Abstract
Objective: To determine if an implanted neuroprosthesis for restoration of an effective cough is less costly than conventional methods of respiratory management. Methods: Nonrandomized clinical trial of participants (N = 14) with spinal cord injury (SCI) using the Cough Stimulator device in the inpatient hospital setting for Cough Stimulator implantation and outpatient hospital or residence for follow-up. A neuroprosthesis was implanted for restoration of an effective cough. The annual costs associated with respiratory management, without (pre implantation) and with (post implantation) the neuroprosthesis, were examined over a 4-year period. Results: The total cost related to implantation of the Cough Stimulator was $59,891, with no maintenance costs over subsequent years. The incidence of respiratory tract infections and the need for caregiver support fell significantly following implantation. The costs associated with respiratory tract infections fell significantly from a mean of $36,406 ± 11,855/year to $13,284 ± 7,035/year (p < .05) pre and post implantation, respectively. Costs fell further to $8,817 ± 5,990 and $4,467 ± 4,404 following the 2nd and 3rd years post implantation (p < .05), respectively. The costs associated with caregiver support fell significantly from $25,312 ± 8,019/year to $2,630 ± 2,233/year (p < .05) pre and post implantation, respectively, and remained low in subsequent years (p < .05). Other costs related to secretion management fell significantly and remained low in subsequent years (p < .05). Break-even analysis demonstrated that this point was reached in the first year. Conclusion: The results of this investigation demonstrate that implantation and use of the Cough Stimulator resulted in significant reductions in the overall costs of respiratory management in this patient population.
Keywords: cough, economics, expiratory muscles, rehabilitation, spinal cord stimulation
The costs of care for patients with spinal cord injury (SCI) remain exceedingly high, with estimates for high tetraplegia (C1–C4) and low tetraplegia (C5–C8) greater than $180,000/year and $113,000/year in the second and subsequent years following acute injury, respectively.1 Patients surviving the first year following injury suffer from multiple complications, among which respiratory problems are the most debilitating, costly, and life-threatening. In fact, respiratory complications are the major cause of morbidity and mortality in cervical SCI and have high associated costs of health care.1,2
Following the first year of injury, Meyers et al3 found that respiratory complications were the primary reasons for hospitalization. Recent studies also demonstrate that respiratory-related issues are very common reasons for hospitalization. According to the University of Alabama-Birmingham (UAB) statistics, ~30% of individuals with SCI experience one or more hospitalizations annually, with lengths of stay averaging between 9 and 22 days.1,3–7 Moreover, management of respiratory issues necessitating hospitalization can easily exceed $50,000.8
The costs of management of respiratory-related illness is very high in the SCI population, and the development of pneumonia is associated with significant mortality.9–13 Based upon data from the National SCI Database, the complications that have the greatest impact on reduced life expectancy were pneumonia and septicemia.1 It has been estimated that persons with complete high tetraplegia have more than 150 times greater risk of death from pneumonia than the general population.14 It is likely that a major factor leading to the development of respiratory tract infections in the SCI population is expiratory muscle paralysis and the consequent lack of an effective cough.2
In previous studies, we demonstrated that the expiratory muscles can be activated using electrical stimulation techniques to restore an effective cough mechanism.15–18 Moreover, use of this method in subjects with SCI results in a significant reduction in the incidence of respiratory tract infections and need for caregiver support related to secretion management.17,18 In this study, we assessed the impact of the use of the Cough Stimulator on overall costs related to respiratory care. It is our hypothesis that, despite the substantial initial costs of the device and surgical implantation, use of this device is a cost-effective means of respiratory management in the care of patients with SCI and an impaired cough.
Methods
This study was approved by the Institutional Review Board, the National Institute of Neurological Disorders and Stroke, and the US Food and Drug Administration. Informed consent was obtained from each subject before enrollment in the study.
Each subject in this study had experienced a traumatic cervical or thoracic SCI and had significant expiratory muscle weakness secondary to expiratory muscle paresis. Each subject had undergone implantation of the Cough Stimulator to restore an effective cough (see ref. 15 for details). Subjects were instructed to apply electrical stimulation to activate their expiratory muscles every 30 seconds for 5 to 10 minutes, 2 to 3 times per day. Subjects also used the device on an as-needed basis for evacuation of secretions.
