Abstract
Background
Evidence indicates that systolic blood pressure (SBP) reduction may reduce hematoma expansion in patients with intracerebral hemorrhage (ICH) who are initially seen with acute hypertensive response.
Objective
To explore the relationship between different variables of SBP reduction and hematoma expansion, perihematomal edema, and 3-month outcome among patients with ICH.
Design
Post hoc analysis of a traditional phase 1 dose-escalation multicenter prospective study.
Setting
Emergency departments and intensive care units.
Patients
Patients having ICH with an elevated SBP of at least 170 mm Hg who were seen within 6 hours of symptom onset.
Intervention
Systolic blood pressure reduction using intravenous nicardipine hydrochloride targeting 3 tiers of sequentially escalating SBP reduction goals (170–199, 140–169, or 110–139 mm Hg).
Main Outcome Measures
We evaluated the effect of SBP reduction (relative to initial SBP) on the following: hematoma expansion (defined as an increased intraparenchymal hemorrhage volume >33% on 24-hour vs baseline computed tomographic [CT] images), higher perihematomal edema ratio (defined as a >40% increased ratio of edema volume to hematoma volume on 24-hour vs baseline CT images), and poor 3-month outcome (defined as a modified Rankin scale score of 4–6).
Results
Sixty patients (mean [SD] age, 62.0 [15.1] years; 34 men) were recruited (18, 20, and 22 patients in each of the 3 SBP reduction goal tiers). The median area under the curve (AUC) (calculated as the area between the hourly SBP measurements over 24 hours and the baseline SBP) was 1360 (minimum, 3643; maximum, 45) U. Comparing patients having less vs more aggressive SBP reduction based on 24-hour AUC analysis, frequencies were 32% vs 17% for hematoma expansion, 61% vs 40% for higher perihematomal edema ratio, and 46% vs 38% for poor 3-month outcome (P>.05 for all). The median SBP reductions were 54 mm Hg at 6 hours and 62 mm Hg at 6 hours from treatment initiation. Comparing patients having equal to or less vs more than the median SBP reduction at 2 hours, frequencies were 21% vs 31% for hematoma expansion, 42% vs 57% for higher perihematomal edema ratio, and 35% vs 48% for poor 3-month outcome (P>.05 for all).
Conclusions
We found no significant relationship between SBP reduction and any of the outcomes measured herein; however, the Antihypertensive Treatment of Acute Cerebral Hemorrhage study was primarily a safety study and was not powered for such end points. The consistent favorable direction of these associations supports further studies with an adequately powered randomized controlled design to evaluate the efficacy of aggressive pharmacologic SBP reduction.
Acute hypertensive response is elevation of blood pressure above normal and premorbid values that initially occurs within the first 24 hours of symptom onset in patients with intracerebral hemorrhage (ICH).1,2 In an analysis of 45 330 patients with ICH,3 75% of patients had systolic blood pressure (SBP) exceeding 140 mm Hg at presentation, and 20% had SBP exceeding 180 mm Hg. Elevated SBP is associated with hematoma expansion4 and poor outcome5; however, the cause-and-effect relationship is unknown. Systolic blood pressure reduction may reduce the rate of hematoma expansion, although conclusive evidence is unavailable.1 However, recent evidence suggests that SBP reduction may be tolerated because of reduced metabolism (hibernation)6 and preserved autoregulation7,8 in the perihematoma region.
Results of the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) suggested that early intensive blood pressure reduction seems to attenuate hematoma expansion in patients with ICH.9 Concurrently, an open-label pilot study funded by the National Institute of Neurological Disorders and Stroke was conducted to demonstrate the tolerability and safety of antihypertensive treatment goals using intravenous nicardipine hydrochloride (18–24 hours after onset) for acute hypertension response associated with spontaneous ICH as described previously.10 Briefly, 3 levels of treatment goals of increasing intensity were evaluated in a stepwise fashion. The observed proportions of the 2 primary safety end points, neurologic deterioration and serious adverse events, were below the prespecified safety thresholds, and the 3-month mortality was lower than expected (approximately 20%) in all SBP tiers.11 The detailed SBP recordings collected as part of the trial protocol allowed an opportunity to study the effect of SBP reduction (relative to initial SBP) independent of tiers on several secondary end points in the study. We determined the effect of SBP reduction on hematoma expansion, perihematomal edema, and 3-month outcome among recruited patients.10
METHODS
STUDY DESIGN
Briefly, 3 levels of treatment goals of increasing intensity were evaluated in a stepwise fashion in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) study. For the first 24 hours after symptom onset, the goal was to reduce and maintain SBP between 170 and 200 mm Hg for the first treatment tier, between 140 and 170 mm Hg for the second treatment tier, and between 110 and 140 mm Hg for the third treatment tier. The nicardipine infusion was started within 6 hours of symptom onset and continued until 24 hours after onset of ICH. Nicardipine was administered as a continuous intravenous infusion with a starting dose of 5 mg/h. The dose was then increased by 2.5 mg/h every 15 minutes as needed, up to a maximum of 15 mg/h. If the SBP fell below the specified levels, intravenous nicardipine was reduced by 2.5 mg/h every 15 minutes until the drug was discontinued.
