Abstract
Aim
We evaluated the direct high-throughput multiple genetic detection system (dHMGS) for Helicobacter pylori in gastric biopsies.
Materials & methods
One hundred and thirty-three specimens were concurrently analyzed by dHMGS, rapid urease test, culture and sequencing.
Results
dHMGS was highly sensitive and specific for H. pylori identification compared with culture and rapid urease test. The correlation coefficient of the quantitative standard curve was R2 = 0.983. A significant difference in the relative H. pylori DNA abundance was found in different gastroduodenal diseases. Concordance rates between dHMGS and sequencing for resistance mutations were 97.1, 100.0, 85.3 and 97.1%, respectively. Finally, dHMGS could efficiently distinguish mixed infection in biopsy specimens.
Conclusion
The dHMGS could efficiently diagnose and quantify H. pylori burden in biopsies, simultaneously screening for virulence, antibiotic resistance and presence of the multistrain infections.
Keywords: direct high-throughput multiple genetic detection system, gastric biopsy specimens, Helicobacter pylori, identification, mixed infection, quantitative analysis, resistance, virulence
Helicobacter pylori infection affects more than half of the world’s population, and is closely associated with gastroduodenal diseases including chronic atrophic gastritis (CAG), chronic superficial gastritis (CSG), peptic ulcer diseases (PUD) and gastric carcinoma [1]. While only some H. pylori infected patients eventually develop these severe gastroduodenal diseases pathologies, it is still not clear to what degree the magnitude of bacterial burden and/or expression of virulence genes contributes to the development of these complications [2,3]. Furthermore, the H. pylori drug resistance and the mixed infection rate seriously increased the complexity and difficulty in clinical diagnosis and treatment [4–6]. Incorporation of these readouts into the rapid, routine diagnostic method would not only inform the physician and patients about the potential risks, but in retrospect, allow the medical community to study and more accurately ascribe risks of specific gastroduodenal diseases.
Our group has assessed high-throughput multiple genetic detection system (HMGS), which could simultaneously detect and analyze a set of genes of H. pylori, including 16S rRNA and ureC genes for H. pylori identification, ten virulence genotypes and four main drug resistance genes in isolated strains. This study showed that HMGS platform was ideally optimized for these applications [7]. While highly accurate in H. pylori detection, and suitable for broad genetic screening for virulence and antibiotic resistance, the method was still culture based. Apart from the requirement of an additional 48-h+ culture time, it could not accurately estimate the bacterial load in biopsy material or accurately detect mixed infection with multistrains due to random selections of cultured colonies for genetic analysis. Finally, the isolation rate in culture was low (20–25%) [7], which could result in increased potential for false-negative detections. Thus, we explored possibility of bypassing the culture step and HMGS application for direct detection of H. pylori in biopsy materials (dHMGS; direct high-throughput multiple genetic detection system) with simultaneous evaluation of multiple parameters. In this study, we utilized 21-parameter dHMGS and further evaluated its usefulness for direct analysis of clinical gastric mucosa biopsy specimens, as well as for quantification of bacterial loads and diagnosis of the mixed (multistrain) H. pylori infection.
Materials & methods
Ethics statement
The study has been approved by Ethics Committee for human studies of Huadong Hospital and Shanghai Tongji Hospital and was conducted in compliance with standards of ethical conduct. All participants provided written informed consent and have signed the informed consent forms (the Ethics Approval Number: [2013]-077).
The inclusion & exclusion criteria
All patients underwent upper gastrointestinal endoscopy due to recurrent upper gastrointestinal symptoms of heartburn, acid reflux, vomiting, abdominal distention or abdominal pain. Patients who received antibiotics therapy for H. pylori within 1 month preceding the study, experienced subtotal gastrostomy, suffered from severe cardiovascular and respiratory diseases or recently consumed NSAID or alcohol, were excluded.
Patients & gastric biopsies
Patients from Huadong Hospital and Tongji Hospital from July 2015 to February 2016 were enrolled. The gastric biopsy specimens were taken from two parts of antrum 2–3 cm front of the pylorus of the patients undergoing endoscopy with their consent, and put in transferring nutrient solution (10% glucose, 2.8% brinell gravy powder, 10% calf serum). The two biopsies were homogenized, combined together into one sample, and then divided into three portions for the following tests: culture, rapid urease test (RUT), conventional PCR and dHMGS. Among them, 133 specimens, which had the clear clinical information, were used in this study. Of all these 133 patients, 72 (54.1%) were males, aged 25–87 years; 61 (45.9%) were females, aged 21–88 years. Forty strains were isolated from 133 biopsy specimens.
Culture
Each biopsy specimen was homogenized by TissueLyser (Jingxin Co., Ltd, Shanghai, China) in 1 ml 0.9% sterile saline solution, and then cultured as described previously [7].
Rapid urease test
Gastric biopsy specimens were placed into semi-solid 2% agar (H. pylori rapid detection kit, Huiyi Biology Companies, Shanghai, China) with a sterile needle and incubated at room temperature. Thirty minutes later, observing whether the indicator discolored; H. pylori infection would be identified if the color changed from yellow to red. All procedures were conducted by the manufacturers’ instructions.
DNA extraction & sequencing
The biopsy specimen homogenates were used to obtain total DNA containing both the patient and microbial DNA. DNA was extracted using genomic DNA extraction kit (Tiangen Biotech Co., Ltd, Beijing, China) following manufacturer’s instructions. Detailed information on DNA extraction, conventional PCR and sequencing procedures has been provided in our published work [7].
dHMGS primers
Twenty one H. pylori primer pairs including identification genes 16S rRNA and ureC, ten virulence genotypes and variants of four main drug resistance genes were included in HMGS assay. In addition, primers for human β-globin gene were included as internal DNA loading control. The primer sequences for all these target genes and the size of the resulting amplicons were published previously [7]. The sequence of the 199-bp housekeeping gene β-globin had the primer pair as follows:
Forwa rd (5′-AGGTGACACTATAGAATACTCTTATCTTCCTCCCACAGCT-3′);
Reverse (5′-GTACGACTCACTATAGGGAAGAAAGCGAGCTTAGTGATACT-3′).
