Abstract
Background: Injury to the spinal cord produces immediate, adaptive inflammatory responses that can exacerbate the initial injury and lead to secondary damage. Thus far, researchers and clinicians have focused on modulating acute inflammation to preserve sensorimotor function. However, this singular approach risks overlooking how chronic inflammation negatively impacts the broader health of persons with a spinal cord injury (SCI). Objective: The aim of this monograph was to discuss interrelated processes causing persistent inflammatory stress after SCI, along with associated health risks. We review archetypal factors that contribute to a chronic inflammatory state, including response to injury, acute infection, and autonomic dysreflexia. Secondary complications producing and exacerbating inflammation are also discussed, including pain, depression, obesity, and injury to the integumentary and skeletal systems. Finally, we discuss the role of bacteria and the gut microbiome in this process and then conclude with a discussion on how a pro-inflammatory phenotype promotes an elevated risk for cardiovascular disease after injury. Conclusions: Effectively managing chronic inflammation should be a high priority for clinicians and researchers who seek to improve the health and life quality of persons with SCI. Chronic inflammation worsens secondary medical complications and amplifies the risk for cardiometabolic disorders after injury, directly impacting both the quality of life and mortality risk after SCI. Inflammation can worsen pain and depression and even hinder neurological recovery. It is, therefore, imperative that countermeasures to chronic inflammation are routinely considered from the point of initial injury and proceeding throughout the lifespan of the individual with SCI.
Keywords: cardiometabolic disorders, chronic inflammation, inflammatory stress, spinal cord injury
Inflammation is the customary adaptive response to injurious stimuli and conditions, including acute infection and tissue damage. Under the best of conditions, the near-term inflammatory response is characterized by activation of host defense against infection, followed by initiation of tissue repair. The inflammatory cascade is typically resolved through integrated feedback mechanisms that restore homeostatic balance. However, when infection or damage persist – or the immune response is directed against the host – chronic inflammation and tissue damage ensue (Figure 1). Paradoxically, the chronic inflammatory response may promote ongoing tissue damage while simultaneously engaged in healing and repair. The molecular and cellular actions that constitute simultaneous repair and damage are less well understood than those of acute inflammation. However, prolonged inflammation is now known to be associated with a myriad of disorders that include type 2 diabetes and all-cause cardiovascular diseases. These chronic inflammatory disorders may not be triggered by the conventional progenitors of infection and injury, but rather caused by sustained tissue malfunction and persistent activation of endocrine and paracrine signaling, which ultimately block reestablishment of preinjury tissue function and systemic homeostasis.
Figure 1.
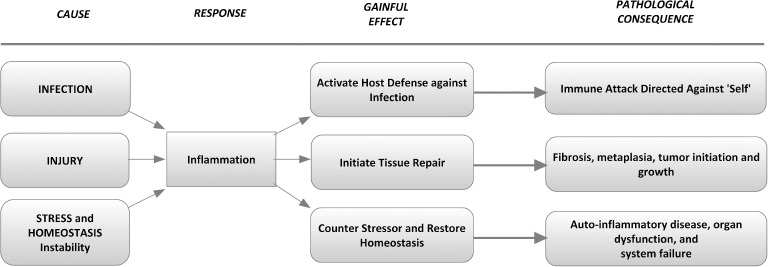
Cause, effect, and consequence of the inflammatory response.
Spinal cord injury (SCI) is a condition known to be characterized by extensive pro-inflammatory activity.1 Acute inflammation occurs at the time of injury, but residual inflammation can persist beyond the initial insult indefinitely.2 Chronic inflammation also results from common secondary complications of SCI (eg, pulmonary infections, urinary tract infections, and pressure ulcers).1,3,4 Thus far, SCI research has primarily focused on modulating acute inflammation occurring within the spinal cord to decrease secondary damage and preserve sensorimotor function.5,6 However, systemic inflammation impacts all body systems, not just one. As such, this monocular approach risks overlooking how chronic inflammation, by its very nature, may negatively impact the health of this population.
Evidence of heightened cardiometabolic disease risk after SCI7,8 underscores an interest in post-SCI pro-inflammatory activity as both a biomarker and instigator of future tissue damage and dysfunction. Chronic low-grade inflammation is an established risk factor for metabolic disorders in persons without SCI.9 Elevated values of C-reactive protein (CRP), a clinical marker of cardiovascular risk and systemic inflammation,10,11 have repeatedly been demonstrated in persons with SCI.8,12 These values correspond with high cardiovascular disease risk, per defined guidelines of the American Heart Association,10,12 and are higher in persons with tetraplegia than paraplegia.12 Although survival rates after SCI have greatly improved,13 persons aging with SCI face an increased risk for secondary health complications such as cardiovascular disease.14 Therefore, it is critical that effective treatments for chronic inflammation be developed to lessen the long-term medical risks that have been documented.15,16
We review the factors that contribute to a chronic inflammatory state after SCI. A recent review by Allison and Ditor described physiological mechanisms responsible for creating and sustaining immune dysfunction and chronic inflammation after SCI.1 Our review builds on this work and considers both the gut microbiome and a pro-inflammatory phenotype as both cause and consequence of these risks.
Processes Contributing to Persistent Inflammatory Stress
Response to cord injury
Injury to the spinal cord produces an immediate inflammatory response occurring within hours of the initial insult17,18 (Figure 219). This neuroinflammation can be reparative or exacerbate the secondary injury, depending on the specific immune cells involved and the temporal response of those cells.2,5,6,20,21 Suppression of acute inflammation is a legitimate clinical goal to preserve function after injury, but current treatment approaches have been unable to keep neuroinflammation from persisting through the chronic phase of injury.2
Figure 2.
