Abstract
Objectives: To determine whether the use of a powered exoskeleton can improve parameters of physical activity as determined by walking time, stand up time, and number of steps in persons with spinal cord injury (SCI). Methods: Three men with complete (1 C5 AIS A and 2 T4 AIS A) and one man with incomplete (C5 AIS D) SCI participated in a clinical rehabilitation program. In the training program, the participants walked once weekly using a powered exoskeleton (Ekso) for approximately 1 hour over the course of 10 to 15 weeks. Walking time, stand up time, ratio of walking to stand up time, and number of steps were determined. Oxygen uptake (L/min), energy expenditure, and body composition were measured in one participant after training. Results: Over the course of 10 to 15 weeks, the maximum walking time increased from 12 to 57 minutes and the number of steps increased from 59 to 2,284 steps. At the end of the training, the 4 participants were able to exercise for 26 to 59 minutes. For one participant, oxygen uptake increased from 0.27 L/min during rest to 0.55 L/min during walking. Maximum walking speed was 0.24 m/s, and delta energy expenditure increased by 1.4 kcal/min during walking. Body composition showed a modest decrease in absolute fat mass in one participant. Conclusion: Exoskeleton training may improve parameters of physical activity after SCI by increasing the number of steps and walking time. Other benefits may include increasing energy expenditure and improving the profile of body composition.
Keywords: energy expenditure, exoskeleton, physical activity, spinal cord injury
Spinal cord injury (SCI) results in physical impairment and limited mobility; this can lead to subsequent chronic comorbidities in survivors confined to lifelong wheelchair status. These comorbidities may include psychosomatic, cardiovascular, and metabolic consequences, as well as significant socioeconomic burdens.1–5 The economic burdens for persons with SCI, their families, and society are increasing at an alarming rate, especially with decreasing mortality as a result of advances in medical interventions.6 The restoration of mobility may serve as a potential rehabilitation intervention that cuts down on several SCI comorbidities and the associated economic burdens.7 Several attempts have been made to restore and improve locomotion following SCI with long-leg braces, hip-knee-ankle foot orthoses, Parastep powered by functional electrical stimulation, body weight–supported treadmill training, and robotic treadmills. Several of these techniques were considered interventions to counterbalance changes in body composition, reduced levels of physical activity, and abnormal lipid and carbohydrate profiles.7–14 Unfortunately, most of these rehabilitation interventions require metabolic energy demands that are too great for individuals with SCI to tolerate. This results in the failure of individuals to pursue these interventions, and therefore they are unlikely to meet the recommended guidelines for improving physical activity after SCI.
The American College of Sports Medicine recommends 30 minutes per day of exercise training for 5 days per week to reduce cardiometabolic risk factors in the general population.15 Recent guidelines for persons with SCI have recommended a minimum of 20 to 30 minutes per day of moderate to vigorous exercise intensity to maintain cardiovascular health.16 Moreover, greater daily leisure time physical activity is associated with lower chronic disease risk in adults with SCI.17–20 Reduced levels of physical activity have been shown to be a problem with aging and lead to a high risk of obesity after SCI.17–20 Two-thirds of the SCI population are considered either overweight or obese.21 Moreover, the alarming prevalence of obesity may be due more to the reduced level of physical activity than to an overconsumption in daily caloric intake.22 A recent study showed that 56% of the studied SCI sample do not meet the recommended daily caloric intake.22 Despite of this observation, body composition assessment studies have revealed that individuals with SCI have 30% to 40% fat mass on average, with a greater tendency for central obesity characterized by deposition of visceral adipose tissue.21–23 Therefore, finding a rehabilitation intervention that can promote increased physical activity is of paramount significance to this population.
Powered exoskeletons using robotic suits have recently been introduced for the rehabilitation of persons with SCI.24–33 Exoskeletons offer a unique opportunity for persons with SCI to experience standing and walking at a low metabolic cost.24,26,29,31 Evidence suggests that exoskeleton-assisted walking can decrease spasticity and improve bowel movement.24 Training may also improve the level of physical activity as well as psychological parameters that are likely to interfere with rehabilitation outcomes. A systemic review concluded that exoskeletons provide a safe, easy to use, and practical method of neurorehabilitation in persons with SCI.24 Previous studies reported that a frequency of 2 to 3 times per week or more for 1 to 2 hours may be beneficial in the rehabilitation of persons with SCI.25–33 Using exoskeleton-assisted walking to improve the level of physical activity may be appealing to persons with SCI; however, it is unclear whether a frequency less than twice a week (ie, once a weekly) will help improve parameters of physical activity.
