Abstract
Coreopsis tinctoria (snow chrysanthemum) has been reported to exert antihyperlipidemic effects. The present study aimed to identify the active compounds of Coreopsis tinctoria and to investigate the molecular mechanisms underlying its effects on lipid dysregulation by measuring lipid levels, reactive oxygen species, lipid peroxidation and fatty acid synthesis. The present results demonstrated that snow chrysanthemum aqueous extracts significantly reduced serum lipid levels and oxidative stress in vivo. The main compounds that were isolated were identified as flavanomarein (compound 1) and eriodictyol 7-O-β-D glucopyranoside (compound 2). Compounds 1 and 2 demonstrated potent antioxidative properties, including free radical scavenging activity, inhibition of lipid peroxidation, as well as lipid-lowering effects in human HepG2 hepatocellular carcinoma cells treated with free fatty acids (FFAs). Compound 2 was revealed to suppress the elevation of triglyceride levels and inhibit lipid peroxidation following FFA treatment. In addition, it was demonstrated to significantly reduce intracellular levels of reactive oxygen species and improve the mitochondrial membrane potential and adenosine triphosphate levels, thus protecting mitochondrial function in FFA-treated HepG2 cells. Furthermore, compound 2 markedly suppressed the protein expression levels of disulfide-isomerase A3 precursor and fatty acid synthase, thus suppressing FFA-induced lipogenesis in HepG2 cells. In conclusion, the present study identified compound 2 as one of the main active compounds in Coreopsis tinctoria responsible for its lipid-lowering effects. Compound 2 was revealed to possess antihyperlipidemic properties, exerted via reducing oxidative stress, protecting mitochondrial function and suppressing lipogenesis.
Keywords: eriodictyol 7-O-β-D glucopyranoside, snow chrysanthemum aqueous extracts, flavanomarein, hyperlipidemia, oxidative stress, mitochondrial function, lipogenesis
Introduction
Lipid dysregulation serves a critical role in the progression of cardiovascular diseases (1), metabolic syndrome (2) and non-alcoholic fatty liver disease (3). These disorders pose major public health concerns, and are associated with family burden and a high socioeconomic cost (2). Currently available lipid-lowering agents used in the treatment of hyperlipidemia include statins and fibrates; however, these agents have been associated with serious adverse effects, including gastrointestinal disturbances, severe muscle damage and hepatotoxicity (4). Therefore, natural products and herbal medicines with improved safety profiles have garnered attention for the treatment of lipid disorders.
The capitula of Coreopsis tinctoria, also known as snow chrysanthemum, have been used in the form of a tea-like beverage for the prevention of cardiovascular disorders, diarrhea and diabetes in traditional Chinese medicine (5). Coreopsis tinctoria has been revealed to contain high concentrations of flavonoids (6), and it has been reported to exert anti-inflammatory effects (5), to promote pancreatic cell recovery (7,8) and to regulate lipid metabolism in hyperlipidemic mice (9). However, the main active compounds of Coreopsis tinctoria, as well as their exact pharmacologic effects on hyperlipidemia, have yet to be elucidated.
An increasing body of evidence has demonstrated that oxidative stress is a key trigger in the progression of hyperlipidemia (1,10). Lipids are thought to be among the most sensitive biological molecules in terms of reactive oxygen species (ROS) susceptibility (11). In addition, lipid peroxidation is known to disturb the integrity of cellular membranes, leading to leakage of cytoplasmic enzymes, which in turn causes cell death and cell death ultimately drives disease progression (11,12). A previous study has demonstrated that flavonoids have the capacity for anti-oxidative activities by reducing the production of ROS and preventing lipid peroxidation, which may be associated with alleviated hyperlipidemia (13).
The aim of the present study was to identify the main active compounds of Coreopsis tinctoria, to evaluate their antihyperlipidemic properties in vivo, and to investigate the molecular mechanisms underlying their effects on lipid regulation in vitro.
Materials and methods
Materials
Commercially available analytical reagents were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin and penicillin-streptomycin-glutamine were obtained from Beyotime Institute of Biotechnology (Haimen, China). Dimethylsulfoxide (for MTT assay), 2,2-diphenyl-picrylhydrazyl (DPPH), thiobarbituric acid (TBA), bovine serum albumin (BSA), MTT, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and mouse monoclonal anti-GAPDH antibody (1:10,000; cat no. G8795) were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Rabbit monoclonal antifatty acid synthase (FAS; 1:1,000; cat no. 3180S) and rabbit monoclonal anti-protein disulfide-isomerase A3 precursor (ERp57; 1:1,000; cat no. 2881S) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Horseradish peroxide-conjugated goat antimouse immunoglobulin (Ig)G (1:5,000; cat no. sc-2005) and goat anti-rabbit IgG (1:5,000; cat no. sc-2004) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Preparation and analysis of snow chrysanthemum aqueous extract and its main compounds
Snow chrysanthemum, the capitulum of Coreopsis tinctoria, was collected in the Uighur Autonomous Region of Xinjiang Province in September 2012, and was identified by Professor Yu-Hai Guo (China Agricultural University, Beijing, China). A voucher specimen (cat no. 201209) was preserved in the herbarium of the Laboratory of Ethnopharmacology of West China Hospital, West China Medical School of Sichuan University (Sichuan, China). Air-dried snow chrysanthemum (100 g) was ground into a powder and decocted with distilled water (0.8 l) by heating reflux extraction at 98°C for 3 h. Subsequently, the snow chrysanthemum aqueous extract (SCAE) was dried until water content was <10%. The total flavonoid content in SCAE was assessed using a colorimetric method, as previously described (14).