After enrollment in the study, SBP, heart rate, transcutaneous oxygen saturation, and respiratory rate were monitored continuously. A neurologic assessment by a qualified neurologist was performed before initiation of antihypertensive treatment using the National Institutes of Health Stroke Scale score and the Glasgow Coma Scale score. Systolic blood pressure was monitored using an intra-arterial catheter or an automated cuff inflation device per the discretion of the primary physician. The frequency of measurements was as follows: every 5 minutes for the first 15 minutes if intravenous nicardipine was being initiated or adjusted, every 15 minutes if no change was made in the dose of intravenous nicardipine for the first hour, and subsequently every 15 to 30 minutes for the duration of the nicardipine infusion. More frequent measurements were recommended if prominent SBP changes were observed as determined by the treating physician. Hourly minimum and maximum SBP recordings for 18 to 24 hours after initiation of nicardipine were recorded on the case report forms.
Postdischarge follow-up was performed at a mean of 90 (±15) days after enrollment. Patients were assessed for their functional outcome using the modified Rankin scale (mRS)12 by a designated neurologist at each site who was blinded to the treatment tier.
Electronic files of the baseline and 24-hour computed tomographic (CT) images were forwarded to the core laboratory for volumetric analysis. The core laboratory neuroimaging personnel, blinded to the treatment tier and clinical findings, reviewed all CT images and recorded findings on a case report form that included the following: (1) site of hemorrhage, (2) ventricular extension by assessing CT images for the presence or absence of blood in the ventricles, (3) parenchymal hematoma volume calculated by computerized image analysis, and (4) parenchymal edema volume calculated by computerized image analysis. Images were imported into a software program (Image-Pro Express; Media Cybernetics, Silver Spring, Maryland). The regions of hemorrhage and perihematoma edema (rim of hypodensity) were identified, and their borders were approximated on the screen with electronic markers as previously described in other studies.13–16 The numbers of pixels constituting the area of hemorrhage with and without edema were determined. With the linear centimeter scale on each CT image, a calibration square was used to determine the calibration factor (pixels per square centimeter) to obtain real surface area measurements in square centimeters. The surface area was multiplied by the image section thickness (0.5–1.0 cm) to obtain a section volume. Section volumes were then summed to obtain the hematoma and perihematoma edema volume. The perihematoma edema volume was calculated by subtracting the hematoma volume from the hematoma volume plus edema volume.
STATISTICAL ANALYSIS
Baseline SBP was calculated using the mean of minimum and maximum SBPs recorded before initiation of treatment. Systolic blood pressure recordings within each hour during treatment were summarized with a mean SBP, derived from minimum and maximum hourly recordings. For each patient, the area under the curve (AUC) was estimated using the trapezoid method to provide a summary of SBP changes over the 24-hour period relative to the patient’s baseline SBP and was dichotomized at the overall median of 1360 U. The mean SBPs at 2 hours and 6 hours were used to determine SBP reduction relative to the baseline value. The change from baseline (baseline SBP minus posttreatment SBP) was dichotomized at the median values of 54 mm Hg at 2 hours and 62 mm Hg at 6 hours.
The effects of dichotomized risk variables (2-hour SBP, 6-hour SBP, and AUC) were then evaluated for the outcomes of hematoma expansion and higher perihematomal edema ratio at 24 hours and an mRS score of 4 to 6 at 3 months. Hematoma expansion was defined as increased intraparenchymal hemorrhage volume exceeding 33% on 24-hour vs baseline CT images. The cutoff for hematoma expansion is based on that defined by Brott et al,13 which is the size change that is associated with significant neurologic deterioration and exceeds the measurement variation because of differences in image acquisition. The same definition has been used for hematoma expansion in other studies.9,17 Relative edema volume was defined as absolute edema volume divided by hematoma volume, yielding a unitless ratio. The ratio has been used previously to detect serial change in edema volume, which can be obscured if absolute edema volume is used.18 Gebel et al18 also found that changes in relative edema volume over time are not contaminated by subsequent clot expansion or retraction. The percentage change from baseline of this ratio was dichotomized at the median value of 40% because no previous cutoff with prognostic validation exists. The primary clinical outcome was death or moderate to severe disability, defined as an mRS score of 4 to 6 at 3 months following treatment initiation. We chose the mRS because of its high interobserver reliability, superiority to other indexes (eg, Barthel index),19,20 and consistency with previous trials in patients with ICH. The cutoff for dichotomization was based on previous multicenter studies17,21 evaluating the effect of hematoma expansion on clinical outcome. We chose to dichotomize instead of using continuous variables because the relationship between percentage change in hematoma volume or edema volume may not have a linear relationship with clinical outcome (at high volumes because of limited intracranial compliance)22 and because variation in error terms may be higher at lower volumes. We also provided absolute change (in milliliters) in hematoma volume and edema volume at 24 hours according to strata based on SBP change.