Previous exoskeleton trials using either Rewalk or Ekso brands included persons with injuries below the C7 level. This limitation in recruitment was due to the insufficient muscle strength in the upper extremities required to use the assistive device in persons with SCI above C7. It is still unclear whether we can train individuals with a high level of injury to attain independence during exoskeleton walking. The purpose of the current case series is to determine whether exoskeleton walking can improve the level of physical activity as determined by the duration of walking, stand up time, and number of steps.
Methods
The study was conducted as a part of a clinical rehabilitation program, and the data were analyzed retrospectively to determine whether exoskeletal walking once a week would lead to improvement in parameters of physical activity.33 We have limited the frequency to once weekly to ensure long-term compliance and adherence to the program as well as to determine whether there is a motor learning aspect separate from the training effects.34 The current case series report was approved by the local ethics committee at the Hunter Holmes McGuire VA Medical Center.
Study protocol
Four subjects participated in a clinical rehabilitation program that primarily focused on wellness in persons with SCI. The program is implemented in our facility as a part of continuum of care following discharge from rehabilitation and is focused primarily on arm-cycling ergometery and circuit resistance training in persons with SCI.34 The program is extended as long as the participant is willing to come once weekly on Fridays to engage in 1-hour sessions and to ensure long-term adherence.35 Resting and exercise blood pressure as well as heart rate are monitored closely prior to and during any exercise intervention. Participants then perform 10 to 15 minutes of arm-cycling ergometery at a comfortable pace to warm-up. Then participants circulate around 5 stations using the Equalizer Multi-Station exercise system for 45 minutes, specifically designed for wheelchair users as previously described.34 Each participant completes up to 10 repetitions at each station before moving to the next station. Weight is progressed after the participant successfully completes 2 sets of 10 reps during the same visit. All aspects of the exercise program are performed under full supervision.
In the current report, we used a powered exoskeleton (Ekso) to provide locomotion to persons with SCI. A detailed description of the device is published elsewhere.36,37 Participants were part of the clinical rehabilitation program and were invited to take part in training using powered exoskeleton.
To ensure that the participants were safely able to engage in the exoskeleton program, the medical doctor provided a written clearance prior to the participants' enrollment in the program. Moreover, participants who passed the screening phase were asked to have dual energy x-ray absorptiometry (DXA) scans for whole body and both hips. T scores less than −2.5 SD would result in elimination from the program. All participants underwent measurements of body weight and height as previously described.22,23 Hip width (distance between 2 greater trochanters), upper leg length (greater trochanter to the lateral aspect of joint line of the knee joint), and lower leg length (lateral aspect of joint line of the knee joint to the bottom of the foot) were measured to allow appropriate adjustment of the width and legs of the robotic suit.
Based on completeness of injury and the strength of the anterior tibial muscle groups, ankle stiffness of the Ekso unit was adjusted. Upper and lower extremity ranges of motion, strength, and spasticity were assessed prior to enrollment in the program using the modified Ashworth Scale (data not shown). All of the screening items necessary to ensure safety of the participants were addressed prior to their engagement in the rehabilitation program.
The Ekso unit offers a gait-training mode with a range of features. These include a first step mode in which steps are manually controlled by the therapist, a pro-step mode that offers complete assistance, and a pro-step+ mode that provides adaptive and variable assist features. The pro-step+ mode is used mainly for persons with incomplete SCI American Spinal Injury Association Impairment Scale (AIS) C or D. The variable assist feature allows participants to volitionally move their legs and receive the minimal assistance required to complete their stepping.36,37 In the first session, all participants were trained using first step mode until they were able to carefully shift their body weight anterolaterally and achieve quality walking. Participants then progressed to the pro-step or pro-step+ mode; 2 buzzers cued the participants accurately to complete weight shifting prior to stepping.
Prior to training, participants were asked to transfer to an adjustable rehabilitation mat. A research assistant helped fit the participants into the device, starting with the shoes support (distally) and then going up toward the trunk (proximally). The software was adjusted based on the recommendation from the manufacturer and progressed based on the clinical need of each participant (Table 1). Every effort was made to ensure that all straps were snug but not excessively tight to avoid any episode of autonomic dysreflexia. Progression in walking time was based on the participants' performance in the preceding session and their willingness to continue training.
Table 1.