The initial cohort of study participants included 17 subjects. Two subjects died for reasons unrelated to study participation. One patient died consequent to the development of a large retroperitoneal bleed complicated by acute renal failure and sepsis, 1.9 years following implantation. Another patient died consequent to urosepsis, 2.3 years following implantation. One patient developed cellulitis over the receiver site due to improper use of the external transmitter, necessitating removal of the receiver. Each of the remaining 14 subjects used the device for more than 3 years. Following implantation, participants were seen in the clinic at monthly intervals for the first 6 months and at 3-month intervals for the next 6 months. During the second and third years, participants were seen every 6 months. The study team was in contact with the participants on a quarterly basis with phone calls during this latter period.
The incidence of acute respiratory tract infections, defined by a change in the character, color, or amount of respiratory secretions and requiring antibiotic administration, was recorded over a 2-year period prior to implantation of the Cough Stimulator. Subject history and review of the medical records, when available, were used to track the incidence of respiratory infections. Following implantation of the system, the incidence of acute respiratory tract infections was monitored continually in each subject.
The degree of caregiver support was determined as the number of times it was necessary for a caregiver to provide the subject with any form of assistance for secretion clearance including suctioning, manually assisted cough, or use of the insufflator-exsufflator device. Caregiver support was evaluated over a 2-week period prior to implantation of the Cough Stimulator and over the course of subsequent years.
The costs of management of acute respiratory tract infections requiring hospitalization was estimated by determining the average costs for hospitalization of a patient with tetraplegia and admitted to MetroHealth Medical Center with an acute respiratory tract infection, including bronchitis and pneumonia. The average length of stay was 9.1 ± 2.4 days and average charges were $61,118 ± $395. The average cost associated with management of outpatient respiratory tract infections ($405) was determined by data from recent studies.19
The costs associated with caregiver support were estimated by applying the cost of 1 hour of care (~$20/hour for licensed practical nurse) for each instance in which caregiver assistance was required for the management of secretions or other respiratory issues. Use of equipment necessary to manage respiratory secretions is presented in Table 1. The costs of the mechanical insufflator/exsufflator device and Vest System (Hill-Rom) were estimated based on monthly rental costs in the Cleveland area. The cost of the remainder of equipment, such as suction machines, was based upon purchase prices. The frequency of replacement costs of disposable materials was estimated based upon frequency of use of these materials.
Table 1.
Costs of implantation of the Cough Stimulator

Statistical analyses to compare pre-and postimplant costs were performed using analysis of variance and post hoc t test. A p value < .05 was taken as statistically significant.
Results
The demographic and clinical data of the subjects who were implanted and subsequently used the Cough Stimulator are shown in Table 2. Of the 14 subjects, 2 were women. With the exception of one subject, each had experienced a cervical SCI. Each had a poor cough as evidenced by marked reductions in maximum expiratory pressure (24.5% ± 4.3% predicted) and peak expiratory airflow generation (12.0% ± 1.5% predicted). Prior to device implantation, these subjects used a variety of methods for secretion management including assisted cough techniques, manual suctioning, and the insufflator/exsufflator device (Table 2). Following implantation of the Cough Stimulator, the subjects, with 2 exceptions, relied entirely on the use of the neuroprosthesis for secretion management. Both of these subjects used the Cough Stimulator but infrequently continued the use of the insufflator-exsufflator.
Table 2.
Clinical data of the subjects
Costs of implantation of the Cough Stimulator
The actual costs ($US dollars) associated with Cough Stimulator implantation, including presurgical evaluation (lab studies, pulmonary function tests, and radiographic studies), surgical implantation, and follow-up visits, are provided in Table 1. The major costs of the presurgical evaluation included MRI testing of the thoracic spine. Presurgical costs totaled $6,394. Equipment costs including the electrodes and stimulator totaled $9,791. Inpatient admission costs included room charges, lab studies, anesthesia services, operating room supplies, and other miscellaneous items totaling $41,932. Cost of the electrical stimulator hardware was $9,791. The cost associated with follow-up visits was $1,774. Total cost of implantation was $59,891.