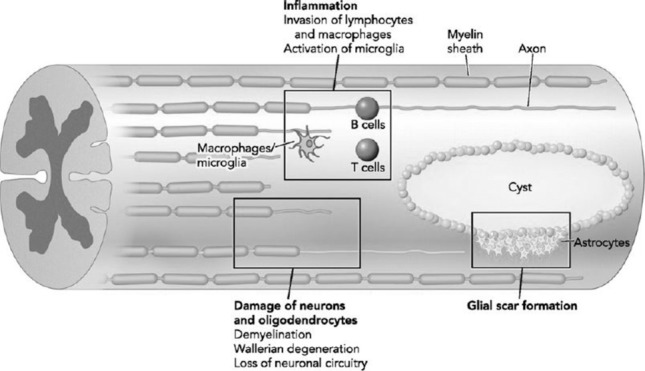
Immune response to acute spinal cord injury. Reproduced with permission from Obermair FJ, Schroter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda). 2008;23:296–304. Copyright © 2008 American Physiological Society.
Autonomic dysfunction
SCI causes dysfunction of the autonomic nervous system22 and immune system.2 This interrelated dysfunction is expressed through pro-inflammation or immune suppression,1,2,22,23 depending on which adrenergic receptor is stimulated by elevated catecholamines.24 Additionally, physiological responses to bouts of autonomic dysfunction can initiate and exacerbate inflammatory stress,25 creating an enabling environment for persistent non-resolving inflammation.1
It is accepted that autonomic dysreflexia triggered by bladder distension or bowel impaction/transit can result in a surge in catecholamines, crisis elevation in arterial blood pressure, reflexive bradycardia, and even myocardial or cerebral ischemia.22,26,27 Sustained elevation in catecholamines and blood pressure during these episodes leads to endothelial damage and inflammatory stress, accelerating pro-atherogenic processes and cardiovascular disease.14,16,25 Although autonomic dysfunction and chronic inflammation likely contribute to the elevated risk for cardiometabolic disorders,1,12,16,28 treatment of inflammation as a metabolic risk factor has not been implemented for this population.
Pain and depression
Pain and depression are common secondary complications of SCI.15,29,30 It is estimated that more than 60% of persons with SCI experience chronic pain,31 and 20% to 30% experience depression after injury.32,33 Pain can be nociceptive or neuropathic in origin,34 is related to depressive symptoms35,36 and impaired sleep,37,38 and may be provoked by the same noxious stimuli that triggers autonomic dysreflexia.39 Research in persons without disability has demonstrated a strong relationship between inflammation and depression40,41 and inflammation and pain,42 suggesting that non-resolving inflammation may worsen these common secondary complications of SCI. A recent clinical trial found that targeting inflammation improved depression after SCI.43 Considering the interrelated nature of inflammation with pain and depression40–42 and the impact these conditions have on health and mortality risk after injury,15,44 chronic inflammation may be a legitimate target in the all-inclusive management of SCI.
Acute infections
Acute infections of the lungs, urinary tract, and pressure ulcers are among the most common of secondary medical complications occurring after SCI,1,3,4,45–47 represent the third highest source of all-cause mortality after injury,45 and can even impact functional neurological recovery.48
Respiratory complications affect approximately 30% to 100% of persons with SCI, depending on the level of injury.49–51 Pneumonia is one of the primary risk factors for increased mortality after SCI52 and is the primary cause of death for both the acute and chronic phases of SCI.4,34,49 It is known that the presence of systemic inflammation worsens respiratory function and that, irrespective of the level or completeness of injury, higher levels of nonspecific acute phase reactant CRP and the inflammatory cytokine IL-6 are associated with reduced pulmonary function after SCI.53
The presence of symptomatic and asymptomatic bacteriuria poses risks for both acute and chronic inflammation. A recent study in persons with SCI reported that roughly 23% of participants had a symptomatic urinary tract infection,45 although nearly 70% of participants had asymptomatic bacteriuria.45 Asymptomatic bacteriuria has been associated with elevated inflammatory biomarkers in other populations54 and thus may explain one source of non-resolving inflammation after SCI.
Pressure ulcers occur in 30% to 80% of persons with SCI.47,55 If left untreated, pressure ulcers can lead to severe infection and bacteremia.4 Pressure ulcers occur in parallel with secondary complications characterized by inflammation and may exacerbate conditions that lead to inflammation. In the former, for example, a higher prevalence of pressure ulcers has been observed in persons with pneumonia.56 In the latter, changing of a bandage and debridement of pressure ulcers can trigger autonomic dysreflexia.55 The concomitant occurrence of secondary conditions after SCI may impede the resolution of inflammation, as chronic inflammation can delay wound healing.57 However, chronic inflammation may also expedite the development of additional complications. In acute SCI settings, elevated pro-inflammatory cytokines have been observed up to a week before the formation of the first pressure ulcer.58 This occurrence may be an indication that the initial, mechanical insult has far-reaching, persistent effects.
Anatomical and lifestyle consequences of SCI contribute to the high prevalence of acute infections in this population. Prompt treatment and proper management of contributing factors (eg, effective bladder management59 and pressure relieving strategies60) are several ways to decrease the incidence of acute infections and potentially reduce the impact of infection on mortality after SCI. However, treating other sources of inflammation (eg, pain, depression, autonomic dysreflexia, and bacteriuria) may be equally important. Persistent inflammation delays wound healing57 and may increase the risk of developing secondary complications characterized by inflammation,58 producing a vicious, self-perpetuating cycle.