Software settings during the course of the exoskeleton training program
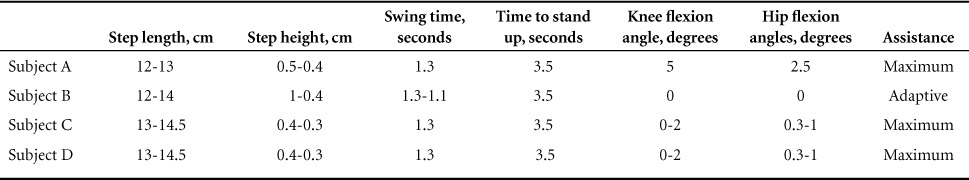
Outcome measures
Blood pressure and heart rate were monitored prior to and following all training sessions, and participants were instructed to communicate any discomfort during training. Following each session, walking time, stepping time, and number of steps were recorded for all participants. A meaningful improvement was determined based on the ability of a participant to walk more than 25 minutes per session. Oxygen uptake (L/min), energy expenditure (EE; kcal/min), and body composition were measured in one participant using portable metabolic cart (COSMED K4b2) and DXA, respectively, as was previously performed.22,38
After arriving into the laboratory, participants were asked to void their bladder. They were then asked to transfer to the mat using a sliding board before being fitted in the Ekso unit. The participants were asked to relax in a sitting position for 5 minutes, and then a breathing mask was snuggly secured to their face to measure oxygen uptake (L/min) and EE using portable COSMED K4b2. Resting EE was collected in a sitting position for 10 minutes to allow the participants a sufficient period to recover after transfer to the mat. The Ekso unit was then placed in standing mode and standing EE was measured for 5 minutes. EE was collected for 6 minutes of starting walking, followed by 6 minutes of walking with a walker, and 6 minutes of walking with bilateral crutches. After the walking period, EE was measured during standing (3 minutes) and sitting (3 minutes) recovery periods. Delta EE was calculated as the total EE during standing or walking minus sitting resting EE using the following equation: Delta EE = standing or walking EE – resting EE.
Results
Eighteen participants were screened for this program. Six did not meet the inclusion criteria set by the manufacturer. Several screening items disqualified participation during the initial evaluation, such as a level of injury above C5, hip width below or outside the recommended guidelines of the manufacturer, and limited hip flexion because of contracture. Other notable items were limited hip and ankle range of motion (ROM) and skin flap.
Twelve participants were included in the program since August 2015; however, only 5 continued throughout the program. Seven individuals decided to discontinue the program due to travel distance, lack of travel funds to support their participation, and lack of family support or caregivers. Data were not included in the current study for any participant who enrolled for less than 4 weeks (n = 1). Four subjects participated in a clinical rehabilitation program that primarily focused on wellness in persons with SCI.
Four individuals with SCI participated in a clinical exoskeletal program that ranged from 10 to 15 weeks. None of the 4 participants experienced any complications during the course of the program. One of the 4 participants missed a single visit over the course of the program due to a conflict with a prescheduled medical appointment. Up to one-fourth to three-eighths inches of leg length discrepancy were noted in 2 of the 4 patients. One participant reported heterotrophic ossification in his left hip joint. The screening items were cleared with the referral physician prior to proceeding with the training. The Ekso unit was adjusted to offset the limited ROM at both the hips and knees. One of the participants was instructed to perform a daily stretching exercise to improve ROM around the ankle prior to enrollment in the study.
Progression in walk time, stand up time, total steps, and walk time to standing up time ratio is presented in Figure 1.Overall, 4 participants showed meaningful improvement in the aforementioned walking variables over the course of the program, especially walking time, stand up time, and number of steps increased as depicted in Figure 1. Physical characteristics, total number of steps, and maximum walking time for the 4 participants are presented in Table 2 and Figure 1A–C.
Figure 1.
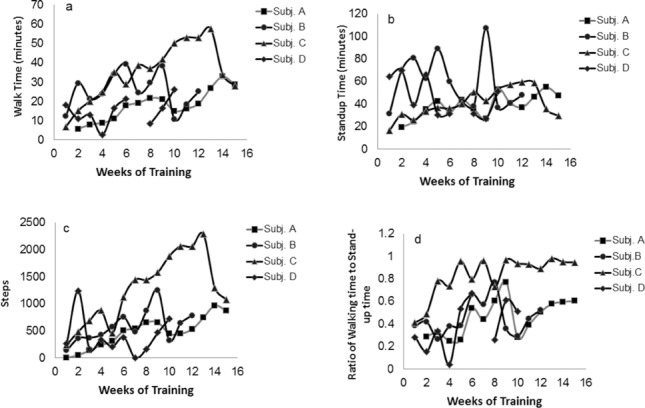
Walking time, stand up time, steps, and walking time to stand up time in 4 participants with SCI over the course of 10 to 15 weeks of powered exoskeleton training.
Table 2.