Its main compounds flavanomarein (compound 1) and eriodictyol 7-O-β-D glucopyranoside (compound 2) were isolated and purified using preparative high-performance liquid chromatography (HPLC; Shimadzu Corporation, Kyoto, Japan). Preparative HPLC was carried out on a SHIMADZU LC-6AD instrument with an SPD-20A detector, using a YMC-Pack ODS-A column (250×20 mm; 5 µm; YMC Co., Ltd., Kyoto, Japan). The dried powders (5 kg) of Coreopsis tinctoria were extracted three times successively with water and 70% ethyl alcohol to obtain the crude extract. The extract was subjected to polyamide resin (Chongqing Change Chemical Co., Ltd., Chongqing, China) column chromatography eluted with water, 30% ethyl alcohol and 70% ethyl alcohol to give three fractions (A-C, respectively). Fraction B was chromatographed over a Sephadex LH-20 column (GE Healthcare Bio-Sciences, Uppsala, Sweden) eluted with 50% methanol to give six fractions 1 to 6. Compound 1 was obtained and further purified by ecrystallization with 100% methanol from fraction 6. Compound 2 was obtained by Prep. HPLC (Shimadazu, YMC-Pack ODS-A; 5 µm, 250×20 mm; Shimadzu Corporation) from fraction 3. The mobile phase was acetonitrile (18%; solvent B): water (82%; solvent A), and the flow rate was 6 ml/min. Compounds 1 and 2 were identified by 1H NMR (600 MHz) and 13C NMR (150 MHz) run on AV II spectrometer (Bruker Corporation, Ettlingen, Germany).
HPLC profiles of SCAE, compound 1 and 2, were analyzed using a reverse column (LC-20A, Inertsil® ODS-SP; 4.6×150 nm; 3.5 µm; Shimadzu Corporation). Equal quantities (20 µl) of SCAE, compounds 1 and 2, were used for analysis. They were eluted at a 1 ml/min flow rate with solvent A, water with 0.1% formic acid, and solvent B (acetonitrile with 0.1% formic acid) at 280 nm. The gradient started from 15% B for the first 5 min, then to 65% by 15 min, and finally to 100% by 20 min at 22°C.
Animals
The animal experiments were approved by the Ethics Committee of the Institutional Animal Care and Treatment Committee of Sichuan University (permit no. 2014002B; Chengdu, China). Male Kunming mice (weight, 18–22 g; age, 4–6 weeks) were provided by the Chengdu Dashuo Experimental Animal Co, Ltd. (Chengdu, China). The mice were housed in controlled temperature (22±1°C) and humidity (55±5%) conditions, under a 12/12 h light/dark cycle with free access to food and water.
Animal experiments
The mice were divided into the following 3 groups (n=10 mice/group): Groups I, II and III. Mice in group I were maintained on a normal pellet diet, whereas mice in groups II and III were maintained on a high-fat diet for the induction of hyperlipidemia, which consisted of the following: Normal diet supplemented with 10% cholesterol, 10% lard, 2% sodium deoxycholic acid and 0.1% propylthiouracil. Following 21 days, group II were treated with 0.5% sodium carboxymethyl cellulose (vehicle). Group III received SCAE (60 mg/kg; compounds 1 and 2). Treatments were given orally twice a day for 42 days. At the end of the study, the mice were sacrificed, and blood, liver and kidney tissue samples were collected for biochemical analysis. Serum was separated by centrifugation at 1,000 × g for 15 min at 4°C, then assays of total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), glutathione peroxidase (GSH-Px) and nitric oxide synthase (NOS) levels were performed. Liver samples were homogenized (10%, w/v) in cold saline, then centrifuged at 1,000 × g for 15 min at 4°C. The supernatant was used for assaying the superoxide dismutase (SOD) and malondialdehyde (MDA) levels. Kidney samples were homogenized (10%, w/v) in cold saline and centrifuged at 1,000 × g for 10 min at 4°C for the lipid peroxidation assay. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology). The commercially available kits used for these measurements included: TC assay kit (cat no. A111-1), TG assay kit (cat no. A110-1), LDL-C assay kit (cat no. A113-1), GSH-Px assay kit (Colorimetric method; cat no. A005), Total NOS assay kit (cat no. A014-2), Total (T-) SOD assay kit (Hydroxylamine method; cat no. A001-1) and MDA assay kit (TBA method; cat no. A003-1; (all from Nanjing Jiancheng Bioengineering Institute, Nanjing, China) kits, according to the manufacturers' protocol. High-density lipoprotein cholesterol (HDL-C) levels were calculated according to the following formula: HDL-C = TC-[(1/5xTG) + LDL-C].
Antioxidant assays
The putative free radical-scavenging properties of SCAE were investigated using DPPH, as previously described (15,16). Various concentrations of compounds 1 and 2 (0, 10, 20, 40, 80 and 160 µmol/l), were added to 500 µmol/l alcoholic DPPH solution. A total of 500 µmol/l alcoholic DPPH solution, without compounds 1 and 2, was used as the control. Following incubation for 30 min in the dark at room temperature, the absorbance of each sample was measured at 517 nm.
Lipid peroxidation was assessed using the TBA method, as previously described (17). Briefly, mouse liver and kidney samples were homogenized (10%, w/v) in cold saline and centrifuged at 1,000 × g for 15 min at 4°C. Then, the liver and kidney tissue homogenates (100 µl, 10%) were mixed with 100 µl compounds 1 or 2 (10, 20, 40, 80 and 160 µmol/l), and ferrous sulfate (8 µl, 70 mmol/l) was added to each mixture. The mixtures were incubated for 30 min at 37°C. Subsequently, 300 µl 20% acetic acid and 300 µl 0.8% TBA in 1.1% sodium dodecyl sulfate was added, and the final mixtures were incubated at 95°C for 60 min. Following cooling, the mixtures were centrifuged at 5,000 × g for 10 min at 4°C and their absorbance was measured at 532 nm (17).
The IC50 value denotes the effective concentration of compounds 1 or 2 used to reduce 50% of available DPPH radicals or inhibit 50% of liver and kidney lipid peroxidation. The IC50 value of compounds 1 and 2 was calculated using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA).