Over the 3-year period of follow-up after implantation of the neuroprosthesis, there were no additional costs. More specifically, there were no receiver or electrode failures. Moreover, the external components were extremely durable; there were no needed replacement costs. The batteries were rechargeable, so they did not require replacement.
Effect of use of the Cough Stimulator on incidence of respiratory tract infections and caregiver support
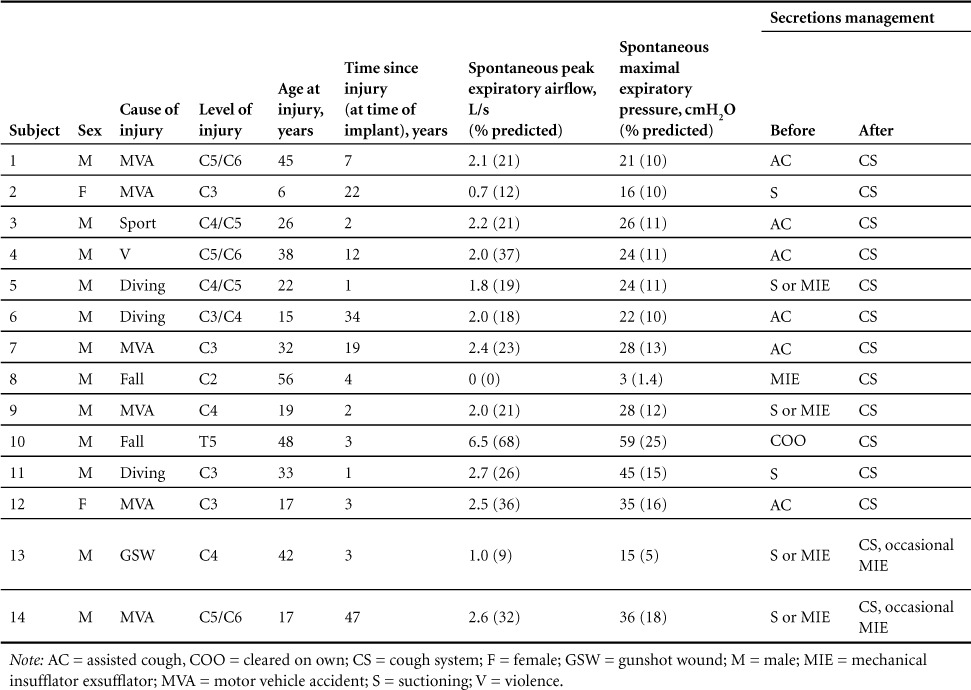
The occurrence of respiratory tract infections, both those requiring hospitalization and those managed on an outpatient basis, and their respective costs before and after device implantation is shown in Figure 1. Prior to implantation of the Cough Stimulator, there were 1.21 ± 0.26 infections/year (upper panel). The incidence of respiratory tract infections fell significantly after the first year (0.36 ± 0.17 infections/year) and remained very low when re-assessed after 2 (0.14 ± 0.10 infections/year) and 3 years (0.21 ± 0.11 infections/year) post implantation. The cost of treating these infections (lower panel) was $36,406 ± 11,855/year before device implantation and fell significantly to $13,284 ± 7,035/year after the first year and fell further to $8,817 ± 5,990/year and $4,467 ± 4,404/year at the second and third years post implantation.
Figure 1.
Incidence of respiratory tract infections pre and post implantation and use of the Cough Stimulator. The incidence of respiratory tract infections fell significantly following restoration of an effective cough (*p < .05 if compared to pre implant). See text for further explanation.