Subjects' physical characteristics and performance during exoskeleton training program
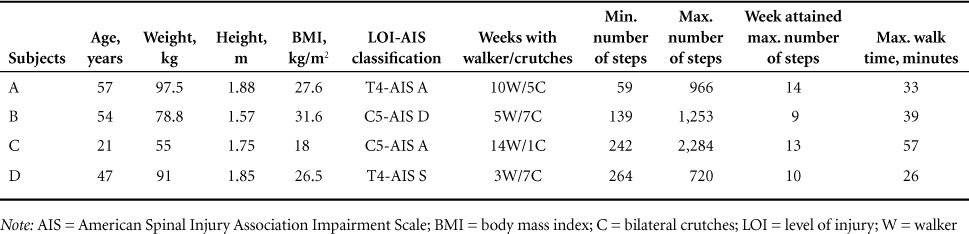
Subject A
Subject A was a 57-year-old male who had a T4 AIS A SCI since 2002 and used a power wheelchair for mobility. The participant used a roller walker for the first 10 weeks before he progressed to bilateral Canadian crutches for the remaining 5 weeks (Table 2). The total number of steps and maximum walking time are shown in Figure 1A–C.
During the last visit, resting oxygen uptake was 0.27 L/min and standing oxygen uptake was 0.4 L/min. After 6 minutes of walking with the walker and then 6 minutes with the crutches, oxygen uptake increased to 0.55 L/min and 0.57 L/min, respectively. Finally, oxygen uptake during standing recovery and sitting recovery was 0.51 L/min and 0.44 L/min, respectively. Figure 2 presents resting EE (R; 1.3 kcal/min), standing (S; 2.05 kcal/min), walking with walker (W; 2.7 kcal/min), walking with crutches (C; 2.75 kcal/min), and both standing (2.5 kcal/min) and sitting (2.1 kcal/min) recovery periods. Delta EE during walking with walker or crutches was 1.4 kcal/min compared to resting; between the first standing period and sitting recovery, it was 1.12 kcal/min.
Figure 2.
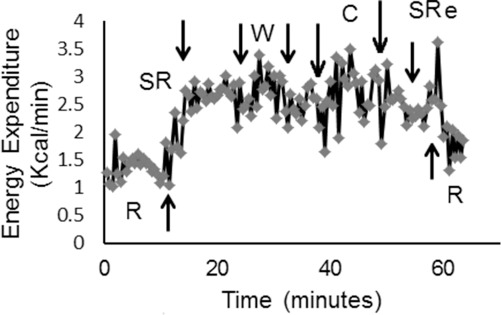
Energy expenditure in an individual with a T4 motor complete SCI during sitting rest (R), standing rest (SR), walking with walker (W), walking with crutches, standing recovery (SRe), and sitting rest (R) in week 12 of the exoskeleton training.
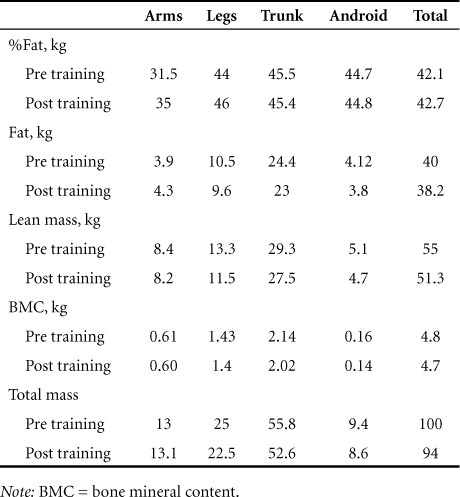
Total body composition assessment by DXA showed decrease in total mass from 99.9 kg to 94.3 kg, lean mass decreased from 55 kg to 51.3 kg, and fat mass decreased from 40 kg to 38.3 kg (Table 3).
Table 3.
Body composition assessment pre training and post training in subject A following the exoskeletal training program
Subject B
Subject B was a 54-year-old male who had a C5 AIS A SCI since 2008 as a result of central cord syndrome. The participant was a community ambulator and could walk with or without a cane; however, he had poor interlimb coordination and tended to fatigue after walking less than 150 feet. During the trial, prostep+ with adaptive and variable assist options was used. The variable assist option was dropped from 100% to 65% over the course of the trial. Subject B used a roller walker for 5 weeks before he progressed to use bilateral Canadian crutches for the remaining 7 weeks (Table 2).
Subject C
Subject C was a 21-year-old male who had a C5 AIS A SCI since 2015. He used a manual wheelchair for mobility. During the study, he used a standard roller walker for 15 weeks. He trialed the use of crutches for one session, however he resumed the use of walker because of limited triceps and hand grip strength on the right side (Table 2). During the 6-minute walk test (62.87 m to 66.7 m), his walking speed increased from 0.14 m/s to 0.24 m/s over the course of the program. The speed needed to cover 10 m ranged from 0.14 m/s to 0.19 m/s and the duration ranged from 52 to 68 seconds. Subject C's average resting blood pressures and heart rate were 85/55 mm Hg and 100 bpm (pre training) and 86/58 mm Hg and 89 bpm (post training).