Because the objective of this study is secondary to the goal of the ATACH study, the analyses are descriptive in nature with presentations of relative risks (RRs) and their 95% confidence intervals (CIs). All calculations were conducted using statistical software (SAS, version 9.1.3; SAS Institute, Cary, North Carolina).
RESULTS
Sixty patients (mean [SD] age, 62.0 [15.1] years; 34 men [57%]) were enrolled, with 18, 20, and 22 patients recruited in each of the 3 SBP reduction goal tiers. The demographic and clinical characteristics of the patients according to SBP reduction strata are summarized in Table 1. Forty patients were enrolled with initial SBP exceeding 200 (minimum, 171; median, 209; maximum, 300; and mean [SD], 213 [25.3]) mm Hg. Of those, 26 (62%) had SBP reduction of at least 62 mm Hg at 6 hours after symptom onset. In contrast, among patients enrolled with initial SBP not exceeding 200 mm Hg, 5 (25%) had such a reduction.
Table 1.
Demographic and Clinical Characteristics of Patients According to Systolic Blood Pressure (SBP) Reduction Strataa
| SBP Reduction at 2 hb | SBP Reduction at 6 hc | Area Under the Curved | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | ≥Median (n=30)e |
<Median (n=30) |
≥Median (n=30)e |
<Median (n=30) |
≤Median (n=30)e |
>Median (n=29)f |
| Age, mean (SD), y | 64.9 (14.9) | 58.8 (14.9) | 62.2 (15.2) | 61.8 (15.2) | 64.3 (13.9) | 59.5 (16.2) |
| Men, No. (%) | 12 (46) | 22 (65) | 18 (56) | 16 (57) | 15 (48) | 19 (66) |
| Race/ethnicity, No. (%) | ||||||
| White | 16 (62) | 15 (44) | 18 (56) | 13 (46) | 19 (61) | 12 (41) |
| African American | 9 (35) | 16 (47) | 12 (38) | 13 (46) | 11 (36) | 14 (48) |
| Initial SBP, mean (SD), mm Hg | 225.9 (27.5) | 199.9 (14.2) | 223.8 (27.9) | 202.0 (16.8) | 226.6 (26.9) | 200.2 (13.0) |
| Initial Glasgow Coma Scale score, median | 15 | 14 | 15 | 14 | 15 | 14 |
| Time from symptom onset, mean (SD), h | ||||||
| To emergency department arrival | 1.7 (1.4) | 1.9 (1.5) | 1.8 (1.5) | 1.7 (1.4) | 1.8 (1.6) | 1.7 (1.2) |
| To treatment initiation | 4.4 (1.7) | 3.9 (1.7) | 4.4 (1.8) | 4.0 (1.6) | 4.5 (1.9) | 3.8 (1.4) |
| Treated within 3 h of symptom onset, No. (%) | 7 (27) | 13 (38) | 11 (34) | 9 (32) | 9 (29) | 11 (38) |
| Oral antihypertensive medications | ||||||
| Previous use, No. (%) | 13 (65) | 18 (69) | 18 (69) | 13 (65) | 18 (75) | 13 (59) |
| Compliant use, No./total No. (%) | 4/13 (31) | 7/18 (39) | 6/18 (33) | 5/13 (39) | 6/18 (33) | 5/13 (39) |
| Diabetes mellitus, No. (%) | 6 (24) | 4 (12) | 5 (17) | 5 (18) | 6 (21) | 4 (14) |
| Hyperlipidemia, No. (%) | 7 (35) | 4 (16) | 7 (32) | 4 (18) | 7 (29) | 4 (19) |