Cell culture and viability assay
The human HepG2 hepatocellular carcinoma cell line was obtained from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (cat no. TCHu72; Shanghai, China). Cells were cultured at 37°C in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, as previously described (18). HepG2 cells (5×103 cells/well) were seeded in each well of 96-well plates (Costar; Corning Incorporated, Corning, NY, USA) and cultured for 24 h at 37°C. Cells were then incubated with compounds 1 or 2 (0, 1, 5, 25, 125 or 625 µmol/l) at 37°C for 24 h. Cells without treatment with compounds 1 and 2 were used as the controls. Cell viability was assessed using an MTT assay, as previously described (18).
Cell lipid accumulation assays
HepG2 cells (4×104 cells/well) were incubated in a 6-well plate (Costar; Corning Incorporated) for 24 h at 37°C. HepG2 cells cultured to 75% confluence were exposed to 1 mmol/l free fatty acids (FFAs) for 24 h to assess hepatic lipid accumulation and lipid peroxidation. The FFA mixture contained 1 mmol/l oleate (cat no. O7501) and 1 mmol/l palmitate (cat no. P9767) (both from Sigma-Aldrich; Merck KGaA) at a ratio of 2:1, and was diluted in the culture medium to obtain the desired final concentration (1 mmol/l). In addition, the FFAs mixture contained BSA (10% w/v; Sigma-Aldrich; Merck KGaA), as previously described (18). HepG2 cells, cultured to 75% confluence, were treated with either DMEM containing BSA (10% w/v; Sigma-Aldrich; Merck KGaA) as a control, or HepG2 cells, cultured to 75% confluence, were treated with 1 mmol/l FFAs alone or together with compounds 1 or 2 (25 µmol/l). A total of 24 h following treatment at 37°C, cells were stained using Oil Red O to assess intracellular lipid droplet accumulation, as previously described by Cui et al (19).
To further investigate the effects of compounds 1 and 2 on intracellular lipid levels, HepG2 cells at 75% confluence were treated for 24 h as aforementioned. FFA-containing medium was removed and the cells were washed twice with PBS. The cells from the various treatment groups were lysed in 1% Triton-X-100 (cat no. T8787; Sigma-Aldrich; Merck KGaA) for 30 min on ice. The cell lysates were determined using a BCA protein assay kit (Beyotime Institute of Biotechnology) and were diluted in 1% Triton-X-100 to obtain the final concentration of 5 mgprot/ml, then prepared for TG level assessments using the Triglyceride Quantification Colorimetric/Fluorometric kit (BioVision, Inc., Milpitas, CA, USA), according to the manufacturer's protocol.
Cell lipid peroxidation assay
To further evaluate the effects of compounds 1 and 2 on intracellular lipid peroxidation, HepG2 cells at 75% confluence were treated with compounds 1 or 2 (25 µmol/l), together with 1 mmol/l FFAs for 24 h. Cell lysates were obtained as aforementioned using 1% Triton-X-100 to assess lipid peroxidation via measuring MDA levels, using a commercially available MDA kit (cat no. A003-4; Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocol.
Intracellular ROS production
HepG2 cells (1×104 cells/well) were incubated in a 24-well plate (Costar; Corning Incorporated) for 24 h at 37°C. HepG2 cells at 75% confluence were plated in 24-well plates and were treated with 1 mmol/l FFAs alone or together with 25 µmol/l compound 2 for 24 h. Subsequently, cells were incubated with 10 µmol/l membrane-permeable oxidation-sensitive fluorescent dye DCFH-DA (cat no. D6883; Sigma-Aldrich; Merck KGaA) for 20 min at 37°C. Stained cells were observed under an Eclipse Ti laser scanning confocal microscope (Nikon Corporation, Tokyo, Japan) and photomicrographs were captured. In addition, HepG2 cells were treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h in a black opaque 96-well microplate (Corning Incorporated). Subsequently, cells were incubated with 10 µmol/l DCFH-DA for 20 min at 37°C. During this process, DCFH-DA is cleaved and oxidized to green fluorescent 2′-7-′-dichlorofluorescein via ROS mediation (DCF; excitation/emission, 488/530 nm), the level of which was measured using the Synergy™ Mx microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Mitochondrial membrane potential (∆Ψm) analysis
HepG2 cells (1×104 cells/well) were incubated in a 24-well plate (Costar; Corning Incorporated) for 24 h at 37°C. HepG2 cells at 75% confluence were treated with 1 mmol/l FFAs alone or together with 25 µmol/l compound 2 for 24 h. Cells were stained with 5 µg/ml JC-1 dye, as a ∆Ψm indicator, for 15 min (20), and then observed under an Eclipse Ti laser scanning confocal microscope (Nikon Corporation). In addition, HepG2 cells (4×104 cells/well) were plated in 6-well plates for 24 h at 37°C, then treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h at 37°C. Cells were harvested by trypsinization, stained with 5 µg/ml JC-1 dye (cat no. M34152; Thermo Fisher Scientific, Inc., Waltham, MA, USA) without cell fixation for 15 min at 37°C, then washed twice with ice-cold PBS and resuspended in 0.5 ml ice-cold FBS-free DMEM. The intensity of fluorescence was determined using a MoFlo Cytomation, Modular flow cytometer (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and the data were analyzed with Summit software version 4.3 (Cytomation, Inc., Fort Collins, CO, USA).
Intracellular adenosine triphosphate (ATP) levels
HepG2 cells (4×104 cells/well) were incubated in a 6-well plate for 24 h at 37°C. HepG2 cells were then treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h. Subsequently, cells were lysed using an ATP assay kit (cat no. A22026; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions, centrifuged at 12,000 × g for 5 min at 4°C, and the supernatants were collected. Protein concentration was determined using a BCA protein assay kit (Beyotime Institute of Biotechnology) and cells were transferred to a black opaque 96-well microplate (Corning Incorporated). Cellular ATP levels were also assessed using the ATP assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) with the Synergy™ Mx microplate reader (BioTek Instruments, Inc.), according to the manufacturer's protocol (21).