Multiplex PCR & capillary electrophoresis
The multiplex quantitative PCR was performed in a mixture containing MgCl2 solution, PCR buffer, forward universal primers, reverse universal primers, Taq polymerase, DNA template and distilled H2O. The mixture was subjected to the amplification. The PCR products were prepared for fragment analysis using the Beckman Coulter GeXP Genetic Analysis system (ABSCIEX, Inc., CA, USA). After the target fragments were respectively separated, the peak height for each gene was reported in the electropherogram and the dye signal strength was measured by fluorescence spectrophotometry in relative fluorescence units of optical fluorescence. The amplified products were considered positive when the value of dye signal was over 2000 relative fluorescence units. The detailed procedures referred to the instruction of GeXP and previous study [7].
Analysis of dHMGS outcomes
The gastric biopsies specimens were considered H. pylori positive when the specific amplification signals simultaneously showed human internal control gene β-globin and H. pylori identification genes 16S rRNA and ureC. When the specific amplification signals showed robust human internal control gene β-globin but did not show any of the 16S rRNA or ureC genes, the specimens were considered H. pylori negative.
Data analysis & statistics
Sensitivity and specificity of diagnostic tests were calculated according to the following formulas: SE = TP/(TP + FN)*100, SP = TN/(TN + FP)*100; PPV, NPV and accuracy was calculated as follows: PPV = TP/P, NPV = TN/N, Accuracy = TP/(TP + TN)*100. FN: False negative; FP: False positive; N: Negative; NPV: Negative predict value; P: Positive; PPV: Positive predict value; SE: Sensitivity; SP: Specificity; TN: True negative; TP: True positive.
All of presented dHMGS dye intensity traces show a representative outcome form an individual run from gastric biopsy specimen selected from total dataset subjected to analysis. Data were statistically analyzed by χ2 test using Stata statistical software package version 12.0 (StataCorp College Station, TX, USA). Additionally, Gwet’s AC1 values were calculated to measure the detection agreement of dHMGS assay with sequencing using AgreeStat version 2011.3 (Advanced Analytics, MD, USA) [8]. The benchmark scales for Gwet’s AC1 values was based on the Landis and Koch’s criteria [9]. The H. pylori distribution of different groups was analyzed by Mann–Whitney rank-sum test for two variables and Kruskal–Wallis H test for more than two variables. All of the above hypothesis tests were two-sided; and a two-tailed p-value of 0.05 or less was considered to indicate statistical significance.
Results
The dHMGS is a highly specific, sensitive & accurate tool for direct detection of H. pylori in biopsy specimens
Our first goal was testing usefulness of dHMGS for direct detection of H. pylori in gastric biopsy specimens. Relative to the outcomes of gene sequencing tests, specificities, predictive values and accuracy of dHMGS for H. pylori identification were calculated, and compared with these values for culturing and RUT, were calculated for all 133 patients (Table 1). The sensitivity and NPV of dHMGS were found to be 97.1 and 96.7% respectively, which were higher than that of culture and RUT. The specificity and PPV were 93.7 and 94.4% respectively, which were higher than RUT and lower than culture. Moreover, dHMGS had the highest accuracy of 95.5% compared with culture and RUT. These data demonstrated that dHMGS was specific for identification of H. pylori, and highly sensitive and accurate technique compared with culture and RUT.
Table 1.
Sensitivity, specificity, positive predict value, negative predict value, accuracy of direct high-throughput multiple genetic detection system to detect Helicobacter pylori compared with rapid urease test, culture and gene sequencing.
| Methods | Gene sequencing
|
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | |||||||
| Culture | Positive | 40 | 0 | 40 | 57.1 | 100.0 | 100.0 | 67.7 | 77.4 |
| Negative | 30 | 63 | 93 | ||||||
| Total | 70 | 63 | 133 | ||||||
|
| |||||||||
| RUT | Positive | 56 | 6 | 62 | 80.0 | 90.5 | 90.3 | 80.3 | 85.0 |
| Negative | 14 | 57 | 71 | ||||||
| Total | 70 | 63 | 133 | ||||||
|
| |||||||||
| dHMGS | Positive | 68 | 4 | 72 | 97.1 | 93.7 | 94.4 | 96.7 | 95.5 |
| Negative | 2 | 59 | 61 | ||||||
| Total | 70 | 63 | 133 | ||||||
dHMGS: Direct high-throughput multiple genetic detection system; NPV: Negative predict value; PPV: Positive predict value; RUT: Rapid urease test.