Integumentary and skeletal injury
The integumentary system is a physical and immune barrier that protects the body from penetration by bacteria and other microorganisms. Breaching of this system by injury to the dermis and epidermis is an obvious source of acute infections in persons with SCI and may ultimately cause subdermal tissue infection. Pressure or shear forces can cause deep tissue injury; this injury produces an inflammatory response and, if left untreated, can progress into a pressure ulcer.61–63 In a survey of wheelchair athletes, nearly 20% of participants reported blisters, lacerations, abrasions, and cuts as common injuries.64 To our knowledge, the prevalence of these skin conditions in the general population of persons with SCI is unknown but would be expected to increase in the years post injury as skin loses collagen content and elasticity during the native course of aging-related immune senescence.
Up to 65% of persons with SCI experience at least one fracture, which is typically attributed to progressive bone loss occurring after injury.65–68 This prevalence does not include occult fractures or microfractures that evade clinical detection. Acute fracture is characterized by inflammation69,70 as the body initiates an inflammatory response to facilitate healing.71 However, unresolved inflammation can impede bone repair.72 Clinically, this may manifest in delayed fracture healing in persons who experience frequent urinary tract infections or in someone who incurs a subsequent wound due to altered mobility following a fracture. Osteoporosis is highly prevalent in other inflammatory conditions, such as chronic obstructive pulmonary disease.73 Extrapolating, this suggests that elevated inflammation may increase the risk of SCI-induced osteoporosis.74 In fact, elevated cytokines have been shown to increase the risk for fractures in nondisabled men.75 Fractures have also been shown to increase hospitalization time, increase the risk for pressure ulcers, and even increase mortality risk after SCI.44,52,65,67,68
Obesity and nutrition-mediated inflammation
It had been estimated that up to 66% of persons with SCI are overweight or obese.76 A recent study involving 2 academic medical and rehabilitation centers reported that 83% of persons with SCI satisfied authoritative guidelines for classification as overweight/obese.8 Obesity negatively influences health and quality of life after injury and has been related to increased rehospitalization rates,77 severe pain,77 and the development of pressure ulcers.78,79
A high-fat and hypercaloric intake,16,80 combined with a decreased energy expenditure,16,81 likely instigates the heightened prevalence of obesity in this population. In turn, obesity and a coincident high-fat diet may further exacerbate the chronic inflammatory state and metabolic risk after SCI.82,83 While once thought of as a benign fat storage disorder, obesity is now recognized as a dynamic process in which fat contributes to immune and pro-inflammatory activity.84 Not surprisingly, CRP is chronically elevated in persons with SCI8,12 and is associated with waist circumference and metabolic syndrome.11 In fact, as many as 76% of people with SCI demonstrate elevated CRP levels,8 a predictive biomarker for metabolic syndrome.11
A recent recommendation suggested that obesity and elevated inflammation be considered as population-specific risk determinants for cardiometabolic syndrome after SCI.8 To address these concerns, clinicians serving the SCI community must adopt proven interventions to treat obesity and cardiometabolic disease in this population, such as the recently adapted Diabetes Prevention Program.83 Prioritizing this treatment for the aging SCI population is necessary because cardiometabolic syndrome has been shown to increase in prevalence as time after injury becomes prolonged.85
Exercise may be a therapeutic countermeasure to obesity and related inflammation after SCI. Exercise has been shown to reduce both inflammatory cytokines and fatigue in obese persons with type 2 diabetes.86 In SCI, a recent behavioral intervention trial reported that promoting physical activity positively impacted both cardiometabolic outcomes and social participation after acute injury.87 Exercise may decrease depression and pain as well as increase muscular strength and aerobic capacity after injury.88,89 For these reasons, detailed exercise recommendations are available for persons with SCI.88,90 Prescribing “exercise as medicine”91,92 may be an effective way to address the inflammatory state and coincident secondary complications after SCI. Indeed, this approach has been suggested for other neurological conditions, such as stroke,93,94 and is a significant component of recently described interventions for persons with SCI.83
Influence of bacteria and the microbiome
Recent research on the gut microbiome has uncovered a novel source of inflammation that may be especially relevant in addressing cardiometabolic risk.95 The role of the gut microbiome in the development of metabolic syndrome has been described and involves several putative mechanisms, including bacterial-derived lipopolysaccharides (LPS) and short-chain fatty acids produced during fermentation of dietary polysaccharides.95
LPS found in the cell wall of Gram-negative bacteria is a highly inflammatory stimulant96; these bacterial wall components can induce “metabolic endotoxemia,” a low-grade inflammatory state that can lead to metabolic syndrome.95,97,98 Elevated LPS levels have been associated with obesity, insulin resistance, diabetes, and cardiovascular disease.97,98 Typically, the gastrointestinal mucosal barrier restricts the passage of resident bacteria; however, breakdown of this barrier can lead to bacterial translocation.99 Bacterial translocation has been shown to occur within 7 days after SCI in rodents.100,101 If bacterial translocation also occurs and persists in humans, it could explain one source of the nonspecific inflammation commonly observed after injury.1 It may also explain part of the elevated risk for cardiometabolic disorders in this population.8,16 Furthermore, if the bacteria are predominantly of the Gram-negative type, LPS-associated gastrointestinal dysmotility102 may explain one cause of gastrointestinal problems commonly observed after SCI.103
The second proposed factor linking the gut microbiome to metabolic syndrome involves short-chain fatty acids (SCFA), which are known to play an important role in regulating inflammation and insulin resistance.104 SCFAs, including butyrate, are also involved in gut-brain signaling through the vagus nerve,105 which has been implicated in regulating metabolic and immune homeostasis.106 Additionally, SCFA levels have been negatively correlated with colonic transit time.107
Although an altered gut microbiome holds potential to impact health outcomes after SCI, this novel source of inflammation has only received limited attention in persons with SCI. To our knowledge, only one study has examined the gut microbiome in humans with SCI. In this study, a decrease in SCFA-producing bacteria was found in persons with differing levels of bowel dysfunction compared to controls.108 An altered microbiome may explain additional secondary complications besides bowel dysfunction. One report described differences in the urinary microbiome between controls without SCI, subjects with SCI, and changes in the SCI urine microbiome with time since injury.109 Because of the relationship between asymptomatic bacteriuria and elevated inflammatory biomarkers,54 incorporating the microbiome in health assessment may provide critical evidence about contributors to chronic inflammation after SCI.