Western blot analysis
HepG2 cells were treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h. Cells were lysed using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) containing 1 mmol/l phenylmethane sulfonylfluoride for 20 min on ice. Subsequently, cell lysates were centrifuged at 12,000 × g for 10 min at 4°C. The protein concentration was determined using a BCA protein assay kit (Beyotime Institute of Biotechnology). Equal amounts (40 µg) of extracted protein samples were separated by 15% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked with 5% non-fat milk for 1 h at room temperature (~22°C), and then incubated with anti-GAPDH, anti-ERp57 and anti-FAS primary antibodies at 4°C overnight. Following washing three times with TBST (TBS containing 0.1% Tween-20; cat no. P0231; Beyotime Institute of Biotechnology), the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit IgG secondary antibodies at room temperature for 2 h. Protein bands were visualized by enhanced chemiluminescence using SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
Statistical analysis
The statistical significance of the differences between groups was assessed using one-way analysis of variance followed by a post hoc Scheffé's test for multiple comparisons. Data are expressed as the mean ± standard deviation of three repeated experiments. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS software version 19.0 (IBM Corp.).
Results
Antihyperlipidemic effects of SCAE
The present results demonstrated that SCAE (60 mg/kg) significantly decreased the serum levels of TC, TG and LDL-C by ~26, 33 and 28%, respectively, whereas it increased the serum levels of HDL-C by >2-fold, compared with the high-fat diet group (P<0.05; Table I). In addition, treatment with SCAE (60 mg/kg) resulted in a significant increase in hepatic SOD and serum GSH-Px concentrations (P<0.05), as well as a significant decrease in hepatic MDA levels (P<0.05) in hyperlipidemic mice maintained on a high-fat diet (Table II).
Table I.
Lipid-lowering effects of SCAE on high-fat diet-induced hyperlipidemic mice.
| Group | TC (mmol/l) | TG (mmol/l) | LDL-C (mmol/l) | HDL-C (mmol/l) |
|---|---|---|---|---|
| I | 2.01±0.51 | 0.73±0.37 | 0.46±0.11 | 1.5±0.13 |
| II | 3.98±0.78a | 1.66±0.61a | 0.99±0.13a | 0.41±0.18a |
| III | 2.96±0.61b | 1.11±0.42b | 0.71±0.12c | 0.82±0.18c |
P<0.001 vs. group I
P<0.05
P<0.001 vs. group II. Data are presented as the mean ± standard deviation (n=10 mice/group). SCAE, snow chrysanthemum aqueous extract; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Group I, normal group; Group II, high-fat diet group; Group III, SCAE group.
Table II.
Antioxidative effects of SCAE on high-fat diet-induced hyperlipidemic mice.
| Group | Serum GSH-Px (U/ml) | Serum NOS (U/ml) | Liver SOD (U/mgprot) | Liver MDA (nmol/mgprot) |
|---|---|---|---|---|
| I | 1,162.76±81.33 | 22.54±2.21 | 61.31±2.85 | 2.18±0.42 |
| II | 776.74±42.10a | 19.33±2.03b | 43.22±2.35a | 4.59±0.61a |
| III | 991.22±22.53c | 21.05±1.43 | 55.9±2.89c | 1.94±0.37c |
P<0.001
P<0.01 vs. group I
P<0.001 vs. group II. Data are presented as the mean ± standard deviation (n=10 mice/group). SCAE, snow chrysanthemum aqueous extract; GSH-Px, glutathione peroxidase; NOS, nitric oxide synthase; SOD, superoxide dismutase; MDA, malondialdehyde; Group I, normal group; Group II, high-fat diet group; Group III, SCAE group.
The main compounds of SCAE were isolated using HPLC and were identified as compound 1 and compound 2 by comparing the NMR results with previous reports (22,23) (Figs. 1 and 2). The antioxidative properties of compounds 1 and 2 were assessed using free radical-scavenging DPPH and lipid peroxidation TBA assays. Compound 2 was revealed to exert more potent antioxidative effects compared with compound 1 (Table III).
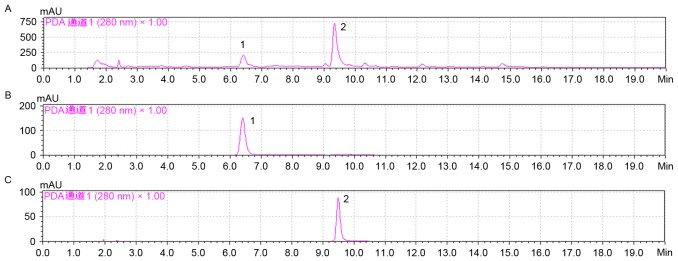
Figure 1.
HPLC profiles of SCAE and its main compounds were analyzed using gradient HPLC and 20 µl of the samples. HPLC profiles of (A) SCAE, (B) compound 1 and (C) compound 2. HPLC, high-performance liquid chromatography; SCAE, snow chrysanthemum aqueous extract; compound 1, flavanomarein; compound 2, eriodictyol 7-O-β-D glucopyranoside; AU, absorbance unit; PDA, photodiode array.
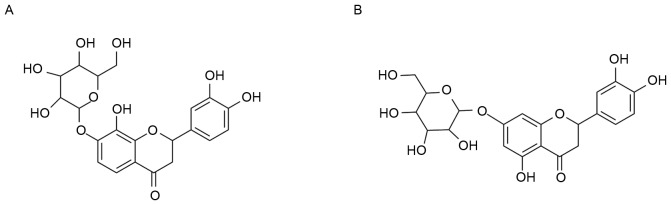
Figure 2.
Chemical structures of the main compounds of snow chrysanthemum aqueous extract, (A) flavanomarein and (B) eriodictyol 7-O-β-D glucopyranoside.
Table III.
IC50 of the antioxidative capabilities of SCAE compounds 1 and 2 in vitro, and in liver and kidney samples isolated from mice.
| IC50 | ||
|---|---|---|
| Assay | Compound 1 (µmol/l) | Compound 2 (µmol/l) |
| DPPH | 44.12±1.18 | 27.02±1.40 |
| TBA (liver) | 61.61±1.68 | 43.22±2.92 |
| TBA (kidney) | 140.97±9.11 | 59.97±3.30 |
Data are presented as the mean ± standard deviation of three samples and of three independent experiments. IC50, half maximal inhibitory concentration; SCAE, snow chrysanthemum aqueous extract; Compound 1, flavanomarein; Compound 2, eriodictyol 7-O-β-D glucopyranoside; DPPH, 2, 2-diphenyl-picrylhydrazyl; TBA, thiobarbituric acid.