The dHMGS can be used for relative quantitation of H. pylori DNA load in gastric mucosa biopsy specimens
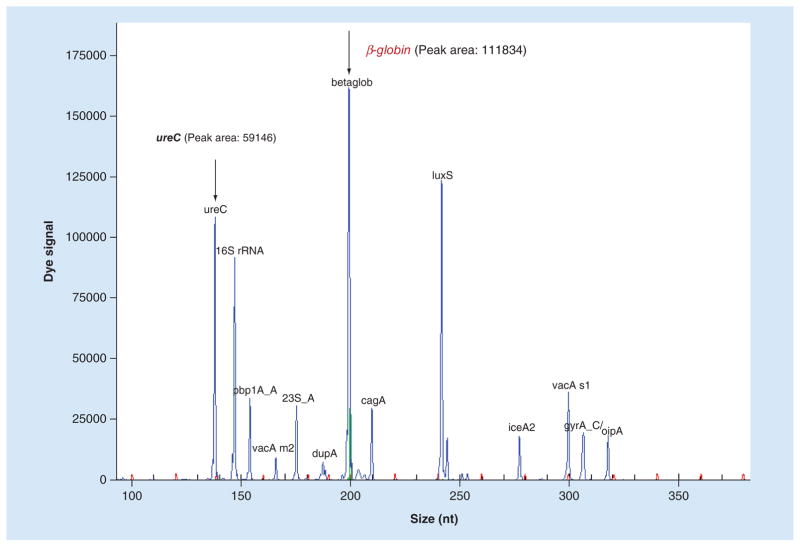
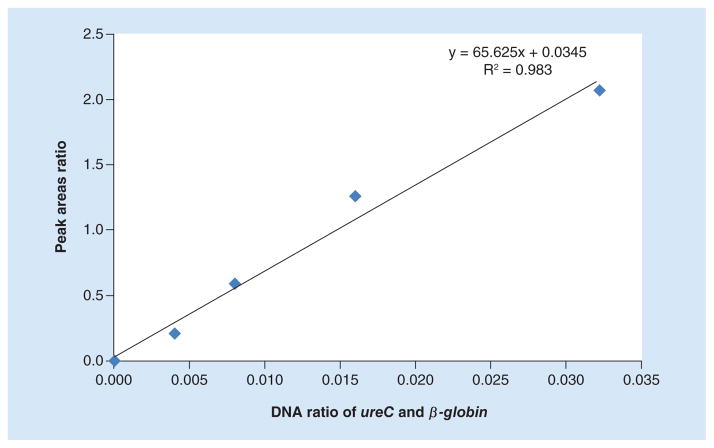
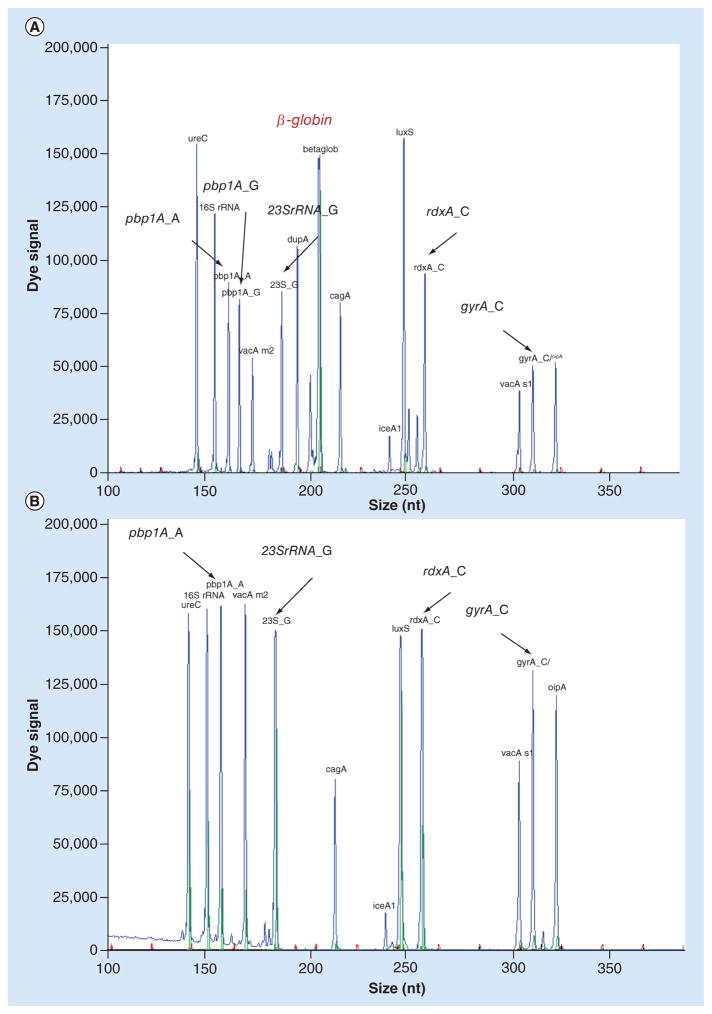
Our next goal was to test the usefulness of dHMGS to estimate bacterial load in the biopsy specimen. Since our analytical panel was design to measure human gene β-globin, an internal control gene for human genome (Figure 1), we could measure relative abundance of H. pylori genetic material within the specimen. A highly conserved and stably expressed gene, ureC, which is present as a single copy in H. pylori, was chosen as the quantitative readout. The relative H. pylori DNA load was determined by the ratio of the peak area between ureC and β-globin based on dHMGS assay in gastric mucosa biopsy specimens. The quantitative function of the ratio of ureC and β-globin gene for clinical specimens was evaluated by the quantitative standard curve. It was obtained the ratio of ureC gene from H. pylori by doubling dilutions from 0 to 0.32 ng and 10 ng β-globin from human genome, and their corresponding dHMGS peak area (Figure 2). The correlation coefficient R2 = 0.983, indicated high fidelity of dHMGS for quantitation of relative abundance of H. pylori in the specimens.
Figure 1. The position and peak area of ureC and β-globin gene in direct high-throughput multiple genetic detection system.
The peak area of ureC and β-globin were 59146 and 111834, respectively. The ratio of the peak area between ureC and β-globin was 0.5289.
Figure 2. The quantitative standard curve of direct high-throughput multiple genetic detection system for biopsy specimens.
The quantitative standard curve obtained by doubling dilutions from 0.004 to 0.032 ng (x-axis) and their corresponding ratio of direct high-throughput multiple genetic detection system peak area (y-axis), 0.21, 0.59, 1.26, 2.05, respectively.