Additional research could identify whether putative changes in the gut microbiome after SCI contribute to metabolic risk in this population and examine whether manipulating the gut microbiome could alter the host's inflammatory,96 metabolic,95 gastrointestinal,102 and even autonomic state110 after SCI. Although LPS and SCFA levels may be modifiable by dietary interventions,97,104 additional research is needed to understand which factors support sustained changes in microbiome composition. Manipulating this novel source of inflammation holds promise for persons with SCI as well as other conditions characterized by chronic inflammation.
Pro-inflammatory phenotype after SCI
As noted, recent attention has focused on blood levels of pro-inflammatory cytokines as a unified forerunner of cardiovascular disease111,112 and a predictor of atherogenesis and cardiovascular events.112 This pro-inflammatory phenotype has now been documented after SCI by several laboratories.8,12,113–118 An expanding body of in vitro studies supports the view that inflammation may directly influence disease processes.112 The pro-atherogenic activity of various serological products raises unique concerns for persons with SCI, as concentrations of blood-borne inflammatory cytokines are typically elevated. In most cases, testing of persons with SCI reveals pathologically elevated levels of CRP,8,12 while elevated IL-6, soluble vascular adhesion molecule (sVCAM)-1, and endothelin-1 have also been reported.118
In addition to suspected elevation of vascular-derived CRP, chronic elevation of plasma-borne CRP and inflammatory cytokines (eg, IL-6) in persons with SCI can be attributed to various antecedents including overt clinical infection, background bacteriuria, excessive fat mass, and skin lesions12,114,116 as well as the common sequelae of activity-induced musculoskeletal injury119–121 and heterotopic ossification.122 Noteworthy are the myriad sources for evolution of inflammatory products, the homogeneous signaling response despite unrelated origins and the finding that their levels after SCI surpass evidence-based cut-scores for elevated cardiovascular disease risk (eg, CRP >3 mg/dL), even without overt evidence of illness or inflammatory disease.8,114,115,118,123
Conclusions
The inflammatory response plays a significant role in both the acute and chronic phases of SCI as an interrelated, multifactorial process that adversely impacts health and quality of life after injury. In the acute phase, inflammation can lead to secondary damage, thus worsening the initial injury. Dysregulation in the autonomic nervous system can foster and produce inflammatory stress. Common secondary complications of SCI, such as acute infection, injury to the integumentary and skeletal systems, and obesity can all produce inflammation. They can also initiate and sustain chronic inflammation, leading to the exacerbation of existing conditions and creating a microenvironment suitable for the development and sustained risk of new medical complications.
Chronic inflammation amplifies the risk for cardiometabolic disorders after injury, although treating inflammation as an independent cardiometabolic risk factor has been widely overlooked for this population. Also, by exacerbating secondary complications, chronic inflammation directly impacts both the quality of life and mortality risk after injury. Research further implicates inflammation as cause for worsened pain and depression and even a hindrance of neurological recovery. Efforts to lessen the impact of secondary medical complications, such as pain, clearly align with the priorities of the SCI community.124,125
For these reasons, effectively managing chronic inflammation after SCI should be a high priority for clinicians and researchers who seek to improve the health and life quality of persons with SCI. It is imperative that chronic inflammation be routinely considered throughout the lifespan of the individual with SCI. Finally, future work is needed to examine whether changes in microbiome composition and the inflammatory phenotype can be manipulated after SCI and which interventions produce the most sustained and impactful changes in inflammation-mediated secondary complications.
Acknowledgments
We would like to thank April Mann for her assistance in technical review of the manuscript.
The authors report no conflicts of interest.