Subject D
Subject D was a 47-year-old male who had a T4 AIS A SCI since 1998. He used a manual wheelchair for mobility. In the study, he used a roller walker for 3 weeks before he progressed to using bilateral Canadian crutches for the remaining 7 weeks (Table 2). The number of steps increased from 264 steps (week 1) to 720 steps (week 10) and maximum walking time was 26 minutes (Figure 1A–C).
Discussion
Restoring locomotion following SCI has become a major rehabilitation goal to offset several SCI-associated comorbidities.5,23 Advancement in robotic technology has allowed achievement of this goal with less metabolic demands compared to previously established orthosis or locomotor interventions.24–28 In the current case series, we demonstrate that exoskeleton training once weekly may be beneficial to improve level of physical activity in persons with SCI. The case series highlights some of the challenges that may result in disqualifying individuals from participating in an established exoskeleton program. Over the course of 10 to 15 weeks, all participants demonstrated improvement in parameters of physical activity including walking time, stand up time, and number of steps. Moreover, 3 of the participants progressed from using a roller walker to bilateral crutches. This finding may highlight the possibility of increasing level of independence and improving walking balance even in persons with complete SCI. Additionally, a person with C5 motor complete SCI was capable of using the robotic exoskeleton without further complications and with improvement in his cardiovascular response to training.
Robotic exoskeletons have evolved, and there are several brands that can meet the increasing demands of persons with SCI to restore walking and engage in community activities.24,26,31,33 The emergence of the exoskeleton as a rehabilitation tool offers longstanding wheelchair users the possibility of restoring ambulation years after SCI. Long sitting time is an independent cardiovascular risk factor and is associated with all-cause mortality.39 Sitting time is likely to contribute to an increase in other SCI-related comorbidities. Exoskeletons may offer an opportunity to restore walking as a means of improving level of physical activity for 1 to 2 hours per day or for 5 to 10 hours per week. Based on the current guidelines, 10,000 steps are needed per day to prevent the development of cardiovascular disease.15 It is unrealistic for persons with motor complete SCI to attain this number of steps per day without increasing the speed of walking of the exoskeleton unit. The maximum number of steps attained was 2,284 steps in 1 hour after 15 weeks of training. This means that a person may need to walk about 5 hours per day to meet the recommended guidelines. The swing time was limited to 1.3 or 1.1 seconds to allow participants enough time to secure walking balance. It is worth noting that shortening the swing time from 1.3 seconds to 1 second improved walking speed from 0.19 m/s to 0.26 m/s. Moreover, shortening the swing time to 0.8 seconds increased walking speed to 0.44 m/s. In follow-up sessions beyond the reported 15 weeks (data not shown), subject C was able to cover 10-meter walking distance in 38 seconds using bilateral crutches after shortening his swing time from 1.3 seconds to 1 second. Moreover, he covered the same distance in 22.5 seconds using bilateral crutches when the swing time was shortened to 0.8 seconds.
Steps increased by 3- to 16-fold following exoskeleton training and were accompanied by an increase in maximum walking duration from 12 to 57 minutes. Participants were able to identify motor learning strategies that allowed better postural control and longer walking duration. As the program progressed, the number of steps per unit time was also increased in all 4 participants. Moreover, 3 of the 4 participants were able to use crutches as an assistive aid instead of the roller walker during training. Despite this improvement, it is not known whether this level of physical activity is accompanied by remarkable changes in body composition or metabolic profile.