Effects of compounds 1 and 2 on lipid accumulation in HepG2 cells
Following treatment of HepG2 cells with compounds 1 and 2, no detectable morphological changes and toxicity were observed (data not shown). Treatment with compounds 1 and 2 (25 µmol/l) was demonstrated to significantly reduce lipid accumulation in FFA-treated HepG2 cells (Fig. 3A and B). In addition, compounds 1 and 2 significantly suppressed the FFA-induced elevation in hepatocellular TG levels to 81 and 62%, respectively (P<0.001; Fig. 3C).
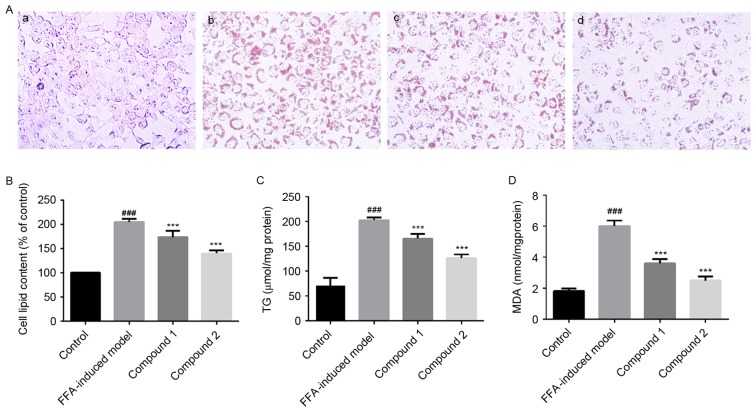
Figure 3.
Compounds 1 and 2 inhibited lipid accumulation, and reduced TG synthesis and lipid peroxidation induced by treatment with FFAs in HepG2 cells. Human HepG2 hepatocellular carcinoma cells were treated with 1 mmol/l FFAs alone or together with compounds 1 and 2 (25 µmol/l) for 24 h. (A) Compounds 1 and 2 inhibited lipid accumulation in HepG2 cells, as demonstrated following staining with Oil Red O. Photomicrographs were captured under ×400 magnification. (a) Control cells; (b) FFA-treated cells; (c) FFA- and compound 1-treated cells; (d) FFA- and compound 2-treated cells. (B) Treatment with compounds 1 and 2 abolished the FFA-induced increase in cell lipid content. (C) Treatment with compounds 1 and 2 significantly reduced TG levels. (D) Lipid peroxidation, assessed using cellular MDA content, was significantly inhibited following treatment with compounds 1 and 2. Data are presented as the mean ± standard deviation. ###P<0.001 vs. control cells; ***P<0.001 vs. FFA-treated cells. Compound 1, flavanomarein; Compound 2, eriodictyol 7-O-β-D glucopyranoside; TG, triglyceride; FFA, free fatty acid; MDA, malondialdehyde.
Effects of compounds 1 and 2 on lipid peroxidation in HepG2 cells
As presented in Fig. 3D, lipid peroxidation was significantly enhanced in HepG2 cells exposed to 1 mmol/l FFAs compared with control cells. However, treatment with compounds 1 and 2 was revealed to significantly inhibit hepatic lipid peroxidation (P<0.001). Notably, compound 2 appeared to exert more potent effects on hepatic lipid accumulation and peroxidation compared with compound 1, thus suggesting that compound 2 may be characterized by higher biological activity.
Effects of compound 2 on ROS production in HepG2 cells
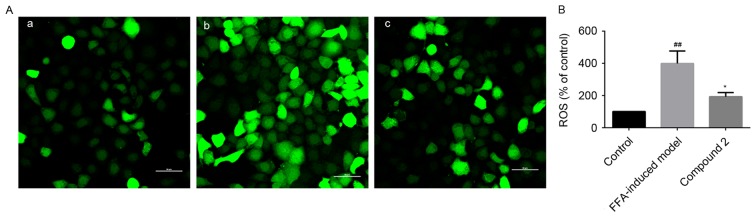
HepG2 cells exposed to FFAs exhibited increased intracellular ROS production, as demonstrated by the increased ROS-mediated oxidation of the acetate moieties of DCFH-DA to green DCF. DCF fluorescence intensity was revealed to be increased by 4-fold in HepG2 cells exposed to FFAs compared with control cells. Notably, compound 2 was demonstrated to significantly suppress the FFA-induced increase in hepatic ROS generation (Fig. 4).
Figure 4.
Compound 2 inhibited FFA-induced ROS production in HepG2 cells. Human HepG2 hepatocellular carcinoma cells were treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h. (A) DCF green fluorescence was visualized in (a) Control, (b) FFA-treated and (c) FFA- and compound 2-treated cells. Photomicrographs were captured under ×400 magnification. (B) DCF fluorescence intensity was quantified in control, FFA-treated and FFA- and compound 2-treated cells. Data are presented as the mean ± standard deviation. ##P<0.01 vs. control cells; *P<0.05 vs. FFA-treated cells. Compound 2, eriodictyol 7-O-β-D glucopyranoside; FFA, free fatty acid; ROS, reactive oxygen species; DCF, 2′-7′-dichlorofluorescein.