| Initial hematoma volume, mean (SD), mL | 15.6 (17.5) | 11.4 (9.5) | 14.4 (17.6) | 12.7 (10.0) | 14.4 (15.7) | 12.6 (9.2) |
| Initial edema volume, mean (SD), mL | 9.1 (14.2) | 6.5 (5.7) | 7.9 (13.6) | 7.7 (6.9) | 7.2 (7.6) | 8.4 (13.6) |
| Nicardipine hydrochloride, mean (SD) | ||||||
| Duration of infusion, h | 39.5 (36.4) | 23.6 (21.4) | 42.5 (37.9) | 31.3 (16.6) | 42.8 (35.1) | 20.2 (20.4) |
| Dose, mg/h | 7.4 (4.8) | 7.5 (4.2) | 7.7 (4.6) | 7.1 (4.4) | 7.9 (4.8) | 6.8 (4.0) |
| Neurologic deterioration within 24 h, No. (%) | 6 (23) | 5 (15) | 8 (25) | 3 (11) | 8 (26) | 3 (10) |
| Absolute hematoma volume change, mean (SD), mL | 2.4 (17.5) | 4.6 (14.8) | 2.9 (15.9) | 4.0 (16.7) | 0.7 (16.1) | 6.4 (16.0) |
| Hematoma expansion, No. (%) | ||||||
| Symptomatic | 2 (8) | 3 (9) | 4 (13) | 1 (4) | 3 (10) | 2 (7) |
| Asymptomatic | 3 (12) | 8 (24) | 3 (9) | 8 (29) | 3 (10) | 8 (28) |
| Edema volume change, mean (SD) | ||||||
| Absolute, mL | 7.8 (20.2) | 11.2 (20.5) | 7.7 (19.1) | 11.3 (21.5) | 6.0 (19.2) | 12.8 (21.0) |
| Relative | 0.3 (0.8) | 0.5 (0.5) | 0.3 (0.4) | 0.5 (0.9) | 0.2 (0.4) | 0.6 (0.8) |
| Mortality, No. (%) | ||||||
| In hospital | 1 (4) | 3 (9) | 2 (6) | 2 (7) | 1 (3) | 3 (10) |
| 3 mo | 5 (19) | 5 (15) | 3 (9) | 7 (25) | 5 (16) | 5 (17) |
The percentages are the number of the population above or below the median; some values were missing from the variables.
Median reduction was 54 mm Hg.
Median reduction was 62 mm Hg.
Median area under the curve was 1360.
Indicates greater SBP reduction from baseline.
One subject had insufficient SBP values to estimate the 24-hour area under the curve.
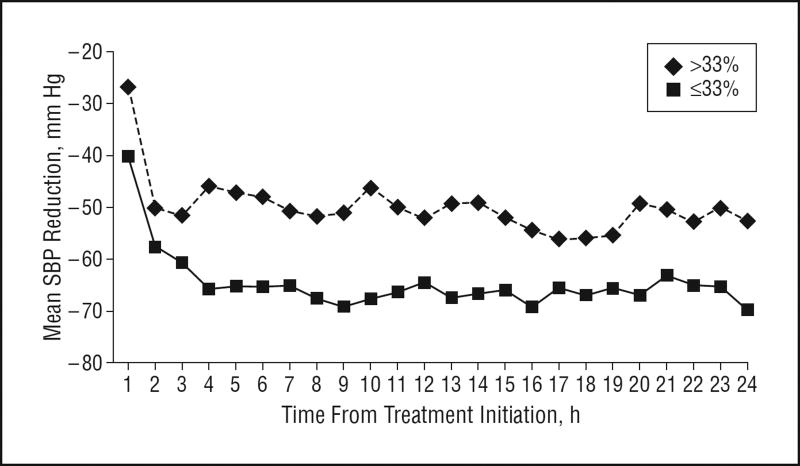
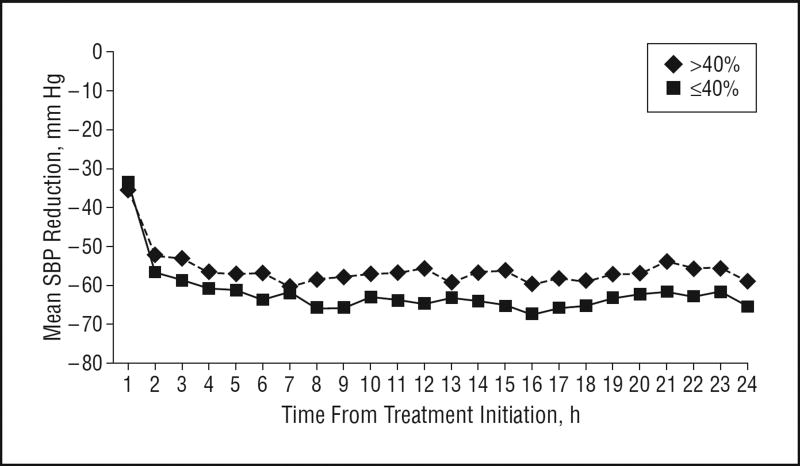
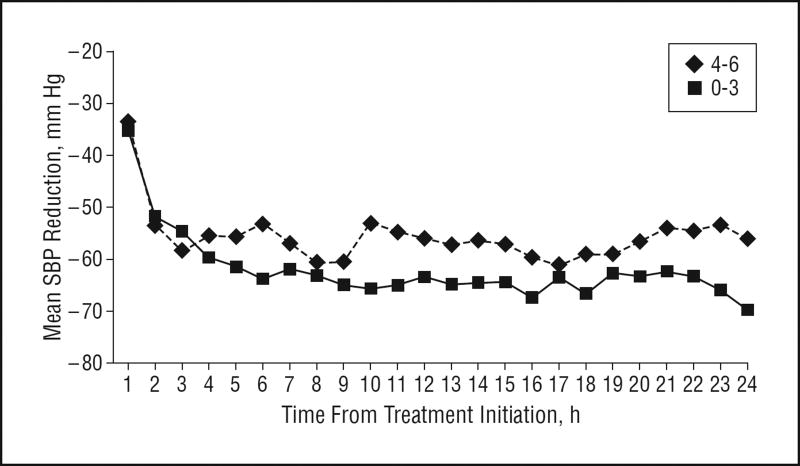
Overall, the percentage change in hematoma volume ranged from −98.2% to 1515.8% (median, 3.3%); the percentage change in relative edema volume ranged from −58.9% to 582.4% (median, 40%). Of 60 patients, 8 were missing the 3-month mRS score. The score distribution among the remaining 52 patients was 0 (2 patients [4%]), 1 (11 patients [21%]), 2 (12 patients [23%]), 3 (5 patients [10%]), 4 (8 patients [15%]), 5 (4 patients [8%]), and 6 (10 patients [19%]). Figures 1, 2, and 3 show the differences in SBP change among patients according to hematoma expansion, higher perihematomal edema ratio, and poor 3-month outcome. Throughout the 24-hour treatment period, SBP reduction was more prominent in patients without hematoma expansion and was less prominent in patients with an mRS score of 4 to 6.
Figure 1.