Persons with SCI are at the lowest spectrum of physical activity compared to the general population,17–19,40 so rehabilitation strategies that may help to promote physical activity are highly recommended. The use of an exoskeleton has been shown to increase oxygen uptake, decrease whole body fat mass, enhance cardiovascular response, and improve gut motility.24,26,31,32 Moreover, anecdotal evidence suggests that exoskeleton training may improve quality of life and mental health in persons with SCI. Increasing levels of physical activity may be accompanied by increasing EE. Participants with SCI have lower daily EE compared to the general population, which exposes them to high risk of developing obesity.40 Because subject A was close to the weight cutoff that was set by the manufacturer (~100 kg), EE was measured to determine whether exoskeleton training increased EE to help maintain or lose weight. Delta EE increased by 1.4 kcal/min, on average, during walking with either walker or crutches. This very low delta EE is attributed to the slow walking speed and to the fact that the exoskeleton is motorized so that the legs move passively. Mathematically, this can be translated to 84 kcal in 1 hour or 252 to 420 kcal per week assuming a frequency of 3 to 5 sessions per week. In 1 month, a participant may expend on average between 1,008 and 1,680 kcal. Assuming that 1 kg of weight is ~7,000 kcal, a person with SCI may lose up to 0.13 to 0.22 kg a month and 1.5 to 2.6 kg per year. It appears that there is a discrepancy between our mathematical calculation and the fact the subject A lost about 1.7 kg of fat mass after 3 months of training. This 1.7 kg loss in fat mass is less than 5% of the baseline value and can be considered within the measurement error of repeated DXA scans. We were not expecting to detect changes in any of the body composition variables based on this frequency of training. This may be in accordance with the recent findings that showed once weekly surface electrical stimulation resulted in an increase in leg lean mass and fatigue resistance without impacting whole body and regional fat mass.41
The once weekly frequency was chosen based on a number of factors. Initially, there was limited clinical staff able to conduct this training program. We sought to establish a training protocol and to understand the pros and cons that are associated with using powered exoskeletons before conducting a study. The current study provided our group with a clear understanding of the criteria necessary to recruit appropriate candidates for future training studies. Another factor that was considered was compliance and adherence rate during this initial phase of exoskeleton training.35 Travel money or reimbursement was not available; participants had to rely on their own funding to ensure long-term compliance. It is unclear whether exoskeleton training for 2 to 3 times a week may reverse the process of skeletal muscle atrophy and osteoporosis after SCI, partially because of the low loading force transmitted to the leg during walking. Therefore, future trials may need to investigate the appropriate frequency of exoskeletal training necessary to counteract several of the health-related consequences after SCI. It is premature to speculate on the exact frequency necessary in a clinical rehabilitation program. The aforementioned factors may be used to set up the frequency until the establishment of clear guidelines.
Detrimental changes in skeletal muscle following SCI may be responsible for several of the metabolic changes as well as the decreased basal metabolic rate.5,42 There is no available evidence that exoskeleton loading restores lean mass or skeletal muscle size following training. We have previously demonstrated that low-intensity progressive resistance training evoked by surface neuromuscular electrical stimulation (NMES) is accompanied by muscle hypertrophy, increased fatigue resistance, decreased ectopic adipose tissue, and improved metabolic profile in persons with SCI.41,43 Therefore, a hybrid approach using exoskeleton and NMES training may be necessary to facilitate improvement in the level of physical activity and provide other gains in body composition and metabolic profile. This hybrid strategy has been shown to be successful with 4 healthy volunteers as well as individuals with SCI and provides a balanced therapeutic approach to induce locomotion.44,45
Despite the progress in parameters of physical activity, a trainer was always needed to walk behind the participant in case of emergency. Each participant needed up to 80% to 100% support at the beginning of training that dropped to 20% to 30% support toward the end of the training. This is an important consideration, because reducing the level of assistance has been shown to increase ground reaction force during the gait cycle in persons with SCI.33 The support varied initially based on the level of injury and the ability of the subject to provide trunk control. Toward the end of training, participants required only minimum assistance or guard support that focused primarily on cueing them to maintain upright posture and not to lean forward.
Limitations
The current case series demonstrated several successful events, confirming that exoskeleton training can be carried out safely in a clinical environment. A major limitation of the current case series is that there is no established protocol. Therefore, these findings should be generalized with caution. Compared to other studies, the frequency selected in this study is unlikely to be sufficient for changes in body composition or metabolic profile; however, there is evidence that powered exoskeleton training may promote the level of physical activity in persons with SCI. The training duration varied from 10 to 15 weeks and was primarily based on the availability of the participants. This may not apply to other settings or other clinical practices. Clinical trials are currently underway investigating the efficacy of using powered exoskeletons on various health-related aspects after SCI. Therefore, the current case series should serve as a basis for other clinical sites to establish their exoskeleton rehabilitation protocol.
Clinical implications of the current findings
It is important to maintain musculoskeletal health below the level of injury. Joint contractures and leg length discrepancy can keep participants from restoring ambulation after SCI. Therefore, appropriate exercise interventions that are likely to maintain muscle flexibility and muscle size and load the paralyzed extremities are necessary for persons with SCI. Participants with high level of injury, C5 and below, may benefit from this training program using the appropriate assistive device similar to platform walker, followed by standard roller walker, and then using bilateral crutches through cuffing their hands. It is highly recommended to have at least 2 to 3 trained staff before establishing a clinical rehabilitation program.
Conclusion
A powered exoskeleton may serve as a potential rehabilitation tool to improve parameters of physical activity as determined by the increasing number of steps, walking time, and stand up time. In this study, we were able to meet the current SCI recommended guidelines of implementing moderate exercise intensity for 20 to 30 minutes per day. Despite the limitations of the current series, using a powered exoskeleton as a rehabilitation tool to restore walking may serve as a countermeasure to several SCI-associated comorbidities. Several studies are warranted to establish this observation and to focus on combining exoskeleton training with other interventions that may promote increases in lean mass similar to functional electrical stimulation.