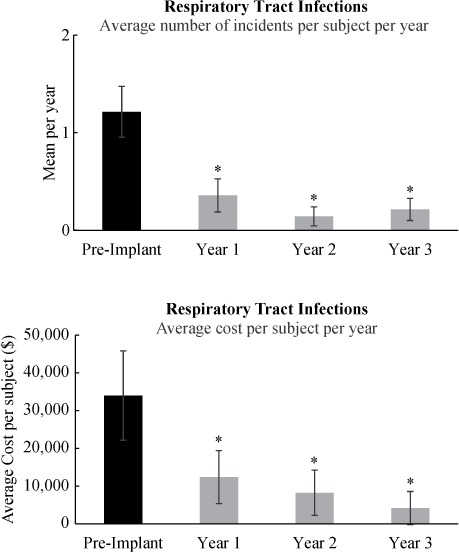
The number of times caregiver support was required for secretion management, expressed in mean number of times/week, is shown in Figure 2 (upper panel). Prior to implantation of the Cough Stimulator, all patients needed caregiver support for secretion management on average 26.8 ± 8.3 times/week. Following implantation, caregiver support for secretion management fell significantly after the first year to 2.8 ± 2.4 times/week (p < .05) and fell further by years 2 (1.6 ± 1.2 times/week) and 3 (1.5 ± 1.2 times/week) (p < .01). Twelve out of 14 subjects did not require caregiver support for secretion management following use of the Cough Stimulator. The costs associated with caregiver support before and after implantation of the Cough Stimulator are provided in Figure 2 (lower panel). Mean costs were $25,312 ± 8,019/year pre implant, they fell significantly to $2,630 ± 2,233/year (p < .01) after the first year, and they remained low in subsequent years (p < .01).
Figure 2.
Average need for caregiver support pre and post implantation and use of the Cough Stimulator. The need for caregiver support fell significantly following restoration of an effective cough (*p < .05 if compared to pre implant). See text for further explanation.
Other costs related to secretion management
In addition to caregiver support, costs encountered by subjects for secretion management included various equipment items including suction machines and insufflator-exsufflator machines and associated supplies (tubing, gloves, etc). These costs totaled of $7,201/year pre implant and fell to $1,101/year following the first and subsequent years (p < .05).
Break-even analysis
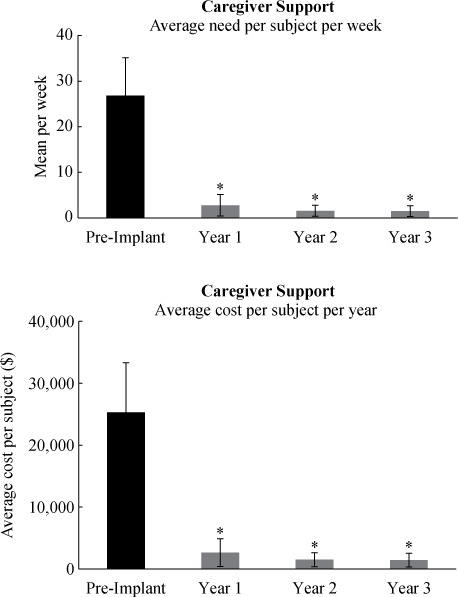
The estimated cumulative costs for secretion management in subjects with SCI (including equipment, caregiver costs, and expenses associated with management of respiratory tract infections) before and after implantation of the Cough Stimulator are shown in Figure 3. Before implantation, total mean costs were $68,919/year. After the first year post implantation, total costs were not significantly different at $76,906/year (p < .05) as the costs of implantation were offset by the large reduction in costs of secretion management and decreased incidence of respiratory infections, essentially reaching the break-even point. Substantial cost savings were realized following years 2 ($11,409/year) and 3 ($6,960/year) due to the marked reductions in the need for caregiver support and incidence of respiratory tract infections. Since the neuroprosthesis is extremely durable and requires minimal maintenance costs, it is expected that these savings will continue indefinitely, limited only by the life expectancy of these patients.
Figure 3.
Estimated costs for respiratory management pre and post implantation and use of the Cough Stimulator. The actual costs of implantation are added to the costs of respiratory management in year 1. Total costs after year 1 were not significantly different compared to total costs pre implantation due to a significant reduction in the usual costs of respiratory management. Overall costs were significantly lower in years 2 and 3 during which subjects continued to use the Cough Stimulator (*p < .05 if compared to pre implant). See text for further explanation.