REFERENCES
- 1. Allison DJ, Ditor DS.. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015; 53 1: 14– 18. [DOI] [PubMed] [Google Scholar]
- 2. Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG.. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol. 2014; 258: 121– 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKinley WO, Gittler MS, Kirshblum SC, Stiens SA, Groah SL.. Spinal cord injury medicine. 2. Medical complications after spinal cord injury: Identification and management. Arch Phys Med Rehabil. 2002; 83 3 suppl 1: S58– 64, S90– 58. [DOI] [PubMed] [Google Scholar]
- 4. Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis. 1997; 25 6: 1285– 1290; quiz 1291–1282. [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS.. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003; 184 1: 456– 463. [DOI] [PubMed] [Google Scholar]
- 6. David S, Lopez-Vales R, Wee Yong V.. Harmful and beneficial effects of inflammation after spinal cord injury: Potential therapeutic implications. Handbook Clin Neurol. 2012; 109: 485– 502. [DOI] [PubMed] [Google Scholar]
- 7. Chopra AS, Miyatani M, Craven BC.. Cardiovascular disease risk in individuals with chronic spinal cord injury: Prevalence of untreated risk factors and poor adherence to treatment guidelines. J Spinal Cord Med. 2016: 1– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nash MS, Tractenberg RE, Mendez AJ, . et al. Cardiometabolic syndrome in people with spinal cord injury/disease: Guideline-derived and non-guideline risk components in a pooled sample. Arch Phys Med Rehabil. 2016; 97 10: 1696– 1705. [DOI] [PubMed] [Google Scholar]
- 9. Cooke AA, Connaughton RM, Lyons CL, McMorrow AM, Roche HM.. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016; 785: 207– 214. [DOI] [PubMed] [Google Scholar]
- 10. Pearson TA, Mensah GA, Alexander RW, . et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107 3: 499– 511. [DOI] [PubMed] [Google Scholar]
- 11. Mirhafez SR, Ebrahimi M, Saberi Karimian M, . et al. Serum high-sensitivity C-reactive protein as a biomarker in patients with metabolic syndrome: Evidence-based study with 7284 subjects. Eur J Clin Nutr. 2016; 70 11: 1298– 1304. [DOI] [PubMed] [Google Scholar]
- 12. Gibson AE, Buchholz AC, Martin Ginis KA.. C-Reactive protein in adults with chronic spinal cord injury: Increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord. 2008; 46 9: 616– 621. [DOI] [PubMed] [Google Scholar]
- 13. Krause JS, Cao Y, DeVivo MJ, DiPiro ND.. Risk and protective factors for cause-specific mortality after spinal cord injury. Arch Phys Med Rehabil. 2016; 97 10: 1669– 1678. [DOI] [PubMed] [Google Scholar]
- 14. Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil.. 2007; 86 2: 142– 152. [DOI] [PubMed] [Google Scholar]
- 15. Cragg JJ, Noonan VK, Noreau L, Borisoff JF, Kramer JK.. Neuropathic pain, depression, and cardiovascular disease: A national multicenter study. Neuroepidemiology. 2015; 44 3: 130– 137. [DOI] [PubMed] [Google Scholar]
- 16. Nash MS, Cowan RE, Kressler J.. Evidence-based and heuristic approaches for customization of care in cardiometabolic syndrome after spinal cord injury. J Spinal Cord Med. 2012; 35 5: 278– 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dusart I, Schwab ME.. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994; 6 5: 712– 724. [DOI] [PubMed] [Google Scholar]
- 18. Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ.. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010; 133 Pt 2: 433– 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Obermair FJ, Schroter A, Thallmair M.. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda). 2008; 23: 296– 304. 21. [DOI] [PubMed] [Google Scholar]
- 20. Bethea JR, Dietrich WD.. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002; 15 3: 355– 360. [DOI] [PubMed] [Google Scholar]
- 21. Donnelly DJ, Popovich PG.. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008; 209 2: 378– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garstang SV, Miller-Smith SA.. Autonomic nervous system dysfunction after spinal cord injury. Phys Med Rehabil Clin N Am. 2007; 18 2: 275– 296, vi– vii. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Guan Z, Reader B, . et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013; 33 32: 12970– 12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madden KS, Sanders VM, Felten DL.. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995; 35: 417– 448. [DOI] [PubMed] [Google Scholar]
- 25. Black PH, Garbutt LD.. Stress, inflammation and cardiovascular disease. J Psychosom Res. 2002; 52 1: 1– 23. [DOI] [PubMed] [Google Scholar]
- 26. Wan D, Krassioukov AV.. Life-threatening outcomes associated with autonomic dysreflexia: A clinical review. J Spinal Cord Med. 2014; 37 1: 2– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hou S, Rabchevsky AG.. Autonomic consequences of spinal cord injury. Compr Physiol. 2014; 4 4: 1419– 1453. [DOI] [PubMed] [Google Scholar]
- 28. Cowan RE, Nash MS.. Cardiovascular disease, SCI and exercise: Unique risks and focused countermeasures. Disabil Rehabil. 2010; 32 26: 2228– 2236. [DOI] [PubMed] [Google Scholar]
- 29. Widerstrom-Noga E. Multidimensional clinical pain phenotypes after spinal cord injury. Pain Manag. 2012; 2 5: 467– 478. [DOI] [PubMed] [Google Scholar]
- 30. Ataoglu E, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S.. Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord. 2013; 51 1: 23– 26. [DOI] [PubMed] [Google Scholar]
- 31. Felix ER, Cruz-Almeida Y, Widerstrom-Noga EG.. Chronic pain after spinal cord injury: what characteristics make some pains more disturbing than others? J Rehabil Res Dev. 2007; 44 5: 703– 715. [DOI] [PubMed] [Google Scholar]
- 32. Fann JR, Bombardier CH, Richards JS, Tate DG, Wilson CS, Temkin N.. Depression after spinal cord injury: Comorbidities, mental health service use, and adequacy of treatment. Arch Phys Med Rehabil. 2011; 92 3: 352– 360. [DOI] [PubMed] [Google Scholar]