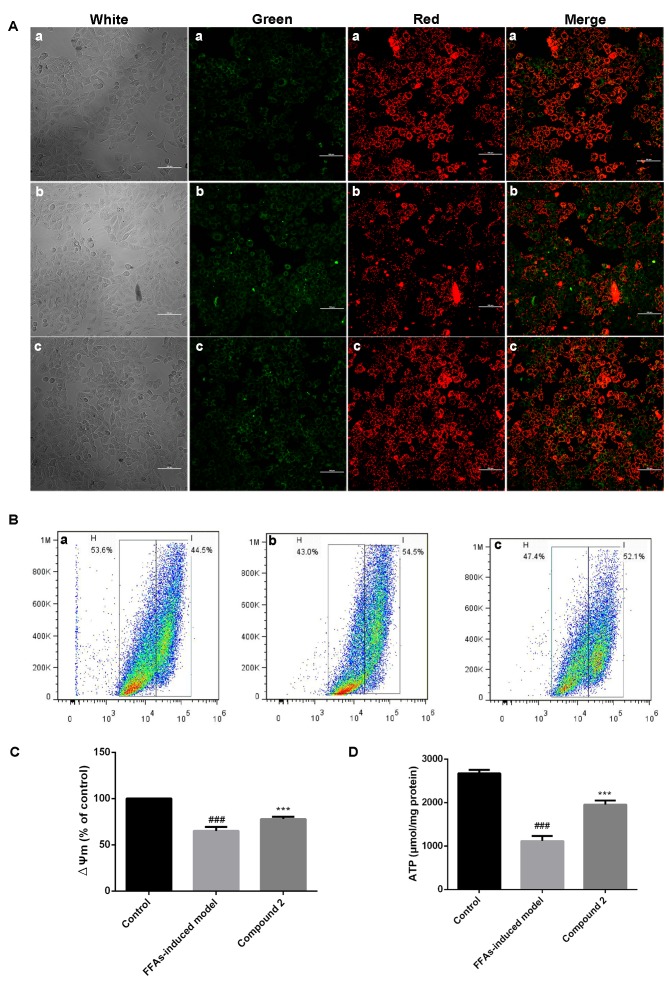
Effects of compound 2 on ∆Ψm
As presented in Fig. 5A, HepG2 cells exposed to FFAs demonstrated decreased ∆Ψm, whereas treatment with compound 2 was revealed to reverse the FFA-induced ∆Ψm decrease. Flow cytometric analysis also demonstrated that HepG2 cells exposed to FFAs exhibited a significant decrease (35%) in ∆Ψm, which was significantly attenuated following treatment with compound 2 (Fig. 5B and C).
Figure 5.
Compound 2 prevented the ∆Ψm collapse and maintained cellular ATP levels in FFA-treated HepG2 cells. Human HepG2 hepatocellular carcinoma cells were treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h. (A) JC-1 staining was used to assess ∆Ψm. Red fluorescence indicates high ∆Ψm, whereas green fluorescence indicates low ∆Ψm. (a) Control cells; (b) FFA-treated cells; (c) FFA- and compound 2-treated cells. Photomicrographs were captured under ×400 magnification. (B) Flow cytometric analysis of ∆Ψm, assessed using JC-1 fluorescence. (a) Control cells; (b) FFA-treated cells; (c) FFA- and compound 2-treated cells. (C) Relative ∆Ψm levels were quantified in control, FFA- and FFA- and compound 2-treated cells. (D) Intracellular ATP levels were assessed in control, FFA- and FFA- and compound 2-treated cells. Data are presented as the mean ± standard deviation. ###P<0.001 vs. control cells; ***P<0.001 vs. FFA-treated cells. Compound 2, eriodictyol 7-O-β-D glucopyranoside; ∆Ψm, mitochondrial membrane potential; ATP, adenosine triphosphate; FFA, free fatty acid.
Effects of compound 2 on intracellular ATP levels
Following exposure of HepG2 cells to FFAs, intracellular ATP levels were significantly decreased, whereas treatment with compound 2 was revealed to counter act the FFA-induced decrease in ATP levels (Fig. 5D). These findings suggested that compound 2 may ameliorate hepatic lipid accumulation due to its protective effects on mitochondrial function, exerted through the reduction in ROS production and the regulation of ∆Ψm and ATP production.
Effects of compound 2 on the expression of lipogenesis-associated proteins
As presented in Fig. 6, following exposure to FFAs for 24 h, the expression levels of the lipogenesis-associated proteins ERp57 and FAS appeared to be upregulated. Compound 2 was demonstrated to markedly attenuate the FFA-induced upregulation in ERp57 and FAS expression. These results suggested that compound 2 may suppress hepatic lipid accumulation through the suppression of lipogenesis, via downregulating the expression of proteins involved in lipogenesis, including ERp57 and FAS.
Figure 6.
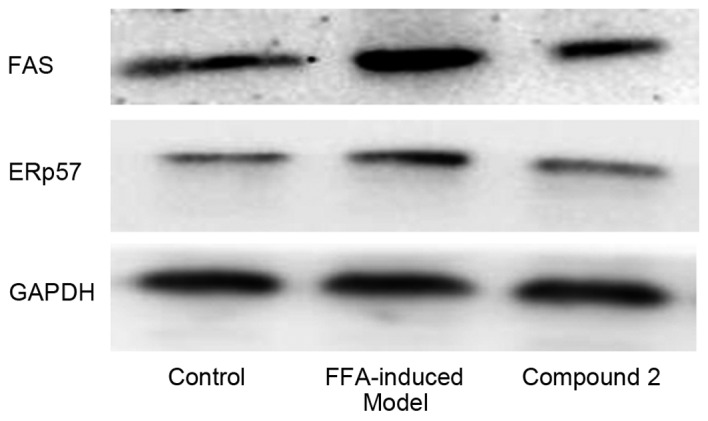
Compound 2 downregulated the protein expression of ERp57 and FAS in HepG2 cells treated with FFAs. Human HepG2 hepatocellular carcinoma cells were treated with 1 mmol/l FFAs alone or together with compound 2 (25 µmol/l) for 24 h. ERp57 and FAS protein expression levels were evaluated using western blot analysis. The results are representative of three independent experiments. Compound 2, eriodictyol 7-O-β-D glucopyranoside; ERp57, disulfide-isomerase A3 precursor; FAS, fatty acid synthase; FFA, free fatty acid.