Discussion
The results of this investigation demonstrate that implantation and use of the Cough Stimulator resulted in a significant reduction in the incidence of respiratory tract infections and the need for caregiver support in patients with SCI. The reduction in the incidence of respiratory tract infections was associated with a significant reduction in the need for hospitalizations and costs associated with caregiver support. As a consequence, implementation of the Cough Stimulator was associated with substantial and significant reductions in overall costs of care. In fact, the break-even point was achieved within the first year of use. Substantial cost savings were achieved in subsequent years compared to preimplant levels.
The Cough Stimulator appears to be cost effective. Benefits in addition to the reduced incidence of respiratory tract infections and need for caregiver support should also be considered in patients who may be candidates for this technique. In previous studies, we demonstrated that this technique resulted in significant improvements in life quality.17,18 Specifically, secretion management interfered less with family life and other activities. Subjects experienced reduced level of stress, less embarrassment related to use of assisted cough techniques, and reductions in their perceptions of financial difficulties related to secretion management.
Obviously, progressive cost savings are much less likely if the subject stops using the device. In this regard, we assessed long-term use of the device following the initial year of close follow-up. All subjects responded that they continued to use the device on a regular basis.18 Verifying subject history, we also found that mean maximum airway pressures were unchanged at the mean 4.6 year compared to the 1-year follow-up point. If the subjects were not using the device, the expiratory muscles would have atrophied, resulting in a reduction in pressure generation. The fact that these subjects continued to use the device on a long-term basis suggests that this device has a high degree of clinical utility.
Comparison to other neuroprotheses
The results of this study compare favorably to the economics of the implanted neuroprosthesis for bladder and bowel management.20 In a study in which the financial benefits of that system were assessed, a similar number of patients were evaluated retrospectively by interviews 9 months to 5 years following implantation. Annual costs of bladder and bowel care, with and without the prosthesis, were projected over 10 years. While clearly cost effective, the economic break-even point was found to be much longer than the Cough Stimulator, at ~5 years.
Although there are no specific investigations regarding the economics of diaphragm pacing, this neuroprosthesis is also cost effective.21–23 Following withdrawal of mechanical ventilation, many patients can be transferred to less intensive and less expensive care settings. Moreover, there is less need for nursing care, and the costs of respiratory tubing, filters, and other supplies associated with ventilators are eliminated. Like the Cough Stimulator, once in place there are minimal maintenance costs associated with use of diaphragm pacemakers.
Study limitations
The actual incidence of hospitalizations for management of respiratory issues in patients with SCI following the first year of injury is unknown. Although there are several previous investigations that have addressed this issue, they suffer from various methodological limitations including (a) reliance on remote subject surveys and therefore limited by recall bias and (b) reports that are limited to a single health care center, with (c) variable lengths of time since injury, and that (d) do not distinguish the specific causes of hospitalization or the level of SCI.4,24–28 In one study, Dryden followed 233 patients with SCI, 117 of whom had cervical injuries, over a 6-year period.29 There were a total of 196 rehospitalizations, of which ~34% were admitted with pneumonia. Other studies of patients with chronic SCI have estimated the frequency of hospitalization for respiratory-related illnesses at ~3.5% to 10%.30,31
If these data are correct, it is likely that the subjects who elected to participate in the current investigation had a higher rate of infections than those in the general SCI population. It is also likely that patients with recurrent infections were more likely to be attracted to the use of the Cough Stimulator, resulting in some selection bias. These patients were also in a better position to benefit from use of this neuroprosthesis.
In some instances, the actual occurrence of respiratory tract infections depended upon the subject's recall and therefore may have lacked precision. However, when subjects indicated that they were hospitalized for respiratory issues, hospital records were always obtained and evaluated. Moreover, there was frequent subject contact throughout the study period preventing any major inaccuracies.
An additional limitation of this study is the fact that caregiver support was monitored for only 2 weeks prior to implantation of the cough system. It is possible that this duration was not entirely reflective of their usual needs.