Direct-biopsy dHMGS detected correlation of H. pylori DNA load & clinical characteristics
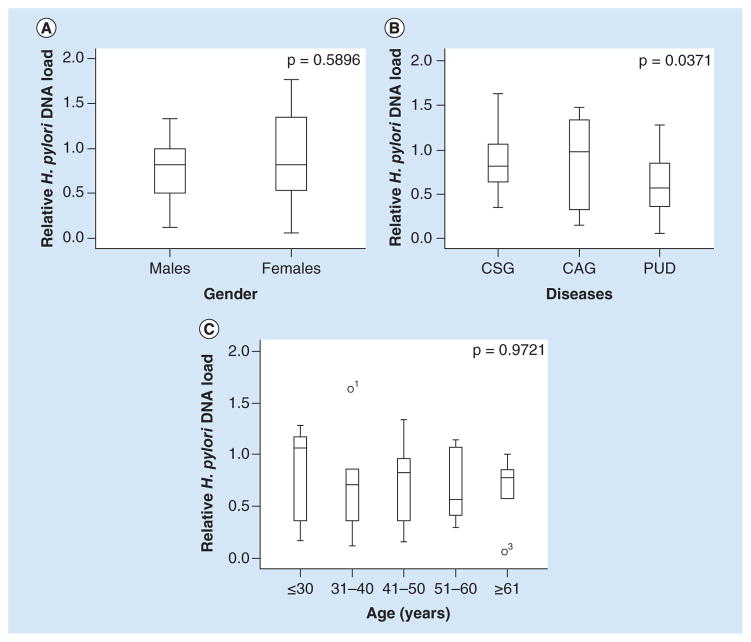
In order to evaluate the correlation of the H. pylori DNA load and clinical characteristics, the relative H. pylori DNA load within different groups were compared. As shown in Figure 3A, the relative H. pylori DNA load in females appeared higher than that of males, but the difference reached no statistical significance (p = 0.5896). Interestingly, the relative H. pylori DNA load was the highest in CSG group, with the mean relative H. pylori DNA of 1.0025. A significant statistical difference was observed between three major types of diagnosed gastroduodenal diseases (CSG, CAG and PUD) (p = 0.0371, Figure 3B). In addition, the relative H. pylori DNA load of 31–40 ages was the highest among all the age groups. However, the relative H. pylori DNA load of different ages had no statistically significant difference (p = 0.9721, Figure 3C).
Figure 3. Correlation of Helicobacter pylori DNA load and clinical characteristics.
(A) Helicobacter pylori DNA load in gender groups; p = 0.5896 (p > 0.05). (B) Helicobacter pylori DNA load in different gastroduodenal diseases including CSG, CAG, PUD, p = 0.0371 (p < 0.05). (C) Helicobacter pylori DNA load among different age groups; p = 0.9721 (p > 0.05). y-axis represents the relative H. pylori DNA level while x-axis represents different parameters.
CAG: Chronic atrophic gastritis; CSG: Chronic superficial gastritis; PUD: Peptic ulcer disease.
dHMGS & sequencing show high concordance rate in detection of specific resistance gene mutations in biopsy specimens
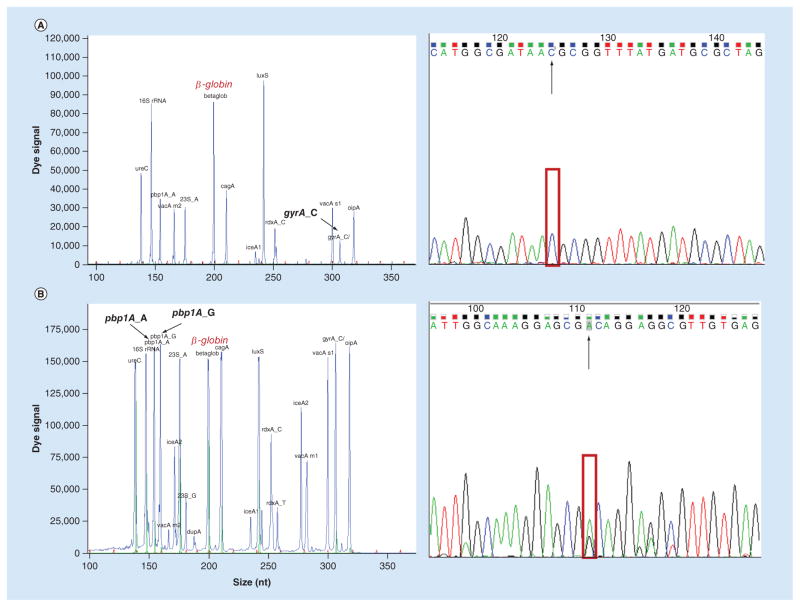
All the sites of resistance genes 23S rRNA (A2143G), rdxA (C148T), pbp1A (A1777G) and gyrA (C261A/G) for clarithromycin (CLA), metronidazole (MTZ), levofloxacin (LEV) and amoxicillin (AMX) were detected by dHMGS from clinical gastric mucosa biopsy specimens directly (Supplementary Figure 1C). Furthermore, the outcomes of dHMGS and sequencing were compared to determine if dHMGS would show the remarkable fidelity. As was shown in Figure 4, the specific peaks were observed in both outcomes of dHMGS and sequencing in the main sites of drug resistance genes. The concordance of dHMGS and sequencing for detection of 23S rRNA, rdxA, pbp1A and gyrA were 97.1, 100.0, 85.3 and 97.1%, respectively. Furthermore, the AC1 values of dHMGS assay and sequencing for detecting the four resistance genes were 0.954, 1.000, 0.820 and 0.957, respectively, which all reached ‘outstanding standards’ based on the Landis and Koch’s criteria (0.81–1 as outstanding). Thus, these results demonstrated that dHMGS has excellent concordance with sequencing in detecting resistance genes of biopsy specimens.
Figure 4. Concordance of direct high-throughput multiple genetic detection system and resistance mutation for antibiotics in biopsy specimens (see facing page).