- 33. Williams R, Murray A.. Prevalence of depression after spinal cord injury: A meta-analysis. Arch Phys Med Rehabil. 2015; 96 1: 133– 140. [DOI] [PubMed] [Google Scholar]
- 34. Sezer N, Akkus S, Ugurlu FG.. Chronic complications of spinal cord injury. World J Orthop. 2015; 6 1: 24– 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang JC, Chan RC, Tsai YA, . et al. The influence of shoulder pain on functional limitation, perceived health, and depressive mood in patients with traumatic paraplegia. J Spinal Cord Med. 2015; 38 5: 587– 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cruz-Almeida Y, Felix ER, Martinez-Arizala A, Widerstrom-Noga EG.. Pain symptom profiles in persons with spinal cord injury. Pain Med (Malden, Mass.). 2009; 10 7: 1246– 1259. [DOI] [PubMed] [Google Scholar]
- 37. Avluk OC, Gurcay E, Gurcay AG, Karaahmet OZ, Tamkan U, Cakci A.. Effects of chronic pain on function, depression, and sleep among patients with traumatic spinal cord injury. Ann Saudi Med. 2014; 34 3: 211– 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP.. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001; 82 11: 1571– 1577. [DOI] [PubMed] [Google Scholar]
- 39. Widerstrom-Noga E, Cruz-Almeida Y, Krassioukov A.. Is there a relationship between chronic pain and autonomic dysreflexia in persons with cervical spinal cord injury? J Neurotrauma. 2004; 21 2: 195– 204. [DOI] [PubMed] [Google Scholar]
- 40. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW.. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9 1: 46– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer CW, Elfving B, Lund S, Wegener G.. Behavioral and systemic consequences of long-term inflammatory challenge. J Neuroimmunol. 2015; 288: 40– 46. [DOI] [PubMed] [Google Scholar]
- 42. White FA, Bhangoo SK, Miller RJ.. Chemokines: Integrators of pain and inflammation. Nat Rev Drug Discov. 2005; 4 10: 834– 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allison DJ, Ditor DS.. Targeting inflammation to influence mood following spinal cord injury: A randomized clinical trial. J Neuroinflammation. 2015; 12: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krause JS, Carter RE, Pickelsimer EE, Wilson D.. A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil. 2008; 89 8: 1482– 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Togan T, Azap OK, Durukan E, Arslan H.. The prevalence, etiologic agents and risk factors for urinary tract infection among spinal cord injury patients. Jundishapur J Microbiol. 2014; 7 1: e8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grossman RG, Frankowski RF, Burau KD, . et al. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine. 2012; 17 suppl 1: 119– 128. [DOI] [PubMed] [Google Scholar]
- 47. Saunders LL, Krause JS, Acuna J.. Association of race, socioeconomic status, and health care access with pressure ulcers after spinal cord injury. Arch Phys Med Rehabil. 2012; 93 6: 972– 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Failli V, Kopp MA, Gericke C, . et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain. 2012; 135 Pt 11: 3238– 3250. [DOI] [PubMed] [Google Scholar]
- 49. Galeiras Vazquez R, Rascado Sedes P, Mourelo Farina M, Montoto Marques A, Ferreiro Velasco ME.. Respiratory management in the patient with spinal cord injury. BioMed Res Int. 2013; 2013: 168757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winslow C, Rozovsky J.. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003; 82 10: 803– 814. [DOI] [PubMed] [Google Scholar]
- 51. Jackson AB, Groomes TE.. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994; 75 3: 270– 275. [DOI] [PubMed] [Google Scholar]
- 52. Cao Y, Krause JS, DiPiro N.. Risk factors for mortality after spinal cord injury in the USA. Spinal Cord. 2013; 51 5: 413– 418. [DOI] [PubMed] [Google Scholar]
- 53. Garshick E, Stolzmann KL, Gagnon DR, Morse LR, Brown R.. Systemic inflammation and reduced pulmonary function in chronic spinal cord injury. PM R. 2011; 3 5: 433– 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prio TK, Bruunsgaard H, Roge B, Pedersen BK.. Asymptomatic bacteriuria in elderly humans is associated with increased levels of circulating TNF receptors and elevated numbers of neutrophils. Exp Gerontol. 2002; 37 5: 693– 699. [DOI] [PubMed] [Google Scholar]
- 55. Parikh RP, Matthew Franzen M, Cecille Pope C, Lisa Gould L.. Autonomic dysreflexia: Be aware and be prepared. Wounds. 2012; 24 6: 160– 167. [PubMed] [Google Scholar]
- 56. Krishnan S, Karg PE, Boninger ML, Brienza DM.. Association between presence of pneumonia and pressure ulcer formation following traumatic spinal cord injury. J Spinal Cord Med. 2016: 1– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yuan R, Geng S, Chen K, Diao N, Chu HW, Li L.. Low-grade inflammatory polarization of monocytes impairs wound healing. J Pathol. 2016; 238 4: 571– 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krishnan S, Karg PE, Boninger ML, . et al. Early detection of pressure ulcer development following traumatic spinal cord injury using inflammatory mediators. Arch Phys Med Rehabil. 2016; 97 10: 1656– 1662. [DOI] [PubMed] [Google Scholar]
- 59. Singh R, Rohilla RK, Sangwan K, Siwach R, Magu NK, Sangwan SS.. Bladder management methods and urological complications in spinal cord injury patients. Indian J Orthop. 2011; 45 2: 141– 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baron J, Swaine J, Presseau J, . et al. Self-management interventions to improve skin care for pressure ulcer prevention in people with spinal cord injuries: A systematic review protocol. Syst Rev. 2016; 5 1: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aoi N, Yoshimura K, Kadono T, . et al. Ultrasound assessment of deep tissue injury in pressure ulcers: Possible prediction of pressure ulcer progression. Plast Reconstr Surg. 2009; 124 2: 540– 550. [DOI] [PubMed] [Google Scholar]