Mean systolic blood pressure (SBP) reduction over 24 hours according to hematoma expansion (≤33% vs >33%).
Figure 2.
Mean systolic blood pressure (SBP) reduction over 24 hours according to higher perihematomal edema ratio (≤40% vs >40%).
Figure 3.
Mean systolic blood pressure (SBP) reduction over 24 hours according to poor 3-month outcome (modified Rankin scale score of 0–3 vs 4–6).
Table 2 gives the magnitude of the relationship between different variables of SBP reduction and the outcome measures. The median AUC was 1360 (minimum, 45; maximum, 3643) U. Comparing patients having more vs less aggressive SBP reduction based on 3-hour AUC analysis, frequencies were 32% vs 17% for hematoma expansion, 61% vs 40% for higher perihematomal edema ratio, and 46% vs 38% for poor 3-month outcome (P>.05 for all). Comparing patients having less vs more than the median SBP reduction at 6 hours, frequencies were 21% vs 31% for hematoma expansion, 42% vs 57% for higher perihematomal edema ratio, and 35% vs 48% for poor 3-month outcome (P>.05 for all). Absolute increase in hematoma volume at 24 hours was nonsignificantly higher (Table 1) among patients having less aggressive SBP reduction based on AUC analysis (mean, 6.4 mL) compared with those having more aggressive SBP reduction (0.7 mL). Similarly, absolute increase in edema volume at 24 hours was higher among patients having less aggressive SBP reduction (6.0 vs 12.8 mL). There were absolute risk reductions in poor 3-month outcome of 22% (RR, 0.61; 95% CI, 0.33–1.16) among patients who did not have hematoma expansion and 12% (RR, 0.74; 0.35–1.54) among patients who did not have higher perihematomal edema ratio.
Table 2.
Relationship Between Systolic Blood Pressure (SBP) Reduction and Outcome Measures
| SBP Reduction at 2 ha | SBP Reduction at 6 hb | Area Under the Curvec | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| No./Total No. (%) | No./Total No. (%) | No./Total No. (%) | |||||||
|
|
|
|
|||||||
| Variable | ≥Mediand | <Median | RR (95% CI) | ≥Mediand | <Median | RR (95% CI) | ≤Mediand | >Median | RR (95% CI) |
| Hematoma expansion | 7/29 (24) | 8/29 (28) | 0.88 (0.37–2.10) | 6/29 (21) | 9/29 (31) | 0.67 (0.27–1.63) | 5/29 (17) | 9/28 (32) | 0.54 (0.21–1.40) |
| Higher perihematomal edema ratio | 10/26 (38) | 17/28 (61) | 0.63 (0.36–1.12) | 11/26 (42) | 16/28 (57) | 0.74 (0.43–1.29) | 10/25 (40) | 17/28 (61) | 0.66 (0.37–1.16) |
| Poor 3-month outcome | 11/26 (42) | 11/27 (41) | 1.04 (0.55–1.97) | 9/26 (35) | 13/27 (48) | 0.72 (0.37–1.39) | 10/26 (38) | 12/26 (46) | 1.20 (0.63–2.27) |
Abbreviations: CI, confidence interval; RR, relative risk.