(A) Resistance genes gyrA_C had the wild-type peak at 306 bp, were detected by direct high-throughput multiple genetic detection system, as well as showed the peak of base C in the sequencing result. (B) The peaks of wild-type and mutant resistance genes pbp1A_A/G were appeared in direct high-throughput multiple genetic detection system at 175 and 180 bp, respectively, as well as showed both the peaks of base A and G in the sequencing result.
dHMGS can efficiently distinguish multistrain infection in gastric biopsy specimens by detecting multiple variants of H. pylori-resistance genes
To evaluate the performance of dHMGS in biopsy specimens, 40 gastric biopsies with the corresponding isolated strains were detected. We compared the resistance gene products of 23S rRNA, rdxA, pbp1A and gyrA for CLA, MTZ, LEV and AMX between 40 infected specimens and their corresponding isolated strains using dHMGS (Table 2). All single gene peaks of 23S rRNA, rdxA, pbp1A and gyrA, respectively, appeared in 40 isolated stains. While, among the corresponding 40 biopsy specimens, there were 35, 39, 32 and 36 single resistance gene peaks for 23S rRNA, rdxA, pbp1A and gyrA that appeared, respectively. Among them, 34 out of 35, 38 out of 39, 30 out of 32 and 36 out of 36 single peaks for four resistance genes were consistent with these of corresponding isolates. Besides, we found 5, 1, 8 and 4 mixed resistance gene peaks (both wide-type and mutant genotypes) for four resistance genes that were detected in biopsy specimens but only single resistance gene peaks in the corresponding isolated strains. As shown in Figure 5, the peaks of wild-type pbp1A_A and mutant genotypes pbp1A_A/G were simultaneously detected in the biopsy specimen (Figure 5A), while only the peak of wild-type pbp1A_A was detected in the corresponding isolated strains (Figure 5B). Thus, dHMGS could efficiently detect H. pylori-resistance genes and distinguish mixed infection in gastric biopsy specimens.
Table 2.
The detection of resistance genes of Helicobacter pylori in biopsy and corresponding isolates by direct high-throughput multiple genetic detection system.
| Antibiotics | Gene | Isolates | Biopsy
|
||
|---|---|---|---|---|---|
| WT | MT | Mixed | |||
| CLA | 23S rRNA | 2143_A | 27 | 1 | 2 |
| 2143_G | 0 | 7 | 3 | ||
|
| |||||
| MTZ | rdxA | 148_C | 37 | 0 | 0 |
| 148_T | 1 | 1 | 1 | ||
|
| |||||
| AMX | pbp1A | 1777_A | 25 | 1 | 7 |
| 1777_G | 1 | 5 | 1 | ||
|
| |||||
| LEV | gyrA | 261_C/T | 30 | 0 | 2 |
| 261_G/A | 0 | 6 | 2 | ||
The performance parameters have been calculated using formulas described in the ‘Materials & methods’ section. AMX: Amoxicillin; CLA: Clarithromycin; LEV: Levofloxacin; MT: Mutant; MTZ: Metronidazole; WT: Wild-type.
Figure 5. Direct high-throughput multiple genetic detection system can efficiently distinguish multistrain infection in gastric biopsy specimens.
(A) The peaks of wild-type pbp1A_A and mutant resistance genes pbp1A_A/G were appeared at 154 and 159 bp in biopsy specimen. (B) The peak of wild-type pbp1A_A appeared at 154 bp in the corresponding isolated strains. 23S rRNA_G, rdxA_C and gyrA_C had the single peak in biopsy specimen and corresponding isolated strains, appeared at 180, 253 and 306 bp, respectively. Human internal control β-globin had the specific peak appeared at 199 bp in biopsy specimens.
Discussion
We had recently showed that HMGS was a highly specific and rapid method for simultaneous H. pylori identification and analysis of virulence as well as antibiotic resistance in cultured isolates from gastroduodenal diseases patients’ biopsies [7]. Here, we assessed the usefulness of HMGS for H. pylori detection, quantification and virulence, and resistance analysis in genetic material directly collected from biopsy specimens (referred to as dHMGS). We have found that similarly to the culture-based HMGS, the dHMGS was highly sensitive, specific and accurate in detection of H. pylori from biopsy specimens and ideal for rapid analysis of genetic properties of the infecting strains. Furthermore, while combining all the previously described advantages of HMGS [7,10], dHMGS bypasses culture step, while adding the benefit of bacterial burden quantification and possibility to detect multistrain coinfections.
Culture is generally considered as the golden standard for the detection of H. pylori infection, and it is the precondition to perform antibiotics susceptibility test (AST), which would provide the information to guide choice of antibiotics to effectively treat H. pylori infection. While H. pylori culture is still a standard for demonstrating a finite proof of H. pylori infection, our studies and others have shown that due to technical challenges associated with cultures, likelihood of false-negative conclusions is quite significant and the sensitivity of detection based on culture method alone is low [11–13]. Therefore, for the purpose of this study, we used the sequencing method to calculate a specificity and sensitivity of H. pylori detections. However, the relatively low rate of positive cultures may frequently lead to false-negative results (as demonstrated here by comparative analysis of multiple methods) and cultures are time-consuming, and thereby do not fulfill the needs of rapid clinical diagnosis required for selection of optimal treatment [11–13]. Furthermore, cultures are not optimal for quantification of bacterial burdens or detection of multistrain infections. Hence, we sought to develop a new method to detect and analyze H. pylori from biopsy specimens directly, which would allow bypassing shortcomings of culture-based methods and accelerate precise diagnosis of H. pylori infection.
Except for culture, RUT is another common diagnosis method for H. pylori diagnosis, while sequencing is considered the gold standard for highly specific and sensitive mean of detection of the microbial genetic material [14]. We evaluated sensitivities, specificities, predictive values and accuracy of mucosa biopsy dHMGS by comparing its outcomes with those of diagnostic cultures, RUT and sequencing.
The sensitivity and NPV of dHMGS were higher than that of culture and RUT. The specificity and PPV were higher than RUT and lower than culture. Moreover, dHMGS has the highest accuracy compared with culture and RUT. dHMGS yielded virtually similar outcomes as the sequencing, however, at a much lower cost and much quicker than sequencing. Also when compared retrospectively dHMGS with HMGS conducted on cultured isolates [10], the sensitivity and specificity was comparable, indicating that for the purpose of HMGS analysis, the culture step is dispensable and could be bypassed. Furthermore, 100% of samples that cultured positively for H. pylori (40 cases) were all detected by dHMGS, and samples that were culture negative but RUT positive (16 cases) also have been verified to be H. pylori positive by dHMGS. Finally, we had other positive detections by dHMGS (12 cases) which all together raised concerns about limitations and lack of sensitivity of the traditional approach. Therefore, these results demonstrate that dHMGS is highly sensitive and specific for H. pylori diagnosis, and could be a very useful supplement for the conventional methods, especially for the culture-negative patients.