- 62. Berlowitz DR, Brienza DM.. Are all pressure ulcers the result of deep tissue injury? A review of the literature. Ostomy/wound Manag. 2007; 53 10: 34– 38. [PubMed] [Google Scholar]
- 63. Gefen A, Farid KJ, Shaywitz I.. A review of deep tissue injury development, detection, and prevention: Shear savvy. Ostomy/wound Manag. 2013; 59 2: 26– 35. [PubMed] [Google Scholar]
- 64. Curtis KA, Dillon DA.. Survey of wheelchair athletic injuries: Common patterns and prevention. Paraplegia. 1985; 23 3: 170– 175. [DOI] [PubMed] [Google Scholar]
- 65. Edwards WB, Schnitzer TJ.. Bone imaging and fracture risk after spinal cord injury. Curr Osteoporos Rep. 2015; 13 5: 310– 317. [DOI] [PubMed] [Google Scholar]
- 66. Bauman WA, Cardozo CP.. Osteoporosis in individuals with spinal cord injury. PM R. 2015; 7 2: 188– 201; quiz 201. [DOI] [PubMed] [Google Scholar]
- 67. Hammond ER, Metcalf HM, McDonald JW, Sadowsky CL.. Bone mass in individuals with chronic spinal cord injury: Associations with activity-based therapy, neurologic and functional status, a retrospective study. Arch Phys Med Rehabil. 2014; 95 12: 2342– 2349. [DOI] [PubMed] [Google Scholar]
- 68. Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K.. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015; 53 9: 701– 704. [DOI] [PubMed] [Google Scholar]
- 69. Li H, Liu J, Yao J, Zhong J, Guo L, Sun T.. Fracture initiates systemic inflammatory response syndrome through recruiting polymorphonuclear leucocytes. Immunol Res. 2016; 64 4: 1053– 1059. [DOI] [PubMed] [Google Scholar]
- 70. Hauser CJ, Zhou X, Joshi P, . et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma. 1997; 42 5: 895– 903; discussion 903–894. [DOI] [PubMed] [Google Scholar]
- 71. Hoff P, Gaber T, Strehl C, . et al. Immunological characterization of the early human fracture hematoma. Immunol Res. 2016. [DOI] [PubMed] [Google Scholar]
- 72. Loi F, Cordova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB.. Inflammation, fracture and bone repair. Bone. 2016; 86: 119– 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okazaki R, Watanabe R, Inoue D.. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab. 2016; 23 3: 111– 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Troy KL, Morse LR.. Measurement of bone: Diagnosis of SCI-induced osteoporosis and fracture risk prediction. Top Spinal Cord Injury Rehabil. 2015; 21 4: 267– 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cauley JA, Barbour KE, Harrison SL, . et al. Inflammatory markers and the risk of hip and vertebral fractures in men: The osteoporotic fractures in men (MrOS). J Bone Miner Res. 2016; 31 12: 2129– 2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rajan S, McNeely MJ, Warms C, Goldstein B.. Clinical assessment and management of obesity in individuals with spinal cord injury: A review. J Spinal Cord Med. 2008; 31 4: 361– 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Y, Cao Y, Allen V, Richards JS.. Weight matters: Physical and psychosocial well being of persons with spinal cord injury in relation to body mass index. Arch Phys Med Rehabil. 2011; 92 3: 391– 398. [DOI] [PubMed] [Google Scholar]
- 78. Li C, DiPiro ND, Cao Y, Szlachcic Y, Krause J.. The association between metabolic syndrome and pressure ulcers among individuals living with spinal cord injury. Spinal Cord. 2016; 54 11: 967– 972. [DOI] [PubMed] [Google Scholar]
- 79. Elsner JJ, Gefen A.. Is obesity a risk factor for deep tissue injury in patients with spinal cord injury? J Biomech. 2008; 41 16: 3322– 3331. [DOI] [PubMed] [Google Scholar]
- 80. Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ.. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia. 1992; 30 12: 880– 889. [DOI] [PubMed] [Google Scholar]
- 81. Buchholz AC, Pencharz PB.. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004; 7 6: 635– 639. [DOI] [PubMed] [Google Scholar]
- 82. De Lorenzo A, Bernardini S, Gualtieri P, . et al. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: A randomized controlled trial for nutrigenomic approach in cardiometabolic risk [published online ahead of print October 5, 2016]. Acta Diabetol. [DOI] [PubMed]
- 83. Nash MS, Kressler J.. Model programs to address obesity and cardiometabolic disease: Interventions for suboptimal nutrition and sedentary lifestyles. Arch Phys Med Rehabil. 2016; 97 9 suppl: S238– S246. [DOI] [PubMed] [Google Scholar]
- 84. Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016; 5: 47– 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Szlachcic Y, Adkins RH, Govindarajan S, Cao Y, Krause JS.. Cardiometabolic changes and disparities among persons with spinal cord injury: A 17-year cohort study. Top Spinal Cord Inj Rehabil. 2014; 20 2: 96– 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abd El-Kader SM, Al-Jiffri OH, Al-Shreef FM.. Aerobic exercises alleviate symptoms of fatigue related to inflammatory cytokines in obese patients with type 2 diabetes. Afr Health Sci. 2015; 15 4: 1142– 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nooijen CF, Stam HJ, Sluis T, Valent L, Twisk J, van den Berg-Emons RJ.. A behavioral intervention promoting physical activity in people with subacute spinal cord injury: Secondary effects on health, social participation and quality of life [published online ahead of print July 4, 2016]. Clin Rehabil [DOI] [PubMed]
- 88. Tweedy SM, Beckman EM, Geraghty TJ, . et al. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury [published online ahead of print October 31, 2016]. J Sci Med Sport. [DOI] [PubMed]
- 89. DiPiro ND, Embry AE, Fritz SL, Middleton A, Krause JS, Gregory CM.. Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal Cord. 2016; 54 9: 675– 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jacobs PL, Nash MS.. Exercise recommendations for individuals with spinal cord injury. Sports Med (Auckland). 2004; 34 11: 727– 751. [DOI] [PubMed] [Google Scholar]
- 91. Pedersen BK, Saltin B.. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015; 25 suppl 3: 1– 72. [DOI] [PubMed] [Google Scholar]