Median SBP reduction was 54 mm Hg.
Median SBP reduction was 62 mm Hg.
Median area under the curve was 1360h.
Indicates greater SBP reduction from baseline.
Twenty patients were treated within 3.1 hours of symptom onset. They had the same or lower frequencies of hematoma expansion (26%), higher perihematomal edema ratio (33%), and poor 3-month outcome (27%) compared with those who were treated later (26%, 58%, and 47%, respectively). The sample size of 20 patients was too small to obtain RRs with reasonable CIs; however, the trend was similar to that of the entire cohort, with more pronounced risk reduction with greater SBP reduction (data not shown).
COMMENT
Previous studies4,5 of ICH have been based predominantly on initial recording, and because SBP treatment and targets were heterogeneous, considerable variability may have occurred that is not represented by the SBP values analyzed in those studies. The multicenter ATACH study prospectively recruited patients within a well-defined time frame and collected SBP data systematically throughout the study. However, because the primary trial objective was tolerability and safety, the analysis was retrospective. In the post hoc ATACH analysis, we generally found that greater SBP reduction at all time points within 24 hours of symptom onset was associated with reduction in hematoma expansion and lower rates of death and disability. The cause-and-effect relationship between SBP measures and the study end points is unclear.
It is possible that patients having hematoma expansion had been seen earlier and had smaller hematomas, while patients who were seen later had already had expansion before presentation and had larger baseline hematomas. The differential SBP change may be related to variation in baseline hematoma volume and to time to presentation, both of which affect hematoma expansion,23,24 However, initial SBP, baseline hematoma volume, and time between symptom onset and emergency department arrival were similar between strata defined by SBP change.
An alternative interpretation of the results is that persistent SBP elevation is a consequence of hematoma expansion and increasing mass effect. The differences in SBP change between the 2 strata defining the presence or absence of hematoma expansion (Figure 1) and death and disability (Figure 3) are obvious at an early stage, presumably preceding the end points under consideration. Four of 7 symptomatic hematoma expansions in the ATACH study11 occurred after 12 hours. Furthermore, in previous experimental25,26 and clinical27 studies, SBP increase during ICH or transtentorial herniation is transient and small. However, definite evidence to identify a causal relationship between SBP change and our study end points is unavailable.
Findings in previous case series have suggested that a high proportion of patients with ICH who experience hematoma expansion have poorly controlled SBP elevation. Chen et al28 reported persistent hypertension in 6 of 8 patients before hematoma expansion. An SBP of at least 195 mm Hg was recorded during the first 6 hours after symptom onset in 5 of 6 patients with hematoma expansion described by Broderick et al.23 In 186 patients with ICH, Kazui et al4 identified several factors associated with hematoma expansion, including history of brain infarction, liver disease, SBP on admission of at least 200 mm Hg, and fasting plasma glucose level of at least 141 mg/dL (to convert glucose level to millimoles per liter, multiply by 0.0555). Maruishi et al29 investigated the effects of serial SBP changes among 57 patients admitted within 6 hours of symptom onset of ICH and found that patients with hematoma expansion were significantly more likely to have increased SBP. Results of other studies have suggested an inconsistent relationship between SBP and hematoma expansion. In a post hoc analysis of 65 patients evaluated within 3 hours of symptom onset of ICH30 and not undergoing surgery, peak SBP was higher among those with (205 mm Hg) vs without (198 mm Hg) hematoma expansion. In the multivariate analysis, peak SBP demonstrated a trend (P=.18) to association with hematoma expansion, but the relationship did not achieve statistical significance. In an exploratory analysis from a randomized study24 of recombinant factor VII among 382 patients with ICH, elevated SBP of at least 170 mm Hg was associated with hematoma expansion (P=.08) in univariate analysis. In multivariate analysis, treatment with recombinant factor VII and longer time from symptom onset to baseline CT image were related to decreased hematoma expansion. Systolic blood pressure did not achieve statistical significance.
In April 2007, INTERACT (Trial Registration: clinicaltrials.gov Identifier: NCT00226096) completed recruitment, randomizing 404 patients within 6 hours of ICH symptom onset.9 INTERACT randomized patients with ICH and elevated SBP (150 to <220 mm Hg) to intensive SBP management (goal SBP, <140 mm Hg) or to American Heart Association guideline–based SBP management (goal SBP, <180 mm Hg)31,32 using available antihypertensive agents. Compared with patients randomized to American Heart Association guideline–based SBP management, patients randomized to intensive SBP management had a mean 14-mm Hg lower SBP for the first hour and a mean 10-mm Hg lower SBP for 1 to 24 hours. The rates of hematoma expansion (>33% increase at 24 hours or 12.5 mL) were 15% among patients randomized to intensive SBP management and 23% among patients randomized to American Heart Association guideline–based SBP management (P=.05); the mean proportional increases were 14% and 36%, respectively (P=.06). Patients recruited within 3 hours of symptom onset and patients with initial SBP of at least 181 mm Hg seemed to benefit most from intensive SBP reduction. No significant difference in 3-month mortality was observed between the groups, although the study was not powered to demonstrate a difference in rates of clinical outcomes.