The clinical manifestations of the H. pylori infection are associated with bacterial load [15]. In our previous study, we used the single copy gene ureC to quantify the H. pylori [10]. In this study, the human genome β-globin was added as the internal control to further normalize the H. pylori load. The relative H. pylori load was determined by the ratio of the peak area between ureC and β-globin from the gastric mucosa biopsy specimens. The correlation coefficient of the quantitative standard curve was R2 = 0.983, indicating that the quantitative relationship in this assay follows linear regression pattern which makes dHMGS an idea tool for precise quantification of bacterial burden in biopsy specimens. Based on quantitation we may potentially define a ‘threshold’ that would correspond with positive cultures, a certain range for ‘below the limit of culture detection’ within the standard curve that is still very likely to be positive albeit undetectable by culture. For example, in this study, we found one sample which was sequencing negative but dHMGS positive (Table 1); the further quantitation analysis of this sample revealed that its relative DNA load was 0.023, which was much lower than the median DNA loads of all biopsy specimens (0.533). This amount of DNA was likely insufficient for robust sequencing, but sufficient to be amplified and generate H. pylori signal in dHMGS assay. As dHMGS has a bright future to become routinely utilized in clinical diagnostics, we anticipate to further standardize limits of detection, link the magnitude of quantitative signal with probabilities of successful cultures and correct/adjust the outcomes analysis based on DNA yield. Developing such thresholds and various CIs will further improve the accuracy of dHMGS; future studies looking broader into the quantitative outcomes of dHMGS are likely to address this point. While we believe that dHMGS offers one of the most, if not the most accurate method to quantify the H. pylori bacterial burden in biopsy material, there are a few potential sources of variation that should be considered when biopsy readouts are extrapolated to assess overall level of H. pylori burden in the infected patient. The variable depth of a biopsy and variation in mucosal surface area in individual biopsy specimen, which in turn, might affect bacterial burden estimation normalized to the level of human housekeeping gene in the sample is a possible source of variation. However, dHMGS using molecular standard and using the whole DNA extracted from the entire biopsy material, while not free of variation, is less likely to be biased by other sources of variation affecting culture and histology-based detection methods. These variations in surface to volume ratios can be further alleviated by use of the pooled samples from multiple biopsies from the same patient, and by strict adherence to the standard operating procedure, including use of the same size of forceps while collecting biopsy material from the entire cohort of patients.
In addition to diagnostic function of dHMGS, we assessed usefulness of dHMGS in detecting the association between the relative H. pylori load and the clinical characteristics in different patient groups. While we have not detected significant difference in bacterial burdens between male and female patients, we were able to detect significantly higher relative H. pylori load in CSG group compared with CAG and PUD groups. These findings were not always consistent with previous reports [10], we believe that thanks to improved quantitative resolution of dHMGS with the human internal control, we were able to observe significantly higher microbial burdens in patients with CSG, and lower in CAG and PUD. In support of our findings, Konturek’s study has found that the chronic inflammation and associated with it gland atrophy and lymphoid tissue formation in CAG and PUD could lead to the decrease in the H. pylori load or even its eventual absence [16]. In addition, bacteria may hide themselves within the mucosal cells and without producing urease in the lumen [17,18]. Other studies were proposed due to changing conditions in gastric mucosa, which is an ‘environmental niche’ for H. pylori, the growth conditions were less conducive, while control of bacterial growth by the immune reactions could become more pronounced over time [19,20]. Finally, this study like our previous study found that the differences in bacterial load could be detected between different age groups. While, these two studies are not exactly comparable due to the different age subgroups and the presence versus absence of the internal control gene β-globin [10]. Our most recent results are consistent with Shukla’s study in which the real-time PCR was used to quantify the H. pylori load in patient from various age intervals [18]. Together, these findings support our notion that dHMGS through its improved quantitative resolution will allow us to detect previously unknown relationships between clinical characteristics and magnitude of H. pylori bacterial burdens and confirm those that are already known.
Expression of certain virulence factors have been reported to be correlated with the occurrence and development of H. pylori-related diseases [21]. In the present study, all ten kinds of H. pylori virulence genotypes could be consistently and reliably detected by dHMGS using gastric mucosa biopsy specimens (Supplementary Figure 1A & B). We anticipated that the assessment of the virulence genotypes in biopsy specimens would provide a useful tool to provide more comprehensive ‘profiling’ of H. pylori in the infected patients. Meanwhile, the increasing H. pylori drug resistance has become the major factor seriously impacting the efficacy of clinical treatment [22–24]. Our previous study using AST showed that the single and multiple resistance rates to CLA, MTZ, LEV and AMX in Shanghai area were extremely high [7]. However, the current treatment of infection continues to be empirical, due to the low-positive culture rate and long waiting period of AST results [25,26]. Since, dHMGS is very rapid, compared with all culture-based method for antibiotic resistance detection with or without AST, we evaluated efficiency of dHMGS in detecting major mutation variants in relation to culture-based HMGS. All main gene mutation sites of 23S rRNA (A2143G), rdxA (C148T), gyrA (C261A/G) and pbp1A (A1777G) for CLA, MTZ, LEV and AMX drug resistance could be directly detected by dHMGS in biopsy specimens for each positive detections (Supplementary Figure 1C), demonstrating that dHMGS can be used to rapidly and accurately diagnose genetic antibiotic resistance without need for culture.