Acknowledgments
We would like to thank all the participants who participated in this clinical training program. We would also like to thank Hunter Holmes McGuire Research Institute and Spinal Cord Injury Services and Disorders for providing the environment to conduct clinical human research trials.
The authors declare no conflicts of interest.
REFERENCES
- 1. Kocina P. Body composition of spinal cord injury adults. Sports Med. 1997; 23 1: 48– 60. [DOI] [PubMed] [Google Scholar]
- 2. Bauman WA, Spungen AM.. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001; 24 4: 266– 277. [DOI] [PubMed] [Google Scholar]
- 3. Bauman WA, Spungen AM.. Coronary heart disease in individuals with spinal cord injury: Assessment of risk factors. Spinal Cord. 2008; 46 7: 466– 476. [DOI] [PubMed] [Google Scholar]
- 4. Dolbow DR, Gorgey AS, Daniels JA, Adler RA, Moore JR, Gater DR Jr.. The effects of spinal cord injury and exercise on bone mass: A literature review. Spinal Cord. 2012; 50 11: 170– 171. [DOI] [PubMed] [Google Scholar]
- 5. Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR.. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014; 37 6: 693– 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strauss D, DeVivo M, Shavelle R, Brooks J, Paculdo D.. Economic factors and longevity in spinal cord injury: A reappraisal. Arch Phys Med Rehabil. 2008; 89 3: 572– 574. [DOI] [PubMed] [Google Scholar]
- 7. Dobkin B, Apple D, Barbeau H, . et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006; 66: 484– 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbeau H. Locomotor training in neurorehabilitation: Emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003; 17: 3– 11. [DOI] [PubMed] [Google Scholar]
- 9. Giangregorio LM, Hicks AL, Webber CE, . et al. Body weight supported treadmill training in acute spinal cord injury: Impact on muscle and bone. Spinal Cord. 2005; 43: 649– 657. [DOI] [PubMed] [Google Scholar]
- 10. Hornby TG, Zemon DH, Campbell D.. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005; 85: 52– 66. [PubMed] [Google Scholar]
- 11. Maxwell JL, Granat MH, Baardman G, Hermens HJ.. Demand for and use of functional electrical stimulation systems and conventional orthoses in the spinal lesioned community of the UK. Artif Organs. 1999; 23 5: 410– 412. [DOI] [PubMed] [Google Scholar]
- 12. Ambrosia RD, Solomonow M, Baratta R.. Current status of walking orthosis for thoracic paraplegics. Iowa Orthop J. 1995; 15: 174– 181. [PMC free article] [PubMed] [Google Scholar]
- 13. Gorgey AS, Poarch H, Miller J, Castillo T, Gater DR.. Locomotor and resistance training restore walking in an elderly person with a chronic incomplete spinal cord injury. NeuroRehabilitation. 2010; 26 2: 127– 133. [DOI] [PubMed] [Google Scholar]
- 14. Gorgey AS, Poarch H, Harnish C, Miller JM, Dolbow D, Gater DR.. Acute effects of locomotor training on neuromuscular and metabolic profile after incomplete spinal cord injury. NeuroRehabilitation. 2011; 29 1: 79– 83. [DOI] [PubMed] [Google Scholar]
- 15. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, . et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007; 39 8: 1423– 1434. [DOI] [PubMed] [Google Scholar]
- 16. Evans N, Wingo B, Sasso E, Hicks A, Gorgey AS, Harness E.. exercise recommendations and considerations for persons with spinal cord injury. Arch Phys Med Rehabil. 2015; 96 9: 1749– 1750. [DOI] [PubMed] [Google Scholar]
- 17. Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res. 2003; 11 4: 563– 570. [DOI] [PubMed] [Google Scholar]
- 18. Buchholz AC, Martin Ginis KA, Bray SR, . et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metabol. 2009; 34 4: 640– 647. [DOI] [PubMed] [Google Scholar]
- 19. Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL.. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: A systematic review. Spinal Cord. 2011; 49 11: 1103– 1127. [DOI] [PubMed] [Google Scholar]
- 20. Hetz SP, Latimer AE, Buchholz AC, Martin Ginis KA; SHAPE-SCI Research Group. . Increased participation in activities of daily living is associated with lower cholesterol levels in people with spinal cord injury. Arch Phys Med Rehabil. 2009; 90 10: 1755– 1759. [DOI] [PubMed] [Google Scholar]
- 21. Gorgey AS, Gater DR Jr.. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007; 12 4: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorgey AS, Caudill C, Sistrun S, . et al. frequency of dietary recalls, nutritional assessment, and body composition assessment in men with chronic spinal cord injury. Arch Phys Med Rehabil. 2015; 96 9: 1646– 1653. [DOI] [PubMed] [Google Scholar]
- 23. Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metabol. 2011; 36 1: 107– 114. [DOI] [PubMed] [Google Scholar]