Future directions
We anticipate that the Cough Stimulator can be implanted using minimally invasive techniques. Based upon recent animal studies, large positive airway pressures and peak airflow rates, in the same range as those achieved with the disc electrodes, can be achieved with wire electrodes.32,33 These electrodes can be inserted with very small incisions and performed on an outpatient basis resulting in substantial cost savings, compared to the disc electrodes used in the present study. Use of minimally invasive techniques would bring the break-even point well within the first year of use by reducing costs of operating room and anesthesia services and eliminating hospital bed charges (estimated reduction of ~$20,000).
Conclusion
The neuroprosthesis to restore cough can substantially reduce the costs of respiratory management in individuals with SCI. In fact, it appears that the break-even point in terms of overall cost occurs rapidly in the first year of use. When extrapolated more broadly to eligible SCI patients worldwide, use of this device would substantially impact the overall costs of care.
It is important to emphasize that these cost savings were associated with significant improvements in life quality. Given the high incidence of respiratory complications in the SCI population, it appears likely that use of the Cough Stimulator would reduce long-term morbidity and mortality, as well.
Acknowledgments
This work was supported by the NIH-NINDS (R01NS049516), Neilson Foundation (278855), NCRR (M01RR000080 and UL1RR024989), and NCATS (UL1TR000439) and is covered by FDA IDE G980267. This investigation was approved by the Institutional Review Board of MetroHealth Medical Center (IRB98-00091). Clinical Trials Registry: NCT00116337.
Dr. DiMarco holds two United States Patents for technology related to the content of this paper: Method and Apparatus for Electrical Activation of the Expiratory Muscles to Restore Cough (5,999,855); Bipolar Spinal Cord Stimulation to Activate the Expiratory Muscles to Restore Cough (8,751,004).
REFERENCES
- 1. National Spinal Cord Injury Statistical Center , Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2015. https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202015.pdf. Accessed August 18, 2016. [Google Scholar]
- 2. Marsolais EB, Boninger ML, McCormick PC, . et al. Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals. J Spinal Cord Med. 2005; 28 3: 259– 293. [DOI] [PubMed] [Google Scholar]
- 3. Meyers AR, Feltin M, Master RJ, . et al. Rehospitalization and spinal cord injury: Cross-sectional survey of adults living independently. Arch Phys Med Rehabil. 1985; 66 10: 704– 708. [PubMed] [Google Scholar]
- 4. Cardenas DD, Hoffman JM, Kirshblum S, McKinley W.. Etiology and incidence of rehospitalization after traumatic spinal cord injury: A multicenter analysis. Arch Phys Med Rehabil. 2004; 85 11: 1757– 1763. [DOI] [PubMed] [Google Scholar]
- 5. Samsa GP, Landsman PB, Hamilton B.. Inpatient hospital utilization among veterans with traumatic spinal cord injury. Arch Phys Med Rehabil. 1996; 77 10: 1037– 1043. [DOI] [PubMed] [Google Scholar]
- 6. Middleton JW, Lim K, Taylor L, Soden R, Rutkowski S.. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord. 2004; 42 6: 359– 367. [DOI] [PubMed] [Google Scholar]
- 7. Young A, Webster B, Giunti G, Pransky G, Nesathurai S.. Rehospitalization following compensable work-related tetraplegia. Spinal Cord. 2006; 44 6: 374– 382. [DOI] [PubMed] [Google Scholar]
- 8. French DD, Campbell RR, Sabharwal S, Nelson AL, Palacios PA, Gavin-Dreschnack D.. Health care costs for patients with chronic spinal cord injury in the Veterans Health Administration. J Spinal Cord Med. 2007; 30 5: 477– 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabadi MH, Mayanna SK, Vincent AS.. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord. 2013; 51 10: 784– 788. [DOI] [PubMed] [Google Scholar]
- 10. Osterthun R, Post MW, van Asbeck FW, van Leeuwen CM, van Koppenhagen CF.. Causes of death following spinal cord injury during inpatient rehabilitation and the first five years after discharge. A Dutch cohort study. Spinal Cord. 2014; 52 6: 483– 488. [DOI] [PubMed] [Google Scholar]
- 11. DeVivo MJ, Krause JS, Lammertse DP.. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999; 80 11: 1411– 1419. [DOI] [PubMed] [Google Scholar]