- 92. Cowan R, Malone L, Nash M.. Exercise is Medicine™: Exercise prescription after SCI to manage cardiovascular disease risk factors. Top Spinal Cord Inj Rehabil. 2009; 14 3: 69– 83. [Google Scholar]
- 93. Coelho Junior HJ, Gambassi BB, Diniz TA, . et al. Inflammatory mechanisms associated with skeletal muscle sequelae after stroke: Role of physical exercise. Mediators Inflamm. 2016; 2016: 3957958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ploughman M, Kelly LP.. Four birds with one stone? Reparative, neuroplastic, cardiorespiratory, and metabolic benefits of aerobic exercise poststroke. Curr Opin Neurol. 2016; 29 6: 684– 692. [DOI] [PubMed] [Google Scholar]
- 95. Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A.. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014; 20 43: 16079– 16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carvalho BM, Saad MJ.. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm. 2013; 2013: 986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cani PD, Amar J, Iglesias MA, . et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007; 56 7: 1761– 1772. [DOI] [PubMed] [Google Scholar]
- 98. Neves AL, Coelho J, Couto L, Leite-Moreira A, Roncon-Albuquerque R.. Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J Molec Endocrinol. 2013; 51 2: R51– R64. [DOI] [PubMed] [Google Scholar]
- 99. MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull. 2004; 71: 1– 11. [DOI] [PubMed] [Google Scholar]
- 100. Liu J, An H, Jiang D, . et al. Study of bacterial translocation from gut after paraplegia caused by spinal cord injury in rats. Spine. 2004; 29 2: 164– 169. [DOI] [PubMed] [Google Scholar]
- 101. Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG.. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016; 213 12: 2603– 2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De Winter BY, De Man JG.. Interplay between inflammation, immune system and neuronal pathways: Effect on gastrointestinal motility. World J Gastroenterol. 2010; 16 44: 5523– 5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Williams RE 3rd, Bauman WA, Spungen AM, . et al. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord. 2012; 50 1: 81– 84. [DOI] [PubMed] [Google Scholar]
- 104. Puddu A, Sanguineti R, Montecucco F, Viviani GL.. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014; 2014: 162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Forsythe P, Bienenstock J, Kunze WA.. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014; 817: 115– 133. [DOI] [PubMed] [Google Scholar]
- 106. Pavlov VA, Tracey KJ.. The vagus nerve and the inflammatory reflex — linking immunity and metabolism. Nat Rev Endocrinol. 2012; 8 12: 743– 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ringel-Kulka T, Choi CH, Temas D, . et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015; 110 9: 1339– 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gungor B, Adiguzel E, Gursel I, Yilmaz B, Gursel M.. Intestinal microbiota in patients with spinal cord injury. PloS one. 2016; 11 1: e0145878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fouts DE, Pieper R, Szpakowski S, . et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Trans Med. 2012; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K.. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett. 2005; 389 2: 109– 114. [DOI] [PubMed] [Google Scholar]
- 111. Galkina E, Ley K.. Immune and inflammatory mechanisms of atherosclerosis (*). Ann Rev Immunol. 2009; 27: 165– 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hansson GK, Libby P.. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006; 6 7: 508– 519. [DOI] [PubMed] [Google Scholar]
- 113. Davies AL, Hayes KC, Dekaban GA.. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007; 88 11: 1384– 1393. [DOI] [PubMed] [Google Scholar]
- 114. Frost F, Roach MJ, Kushner I, Schreiber P.. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005; 86 2: 312– 317. [DOI] [PubMed] [Google Scholar]
- 115. Liang H, Mojtahedi MC, Chen D, Braunschweig CL.. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008; 89 1: 36– 41. [DOI] [PubMed] [Google Scholar]
- 116. Manns PJ, McCubbin JA, Williams DP.. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005; 86 6: 1176– 1181. [DOI] [PubMed] [Google Scholar]
- 117. Morse LR, Stolzmann K, Nguyen HP, . et al. Association between mobility mode and C-reactive protein levels in men with chronic spinal cord injury. Arch Phys Med Rehabil. 2008; 89 4: 726– 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY.. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007; 106 11: 919– 928. [DOI] [PubMed] [Google Scholar]
- 119. Carp SJ, Barr AE, Barbe MF.. Serum biomarkers as signals for risk and severity of work-related musculoskeletal injury. Biomark Med. 2008; 2 1: 67– 79. [DOI] [PubMed] [Google Scholar]
- 120. Matute Wilander A, Karedal M, Axmon A, Nordander C.. Inflammatory biomarkers in serum in subjects with and without work related neck/shoulder complaints. BMC Musculoskelet Disord. 2014; 15: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pritchett JW. C-reactive protein levels determine the severity of soft-tissue injuries. Am J Orthop (Belle Mead, NJ). 1996; 25 11: 759– 761. [PubMed] [Google Scholar]
- 122. Estrores IM, Harrington A, Banovac K.. C-reactive protein and erythrocyte sedimentation rate in patients with heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2004; 27 5: 434– 437. [DOI] [PubMed] [Google Scholar]
- 123. Greenland P, Alpert JS, Beller GA, . et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am College Cardiol. 2010; 56 25: e50– 103. [DOI] [PubMed] [Google Scholar]
- 124. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004; 21 10: 1371– 1383. [DOI] [PubMed] [Google Scholar]
- 125. Lo C, Tran Y, Anderson K, Craig A, Middleton J.. Functional priorities in persons with spinal cord injury: Using discrete choice experiments to determine preferences. J Neurotrauma. 2016; 33 21: 1958– 1968. [DOI] [PubMed] [Google Scholar]