Sanger sequencing is a method that represents a universal standard for precise gene detection [27]. In this study, we demonstrated that dHMGS could efficiently distinguish the mixed resistance genotypes by showing distinct wild-type and mutant peaks simultaneously within a single biopsy specimen. In contrast, the sequencing result could only report one dominant peak of the specific gene site (Figure 4B). Therefore, dHMGS might be more suitable and direct for detection of mixed resistance infection than the sequencing method.
To evaluate the performance of dHMGS in biopsy specimens, 40 gastric biopsies with the corresponding isolated strains were detected. The comparison of the resistance genotype from biopsy specimens and the corresponding isolated strains showed that most of them were consistent (Table 2). Interestingly, besides single resistance genotype, dHMGS could also detect mixed resistance genotype (both wild-type and mutant genotypes) simultaneously from same biopsy specimens by appearing in two distinct peaks (Figure 5A), which indicated that these patients may have mixed infection with the heteroresistant strains. Several studies showed that the mixed infection rate in some regions could reach around 20%, which seriously increased the complex and difficulty in clinical diagnosis and treatment [6,28,29]. Therefore, the resistance analysis using biopsy specimens would be more accurate and objective than using isolated strains. Generally, these results demonstrated that dHMGS is a very promising method to distinguish the multistrain infection from biopsy specimens directly, which would provide valuable clue for clinical diagnosis, treatment and monitoring.
One limitation of the present study was that biopsies were collected from antrum alone. It remains unanswered whether our results would differ if our specimens were collected from both gastric antrum and gastric body of the enrolled patients. Future studies are needed to explore how the H. pylori loads differ between these two locations in different subgroups of patients. While the main goal of present report is to share dHMGS with the scientific community and encourage its use by other centers, we plan to use dHMGS technology to conduct long-term follow-up study on a larger cohort of patients. Such studies will help to dissect the effects of the increasing drug resistance rate (both genotypic and phenotypic resistance) on the eradication/relapse rate in patients treated for H. pylori infection.
Conclusion & future perspective
In summary, our new application of HMGS to directly detect and analyze H. pylori in biopsy specimens (dHMGS) showed high efficiency and improved sensitivity for diagnosis of H. pylori compared with classical culture and RUT. dHMGS was equally sensitive or nearly as sensitive as biopsy material sequencing or HMGS of cultured micro-organisms. In addition to greater sensitivity and accuracy, dHMGS could simultaneously quantify H. pylori burden in the collected biopsy material, screen for virulence and drug resistance genes, and allow for potential detection of multistrain H. pylori infection. Thus, dHMGS shows significant advantage and promise as a very rapid, highly informative and precise diagnostic tool in clinical practice of H. pylori infections.
Supplementary Material
Executive summary.
Helicobacter pylori infection is closely associated with gastroduodenal diseases including chronic atrophic gastritis, chronic superficial gastritis, peptic ulcer diseases and gastric carcinoma.
High bacterial burden and expression of the virulence factors contribute to certain outcomes of gastroduodenal diseases linked to H. pylori infection.
The H. pylori drug resistance and especially the mixed infection rate seriously increased the complex and difficulty in clinical diagnosis and treatment.
High-throughput multiplex genetic detection system (direct high-throughput multiple genetic detection system; dHMGS) was established for direct identification of H. pylori with concomitant analysis of quantification, virulence, drug resistance and mixed infection in biopsy specimens.
Materials & methods
One hundred and thirty-three gastric biopsy specimens were screened by dHMGS, rapid urease test, culture and Sanger sequencing, and outcomes from these methods were analyzed and compared.
The correlation of relative quantitation of H. pylori DNA load by dHMGS in gastric mucosa biopsy specimens and clinical characteristics was analyzed using the χ2 test.
The AC1 values of dHMGS assay and Sanger sequencing for the four resistance genes were analyzed based on the Landis and Koch’s criteria.
Results
dHMGS proofed a highly specific (97.1%), sensitive (93.7%) and accurate (95.5%) tool for direct detection of H. pylori in biopsy specimens compared with culture and rapid urease test.
dHMGS showed high fidelity (R2 = 0.983) for quantitation of relative abundance of H. pylori in biopsy specimens.
Significant difference in the relative H. pylori DNA abundance was found in different gastroduodenal diseases (p = 0.0213).
The AC1 values of dHMGS assay and Sanger sequencing for detecting the four resistance genes were 0.954, 1.000, 0.820 and 0.957, respectively, which demonstrated that dHMGS has excellent concordance with sequencing in detecting resistance genes of biopsy specimens.
dHMGS can efficiently distinguish the mixed resistance genotypes within a single biopsy specimen.
Discussion
The new application of HMGS (dHMGS) could efficiently diagnose and quantify H. pylori burden, simultaneously screening for virulence, drug resistance and possible detection of the multistrain infections in biopsy specimens.
