonies (right). Data are mean ± SD, *P<0.05, **P<0.01. The data represent three independent experiments.
Discussion
Hepatocellular cancer (HCC) is a leading cause of cancer-related death worldwide, and the incidence of HCC is increasing in many parts of the world (23). Sorafenib is one of multiple targeted agents utilized by oncologists, and is FDA approved for treatment of a wide range of human cancers, including kidney, melanoma, prostate, ovarian, pancreatic, lung cancer and HCC. However, only rarely do sorafenib treated tumors regress completely, and the therapeutic effects of the drug are often temporary, most patients ultimately develop drug resistance and suffer relapses (24). Therefore, more effective therapeutic strategies and precision medicines to improve the prognosis of HCC patients with sorafenib resistance are urgently needed. Recent studies have shown that some mechanism was found in sorafenib-resistant cell lines. A tumor suppressor gene angiopoietin-like protein 1 (ANGPTL1) positively correlates with sorafenib sensitivity in HCC cells and human HCC tissues. ANGPTL1 directly interacts with and inactivates the MET receptor, which contributes to Slug suppression through inhibition of the extracellular receptor kinase/protein kinase B (ERK/AKT)-dependent early growth response protein 1 (Egr-1) pathway. ANGPTL1 may serve as a novel MET receptor inhibitor for advanced HCC therapy (25). Another study found PROX1 positively correlates with sorafenib resistance in HCC cells and may be a new potential strategy for improving sorafenib efficacy (26).
PD-L1, a ligand of PD1, is highly upregulated on many kinds of tumor cells, including melanoma, ovarian, and lung cancers. Because programmed death-1 (PD-1) and PD-L1 blockade have yielded promising clinical effects, understanding the regulatory mechanism of PD-L1 may identify biomarkers and/or develop combinatorial strategies for clinical use. Recently, PD-L1 overexpression also was reported in resistant cells. PD-L1 was expressed at a high level in SCLC cells (H69 and H82) resistant to cisplatin versus parental cells (10). Bishop et al showed that ENZ resistance is associated with high frequency of PD-1/L1 therapy targets, both in the tumor and circulating immune cells (9). Cancer epigenetics involves the causes of CpG island hypermethylation in tumor suppressor genes leading to transcriptional silencing. DNMT1 is a member of DNA methyltransferase family which includes DNMT1, DNMT3a and DNMT3b. Methylation occurs predominantly at cytosine-C5 in the context of CpG dinucleotides, and is established and maintained by three DNA methyltransferases, DNMT3a, DNMT3b, and DNMT1. DNMT3a and DNMT3b have mostly de novo DNA methylation activity, whereas DNMT1 plays a central role in preserving the patterns of DNA methylation through cell division (27). DNMT1 and DNA methylation is often considered as a key epigenetic regulatory mechanism and potential target for resistance to drugs (28).
Although PD-L1 and DNMT1 has been shown to be overexpressed in human cancer and drug resistance, respectively, whether and how PD-L1/DNMT1 axis affect sorafenib resistance to HCC remains largely unexplored. In this study, we mainly focused on DNMT1, because GEO data analysis showed DNMT1 overexpression in sorafenib resistance HCC cells, but not DNMT3a and 3b (data not shown). In vitro study found DNMT1 was upregulated in sorafenib-resistant HCC cells, which is consistent to GEO assay. Given both DNMT1 and PDL1 were significantly upregulated in sorafenib-resistant HCC cells and positively correlated by GEO assay, we proposed PD-L1 augments DNMT1 expression. We demonstrated that downregulation of PD-L1 in sorafenib-resistant cells might result from deregulation of DNMT1 at both protein and mRNA levels, leading to the upregulation of tumor suppressor CDH1 and the reduction of CDH1 promoter methylation. Moreover, depletion of PD-L1 or DNMT1 enhanced cell growth arrest, suggesting that PD-L1/DNMT1 axis plays a key role in survival and proliferation of sorafenib-resistant HCC