We explored the possibility that increased perihematomal edema contributed to our observed association between lack of SBP reduction and poor outcome. A prospective study33 of 240 consecutive patients within 3 hours of symptom onset of first-ever ischemic stroke (n=190) or ICH (n=50) demonstrated a relationship between high SBP and cerebral edema. Patients underwent CT within 24 hours of stroke onset and 5 days later to determine the presence of brain edema. Edema was defined as midline shift, sulcal effacement, or ventricular compression in patients with acute ischemic stroke or as a hypodense area around the hematoma accompanied by mass effect in patients with ICH.
The main outcome measure, brain edema formation, was present in 78 patients (33%). The 24-hour SBPs were significantly higher in patients with brain edema. In multivariate analysis, the 24-hour SBPs remained significantly associated with brain edema after adjusting for other covariates. The prognostic value of relative perihematomal edema volume is controversial. Gebel et al34 found that higher relative perihematomal edema volumes at baseline or 20 hours after symptom onset of ICH were associated with better functional outcome at 3 months. However, there is some evidence that higher perihematomal edema volume is associated with greater mortality in patients with ICH.14
In conclusion, in a post hoc analysis of the ATACH study, we observed a nonsignificant relationship between magnitude of SBP reduction and hematoma expansion and 3-month outcome. The consistent favorable direction of these associations supports further studies with an adequately powered randomized controlled design to evaluate the efficacy of aggressive pharmacologic SBP reduction.
Acknowledgments
Financial Disclosure: Dr Palesch receives funding from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Funding/Support: This study was supported by grant RO-1-NS44976-01A2 from the National Institute of Neurological Diseases and Stroke.
Antihypertensive Treatment of Acute Cerebral Hemorrhage Study Investigators
Principal Investigators and Coordinators
Adnan I. Qureshi, MD, principal investigator; Haitham H. Hussein, MD, and Nauman Tariq, MD, imaging analysis; Jill Novitzke, RN, BSN, and Haitham H. Hussein, MD, study coordinators; Yuko Y. Palesch, PhD, Reneé Martin, PhD, and Yuan Liu, PhD, statisticians; and Catherine Dillon, data manager.
Chinekwu Anyanwu, MD; Betty Brown, RN; Salvador Cruz-Flores, MD; Kelly Dickerson, RN; As’ad Ehtisham, MD; Mustapha A. Ezzeddine, MD; Eliahu Feen, MD; Joshua N. Goldstein, MD, PhD; Pansy Harris-Lane, RN; Eve Holzemer, RN; Hoda Jradi, MS; Jawad F. Kirmani, MD; Kiwon Lee, MD; Stephan A. Mayer, MD; Ibrahim Mohammad, MD; Yousef M. Mohammad, MD; Christopher Murphy, RN; Noeleen Ostapkovich, RN; Spozhmy Panezai, MD; Nikolaos I. H. Papamitsakis, MD; Augusto Parra, MD; Warren Selman, MD; Jose I. Suarez, MD; Gene Sung, MD; M. Fareed K. Suri, MD; John Terry, MD; Vangie Thomson, RN; and Lauren Wendell.
Coordinating Centers
University of Minnesota, Minneapolis (clinical coordinating center); and Medical University of South Carolina, Charleston (statistical and data coordinating center).
Hospitals and Centers
Boston: Massachusetts General Hospital; Cleveland, OH: Case Western Reserve University; Columbus: Ohio State University; Edison, NJ: JFK Medical Center; Kansas City: Kansas University Medical Center; Newark: University of Medicine and Dentistry of New Jersey; New York, NY: Columbia University Medical Center; Los Angeles: University of Southern California; Saint Louis, MO: Saint Louis University; and Wichita, KS: Via Christi Regional Medical Center.
Footnotes
Author Contributions: Study concept and design: Qureshi and Palesch. Acquisition of data: Qureshi, Novitzke, Cruz-Flores, Ezzeddine, Ehtisham, Goldstein, Hussein, Suri, and Tariq. Analysis and interpretation of data: Qureshi, Palesch, Martin, Cruz-Flores, and Ezzeddine. Drafting of the manuscript: Qureshi, Palesch, Novitzke, and Tariq. Critical revision of the manuscript for important intellectual content: Qureshi, Palesch, Martin, Cruz-Flores, Ezzeddine, Ehtisham, Goldstein, and Suri. Statistical analysis: Palesch and Martin. Obtained funding: Qureshi. Administrative, technical, and material support: Qureshi, Novitzke, Ehtisham, and Goldstein. Study supervision: Palesch, Ezzeddine, and Ehtisham. Image analysis: Goldstein and Tariq.