Discussion
Hyperlipidemia has been identified as an important risk factor for the development of atherosclerosis (1) and acute necrotic pancreatitis (24). Coreopsis tinctoria is a herbal medicine used to regulate lipid metabolism in traditional Chinese medicine (9). However, the exact pharmacological effects of Coreopsis tinctoria, as well as the main active compounds and the molecular mechanisms responsible for these effects, have yet to be elucidated. In the present study, SCAE was demonstrated to decrease serum lipid levels in a mouse model of hyperlipidemia, possibly due to its antioxidative properties. Its main active compounds, compounds 1 and 2, were revealed to decrease lipid accumulation in HepG2 cells, possibly through the reduction of oxidative stress, the protection of mitochondrial function and the suppression of lipogenesis.
Administration of a high-fat diet has been reported to increase fat and cholesterol intake, decrease the β-oxidation of fatty acids and accelerate TG synthesis in rats, resulting in increased TC and TG levels in the bloodstream (25). The present results suggested that the flavonoid-rich SCAE may attenuate lipid disorders and regulate TG levels. The liver is primarily responsible for lipid synthesis, metabolism and transportation (13,26), and hyperlipidemia has been reported to increase hepatic lipid content, thus enhancing ROS generation and lipid peroxidation (27). A previous study revealed that flavonoids may attenuate hyperlipidemia, possibly due to their potent antioxidative effects (25). The present study suggested that SCAE may enhance the endogenous antioxidative defense mechanisms of hepatocytes, thus ameliorating hyperlipidemia, due to its high flavonoid content and potent antioxidative properties.
In the present study, compounds 1 and 2 were the main compounds isolated from SCAE. Treatment of HepG2 cells with FFAs leads to increased lipid accumulation, TG synthesis and lipid peroxidation, and has been used to evaluate the effects of putative lipid-lowering agents on lipid accumulation and lipid peroxidation in vitro (18,28). The present results suggested that compound 2 may be characterized by more potent lipid-lowering capabilities compared with compound 1. In addition, compound 2 appeared to exert stronger antioxidative effects, as demonstrated by its greater capabilities for scavenging free radicals and inhibiting lipid peroxidation compared with compound 1. These results suggested that compound 2 may be the main bioactive compound of SCAE responsible for its lipid-lowering effects, due to its potent antioxidative capabilities.
Mitochondria have been identified as the center of cellular lipid metabolism and one of the main sources of intracellular ROS generation (29,30). Excessive fatty acid metabolism has been associated with increased ROS generation, as well as decreased activity of antioxidant enzymes, ultimately resulting in mitochondrial damage (3,10,25). Malfunctioning mitochondria release higher quantities of ROS (30), thus resulting in a vicious cycle of mitochondrial dysfunction, decreased mitochondrial fatty acid β-oxidation and increased TG synthesis. The present results demonstrated that compound 2 counteracted the FFA-induced increase in intracellular ROS production. Furthermore, it was revealed to prevent the FFA-induced collapse of the ∆Ψm and the decrease in cellular ATP levels, thus suggesting that compound 2 may protect mitochondrial function.
The endoplasmic reticulum (ER) is known to serve a central role in de novo lipogenesis. Oxidative and ER stress have been reported to occur simultaneously or successively, and ER stress has been associated with hepatic lipid accumulation (31). The ER-associated protein ERp57 has been revealed to be upregulated during FFA-induced cellular steatosis, whereas its knockdown significantly reduced lipid accumulation in steatotic cells (31). FAS has been identified as a key enzyme during lipogenesis, as it catalyzes the terminal steps in de novo fatty acid synthesis (32). The present results demonstrated that compound 2 downregulated the protein expression levels of ERp57 and FAS in FFA-treated HepG2 cells. These results suggested that compound 2 may prevent de novo lipogenesis, via suppressing the expression of ERp57 and FAS in hepatocytes.
In conclusion, the present study suggested that compound 2 may be the main active compound of Coreopsis tinctoria, responsible for its lipid-regulating effects. Furthermore, compound 2 was demonstrated to enhance the endogenous antioxidative defense mechanisms of hepatocytes and to protect mitochondria against oxidative damage. In addition, its effects on ER stress reduction and the inhibition of de novo lipogenesis may be involved in the molecular mechanisms underlying the lipid-lowering effects of SCAE. The present results suggested that compound 2 may have potential for the development of novel therapeutic strategies for the treatment of patients with hyperlipidemia.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81673710).
References
- 1.Abliz A, Aji Q, Abdusalam E, Sun X, Abdurahman A, Zhou W, Moore N, Umar A. Effect of Cydonia oblonga Mill. leaf extract on serum lipids and liver function in a rat model of hyperlipidaemia. J Ethnopharmacol. 2014;151:970–974. doi: 10.1016/j.jep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Cignarella A, Bellosta S, Corsini A, Bolego C. Hypolipidemic therapy for the metabolic syndrome. Pharmacol Res. 2006;53:492–500. doi: 10.1016/j.phrs.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irudayaraj SS, Sunil C, Duraipandiyan V, Ignacimuthu S. In vitro antioxidant and antihyperlipidemic activities of Toddaliaasiatica (L) Lam. Leaves in Triton WR-1339 and high fat diet induced hyperlipidemic rats. Food Chem Toxicol. 2013;60:135–140. doi: 10.1016/j.fct.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Shi S, Zhao M, Chai X, Tu P. Coreosides A-D, C14-polyacetylene glycosides from the capitula of Coreopsis tinctoria and its anti-inflammatory activity against COX-2. Fitoterapia. 2013;87:93–97. doi: 10.1016/j.fitote.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Mourboul A, Li ZY. Research advance in medicinal plants from genus Coreopsis. Zhongguo Zhong Yao Za Zhi. 2013;38:2633–2638. (In Chinese) [PubMed] [Google Scholar]