cells. Accordingly, inhibition of PD-L1/DNMT1 axis may contribute to the resistance of molecular-targeted therapy. DNMT1 inhibitor caused a gradual decrease in self-renewal and tumorigenicity, and upregulation of apoptosis- and differentiation-related genes in HCC (29,30). Decitabine is a useful demethylation agent in clinic. It can decrease DNMT1 expression and cell cycle and is commonly used as a single agent to treat patients with MDS and elderly patients with AML (30,31). PDL1 antibodies, such as durvalumab and avelumab, are selective, high-affinity, human antibodies that block PD-L1 binding to PD-1, allowing T cells to recognize and kill tumor cells (7,32). However, these antibodies can only inhibit PD-L1 activity but not expression, which limits the use to overcome HCC resistance to sorafenib. Some transcranial factors were reported to directly regulate PD-L1. MYC regulates CD47 and PD-L1 at the transcriptional level by binding to their promoters in human melanoma (13). Moreover, PD-L1 is a direct target of HIF1-α and blockade of PD-L1 under hypoxia enhanced myeloid-derived suppressor cell-mediated T cell activation. Lo et al showed that inflammation increases PD-L1 expression in tumors through TNF-α-mediated activation of NFκB, leading to transactivation of CSN5 (14). In this study, upregulation of phosphorylation of NFκB-p65 (Ser536) was positively associated with PD-L1 expression in sorafenib resistance HCC cells. Because NFκB knockdown significantly decreased both protein and mRNA levels of PD-L1 and DNMT1, especially, diminishing the binding of PD-L1 promoter, it demonstrated that PD-L1 is one of a direct target of NFκB in sorafenib-resistant cells. Notably, NFκB inhibitor Bay-11 can decrease PD-L1 expression, which can be used to treat resistant cells to a PD-L1 inhibitor. Cell proliferation results showed that both decitabine and Bay 11 sensitize resistant cells to sorafenib treatment, further supporting that cellular function of PD-L1/DNMT1 signaling is required to sustain survival and proliferation of sorafenib-resistant cells. Importantly, combination of decitabine and Bay 11 synergistically inhibited colony formation of HCC resistant cells to sorafenib indicating that dual inactivation of PD-L1 and DNMT1 synergistically disrupts the cell growth and proliferation of sorafenib-resistant HCC cells.
For mechanism study, first we elucidated how PD-L1 regulates the DNMT1 gene. As phosphorylated STAT3 can bind specific DNA elements resulting in transcriptional activation. Previously it was reported that STAT3 binds DNMT1 promoter and positively regulates DNMT1 transcription (33), we proposed that PD-L1 augments DNMT1 expression by STAT3 in sorafenib-resistant HCC cells. We found depletion of PD-L1 significantly downregulated phosphorylation of STAT3 followed by the DNMT1 expression. Then CHIP and promoter reporter assay confirmed that STAT3 transcriptionally regulate DNMT1. Of note, it is likely that STAT3 is downstream of PD-L1. One report suggests that STAT3 as an upstream target regulates PD-L1 expression (34). There may be a feedback loop between STAT3 and PD-L1 which need further investigation.
Taken together, our results identified a mechanistic and functional link between PD-L1 and DNMT1-dependent DNA methylation in HCC cells resistant to sorafenib. These results also established the clinical potential strategy for targeting NFκB/PD-L1/STAT3/DNMT1 axis aimed to improve sorafenib efficacy and overcoming sorafenib resistance.
Acknowledgments
The authors would like to thank Dr Ningling Kang at the Hormel Institute, University of Minnesota for providing HCC cell lines, and Lin Yang at the Mayo Clinic, Rochester, MN, USA for assistance in English editing. This study was supported by grants from Foundation of Jilin Provincial Development and Reform Commission (KY20160002; 3J115AJ73428, Yahui Liu), Jilin University Fund for Excellent Young Teacher (no. 419080500355, Bai Ji), and Youth Fund from the Department of Science and Technology, Jilin Province, China (no. 20140520026JH, Bai Ji).