- 24. Miller LE, Zimmermann AK, Herbert WG.. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med Devices (Auckland, NZ). 2016; 9: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sale P, Russo EF, Russo M, Masiero S, Piccione F, Calabrò RS, Filoni S.. Effects on mobility training and de-adaptations in subjects with spinal cord injury due to a wearable robot: A preliminary report. BMC Neurol. 2016; 16 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans N, Hartigan C, Kandilakis C, Pharo E, Clesson I.. Acute cardiorespiratory and metabolic responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2015; 21 2: 122– 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kozlowski AJ, Bryce TN, Dijkers MP.. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015; 21 2: 110– 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeilig G, Weingarden H, Zwecker M, Dudkiewicz I, Bloch A, Esquenazi A.. Safety and tolerance of the ReWalk exoskeleton suit for ambulation by people with complete spinal cord injury: A pilot study. J Spinal Cord Med. 2012; 35 2: 96– 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang A, Asselin P, Knezevic S, Kornfeld S, Spungen AM.. Assessment of in-hospital walking velocity and level of assistance in a powered exoskeleton in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2015; 21 2: 100– 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartigan C, Kandilakis C, Dalley S, . et al. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehabil. 2015; 21 2: 93– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asselin P, Knezevic S, Kornfeld S, . et al. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015; 52 2: 147– 158. [DOI] [PubMed] [Google Scholar]
- 32. Kressler J, Thomas CK, Field-Fote EC, . et al. Understanding therapeutic benefits of overground bionic ambulation: Exploratory case series in persons with chronic, complete spinal cord injury. Arch Phys Med Rehabil. 2014; 95 10: 1878– 1887.e4 [DOI] [PubMed] [Google Scholar]
- 33. Fineberg DB, Asselin P, Harel NY, . et al. Vertical ground reaction force-based analysis of powered exoskeleton-assisted walking in persons with motor-complete paraplegia. J Spinal Cord Med. 2013; 36 4: 313– 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fisher JA, McNelis MA, Gorgey AS, Dolbow DR, Goetz LL.. Does upper extremity training influence body composition after spinal cord injury? Aging Disabil. 2015; 6 4: 271– 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gorgey AS. Exercise awareness and barriers after spinal cord injury. World J Orthop. 2014; 5 3: 158– 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strausser KA, Kazerooni H.. The development and testing of a human machine interface for a mobile medical exoskeleton. 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS); September 25–30, 2011: San Francisco:p. 4911e6. [Google Scholar]
- 37. Strausser KA, Swift TA, Zoss AB, Kazerooni H, Bennett BC.. Mobile exoskeleton for spinal cord injury: Development and testing. Arlington: ASME; October 31–November 2, 2011; Arlington, VA : p. 419e25. [Google Scholar]
- 38. Gorgey AS, Dolbow DR, Gater DR Jr.. A model of prediction and cross-validation of fat-free mass in men with motor complete spinal cord injury. Arch Phys Med Rehabil. 2012; 93 7: 1240– 1245. [DOI] [PubMed] [Google Scholar]
- 39. Rezende LF, Sá TH, Mielke GI, Viscondi JY, Rey-López JP, Garcia LM.. All-cause mortality attributable to sitting time: Analysis of 54 countries worldwide. Am J Prev Med. 2016; 51 2: 253– 263. [DOI] [PubMed] [Google Scholar]
- 40. Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E.. Lower daily energy expenditure as measured by respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998; 68 6: 1223– 1227. [DOI] [PubMed] [Google Scholar]
- 41. Gorgey AS, Caudill C, Khalil RE.. Effects of once weekly of NMES training on knee extensors fatigue and body composition in a person with spinal cord injury. J Spinal Cord Med. 2016; 39 1: 99– 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biering-Sorensen BO, Kristenson IB, Kjaer M, Biering-Sorensen F.. Muscle after spinal cord injury. Muscle Nerve. 2009; 40 4: 499– 519. [DOI] [PubMed] [Google Scholar]
- 43. Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012; 44 1: 165– 174. [DOI] [PubMed] [Google Scholar]
- 44. del-Ama AJ, Gil-Agudo A, Pons JL, Moreno JC.. Hybrid FES-robot cooperative control of ambulatory gait rehabilitation exoskeleton. J Neuroeng Rehabil. 2014; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Del-Ama AJ, Gil-Agudo A, Pons JL, Moreno JC.. Hybrid gait training with an overground robot for people with incomplete spinal cord injury: A pilot study. Front Hum Neurosci. 2014; 13 8: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]