References
- 1.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118(2):176–187. doi: 10.1161/CIRCULATIONAHA.107.723874. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25(1):32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28(12):2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 5.Dandapani BK, Suzuki S, Kelley RE, Reyes-Iglesias Y, Duncan RC. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995;26(1):21–24. doi: 10.1161/01.str.26.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AI, Hanel RA, Kirmani JF, Yahia AM, Hopkins LN. Cerebral blood flow changes associated with intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13(3):355–370. doi: 10.1016/s1042-3680(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 7.Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57(1):18–24. doi: 10.1212/wnl.57.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52(2):266–272. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Anderson CS, Huang Y, Wang G, et al. INTERACT Investigators. Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6(1):56–66. doi: 10.1385/ncc:6:1:56. [DOI] [PubMed] [Google Scholar]
- 11.Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38(2):637–648. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 14.McCarron MO, Hoffmann KL, DeLong DM, Gray L, Saunders AM, Alberts MJ. Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology. 1999;53(9):2176–2179. doi: 10.1212/wnl.53.9.2176. [DOI] [PubMed] [Google Scholar]
- 15.McCarron MO, McCarron P, Alberts MJ. Location characteristics of early perihaematomal oedema. J Neurol Neurosurg Psychiatry. 2006;77(3):378–380. doi: 10.1136/jnnp.2005.070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman RD, Maldjian JA, Brun NC, Horvath B, Skolnick BE. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by CT scan. AJNR Am J Neuroradiol. 2006;27(3):666–670. [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SM, Broderick J, Hennerici M, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 18.Gebel JM, Jr, Jauch EC, Brott TG, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33(11):2631–2635. doi: 10.1161/01.str.0000035284.12699.84. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke. 1991;22(10):1242–1244. doi: 10.1161/01.str.22.10.1242. [DOI] [PubMed] [Google Scholar]
- 21.Mayer SA, Brun NC, Begtrup K, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 22.Schettini A, Walsh EK. Contribution of brain distortion and displacement to CSF dynamics in experimental brain compression. Am J Physiol. 1991;260(1, pt 2):R172–R178. doi: 10.1152/ajpregu.1991.260.1.R172. [DOI] [PubMed] [Google Scholar]
- 23.Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72(2):195–199. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- 24.Broderick JP, Diringer MN, Hill MD, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38(3):1072–1075. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 1999;27(5):965–971. doi: 10.1097/00003246-199905000-00036. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Wilson DA, Traystman RJ. Treatment of transtentorial herniation unresponsive to hyperventilation using hypertonic saline in dogs: effect on cerebral blood flow and metabolism. J Neurosurg Anesthesiol. 2002;14(1):22–30. doi: 10.1097/00008506-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi AI, Geocadin RG, Suarez JI, Ulatowski JA. Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28(5):1556–1564. doi: 10.1097/00003246-200005000-00049. [DOI] [PubMed] [Google Scholar]
- 28.Chen ST, Chen SD, Hsu CY, Hogan EL. Progression of hypertensive intracerebral hemorrhage. Neurology. 1989;39(11):1509–1514. doi: 10.1212/wnl.39.11.1509. [DOI] [PubMed] [Google Scholar]
- 29.Maruishi M, Shima T, Okada Y, Nishida M, Yamane K. Involvement of fluctuating high blood pressure in the enlargement of spontaneous intracerebral hematoma. Neurol Med Chir (Tokyo) 2001;41(6):300–305. doi: 10.2176/nmc.41.300. [DOI] [PubMed] [Google Scholar]
- 30.Jauch EC, Lindsell CJ, Adeoye O, et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke. 2006;37(8):2061–2065. doi: 10.1161/01.STR.0000229878.93759.a2. [DOI] [PubMed] [Google Scholar]
- 31.Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30(4):905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AI, Harris-Lane P, Kirmani JF, et al. Treatment of acute hypertension in patients with intracerebral hemorrhage using American Heart Association guidelines. Crit Care Med. 2006;34(7):1975–1980. doi: 10.1097/01.CCM.0000220763.85974.E8. [DOI] [PubMed] [Google Scholar]
- 33.Vemmos KN, Tsivgoulis G, Spengos K, et al. Association between 24-h blood pressure monitoring variables and brain oedema in patients with hyperacute stroke. J Hypertens. 2003;21(11):2167–2173. doi: 10.1097/00004872-200311000-00027. [DOI] [PubMed] [Google Scholar]
- 34.Gebel JM, Jr, Jauch EC, Brott TG, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33(11):2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]