References
- 1.Di Maio M, Daniele B, Perrone F. Targeted therapies: Role of sorafenib in HCC patients with compromised liver function. Nat Rev Clin Oncol. 2009;6:505–506. doi: 10.1038/nrclinonc.2009.114. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang TW, Hohmeyer A, Pesic M, Leibold J, von Thun A, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med. 2014;20:1138–1146. doi: 10.1038/nm.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–220. doi: 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- 7.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–3125. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. KEYNOTE-024 Investigators: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. PD-L1 is highly expressed in Enzalutamide-resistant prostate cancer. Oncotarget. 2015;6:234–242. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan F, Pang J, Peng Y, Molina JR, Yang P, Liu S. Elevated cellular PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells. PLoS One. 2016;11:e0162925. doi: 10.1371/journal.pone.0162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, Yokoyama Y, Kossenkov AV, Bradner JE, Conejo-Garcia JR, et al. BET Bromodomain inhibition promotes anti-tumor immunity by suppressing PD-L1 expression. Cell Rep. 2016;16:2829–2837. doi: 10.1016/j.celrep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 15.Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2:S4–S11. doi: 10.1038/ncponc0354. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 16.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M, Fraga MF, Paz MF, Campo E, Colomer D, Novo FJ, Calasanz MJ, Galm O, Guo M, Benitez J, et al. Cancer epigenetics and methylation. Science. 2002;297:1807–1808. doi: 10.1126/science.297.5588.1807d. [DOI] [PubMed] [Google Scholar]
- 18.Spinzi G, Paggi S. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2497–2498. doi: 10.1056/NEJMc081780. author reply 2498–2499. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Li J, Huang T, Duan S, Dai D, Jiang D, Sui X, Li D, Chen Y, Ding F, et al. Meta-analysis of DNA methylation biomarkers in hepatocellular carcinoma. Oncotarget. 2016;7:81255–81267. doi: 10.18632/oncotarget.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Wang Y, Guo Y, Sun S. Down-regulated microRNA-152 induces aberrant DNA methylation in hepatitis B virus-related hepatocellular carcinoma by targeting DNA methyltransferase 1. Hepatology. 2010;52:60–70. doi: 10.1002/hep.23660. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Ruan Y, Wang X, Min L, Shen Z, Sun Y, Qin X. BAY 11–7082, a nuclear factor-κB inhibitor, induces apoptosis and S phase arrest in gastric cancer cells. J Gastroenterol. 2014;49:864–874. doi: 10.1007/s00535-013-0848-4. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:436. doi: 10.1038/nrclinonc.2015.121. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Jin R, Zhao J, Liu J, Ying H, Yan H, Zhou S, Liang Y, Huang D, Liang X, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367:1–11. doi: 10.1016/j.canlet.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ, Yang CY, Sung SY, Su JL. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology. 2016;64:1637–1651. doi: 10.1002/hep.28773. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Ye X, Zhang JB, Ouyang H, Shen Z, Wu Y, Wang W, Wu J, Tao S, Yang X, et al. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene. 2015;34:5524–5535. doi: 10.1038/onc.2015.7. [DOI] [PubMed] [Google Scholar]
- 27.Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min HY, Lee SC, Woo JK, Jung HJ, Park KH, Jeong HM, Hyun SY, Cho J, Lee W, Park JE, et al. Essential role of DNA methyltransferase 1-mediated transcription of insulin-like growth factor 2 in resistance to histone deacetylase inhibitors. Clin Cancer Res. 2017;23:1299–1311. doi: 10.1158/1078-0432.CCR-16-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C, Gong F. DNA methyltransferase 1: A potential gene therapy target for hepatocellular carcinoma? Oncol Res Treat. 2016;39:448–452. doi: 10.1159/000447414. [DOI] [PubMed] [Google Scholar]
- 30.Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, Wilson RK, Baty JD, Duncavage EJ, Tandon B, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–2036. doi: 10.1056/NEJMoa1605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncavage EJ, Uy GL, Petti AA, Miller CA, Lee YS, Tandon B, Gao F, Fronick CC, O'Laughlin M, Fulton RS, et al. Mutational landscape and response are conserved in peripheral blood of AML and MDS patients during decitabine therapy. Blood. 2017;129:1397–1401. doi: 10.1182/blood-2016-10-745273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Hodge JW, Schlom J, Kobayashi H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget. 2017;8:8807–8817. doi: 10.18632/oncotarget.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, Wang HY, Woetmann A, Raghunath PN, Odum N, Wasik MA. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood. 2006;108:1058–1064. doi: 10.1182/blood-2005-08-007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WB, Yen ML, Liu KJ, Hsu PJ, Lin MH, Chen PM, Sudhir PR, Chen CH, Chen CH, Sytwu HK, et al. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Rep. 2015;5:392–404. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]