dHMGS shows significant advantage and promise as a very rapid, highly informative and precise diagnostic tool in clinical practice of H. pylori infections.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
To view the supplementary data that accompany this paper, please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/fmb-2016-0149
Financial & competing interests disclosure
This work was supported by Shanghai science and Technology Committee ‘Lead project’ (grant no. 14411962800 and 16411968000), Shanghai Shenkang Hospital Development Center ‘New frontier technology joint research project’ (grant no. SHDC12013123, SHDC12015107 and SHDC22014003), Shanghai Health Bureau ‘Key research projects’ (grant no. 20134008), Shanghai Science and Technology Committee ‘Natural Science Foundation’ (grant no: 14ZR1413100), Ministry of Science and Technology ‘The National High Technology Research and Development Program of China (863 Program)’ (grant no. 2015AA021107-019). Olszewski MA was supported by VA Merit Grant (grant no. I01BX000656). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Venerito M, Goni E, Malfertheiner P. Helicobacter pylori screening: options and challenges. Expert Rev Gastroenterol Hepatol. 2016;10(4):497–503. doi: 10.1586/17474124.2016.1126507. [DOI] [PubMed] [Google Scholar]
- 2.Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: recent developments and future implications. Microbes Infect. 2003;5(8):693–703. doi: 10.1016/s1286-4579(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastro Hepat. 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20(29):9912–9921. doi: 10.3748/wjg.v20.i29.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JJ, Kim JG, Dong HK. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003;8(3):202–206. doi: 10.1046/j.1523-5378.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 6••.Ben Mansour K, Fendri C, Battikh H, et al. Multiple and mixed Helicobacter pylori infections: comparison of two epidemiological situations in Tunisia and France. Infect Genet Evol. 2016;37:43–48. doi: 10.1016/j.meegid.2015.10.028. Investigation of the mechanisms leading to antibiotic resistance of Helicobacter pylori containing heteroresistant phenotypes from separate patients before H. pylori eradication. [DOI] [PubMed] [Google Scholar]
- 7••.Hu B, Zhao F, Wang S, et al. A highthroughput multiplex genetic detection system for Helicobacter pylori identification, virulence and resistance analysis. Future Microbiol. 2016 doi: 10.2217/fmb-2016-0023. (Epub ahead of print). Our recent study established a novel high-throughput multiple genetic detection system, which could simultaneously detect and analyze a set of genes of H. pylori isolates, including 16S rRNA for H. pylori identification, ten virulence genotypes and four main drug resistance genes and their variants. [DOI] [PubMed] [Google Scholar]
- 8.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(1):29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 9.Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Med Res Methodol. 2013;13:61. doi: 10.1186/1471-2288-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Zhou L, Zhao F, Hu B, et al. A creative Helicobacter pylori diagnosis scheme based on multiple genetic analysis system: qualification and quantitation. Helicobacter. 2015;20(5):343–352. doi: 10.1111/hel.12206. Our previous work developed a creative H. pylori diagnostic scheme (multiple genetic analysis system) based on qualitative and quantitative analysis. [DOI] [PubMed] [Google Scholar]
- 11.Hachem CY, Clarridge JE, Evans DG, Graham DY. Comparison of agar based media for primary isolation of Helicobacter pylori. J Clin Pathol. 1995;48:714–716. doi: 10.1136/jcp.48.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuchi E, Forne M, Quintana S. Comparison of two transport media and three culture media for primary isolation of Helicobacter pylori from gastric biopsies. Clin Microbiol Infect. 2002;8(9):609–610. doi: 10.1046/j.1469-0691.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 13.Cover TL. Perspectives on methodology for in vitro culture of Helicobacter pylori. Methods Mol Biol. 2012;921:11–15. doi: 10.1007/978-1-62703-005-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Wang Y. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. 2015;21(40):11221. doi: 10.3748/wjg.v21.i40.11221. Review of the current options and novel developments of diagnostic tests of H. pylori including histology, culture, rapid urease test as well as molecular methods, and their applications in different clinical conditions or for specific purposes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Gerrits MM, van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6(11):699–709. doi: 10.1016/S1473-3099(06)70627-2. A good review on the treatment of H pylori infection and the clinical relevance, mechanisms and diagnosis of antimicrobial resistance. [DOI] [PubMed] [Google Scholar]
- 16.Konturek PC, Brzozowski T, Konturek SJ, et al. Functional and morphological aspects of Helicobacter pylori-induced gastric cancer in Mongolian gerbils. Eur J Gastroenterol Hepat. 2003;15(7):745–754. doi: 10.1097/01.meg.0000059155.68845.9d. [DOI] [PubMed] [Google Scholar]
- 17.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187(8):1165–1177. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Shukla SK, Prasad KN, Tripathi A, Ghoshal UC, Krishnani N, Nuzhat H. Quantitation of Helicobacter pylori ureC gene and its comparison with different diagnostic techniques and gastric histopathology. J Microbiol Meth. 2011;86(2):231–237. doi: 10.1016/j.mimet.2011.05.012. Develops a real-time quantitative PCR assay to measure H. pylori DNA load in different histopathological and disease parameters, and compared sensitivity and specificity with other diagnostic tests. [DOI] [PubMed] [Google Scholar]
- 19.Haley KP, Gaddy JA. Helicobacter pylori: genomic insight into the host-pathogen interaction. Int J Genomics. 2015;2015:1–8. doi: 10.1155/2015/386905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konturek JW. Discovery by Jaworski of Helicobacter pylori and its pathogenetic role in peptic ulcer, gastritis and gastric cancer. J Physiol Pharmacol. 2003;54(Suppl 3):23–41. [PubMed] [Google Scholar]
- 21.Proença-Modena JL, Acrani GO, Brocchi M. Helicobacter pylori: phenotypes, genotypes and virulence genes. Future Microbiol. 2009;4(2):223–240. doi: 10.2217/17460913.4.2.223. [DOI] [PubMed] [Google Scholar]
- 22.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115(5):1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 23.Katelaris PH. Helicobacter pylori: antibiotic resistance and treatment options. J Gastroenterol Hepatol. 2009;24(7):1155–1157. doi: 10.1111/j.1440-1746.2009.05911.x. [DOI] [PubMed] [Google Scholar]
- 24.Alfizah H, Norazah A, Hamizah R, Ramelah M. Resistotype of Helicobacter pylori isolates: the impact on eradication outcome. J Med Microbiol. 2014;63(Pt 5):703–709. doi: 10.1099/jmm.0.069781-0. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 26.Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol. 2007;21(2):315–324. doi: 10.1016/j.bpg.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Bentley SD, Parkhill J. Genomic perspectives on the evolution and spread of bacterial pathogens. Proc R Soc B Biol. 2015;282(1821):20150488. doi: 10.1098/rspb.2015.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao CY, Lee AY, Huang AH, et al. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect Genet Evol. 2014;23:196–202. doi: 10.1016/j.meegid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 29.De Francesco V, Zullo A, Ierardi E, et al. Phenotypic and genotypic Helicobacter pylori clarithromycin resistance and therapeutic outcome: benefits and limits. J Antimicrob Chemother. 2010;65(2):327–332. doi: 10.1093/jac/dkp445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.