- 12. Burns SP, Weaver FM, Parada JP, . et al. Management of community-acquired pneumonia in persons with spinal cord injury. Spinal Cord. 2004; 42 8: 450– 458. [DOI] [PubMed] [Google Scholar]
- 13. DeVivo MJ, Black KJ, Stover SL.. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993; 74 3: 248– 254. [PubMed] [Google Scholar]
- 14. DeVivo MJ, Stover SL.. Long-term survival and causes of death. : Stover SL, DeLisa JA, Whiteneck GG, Spinal Cord Injury: Clinical Outcomes from the Model Systems. Gaithersburg, MD: Aspen Publishers; 1995: 289– 316. [Google Scholar]
- 15. DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR.. Spinal cord stimulation: A new method to produce cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006; 173 12: 1386– 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR.. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: Results of a National Institutes of Health-sponsored clinical trial. Part I: Methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009; 90 5: 717– 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DiMarco AF, Kowalski KE, Geertman RT, . et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: Results of a National Institutes of Health-sponsored clinical trial. Part II: Clinical outcomes. Arch Phys Med Rehabil. 2009; 90 5: 726– 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT.. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med. 2014; 37 4: 380– 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monte SV, Paolini NM, Slazak EM, Schentag JJ, Paladino JA.. Costs of treating lower respiratory tract infections. Am J Manag Care. 2008; 14 4: 190– 196. [PubMed] [Google Scholar]
- 20. Creasey GH, Dahlberg JE.. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch Phys Med Rehabil. 2001; 82 11: 1520– 1525. [DOI] [PubMed] [Google Scholar]
- 21. DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT.. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002; 166 12: 1604– 1606. [DOI] [PubMed] [Google Scholar]
- 22. DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT.. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005; 127 2: 671– 678. [DOI] [PubMed] [Google Scholar]
- 23. DiMarco AF, Onders RP, Ignagni A, Kowalski KE.. Inspiratory muscle pacing in spinal cord injury: Case report and clinical commentary. J Spinal Cord Med. 2006; 29 2: 95– 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaglal SB, Munce SE, Guilcher SJ, . et al. Health system factors associated with rehospitalizations after traumatic spinal cord injury: A population-based study. Spinal Cord. 2009; 47 8: 604– 609. [DOI] [PubMed] [Google Scholar]
- 25. Franceschini M, Di Clemente B, Rampello A, Nora M, Spizzichino L.. Longitudinal outcome 6 years after spinal cord injury. Spinal Cord. 2003; 41 5: 280– 285. [DOI] [PubMed] [Google Scholar]
- 26. Charlifue S, Lammertse DP, Adkins RH.. Aging with spinal cord injury: Changes in selected health indices and life satisfaction. Arch Phys Med Rehabil. 2004; 85 11: 1848– 1853. [DOI] [PubMed] [Google Scholar]
- 27. Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG.. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998; 36 1: 45– 50. [DOI] [PubMed] [Google Scholar]
- 28. Eastwood EA, Hagglund KJ, Ragnarsson KT, Gordon WA, Marino RJ.. Medical rehabilitation length of stay and outcomes for persons with traumatic spinal cord injury – 1990–1997. Arch Phys Med Rehabil. 1999; 80 11: 1457– 1463. [DOI] [PubMed] [Google Scholar]
- 29. Dryden DM, Saunders LD, Rowe BH, . et al. Utilization of health services following spinal cord injury: A 6-year follow-up study. Spinal Cord. 2004; 42 9: 513– 525. [DOI] [PubMed] [Google Scholar]
- 30. Garshick E, Kelley A, Cohen SA, . et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005; 43 7: 408– 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ.. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999; 80 11: 1402– 1410. [DOI] [PubMed] [Google Scholar]
- 32. Kowalski KE, DiMarco AF.. Comparison of wire and disc leads to activate the expiratory muscles in dogs. J Spinal Cord Med. 2011; 34 6: 600– 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kowalski KE, Kowalski T, DiMarco AF.. Safety assessment of epidural wire electrodes for cough production in a chronic pig model of spinal cord injury. J Neurosci Methods. 2016; 268: 98– 105. [DOI] [PMC free article] [PubMed] [Google Scholar]