- 7.Dias T, Bronze MR, Houghton PJ, Mota-Filipe H, Paulo A. The flavonoid-rich fraction of Coreopsis tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats. J Ethnopharmacol. 2010;132:483–490. doi: 10.1016/j.jep.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Dias T, Liu B, Jones P, Houghton PJ, Mota-Filipe H, Paulo A. Cytoprotective effect of Coreopsis tinctoria extracts and flavonoids on tBHP and cytokine-induced cell injury in pancreatic MIN6 cells. J Ethnopharmacol. 2012;139:485–492. doi: 10.1016/j.jep.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Li YL, Chen X, Xue J, Liu J, Chen X, Wulasihan M. Flavonoids furom Coreopsis tinctoria adjust lipid metabolism in hyperlipidemia animals by down-regulating adipose differentiation-related protein. Lipids Health Dis. 2014;13:193. doi: 10.1186/1476-511X-13-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong G, Qin Y, Huang W, Zhou S, Wu X, Yang X, Zhao Y, Li D. Protective effects of diosgenin in the hyperlipidemic rat model and in human vascular endothelial cells against hydrogen peroxide-induced apoptosis. Chem-Biol Interact. 2010;184:366–375. doi: 10.1016/j.cbi.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 11.El-Demerdash FM, Nasr HM. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Bio. 2014;28:89–93. doi: 10.1016/j.jtemb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Miri R, Saadati H, Ardi P, Firuzi O. Alterations in oxidative stress biomarkers associated with mild hyperlipidemia and smoking. Food Chem Toxicol. 2012;50:920–926. doi: 10.1016/j.fct.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Zarzecki MS, Araujo SM, Bortolotto VC, de Paula MT, Jesse RJ, Prigol M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep. 2014;1:200–208. doi: 10.1016/j.toxrep.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Kang OJ, Gweon OC. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J Funct Foods. 2013;5:80–86. doi: 10.1016/j.jff.2012.08.006. [DOI] [Google Scholar]
- 15.Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res 523–524. 2003:163–172. doi: 10.1016/S0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 16.Hajiaghaalipour F, Kanthimathi MS, Sanusi J, Rajarajeswaran J. White tea (Camellia sinensis) inhibits proliferation of the colon cancer cell line, HT-29, activates caspases and protects DNA of normal cells against oxidative damage. Food Chem. 2015;169:401–410. doi: 10.1016/j.foodchem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Grespan R, Aguiar RP, Giubilei FN, Fuso RR, Damião MJ, Silva EL, Mikcha JG, Hernandes L, Amado C Bersani, Cuman RK. Hepatoprotective effect of pretreatment with Thymus vulgar is Essential Oil in experimental model of acetaminophen-induced injury. Evid Based Complement Alternat Med. 2014;2014:954136. doi: 10.1155/2014/954136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo MS, Hong SW, Yeon SH, Kim YM, Um KA, Kim JH, Kim HJ, Chang KC, Park SW. Magnolia officinalis attenuates free fatty acid-induced lipogenesis via AMPK phosphorylation in hepatocytes. J Ethnopharmacol. 2014;157:140–148. doi: 10.1016/j.jep.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010;2:95–104. [PMC free article] [PubMed] [Google Scholar]
- 20.Sung DK, Chang YS, Kang S, Song HY, Park WS, Lee BH. Comparative evaluation of hypoxic-ischemic brain injury by flow cytometric analysis of mitochondrial membrane potential with JC-1 in neonatal rats. J Neurosci Methods. 2010;193:232–238. doi: 10.1016/j.jneumeth.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Gyamfi D, Everitt HE, Tewfik I, Clemens DL, Patel VB. Hepatic mitochondrial dysfunction induced by fatty acids and ethanol. Free Radic Biol Med. 2012;53:2131–2145. doi: 10.1016/j.freeradbiomed.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Pan J, Zhang S, Yan L, Tai J, Xiao Q, Zou K, Zhou Y, Wu J. Separation of flavanone enantiomers and flavanone glucoside diastereomers from Balanophora involucrata Hook. F. by capillary electrophoresis and reversed-phase high-performance liquid chromatography on a C18 column. J Chromatogr A. 2008;1185:117–129. doi: 10.1016/j.chroma.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Shi S, Zhao M, Tu P. Novel chalcone from Coreopsis tinctoria Nutt. Biochem Systemat Ecol. 2006;34:766–769. doi: 10.1016/j.bse.2006.05.005. [DOI] [Google Scholar]
- 24.Czakó L, Szabolcs A, Vajda A, Csáti S, Venglovecz V, Rakonczay Z, Jr, Hegyi P, Tiszlavicz L, Csont T, Pósa A, et al. Hyperlipidemia induced by a cholesterol-rich diet aggravates necrotizing pancreatitis in rats. Eur J Pharmacol. 2007;572:74–81. doi: 10.1016/j.ejphar.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Feng LJ, Yu CH, Ying KJ, Hua J, Dai XY. Hypolipidemic and antioxidant effects of total flavonoids of Perilla frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int. 2011;44:404–409. doi: 10.1016/j.foodres.2010.09.035. [DOI] [Google Scholar]
- 26.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Peng L, Lu W, Wang Y, Huang X, Gong C, He L, Hong J, Wu S, Jin X. Effect of Eclipta prostrata on lipid metabolism in hyperlipidemic animals. Exp Gerontol. 2015;62:37–44. doi: 10.1016/j.exger.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Zhang L, Gurley E, Studer E, Shang J, Wang T, Wang C, Yan M, Jiang Z, Hylemon PB, et al. Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology. 2008;47:1905–1915. doi: 10.1002/hep.22239. [DOI] [PubMed] [Google Scholar]
- 29.Schönfeld P, Więckowski MR, Lebiedzińska M, Wojtczak L. Mitochondrial fatty acid oxidation and oxidative stress: Lack of reverse electron transfer-associated production of reactive oxygen species. Biochim Biophys Acta. 2010;1797:929–938. doi: 10.1016/j.bbabio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231–241. doi: 10.1016/j.freeradbiomed.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Chan PK, Pan SY, Kwon KH, Ye Y, Chu JH, Fong WF, Tsui WM, Yu ZL. ERp57 is up-regulated in free fatty acids-induced steatotic L-02 cells and human nonalcoholic fatty livers. J Cell Biochem. 2010;110:1447–1456. doi: 10.1002/jcb.22696. [DOI] [PubMed] [Google Scholar]
- 32.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]